Abstract
Stressors activate the hypothalamic-pituitary-adrenal (HPA) axis and immune system eliciting changes in cognitive function, mood and anxiety. An important link between stress and altered behavior is stimulation of the Kynurenine Pathway which generates neuroactive and immunomodulatory kynurenines. Tryptophan entry into this pathway is controlled by rate-limiting indoleamine/tryptophan 2,3-dioxygenases (DOs: Ido1, Ido2, Tdo2). Although implicated as mediating changes in behavior, detecting stress-induced DO expression has proven inconsistent. Thus, C57BL/6J mice were used to characterize DO expression in brain-regions, astrocytes and microglia to characterize restraint-stress-induced DO expression. Stress increased kynurenine in brain and plasma, demonstrating increased DO activity. Of three Ido1 transcripts, only Ido1-v1 expression was increased by stress and within astrocytes, not microglia, indicating transcript- and glial-specificity. Stress increased Ido1-v1 only in frontal cortex and hypothalamus, indicating brain-region specificity. Of eight Ido2 transcripts, Ido2-v3 expression was increased by stress, again only within astrocytes. Likewise, stress increased Tdo2-FL expression in astrocytes, not microglia. Interestingly, Ido2 and Tdo2 transcripts were not correspondingly induced in Ido1-knockout (Ido1KO) mice, suggesting that Ido1 is necessary for the central DO response to acute stress. Unlike acute inflammatory models resulting in DO induction within microglia, only astrocyte DO expression was increased by acute restraint-stress, defining their unique role during stress-dependent activation of the Kynurenine Pathway.
Keywords: Stress, Ido, Tdo, Kynurenine, Astrocyte, Liver
Highlights
-
•
Acute stress increased tryptophan metabolism to kynurenine.
-
•
Stress increased Ido1, Ido2 and Tdo2 expression by astrocytes.
-
•
Stress did not increase Ido2 in astrocytes from Ido1KO mice.
-
•
Stress did not increase Tdo2 in brains fromIdo1KO mice.
-
•
Brain and hepatic Ido1, Ido2 and Tdo2 are differentially regulated by stress.
1. Introduction
An acute stress response is necessary for survival; it facilitates adaptation to external stressors and primes the body for the metabolic, physical and cognitive demands of fight-or-flight (McEwen, 2007). Although classically assessed by HPA axis and sympathetic nervous system (SNS) activation, the stress response also involves precise changes in neuronal plasticity (McEwen, 2007) along with central and peripheral immune activation (Sorrells and Sapolsky, 2007, Sorrells et al., 2009). When the behavioral response to physical, psychological or metabolic stressors becomes maladaptive, profound consequences upsetting physical health and mental wellbeing occur (Chrousos and Gold, 1992). Nearly half of Americans report experiencing at least one psychiatric disorder at some point in their lives, the most common being depression (Kessler et al., 2003, Kessler et al., 1994). Mounting evidence supports a causal link between stress-induced activation of the Kynurenine Pathway and psychiatric disorders, including depression (Myint et al., 2012, O'Farrell and Harkin, 2015, Won and Kim, 2015) and schizophrenia (Chiappelli et al., 2014, Pocivavsek et al., 2016). Stress can also trigger depression, as well as influence the length and severity of depressive episodes (Gold et al., 2015). Similarly, stress is a comorbid factor for schizophrenia (Howes et al., 2016). Thus, advancing our understanding of the mechanism(s) by which the brain responds to stress serves to elucidate the biology underpinning stress-related psychiatric disease.
Tryptophan (Trp) metabolism via the Kynurenine Pathway is initiated by three rate-limiting dioxygenases, DOs (McCusker et al., 2014). Acute predatory stress (Miura et al., 2011), foot shock (Pawlak et al., 2000) and physical restraint or immobilization (Gibney et al., 2014) increase DO activity and mRNA expression in the brain and liver. The general dogma is that glucocorticoids regulate Tdo2 expression, while Ido1 and Ido2 are regulated by inflammatory mediators (Lawson et al., 2016, McCusker et al., 2014). Indeed, early work on stress and the Kynurenine Pathway focused on the activation of hepatic Tdo2 (a.k.a. tryptophan pyrrolase) associated with HPA axis activation (Badawy and Evans, 1973, Nomura, 1965, Shimazu, 1964, Shimazu, 1962). Acute restraint-stress increases hepatic Tdo2 mRNA expression and activity (Gibney et al., 2014) mediated in part by adrenocortical secretions (Nomura, 1965). Acute stress also increases Ido1 expression in the brain (Kiank et al., 2010, Vecchiarelli et al., 2015) and periphery (Kiank et al., 2010). Nevertheless, there is only one report of stress regulating Ido2 expression in the CNS (Browne et al., 2012), although its unique role in immunophysiology is established (Metz et al., 2014).
Stress is associated with immunological changes both in the periphery and CNS (Frank et al., 2015, Sorrells et al., 2009). Acute stress increases brain cytokine levels including TNFα (Madrigal et al., 2002, Ohgidani et al., 2016) and IL-1β (Nguyen et al., 2000, Nguyen et al., 1998). These cytokines synergistically regulate Kynurenine Pathway activity (Fujigaki et al., 2006, Fujigaki et al., 2001). The pro-inflammatory effects of stress in the CNS is well established (Sorrells and Sapolsky, 2007, Sorrells et al., 2009) and it has been demonstrated that cytokine induction is necessary for Ido1 upregulation by stress (Kiank et al., 2010, Liu et al., 2015). Although some work has investigated the regulation of DOs by stress within the CNS and periphery, the cellular origins responsible for DO induction remain undefined.
Kynurenine itself is not considered a neuroactive metabolite (McCusker et al., 2014), albeit increased DO activity is required for depression-like behaviors following stress (Gibney et al., 2014, Liu et al., 2015). Instead, Kyn is further metabolized down the Kynurenine Pathway into other neuroactive metabolites, i.e. kynurenines. This is especially relevant to the CNS owing to the remarkable cellular specificity in the production of kynurenines (McCusker et al., 2014). Most notably, astrocytes are enzymatically equipped to produce kynurenic acid (KynA) a glutamate (NMDA) and acetylcholine (α7nACh) receptor antagonist (Guillemin et al., 2001, Wu et al., 2010), while microglia produce quinolinic acid (QuinA) and 3-hydroxykynurenine (3-HK) which are NMDA receptor agonists (Guillemin et al., 2004, Heyes et al., 1996). A recent study found that chronic unpredictable stress increased central Ido1 and Tdo2 mRNA coincident with increased KynA concentrations, but unchanged 3-HK (Dugan et al., 2016). This would suggest a specific role for astrocytes in stress-induced DO induction.
Herein we report induction of all three DOs by acute restraint-stress specifically within astrocytes. We have expanded on recent work investigating DO-regulation by stress (Vecchiarelli et al., 2015) to include the regulation of recently described alternatively-spliced DO mRNA transcripts (Brooks et al., 2016a, Brooks et al., 2016b). Moreover, since acute stress increases Kyn levels in both plasma and brain (Kennett and Joseph, 1981, Pawlak et al., 2000), we include DO-regulation by stress in liver, the major tryptophan metabolizing organ primarily via Tdo2. Finally, since there is evidence of changes in Ido2 expression within Ido1-knockout (Ido1KO) mice (Fukunaga et al., 2012, Lee et al., 2014), we expanded our study to include stress-induced DO-regulation in brain, astrocytes and liver of Ido1KO mice.
2. Materials and methods
2.1. Animals
C57BL/6J (wild-type) or Ido1KO mice (The Jackson Laboratory, Bar Harbor, ME, USA) were used to establish breeding colonies to supply male mice for experiments. Mice were housed on a reversed 12 h light-dark cycle with ab lib access to food and water. Mice were individually housed at least 1 week prior to experiments. Mice were 14–15 weeks of age at the time of treatment. All animal procedures were approved by the Institutional Animal Care and Use Committee and performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council).
2.2. Study design
2.2.1. Restraint-stress
Restraint-stress was initiated at the onset of the dark cycle (10 a.m.) and maintained for 3 h using ventilated syringes (Steelman et al., 2010). Mice were euthanized 2 h after the cessation of restraint, at which time wild-type mice had a significant reduction in body weight associated with restraint-stress (control change in body weight 0.1 ± 0.1 g vs. restrained −0.9 ± 0.1 g, p < 0.001). A similar response was seen with Ido1KO mice (control 0.4 ± 0.1 g vs. restrained −0.7 ± 0.2 g, p < 0.001).
Following euthanasia, caval blood was collected into heparinized syringes. Samples were centrifuged and plasma collected. Mice were intracardially perfused using cold PBS plus 2 mM EDTA. After perfusion, samples were collected from separate cohorts of mice for either 1) whole brain sampling followed by glia enrichment or 2) brain region and liver collection as described in sections 2.2.2, 2.2.3, respectively.
2.2.2. Brain sampling and glia enrichment: mouse cohort 1
Brains were removed and placed in ice-cold Hank's balanced salt solution then homogenized with a GentleMACS dissociator using Neural Tissue Dissociation kits (130-093-231, Miltenyi Biotec Inc.) per manufacturer instructions. Immediately after homogenization, an aliquot of ‘brain’ was removed and stored in TRIzol (15596018, Ambion by Life Technologies) for later RNA isolation. The 'brain' samples were collected from homogenates before removal of astrocytes and microglia and thus represent expression by all cells and all brain regions in their original proportion. Remaining homogenate was demyelinated by centrifugation in 20% isotonic Percoll (E0414-1L, Sigma-Aldrich, St. Louis, MO). The cell pellet was re-suspended then partitioned into two fractions for positive-selection using either anti-Cd11b (130-093-634) or anti-Glast labeling microbeads (130-095-826). Microglia (Cd11b+) and astrocytes (Glast+) were enriched using MACS MS magnetic separation columns. Microglia and astrocyte populations were suspended in TRIzol and stored at −80 °C for later RNA isolation (Lawson et al., 2011). Glia enrichment was verified by comparing Glast1 and Cd11b mRNA expression to the 'brain' homogenate from which they were derived. Glast1 expression was greater in enriched astrocytes compared to brain in wild-type mice (5.9 ± 0.4-fold, p < 0.001) and Ido1KO mice (12.0 ± 1.0-fold, p < 0.001). Cd11b expression was greater in microglia preparations compared to brain from wild-type mice (265.6 ± 10.8-fold, p < 0.001).
2.2.3. Brain region and liver collection: mouse cohort 2
Brain-regions (prefrontal cortex (PFC), striatum (Stri), hippocampus (Hippo), hypothalamus (Hypo)) and livers were collected and frozen for later RNA extraction. The remaining brain (sans PFC, Stri, Hippo and Hypo) was frozen for analysis of Kyn.
2.3. Gene expression by qPCR
RNA was extracted from brain, astrocytes, microglia, PFC, Stri, Hippo, Hypo and liver, then reverse-transcribed (4368813, Applied Biosystems). Resulting cDNA was used for quantitative polymerase chain reaction (qPCR) using TaqMan Universal PCR Master Mix (4324020, Applied Biosystems). Expression of each test gene were normalized to the reference gene (Gapdh) using the 2−ΔΔCt method (Livak and Schmittgen, 2001). Gene expression in naïve control (Ctrl) brain or Ctrl PFC is set to 1.0, with samples from the same experiments expressed relative to appropriate controls. Gene structure and mRNA transcripts for the DOs are shown in Fig. 1. Assays designed to quantify the various DO transcripts are described in Table 1. PCR was performed with probe-based assays (IDT, Coralville, Iowa). Custom assays were designed using the IDT PrimerQuest® Design Tool. Assays for Kat2 (Mm.PT.49.16077568), Kmo (Mm.PT.56.31570400), Kynu (Mm.PT.56.12643855) and Haao (Mm.PT.58.29327685) were predesigned by IDT. Because of low expression, some transcripts are 'not detected' (i.e. Ct values ‘undetermined’) thus preventing calculation of relative gene expression following induction; for analysis a Ct value of 40.0 is assigned when this occurs.
