Abstract
The aim of the present study was to analyze the risk factors for new-onset chronic kidney disease (CKD) in patients who have received a liver transplant. A total of 190 patients who underwent liver transplantation between March 2001 and January 2015 were followed up, and analyzed retrospectively. Sex, age, primary disease, preoperative laboratory findings (hemoglobin, albumin, creatinine and glomerular filtration rate), surgical approach, blood loss during the surgery and transfusion volume, postoperative complications, and the average levels of calcineurin inhibitors (CNIs) (from liver transplantation to the onset of CKD) were analyzed. In total, 40 patients developed new-onset CKD after transplantation. Clinical data in the new-onset CKD group were compared with the non-CKD group. A χ2 test, t-test and logistic regression analysis were performed using SPSS 17.0 software. The incidence of new-onset CKD after liver transplantation was 21.1%. Renal pathology included IgA nephropathy, hepatitis B virus-associated nephropathy, membranous proliferative glomerulonephritis, focal segmental glomerular sclerosis and cryoglobulinemia-associated renal injury. Among the CKD patients, 85.7% had tubulointerstitial damage. Univariate analysis showed that preoperative renal function, hemoglobin, intraoperative blood loss and transfusion volume, postoperative acute kidney injury, average levels of CNIs, and hypertension were risk factors for new-onset CKD after liver transplantation. Logistic regression analysis showed that preoperative glomerular filtration rate [odds ratio (OR)=0.980, P=0.041], hemoglobin (OR=0.972, P=0.034), average levels of CNIs (OR=1.364, P=0.015) and hypertension (OR=4.833, P=0.048)] were independent risk factors for new-onset CKD. The incidence of new-onset CKD in patients who received liver transplantation was high. The main risk factors were identified to be preoperative glomerular filtration rate, hemoglobin, postoperative average levels of CNIs and hypertension.
Keywords: calcineurin inhibitors, chronic kidney disease, liver transplantation, renal pathology
Introduction
With the increase in the success rate of liver transplantation, giving rise to overall 1- and 5-year patient survival rates of 90 and 75%, respectively (1), more and more patients are developing late complications, such as renal dysfunction. Liver transplant patients have the second highest incidence of chronic kidney disease (CKD) post-surgery, after heart and lung transplant patients (2). Some studies have reported the incidence of CKD is 4.0–27.5% within 1 year after liver transplantation (3). CKD is a major contributor to post-transplant mortality. The relative risk is 3.32 (2.96–3.71) (2). Therefore, it is necessary to identify the causes of new-onset CKD after liver transplantation. Some studies have reported that the causes are complex and may include the preoperative glomerular filtration rate, a history of hypertension, diabetes, the degree of proteinuria, hemoglobin level and the duration of renal impairment (4–6). Calcineurin inhibitor (CNI)-related nephrotoxicity and chronic CNI nephrotoxicity are often observed in liver transplant patients. One study indicated that low-dose tacrolimus combined with everolimus 1 month after liver transplantation could help preserve renal function at 1 year post-liver transplantation (7). However, few studies have been published regarding the intraoperative risk factors for new-onset CKD. The goal of the current study was to analyze the risk factors for new-onset CKD occurring in patients receiving liver transplantation, and to assess how the quality of life and long-term survival of these patients may be improved.
Patients and methods
Patients
Forty patients with new-onset CKD were analyzed retrospectively among 190 patients who underwent liver transplantation between March 2001 and January 2015 at Beijing Tsinghua Changgung Hospital (Beijing, China) or People's Hospital, Peking University (Beijing, 100044); the remaining 150 patients did not develop CKD, and 40 of them were selected randomly, using a random number chart, to serve as the control group. The risk factors for new-onset CKD occurrence were analyzed in patients who received liver transplantation.
Methods
Demographic details, relevant preoperative and postoperative laboratory tests, and average levels of CNIs (during liver transplantation to the onset of CKD) were recorded. All patients were followed up every 3 months after liver transplantation, and urinalysis and kidney function were examined. None of the patients required hemodialysis. Kidney function was assessed by estimated glomerular filtration rate (eGFR), which was calculated with the CKD-EPI equation according to the 2002 KDOQI guidelines (3). CKD is defined as renal impairment (kidney morphology, pathology, imaging, blood or urine composition abnormalities) persisting for >3 months with or without eGFR decrease, and/or eGFR<60 ml/min·1.73 m2 for >3 months with or without renal impairment. New-onset CKD is defined as CKD that occurs after liver transplantation. In patients with hepatitis B virus (HBV), HBsAg, HBsAb, HBeAg, HBeAb, HBcAb and HBV-DNA were tested regularly. Antiviral drugs included entecavir, adefovir dipivoxil and tenofovir. In patients with hepatitis C virus (HCV), hepatitis C antibody and HCV-RNA were examined regularly. The routine treatments included interferon, lamivudine and ribavirin. Prednisone, tacrolimus and mycophenolate mofetil were selected as anti-rejection drugs after liver transplantation.
