INTRODUCTION
Sleep disorders are common in the elderly. It is estimated that 40-70% of elderly experience chronic sleep problems. Higher rates of sleep problems are observed in those elderly with medical and psychiatric comorbidity. The comorbid disorders have additive effects on sleep disturbances, i.e. higher the number of comorbidities, higher the rates of sleep problems.
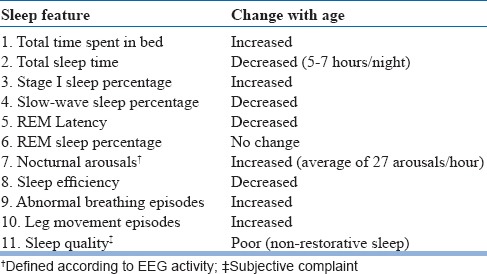
Elderly subjects have initial insomnia, wake up earlier than usual, have higher time spent in bed, have nighttime awakenings, nap more, and have decreased total sleep as compared to younger adults. With increasing age lighter stages of sleep becomes more, whereas, REM and slow-wave sleep reduce; up to 6 years of age there is 2% decrease in slow-wave sleep every decade. Slow-wave sleep does not change much from 60 to 90 years of age. However, sleep efficiency, i.e. the duration of sleep relative to total time in bed, continue to decrease over time. Sleep in the elderly is fragmented, lighter and is characterized by episodes of arousals and awakenings.
Certain changes in circadian physiology occur with age. They include sleeping before the desirable sleep and wake times, earlier clock hour of internal circadian time, alteration in the relationship between the internal circadian time and sleep, reduction in circadian amplitude, and reduced sensitivity to low to moderate light levels. Among the elderly, core body temperature, and cortisol and melatonin rhythms occur at an earlier time. The changes in sleep that occur with age are summarized in Table 1. However, these changes are not the common sleep complaints of the elderly population.
Table 1.
Sleep changes in the elderly
Indian Psychiatric Society (IPS) published Clinical Practice Guideline (CPG) for management of sleep disorders, for the first time in the year 2006 and a revised version in the year 2017. The current version of the CPG is an update of the earlier versions which specifically focuses on management of sleep disorders in the elderly. The current version of the CPG for sleep disorders in elderly must be read in conjunction with the previous versions of CPG for sleep disorders in the adult population. These guidelines provide a broad framework for assessment and management of sleep problems in elderly patients. Most of the recommendations made as part of the guidelines are evidence-based. However, these guidelines should not be considered as a substitute for professional knowledge and clinical judgment. The recommendations made as part of these guidelines are best available options that have to be modified as per the clinical needs of the individual patient and the treatment setting.
SLEEP DISORDERS IN ELDERLY
Insomnia and daytime sleepiness are the common sleep complaints in older individuals (table 2). Among the elderly, the prevalence of initial insomnia are found in 15%–45%, middle insomnia in 20%–65%, late insomnia in 15%–54%, and 10% have poor sleep quality. Those having sleep complaints report poor quality of life and higher rates of depression and anxiety. Cognitive deficits can appear because of chronic sleep problems in the elderly that include attentional impairment, difficulties in short-term memory, increase inresponse time and decreased performance. Furthermore, sleep problems increase mortality in older individuals; lower sleep efficiency (<80%) almost doubles the risk of all-cause mortality. The common primary sleep disorders thatare seen in older adults include chronic insomnia disorder (CID), sleep-disordered breathing (SDB), restless legs syndrome(RLS)/periodic limb movements in sleep (PLMS), REM sleep behavior disorder (RBD) and advanced sleep-wake phase disorder (ASWPD).
Table 2.
Common sleep complaints in the elderly

CHRONIC INSOMNIA DISORDER
Insomnia is commonly defined as recurring problems in sleep initiation (sleep onset insomnia) or maintaining asleep (sleep maintenance insomnia). The former is also called initial insomnia, and the later can be either middle or terminal insomnia. Without distinguishing between primary and secondary, ICSD-3 identifies insomnia disorder, that can be divided on the basis of its duration into transient or short-term (lasting only a few days to three to four weeks) or chronic (persisting for more thanthree months). Clinically (as well as using polysomnographic criteria) insomnia is defined as a sleep latency of more than 30 minutes, remaining awake after sleep onset formore than 30 minutes, sleep efficiency of less than 85%, or less than 6 to 6.5 hours total time asleep, occurring on three nights per week or more. Complaints of only non-restorative sleep are no longer sufficient to diagnose insomnia disorder. The diagnostic criteria for chronic insomnia disorder are in table 3.
Table 3.
Diagnostic criteria for chronic insomnia disorder

Insomnia is the most common sleep complaint in the elderly. Insomnia is seen in 20 to 40% of older adults as compared with 9% to 15% in the general adult population. Sleep maintenance insomnia is more common in elderly than sleep onset insomnia. Insomnia disorder leads to fatigue, mood changes, poor cognition, daytime sleepiness and impairment in functioning. Other consequences of untreated insomnia disorder include increased chances of developing mental illness, accidents, poorer quality of life, and greater healthcare utilization.
Assessment and Evaluation
In the diagnostic evaluation of patients presenting with insomnia, detailed sleep, medical and psychiatric history is essential. Detailed information has to be obtained regarding the initiation of symptoms, its course, and progression. Also, information from the bedpartner is invaluable in arriving at the correct diagnosis. There are several conditions that can be mistaken for insomnia and need to be ruled out before making a diagnosis of insomnia (table 4). Insomnia is associated with several psychiatric disorders (table 5) and medical disorders (table 6) in the elderly. It is pertinent to ask about the prescription as well as non-prescription medications that a person is taking several classes of medications are associated with insomnia (table 7). Sleep log or diary is a primary subjective method which gives nightly information on perceived sleep patterns and quality. It not only aids in the assessment of insomnia but also in monitoring treatment outcomes. Simple forms of sleep diaries may only include information on how much time a person spends in bed, approximate durations of sleeping and waking time, and estimation of sleep efficiency. While starting therapy, sleep diaries are typically filled two weeks prior and continued during treatment and follow up period. An algorithm for the diagnosis of insomnia disorder is presented in figure 1.
Table 4.
Conditions are mistaken for insomnia
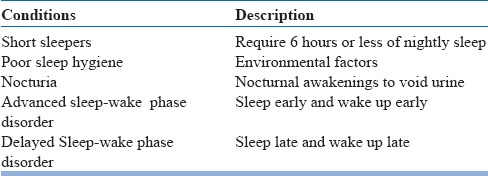
Table 5.
Insomnia in psychiatric disorders
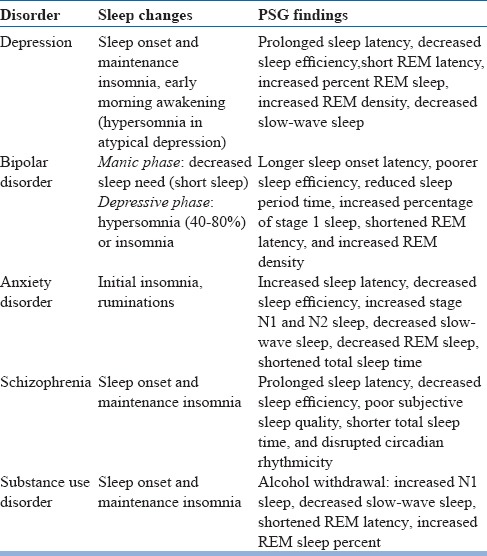
Table 6.
Medical conditions associated with insomnia in elderly
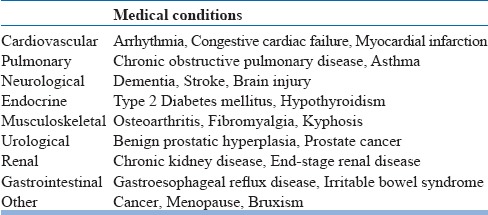
Table 7.