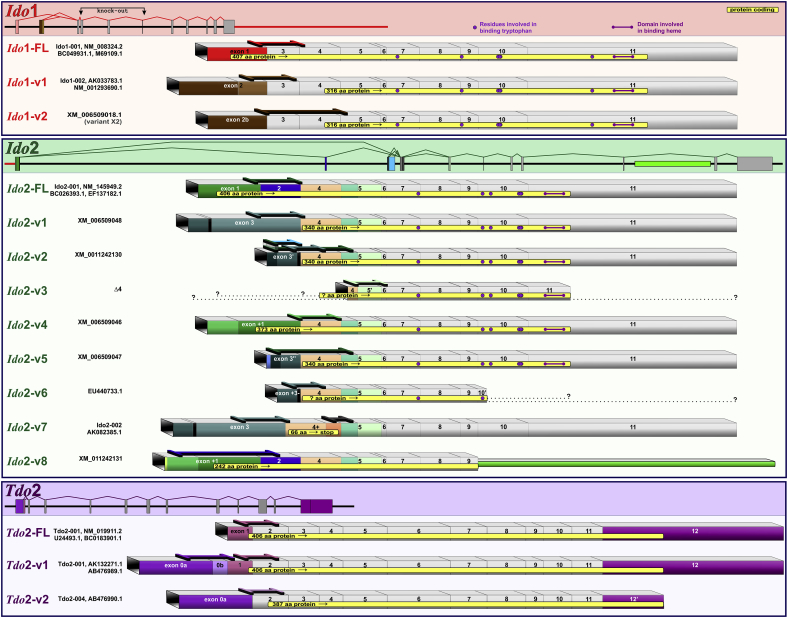
Fig. 1.
Gene structure and transcripts for murine Ido1, Ido2 and Tdo2. Like human DOs, murine DO gene-processing results in the expression of multiple mRNA transcripts. Our previous (Brooks et al., 2016a, Brooks et al., 2016b) and current work clearly show distinct transcripts are utilized to fine-tune regulation of the Kynurenine Pathway.
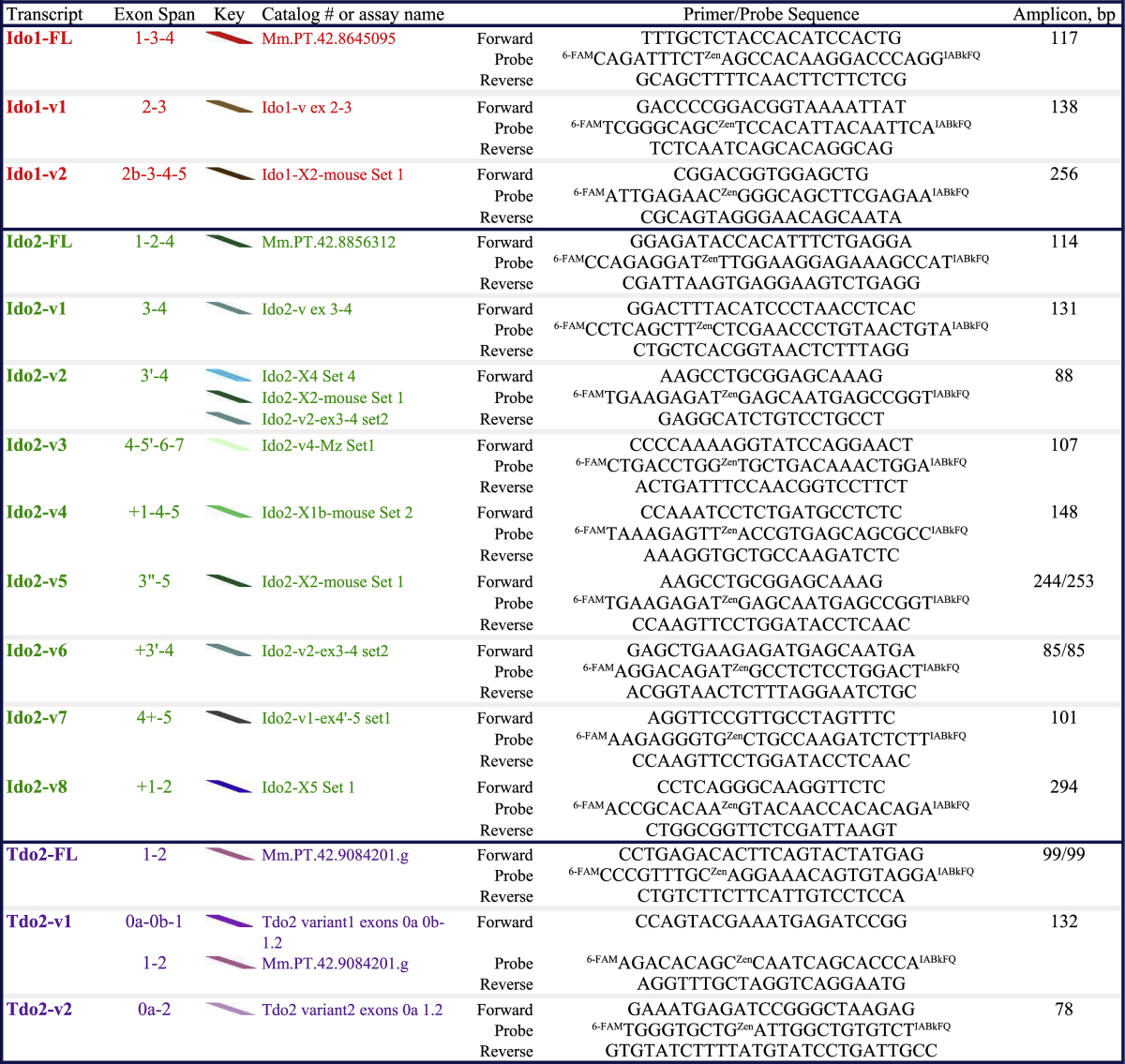
Table 1.
Assay specifics for analysis of murine Ido1, Ido2 and Tdo2 steady-state gene expression.
Ido2-v5 and Ido2-v6 sequences lie within Ido2-v2 (thus it is not possible to design an assay specific to Ido2-v5 or Ido2-v6 that does not also quantify Ido2-v2). We have data indicating distinct differences in the regulation of these three transcripts (Brooks et al., 2016a). Tdo2-FL's complete sequence lies within Tdo2-v1 (thus it is not possible to design an assay specific to Tdo2-FL that does not also quantify Tdo2-v1). Our current and published (Brooks et al., 2016a, Brooks et al., 2016b) data show distinct differences in the expression and regulation of Tdo2-FL and Tdo2-v1, indicating that they are distinctly expressed transcripts. Names for Ido2-v3:Δ4, Tdo2-FL/-v1/-v2 and Tdo2 exon 0a and 0b exon designations are shown in an attempt to agree with published nomenclature (Kanai et al., 2009, Metz et al., 2014). Specifics shown for each qPCR assay include transcript location, catalog numbers (Mm.PT …) or our custom assay names, primer/probe sequences and confirmed amplicon sizes.
2.4. Kynurenine levels by high performance liquid chromatography (HPLC)
Brain samples were suspended in ice cold buffer (0.1 N perchloric acid + 25 μM ascorbic acid) at 200 mg wet weight/ml and disrupted by sonication. Samples were incubated for 30 min then centrifuged at 12,000 g for 5 min at 4 °C. Supernatants were collected and loaded into Spin-X filters (Corning, NY, USA) and centrifuged at 10,000 g for 5 min at 4 °C. Filtrates were used for HPLC analysis. Plasma samples were processed for analysis by HPLC as previously reported (Zhai et al., 2015). HPLC mobile phase consisted of 75 mM monosodium phosphate (pH 4.6), 25 μM EDTA and 0.01% triethylamine prepared in either 4.5 or 6% acetonitrile for brain and plasma extracts, respectively. Chromatogram peaks were integrated using EZChrom SI software (Agilent Technologies, Santa Clara, CA, USA). Kyn standards were made to encompass levels in the samples.
2.5. Statistics
Two-way ANOVA was used to compare the relative expression of genes between astrocytes, microglia and brain, and to compare brain-regions and liver, while T-tests were used to determine the effects of stress. Analysis was performed using SigmaPlot 14.0 software. Significance was set at p ≤ 0.05.
3. Results
3.1. Acute stress increases gene expression of markers for HPA axis activation and inflammation
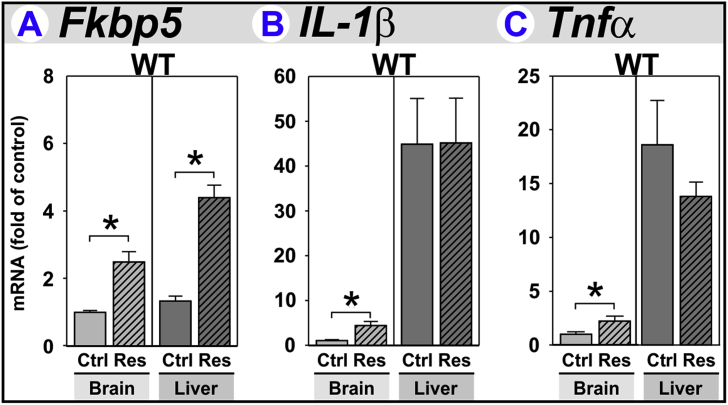
FK506-binding protein 51 (Fkbp5) expression was increased by restraint-stress in both brain and liver (Fig. 2A). These data confirm a HPA axis response (Stechschulte and Sanchez, 2011, Zannas et al., 2016). Some effects of restraint-stress are mediated by increases in pro-inflammatory cytokines. Restraint-stress increased IL-1β (Fig. 2B) and Tnfα (Fig. 2C) expression within brain, confirming an inflammatory response. Although relative expression of IL-1β and Tnfα was higher in liver vs. brain, a stress-induced inflammatory response was not observed in liver. Also, IFNγ gene expression was unaffected by restraint-stress in brain and liver. IFNγ protein concentrations in plasma from naïve and restrained mice were below detection-limits (data not shown).
Fig. 2.
Acute stress increases gene expression of markers for stress and inflammation. Perfused brain and liver tissue obtained from wild-type (WT) control (Ctrl) and restraint-stressed (Res) mice sacrificed 2 h after a 3 h restraint were used to assess (A) HPA activation by quantifying Fkbp5 expression and an inflammatory response by quantifying (B) IL-1β and (C) Tnfα expression. *p < 0.05 Ctrl vs. Res.
3.2. Acute stress increases plasma and brain kynurenine in wild-type, but not Ido1KO mice
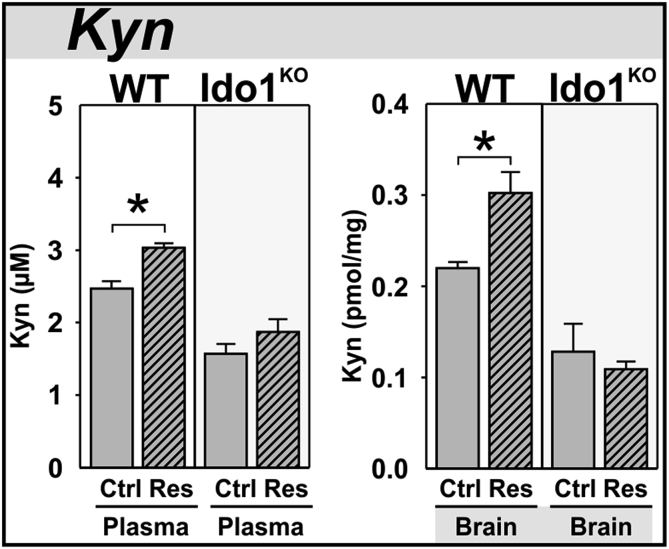
Plasma concentrations of Kyn were increased by restraint-stress in wild-type, but not Ido1KO mice (Fig. 3A). Brain concentrations of Kyn were also increased by stress in wild-type, but not Ido1KO mice (Fig. 3B). Since the DOs are rate-limiting enzymes in the metabolism of Trp to Kyn (McCusker et al., 2014), these data verify restraint-stress activation of the Kynurenine Pathway and the necessity of Ido1 for increased Kyn.
Fig. 3.