Statistical analysis
Statistical analyses were performed with SPSS version 17.0, with P<0.05 considered to indicate a statistically significant difference. Measurement data are expressed as the mean ± standard deviation (SD). Data were estimated by Kolmogorov-Smirnov test for normal distribute test, unpaired Student's t-test was used to compare between groups if the data were estimated to be normally-distributed, and the Mann Whitney U test was used if the data had a non-normal distribution. Count data are expressed as a percentage, and a χ2 test was used to compare between two groups. Logistic regression analysis was used to analyze the contributing factors to new-onset CKD in patients receiving liver transplantation.
Results
Comparison of preoperative clinical data between the new-onset CKD group and the non-CKD group in patients who received liver transplantation
The new-onset CKD patients were followed up for 63±45 months (4–156 months) vs. 42±21 months (6–84 months) in the non-CKD group. The average age in the new-onset CKD group was 51.5±8.8 (28–70) years, compared with 47.4±11.2 (10–60) years in the non-CKD group. Of the 40 patients in the new-onset CKD group, 35 (87.5%) were male.
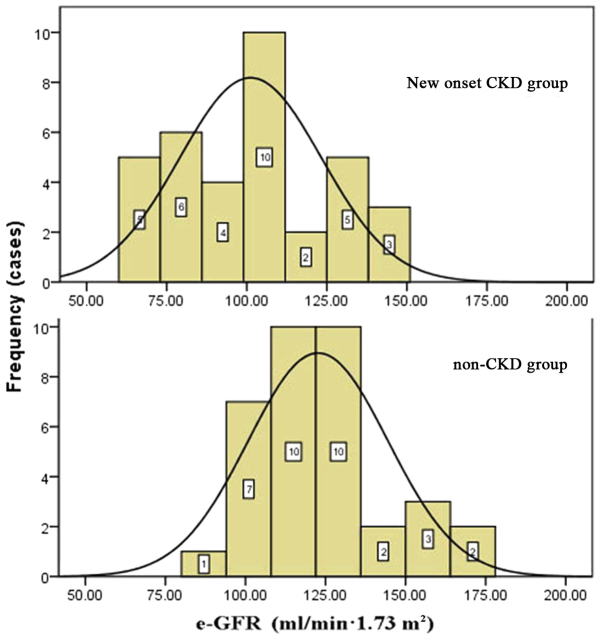
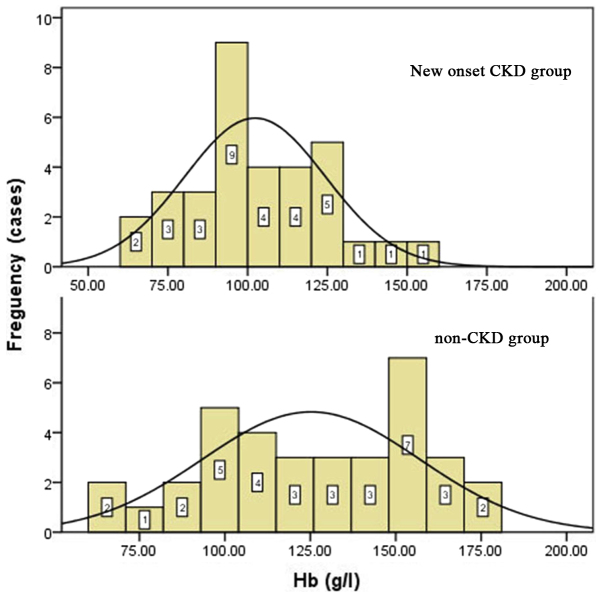
Primary diseases of the liver included HBV-related cirrhosis, alcoholic cirrhosis, liver cancer, HBV-related cirrhosis with hepatocellular carcinoma, primary biliary cirrhosis, cryptogenic cirrhosis and drug-induced hepatitis. There were no statistically significant differences in primary liver diseases between the two groups (P=0.054), as shown in Table I. Preoperative biochemical tests included hemoglobin level, liver function, renal function, and albumin and lipid levels. The preoperative eGFRs and hemoglobin levels of the new-onset CKD group were significantly lower than those in the control group (P<0.05): The mean hemoglobin level was 102.6±21.7 g/l in the new-onset CKD group vs. 121.3±28.7 g/l in non-CKD patients; and the mean eGFR was 105.6±37.1 ml/min·1.73 m2 in the new-onset CKD group vs. 131.6±44.1 ml/min·1.73 m2 in the non-CKD group (Table II; Figs. 1 and 2). None of the patients in the new-onset CKD and non-CKD groups were diagnosed with CKD prior to the liver transplant. Some of the patients in new-onset CKD group seems have lower eGFR but the data were all in normal range.