Medications associated with insomnia
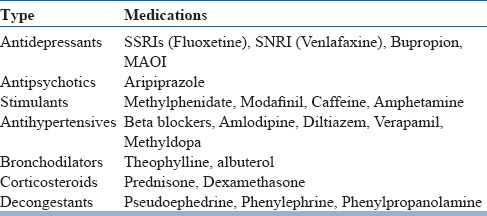
Figure 1.
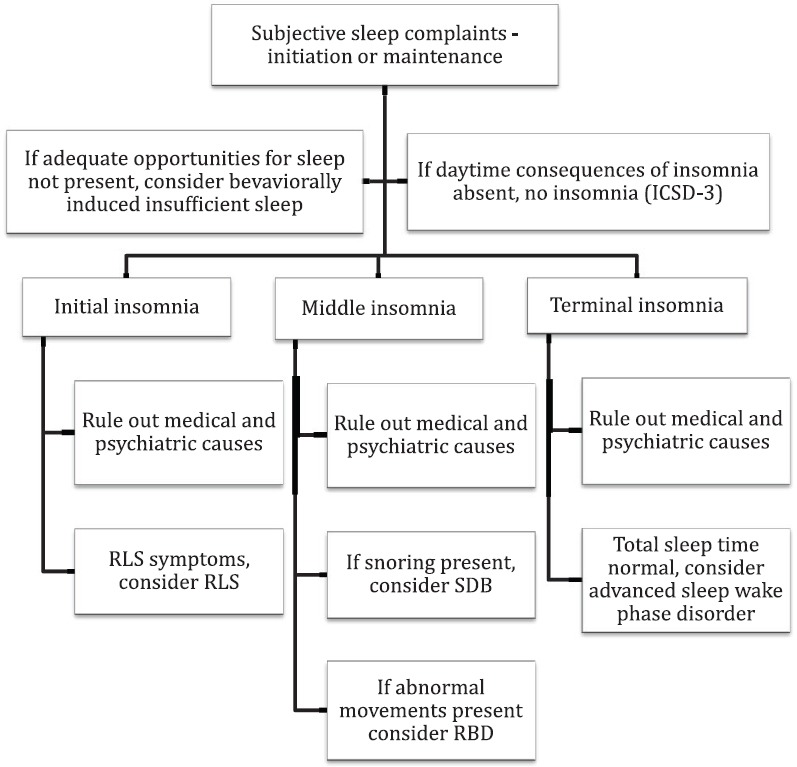
Algorithm for diagnosis of insomnia disorder
There are specific scales and other rating instruments available for assessment of sleep and insomnia symptoms (table 8). These instruments aid in the evaluation of patients with insomnia and may be used in addition to history and examination. Objective methods such as polysomnography (PSG) and actigraphy are used in special laboratories for measuring sleep initiation and maintenance variables. PSG is not indicated routinely in the evaluation of insomnia but may be used to rule out other sleep disorders or in non-responders (i.e. when patients do not respond to the first-line medications). Actigraphy is a small device that is worn as a wristwatch that records movement frequency and is a reliable and valid tool for assessing sleep duration.
Table 8.
Objective assessment scales in insomnia
Non-pharmacological management
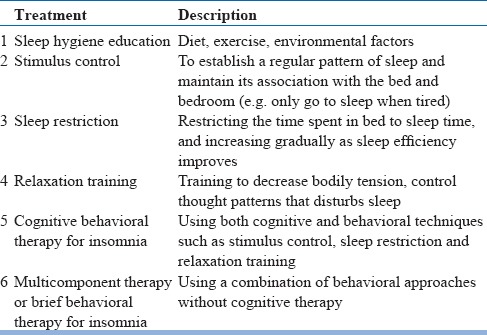
The initial treatment for insomnia in the elderly should include non-pharmacological approaches. Several treatment options for the older individuals are available (see Table 9).
Table 9.
Non-pharmacological treatment for chronic insomnia disorder
Sleep hygiene education can be used which includes certain lifestyle changes such as diet control, regular exercise and reducing stimulant and alcohol use, in addition to controlling environmental factors such as ambient noise, light, and temperature that may disturb sleep. Also, it is advised to avoid frequent daytime napping, late evening exercises, and late heavy dinner. A regular exercise regimen and adequate light exposure during daytime have been found useful forinsomnia in elderly.
Stimulus Control Therapy: The theory behind stimulus control treatment is maladaptive conditioning between the bedroom environment and bedtime with behaviors that are incompatible with sleep. These behaviors include worrying, reading, using a smartphone or watching TV while in bed. Stimulus control aims at reducing the association between these maladaptive behaviors which maintain arousal during bedtime and increasing the association between sleep and sleep-promoting stimuli. This is achieved by restricting the time one is awake in bed by reducing the sleep-interfering activities, and by maintaining a regular sleep-wake schedule. Instructions to patients for stimulus control include:1) Lie on the bed only when feeling sleepy, 2) Avoid any activity that keeps you awake in the bedroom, other than sex, 3) Sleep only on bed in the bedroom, and not on other places such as sofa, 4) To leave the bedroom soon after waking, 5) To come to bedroom only when feel sleepy, 6) Keep the waking up time fixed, irrespective of the hours of sleep during night, and 7) Avoid napping during day.
Sleep Restriction: It includes reducing the duration spent in bed to the actual time asleep, thus creating sleep deprivation and subsequent increase in sleep drive. Before starting sleep restriction therapy, a sleep log for 2 weeks is maintained which gives an estimate of average sleep time versus actual time in bed, i.e. sleep efficiency (SE). The allowed sleep time is the average subjective sleep time but is never less than 5 hours. As SE improves (more than 90%), time in bed is increasedby 15-minute increments until adequate sleep duration is attained.
Relaxation therapy and imagery: Thoughts can be detrimental to sleep and anxiety may cause sleep onset insomnia. Relaxation training originally used to alleviate anxiety is used for the treatment of sleep onset insomnia. Several techniques have been used in the treatment of insomnia that includes 1) Progressive muscle relaxation, 2) Autogenic training (induction of sensations of warmth and heaviness are used to promote somatic relaxation), 3) Imagery (pleasant imagery can be used along with relaxation to improve sleep). Individuals must practice the chosen technique at least twice a day, and itmay require several weeks of practice before the skill is acquired.
Cognitive behavioral therapy for insomnia (CBT-I): This has been specifically developed for insomnia that comprises of cognitive approaches that target cognitive distortions and misconceptions related to insomnia, behavioral approaches such as stimulus control and sleep restriction, and educational approaches such as sleep hygiene. This can be delivered in several different formats either face-to-face individual or in a group setting, through the telephone- or internet-based modules, and also through self-help books. CBT-I in elderly has mild effect for sleep problems and works best for sleep maintenance insomnia. Multi-component therapy involves a different combination of all the treatment approaches listed above. Brief behavioral therapy (BBT) is also used for insomnia in older adults.
Pharmacological management
In the elderly, the ideal hypnotic should 1) be able to induce sleep rapidly, 2) have no adverse effects on normal sleep architecture, 3) demonstrate no significant residual effects, 4) be safe in patients with respiratory and cardiac conditions, 5) have minimal effects on memory, 6) not impair functioning, 7) have no risk of tolerance or rebound insomnia, 8) be safe during overdose, and 9) possess no potential for abuse or dependence. However, there is no ideal hypnotic available till date.
The basic rules of rational pharmacotherapy for insomnia in the elderly includes prescribing the lowest effective dose (which is usually half of adult dose), for shortest possible time (no more than 3–4 weeks), intermittent dosing (two to four times weekly) if possible, using drugs that have shorter elimination half-lives and lesser daytime sedation, and which can be gradually discontinued without causing rebound insomnia.