Acute stress increases plasma and brain kynurenine in wild-type, but not Ido1KOmice. Two hours after a three hour restraint-stress (A) plasma and (B) brain from WT and Ido1KO mice were analyzed for kynurenine (Kyn) by HPLC. *p < 0.05 comparing control (Ctrl) to restraint-stress (Res) samples.
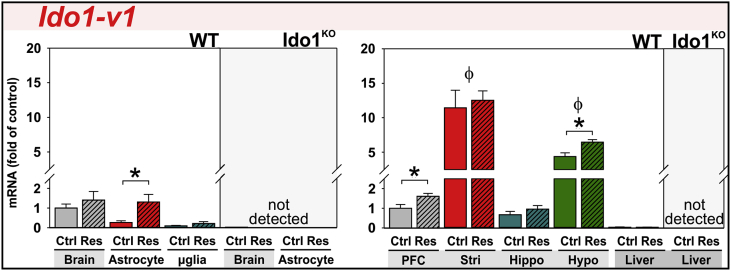
3.3. Acute stress increases Ido1-v1 expression in astrocytes and select brain-regions
Ido1 expression in the mouse brain is reported to both increase and remain unchanged following acute stress. Based on our nomenclature (Fig. 1), these studies used PCR assays amplifying either Ido1-FL (Browne et al., 2012, Kiank et al., 2010) or all Ido1 transcripts simultaneously, Ido1-Tot (Gibney et al., 2014). We independently quantified expression three Ido1 transcripts to refine this issue. In agreement with our previous work (Brooks et al., 2016b), Ido1-FL and Ido1-v2 transcripts were poorly expressed in brains and livers of naïve mice. Their expression was not induced by restraint-stress (data not shown).
Ido1-v1 is well expressed in the mouse brain and numerically, but non-significantly, elevated by restraint-stress (Fig. 4). Initially, this suggests that Ido1-v1 expression is not altered by restraint-stress; however, a more in-depth investigation reveals the nature of Ido1-v1 expression. Ido1-v1 expression is induced 4.8-fold in astrocytes, but not in microglia, isolated from stressed mice. The stress effect is not only cell-type specific but brain-region specific, disguising significant effects when whole-brain is assessed. Ido1-v1 expression was highest in striatum followed by hypothalamus relative to other brain-regions. Restraint-stress increased Ido1-v1 in prefrontal cortex and hypothalamus. As expected, Ido1KO mice did not express Ido1-v1 (Fig. 4), Ido1-FL or Ido1-v2 (not shown). Hepatic expression of Ido1-FL, Ido1-v1 and Ido1-v2 was low and not altered by stress. Thus, Ido1 expression exhibits brain-region, glial and transcript-specificity within naïve and restrained wild-type mice.
Fig. 4.
Acute stress increases Ido1-v1 expression in astrocytes and select brain-regions. Tissue and glia from control (Ctrl) and restraint-stressed (Res) mice sacrificed 2 h after a 3 h restraint were analyzed for Ido1-v1 expression. Expression was quantified in whole-brain, glia, liver and several brain-regions: the prefrontal cortex (PFC), striatum (Stri), hippocampus (Hippo) and hypothalamus (Hypo). Samples from Ido1KO mice did not express any Ido1 transcript. Ido1-FL and Ido1-v2 transcripts were not detected by qPCR. *p < 0.05 comparing Ctrl to Res within tissue or glia. ϕp<0.05 compared to other brain-regions.
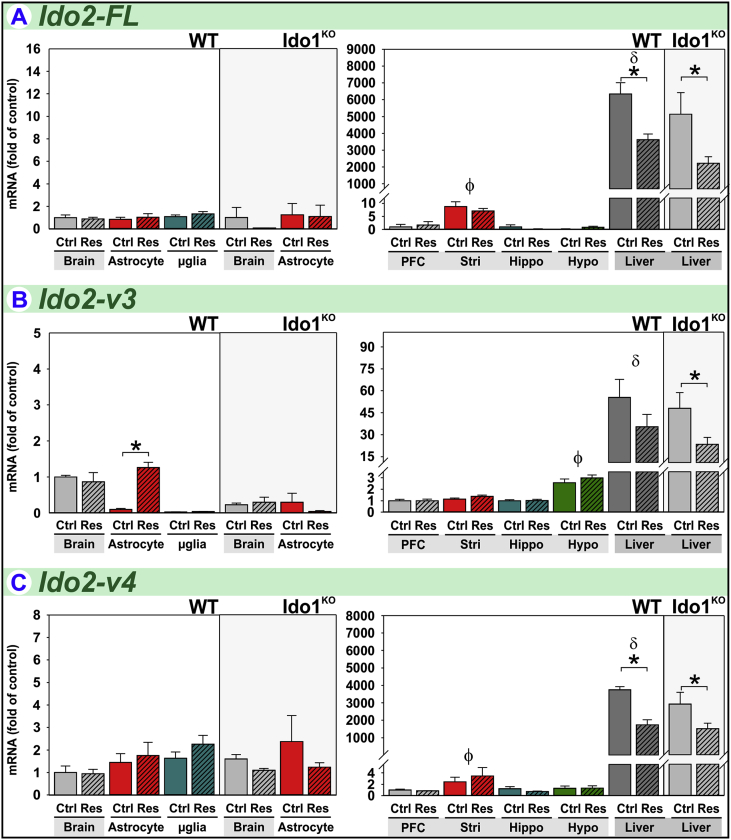
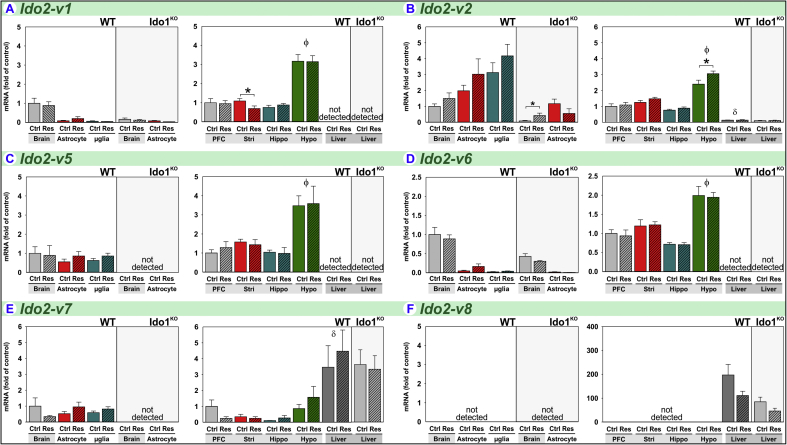
3.4. Acute stress increases Ido2-v3 expression in astrocytes
To our knowledge, there is only one report examining the effect of stress on Ido2 expression. Using an assay that detects all transcripts (Ido2-Tot), forced-swimming-associated stress increased hippocampal Ido2 expression in BALB/c, but not C57BL/6J, mice (Browne et al., 2012). We quantified expression of Ido2 (Fig. 1) for transcript-, tissue- and glial-specific responses between naïve and restrained mice. Of eight Ido2 mRNA variants, restraint-stress remarkably increased the expression of only one Ido2 transcript; this transcript-specificity would make it difficult to detect changes when simultaneously quantifying all transcripts, i.e. Ido2-Tot.
Ido2-FL is poorly expressed in the naïve mouse brain except within the striatum where Ido2-FL expression is 10-fold higher (Fig. 5A) than other brain-regions. In striking contrast, hepatic Ido2-FL is markedly higher in relation to brain-regions. While restraint-stress had no effect on central Ido2-FL, hepatic Ido2-FL expression in wild-type and Ido1KO mice was decreased following restraint.
Fig. 5.
Acute stress increases Ido2-v3 expression in astrocytes. Tissue and glia from control (Ctrl) and restraint-stressed (Res) mice sacrificed 2 h after a 3 h restraint were analyzed for (A) Ido2-FL, (B) Ido2-v3 and (C) Ido2-v4 expression. *p < 0.05 comparing Ctrl to Res within tissue or glia. ϕp<0.05 compared to other brain-regions. δp<0.05 liver compared to all brain-regions.
Ido2-v3 is well expressed in the mouse brain, but stress-induced changes are not realized when analyzing whole-brain or brain-regions (Fig. 5B). The low basal expression in astrocytes and microglia relative to brain indicates that most of the Ido2-v3 in naïve brain is expressed by stress-insensitive cells. Nonetheless, Ido2-v3 expression is increased by stress 13-fold in astrocytes. In Ido1KO mice, Ido2-v3 expression by astrocytes is not increased by stress. In wild-type mice, hepatic expression of Ido2-v3 is greater relative to brain-regions; and hepatic Ido2-v3 is decreased by stress, albeit significant only for Ido1KO mice.
Ido2-v4 is also poorly expressed in the naïve mouse brain except within the striatum (Fig. 5C) where Ido2-v4 expression is highest (Brooks et al., 2016b). Again, hepatic expression of Ido2-v4 is markedly higher in relation to brain-regions. While not regulated by stress within the brain, stress decreased hepatic Ido2-v4 expression in wild-type and Ido1KO mice.
Thus, expression of Ido2-FL, Ido2-v3 and Ido2-v4 are stress sensitive, lowered in liver and Ido2-v3 is elevated in astrocytes. Other Ido2 transcripts exhibit unique patterns of expression across tissues and brain-regions, but their expression is relatively independent of stress (Supplementary Fig. S1).
Compared to wild-type mice, the brains of Ido1KO mice are strikingly deficient in several Ido2 transcripts, including 78% reduction in Ido2-v3 (Fig. 5B), 84% reduction in Ido2-v1, 92% reduction in Ido2-v2, 22% reduction in Ido2-v5, 57% reduction in Ido2-v6 and loss of detectable Ido2-v7 (Fig. S1). This loss of Ido2 in Ido1KO is cell- and tissue-specific, and was previously reported to occur in B-lymphocytes, but not liver (Metz et al., 2014). We confirm their finding showing that relative expression of various Ido2 transcripts is similar in the liver between wild-type and Ido1KO mice (Fig. 5 and Fig. S1). The decrease in Ido2-v3 within brains of Ido1KO mice is not mediated astrocytes, as Ido2-v3 in astrocytes from Ido1KO mice is numerically greater than astrocytes from wild-type mice. Thus, loss of Ido2-v3 occurs in another cell-type within the brain. Additionally, although Ido2-v3 is induced by stress in astrocytes from wild-type mice, it was not increased in Ido1KO mice. Thus, the regulation of Ido2 is transcript-, tissue- and cell-type-specific in wild-type mice, and genetic deletion of Ido1 perturbs central Ido2 expression. Importantly, when interpreting physiologic differences between wild-type and Ido1KO mice, diminished Ido2 expression in the brain should be considered.
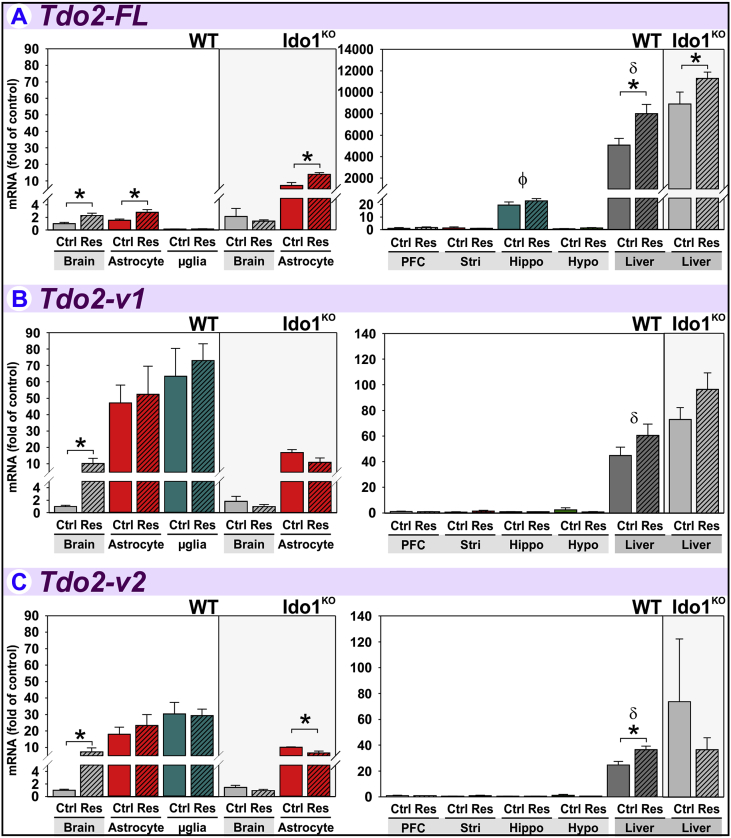
3.5. Acute stress increases Tdo2-FL expression in brain, astrocytes and liver
Tdo2 was the first DO investigated within the context of acute stress with early reports describing increases in hepatic Tdo2 activity (Curzon and Green, 1969, Nemeth, 1976, Nomura, 1965). However, Tdo2 expression in the frontal cortex of the rat brain was unchanged after acute stress despite an increase of Tdo2 in liver using a qPCR assay that quantified all Tdo2 transcripts, i.e. Tdo2-Tot (Gibney et al., 2014). The current report is the first to quantify the regulation of the three known Tdo2 transcripts (Fig. 1) in stressed mice to identify distinct regulatory profiles.