Table I.
Primary liver diseases in new onset CKD and non-CKD groups.
| Factors | New-onset CKD, n (%) (n=40) | Non-CKD, n (n=40) (%) | χ2 | P-value |
|---|---|---|---|---|
| Hepatitis B liver cirrhosis | 16 (40.0) | 9 (22.5) | ||
| Hepatitis C liver cirrhosis | 2 (5.0) | 2 (5.0) | ||
| Alcoholic cirrhosis | 4 (10.0) | 3 (7.5) | ||
| Liver cancer | 3 (7.5) | 4 (10.0) | 16.70 | 0.06 |
| Hepatitis B liver cirrhosis and liver cancer | 12 (30.0) | 20 (50.0) | ||
| Hepatitis C liver cirrhosis and liver cancer | 0 (0) | 1 (2.5) | ||
| Alcoholic cirrhosis and liver cancer | 1 (2.5) | 0 (0) | ||
| Hepatitis B and C liver cirrhosis | 2 (5.0) | 1 (2.5) |
CKD, chronic kidney disease.
Table II.
Preoperative clinical data in new-onset CKD and non-CKD groups.
| Factors | New-onset CKD (n=40) | Non-CKD (n=40) | Z/t | P-value |
|---|---|---|---|---|
| HB (g/l) | 102.6±21.7 | 121.3±28.7 | −3.039 | 0.003a |
| Scr (umol/l) | 77.7±26.1 | 61.4±16.3 | 3.125 | 0.003a |
| eGFR (ml/min·1.73 m2) | 105.6±37.1 | 131.6±44.1 | −2.661 | 0.010a |
| BUN (mmol/l) | 7.9±6.6 | 4.6±1.4 | 1.674b | 0.094 |
| ALT (U/l) | 44.7±48.9 | 41.5±32.0 | 0.135b | 0.893 |
| AST (U/l) | 59.6±35.7 | 53.6±43.3 | 1.355b | 0.175 |
| TBIL (mmol/l) | 136.8±167.4 | 79.4±119.3 | 1.787b | 0.074 |
| TG (mmol/l) | 1.12±0.84 | 0.93±0.44 | 0.270b | 0.787 |
| LDL (mmol/l) | 1.63±1.58 | 1.79±0.71 | −0.503 | 0.619 |
| ALB (g/l) | 35.0±7.1 | 36.6±5.9 | −1.035 | 0.304 |
| Hypertension (%) | 3/40 (7.5) | 3/40 (7.5) | N/A | |
| Diabeties (%) | 2/40 (5) | 2/40 (5) | N/A |
P<0.05 is considered statistically difference. Z
statistical value of non-normal distribution, t is the statistical value of normal distribution. CKD, chronic kidney disease; HB, hemoglobin; Scr, creatinine; eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen; ALT, alanine transaminase; AST, aspartate transaminase; TBIL, total bilirubin; TG, triglyceride; LDL, low density lipoprotein; ALB, albumin.
Figure 1.
Preoperative eGFR distribution in new onset CKD group (above) and Non-CKD group (below). eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease.
Figure 2.
Preoperative hemoglobin distribution in new onset CKD group (above) and non-CKD group (below). CKD, chronic kidney disease.
Surgical methods, intraoperative blood loss, blood transfusion and anhepatic time
13/40 patients (40.6%) in the new-onset CKD group and 22/40 (55%) in the non-CKD group received orthotropic liver transplantation. 19/40 (59.4%) patients in the new-onset CKD group and 18/40 (45%) in the non-CKD group received piggyback orthotropic liver transplantation. There were no significant differences in surgical procedures between the two groups (P=0.263).
