Abstract
Between September 2017 and February 2018, influenza A(H1N1)pdm09, A(H3N2) and B viruses (mainly B/Yamagata, not included in 2017/18 trivalent vaccines) co-circulated in Europe. Interim results from five European studies indicate that, in all age groups, 2017/18 influenza vaccine effectiveness was 25 to 52% against any influenza, 55 to 68% against influenza A(H1N1)pdm09, −42 to 7% against influenza A(H3N2) and 36 to 54% against influenza B. 2017/18 influenza vaccine should be promoted where influenza still circulates.
Keywords: influenza, influenza vaccine effectiveness, influenza vaccination, case control study, multicentre study, Europe
Most countries in the European Union (EU) recommend and fund seasonal influenza vaccine for elderly people and individuals at increased risk of severe influenza [1]. The United Kingdom (UK) commenced the incremental introduction of a universal childhood influenza vaccination programme in 2013/14 using a quadrivalent live attenuated influenza vaccine (LAIV4) for healthy children and quadrivalent inactivated vaccine (QIV) for at-risk children for whom LAIV4 is contraindicated [2].
The trivalent influenza vaccines for the 2017/18 northern hemisphere influenza season include an A/Michigan/45/2015 (H1N1)pdm09-like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus and a B/Brisbane/60/2008-like virus (B/Victoria lineage). The quadrivalent vaccines also contain a B/Phuket/3073/2013-like virus (B/Yamagata lineage) [3].
The early phase of the 2017/18 influenza season in Europe was characterised by the co-circulation of influenza A(H1N1)pdm09, influenza A(H3N2) and influenza B, with different patterns of dominant type or subtype observed between countries [4]. Up to February 2018, most influenza B viruses assigned to a lineage were B/Yamagata viruses, not included in the 2017/18 trivalent vaccine [3,4].
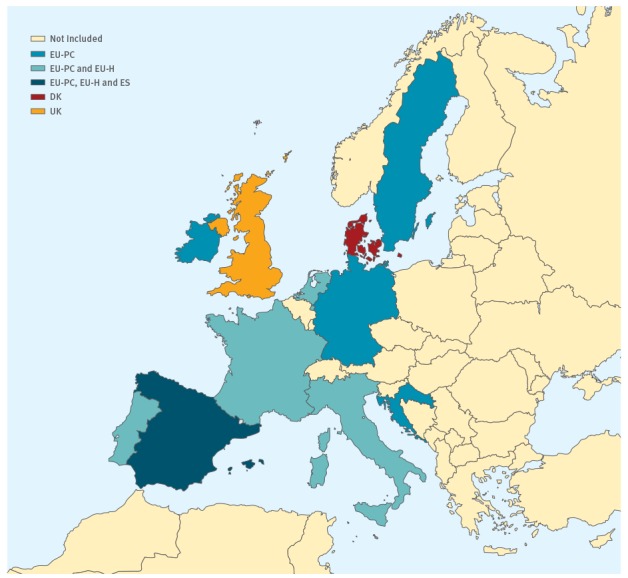
Here we present the interim 2017/18 season influenza vaccine effectiveness (VE) estimates from three single-country studies (UK, Denmark (DK) and Spain (ES)) and two multi-country studies (primary care (EU-PC) and hospital (EU-H) European Influenza - Monitoring Vaccine Effectiveness (I-MOVE/I-MOVE+) networks) (Figure 1).
Figure 1.
European Union countries contributing to the influenza vaccine effectiveness results presented, by study, 2017/18 (n = 21,220)
DK: Denmark study; ES: Spain study; EU-H: European hospital-based multi-country I-MOVE+ study; EU-PC: European primary care-based multi-country I-MOVE/I-MOVE+ study; UK: United Kingdom study.
Study design and estimation of vaccine effectiveness
The methods of these five studies have been described in detail elsewhere [5-9]. Study sites included in EU-PC (Croatia, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain and Sweden) and EU-H (France, Italy, the Netherlands, Portugal and Spain) followed generic protocols for primary care-based or hospital-based studies.
All five studies used a test-negative case control design (TND) [10]. In short, individuals presenting at participating healthcare settings with a pre-determined set of symptoms (including at least one systemic and one respiratory symptom) were swabbed. Samples were tested for influenza using RT-PCR. Individuals testing positive for influenza were classified as cases (by influenza (sub)type), those testing negative as controls.
The ES, UK and EU-PC studies included patients at primary care level (henceforth referred to as medically attended), the EU-H study included patients at hospital level (henceforth referred to as hospitalised), and the DK study included results from primary care and hospital level pooled together. Patients’ inclusion was foreseen to be systematic (or exhaustive) in the ES, EU-PC and EU-H studies and ad hoc in the DK and UK studies. In Spain, 268 of the 833 physicians included in the ES study were also included in the EU-PC study.
The study population included all age groups in all studies except for EU-H, which was confined to individuals 65 years and older.
In all studies, we defined patients as vaccinated with the 2017/18 influenza vaccine if they had been vaccinated at least 14 days (UK) or 15 days (all other studies) before symptom onset. Patients were excluded if they were vaccinated less than 14 (UK) or 15 days (all other studies) before symptom onset or if the date of vaccination was unknown.
In seven EU-PC countries (France, Germany, Ireland, the Netherlands, Portugal, Spain and Sweden), the UK, ES and DK, all or a random sample of positive influenza specimens were selected for genetic sequencing.
VE was computed by comparing the odds of vaccination between cases and controls (VE = (1 − OR) × 100). All studies used logistic regression to adjust VE for measured confounding variables, excluding patients with missing data for covariates in the model (complete case analysis) (Table 1). We computed VE overall and, where possible, by age group and target population (as defined locally in the various studies and study sites) against any influenza, influenza A(H3N2), influenza A(H1N1)pdm09, any influenza B and influenza B/Yamagata.
Table 1. Summary characteristics of the influenza vaccine effectiveness studies included, Europe, influenza season 2017/18 (n = 21,220).