Tdo2-FL expression is increased to 2.3-fold of controls in brain by restraint-stress (Fig. 6A). This effect is paralleled by a 1.8-fold increase within astrocytes. Tdo2-FL expression in microglia is lower than brain and astrocytes and unaffected by stress. Tdo2-FL expression within astrocytes from Ido1KO mice was also doubled by restraint-stress, but not within the brains from which they were derived. Astrocyte Tdo2-FL expression is greater in Ido1KO mice compared to the brain of wild-type mice, suggesting genetic deletion of Ido1 results in specific compensatory Tdo2-FL upregulation within astrocytes. Although stress increased Tdo2-FL expression in brain from wild-type mice, stress did not increase Tdo2-FL in brain of Ido1KO mice. This finding suggests another brain cell-type, possibly neurons (Lawson et al., 2016), expressing Tdo2-FL is only stress-sensitive when Ido1 is intact. Tdo2-FL is considerably higher in the liver relative to brain-regions and increased by stress in both wild-type and Ido1KO mice.
Fig. 6.
Acute stress increases Tdo2-FL expression in liver, brain and astrocytes. Tissue and glia from control (Ctrl) and restraint-stressed (Res) mice sacrificed 2 h after a 3 h restraint were analyzed for (A) Tdo2-FL, (B) Tdo2-v1 and (C) Tdo2-v2 expression. *p < 0.05 comparing Ctrl to Res within tissue or glia. ϕp<0.05 compared to other brain-regions. δp<0.05 liver compared to all brain-regions.
Tdo2-v1 is increased to 10.1-fold of controls by restraint-stress in brains from wild-type mice (Fig. 6B). Although Tdo2-v1 expression is greater in astrocytes and microglia relative to whole-brain, its expression in these cells is unaffected by restraint. These data suggest the stress-induced increase in brain Tdo2-v1 is mediated by neurons (or other glia) which also express Tdo2 (Lawson et al., 2016). Unlike Tdo2-FL, Tdo2-v1 expression is similar across brain-regions. Thus, these data illustrate a unique expression pattern vs. Tdo2-FL (despite the entire Tdo2-FL sequence encompassed within Tdo2-v1, Fig. 1). Like Tdo2-FL, there is no increase in brain Tdo2-v1 expression in Ido1KO mice, indicating a requirement of Ido1 for brain Tdo2-v1 induction. In wild-type mice, hepatic Tdo2-v1 expression is greater than in brain-regions, but not significantly affected by restraint-stress.
Tdo2-v2 expression in brain of wild-type mice is increased 7.3-fold by restraint-stress (Fig. 6C). Tdo2-v2 expression in both astrocytes and microglia is greater than brain levels, yet unaffected by restraint. Like Tdo2-v1, these data suggest the stress-induced increase in brain Tdo2-v2 is mediated by neurons or other glia. Tdo2-v2 expression in astrocytes from Ido1KO mice is decreased slightly but significantly by restraint. Tdo2-v2 expression is similar across brain-regions. In wild-type mice, Tdo2-v2 levels in liver were greater than brain-regions and increased by restraint.
Therefore, all three Tdo2 transcripts are increased by restraint-stress in brains of wild-type mice. The increase in brain Tdo2-FL parallels the increase in astrocytes, whereas the increases in Tdo2-v1 and Tdo2-v2 are mediated by unidentified cell-types. Only Tdo2-FL and Tdo2-v2 are increased by restraint-stress in liver relative to control mice. Thus, there is transcript-, tissue- and cellular-specificity of Tdo2 expression and regulation by restraint-stress.
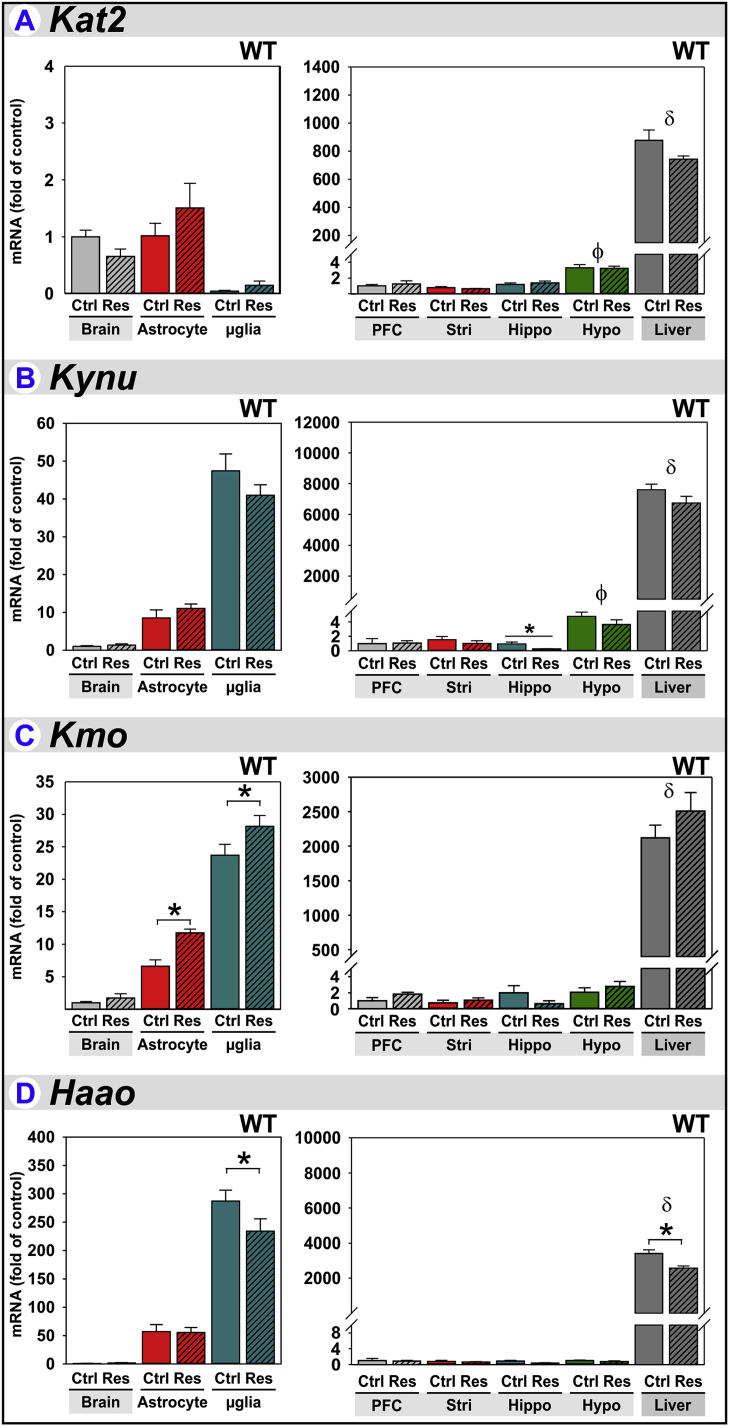
3.6. Other Kynurenine Pathway related gene expression & regulation by stress
The DOs are rate-limiting for Trp metabolism to Kyn, but Kyn itself is further metabolized into neuro- and immune-active kynurenines in a cell-specific manner. This specificity is achieved by differential expression of enzymatic machinery downstream of the DOs (Dantzer et al., 2008). Thus, we expanded our analysis to include enzymes further along the Kynurenine Pathway.
Kat2 (kynurenine-2-oxoglutarate aminotransferase) converts Kyn to KynA. Kat2 expression is enriched in astrocytes compared to microglia and highly expressed in liver. Within brain regions, Kat2 expression is highest in the hypothalamus; however, Kat2 expression is unaffected by stress (Fig. 7A). These data confirm previous reports of astrocyte enrichment of Kat2 (Guidetti et al., 2007, Kiss et al., 2003, Potter et al., 2010).
Fig. 7.
Other Kynurenine Pathway related gene expression & regulation by stress. Tissue and glia from control (Ctrl) and restraint-stressed (Res) mice sacrificed 2 h after a 3 h restraint were analyzed for (A) Kat2, (B) Kynu, (C) Kmo and (D) Haao expression. *p < 0.05 comparing Ctrl to Res within tissue or glia. ϕp<0.05 compared to other brain-regions. δp<0.05 liver compared to all brain-regions.
Kynu (kynureninase) initiates Kyn metabolism to QuinA. Kynu expression is greater in microglia relative to astrocytes and considerably higher in liver (Fig. 7B). These data confirming previous reports of microglial enrichment of this enzyme within the brain (Guillemin et al., 2003, Guillemin et al., 2001). Kynu expression is highest in the hypothalamus with expression largely unaffected by stress, the exception being a decrease in the hippocampus.
Kmo (kynurenine 3-monooxygenase) also initiates Kyn metabolism to QuinA. Kmo expression is greater in microglia relative to astrocytes, but again considerably higher in liver. These data confirm previous reports of microglial enrichment of this enzyme in the brain (Guillemin et al., 2003, Guillemin et al., 2001). Kmo expression is increased ∼50% in astrocytes and ∼20% in microglia by stress (Fig. 7C).
Haao (3-hydroxyanthranilate 3,4-dioxygenase) acts downstream of Kynu and Kmo to complete QuinA synthesis. Haao expression is greater in microglia relative to astrocytes, but again considerably higher in liver. Haao expression is slightly but significantly decreased by stress in both microglia and liver (Fig. 7D). Again, these data confirm preferential expression of Haao by microglia in the brain (Guillemin et al., 2001, Heyes et al., 1996).
The extremely high levels of Tdo2, Ido2 and especially the downstream enzymes within the liver (relative to brain), attest to its ability to efficiently generate niacin and NAD (Bender and Olufunwa, 1988). Overall, since the DOs are considered rate-limiting in the Kynurenine Pathway, these changes in downstream enzymes probably do not result in an overall shift in the relative ability to produce downstream kynurenines; that is to say astrocytes will generate primarily KynA and microglia QuinA as limited by Kyn production by the DOs.
4. Discussion
Acute restraint-stress increased the expression of all three DOs within the mouse brain in a transcript-, tissue- and cell-type specific manner. Brain and plasma levels of Kyn were found to be increased in stressed mice relative to controls demonstrating a functional increase in DO activity following acute restraint-stress. Remarkably, Ido1-v1, Ido2-v3 and Tdo-FL expression was increased by stress in astrocytes, but not microglia. However, in comparison to wild-type mice, we found aberrant Ido2 and Tdo2 expression in Ido1KO mice demonstrating a necessity of Ido1 for normal Ido2 and Tdo2 regulation within the brain. The expression patterns of downstream Kynurenine Pathway enzymes confirm the hypothesis that astrocytes are central producers of KynA while microglia are equipped to produce primarily QuinA. Since only astrocyte DO expression was increased by stress, these finding further implicate a key role for Kyn and KynA within the neurobiology of stress-induced behaviors.