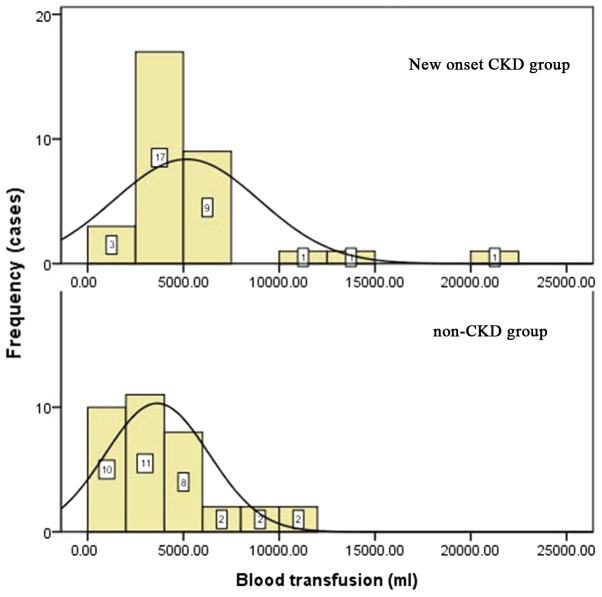
Blood loss in the new-onset CKD group was 5,697±7,749 ml vs. 2,268±2,185 ml in the control group (P=0.000), and intraoperative blood transfusion in the new-onset CKD group was 5,181±3,780 ml vs. 3,754±2,902 ml in the control group (P=0.031). Blood loss and intraoperative blood transfusion were different between the two groups. The anhepatic time was 63.3±15.6 min in the new-onset CKD group vs. 69.2±18.0 min in the non-CKD group, and there was no significant difference between the two groups (P=0.611). The blood transfusion volumes during surgery are shown in Fig. 3.
Figure 3.
Intraoperative blood transfusion in new-onset CKD group (above) and non-CKD group (below). CKD, chronic kidney disease.
Postoperative incidence of acute kidney injury (AKI)
AKI after liver transplantation was defined as a serum creatinine level elevated to ≥26.4 µmol/l, or increased to ≥50% of the baseline within one 48 h period during 1 month following liver transplantation. 16/40 (40%) patients in the new-onset CKD group developed postoperative AKI vs. no patients in the non-CKD group, as shown in Table III.
Table III.
Postoperative clinical data on new-onset CKD and non-CKD patients who received liver transplantation.
| Factors | New-onset CKD | Non-CKD | t/χ2 | P-value |
|---|---|---|---|---|
| AKI (%) | 16 (40.0) | 0 (0.0) | 17.797 | <0.001a |
| Tacrolimus (ng/ml) | 14.0±4.5 | 11.0±2.8 | 3.334b | 0.001a |
| Postoperative hypertension (%) | 14 (35.0) | 5 (12.5) | 4.234 | 0.040a |
| Postoperative diabetes (%) | 13 (32.5) | 10 (25.0) | 1.491 | 0.222 |
P<0.05 is considered to indicate a statistically significant difference. t
statistical value in normal distribution, χ2 is the statistical value in categorical variables. CKD, chronic kidney disease; AKI, acute kidney injury.
Average levels of CNIs from liver transplantation to the onset of CKD
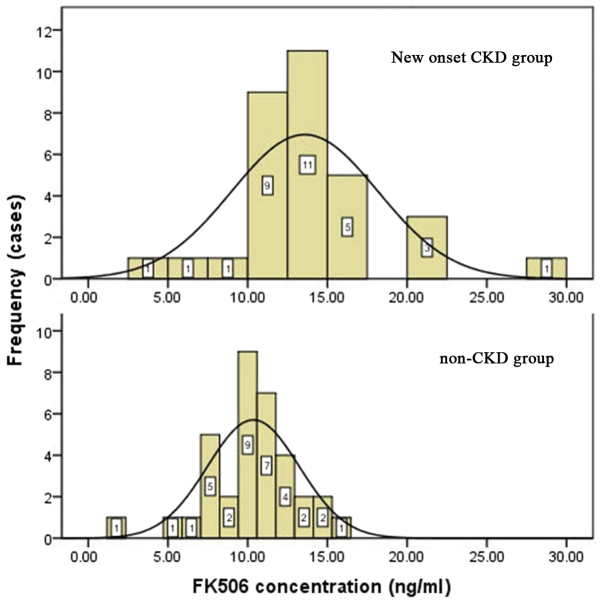
We calculated the average levels of CNIs from liver transplantation to the onset of CKD. The mean concentration of tacrolimus in the new-onset CKD group was 14.0±4.5 ng/ml vs. 11.0±2.8 ng/ml in the control group (P=0.001), as shown in Table III and Fig. 4.
Figure 4.
The postoperative plasma concentration of FK506 in new onset CKD group (above) and non-CKD group (below). CKD, chronic kidney disease.