| ES | UK | EU-PC | DK | EU-H | |
|---|---|---|---|---|---|
| Study period | 30 Oct 2017 to 21 Jan 2018 | 1 Oct 2017 to 14 Jan 2018 | 25 Sep 2017 to 26 Jan 2018 | 1 Dec 2017 to 5 Feb 2018 | 25 Oct 2017 to 4 Feb 2018 |
| Setting | Primary care | Primary care | Primary care | Primary care and hospital | Hospital |
| Location | Spain | England, Scotland, Northern Ireland and Wales | Croatia, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain and Sweden | Denmark | France, Italy, the Netherlands, Portugal and Spain |
| Study design | TND | TND | TND | TND | TND |
| Data source | Sentinel physicians and laboratory | Sentinel physicians and laboratory | Sentinel physicians and laboratorya | Data linkage of Danish Microbiology Database, the Danish Vaccination Register and the Danish National Hospital Register | Hospital charts, vaccine registers, interviews with GPs/pharmacists, laboratory |
| Age group of study population | All | All | ≥ 6 months | ≥ 18 years | ≥ 65 years |
| Case definition | ILI | ILI | ILI | ILI | SARI |
| Selection of patients | Systematic | At practitioner's discretion | Systematic | At practitioner's discretion | Exhaustive |
| Vaccine types usedb | Mostly TIV (no individual data) | In children: 19% TIV, 77% LAIV4 nasal spray, 3% unknown In adults: 100% unknown |
67% TIV, 17% unknown, 8% TIV adjuvanted, 4% QIV, 3% TIV intradermal, 1% LAIV4 nasal spray | Only TIV (no individual data) | 57% TIV, 25% unknown, 8% TIV adjuvanted, 10% TIV intradermal |
| Variables of adjustment | Age (RCS), sex, presence of chronic conditions, onset date, region | Age group, sex, onset date, pilot area for child vaccination programme, surveillance scheme | Age (RCS), sex, presence of chronic conditions, onset date and study site | Age group, sex, presence of chronic conditions, onset date | Age (RCS), lung diseases, heart diseases, diabetes mellitus, obesity (BMI ≥ 30), renal diseases, cancer and hospitalisation in the past 12 months, onset date, study site |
DK: Denmark study; ES: Spain study; EU-H: European hospital-based multi-country I-MOVE+ study; EU-PC: European primary care-based multi-country I-MOVE/I-MOVE+ study; GP: general practitioner; ILI: influenza-like illness; LAIV4: quadrivalent live attenuated influenza vaccines; LRI: lower respiratory infection; SARI: severe acute respiratory infection; TIV: trivalent inactivated vaccines; TND: test-negative design; RCS: restricted cubic spline; UK: United Kingdom study.
a In Spain, 268 of the 833 physicians included in the ES study were also included in the EU-PC study.
b Vaccines were egg-propagated, non-adjuvanted and administered intramuscularly unless otherwise specified.
If the number of events per parameter was lower than 10, we conducted a sensitivity analysis using penalised logistic regression to assess small sample bias [11].
Results
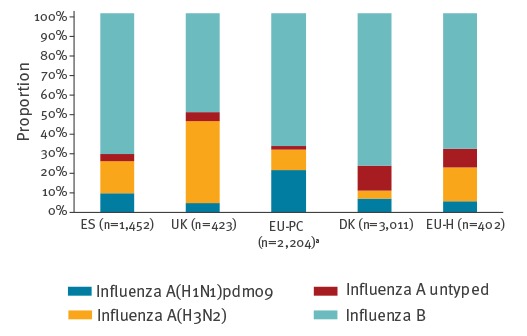
Between September 2017 and February 2018, the number of patients included in the VE analysis by study was 2,399 (1,452 cases) in the ES, 1,331 (421 cases) in the UK, 4,652 (2,103 cases) in the EU-PC, 11,907 (3,011 cases) in the DK and 931 (385 cases) in the EU-H study. Overall, more than two thirds of cases were positive for influenza B viruses in all studies except UK, where influenza A and B viruses were detected in similar proportions (51% (214/423) and 49% (209/423), respectively) (Figure 2). Where subtyped, influenza A viruses were mainly A(H3N2) in ES (62% (233/375) of subtyped influenza A specimens), UK (90% (174/194)) and EU-H (74% (68/92)), and mainly A(H1N1)pdm09 in DK (56% (145/257)) and EU-PC (67% (469/698)).
Figure 2.
Proportion of influenza (sub)types by study, Europe, 2017/18 (n = 6,979)
DK: Denmark study; ES: Spain study; UK: United Kingdom study; EU-H: European hospital-based multi-country I-MOVE+ study; EU-PC: European primary care-based multi-country I-MOVE/I-MOVE+ study.
a Includes two influenza A(H1N1)pdm09/B co-infections; 98 of influenza A(H1N1)pdm09, 122 of A(H3N2) and 454 of B cases were also in ES.
The graph includes individuals excluded from the VE analysis due to missing covariates.
Any influenza
Among all ages, VE against any medically attended influenza ranged between 25% (95% confidence interval (CI): −10 to 48) in the UK study and 52% (95% CI: 29 to 67) in the ES study. In UK, VE of the LAIV4 was 53% (95% CI: −56 to 86) in children and VE of the inactivated vaccine was 18% (95% CI: −23 to 45) in adults (Table 2). Among the target groups for influenza vaccination, the VE was 36% (95% CI: 13 to 53) in EU-PC and 40% (95% CI: 1 to 63) in the ES study. In EU-H, VE against any hospitalised influenza in patients aged 65 years and older was 35% (95% CI: 13 to 51).
Table 2. Interim adjusted seasonal vaccine effectiveness against any laboratory-confirmed influenza, influenza A(H1N1)pdm09, A(H3N2) and B, by age group, target group for vaccination and by study, Europe, influenza season 2017/18.
| Influenza type/subtype and study site | Setting | Study population | Cases | Controls | Adjusted VE | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Vacc | % | All | Vacc | % | |||||
| Influenza A + B | ||||||||||
| ES | PC | All ages | 1,452 | 98 | 7 | 947 | 75 | 8 | 52 | 29 to 67 |
| 0–14 years | 589 | 15 | 3 | 370 | 13 | 4 | 78 | 45 to 91 | ||
| 15–64 years | 762 | 44 | 6 | 511 | 40 | 8 | 54 | 23 to 73 | ||
| ≥ 65 years | 101 | 42 | 42 | 66 | 22 | 33 | 21 | −93 to 68 | ||
| Target groupa | 278 | 72 | 26 | 180 | 50 | 28 | 40 | 1 to 63 | ||
| UK | PC | All ages | 421 | 93 | 22 | 910 | 190 | 21 | 25 | −10 to 48 |
| 2–17 years (LAIV4) | 69 | 5 | 7 | 166 | 19 | 11 | 53 | −56 to 86 | ||
| ≥ 18 years (IIV) | 347 | 87 | 25 | 630 | 163 | 26 | 18 | −23 to 45 | ||
| EU-PC | PC | All ages | 2,103 | 210 | 10 | 2,549 | 272 | 11 | 38 | 20 to 52 |
| 0–17 years | 846 | 26 | 3 | 998 | 35 | 4 | 59 | 23 to 78 | ||
| 18–64 years | 1,021 | 74 | 7 | 1,288 | 109 | 8 | 34 | 5 to 54 | ||
| ≥ 65 years | 234 | 110 | 47 | 262 | 128 | 49 | 44 | 8 to 66 | ||