4.1. Acute stress increases gene expression of markers for stress and inflammation
The induction of Fkbp5 by restraint-stress in brain and liver (Fig. 2A) confirm glucocorticoid receptor activation (Stechschulte and Sanchez, 2011, Zannas et al., 2016). However, restraint-stress increased brain, but not liver, expression of pro-inflammatory cytokines, Tnfα and IL-1β (Fig. 2B–C), confirming previous reports of increases in brain IL-1β (Nguyen et al., 2000, Nguyen et al., 1998) and circulating TNFα and IL-1β following stress, without coincident changes in the liver (Ohta et al., 2016). While both the HPA axis and autonomic nervous system contribute to the induction of peripheral and central pro-inflammatory cytokines following stress (Sorrells and Sapolsky, 2007, Sorrells et al., 2009), the pro-inflammatory cytokines TNFα (Ando and Dunn, 1999) and IL-1β (Dunn, 1992) also increase glucocorticoid release. Extending our understanding by which inflammatory mediators are regulated by the stress response (or vice versa) is out of the scope of this study; however, we are interested in how these confirmed stress responses affect the Kynurenine Pathway.
4.2. Acute stress increases plasma and brain kynurenine in wild-type, but not Ido1KO mice
Following acute stress, increases in central and peripheral Kyn concentrations have been independently verified (Kennett and Joseph, 1981, Kiank et al., 2010, Miura et al., 2013, Miura et al., 2011, Ohta et al., 2016, Pawlak et al., 2000). Our data reaffirm acute-stress increases both brain and plasma Kyn and further demonstrates the necessity of Ido1 for restraint-stress-induced increase in Kyn (Fig. 3A–B). Although Ido1 is intimately involved in elevated Kyn levels associated with inflammation (O'Connor et al., 2009b), Ido2 and Tdo2 also have this capability (Lawson et al., 2016). Thus, the inability of stress to induce Ido2-v3 and Tdo2 variants in Ido1KO mice may also be involved in the lack of Kyn induction in Ido1KO mice.
4.3. Acute stress increases Ido1-v1 expression in astrocytes and select brain-regions
Ido1 expression is increased by inflammatory mediators (Brooks et al., 2016b, McCusker et al., 2014, Wang et al., 2010) and synergistically by inflammatory mediators plus corticosteroids, although in a transcript-specific manner (Brooks et al., 2016b). Within the brain and liver of naïve mice, Ido1-FL and Ido1-v2 expression was extremely low, similar to our previous report (Brooks et al., 2016b). Ido1-FL and Ido1-v2 expression were not increased by restraint-stress in the current study; however, a previous study observed a 14-fold increase in whole-brain Ido1-FL following acute combined acoustic and restraint-stress of female BALB/c mice (Kiank et al., 2010). Whether the difference between that of Kiank and our study is due to a stronger stressor (acoustic + restraint vs. restraint), different mouse strain (BALB/c vs. C57BL/6J) or sex (female vs. male) is open to debate. Basal Ido1 expression by astrocytes from BALB/c mice may be greater than that of C57BL mice, permitting detection of Ido1 induction in whole brain by stress. Independent of this, Ido1-FL and Ido1-v2 are inducible in vivo by inflammatory signals such as LPS (Brooks et al., 2016a, O'Connor et al., 2009b) and Ido1-FL induction in the mouse brain by mycobacterium infection is IFNγ-dependent (O'Connor et al., 2009a). Since IFNγ was not increased in our acute-stress model, Ido1-FL was not induced. Thus, our data clearly suggest that neither Ido1-FL nor Ido1-v2 mediate the stress-induced Kyn levels in the brain of C57BL/6J mice.
Ido1-v1 is the major Ido1 transcript within the naïve mouse brain, and its expression is increased by LPS in vivo (Brooks et al., 2016a) and IFNγ ex vivo (Brooks et al., 2016b). IFNγ also induces Ido1-Tot expression in astrocytes (Kwidzinski et al., 2005) and microglia (Yadav et al., 2007). However, the current data suggest IFNγ-independent stimuli must be responsible for Ido1-v1 induction in astrocytes by stress. Although Ido-v1 was not increased by stress in whole-brain, its expression was increased by stress in the frontal cortex and hypothalamus (Fig. 4), likely within astrocytes. The significance of the cortex and hypothalamus sensitivity to restraint-induced Ido1-v1 induction relative to behavior remains to be determined.
Astrocytes are a heterogeneous population of related cells (Guillemin and Brew, 2010) varying in functionality across brain-regions (Sery et al., 2015). Previous reports assessing Ido1-Tot indicated primary cultures of murine astrocytes do not express Ido1 (Kwidzinski et al., 2005). By contrast, we detected Ido1-v1 expression in freshly isolated astrocytes, albeit at lower levels compared to the brains from which they were isolated. Thus, the distribution of Ido1 transcripts is cell-type specific. Astrocytes are not the major source of central Ido1-v1, but a 4.8-fold induction by stress could be critical to changes in astrocyte Kyn and KynA production.
We detected minimal expression of Ido1 transcripts in liver which were unaffected by restraint-stress; however, Ohta found increased hepatic Ido1-Tot following 6 h of water-immersion restraint-stress. This was associated with increased hepatic and serum IFNγ (Ohta et al., 2016). Thus, it is possible that our acute stress was insufficient for inducing IFNγ necessary for hepatic Ido1 induction.
4.4. Acute stress increases Ido2-v3 expression in astrocytes
Ido2 expression in the brain and liver is consistent with our previous report (Brooks et al., 2016b). For example, Ido2-FL is enriched in the striatum, yet its expression is highest in liver (Fig. 5A). Sets of Ido2 transcripts share expression patterns: Ido2-FL and Ido2-v4 are enriched in striatum, while Ido2-v1, Ido2-v2, Ido2-v3, Ido2-v5 and Ido2-v6 are enriched in hypothalamus, but Ido2-v8 was not detected in the brain (Fig. 5 and Fig. S1). The cell-types responsible for these differences are unknown. Cells within distinct brain-regions must either differently splice Ido2 pre-RNA to generate different transcripts or utilize different promoter regions to initiate transcription thereby generating different transcripts. The same can be said for Ido1 and Tdo2 transcript profiles, albeit Ido2 is the most complex.
Mazarei found enriched expression of both Ido1-Tot and Ido2-Tot in striatum and that Ido1KO mice were deficient in Ido2-Tot within the striatum (Mazarei et al., 2013), reflecting our expression pattern for Ido1-v1 or Ido2-FL, Ido2-v4 and Ido2-v6, respectively (Fig. 4, Fig. 5 and Fig. S1). Moreover, unlike wild-type mice, Ido2-v3 expression was not increased by stress in Ido1KO mice (Fig. 5B), further highlighting the dysregulation of Ido2 within the brain in the absence of Ido1. These findings raise the issue as to whether behavioral changes seen with Ido1KO mice may also be dependent on reduced basal Ido2 expression and the non-inducible nature of Ido2 in Ido1KO mice. This is important, as Ido1 is considered anti-inflammatory, whereas Ido2 acts as a pro-inflammatory facilitator most likely via an enzymatic-independent mechanism (Merlo and Mandik-Nayak, 2016).
Astrocytes and microglia also differentially express Ido2 transcripts. Ido2-v3 (Fig. 5B) was the only Ido2 transcript upregulated by stress and this only occurred within astrocytes. When cloned and overexpressed, the enzymatic activity of the protein encoded by Ido2-v3 was less than that from Ido2-FL (Metz et al., 2014). Thus, the enzymatic or non-enzymatic role that stress-responsive Ido2-v3 plays in animal behavior, brain function and Kyn production remains to be determined.
In the current study, both astrocytes and microglia were found to express all three DOs, albeit to varying degrees. However, the DOs may not be universally co-expressed. As an example, within the liver Ido2 and Tdo2 are constitutively expressed by mature hepatocytes (Fukunaga et al., 2012, Inayoshi et al., 2005), whereas low but interferon-γ-inducible Ido1 expression is found within hepatic stellate cells (Kumar et al., 2016). This pattern may explain the abundant relative expression of Ido2 and Tdo2 compared to Ido1 found by liver in the current study. One of the critical issues under investigation by several laboratories is the need for this segregation. The three DOs have redundant enzymatic activity (i.e. Trp→Kyn metabolism) but they also have non-redundant functions such as Ido1-non-enzymatic mediated self-tolerance (Pallotta et al., 2011) and Ido2-pro-inflammatory mediation of autoimmunity (Merlo and Mandik-Nayak, 2016). Clearly in the current work, restraint-stress induces specific Ido1, Ido2 and Tdo2 transcripts within astrocytes while decreasing Ido2 and increasing Tdo2 in liver. Whether these regulatory profiles result in distinct cellular functions is unknown and warrants consideration. Compared to brain, liver greatly over-expresses Ido2-FL, Ido2-v3, Ido2-v4 and Ido2-v8, consistent with previous reports of abundant hepatic Ido2 protein (Fukunaga et al., 2012) and mRNA (Brooks et al., 2016b). In contrast, Ido2-v1, Ido2-v2, Ido2-v5 and Ido2-v6 expression levels are higher in all brain-regions compared to liver (Fig. 5 and Fig. S1). These data clearly illustrate the utilization of alternative-gene processing to produce distinct Ido2 transcript profiles. The ability to fine-tune the Ido2 transcriptome endows the brain and liver with precise regulatory control for responding differentially to stressors.
Hepatic Ido2 expression did not appear to differ significantly between wild-type and Ido1KO mice, although Kolodziej (2013) found decreased Ido2 expression in inguinal lymph nodes of Ido1KO mice. Nevertheless, stress resulted in decreased hepatic Ido2-FL, Ido2-v3 and Ido2-v4 and striatal Ido2-v1. Again, the functional consequences of these tissue-specific changes on behavior remain undetermined.
Determining the mechanism behind perturbed (diminished) expression of specific Ido2 transcripts in Ido1KO mice is outside the scope of the current study. Ball et al. hypothesized that, since Ido1 and Ido2 are adjacent on mouse (and human) chromosome 8 (Tdo2 is on murine chromosome 3), the excision of the 3 exons and intervening introns of the Ido1 gene (Fig. 1 top) may perturb Ido2 expression by disrupting cis-regulatory elements (Ball et al., 2007). The Ido1 gene (spanning 13 kbp) is located 5′ of Ido2 (spanning 22 kbp) with less than 8 kbp's separating the two genes on both the mouse and human chromosomes. While both promoters and enhancer elements are short DNA sequences (typically 100 bp and 50–1500 bp, respectively), promoters are generally very near the transcription start site (TSS). In contrast, enhancer elements may be located far (>100,000 bp) upstream of the TSS (Kulaeva et al., 2012). Thus, enhancers controlling Ido2 expression could easily reside within the upstream Ido1 gene. Thus far, we are unaware of any reports identifying upstream regulatory elements involved in Ido2 expression. However, future studies utilizing the commercially available Ido1KO mouse model should consider the involvement of both altered Ido2 expression (current work and Ball et al., 2007, Metz et al., 2014) and activity (Metz et al., 2014).
Similar to murine Ido2, alternative transcripts for human IDO2 have been described with transcripts lacking various exons or initiating at exon 1 or 2 (Ensembl, 2016a, Metz et al., 2007). Human IDO2 transcripts containing exon 10 are widely expressed across tissues including liver and brain, but those amplified with a forward primer in exon 1a plus reverse primers in either exon 8 or exon 10 are limited to placenta and brain (Metz et al., 2007). Although not confirmed by qPCR analysis, alternate transcripts for human IDO1 and TDO2 have been described (Ensembl, 2016b, Ensembl, 2016c). Thus, DO expression and regulation in humans is most likely also transcript-, tissue- and cell-specific. Characterization of human DO transcript regulation by inflammatory or stress-related signals has not been reported.