The first line drugsfor chronic insomnia disorder includes nonbenzodiazepine Z-drugs, benzodiazepines and melatonin receptor agonist ramelteon (table 10). Other medications include orexin receptor antagonist suvorexant, the antidepressants such as doxepin, antihistamines, antipsychotics, and melatonin. There is no evidence of superior efficacy of either of the agents in insomnia treatment.
Table 10.
First line medications† for chronic insomnia in elderly
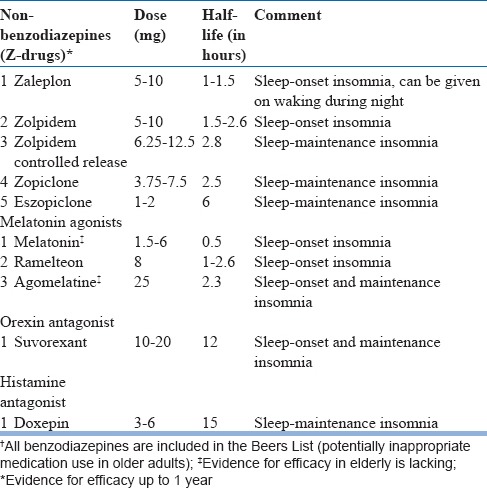
Non-benzodiazepine benzodiazepine receptor agonists: The Z-drugs in this group include zolpidem, zaleplon, zopiclone, and eszopiclone. These medications target the GABA-A receptor complexes and have preferential affinity for the α-1 subunit. For sleep onset insomnia, medications with a shorter half-life (zaleplon or zolpidem) are preferred, whereas, for sleep maintenance insomnia, those with a longer half-life (zolpidem sustained release or eszopiclone) are needed. Zaleplon can be used specifically for the treatment of mid-night awakenings because of its short half-life and ensures 4 hours of sleep. Owing to less adverse effects, Z-drugs are considered as the first-line agents for chronic insomnia wherever available.
Melatonin agonists: Ramelteon, a selective melatonin MT1 and MT2 receptor agonist, has been used for chronic insomnia at 4 to 8 mg/day. It probably acts through suprachiasmatic nucleus by influencing homeostatic sleep signaling. Ramelteon reduces sleep onset latency and total sleep timein older adults with insomnia and wastolerated well up to one year. Studies suggest that low doses of melatonin (0.5-6 mg) improve initial sleep quality in older adults with insomnia. There is some evidence to suggest that agomelatine at 25 mg isuseful for the treatment of insomnia in the elderly.
Orexin receptor antagonist: Suvorexant is a newer medication for treating insomnia that blocks orexin-mediated wake signaling. It improves initial and maintenance insomnia at 10-20 mg dosage. Suvorexant is usually taken on an empty stomach so that sleep onset is faster. It is recommended that it is taken at a time when one plans to sleep for at least 7 hours continuously. Common side effects include somnolence, fatigue, and headache. Suvorexant improves sleep onset and maintenance and is tolerated well bythe elderly.
Very low dose doxepin: Doxepin 3-6 mg through its H1 antagonism action has been found to be safe and effective in the treatment of sleep maintenance insomnia in the elderly. It improves sleep maintenance, total sleep time and sleep quality, but has no effect on sleep onset. There are no residual symptoms the next day. However, long-term studies are lacking.
Several benzodiazepines such as estazolam, temazepam, triazolam, flurazepam have been used for the treatment of chronic insomnia. In absence of benzodiazepines approved as hypnotics, other agents such as lorazepam, oxazepam, clonazepam or diazepam can be used. The strategy is to use long-acting benzodiazepines if comorbid anxiety is present. In other situations, either short- or intermediate-acting agent are preferred for sleep onset and sleep maintenance insomnia, respectively. However, benzodiazepines should be used with caution in the elderly, as it can lead to falls and fractures, motor vehicle accidents, cognitive decline, delirium, dependence and are best avoided if possible. Sedating tricyclic antidepressants may be used as hypnotics in low doses, e.g. amitriptyline 10-50 mg, imipramine 25-50 mg. Other commonly used medication for insomnia, mostly available as over-the-counter agents includes diphenhydramine (50-100 mg) and promethazine (25-100 mg). However, these medications are best avoided in elderly and are included in Beers list for potentially inappropriate medication use in the elderly.
Trazodone 50 mg improves sleep parameters in Alzheimer's disease and is tolerated well. Mirtazapine (7.5-15 mg) and esmirtazapine (1.5-4.5 mg) has also been reported to be effective in the treatment of insomnia. Atypical antipsychotics such as a low dose of quetiapine (25-100 mg) may be useful in some patients with insomnia if other agents are not effective. Tiagabine, a selective inhibitor of GABA transporter, at 4-6 mg dose increase slow-wave sleep and was well tolerated in elderly. Gabapentin (100-600 mg) and pregabalin (150-300 mg) may sometimes be used for insomnia, specifically for those with comorbid neuropathic pain symptoms.
SLEEP-DISORDERED BREATHING (SDB)
SDB is an umbrella term encompassing obstructive sleep apnoea (OSA), primary or secondary centralsleep apnoea (CSA), high-altitude periodic breathing, Cheyne–Stokes respiration, nonobstructive hypoventilation, or hypoxemia disorders secondary to pulmonary parenchymal, vascular, neuromuscularor chest wall disorders. OSA, which is the most common SDB, is characterized by a distinctive snoring pattern caused by intermittent airway collapse. In this condition, there are of periods of loud snoring or brief gasping followed by cessation of respiration lasting 20 to 30 seconds. This leads to sleep arousals, possibly due to arterial oxygen desaturation. Often the patient is not aware of his or her snoring and nighttime arousals, and a subset of patients complain of excessive daytime sleepiness (EDS).
In patients having SDB, apneas (complete cessation of respiration) and/or hypopneas (30% reduction in airflow with 4% oxygen desaturation) occur during sleep. SBD is diagnosed when these episodes last more than 10 sec and occurseveral times during the night, leading to frequent arousals and fall in oxygen saturation. The total number of apnoea and hypopnea episodes per hour of sleep is known as apnea-hypopnea index (AHI). For the diagnosis of SBD, an AHI morethan 5 is required. SDB in > 65-year-olds is found in >20%(as defined by AHI ≥10) and can be up to 60% in frail elderly patients (Netzer et al. 2016). The known risk factors for SDB in older adults include male gender, positive family history, smoking, several craniofacial abnormalities such as retrognathia, retrusion of the maxilla, macroglossia, central obesity, concomitant pulmonary diseases that reduce the chest wall compliance or any condition reducing diaphragmatic movement. Therisk of SBD becomes more if there is alcohol consumption sedating medication use. In elderly patients with severe SDB (AHI≥30), cognitive impairments are frequent that includes poor attention and recall, slowed response time and impairments in executive function.
Central sleep apnea (CSA) is characterized by loss of ventilatory drive leading to stoppage of breathing (at least 5 or more per hour) during sleep. On the basis of etiology, CSA in the older population can be broadly classified as primary CSA, Cheyne–Stokes breathing pattern inheart failure, and CSA in neurodegenerative disease and stroke.
Assessment and Evaluation
Evaluation of SDB includes a complete sleep history that covers the history of snoring, snorting, non-refreshing sleep, nocturia >2/night, nocturnal gastro-esophageal reflux, sleep-talking, increased dreaming, daytime fatigue and in a subset of patients, excessive daytime sleepiness. Preferably, in all cases, parallel information must be obtained from bed-partners who complain of witnessed pauses in breath during sleep. Also, details of medical and psychiatric disorders, prescribed medications, alcohol use, as well as cognitive impairment should be elicited. Formal evaluation for OSA should be considered in elderly patients who exhibit typical symptoms, but also should be considered in patients with comorbid cardiovascular risk factors or atypical symptoms such as nocturia, un explainedfalls, automobile accidents, or cognitive decline.