| Target groupa | 554 | 172 | 31 | 713 | 217 | 30 | 36 | 13 to 53 | ||
| DK | PC and hospital | All ages | 3,011 | 593 | 20 | 8,896 | 2299 | 26 | 34 | 25 to 41 |
| 18–64 years | 1,564 | 136 | 9 | 3,462 | 538 | 16 | 47 | 35 to 58 | ||
| ≥ 65 years | 934 | 447 | 48 | 3,089 | 1,688 | 55 | 23 | 10 to 34 | ||
| EU-H | Hospital | ≥ 65 years | 385 | 200 | 52 | 546 | 332 | 61 | 35 | 13 to 51 |
| Influenza A(H1N1)pdm09 | ||||||||||
| EU-PC | PC | All ages | 444 | 14 | 3 | 1,999 | 195 | 10 | 68 | 42 to 83 |
| 18–64 years | 203 | 7 | 3 | 955 | 77 | 8 | 63 | 12 to 84 | ||
| DK | PC and hospital | All ages | 214 | 18 | 8 | 8,896 | 2299 | 26 | 55 | 23 to 74 |
| 18–64 years | 119 | 7 | 6 | 3,462 | 538 | 16 | 60 | 13 to 82 | ||
| ≥ 65 years | 26 | 11 | 42 | 3,089 | 1,688 | 55 | 37 | −40 to 72 | ||
| Influenza A(H3N2) | ||||||||||
| ES | PC | All ages | 233 | 22 | 9 | 947 | 75 | 8 | 7 | −74 to 51 |
| UK | PC | All ages | 194 | 58 | 30 | 910 | 190 | 21 | −27 | −111 to 24 |
| EU-PC | PC | All ages | 220 | 35 | 16 | 1,505 | 147 | 10 | −16 | −96 to 31 |
| 18–64 years | 140 | 9 | 6 | 771 | 66 | 9 | 27 | −62 to 67 | ||
| DK | PC and hospital | All ages | 122 | 53 | 43 | 8,896 | 2,299 | 26 | -42 | −116 to 7 |
| 18–64 years | 45 | 6 | 13 | 3,462 | 538 | 16 | 21 | −95 to 68 | ||
| ≥ 65 years | 67 | 45 | 67 | 3,089 | 1,688 | 55 | −65 | −178 to 2 | ||
| EU-H | Hospital | ≥ 65 years | 60 | 38 | 63 | 242 | 154 | 64 | −1 | −93 to 47 |
| Any influenza B | ||||||||||
| ES | PC | All ages | 1,022 | 72 | 7 | 947 | 75 | 8 | 52 | 27 to 68 |
| 0–17 years | 440 | 10 | 2 | 370 | 13 | 4 | 83 | 54 to 94 | ||
| 18–64 years | 503 | 31 | 6 | 511 | 40 | 8 | 51 | 13 to 72 | ||
| ≥ 65 years | 79 | 31 | 39 | 66 | 22 | 33 | 15 | −114 to 66 | ||
| Target groupa | 207 | 53 | 26 | 180 | 50 | 28 | 38 | −5 to 63 | ||
| UK | PC | All ages | 209 | 33 | 16 | 910 | 190 | 21 | 54 | 24 to 72 |
| EU-PC | PC | All ages | 1,368 | 150 | 11 | 2,510 | 269 | 11 | 39 | 19 to 54 |
| 0–17 years | 562 | 21 | 4 | 980 | 35 | 4 | 58 | 15 to 79 | ||
| 18–64 years | 643 | 55 | 9 | 1,279 | 108 | 8 | 27 | −9 to 51 | ||
| ≥ 65 years | 161 | 74 | 46 | 250 | 126 | 50 | 54 | 20 to 73 | ||
| Target groupa | 382 | 125 | 33 | 696 | 215 | 31 | 39 | 14 to 56 | ||
| DK | PC and hospital | All ages | 2,298 | 437 | 19 | 8,896 | 2,299 | 26 | 36 | 27 to 44 |
| 18–64 years | 1,220 | 111 | 9 | 3,462 | 538 | 16 | 44 | 30 to 56 | ||
| ≥ 65 years | 701 | 319 | 46 | 3,089 | 1,688 | 55 | 28 | 14 to 39 | ||
| EU-H | Hospital | ≥ 65 years | 249 | 131 | 53 | 524 | 321 | 61 | 34 | 8 to 52 |
| Influenza B/Yamagata | ||||||||||
| ES | PC | All ages | 84 | 4 | 5 | 993 | 81 | 8 | 77 | 14 to 94 |
| EU-PCb | PC | All ages | 395 | 34 | 9 | 2,065 | 206 | 10 | 49 | 19 to 67 |
CI: confidence interval; DK: Denmark study; ES: Spain study; EU-H: European hospital-based multi-country I-MOVE+ study; EU-PC: European primary care-based multi-country I-MOVE/I-MOVE+ study; PC: primary care; UK: United Kingdom study; Vacc: vaccinated; VE: vaccine effectiveness.
a Groups targeted by seasonal influenza vaccination as defined locally in the studies and study sites.
b Only includes study sites where lineage was available for all samples or where lineage was determined systematically.
Study sites included in the EU-H analysis: France, Italy, Navarra, the Netherlands, Portugal and Spain (except for influenza A(H3N2) analysis: Navarra and Spain only).
Study sites included in EU-PC analysis for all influenza and influenza B: Croatia, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain, Sweden. For analysis against A(H1N1)pdm09: France, Germany, Italy and Spain were included. For analysis against A(H3N2): France, Germany, Ireland, Spain and Sweden were included.
Influenza A(H1N1)pdm09
All 76 influenza A(H1N1)pdm09 viruses sequenced belonged to clade 6B.1 (A/Michigan/45/2015) (Table 2).
VE against influenza A(H1N1)pdm09 was 68% (95% CI: 42 to 83) and 55% (95% CI: 23 to 74) among all ages in the EU-PC and DK studies, respectively. Among 18–64 year-olds, it was 63% (95% CI: 12 to 84) and 60% (95% CI: 13 to 82) in the EU-PC and DK studies, respectively. Among those aged 65 years and older, it was 37% (95% CI: −40 to 72) in the DK study (Table 2).
Influenza A(H3N2)
Of the 204 influenza A(H3N2) viruses sequenced, 63% (n = 129) belonged to genetic clade 3C.2a, 35% (n = 72) to 3C.2a1 and 1% (n = 3) to 3C.3a (Table 3).
Table 3. Influenza viruses characterised by clade and study site, Europe, influenza season 2017/18 (n = 886).
| Clade | ES a | UK | EU-PCb | DKc | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Total influenza A(H1N1) | n = 142 | n = 20 | n = 469 | n = 113 | |||||
| Sequenced | 28 | 100 | 10 | 100 | 25 | 100 | 23 | 100 | |
| A/Michigan/45/2015 | 6B.1 | 28 | 100 | 10 | 100 | 25 | 100 | 23 | 100 |
| Total influenza A(H3N2) | n = 233 | n = 174 | n = 229 | n = 144 | |||||
| Sequenced | 51 | 100 | 59 | 100 | 43 | 100 | 51 | 100 | |
| A/HongKong/4801/2014 | 3C.2a | 20 | 39 | 46 | 78 | 27 | 63 | 36 | 71 |
| A/Singapore/INFIMH-16–0019/2016 | 3C.2a1 | 31 | 61 | 10 | 17 | 16 | 37 | 15 | 29 |
| A/Switzerland/9715293/2013 | 3C.3a | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 |
| Total influenza B | n = 1,022 | n = 209 | n = 1,469 | n = 625 | |||||
| Sequenced | 164 | 100 | 116 | 100 | 207 | 100 | 109 | 100 | |
| B/Yamagata | 136 | 83 | 116 | 100 | 198 | 96 | 109 | 100 | |
| B/Phuket/3073/2013 | 3 | 136 | 100 | 0 | 0 | 198 | 100 | 109 | 100 |
| B/Victoria | 28 | 17 | 0 | 0 | 9 | 4 | 0 | 0 | |
| B/Norway/2409/2017 | 1A Δ(K162, N163) | 20 | 71 | 0 | 0 | 5 | 56 | 0 | 0 |
| B/Brisbane/60/2008 | 1A | 8 | 29 | 0 | 0 | 4 | 44 | 0 | 0 |
a 50 specimens from ES are also included in EU-PC data.
b The specimens sequenced from Spain are originating from the entire National Influenza Surveillance System between weeks 44/2017 and 03/2018.
c Sequence information is based on a sub-sample of influenza-positive samples received for surveillance at the National Influenza Center Denmark from week 40/2017 to 4/2018.