4.5. Acute stress increases Tdo2-FL expression in brain, astrocytes and liver
Our data (Fig. 6A–C) support previous reports of abundant hepatic expression of Tdo2 relative to the brain (Brooks et al., 2016b, Kanai et al., 2009) as well as the upregulation of Tdo2 mRNA in liver by restraint-stress (Gibney et al., 2014, Ohta et al., 2016) and in brain-slice cultures by glucocorticoids (Brooks et al., 2016b). However, this is the first report describing the stress-induced upregulation of hepatic Tdo2-FL and Tdo2-v2 and of all three functionally characterized Tdo2 transcripts (Kanai et al., 2009) within whole-brain. However, stress did not induce Tdo2 transcripts in selected brain-regions (PFC, Stri, Hippo and Hypo). Similarly, Gibney reported no acute stress-induced change in Tdo2-Tot mRNA within the PFC (Gibney et al., 2014), further implicating other brain-regions such as the cerebellum or brain stem (Kanai et al., 2009, Mazarei et al., 2013, Pawlak et al., 2000) in the central Tdo2 transcriptional response to acute restraint-stress.
Shimazu first reported increased hepatic Tdo2 activity (in vitro conversion of Trp to Kyn by liver homogenate) following peripheral administration of corticosterone, or by stimulation of the hypothalamic sympathetic nucleus, both effects seemingly independent of adrenal secretions. However, hepatic Tdo2 enzymatic activity was greater when the hypothalamus of animals with intact adrenals were stimulated (Shimazu, 1964). Indeed, the addition of the synthetic glucocorticoid dexamethasone to primary hepatocytes increases Tdo2 activity (Nakamura et al., 1980, Noda et al., 1983) and mRNA levels (Nakamura et al., 1987, Niimi et al., 1983, Ott et al., 2015). Acute stress also increases hepatic Tdo2 activity (Curzon and Green, 1969, Gibney et al., 2014, Nomura, 1965, Ohta et al., 2016), an effect either reportedly requiring (Curzon and Green, 1969) or only partly moderated by adrenal secretions (Nomura, 1965, Ohta et al., 2016). Collectively, these data suggest hepatic Tdo2 activity is increased in a glucocorticoid-dependent (stress, corticosterone-induced) and glucocorticoid-independent (direct hypothalamic stimulation) manner.
Whether glucocorticoids directly mediate the upregulation of all three Tdo2 transcripts in the brain is unlikely since dexamethasone only upregulated Tdo2-FL in brain-slice cultures (Brooks et al., 2016b). Thus, Tdo2-FL induction likely represents the adrenal-dependent transcript in the aforementioned studies, whereas Tdo2-v1 and Tdo2-v2 represent the adrenal/glucocorticoid-independent transcripts. Again, it appears that tissues utilize different DO transcripts to fine-tune responses to specific physiologic inputs. Defining the Tdo2 transcriptome within specific hepatic cell-types is needed to completely understand Tdo2 (and Ido) regulation in response to stress.
Following acute stress, only the glucocorticoid-responsive Tdo2-FL transcript was induced within astrocytes. Curiously, the stress-induced increase in Tdo2-FL was preserved in astrocytes of Ido1KO mice but not within whole-brain homogenate of Ido1KO mice (Fig. 6A). Thus, although genetic deletion of Ido1 blocks stress-induced increases of astrocyte Ido2-v3 (Fig. 5B), astrocyte Tdo2-FL expression is still increased by stress in Ido1KO mice (Fig. 6A). Hence, in astrocytes, the induction of Ido2 is Ido1-dependent, but the induction of Tdo2 is Ido1-independent. Tdo2 (unknown transcript) is present primarily in neurons and astrocytes (McCusker et al., 2014). The stress-induced increase in Tdo2-v1 and Tdo2-v2 in whole brain is not seen in astrocytes (or microglia). Presumably, this induction is occurring within neurons and surprising requires Ido1 (Fig. 6). Elevated Tdo2 in neurons and astrocytes, along with elevated KynA, is a hallmark of schizophrenia (Erhardt et al., 2016, Miller et al., 2006). Stress-induced Tdo2 may provide the comorbidity link between stress and schizophrenic development and psychosis (Howes et al., 2016, Weidenauer et al., 2016).
4.6. Downstream Kynurenine Pathway genes and their regulation by stress
The enzymatic machinery downstream of the DOs define the cellular-specificity of Kyn metabolism into neuroactive kynurenines (Dantzer et al., 2008, McCusker et al., 2014). Astrocytes expressed significantly more Kat2 and less Kynu, Kmo and Haao than microglia (Fig. 7A–D). Thus, downstream of the DOs, the relative expression levels of Kynurenine Pathway enzymes within astrocytes and microglia support current dogma which holds that astrocytes predominately produce KynA (Guidetti et al., 2007, Herédi et al., 2016, Kiss et al., 2003, McCusker et al., 2014, Potter et al., 2010) and microglia produce 3-HK and QuinA (Guillemin et al., 2001, Heyes et al., 1996, McCusker et al., 2014). Although there are 3 well-characterized Kat enzymes, Kat2 is believed to account for the majority of KynA production in the mammalian brain (Rossi et al., 2008). Whether Kat1, Kat2 or Kat3 or their mRNA isoforms are differentially regulated in the brain or liver in response to stress is not known and outside the scope of the current project.
Downstream Kyn Pathway enzymes are spatially and temporally regulated in the brain by inflammation (Parrott et al., 2016) and acute stress (Vecchiarelli et al., 2015). Following acute stress, we found minor changes in the expression of these enzymes, such as the increase in astrocyte and microglia KMO. Nonetheless, the DOs are considered rate-limiting and DO expression was increased by acute stress in astrocytes (Fig. 4, Fig. 5, Fig. 6). Both acute and chronic stress increase brain KynA (Dugan et al., 2016, Pawlak et al., 2000) despite unchanging Kat2 expression (Fig. 7). These findings suggest that the increase in KynA is controlled by elevated astrocyte DO expression without a necessary increase in Kat2. In contrast to the mild inductions by stress, acute LPS-induced inflammatory responses cause 2–5 fold increases in KMO expression (dependent on brain region) without induction of Haao or Kynu in the mouse brain and small changes in Kat2 expression (Parrott et al., 2016). These inflammatory responses suggest more robust changes in microglial downstream enzymes during neuroinflammation compared to stress.
The relevance of these findings to human well-being remains conjectural. The Kynurenine Pathway is implicated in mediating symptoms of depression induced by chronic stress and inflammation (O'Farrell and Harkin, 2015). Increased plasma Kyn and cerebral spinal fluid levels of Kyn and QuinA are associated with depression symptomology in patients treated with IFNα (Raison et al., 2010). In adolescents, the peripheral Kyn:Trp ratio correlates with anhedonia scores (Gabbay et al., 2012) and increased suicidality (Bradley et al., 2015). These findings have been largely attributed to altered IDO1 activity (McCusker et al., 2014) with little known regarding the role of IDO2. In contrast, evidence is building for TDO2 involvement in human mental health.
White-matter astrocyte TDO2 immune staining was increased in post mortem frontal cortex and anterior cingulate samples of Schizophrenic patients compared to controls (Miller et al., 2006, Miller et al., 2004). Schizophrenic patients have elevated salivary (Chiappelli et al., 2014), cerebrospinal fluid (Schwarcz et al., 2001) and frontal cortex levels of KynA (Schwarcz et al., 2001). Tdo2 mRNA expression in frontal cortex (Miller et al., 2004) and anterior cingulate (Miller et al., 2006) are greater post mortem in Schizophrenic patients than controls. One hallmark of Schizophrenia is the reduced ability of a ‘prepulse’ (or weak cue) to inhibit the natural startle response to a subsequent stronger stimulus (Kumari et al., 2000). Prepulse inhibition is also disrupted by increased brain KynA following peripheral administration of Kyn to rodents (Erhardt et al., 2004). Within the brain, KynA reduces neurotransmitter activity by antagonizing α7nACh (Wu et al., 2010) and NMDA receptors (Guillemin et al., 2001). Acute stress increases urinary (Francesconi et al., 1972) and salivary (Chiappelli et al., 2014) KynA levels in healthy volunteers. Acute stress also increases salivary KynA in Schizophrenic patients and greater KynA levels correlated with greater disease severity (Chiappelli et al., 2014). However, we are unaware of studies quantifying stress effects on central levels of KynA in humans. Nonetheless, mitigating stress (Chiappelli et al., 2014, Weidenauer et al., 2016) and targeting astrocyte DOs and/or KAT are proposed as a therapeutic approaches for treating Schizophrenia (Erhardt et al., 2016, O'Farrell and Harkin, 2015, Pocivavsek et al., 2016). While astrocyte Tdo2 is stress-sensitive in rodents and implicated in mediating increased central KynA in patients and rodent models of Schizophrenia, the expression of alternative DO transcript in humans following stress and in the pathogenesis of Schizophrenia remain totally un-defined.
5. Conclusion
Although several studies have implicated the Kynurenine Pathway in stress-induced depression-like behavior, the cellular-origin and transcript-specificity of the DOs remained undefined. Herein, we report that all three DOs were upregulated by stress in a cell- and transcript-specific manner. Specifically, Ido1-v1, Ido2-v3 and Tdo2-FL were all increased in astrocytes. In contrast, brain Tdo2-v1 and Tdo2-v2 were upregulated by stress, a response that is independent of astrocyte or microglial expression. When only investigating whole-brain or brain-regions these subtle but critical changes are easily overlooked. Remarkably, stress did not increase several DO transcripts in brain of Ido1KO mice, suggesting an Ido1 requirement for their induction. Thus, our data highlight a significant perturbation of Ido2 and Tdo2 regulation within the brain of Ido1KO mice. The specific role for astrocytes in acute stress is supported by reports of stress-induced increases in central kynurenic acid (KynA) (Dugan et al., 2016, Kiank et al., 2010, Pawlak et al., 2000), the major downstream Kynurenine Pathway product produced by astrocytes (Guidetti et al., 2007, Kiss et al., 2003, Zmarowski et al., 2009). Future studies examining the effect of stress on the Kynurenine Pathway should consider the role of all three DOs and examine cell-specific changes. Undoubtedly, a single qPCR assay will frequently miss cell-specific changes in DO expression.
Acknowledgments
This work was supported by the National Institutes of Health: RO1 MH101145 to RHM and R01 SUB UT 00000712 to KWK.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ynstr.2017.02.002.
Contributor Information
Carlos R. Dostal, Email: cdostal2@illinois.edu.
Megan Carson Sulzer, Email: carsonsulzerm@gmail.com.
Keith W. Kelley, Email: kwkelley@illinois.edu.
Gregory G. Freund, Email: freun@illinois.edu.
Robert H. McCusker, Email: rmccuske@illinois.edu.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Fig. S1.
Additional Ido2 transcripts and their expression following acute stress. Tissue and glia from control (Ctrl) and restraint-stressed (Res) mice sacrificed 2 h after a 3 h restraint were analyzed for additional Ido2 transcripts. (A) Ido2-v1 expression in brain is greater than astrocyte and microglia levels, indicating these cell-types are not the major source of Ido2-v1. Hypothalamic Ido2-v1 expression was greater than all other brain-regions. Stress slightly decreased Ido2-v1 relative to controls in the striatum. Ido2-v1 expression was not detectable in the liver. (B) Ido2-v2 expression was greater in microglia than brain with brain and astrocytes having similar expression levels, indicating that microglia and astrocytes are a major source of central Ido2-v2. In Ido1KO mice, Ido2-v2 was greater in astrocytes compared to brain, and increased in the brain following restraint-stress relative to Ctrl. Within brain-regions, Ido2-v2 expression differed with Hypo > PFC = Stri > Hippo > liver. Hypo Ido2-v2 was increased ∼28% by stress relative to Ctrl. (C) Ido2-v5 expression did not differ in brain, astrocytes or microglia of Ctrl or stressed mice, indicating a uniform expression pattern for this transcript. Ido2-v5 expression in the hypothalamus was greater than all other brain-regions. Hepatic Ido2-v5 expression was not detected. Ido2-v5 expression was not detected in brain or astrocytes of Ido1KO mice. (D) Brain Ido2-v6 expression was greater than astrocyte and microglia, indicating these cell-types are not the major source of brain Ido2-v6. In Ido1KO mice, brain Ido2-v6 expression was also greater than astrocyte levels. Ido2-v6 expression was greater in Hypo than all other brain-regions. Hepatic Ido2-v6 was not detectable. (E) Ido2-v7 expression did not differ in brain, astrocytes or microglia of Ctrl or stressed mice or across brain-regions, indicating a uniform expression pattern for this transcript. Ido2-v7 was not detected in Ido1KO mice brain or astrocytes. Hepatic Ido2-v7 expression levels were greater than all other brain-regions. (F) Ido2-v8 expression was only detected in liver. *p < 0.05 comparing Ctrl to Res samples within tissue or glia. ϕp<0.05 compared to other brain-regions. δp<0.05 liver compared to all brain-regions.