Postoperative complications
Postoperative complications included postoperative hypertension and diabetes. 14/40 (35.0%) patients developed hypertension in the new-onset CKD group vs. 5/40 (12.5%) patients in the control group (P=0.040); 13/40 (32.5%) patients developed diabetes in the new-onset CKD group vs. 9/40 (25.0%) patients in the control group (P=0.222), as shown in Table III. There was no recurrence of HBV and HCV post-liver transplantation within the follow-up period, which was 63±45 months in the new-onset CKD group and 42±21 months in the control group.
Risk factors for new-onset CKD in patients receiving liver transplantation, identified by logistic regression analysis
Our data, analyzed by univariate analysis, indicated that preoperative eGFR (P=0.010), preoperative hemoglobin (P=0.003), intraoperative blood loss (P=0.000), intraoperative blood transfusion (P=0.031), postoperative AKI (P=0.000), postoperative average levels of CNIs (P=0.001) and postoperative hypertension (P=0.040) were associated with new-onset CKD occurrence in patients who had undergone liver transplantation, as shown in Table IV. By multivariate logistic regression analysis, we showed that preoperative eGFR, hemoglobin level, average postoperative levels of CNIs and postoperative hypertension were independent risk factors for new-onset CKD occurrence (Table V).
Table IV.
Risk factors for new onset CKD in patients that received liver transplantation by univariate logistic regression analysis.
| Factors | b | OR | 95% CI | P-value |
|---|---|---|---|---|
| eGFR | −0.017 | 0.983 | 0.970–0.997 | 0.010 |
| HGB | −0.029 | 0.972 | 0.952–0.992 | 0.003 |
| Intraoperative blood loss | 0.000 | 1.000 | 1.000–1.001 | <0.001 |
| Intraoperative blood transfusion | 0.027 | 1.003 | 1.000–1.010 | 0.031 |
| Acute kidney injury | 0.115 | 1.010 | 1.001–1.021 | <0.001 |
| Calcineurin concentration | 0.261 | 1.299 | 1.082–1.559 | 0.001 |
| Postoperative hypertension | 1.212 | 3.360 | 1.063–10.620 | 0.040 |
P<0.05 is considered statistically significant. CKD, chronic kidney disease; OR, odds ratio; eGFR, estimated glomerular filtration rate; HB, hemoglobin; b, regression of coefficient.
Table V.
Risk factors for new onset CKD in patients that received liver transplantation by multivariate logistic regression analysis.
| Items | b | OR | 95% CI | P-value |
|---|---|---|---|---|
| eGFR | −0.020 | 0.980 | 0.962–0.999 | 0.041 |
| HGB | −0.029 | 0.972 | 0.946–0.998 | 0.034 |
| Calcineurin concentration | 0.311 | 1.364 | 1.063–1.751 | 0.015 |
| Postoperative Hypertension | 1.575 | 4.833 | 1.014–23.03 | 0.048 |
| Acute kidney injury | 0.132 | 2.015 | 1.010–12.13 | 0.065 |
| Intraoperative blood loss | 1.578 | 4.845 | 0.540–43.48 | 0.159 |
| Intraoperative blood transfusion | 1.748 | 5.741 | 0.643–51.28 | 0.118 |
P<0.05 is considered to indicate a statistically significant difference. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HB, hemoglobin; OR, odds ratio; CI, confidence interval; b, regression of coefficient.
Renal pathology of patients with new-onset CKD following liver transplantation
Out of 40 patients in the new-onset CKD group, only 7 patients gave consent for renal biopsy. 6/7 (85.7%) patients had tubulointerstitial damage, which was associated with CNIs. Some exhibited glomerular diseases, including IgA nephropathy, HBV-associated nephropathy, membranous proliferative glomerulonephritis, focal segmental glomerulosclerosis (FSGS) and cryoglobulinemia-associated kidney damage, as shown in Table VI.
Table VI.
Clinical and pathological data of 7 new onset CKD patients that received liver transplantation.