Among all ages, VE against influenza A(H3N2) ranged from −42% (95% CI: −116 to 7) in the DK and 7% (95% CI: −74 to 51) in the ES study. VE against hospitalisation for influenza A(H3N2) in patients aged 65 years and older was −1% (95% CI: −93 to 47) in EU-H (Table 2).
Influenza B
Of the 596 influenza B viruses sequenced, 94% (n = 559) were B/Yamagata (all belonging to clade 3 influenza B/Phuket/3073/2013) and 6% (n = 37) were influenza B/Victoria (25 belonging to clade 1A Δ(K162, N163) and 12 belonging to clade 1A) (Table 3).
Among all ages, VE against influenza B ranged between 36% (95% CI: 27 to 44) in the DK and 54% (95% CI: 24 to 72) in the UK study. Age group-specific VE was lowest at 15% (95% CI: −114 to 66) among those aged 65 years and older and highest at 83% (95%CI: 54 to 94) in the 0–14-year age group in the ES study. VE was 34% (95% CI: 8 to 52) against hospitalised influenza B in EU-H and 28% (95% CI: 14 to 39) against medically attended and hospitalised influenza B in the DK study among those aged 65 years and older (Table 2). VE against influenza B/Yamagata was 77% (95% CI: 14 to 94) in the ES study and 49% (95% CI: 19 to 67) in EU-PC (Table 2).
Sensitivity analyses
For all of the above analyses, sensitivity analyses for small sample size gave similar results (absolute difference ranging between 1% and 6%).
Discussion
Interim results from five established influenza VE studies across Europe indicate that 2017/18 VE against all influenza ranged between 25 and 52% among all ages and between 36 and 40% in the targeted groups. VE was moderate to good against influenza A(H1N1)pdm09 among all ages (55 to 68%), poor against influenza A(H3N2) with all point estimates below 8% for all ages, and moderate against influenza B, with point estimates between 39 and 52% for all ages.
The good VE against medically attended influenza A(H1N1)pdm09 is consistent with historical data [12]. However, during the last influenza A(H1N1)pdm09 season in Europe (2015/16), the EU-PC VE of 33% against influenza A(H1N1)pdm09 in all age groups was lower than what we report here [13]. In the 2015/16 season, the influenza vaccine strain A/California/7/2009 (H1N1)pdm09 differed from the circulating strains which mainly belonged to the genetic subgroup 6B.1 (represented by A/Michigan/45/2015 (H1N1)pdm09). This 6B.1 strain was included in the 2017/18 vaccine and was identified in all A(H1N1)pdm09 samples sequenced in the study sites. The change in vaccine strain may have led to a better VE against A(H1N1)pdm09. More precise end-of-season estimates and results at the hospital level will help investigate this hypothesis.
The influenza A(H3N2) component included in the 2017/18 northern hemisphere vaccine was the same as in the 2016/17 northern hemisphere vaccine [14]. As anticipated based on EU-H 2016/17 results [15] and 2017 interim results from Australia [16], and as already reported in other published early estimates for the northern hemisphere [17], the VE against influenza A(H3N2) was low in participating study sites. In our studies, 63% of sequenced influenza A(H3N2) viruses belonged to the A/HongKong/4801/2014 vaccine strain genetic group (3C.2a) and 35% to the A/Singapore/INFIMH-16–0019/2016 clade (3C.2a1), which is the selected strain in the 2018 southern hemisphere and 2018/19 northern hemisphere influenza vaccines [18]. Small sample size limited VE estimation by clade and subclade, which will be a priority for end-of-season analyses. Our results further support the need for more effective interventions in older people, in whom the burden of influenza A(H3N2) is most notable and the VE, including against severe outcome, is the lowest [19]. Based upon recent cost-effectiveness work undertaken by Public Health England, the UK Joint Committee on Vaccination and Immunisation has advised that use of adjuvanted trivalent inactivated vaccines (TIV) in those aged 65 years and older would be both more effective and cost-effective than the non-adjuvanted trivalent or quadrivalent vaccines currently in use [20].
The interim VE against medically attended influenza B was moderate in the studies included here (36% to 54% among all ages), similar to recently published estimates from northern hemisphere countries [17,21,22]. It was moderate to good against medically attended influenza B in children (58% in EU PC and 83% in ES) and poorer at 34% against hospitalised outcome among adults 65 years and older. The vast majority (94%) of sequenced influenza B samples were of the B/Yamagata lineage, which was not included in the 2017/18 northern hemisphere TIV. VE was 77% and 49% against influenza B/Yamagata in the ES and EU-PC studies, respectively, suggesting important cross-lineage protection.
The UK study was the only one to provide VE estimates for the quadrivalent vaccines. Vaccine effectiveness against any influenza among children was similar in the UK study (53%), where children receive LAIV4, and in the EU-PC study (59%), where most vaccinated children received TIV. However, it is difficult to compare these estimates against any influenza since the relative proportion of circulating (sub)types was different in the UK, where there was a higher proportion of circulating influenza A(H3N2) viruses, compared with most countries participating in the EU-PC study. In past seasons where circulating and vaccine lineages were different, contradictory results were observed [13,23,24]. Partial, but not full cross-protection between mismatched influenza B lineages has been suggested by two systematic reviews [25,26]. More precise end-of-season estimates by lineage, age group and vaccine type would be of added value to discuss cross-lineage protection and the added protection conferred by quadrivalent vaccines. Such information is relevant at a time when QIV is available in most European countries [27] and preferentially recommended in some [28].
End-of-season analyses are needed to verify the conclusions from these interim season results. A larger sample size should allow more precise estimates, especially in stratified analyses. Recent publications suggest a potentially strong (boosting or lowering) effect of previous vaccination on VE estimates [29,30] and end-of-season analyses should take this into account. Although TND is a well-recognised study design to measure VE, we cannot rule out bias from unmeasured confounding.
These early VE results from five studies were included in the Global Influenza VE (GIVE) report to help inform the World Health Organization vaccine strain selection committee meeting on 22 February 2018. For the 2018/19 northern hemisphere trivalent vaccine, this selection committee recommended to include the same influenza A(H1N1) component as in the 2017/18 northern hemisphere vaccine, an A/Singapore/INFIMH-16–0019/2016 (H3N2)-like virus and a B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) [18].