Findings on physical examination that supports OSA include: 1) neck circumference >40 cm in males and 37 cm in females, measured at cricothyroid level, 2) body mass index (BMI) >25 kg/mm2, 3) low-lying soft palate, 4) elongated or large uvula, 5) large tongue (often noted by the presence of tongue edge crenations), or 6) large tonsils or narrow distance between the tonsillar pillars. In older adults, an additional risk factor is an edentulous state, i.e. when patients remove their dentures at night, this leads to a reduction in the vertical dimension that increases the occurrence of upper airway obstructive events.
Screening tools, such as the Berlin Questionnaire can be used for estimation of OSA risk, which has been validated for Indian population. Epworth Sleepiness Scale may be used to document sleepiness.
The SDB is diagnosed based on overnight sleep recording, i.e. nocturnal PSG. A full night polysomnography in the sleep laboratory is considered as the gold standard method for diagnosis of SDB. In this method, sleep data of entire night is recorded and is scored by a trained scorer. The subsequent night is used to manually titrate the pressure of PAP device. Thus, each study requires at least two nights. However, in extreme cases where pre-test clinical data suggests severe OSA and patient is not having any other comorbidity, split-night study may be done. During “split study” the initial half of the night is used for diagnosis, whereas, the second half is used to titrate positive arterial pressure (PAP) if the OSA is severe. The limited-channel home-based testing (respiratory polygraphy) has also been studied in making the diagnosisof SDB. However, this must be used cautiously only in selected cases as it provides data only regarding sleep discorded breathing and can miss other information which may be important for patient's management.
Non-pharmacological management
Treatments for SDB depend on the symptoms and disease severity, and the presence of cardiovascularor metabolic disease. Treatment options in OSA are summarized in table 11. Treatment for mild OSA (AHI 6-15) without any other medical comorbidity includes lifestyle modification strategies such as regular exercise and weightloss, smoking cessation and reducing caffeine, alcohol, and avoiding the use of sedatives and other recreational drugs, and optimizing sleep and medical management of comorbidities. These patients should be followed up every three months to clinically assess symptoms and progress of weight reduction.
Table 11.
Treatment options for OSA
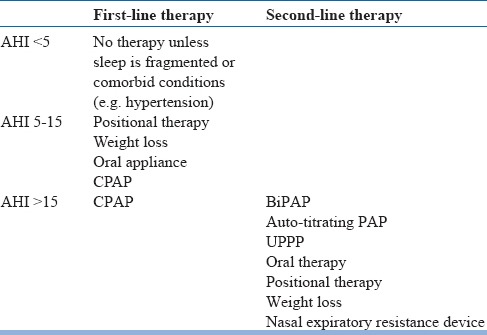
Inmild-to-moderate SDB, positional measures and oral mandibular advancement splints are used. Positional therapy consists of techniques intended to increase the time spent in the lateral sleeping position as opposed to the supine position. These include using pillows to prop up while lying or even sewing a ball into the back of the shirt to make the supine position more uncomfortable. There are sleep shirts available to facilitate lateral positional therapy. Positional therapy is indicated specifically for positional sleep apnea. In a meta-analysis, both continuous positive airway pressure (CPAP) and positional modification techniques were effectivein reducing AHI in those with positional OSA; however, the former was better.
Oral appliances are helpful in improving respiration and sleep quality in patients with OSA. It is recommended that for OSA in adults, a custom, titratable appliance is prescribed in consultation with a qualified dentist. However, their effectiveness in elderly is poorly studied.
In moderate to severe cases of OSA or symptomatic cases (associated with other comorbidities) mainstay of therapy is positive airway pressure devices and it is considered the gold standard treatment. Usually, manual CPAP device is preferred. Bilevel PAP device is advisable when the patient is not able to tolerate CPAP pressure or when PAP pressure requirement is beyond 20 cmH2O. In a network meta-analysis, CPAP was found to the most effective treatment for OSA. Older adults whoadhere to at least three months of CPAP treatment show improvements in cognitive functions such as psychomotor speed, executive functioning, and non-verbal delayed recall. Auto-PAP has limited efficacy in the management of OSA.
Surgical modifications of upper airway include procedures such as maxillomandibular advancement (MMA), laser-assisted uvulopalatoplasty (LAUP), uvulopharyngopalatoplasty (UPPP) and radiofrequency ablation (RFA). In a meta-analysis, MMA resulted in improvements in the AHI, whereas, pharyngeal surgeries had less consistent outcomes. Several studies have found good results with newer pharyngeal surgeries and multi-level techniques, which needs replication. However, due to limited data, recurrence rates falling close to 50%, surgical methods are restricted to only a selected group of patients with major anatomical issues and PAP failure.
Hypnotics and other sedating medications worsen SDB by inducing or worsening OSA and CSA. These medications should be avoided if SBD is suspected.
Sometimes, modafinil or armodafinil can be used if daytime sleepiness persists in spite of compliance to PAP therapy.
SLEEP-RELATED MOVEMENT DISORDERS
Sleep-related movement disordersinclude conditions that have stereotyped movements that disrupt sleep. The common presenting features are initial or maintenance insomnia. It includes restless leg syndrome (RLS), periodic limb movement disorder (PLMD) and REM sleep behavior disorder (RBD).
RESTLESS LEGS SYNDROME (RLS)
Restless legs syndrome (RLS) (also called Willis-Ekbom disease) is featured by dysesthesia in the legs which are usually described as bubbly or a “creepy and crawly” sensation, tingling, tickling, just restlessness, stretching sensation in the leg muscles that occurs during rest and is relieved by movement. Indian data suggests that most of the patients adopt one of the four strategies: moving legs, coming out of bed and walking, asking somebody to massage their legs and tying a rope on their legs. These complaints are almost always seen at night. A number of conditions may be mistaken for RLS, so they should be ruled out carefully, before diagnosing RLS. The prevalence of RLS in >65-year-olds is 10-35%.
Certain medications are seen in those having RLS. These are commonly seen in elderly, increasing the chances of having RLS. These include iron deficiency, uremia, neuropathies and cardiovascular disorders. Hence, these disorders must be ruled out in all patients of RLS. Serum ferritin level <50 μg/L has been found to correlate with severe RLS symptoms in comparison with serum iron levels, total iron binding capacity (TIBC) or transferrin saturation percentage. The prevalence of RLS is typically between 15to 40% in patients with uremia. It affects hemodialysis, as patients are required to lie still during the procedure. There is some evidence to suggest that RLS is associated with diabetic or alcoholic neuropathy and spinocerebellar ataxia type 3.
Assessment and Evaluation
Diagnosis of RLS is purely clinical and depends upon the information provided by the patient (table 12). All the criteria need to be met to diagnose RLS. Also, the RLS mimics (table 13) need to be differentiated. Additional supportive criteria include treatment response with dopaminergic agents (60-75%). Iron deficiency frequently coexists and needs investigation. Low serum ferritin (<50 μg/L) is a sensitive marker for iron deficiency. The recommended investigations in elderly include hemogram, serum ferritin, blood urea and serum creatinine in all cases, and if possible, serum folate, vitamin B12, and magnesium levels.
Table 12.