| Item no. | Sex | Age | PLD | P-HB | P-eGFR | IBT | P-FK506 | P-AKI | P-HTN | P-RBT | RP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 56 | HBC/LC | 93.3 | 100.3 | 3,800 | 4.0 | + | − | 3 months | FSGS/ATI |
| 2 | Male | 62 | HBC | 96.9 | 87.6 | 4,000 | 17.0 | − | + | 4 months | IgA/CFRI |
| 3 | Male | 50 | HBC | 121.2 | 146.2 | 6,500 | 15.9 | − | + | 2 years | STIN/CFRI |
| 4 | Male | 56 | HBC/LC | 112.6 | 137.2 | 2,800 | 17.4 | − | + | 3 years | IgA/CGKD/STIN |
| 5 | Male | 50 | HBC | 89.7 | 80.4 | 3,500 | 14.3 | − | + | 6 years | IgA/CGKD/STIN |
| 6 | Male | 28 | HCC | 114.3 | 171.6 | 3,800 | 9.1 | − | − | 7 years | MPGN/CFRI/STIN |
| 7 | Male | 43 | HBC | 94.8 | 88.1 | 3,600 | 6.0 | − | + | 9 years | MPGH |
+, positive; -, negative; CKD, chronic kidney disease; PLD, primary liver disease; P-HB, preoperative HB (g/l); P-eGFR, preoperative eGFR (ml/min·1.73 m2); IBT, intraoperative blood transfusion (ml); P-FK506, postoperative average FK506 concentration (ng/ml); P-AKI, postoperative AKI; P-HTN, postoperative hypertension; P-RBT, postoperative renal biopsy time; RP, renal pathology; HBC, hepatitis B cirrhosis; LC, liver cancer; FSGS, focal segmental glomerulosclerosis; ATI, acute tubular injury; IgA, IgA nephropathy; CFRI, chronic FK506 renal injury; STIN, subacute tubular interstitial nephropathy; CGKD, cryoglobulinemia kidney damage; HCC, hepatitis C cirrhosis; MPGN, membranous proliferative glomerulonephritis.
Discussion
CKD significantly impacts long-term prognosis and is a common complication in patients who have received a liver transplant. Studies (3) have reported that the incidence rates of CKD are 4.0–27.5% within 1 year and 30–50% within 10 years post-surgery (8). In this study, 190 patients who underwent liver transplantation were followed up for 4–156 months; 40/190 (21.1%) patients developed new-onset CKD, which was significantly higher than the general population.
There have been few studies regarding intraoperative risk factors for new-onset CKD. In the present study, we analyzed surgical methods (orthotopic liver transplantation or piggyback orthotopic liver transplantation), anhepatic time, intraoperative blood loss and blood transfusion, and the results indicates that the volume of intraoperative blood loss and blood transfusion was a risk factor for new-onset CKD occurrence. The kidneys are sensitive to ischemia, especially in patients with impaired renal function, who are less tolerant of intraoperative hemodynamic instability. With an increase in intraoperative blood loss and blood transfusion leading to a decrease in renal perfusion pressure and glomerular filtration rate, renal ischemia-reperfusion injury is aggravated. That eventually leads to inflammatory responses, such as leukocyte infiltration, interstitial edema and a decrease in microvascular blood flow, which conversely exacerbates ischemic reperfusion injury (9).
Previous data also indicated that the dose-dependent nephrotoxicity of CNIs also plays an important role in new-onset CKD (10). Morard et al (11) reported that FK506 concentrations of ≥10 and ≥8 ng/ml at 1 and 5 years post-liver transplantation, respectively, were independent risk factors for the occurrence of CKD. In this study, we analyzed the average plasma FK506 concentration between the time of liver transplantation and the onset of CKD. Our results showed that the average plasma FK506 concentration was 14.0±4.5 ng/ml in the new-onset CKD group, in contrast to 11.0±2.8 ng/ml in the control group (P=0.001). Our multivariate analysis indicated that the average plasma concentration of FK506 from the time of liver transplantation to the onset of CKD was an independent risk factor for new-onset CKD in patients who had received liver transplantation. Calcineurin stimulates the secretion of endothelin by vascular endothelial cells, the release of angiotensin II and the overexpression of transforming growth factor-β. This process is accompanied by weakening of matrix degradation enzyme activity, which causes excessive contraction of glomerular arterioles, hyalinosis, chronic thromboembolism and excessive synthesis of the extracellular matrix. Finally, it leads to tubular atrophy, interstitial fibrosis, and a decrease in renal blood flow and glomerular filtration rate. The severity of nephrotoxicity is mainly due to the long-term use of calcineurin drugs. In addition, the side effects of CNIs, such as hypertension, diabetes, hyperlipidemia and hyperuricemia, also increase renal damage.
Our study also showed that preoperative low glomerular filtration rate and low hemoglobin are risk factors for new-onset CKD in patients who have received a liver transplant. These patients are more prone to ischemia-reperfusion injury during and after surgery because of the trauma and circulation instability.