In the context of an influenza season with co-circulation of influenza A(H3N2), influenza A(H1N1)pdm09 and influenza B viruses mismatched with the trivalent vaccine, results from these five EU studies indicate a moderate VE against all influenza. Vaccination continues to be the most effective preventive measure against influenza and uptake of the 2017/18 trivalent or quadrivalent influenza vaccines should still be promoted in countries with ongoing virus circulation. In particular in settings with evidence of influenza A(H3N2) virus circulation, prophylactic use of antiviral drugs, administered according to country-specific guidelines, could help prevent severe outcomes [31]. Based on our results and in the absence of major antigenic drift, we may expect a good protection of the 2018/19 northern hemisphere seasonal influenza vaccine against influenza A(H1N1) and B viruses. Monitoring the effectiveness of the 2018 southern influenza vaccine against influenza A(H3N2) viruses will be important to prepare for the next influenza season in the northern hemisphere.
Acknowledgements
Funding: The five studies have received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 634446 to conduct the study in individuals aged 65 years or more.
ECDC has contributed to fund some study sites of the EU-PC study under the Framework contract No ECDC/2014/026 for the individuals aged less than 65 years.
All study teams are very grateful to all patients, general practitioners, paediatricians, hospital teams, laboratory teams, regional epidemiologists who have contributed to the studies.
We acknowledge the authors, originating and submitting laboratories of the sequences from GISAID’s EpiFlu Database used for this study. All submitters of data may be contacted directly via the GISAID website www.gisaid.org
I-MOVE/I-MOVE+ group: Croatia
EU-PC study:
Bernard Kaic, Croatian Institute of Public Health, Zagreb
Sanja Kurecic Filipovic, Croatian Institute of Public Health, Zagreb
Vesna Visekruna-Vucina, Croatian Institute of Public Health, Zagreb
Iva Pem Novosel, Croatian Institute of Public Health, Zagreb
Zvjezdana Lovric, Croatian Institute of Public Health, Zagreb
Goranka Petrović, Croatian Institute of Public Health, Zagreb
Denmark
DK study:
Tyra Grove Krause, Department of Infectious Disease Epidemiology and Prevention, Statens Serum Institut, Artillerivej 5, 2300 Copenhagen S, Denmark
Thea Kølsen Fische, Department of Virus and Microbiological Special diagnostics, National Influenza Center, Statens Serum Institut, Artillerivej 5, 2300 Copenhagen S, Denmark
France
EU-PC and EU-H studies:
Bruno Lina, Laboratoire de Virologie, CNR des virus des infections respiratoires, Institut des Agents Infectieux, Groupement Hospitalier Nord des HCL, Lyon, France; Laboratoire Virpath, CIRI Inserm U1111, CNRS 5308, ENS, UCBL, Faculté de Médecine LYON Est, Université de Lyon, Lyon.
EU-PC study:
Alessandra Falchi, EA7310, Laboratoire de Virologie, Université de Corse-Inserm, FR- 20250, Corte, France
Ana-Maria Vilcu, Cécile Souty, Thierry Blanchon, Sorbonne Université, INSERM, Institut Pierre Louis d’Epidémiologie et de Santé Publique (IPLESP), F75012 Paris.
Sylvie van der Werf, Unité de Génétique Moléculaire des Virus à ARN, UMR 3569 CNRS, Université Paris Diderot SPC, Institut Pasteur, Paris; CNR des virus des infections respiratoires, Institut Pasteur, Paris
Vincent Enouf, Unité de Génétique Moléculaire des Virus à ARN, UMR 3569 CNRS, Université Paris Diderot SPC, Institut Pasteur, Paris ; CNR des virus des infections respiratoires, Institut Pasteur, Paris.
Sylvie Behillil, Unité de Génétique Moléculaire des Virus à ARN, UMR 3569 CNRS, Université Paris Diderot SPC, Institut Pasteur, Paris; CNR des virus des infections respiratoires, Institut Pasteur, Paris.
Martine Valette, Laboratoire de Virologie, CNR des virus des infections respiratoires, Institut des Agents Infectieux, Groupement Hospitalier Nord des HCL, Lyon.
Sibylle Bernard-Stoecklin, Santé publique France, Paris.
Daniel Lévy-Bruhl, Santé publique France, Paris.
EU-H study:
Odile Launay, Innovative clinical research network in vaccinology, I-REIVAC, CIC Cochin-Pasteur, Paris
Pierre Loulergue, I-REIVAC, CIC Cochin-Pasteur, Paris
Nezha Lenzi, I-REIVAC, Paris
Zineb Lesieur, I-REIVAC, Paris
Anne-Sophie L'Honneur, service de Virologie, Hôpital Cochin, Paris
Florence Galtier: CIC 1411, hôpital St Eloi, CHU de Montpellier ; Inserm, F-CRIN, I-REIVAC
Camille Agostini, CIC 1411, hôpital St Eloi, CHU de Montpellier
Chris Serrand, CIC 1411, hôpital St Eloi, CHU de Montpellier
Corinne Merle, Service des Maladies Infectieuses et Tropicales, Hôpital Gui de Chauliac, CHU de Montpellier
Vincent Foulongne, Pathogenesis and Control of Chronic Infections, Univ. Montpellier, INSERM, EFS, CHU Montpellier, Montpellier, France
Philippe Vanhems, I-REIVAC; Hôpital Edouard Herriot, Lyon
Fabrice Lainé, I-REIVAC; CIC 1414, Hôpital Pontchaillou, Rennes
Gisèle Lagathu, laboratoire de virologie, C.H.U de Rennes
Fabrice Carrat, UPMC Univ Paris 06, IPLESP UMRS 1136, Public health department, Hôpital Saint-Antoine, Paris
Germany
EU-PC study:
Silke Buda, Department for Infectious Disease Epidemiology, Robert Koch Institute, Berlin
Ute Preuss, Department for Infectious Disease Epidemiology, Robert Koch Institute, Berlin
Kerstin Prahm, Department for Infectious Disease Epidemiology, Robert Koch Institute, Berlin
Brunhilde Schweiger, National Reference Center for Influenza, Robert Koch Institute, Berlin
Marianne Wedde, National Reference Center for Influenza, Robert Koch Institute, Berlin
Alla Heider, National Reference Center for Influenza, Robert Koch Institute, Berlin
Maria Martin, National Reference Center for Influenza, Robert Koch Institute, Berlin
Barbara Biere, National Reference Center for Influenza, Robert Koch Institute, Berlin
Ralf Duerrwald, National Reference Center for Influenza, Robert Koch Institute, Berlin
Ireland
EU-PC study:
Lisa Domegan, HSE-Health Protection Surveillance Centre, Dublin
Laura Coughlan, HSE-Health Protection Surveillance Centre, Dublin
Joan O’Donnell, HSE-Health Protection Surveillance Centre, Dublin
Michael Joyce, Irish College of General Practitioners, Dublin
Claire Collins, Irish College of General Practitioners, Dublin
Linda Dunford, National Virus Reference Laboratory, University College Dublin
Joanne Moran, National Virus Reference Laboratory, University College Dublin
Grainne Tuite, National Virus Reference Laboratory, University College Dublin
Margaret Duffy, National Virus Reference Laboratory, University College Dublin
Jeff Connell, National Virus Reference Laboratory, University College Dublin
Cillian de Gascun, National Virus Reference Laboratory, University College Dublin
Italy
Italian Influenza Surveillance System:
Caterina Rizzo, Istituto Superiore di Sanità, Rome
Antonino Bella, Istituto Superiore di Sanità, Rome
Valeria Alfonsi, Istituto Superiore di Sanità, Rome
Maria Rita Castrucci, National Influenza Center, Istituto Superiore di Sanità, Rome
Simona Puzelli, National Influenza Center, Istituto Superiore di Sanità, Rome
EU-PC study:
Elisabetta Pagani, Regional Reference Laboratory for influenza, TRENTINO ALTO ADIGE, Bolzano
Valeria Ghisetti, Regional Reference Laboratory for influenza, PIEMONTE
Elena Pariani, Regional Reference Laboratory for influenza, LOMBARDIA, Milano