Diagnostic Criteria for RLS/WED
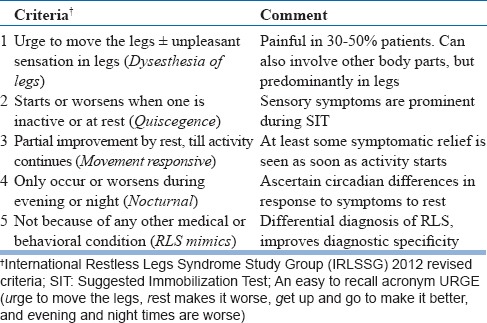
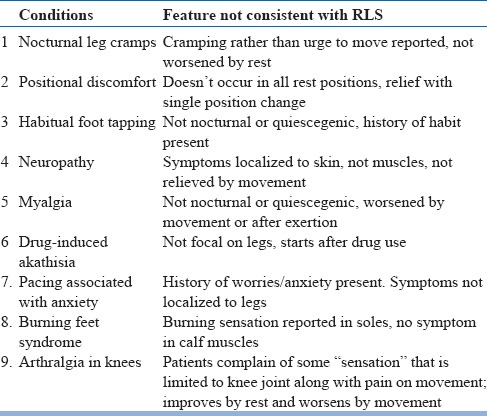
Table 13.
RLS Mimics
Salient features of patient workup have been summarized in table 14.
Table 14.
Work up of a suspected case of RLS at baseline and on each follow-up visit

International Restless Legs Scale (IRLS) is a 10-item measure of disease severity in RLS that is easy to use and has been used extensively. Actigraphy or polysomnographic assessment is usually not required.
The Suggested Immobilization Test (SIT) was developed for evaluation of RLS, however, it has limited clinical utility. In the standard procedure of the SIT, the patient lies down with legs straight for one hour starting at 9pm. Patients are encouraged to resist any urge to move during the SIT. Also, the SIT gives information on the propensity to symptom onset because immobilization provokes RLS symptoms. A modification of SIT, Multiple Suggested Immobilization Test (m-SIT) is also validated which consists of several tests in the afternoon and evening hours.
Non-pharmacological Treatment
Several dietary and lifestyle modifications are useful to control RLS. Patients are instructed to take a hot shower or massage their legs before bedtime, have regular sleeping and waking timings, regular exercise, and avoiding sleep deprivation and caffeine, cigarettes, andalcohol. Several over-the-counter medications such as antihistamines worsen RLS symptoms and are better avoided. Other medications such as antipsychotics, antiemetics, antidepressants, beta blockers, some anticonvulsants and lithium are known to exacerbate RLS and should be discontinued if possible.
Pharmacological Treatment
Pharmacotherapy is indicated when RLS symptoms impair functioning, sleep, and quality of life. The medication options in RLS are summarized in table 15. The first-line treatment of primary RLS includes non-ergot based dopamine agonists (ropinirole, pramipexole, and rotigotine), which have the highest level of evidence for efficacy and safety. The dopamine precursors (levodopa) and ergot-based dopamine agonists (e.g. cabergoline) are effective, but more likely to cause augmentation, hence, are considered as second-line agents. In general, longer acting dopamine agents are preferred and the dopaminergic load should be decreased by prescribing the lowest dose that is effective for the shortest time.
Table 15.
Pharmacotherapy of RLS

One of the major problem during treatment with short-acting dopaminergic agents is augmentation, which is characterized by either worsening of the severity of symptomsor by an earlier onset of symptoms as compared to baseline. This condition worsened by increasing the dose of dopaminergic agents. This must be differentiated from the end of dose phenomenon. The Max Planck Institute's operationalcriteria for augmentation is summarized in table 16.
Table 16.
Max Planck Institute criteria for augmentation

If augmentation limits the use of dopamine agonists, non-dopaminergic agents such as gabapentin and pregabalin are preferred agents and have a fair level of evidence for their efficacy. In some recent guidelines, use of these drugs is considered as first-line to prevent the development of augmentation. Specifically, gabapentin enacarbil (the pro-drug of gabapentin) is preferred considering its longer half-life. Use of opioids is limited by their possible abuse potential but may be usefulin non-responders, specifically those having severe augmentation features. Benzodiazepines such as clonazepam do not reduce PLMS, but improves sleep; therefore, they are indicated if sleep disturbance is prominent.
For secondary RLS, the underlying condition needs treatment along with specific treatment with dopamine agonists. Iron therapy is indicated in those with lower ferritin levels. Oral supplementation or intravenous formulations are indicated so that the ferritin levels increase to more than 50 μg/L. It is not effective if ferritin levels are higher than 100 μg/L and anemia is absent. In those with RLS associated with end-stage renal disease, dialysis improves the symptoms.
RAPID EYE MOVEMENT SLEEP BEHAVIOR DISORDER (RBD)
The prevalence of RBD is 0.5% in the general population. Among the elderly, 7% have RBD. It is predominantly seen in males after the age of 50 years. The plausible reasons for lower rates in females are summarized in table 17.
Table 17.
Plausible reasons for lower rates of RBD in females

RBD is featured by dream enactment behavior (DEB) that ranges from simple to complex vocalizations and motoric behaviors that occur during REM sleep. These behaviors occur because there are periods where there is no loss of muscle tone that is typically found in REM sleep, i.e. REM sleep without atonia (RSWA). Typically, RBD behaviors are more prominent during the latter part of the night when REM sleep predominates. Common RBD behaviors are walking, speaking and eating. Occasionally, they can be violent and the patient or their bed partner may be hurt; however, most are unaware of these actions. It is likely that some patients with RBD involve impressive and obvious DEB, whereas, most DEB may be unobtrusive movements limited to the hands, otherwise known as hand babbling.
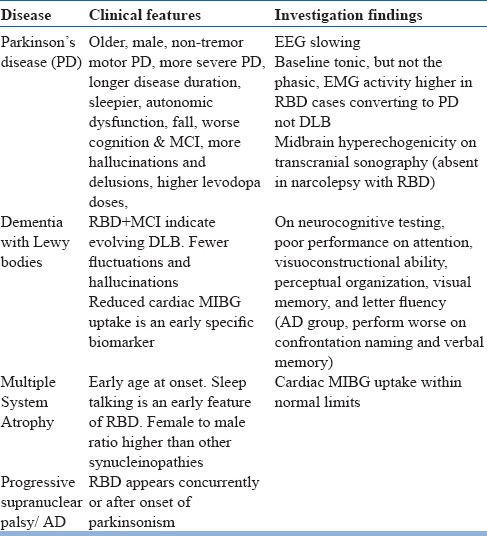
Idiopathic RBD is linked to neurodegenerative disorders, specifically with alpha-synucleinopathies (e.g. Parkinson's disease, Lewy Body Dementia, multiple system atrophy) (see table 18). Features that suggest the possibility of neurodegenerative disorders include abnormal smell or vision, constipation, orthostasis and motor slowing. Several medications and drugs are also associated with RBD (see table 19).
Table 18.
Secondary causes of RBD
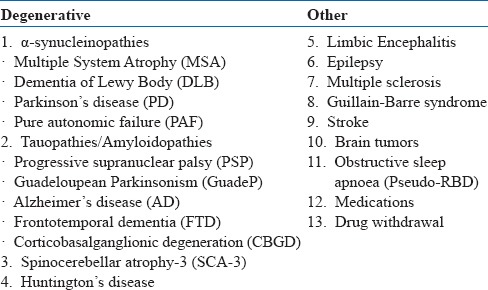
Table 19.
Drugs associated with RBD
Assessment and Evaluation
The observations of a bed partner or someone who has witnessed the patient's sleep are invaluable. In patientswithout a bed partner, clues suggesting possible RBD include vivid or terrifying dreams, falling out of bed, or unexplained nocturnal bruising. RBD can be accurately identified with the following screening question: “Has anyone ever told that you act out your dreams (e.g. punching or flailing arms in theair or screaming and shouting in your sleep?)”. RBD need to be distinguished from NREM sleep disorders. One clue which favors RBD is that patients are alert and immediately have a vivid dream recall. Another clue is the phenomenon of isomorphism, i.e. the DEB correlates with a recalled dream, in contrast to confusion and little recall after NREM sleep disorder. A 16-item Mayo Sleep Questionnaire (MSQ) can be used to screen for RBD and other sleep disorders. All patients with dream enactment history should be screened for OSA, which can mimic RBD. The differential diagnosis of RBD is summarized in table 20. Detailed neurological examination in patients with RBD is summarized in table 21. Early features of neurodegenerative disorders having RBD is presented in table 22.