Several studies have analyzed the postoperative risk factors for CKD in patients who have received liver transplantation. AKI was associated with the occurrence of CKD (12–14). The occurrence of CKD in liver transplant patients who had AKI was several times higher than in those patients who did not have concomitant AKI (15). Tinti et al (16) retrospectively studied 24 patients who had received liver transplantation, 9 of which developed postoperative AKI. The incidence rates of CKD were 44.4 and 6.7% in the AKI group and the non-AKI group, respectively. Our univariate analysis showed that postoperative AKI was a risk factor for new-onset CKD occurrence in patients who had received liver transplantation. The rate of AKI was 100% in the new-onset CKD group. In contrast, it was only 40.7% in the non-CKD group (P=0.000).
In the general population, elevated blood pressure may cause various injuries, including glomerular endothelial and epithelial cell injuries, and an increase in the activity of renin-angiotensin-aldosterone system. This eventually leads to damage to kidney function and proteinuria. Hypertension is commonly seen among patients with chronic glomerulonephritis, especially in FSGS. Some studies have reported that postoperative hypertension and diabetes are risk factors for CKD among patients who have received liver transplantation. Shao et al (10) retrospectively analyzed 772 liver transplant patients, and showed that post-operative hypertension (OR=2.230, 95% CI: 1.059–4.696, P<0.05) was a risk factor for CKD, as determined by logistic regression. Our study consistently showed that postoperative hypertension was an independent risk factor for new-onset CKD occurrence. However, there was no statistical difference in the postoperative occurrence of diabetes between the new-onset CKD group and the non-CKD group, according to our data. This discrepancy may possibly be attributed to the short history of diabetes. However, our results will be further confirmed by future studies with larger number of patients.
In the past, very few CKD patients who received liver transplantation underwent renal biopsy. Several studies showed that the renal pathology in CKD patients was complex and diverse, including CNI-induced renal damage, hypertensive renal damage, diabetic nephropathy, HBV-associated membranous nephropathy, IgA nephropathy, thrombotic microangiopathy, FSGS, membranous proliferative glomerulonephritis, cryoglobulinemia-associated renal damage, acute tubular necrosis, acute interstitial nephritis, amyloidosis, anti-glomerular basement membrane nephropathy, crescent glomerulonephritis and hydroxyethyl starch-associated tubule and interstitial lesions (11,17,18). In our study, 7 out of the 40 new-onset CKD patients underwent renal biopsy, among which 6 patients had acute or chronic tubular and interstitial damage. On the contrary, glomerular diseases included IgA nephropathy, membranous proliferative glomerulonephritis associated with HBV, membranous proliferative glomerulonephritis, FSGS and cryoglobulinemia associated with renal damage.
In conclusion, CKD is a common complication in patients who have received liver transplantation, and can affect the long-term prognosis of these patients. The preoperative glomerular filtration rate, hemoglobin level, average level of CNIs and postoperative hypertension are major risk factors for new-onset CKD following liver transplantation. Considering the severe shortage of liver donors, it is important to prevent new-onset CKD and improve survival by more carefully monitoring renal disease and function. Anemia correction, careful monitoring of CNI concentration and the prevention of AKI are of great significance for liver transplant patients with regard to avoiding new-onset CKD. We will examine more kidney biopsy samples among new-onset CKD patients post-liver transplant in order to better analyze the contribution of various risk factors to renal pathology in a future study. Finally, our study provided evidence to support the development of a better follow-up system to improve survival and quality of life for liver transplant patients.
Our sample size was limited, some of the conclusions were failed to be further discussed, such as the number of patients in the new-onset CKD groups were not sufficient to assess the severity of CKD. We will expand the sample size for future studies.