Fausto Baldanti, Regional Reference Laboratory for influenza, LOMBARDIA, Pavia
Giorgio Palù, Regional Reference Laboratory for influenza, VENETO
Pierlanfranco D'Agaro, Regional Reference Laboratory for influenza, FRIULI VENEZIA GIULIA
Filippo Ansaldi, Regional Reference Laboratory for influenza, LIGURIA
Paola Affanni, Regional Reference Laboratory for influenza, EMILIA-ROMAGNA
Gian Maria Rossolini, Regional Reference Laboratory for influenza, TOSCANA
Barbara Camilloni, Regional Reference Laboratory for influenza, UMBRIA
Patrizia Bagnarelli, Regional Reference Laboratory for influenza, MARCHE
Maurizio Sanguinetti, Regional Reference Laboratory for influenza, LAZIO
Luigi Atripaldi, Regional Reference Laboratory for influenza, CAMPANIA
Maria Chironna, Regional Reference Laboratory for influenza, PUGLIA
Caterina Serra, Regional Reference Laboratory for influenza, SARDEGNA
Francesco Vitale, Regional Reference Laboratory for influenza, SICILIA
EU-H study:
Maria Chironna, Department of Biomedical Science and Human Oncology, Bari hospital, Bari
Cinzia Germinario, Department of Biomedical Science and Human Oncology, Bari hospital, Bari
Andrea Orsi, San Martino Polyclinic Hospital, University of Genoa, Genoa
Filippo Ansaldi, San Martino Polyclinic Hospital, University of Genoa, Genoa
Ilaria Manini, Department of Molecular and Developmental Medicine, University of Siena, Siena
Emanuele Montomoli, Department of Molecular and Developmental Medicine, University of Siena, Siena
Christian Napoli, Sant’Andrea Hospital, Sapienza University of Rome, Rome
Giovanni Battista Orsi, Sant’Andrea Hospital, Sapienza University of Rome, Rome
Navarra:
EU-H study:
Itziar Casado, Instituto de Salud Pública de Navarra, IdiSNA, CIBERESP, Pamplona
Jesús Castilla, Instituto de Salud Pública de Navarra, IdiSNA, CIBERESP, Pamplona
Leticia Fernandino, Instituto de Salud Pública de Navarra, IdiSNA, CIBERESP, Pamplona
Iván Martínez-Baz, Instituto de Salud Pública de Navarra, IdiSNA, CIBERESP, Pamplona
Guillermo Ezpeleta, Instituto de Salud Pública de Navarra, IdiSNA, CIBERESP, Pamplona
Ana Navascués, Complejo Hospitalario de Navarra, IdiSNA, Pamplona
Alejandra Pérez-García, Complejo Hospitalario de Navarra, IdiSNA, Pamplona
Aitziber Aguinaga, Complejo Hospitalario de Navarra, IdiSNA, Pamplona
Carmen Ezpeleta, Complejo Hospitalario de Navarra, IdiSNA, Pamplona
The Netherlands
EU-PC and EU-H studies:
Adam Meijer, National Institute for Public Health and the Environment (RIVM), Bilthoven
Sharon van den Brink, National Institute for Public Health and the Environment (RIVM), Bilthoven
Wim van der Hoek, National Institute for Public Health and the Environment (RIVM), Bilthoven
Gabriel Goderski, National Institute for Public Health and the Environment (RIVM), Bilthoven
Lisa Wijsman, National Institute for Public Health and the Environment (RIVM), Bilthoven
EU-PC study:
Mariam Bagheri, National Institute for Public Health and the Environment (RIVM), Bilthoven
Frederika Dijkstra, National Institute for Public Health and the Environment (RIVM), Bilthoven
Marit de Lange, National Institute for Public Health and the Environment (RIVM), Bilthoven
Ton Marzec, National Institute for Public Health and the Environment (RIVM), Bilthoven
Pieter Overduin, National Institute for Public Health and the Environment (RIVM), Bilthoven
Anne Teirlinck, National Institute for Public Health and the Environment (RIVM), Bilthoven
Erny Wentink, Netherlands Institute for Health Services Research (NIVEL), Utrecht
Gé Donker, Netherlands Institute for Health Services Research (NIVEL), Utrecht
EU-H study:
Sierk Marbus, National Institute for Public Health and the Environment (RIVM), Bilthoven
Rianne van Gageldonk- Lafeber, National Institute for Public Health and the Environment (RIVM), Bilthoven
Peter Schneeberger, Jeroen Bosch Hospital, 's Hertogenbosch
Jan Jelrik van Oosterheert, University Medical Center Utrecht, Utrecht
Valentijn Schweitzer, University Medical Center Utrecht, Utrecht
Geert Groeneveld, Leiden University Medical Center, Leiden
Portugal
EU-PC and EU-H studies:
Baltazar Nunes, Departamento de Epidemiologia, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon
Ausenda Machado, Departamento de Epidemiologia, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon
Ana Paula Rodrigues, Departamento de Epidemiologia, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon
Verónica Gomez, Departamento de Epidemiologia, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon
Irina Kislaya, Departamento de Epidemiologia, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon
EU-PC study:
Raquel Guiomar, Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon
Pedro Pechirra, Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon
Paula Cristóvão, Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon
Inês Costa, Departamento de Doenças Infeciosas, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon
EU-H study:
António Panarra, Centro Hospitalar de Lisboa Central, Lisbon
Rita Côrte-Real, Centro Hospitalar de Lisboa Central, Lisbon
José Poças, Centro Hospitalar de Setúbal, Setúbal
Maria João Peres, Centro Hospitalar de Setúbal, Setúbal
Spain
The cycEVA work group
Amparo Larrauri:, National Centre of Epidemiology, Institute of Health Carlos III, CIBERESP
Alin Gherasim, National Centre of Epidemiology, Institute of Health Carlos III, CIBERESP
Francisco Pozo, Inmaculada Casas National Centre for Microbiology, National Influenza Reference Laboratory,
HO-National Influenza Centre, Institute of Health Carlos III
Luis García Comas, Dirección General de Salud Pública, Comunidad de Madrid
María Esther Insua Marisquerena, Dirección General de Salud Pública, Comunidad de Madrid
Juan Carlos Galán, Laboratorio Hospital Ramón y Cajal
Mª Dolores Folgueira, Laboratorio Hospital Doce de Octubre
Fernando Gonzalez Carril, Departamento de Salud, Gobierno del País Vasco,
Rosa Sancho Martínez, Departamento de Salud, Gobierno del País Vasco
Gustavo Cilla Laboratorio Hospital Donostia, CIBERER
Jesús Castilla, Navarra Institute for Health Research IdiSNA, Pamplona, CIBERESP
Manuel García Cenoz, Navarra Institute for Health Research IdiSNA, Pamplona, CIBERESP
Ana Navascués, Complejo Hospitalario de Navarra
Carmen Quiñones Rubio, Dirección General de Salud Pública y Consumo de La Rioja
Eva Martinez Ochoa, Dirección General de Salud Pública y Consumo de La Rioja
Miriam Blasco, Laboratorio Hospital San Pedro de Logroño
Jaume Gimenez Duran, Servicio de Epidemiología, Dirección General de Salud Pública, Mallorca
Juana Maria Vanrell, Servicio de Epidemiología, Dirección General de Salud Pública, Mallorca
Jordi Reina, Laboratorio del Hospital Son Espases, Mallorca
Daniel Castrillejo, Servicio de Epidemiología. DGSC, Consejería de Bienestar Social y Sanidad, Ciudad Autónoma
e Melilla
The Spanish Influenza Sentinel Surveillance System:
Amparo Larrauri, National Centre of Epidemiology, Institute of Health Carlos III, CIBERESP
Alin Manuel Gherasim, National Centre of Epidemiology, Institute of Health Carlos III, CIBERESP
Concha Delgado, National Centre of Epidemiology, Institute of Health Carlos III, CIBERESP
Jesus Oliva National Centre of Epidemiology, Institute of Health Carlos III, CIBERESP Francisco Pozo, National Centre for Microbiology, National Influenza Reference Laboratory, WHO-National Influenza Centre, Institute of Health Carlos III.