Table 20.
Differential diagnosis of RBD in elderly
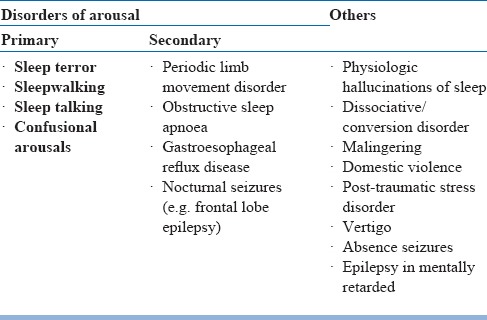
Table 21.
Neurological history and examination: salient features
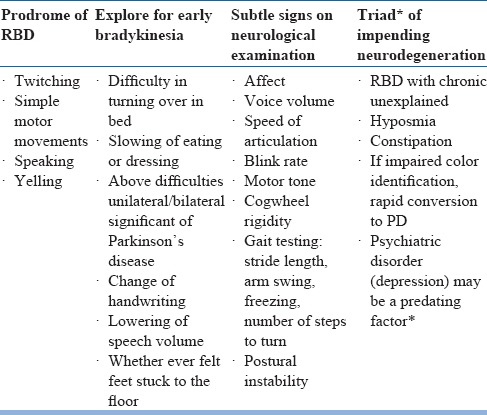
Table 22.
Early features of neurodegenerative disorders that present as RBD
Although history may suggest RBD, PSG is often required for definitive diagnosis. The diagnosis of RSWA is based on EMG findings during REM sleep. To diagnose RBD, EMG abnormalities include increase in muscle tone and/or phase twitch during REM sleep. The duration of RBD may have a wide variation, however, most occur less than once weekly and last for less than 2 minutes.
Approach to a patient with RBD is depicted in figure 2.
Figure 2.
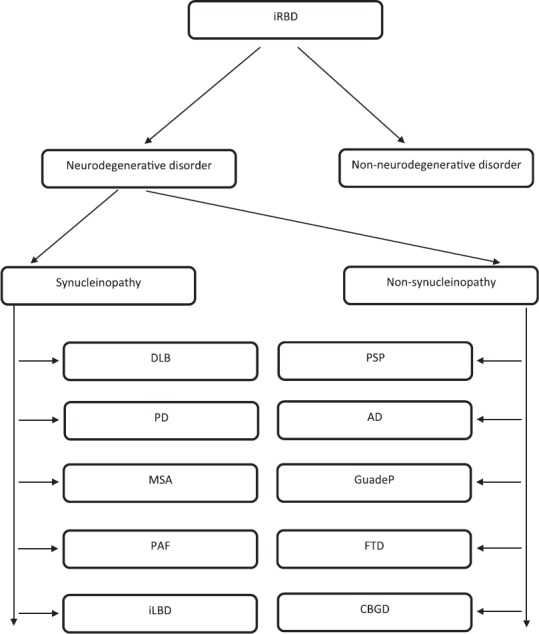
Flowchart showing differential diagnosis, progression monitoring and treatment decision tree in idiopathic RBD (iRBD)
Non-pharmacological Treatment
The primary goals of treatment in RBD include preventing injury to the patient and their bed partner during sleep andminimizing sleep disruption.
Injury-preventing techniques: These includes modification of environment such as sleeping on the floor to avoid falling from bed, padding corners of furniture, keeping the window and door locked at night and removing potentially dangerous objects from the room. Using heavy curtains on bedroom windows reduces sleep disruption. The bed partner may be asked to sleep separately till the condition improves.
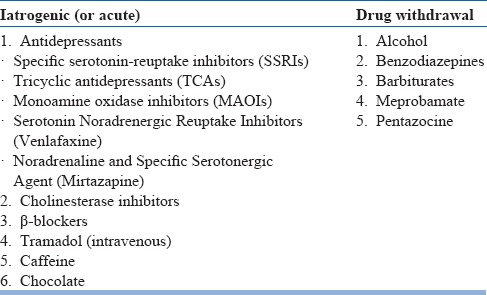
Discontinue medications known to exacerbate RBD: Medications that are known to exacerbate RBD should be discontinued if possible. These include antidepressants (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants and monoamine oxidase inhibitors), beta-blockers (atenolol, bisoprolol), tramadol and cholinesterase inhibitors. If there is comorbid depression, bupropion is preferred as it doesn't worsen RBD.
Treat underlying cause: If any specific underlying cause is found (e.g. brain stem tumor), it requires specific treatment.
Prognostic counseling: Patients need to be educated regarding the possibility of RBD being an early marker of neurodegenerative disease.
Bed alarm: A bed alarm can be used for the treatment of RBD. It consists of a pressure sensor that is placed on the shoulders which get activated during dream enactment. At that time a pre-recorded message is played which tells them that it is a dream, thereby calming the patient and they go back to sleep.
Pharmacological Treatment
First-line treatment: The preferred treatment for RBD is low-dose clonazepam at doses from 0.5-1 mg/day. It is a long-acting benzodiazepine that reduces abnormal motor behavior in 90% of subjects (table 23). However, there are concerns regarding its use in elderly such as residual sleepiness, unsteadiness leading to falls and fractures, delirium and cognitive impairment, which limits its use in frail patients. Also, it should be avoided in RBD patients with comorbid OSA, as it is potential respiratory suppressant. Furthermore, clonazepam being a cytochrome P450 substrate, drug interactions needs consideration.
Table 23.
Drugs used in treatment of RBD
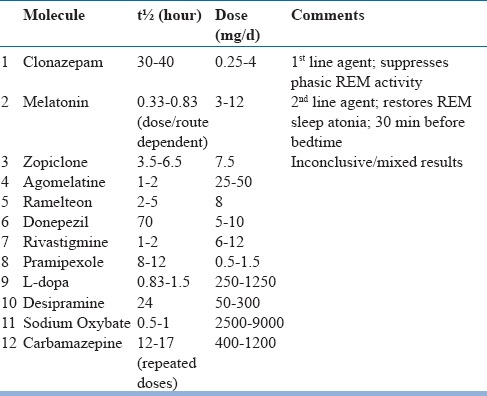
Melatonin at high-doses is also as effective as clonazepam for RBD and can be considered as an alternative first-line agent. It is probably better tolerated, specifically in those with neurodegenerative disease. The recommended dose is 3-15 mg at bedtime (median effective dose is 6 mg). Other melatonergic agents such as agomelatine (25–50 mg) or ramelteon (8 mg) at bedtime may be helpful, but evidence for their efficacy is low.
Second-line drugs: Medications such as dopaminergic agonists (pramipexole) and acetylcholinesterase inhibitors (rivastigmine) have been used with variable success in patients with RBD and may be considered in treatment-refractory patients as second-line agents. Other agents that have been used include temazepam, lorazepam, zolpidem, zopiclone, cannabinoids, and sodium oxybate.