Overall these additional Ido2 transcripts are relatively impervious to stress. However, Ido2 transcripts fall into two general categories: 1) hepatic expression > than brain (Ido2-FL, Ido2-v3, Ido2-v4 (Fig. 5), Ido2-v7 and Ido2-v8 (Fig. S1); 2) brain expression > than liver (Ido2-v1, Ido2-v2, Ido2-v5 and Ido2-v6 (Fig. S1). This illustrates a distinct regulatory mechanism driving the expression of Ido2, albeit with as yet unrecognized functional consequences.
References
- Ando T., Dunn A.J. Mouse tumor necrosis factor-alpha increases brain tryptophan concentrations and norepinephrine metabolism while activating the HPA axis in mice. Neuroimmunomodulation. 1999;6:319–329. doi: 10.1159/000026391. [DOI] [PubMed] [Google Scholar]
- Badawy A.A.-B., Evans M. The mechanism of inhibition of rat liver tryptophan pyrrolase activity by 4-hydroxypyrazolo[3,4-d]pyrimidine (Allopurinol) Biochem. J. 1973;133:585–591. doi: 10.1042/bj1330585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball H.J., Sanchez-Perez A., Weiser S., Austin C.J.D., Astelbauer F., Miu J., McQuillan J.A., Stocker R., Jermiin L.S., Hunt N.H. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Bender D.A., Olufunwa R. Utilization of tryptophan, nicotinamide and nicotinic acid as precursors for nicotinamide nucleotide synthesis in isolated rat liver cells. Br. J. Nutr. 1988;59:279–287. doi: 10.1079/bjn19880035. [DOI] [PubMed] [Google Scholar]
- Bradley K.A.L., Case J.A.C., Khan O., Ricart T., Hanna A., Alonso C.M., Gabbay V. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res. 2015;227:206–212. doi: 10.1016/j.psychres.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A.K., Lawson M.A., Rytych J.L., Yu K.C., Janda T.M., Steelman A.J., McCusker R.H. Immunomodulatory factors galectin-9 and interferon-gamma synergize to induce expression of rate-limiting enzymes of the kynurenine pathway in the mouse hippocampus. Front. Immunol. 2016;7:1–16. doi: 10.3389/fimmu.2016.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A.K., Lawson M.A., Smith R.A., Janda T.M., Kelley K.W., McCusker R.H. Interactions between inflammatory mediators and corticosteroids regulate transcription of genes within the Kynurenine Pathway in the mouse hippocampus. J. Neuroinflammation. 2016;13:1–16. doi: 10.1186/s12974-016-0563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne C.A., O'Brien F.E., Connor T.J., Dinan T.G., Cryan J.F. Differential lipopolysaccharide-induced immune alterations in the hippocampus of two mouse strains: effects of stress. Neuroscience. 2012;225:237–248. doi: 10.1016/j.neuroscience.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Chiappelli J., Pocivavsek A., Nugent K.L., Notarangelo F.M., Kochunov P., Rowland L.M., Schwarcz R., Hong L.E. Stress-induced increase in kynurenic acid as a potential biomarker for patients with schizophrenia and distress intolerance. JAMA psychiatry. 2014;71:761–768. doi: 10.1001/jamapsychiatry.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G.P., Gold P.W. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. Jama. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Curzon G., Green A.R. Effects of immobilization on rat liver tryptophan pyrrolase and brain 5-hydroxytryptamine metabolism. Br. J. Pharmacol. 1969;37:689–697. doi: 10.1111/j.1476-5381.1969.tb08507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan A.M., Parrott J.M., Redus L., Hensler J.G., O'Connor J.C. Low-level stress induces production of neuroprotective factors in wild-type but not BDNF+/- mice: interleukin-10 and kynurenic acid. Int. J. Neuropsychopharmacol. 2016;19:1–5. doi: 10.1093/ijnp/pyv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A.J. The role of interleukin-1 and tumor necrosis factor alpha in the neurochemical and neuroendocrine responses to endotoxin. Brain Res. Bull. 1992;29:807–812. doi: 10.1016/0361-9230(92)90148-q. [DOI] [PubMed] [Google Scholar]
- Ensembl . 2016. Indoleamine 2,3-dioxygenase 2 [WWW Document]http://useast.ensembl.org/Homo_sapiens/Gene/Summary?g=ENSG00000188676;r=8:39792133–39873910 Ensembl release 87-Dec 2016. [Google Scholar]
- Ensembl . 2016. Indoleamine 2,3-dioxygenase 1 [WWW Document]http://useast.ensembl.org/Homo_sapiens/Gene/Summary?g=ENSG00000131203;r=8:39759794–39785963 Ensembl release 87-Dec 2016. [Google Scholar]
- Ensembl . 2016. Tryptophan 2,3-dioxygenase [WWW Document]http://useast.ensembl.org/Homo_sapiens/Gene/Summary?db=core;g=ENSG00000151790;r=4:156775890–156841558 Ensembl release 87-Dec 2016. [Google Scholar]
- Erhardt S., Schwieler L., Emanuelsson C., Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol. Psychiatry. 2004;56:255–260. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Erhardt S., Schwieler L., Imbeault S., Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology. 2016;112:297–306. doi: 10.1016/j.neuropharm.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Francesconi R.P., Boyd A.E., III, Mager M. Human tryptophan and tyrosine metabolism: effects of acute exposure to cold stress. J. Appl. Physiol. 1972;33:165–169. doi: 10.1152/jappl.1972.33.2.165. [DOI] [PubMed] [Google Scholar]
- Frank M.G., Weber M.D., Watkins L.R., Maier S.F. Stress-induced neuroinflammatory priming: a liability factor in the etiology of psychiatric disorders. Neurobiol. Stress. 2015;4:62–70. doi: 10.1016/j.ynstr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujigaki H., Saito K., Fujigaki S., Takemura M., Sudo K., Ishiguro H., Seishima M. The signal transducer and activator of transcription 1 and interferon regulatory factor 1α are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kB. J. Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- Fujigaki S., Saito K., Sekikawa K., Tone S., Takikawa O., Fujii H., Wada H., Noma A., Seishima M. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism. Eur. J. Immunol. 2001;31:2313–2318. doi: 10.1002/1521-4141(200108)31:8<2313::aid-immu2313>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Yamamoto Y., Kawasoe M., Arioka Y., Murakami Y., Hoshi M., Saito K. Studies on tissue and cellular distribution of indoleamine 2,3-dioxygenase 2: the absence of IDO1 upregulates IDO2 expression in the epididymis. J. Histochem. Cytochem. 2012;60:854–860. doi: 10.1369/0022155412458926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V., Ely B.A., Babb J., Liebes L. The possible role of the kynurenine pathway in anhedonia in adolescents. J. Neural Transm. 2012;119:253–260. doi: 10.1007/s00702-011-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney S.M., Fagan E.M., Waldron A., O'Byrne J., Connor T.J., Harkin A. Inhibition of stress-induced hepatic tryptophan 2, 3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int. J. Neuropsychopharmacol. 2014;17:917–928. doi: 10.1017/S1461145713001673. [DOI] [PubMed] [Google Scholar]
- Gold P.W., Machado-Vieira R., Pavlatou M.G. Clinical and biochemical manifestation of depression: relation to the neurobiology of stress. Neural Plast. 2015;2015:11. doi: 10.1155/2015/581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P., Hoffman G.E., Melendez-Ferro M., Albuquerque E.X., Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- Guillemin G.J., Brew B.J. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J. Leukoc. Biol. 2010;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Guillemin G.J., Kerr S.J., Smythe G.A., Smith D.G., Kapoor V., Armati P.J., Croitoru J., Brew B.J. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J. Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- Guillemin G.J., Smith D.G., Smythe G.A., Armati P.J., Brew B.J. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv. Exp. Med. Biol. 2003;527:105–112. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- Guillemin G.J., Smythe G., Takikawa O., Brew B.J. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2004:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- Herédi J., Berkó A.M., Jankovics F., Iwamori T., Iwamori N., Ono E., Horváth S., Kis Z., Toldi J., Vécsei L., Gellért L. Astrocytic and neuronal localization of kynurenine aminotransferase-2 in the adult mouse brain. Brain Struct. Funct. 2016 doi: 10.1007/s00429-016-1299-5. [DOI] [PubMed] [Google Scholar]
- Heyes M.P., Achim C.L., Wiley C.A., Major E.O., Saito K., Markey S.P. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem. J. 1996;320(Pt 2):595–597. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O.D., McCutcheon R., Owen M.J., Murray R. The role of genes, stress and dopamine in the development of schizophrenia. Biol. Psychiatry. 2016;81:9–20. doi: 10.1016/j.biopsych.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inayoshi Y., Kaneoka H., Machida Y., Terajima M., Dohda T., Miyake K., Iijima S. Repression of GR-mediated expression of the tryptophan oxygenase gene by the SWI/SNF complex during liver development. J. Biochem. 2005;138:457–465. doi: 10.1093/jb/mvi147. [DOI] [PubMed] [Google Scholar]
- Kanai M., Nakamura T., Funakoshi H. Identification and characterization of novel variants of the tryptophan 2,3-dioxygenase gene: differential regulation in the mouse nervous system during development. Neurosci. Res. 2009;64:111–117. doi: 10.1016/j.neures.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Kennett G.A., Joseph M.H. The functional importance of increased brain tryptophan in the serotonergic response to restraint stress. Neuropharmacology. 1981;20:39–43. doi: 10.1016/0028-3908(81)90039-3. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Koretz D., Merikangas K.R., Rush A.J., Walters E.E., Wang P.S. The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Mcgonagle K.A., Zhao S., Nelson C.B., Hughes M., Eshleman S., Wittchen H., Kendler K.S. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch. Gen. Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kiank C., Zeden J.-P., Drude S., Domanska G., Fusch G., Otten W., Schuett C. Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS One. 2010;5:e11825. doi: 10.1371/journal.pone.0011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss C., Ceresoli-Borroni G., Guidetti P., Zielke C.L., Zielke H.R., Schwarcz R. Kynurenate production by cultured human astrocytes. J. Neural Transm. 2003;110:1–14. doi: 10.1007/s00702-002-0770-z. [DOI] [PubMed] [Google Scholar]
- Kolodziej L. Investigation of the kynurenine pathway in Indoleamine 2, 3 dioxygenase deficient mice with inflammatory arthritis. Transgenic Res. 2013;22:1049–1054. doi: 10.1007/s11248-013-9696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaeva O.I., Nizovtseva E.V., Polikanov Y.S., Ulianov S.V., Studitsky V.M. Distant activation of transcription: mechanisms of enhancer action. Mol. Cell. Biol. 2012;32:4892–4897. doi: 10.1128/MCB.01127-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Wang J., Thomson A.W., Gandhi C.R. Hepatic stellate cells increase the immunosuppressive function of natural Foxp3+ regulatory T cells via IDO-induced AhR activation. J. Leukoc. Biol. 2016;101:1–10. doi: 10.1189/jlb.2A0516-239R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V., Soni W., Mathews V.M., Sharma T. Prepulse inhibition of the startle response in men with schizophrenia. Arch. Gen. Psychiatry. 2000;57:609–614. doi: 10.1001/archpsyc.57.6.609. [DOI] [PubMed] [Google Scholar]
- Kwidzinski E., Bunse J., Aktas O., Richter D., Mutlu L., Zipp F., Nitsch R., Bechmann I. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- Lawson M., Kelley K.W., Dantzer R. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain. Behav. Immun. 2011;25:1569–1575. doi: 10.1016/j.bbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M.A., Dostal C.R., Brooks A.K., McCusker R.H. The Kynurenine (Kyn) Pathway and Neuroinflammation: the Confused Brain. In: Opp M.R., editor. Primer of PsychoNeuroImmunology Research; Los Angeles, CA: 2016. pp. 95–101. [Google Scholar]