Inmaculada Casas, National Centre for Microbiology, National Influenza Reference Laboratory, WHO-National Influenza Centre, Institute of Health Carlos III.
The Spanish IMOVE+ study: the hospitals component:
Amparo Larrauri, National Centre of Epidemiology, Institute of Health Carlos III, CIBERESP
Alin Manuel Gherasim, National Centre of Epidemiology, Institute of Health Carlos III, CIBERESP
Francisco Pozo, National Centre for Microbiology, National Influenza Reference Laboratory, WHO-National Influenza Centre, Institute of Health Carlos III.
Inmaculada Casas, National Centre for Microbiology, National Influenza Reference Laboratory, WHO-National Influenza Centre, Institute of Health Carlos III.
Miriam García Dirección General de Salud Pública, Aragón;
Miriam Latorre, Hospital Universitario Miguel Servet, Zaragoza
Ana María Milagro Beamonte, Hospital Universitario Miguel Servet, Zaragoza
Ana Martinez Sapiña, Hospital Universitario Miguel Servet, Zaragoza
Madalen Oribe Amores, Subdirección de Salud Pública Gipuzkoa, País Vasco
Amaia Aizpurúa, Laboratorio Hospital Donostia, CIBERER
Gustavo Cilla, Laboratorio Hospital Donostia, CIBERER
Milagrosa Montes, Laboratorio Hospital Donostia, CIBERER
Sweden
EU-PC study:
Katherina Zakikhany, The Public Health Agency of Sweden, Stockholm
Mia Brytting, The Public Health Agency of Sweden, Stockholm
Åsa Wiman, The Public Health Agency of Sweden, Stockholm
Annasara Carnahan, The Public Health Agency of Sweden, Stockholm
United Kingdom
UK study:
Richard Pebody, Public Health England, London
Fiona Warburton, Public Health England, London
Abdelmajid Djennad, Public Health England, London
Joanna Ellis, Public Health England, London
Nick Andrews, Public Health England, London
Diogo Marques, Health Protection Scotland, Glasgow
Simon Cottrell, Public Health Wales, Cardiff
Arlene Reynolds, Health Protection Scotland, Glasgow
Rory Gunson, West of Scotland Specialist Virology Centre, Glasgow
Monica Galiano, Public Health England, London
Angie Lackenby, Public Health England, London
Chris Robertson, University of Strathclyde, Glasgow
Mark O’Doherty, Public Health Agency Northern Ireland, Belfast
Mary Sinnathamby, Public Health England, London
Ivelina Yonova, University of Surrey, Guildford, Royal College of General Practitioners, London
Catherine Moore, Public Health Wales, Cardiff
Muhammed Sartaj, Public Health Agency Northern Ireland, Belfast
Simon de Lusignan, University of Surrey, Guildford, Royal College of General Practitioners, London
Jim McMenamin, Health Protection Scotland, Glasgow
Maria Zambon, Public Health England, London
EpiConcept
EU-PC and EU-H studies: Marta Valenciano, Alain Moren, EpiConcept, Paris
EU-PC study: Esther Kissling, EpiConcept, Paris
EU-H study: Marc Rondy, EpiConcept, Paris
European Centre for Disease Prevention and Control (ECDC)
Pasi Penttinen, European Centre for Disease Prevention and Control
Conflict of interest: None
Authors’ contributions: Marc Rondy: coordination I-MOVE+ hospital network, study design, analysis of hospital data, interpretation of results, manuscript writing. Esther Kissling: coordination I-MOVE/I-MOVE+ primary care network, study design, analysis of primary care data, interpretation of results, manuscript writing. Both authors contributed equally to the study and manuscript. Hanne-Dorthe Emborg, Alin Gherasim, Richard Pebody, Ramona Trebbien, Amparo Larrauri and Jim McMenamin: coordination of their respective studies, data analysis and interpretation of results. Read, contributed to and approved the final version of the manuscript. Francisco Pozo: coordinated the I-MOVE/I-MOVE+ virological analysis of the primary care study. European IVE group: Primary care and hospital sites at national/regional level: data collection, data validation, results interpretation, review of manuscript.
Laboratories: virological analysis, genetic characterisation, interpretation of results, review of manuscript.
Alain Moren, Marta Valenciano: study design, coordination of I-MOVE/I-MOVE+ network, interpretation of results, contribution to manuscript writing. Pasi Penttinen: study design, interpretation of results, review of manuscript.