CIRCADIAN RHYTHM SLEEP-WAKE DISORDERS (CRSWD)
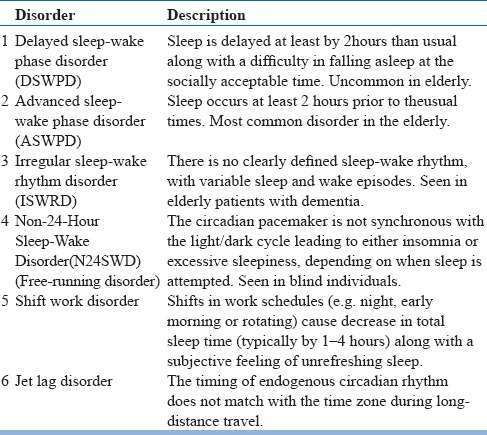
Sleep and circadian rhythm changes with aging, as well as less exposure to light and reduced activity level, are factors leading to circadian rhythm sleep-wake disorders (CRSWDs) in the elderly (see table 24). Sleep quality is optimal if there is a match between the intended sleep times with thetiming of endogenous circadian rhythm. CRSWDs can occur if the timings of central circadian clock changes or the timing of endogenous circadian rhythm don't match with the daily social and physical environment. The underlying cause for CRSWDs is related to circadian timing alterations, however, the clinical presentation depends on the physiological, behavioral and environmental factors. Shift work and Jet lag disorder are extrinsic, whereas, rest are intrinsic disorders.
Table 24.
Circadian rhythm sleep-wake disorders
In the elderly advanced sleep-wake phase disorder (ASWPD) is most common, which is characterized by an advancement of the sleep-wake cycle. It presents as regular and involuntary sleep and wake times which occur several hours before the usual times. In a large multicenter study on subjects more than 65 years, 20% experienced early morning awakening.
Evaluation and assessment
To diagnose CRSWDs, clinical sleep history, monitoring with a sleep diary, and if possible actigraphy for at least one week is required. Patients with ASWPD typically report sleepiness during the early evening, early sleep onset (between 6-9 PM), and spontaneous early morning awakening (2-5 AM). Even if they resist evening sleepiness and sleep at conventional bedtime, they still wakeup early and have daytime sleepiness. A sleep log or actigraphy monitoring is required to demonstrate a pattern of advancement in the regular sleep time for at least one week. Psychiatric disorders that cause early morning awakening such as depression and bipolar disorder need to be ruled out. Morningness-Eveningness Questionnaire (MEQ) can be used to characterize the chronotype of an individual, which shows a definite ‘morning type’. Polysomnography is not always needed for diagnosis of CRSD, but may be indicated to rule out other comorbid conditions such as OSA or PLMS. If PSG is performed, it is to be done at the usual sleep time of the patient.
To study the phase and amplitude of circadian rhythm, dim light melatonin onset (DLMO) and nadir core body temperature (Tmin) can be used. DLMO is considered as the most reliable among all. It is the timewhen endogenous melatonin levels start rising in dim light, i.e. approximately 2–3 hours before the usual bedtime. DLMO is used to estimate the circadian timing of individual so that melatonin and other treatment can be administered at appropriate times. The intrinsic circadian period can be estimated under free-running conditions, where the effects of light are controlled.
Management of Advanced sleep-wake phase disorder (ASWPD)
Treatment of ASWPD involves delaying the sleep-wake timing through chronotherapy, timed-melatonin, and timed-light exposure. Sleep hygiene is not of much use, except for avoiding long evening naps which may improve nocturnal sleep. The co-morbid conditions that may contribute to sleep complaints, especially depression should be treated.
Nonpharmacological treatment
Light therapy: The principle involves reducing morning light that advances phase and increasing evening light which delays phase. Patients are instructed to avoid going out and stay in a dark room during the early morning and to wear sunglasses while going out. Also, they are instructed to remain outdoors in the late afternoon and early evening and use bright lights inside the home during the evening. Bright light is recommended in the evening, between 7 to 9 PM (at least 5000 lux for 2 hours) todelay the circadian phase.
Chronotherapy: The goal in the treatment of ASWPD is a correction of abnormal early sleep phase. Most patients have difficulty delaying their sleep schedule, but find it easier to advance. Therefore, in some persons, advancing around the clock is preferred. In ASWPD, typically timing of sleep is advanced by 3 hours every 2 days until the expected time is reached. However, such schedules may not be liked by many and can cause disruptions in routine work.
Pharmacological Treatment
Melatonin: Phase delay can be induced by administration of melatonin in the early morning; the optimal time is in the early morning following spontaneous awakening. Light exposure should be minimized so that phase delay with melatonin is not countered by the phase advance effect of early morning light. It is useful at doses ranging from 0.5 to 10 mg.
CONCLUSION
Sleep in the elderly gets lighter and fragmented, however, very few otherwise healthy older people complain about their sleep problem. In the majority, sleep disruptions are associated with medical and psychiatric disorders, use of medications, changes in circadian rhythm and other sleep disorders such as sleep disordered breathing and REM behavior disorder. It is important to identify various causes of sleep disturbances in the elderly and to start treatment at the earliest. Furthermore, the factors that maintain disrupted sleep are targeted using nonpharmacological and behavioral techniques, along with medications if needed. This would enhance the quality and duration of sleep, and improve the overall quality of life in the elderly.
REFERENCES
- 1.Abbott SM, Reid KJ, Zee PC. Circadian Rhythm Sleep-Wake Disorders. Psychiatr Clin North Am. 2015;38(4):805–23. doi: 10.1016/j.psc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. International Restless Legs Syndrome Study Group. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014;15(8):860–73. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Altınyazar V, Kiylioglu N. Insomnia and dementia: is agomelatine treatment helpful? Case report and review of the literature. Ther Adv Psychopharmacol. 2016;6(4):263–8. doi: 10.1177/2045125316646064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes H, Edwards BA, Joosten SA, Naughton MT, Hamilton GS, Dabscheck E. Positional modification techniques for supine obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med Rev. 2016 doi: 10.1016/j.smrv.2016.11.004. S1087-0792(16)30138-1. [DOI] [PubMed] [Google Scholar]
- 5.Camargos EF, Louzada LL, Quintas JL, Naves JO, Louzada FM, Nóbrega OT. Trazodone improves sleep parameters in Alzheimer disease patients: a randomized, double-blind, and placebo-controlled study. Am J Geriatr Psychiatry. 2014;22(12):1565–74. doi: 10.1016/j.jagp.2013.12.174. [DOI] [PubMed] [Google Scholar]
- 6.Caples SM, Rowley JA, Prinsell JR, Pallanch JF, Elamin MB, Katz SG, Harwick JD. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33(10):1396–407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: a systematic review and meta-analysis. Psychother Psychosom. 2012;81(4):206–16. doi: 10.1159/000335379. [DOI] [PubMed] [Google Scholar]
- 8.Dhyani M, Rajput R, Gupta R. Hindi translation and validation of dysfunctional beliefs and attitudes about sleep (DBAS - 16) Ind Psychiatry J. 2013;22(1):80–5. doi: 10.4103/0972-6748.123639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Borreguero D, Allen RP, Kohnen R, Högl B, Trenkwalder C, Oertel W, et al. International Restless Legs Syndrome Study Group. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: report from a World Association of Sleep Medicine-International Restless Legs Syndrome Study Group consensus conference at the Max Planck Institute. Sleep Med. 2007;8(5):520–30. doi: 10.1016/j.sleep.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Borreguero D, Cano-Pumarega I. New concepts in the management of restless legs syndrome. BMJ. 2017;356:j104. doi: 10.1136/bmj.j104. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Borreguero D, Kohnen R, Boothby L, Tzonova D, Larrosa O, Dunkl E. Validation of the multiple suggested immobilization test: a test for the assessment of severity of restless legs syndrome (Willis-Ekbom disease) Sleep. 2013;36(7):1101–9. doi: 10.5665/sleep.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Borreguero D, Silber MH, Winkelman JW, Högl B, Bainbridge J, Buchfuhrer M, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016;21:1–11. doi: 10.1016/j.sleep.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Gupta R, Ahmad S, Dhar M, Goel D, Lahan V. Clinical presentation of restless legs syndrome: does the gender matter? Sleep Biol Rhythms. 2014a;12:180–186. [Google Scholar]