- Lee Y.-K., Lee H.B., Shin D.-M., Kang M.J., Yi E.C., Noh S., Lee J., Lee C., Min C.-K., Choi E.Y. Heme-binding-mediated negative regulation of the tryptophan metabolic enzyme indoleamine 2,3-dioxygenase 1 (IDO1) by IDO2. Exp. Mol. Med. 2014;46:e121. doi: 10.1038/emm.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-N., Peng Y.-L., Lei-Liu, Wu T.-Y., Zhang Y., Lian Y.-J., Yang Y.-Y., Kelley K.W., Jiang C.-L., Wang Y.-X. TNFα mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur. Cytokine Netw. 2015;26:15–25. doi: 10.1684/ecn.2015.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madrigal J.L.M., Hurtado O., Moro M.A., Lizasoain I., Lorenzo P., Castrillo A., Boscá L., Leza J.C. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002;26:155–163. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Mazarei G., Budac D.P., Lu G., Lee H., Möller T., Leavitt B.R. The absence of indoleamine 2,3-dioxygenase expression protects against NMDA receptor-mediated excitotoxicity in mouse brain. Exp. Neurol. 2013;249:144–148. doi: 10.1016/j.expneurol.2013.08.007. [DOI] [PubMed] [Google Scholar]
- McCusker R.H., Kavelaars A., Heijnen C.J., Dantzer R., Kelley K.W. Depression, inflammation and tryptophan metabolism. In: Kusnecov A., Anisman H., editors. The Wiley-Blackwell Handbook of Psychoneuroimmunology. John Wiley & Sons, Ltd; 2014. pp. 448–468. [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Merlo L.M.F., Mandik-Nayak L. IDO2: a pathogenic mediator of inflammatory autoimmunity. Clin. Med. Insights Pathol. 2016;9:21–28. doi: 10.4137/CPath.S39930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz R., DuHadaway J.B., Kamasani U., Laury-Kleintop L., Muller A.J., Prendergast G.C. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- Metz R., Smith C., DuHadaway J.B., Chandler P., Baban B., Merlo L.M.F., Pigott E., Keough M.P., Rust S., Mellor A.L., Mandik-Nayak L., Muller A.J., Prendergast G.C. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int. Immunol. 2014;26:357–367. doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.L., Llenos I.C., Dulay J.R., Barillo M.M., Yolken R.H., Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol. Dis. 2004;15:618–629. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Miller C.L., Llenos I.C., Dulay J.R., Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Miura H., Ando Y., Noda Y., Isobe K., Ozaki N. Long-lasting effects of inescapable-predator stress on brain tryptophan metabolism and the behavior of juvenile mice. Stress. 2011;14:262–272. doi: 10.3109/10253890.2010.541539. [DOI] [PubMed] [Google Scholar]
- Miura H., Ando Y., Noda Y., Ozaki N., Isobe K. Effects of minocycline on changes in brain tryptophan metabolism and the behavior of juvenile mice elicited by inescapable-predator stress. J. Trauma. Stress Disord. Treat. 2013;2:1–7. [Google Scholar]
- Myint A.M., Schwarz M.J., Müller N. The role of the kynurenine metabolism in major depression. J. Neural Transm. 2012;119:245–251. doi: 10.1007/s00702-011-0741-3. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Niimi S., Nawa K., Noda C., Ichihara A., Takagi Y., Anai M., Sakaki Y. Multihormonal regulation of transcription of the tryptophan 2,3-dioxygenase gene in primary cultures of adult rat hepatocytes with special reference to the presence of a transcriptional protein mediating the action of glucocorticoids. J. Biol. Chem. 1987;262:727–733. [PubMed] [Google Scholar]
- Nakamura T., Shinno H., Ichihara A. Insulin and glucagon as a new regulator system for tryptophan oxygenase activity demonstrated in primary cultured rat hepatocytes. J. Biol. Chem. 1980;255:7533–7535. [PubMed] [Google Scholar]
- Nemeth S. The effect of stress on the activity of hepatic tryptophan pyrrolase, of tyrosine aminotransferase in various organs and on the level of tryptophan in the liver and plasma of rats. Physiol. Bohemoslov. 1976;26:557–563. [PubMed] [Google Scholar]
- Nguyen K.T., Deak T., Owens S.M., Kohno T., Fleshner M., Watkins L.R., Maier S.F. Exposure to acute stress induces brain Interleukin-1β protein in the rat. J. Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K.T., Deak T., Will M.J., Hansen M.K., Hunsaker B.N., Fleshner M., Watkins L.R., Maier S.F. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res. 2000;859:193–201. doi: 10.1016/s0006-8993(99)02443-9. [DOI] [PubMed] [Google Scholar]
- Niimi S., Nakamura T., Nawa K., Ichihara A. Hormonal regulation of translatable mRNA of tryptophan 2,3-dioxygenase in primary cultures of adult rat hepatocytes. J. Biochem. 1983;94:1697–1706. [PubMed] [Google Scholar]
- Noda C., Nakamura T., Ichihara A. alpha-Adrenergic regulation of enzymes of amino acid metabolism in primary cultures of adult rat hepatocytes. J. Biol. Chem. 1983;258:1520–1525. [PubMed] [Google Scholar]
- Nomura J. Effects of stress and psychotropic drugs on rat liver tryptophan. Endocrinology. 1965;76:1190–1194. doi: 10.1210/endo-76-6-1190. [DOI] [PubMed] [Google Scholar]
- O'Connor J.C., André C., Wang Y., Lawson M., Szegedi S.S., Lestage J., Castanon N., Kelley K.W., Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J. Neurosci. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor J.C., Lawson M.A., André C., Moreau M., Lestage J., Castanon N., Kelley K.W., Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell K., Harkin A. Stress-related regulation of the kynurenine pathway: relevance to neuropsychiatric and degenerative disorders. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Ohgidani M., Kato T.A., Sagata N., Hayakawa K., Shimokawa N., Sato-Kasai M., Kanba S. TNF-α from hippocampal microglia induces working memory deficits by acute stress in mice. Brain. Behav. Immun. 2016;55:17–24. doi: 10.1016/j.bbi.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Kubo H., Yashiro K., Ohashi K., Tsuzuki Y., Wada N., Yamamoto Y., Saito K. Effect of water-immersion restraint stress on tryptophan catabolism through the kynurenine pathway in rat tissues. J. Physiol. Sci. 2016:1–12. doi: 10.1007/s12576-016-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Litzenburger U.M., Rauschenbach K.J., Bunse L., Ochs K., Sahm F., Pusch S., Opitz C.A., Blaes J., von Deimling A., Wick W., Platten M. Suppression of TDO-mediated tryptophan catabolism in glioblastoma cells by a steroid-responsive FKBP52-dependent pathway. Glia. 2015;63:78–90. doi: 10.1002/glia.22734. [DOI] [PubMed] [Google Scholar]
- Pallotta M.T., Orabona C., Volpi C., Vacca C., Belladonna M.L., Bianchi R., Servillo G., Brunacci C., Calvitti M., Bicciato S., Mazza E.M.C., Boon L., Grassi F., Fioretti M.C., Fallarino F., Puccetti P., Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- Parrott J.M., Redus L., O'Connor J.C. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J. Neuroinflammation. 2016;13:1–15. doi: 10.1186/s12974-016-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak D., Takada Y., Urano T., Takada A. Serotonergic and kynurenic pathways in rats exposed to foot shock. Brain Res. Bull. 2000;52:197–205. doi: 10.1016/s0361-9230(00)00252-5. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A., Notarangelo F.M., Wu H.-Q., Bruno J.P., Schwarcz R. Astrocytes as pharmacological targets in the treatment of schizophrenia: focus on kynurenic acid. In: Pletnikov M.V., Waddington J.L., editors. Handbook of Behavioral Neuroscience. Elsevier; 2016. pp. 423–443. [Google Scholar]
- Potter M.C., Elmer G.I., Bergeron R., Albuquerque E.X., Guidetti P., Wu H.-Q., Schwarcz R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35:1734–1742. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Dantzer R., Kelley K.W., Lawson M.A., Woolwine B.J., Vogt G., Spivey J.R., Saito K., Miller A.H. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F., Schwarcz R., Rizzi M. Curiosity to kill the KAT (kynurenine aminotransferase): structural insights into brain kynurenic acid synthesis. Curr. Opin. Struct. Biol. 2008;18:748–755. doi: 10.1016/j.sbi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Rassoulpour A., Wu H.Q., Medoff D., Tamminga C.A., Roberts R.C. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Sery O., Sultana N., Kashem M.A., Pow D.V., Balcar V.J. GLAST but not least — distribution, function, genetics and epigenetics of L-glutamate transport in brain—focus on GLAST/EAAT1. Neurochem. Res. 2015:2461–2472. doi: 10.1007/s11064-015-1605-2. [DOI] [PubMed] [Google Scholar]
- Shimazu T. Role of hypothalamus in the induction of tryptophan pyrrolase activity in rabbit liver. J. Biochem. 1964;55:163–171. [PubMed] [Google Scholar]
- Shimazu T. The effect of electric stimulation of hypothalamus on rabbit liver tryptophan pyrrolase. Biochim. Biophys. Acta. 1962;65:373–375. doi: 10.1016/0006-3002(62)91064-8. [DOI] [PubMed] [Google Scholar]
- Sorrells S.F., Caso J.R., Munhoz C.D., Sapolsky R.M. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S.F., Sapolsky R.M. An inflammatory review of glucocorticoid actions in the CNS. Brain. Behav. Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechschulte L.A., Sanchez E.R. FKBP51-A selective modulator of glucocorticoid and androgen sensitivity. Curr. Opin. Pharmacol. 2011;11:332–337. doi: 10.1016/j.coph.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman A.J., Alford E., Young C.R., Welsh T.H., Meagher M.W., Welsh C.J.R. Restraint stress fails to render C57BL/6 mice susceptible to theiler's virus-induced demyelination. Neuroimmunomodulation. 2010;17:109–119. doi: 10.1159/000258694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli H.A., Gandhi C.P., Hill M.N. Acute psychological stress modulates the expression of enzymes involved in the Kynurenine Pathway throughout corticolimbic circuits in adult male rats. Neural Plast. 2015;2016:1–12. doi: 10.1155/2016/7215684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lawson M., Dantzer R., Kelley K.W. LPS-induced indoleamine 2,3-dioxygenase is regulated in an interferon-gamma-independent manner by a JNK signaling pathway in primary murine microglia. Brain. Behav. Immun. 2010;24:201–209. doi: 10.1016/j.bbi.2009.06.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenauer A., Bauer M., Sauerzopf U., Bartova L., Praschak-Rieder N., Sitte H.H., Kasper S., Willeit M. Making sense of: sensitization in schizophrenia. Int. J. Neuropsychopharmacol. 2016;00:1–10. doi: 10.1093/ijnp/pyw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won E., Kim Y.-K. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr. Neuropharmacol. 2015:1–8. doi: 10.2174/1570159X14666151208113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-Q., Pereira E.F.R., Bruno J.P., Pellicciari R., Albuquerque E.X., Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J. Mol. Neurosci. 2010;40:204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M.C., Burudi E.M.E., Alirezaei M., Flynn C.C., Watry D.D., Lanigan C.M., Fox H.S. IFN-gamma-induced Ido and Wrs expression in microglia is differentially regulated by IL-4. Glia. 2007;55:1416–1425. doi: 10.1002/glia.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas A.S., Wiechmann T., Gassen N.C., Binder E.B. Gene–stress–epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology. 2016;41:261–274. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L., Dey M., Lauing K.L., Gritsina G., Kaur R., Lukas R.V., Nicholas M.K., Rademaker A.W., Dostal C.R., McCusker R.H., Raizer J.J., Parsa A.T., Block O., Wainwright D.A. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J. Clin. Neurosci. 2015;22:1964–1968. doi: 10.1016/j.jocn.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmarowski A., Wu H.Q., Brooks J.M., Potter M.C., Pellicciari R., Schwarcz R., Bruno J.P. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur. J. Neurosci. 2009;29:529–538. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]