Reference list
- 1.European Centre for Disease Prevention and Control (ECDC). Seasonal influenza vaccination and antiviral use in Europe. Overview of vaccination recommendations and coverage rates in the EU Member States for the 2013-14 and 2014-15 influenza seasons. VENICE technical report [Internet]. Stockholm: ECDC; 2016. Available from: http://venice.cineca.org/Seasonal-influenza-vaccination-antiviral-use-europe.pdf
- 2.Hakin B, Cosford P, Harvey F. The flu immunisation programme 2013/14– extension to children. London: Department of Health; 2013. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/225360/Children_s_flu_letter_2013.pdf
- 3.World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2017-2018 northern hemisphere influenza season. Geneva: WHO; 2017. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/2017_18_north/en/
- 4.European Centre for Disease Prevention and Control (ECDC), World Health Organization Regional Office for Europe (WHO/Europe). Flu News Europe. Summary week 7/2018 (12-18 February 2018). Stockholm: ECDC, Copenhagen: WHO/Europe; 2018. Available from: http://flunewseurope.org/Archives
- 5.I-MOVE+. Protocol for hospital-based test negative case control studies to measure seasonal influenza vaccine effectiveness against influenza laboratory confirmed SARI hospitalisation among the elderly across the European Union and European Economic Area Member States. Paris: EpiConcept; 2016. Available from: https://drive.google.com/file/d/0B54XpZN4SY65OGRSTHlvOVZTMFE/view
- 6.European Centre for Disease Prevention and Control (ECDC). Protocol for case control studies to measure pandemic and seasonal vaccine effectiveness in the European Union and European Economic Area. Stockholm: ECDC; 2009. Available from: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0907_TED_Influenza_AH1N1_Measuring_Influenza_Vaccine_Effectiveness_Protocol_Case_Control_Studies.pdf
- 7. Jimenez-Jorge S, de Mateo S, Delgado-Sanz C, Pozo F, Casas I, Garcia-Cenoz M, et al. Estimating influenza vaccine effectiveness in Spain using sentinel surveillance data. Euro Surveill. 2015;20(28):21187. 10.2807/1560-7917.ES2015.20.28.21187 [DOI] [PubMed] [Google Scholar]
- 8. Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Euro Surveill. 2016;21(38):30348. 10.2807/1560-7917.ES.2016.21.38.30348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emborg HD, Krause TG, Nielsen L, Thomsen MK, Christiansen CB, Skov MN, et al. Influenza vaccine effectiveness in adults 65 years and older, Denmark, 2015/16 - a rapid epidemiological and virological assessment. Euro Surveill. 2016;21(14):30189. 10.2807/1560-7917.ES.2016.21.14.30189 [DOI] [PubMed] [Google Scholar]
- 10. Rodrigues L, Kirkwood BR. Case-control designs in the study of common diseases: updates on the demise of the rare disease assumption and the choice of sampling scheme for controls. Int J Epidemiol. 1990;19(1):205-13. 10.1093/ije/19.1.205 [DOI] [PubMed] [Google Scholar]
- 11. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373-9. 10.1016/S0895-4356(96)00236-3 [DOI] [PubMed] [Google Scholar]
- 12. Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942-51. 10.1016/S1473-3099(16)00129-8 [DOI] [PubMed] [Google Scholar]
- 13. Kissling E, Valenciano M, Pozo F, Vilcu A-M, Reuss A, Rizzo C, et al. 2015/16 I-MOVE/I-MOVE+ multicentre case control study in Europe: moderate vaccine effectiveness estimates against influenza A(H1N1)pdm09 and low estimates against lineage mismatched influenza B among children. Influenza Other Respir Viruses. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2016-2017 northern hemisphere influenza season. Geneva: WHO; 2016. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/2016_17_north/en/
- 15. Rondy M, Gherasim A, Casado I, Launay O, Rizzo C, Pitigoi D, et al. Low 2016/17 season vaccine effectiveness against hospitalised influenza A(H3N2) among elderly: awareness warranted for 2017/18 season. Euro Surveill. 2017;22(41):17-00645. 10.2807/1560-7917.ES.2017.22.41.17-00645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sullivan SG, Chilver MB, Carville KS, Deng Y-M, Grant KA, Higgins G, et al. Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017. Euro Surveill. 2017;22(43):17-00707. 10.2807/1560-7917.ES.2017.22.43.17-00707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skowronski DM, Chambers C, De Serres G, Dickinson JA, Winter A-L, Hickman R, et al. Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018. Euro Surveill. 2018;23(5):18-00035. 10.2807/1560-7917.ES.2018.23.5.18-00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2018-2019 northern hemisphere influenza season. Geneva: WHO; 2018. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/201802_recommendation.pdf?ua=1
- 19. Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: A systematic review and meta-analysis of test-negative design case-control studies. J Infect. 2017;75(5):381-94. 10.1016/j.jinf.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Public Health England (PHE). Summary of data to support the choice of influenza vaccination for adults in primary care. London: PHE; 2018. Available from: https://www.gov.uk/government/publications/flu-vaccination-supporting-data-for-adult-vaccines/summary-of-data-to-support-the-choice-of-influenza-vaccination-for-adults-in-primary-care
- 21. Flannery B, Chung JR, Belongia EA, McLean HQ, Gaglani M, Murthy K, et al. Interim Estimates of 2017-18 Seasonal Influenza Vaccine Effectiveness - United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67(6):180-5. 10.15585/mmwr.mm6706a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castilla J, Navascués A, Casado I, Pérez-García A, Aguinaga A, Ezpeleta G, et al. Interim effectiveness of trivalent influenza vaccine in a season dominated by lineage mismatched influenza B, northern Spain, 2017/18. Euro Surveill. 2018;23(7):18-00057. 10.2807/1560-7917.ES.2018.23.7.18-00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skowronski DM, Janjua NZ, Sabaiduc S, De Serres G, Winter A-L, Gubbay JB, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011-2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis. 2014;210(1):126-37. 10.1093/infdis/jiu048 [DOI] [PubMed] [Google Scholar]
- 24. Rondy M, Larrauri A, Casado I, Alfonsi V, Pitigoi D, Launay O, et al. 2015/16 seasonal vaccine effectiveness against hospitalisation with influenza A(H1N1)pdm09 and B among elderly people in Europe: results from the I-MOVE+ project. Euro Surveill. 2017;22(30):30580. 10.2807/1560-7917.ES.2017.22.30.30580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11(1):153. 10.1186/1741-7015-11-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DiazGranados CA, Denis M, Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: a systematic review with meta-analyses of controlled trials. Vaccine. 2012;31(1):49-57. 10.1016/j.vaccine.2012.10.084 [DOI] [PubMed] [Google Scholar]
- 27.European Centre for Disease Prevention and Control (ECDC). Types of seasonal influenza vaccine. Stockholm: ECDC. [Accessed 28 Feb 2018]. Available from: https://ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/types-of-seasonal-influenza-vaccine
- 28.Impfkommission S. (STIKO). Wissenschaftliche Begründung für die Empfehlung des quadrivalenten saisonalen Influenzaimpfstoffs. [Scientific reason for recommending the quadrivalent seasonal influenza vaccine]. Epidemiologisches Bulletin. 2018;2. German. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2018/Ausgaben/02_18.pdf?__blob=publicationFile
- 29. Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. 2017;16(7):1-14. 10.1080/14760584.2017.1334554 [DOI] [PubMed] [Google Scholar]
- 30. Rondy M, Launay O, Castilla J, Costanzo S, Puig-Barberà J, Gefenaite G, et al. InNHOVE/I-MOVE+working group Repeated seasonal influenza vaccination among elderly in Europe: Effects on laboratory confirmed hospitalised influenza. Vaccine. 2017;35(34):4298-306. 10.1016/j.vaccine.2017.06.088 [DOI] [PubMed] [Google Scholar]
- 31.European Centre for Disease Prevention and Control (ECDC). Expert opinion on neuraminidase inhibitors for the prevention and treatment of influenza – review of recent systematic reviews and meta-analyses. Stockholm: ECDC; 2017. Available from: https://ecdc.europa.eu/sites/portal/files/documents/Scientific-advice-neuraminidase-inhibitors-2017.pdf