- 14.Gupta R, Ali R, Dhyani M, Das S, Pundir A. Hindi translation of Berlin questionnaire and its validation as a screening instrument for obstructive sleep apnea (OSA) J Neurosci Rural Practice. 2016;7:244–249. doi: 10.4103/0976-3147.176187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta R, Das S, Gujar K, Mishra KK, Gaur N, Majid A. Clinical Practice Guidelines for Sleep Disorders. Indian Journal of Psychiatry. 2017 Jan;59(Suppl 1):S116–S138. doi: 10.4103/0019-5545.196978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta R, Goel D, Ahmed S, Dhar M, Lahan V. What patients do to counteract the symptoms of Willis-Ekbom disease RLS/WED): Effect of gender and severity of illness. Ann Indian Acad Neurol. 2014b;17:405–8. doi: 10.4103/0972-2327.144010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hening WA, Allen RP, Earley CJ, Picchietti DL, Silber MH. Restless Legs Syndrome Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. An update on thedopaminergic treatment of restless legs syndrome andperiodic limb movement disorder. Sleep. 2004;27:560–83. doi: 10.1093/sleep/27.3.560. [DOI] [PubMed] [Google Scholar]
- 18.Herring WJ, Connor KM, Snyder E, Snavely DB, Zhang Y, Hutzelmann J, et al. Suvorexant in elderly patients with insomnia: pooled analyses of data from phase III randomized controlled clinical trials. Am J Geriatr Psychiatry. 2017;25(7):791–802. doi: 10.1016/j.jagp.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Horne JA, Ostberg O. A self-assessment questionnaire to determinemorningness-eveningness in human circadian rhythms. Int JChronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 20.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10(3):169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Joshi S. Nonpharmacologic therapy for insomnia in the elderly. Clin Geriatr Med. 2008;24(1):107–19. doi: 10.1016/j.cger.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Jung Y, St Louis EK. Treatment of REM sleep behavior disorder. Curr Treat Options Neurol. 2016;18(11):50. doi: 10.1007/s11940-016-0433-2. [DOI] [PubMed] [Google Scholar]
- 24.Keijzer H, Smits MG, Duffy JF, Curfs LM. Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med Rev. 2014;18(4):333–9. doi: 10.1016/j.smrv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Lahan V, Gupta R. Translation and validation of the insomnia severity index in hindi language. Indian J Psychol Med. 2011;33(2):172–6. doi: 10.4103/0253-7176.92060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lankford A, Rogowski R, Essink B, Ludington E, Heith Durrence H, Roth T. Efficacy and safety of doxepin 6 mg in a four-week outpatient trial of elderly adults with chronic primary insomnia. Sleep Med. 2012;13(2):133–8. doi: 10.1016/j.sleep.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Liu T, Li W, Zhou H, Wang Z. Verifying the relative efficacy between continuous positive airway pressure therapy and its alternatives for obstructive sleep apnea: a network meta-analysis. Front Neurol. 2017;8:289. doi: 10.3389/fneur.2017.00289. doi: 10.3389/fneur.2017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markota M, Rummans TA, Bostwick JM, Lapid MI. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632–9. doi: 10.1016/j.mayocp.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 29.McGrane IR, Leung JG, St Louis EK, Boeve BF. Melatonin therapy for REM sleep behavior disorder: a critical review of evidence. Sleep Med. 2015;16(1):19–26. doi: 10.1016/j.sleep.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaud M, Paquet J, Lavigne G, Desautels A, Montplaisir J. Sleep laboratory diagnosis of restless legs syndrome. Eur Neurol. 2002;48(2):108–13. doi: 10.1159/000062996. [DOI] [PubMed] [Google Scholar]
- 31.Milligan SA, Chesson AL. Restless legs syndrome in the older adult: diagnosis and management. Drugs Aging. 2002;19(10):741–51. doi: 10.2165/00002512-200219100-00003. [DOI] [PubMed] [Google Scholar]
- 32.Montgomery P, Dennis J. A systematic review of non-pharmacological therapies for sleep problems in later life. Sleep Med Rev. 2004;8(1):47–62. doi: 10.1016/S1087-0792(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+ Cochrane Database Syst Rev. 2003;(1):CD003161. doi: 10.1002/14651858.CD003161. [DOI] [PubMed] [Google Scholar]
- 34.Netzer NC, Ancoli-Israel S, Bliwise DL, Fulda S, Roffe C, Almeida F, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. Eur Respir J. 2016;48(4):992–1018. doi: 10.1183/13993003.01975-2015. [DOI] [PubMed] [Google Scholar]
- 35.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 36.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 37.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 38.Olde Rikkert MG, Rigaud AS. Melatonin in elderly patients with insomnia. A systematic review. Z Gerontol Geriatr. 2001;34(6):491–7. doi: 10.1007/s003910170025. [DOI] [PubMed] [Google Scholar]
- 39.Patel KV, Aspesi AV, Evoy KE. Suvorexant: a dual orexin receptor antagonist for the treatment of sleep onset and sleep maintenance insomnia. Ann Pharmacother. 2015;49(4):477–83. doi: 10.1177/1060028015570467. [DOI] [PubMed] [Google Scholar]
- 40.Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J Clin Sleep Med. 2015;11(7):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson GS, Zammit G, Wang-Weigand S, Zhang J. Safety and subjective sleep effects of ramelteon administration in adults and older adults with chronic primary insomnia: a 1-year, open-label study. J Clin Psychiatry. 2009;70(4):467–76. doi: 10.4088/jcp.07m03834. [DOI] [PubMed] [Google Scholar]
- 42.Rojas-Fernandez CH, Chen Y. Use of ultra-low-dose (≤6 mg) doxepin for treatment of insomnia in older people. Can Pharm J (Ott) 2014;147(5):281–9. doi: 10.1177/1715163514543856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roth T, Wright KP, Jr, Walsh J. Effect of tiagabine on sleep in elderly subjects with primary insomnia: a randomized, double-blind, placebo-controlled study. Sleep. 2006;29(3):335–41. doi: 10.1093/sleep/29.3.335. [DOI] [PubMed] [Google Scholar]
- 44.Russell T, Duntley S. Sleep disordered breathing in the elderly. Am J Med. 2011;124(12):1123–6. doi: 10.1016/j.amjmed.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Ruwe F, IJzerman-Boon P, Roth T, Zammit G, Ivgy-May N. A phase 2 randomized dose-finding study with esmirtazapine in patients with primary insomnia. J Clin Psychopharmacol. 2016;36(5):457–64. doi: 10.1097/JCP.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 46.Savarese M, Carnicelli M, Cardinali V, Mogavero MP, Federico F. Subjective hypnotic efficacy of trazodone and mirtazapine in patients with chronic insomnia: a retrospective, comparative study. Arch Ital Biol. 2015;153(2-3):231–8. doi: 10.12871/0003982920152348. [DOI] [PubMed] [Google Scholar]
- 47.Seyffert M, Lagisetty P, Landgraf J, Chopra V, Pfeiffer PN, Conte ML, Rogers MA. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139. doi: 10.1371/journal.pone.0149139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma S, Trivedi JK. Shiv Gautam, Ajit Avasthi. Guidelines for the treatment of sleep disorders. Published by Indian Psychiatric Society. 2006;II:232–264. [Google Scholar]
- 49.Thompson W, Quay TA, Rojas-Fernandez C, Farrell B, Bjerre LM. Atypical antipsychotics for insomnia: a systematic review. Sleep Med. 2016;22:13–7. doi: 10.1016/j.sleep.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 51.Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 52.Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51(8):1085–91. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhu Y, Long H, Jian F, Lin J, Zhu J, Gao M, Lai W. The effectiveness of oral appliances for obstructive sleep apnea syndrome: A meta-analysis. J Dent. 2015;43(12):1394–402. doi: 10.1016/j.jdent.2015.10.008. [DOI] [PubMed] [Google Scholar]