References
- 1.Kim WR, Smith JM, Skeans MA, Schladt DP, Schnitzler MA, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2012 annual data report: Liver. Am J Transplant. 2014;14(Suppl 1):S69–S96. doi: 10.1111/ajt.12581. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Bari K. Chronic kidney disease and related long-term complications following liver transplantation. Adv Chronic Kidney Dis. 2015;225:404–411. doi: 10.1053/j.ackd.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber ML, Ibrahim HN, Lake JR. Renal dysfunction in liver transplant recipients: Evaluation of the critical issues. Liver Transpl. 2012;18:1290–1301. doi: 10.1002/lt.23522. [DOI] [PubMed] [Google Scholar]
- 4.Ruebner R, Goldberg D, Abt PL, Bahirwani R, Levine M, Sawinski D, Bloom RD, Reese PP. Risk of end-stage renal disease among liver transplant recipients with pretransplant renal dysfunction. Am J Transplant. 2012;12:2958–2965. doi: 10.1111/j.1600-6143.2012.04177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Israni AK, Xiong H, Liu J, Salkowski N, Trotter JF, Snyder JJ, Kasiske BL. Predicting end-stage renal disease after liver transplant. Am J Transplant. 2013;13:1782–1792. doi: 10.1111/ajt.12257. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Goodrich NP, Schaubel DE, Guidinger MK, Merion RM. Patient-specific prediction of ESRD after liver transplantation. J Am Soc Nephrol. 2013;24:2045–2052. doi: 10.1681/ASN.2013040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, Settmacher U, Heyne N, Clavien PA, Muehlbacher F, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation-PROTECT. Am J Transplant. 2012;12:1855–1865. doi: 10.1111/j.1600-6143.2012.04049.x. [DOI] [PubMed] [Google Scholar]
- 8.Fabrizi F, Dixit V, Martin P, Messa P. Chronic kidney disease after liver transplantation: Recent evidence. Int J Artif Organs. 2010;33:803–811. [PubMed] [Google Scholar]
- 9.Li B, Chen B, Zhang G, Wang K, Zhou L, Hu S. Cell apoptosis and Fas gene expression in liver and renal tissues after ischemia-reperfusion injury in liver transplantation; Transplant Proc; 2010; pp. 1550–1556. [DOI] [PubMed] [Google Scholar]
- 10.Shao ZY, Yan LN, Wang WT, Li B, Wen TF, Yang JY, Xu MQ, Zhao JC, Wei YG. Prophylaxis of chronic kidney disease after liver transplantation - experience from west China. World J Gastroenterol. 2012;18:991–998. doi: 10.3748/wjg.v18.i9.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morard I, Mentha G, Spahr L, Majno P, Hadengue A, Huber O, Morel P, Giostra E. Long-term renal function after liver transplantation is related to calcineurin inhibitors blood levels. Clin Transplant. 2006;20:96–101. doi: 10.1111/j.1399-0012.2005.00447.x. [DOI] [PubMed] [Google Scholar]
- 12.Kubal C, Cockwell P, Gunson B, Jesky M, Hanvesakul R, Dronavalli V, Bonser RS, Neil D. Chronic kidney disease after nonrenal solid organ transplantation: A histological assessment and utility of chronic allograft damage index scoring. Transplantation. 2012;93:406–411. doi: 10.1097/TP.0b013e318240e984. [DOI] [PubMed] [Google Scholar]
- 13.Cantarovich M, Tchervenkov J, Paraskevas S, Ghali P, Wong P, Deschênes M, Chaudhury P, Hassanain M, Vrochides D, Metrakos P, Barkun J. Early changes in kidney function predict long-term chronic kidney disease and mortality in patients after liver transplantation. Transplantation. 2011;92:1358–1363. doi: 10.1097/TP.0b013e3182384aff. [DOI] [PubMed] [Google Scholar]
- 14.Lee JP, Heo NJ, Joo KW, Yi NJ, Suh KS, Moon KC, Kim SG, Kim YS. Risk factors for consequent kidney impairment and differential impact of liver transplantation on renal function. Nephrol Dial Transplant. 2010;25:2772–2785. doi: 10.1093/ndt/gfq093. [DOI] [PubMed] [Google Scholar]
- 15.Velidedeoglu E, Bloom RD, Crawford MD, Desai NM, Campos L, Abt PL, Markmann JW, Mange KC, Olthoff KM, Shaked A, Markmann JF. Early kidney dysfunction post liver transplantation predicts late chronic kidney disease. Transplantation. 2004;77:553–556. doi: 10.1097/01.TP.0000114609.99558.41. [DOI] [PubMed] [Google Scholar]
- 16.Tinti F, Umbro I, Mecule A, Rossi M, Merli M, Nofroni I, Corradini SG, Poli L, Pugliese F, Ruberto F, et al. RIFLE criteria and hepatic function in the assessment of acute renal failure in liver transplantation. Transplant Proc. 2010;42:1233–1236. doi: 10.1016/j.transproceed.2010.03.128. [DOI] [PubMed] [Google Scholar]
- 17.Kamar N, Guilbeau-Frugier C, Servais A, Tack I, Thervet E, Cointault O, Esposito L, Guitard J, Lavayssière L, Muscari F, et al. Kidney histology and function in liver transplant patients. Nephrol Dial Transplant. 2011;26:2355–2361. doi: 10.1093/ndt/gfq718. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Akalin E, Dikman S, Gagliardi R, Schiano T, Bromberg J, Murphy B, de Boccardo G. The variable pathology of kidney disease after liver transplantation. Transplantation. 2010;89:215–221. doi: 10.1097/TP.0b013e3181c353e5. [DOI] [PubMed] [Google Scholar]