Abstract
Background:
Ganoderma lucidum from Ganodermataceae family is a kind of mushroom known to have various therapeutic properties such as lowering high blood sugar and high blood pressure, boosting the immune system as well as its antibacterial and antioxidant effects.
Materials and Methods:
this study investigated the oxidative stability, microbial and sensory properties of sausage at three different treatments; (i) 1% w/w Ganoderma lucidum powder (GLP) without nitrite as a food preservative (P), (ii) 0.5% w/w GLP with 80 ppm nitrite (N + P), and (iii) sausage with 120 ppm nitrate (N). Lipid oxidation was evaluated using peroxide value (PV) and thiobarbituric acid reactive species. Antimicrobial properties were assessed by total plate count (TPC), yeasts and molds, coliforms, Clostridium perfringens, and Staphylococcus aureus. Sensory assessment was evaluated by nine-point hedonic procedure.
Results:
Samples in N + P treatment showed lower PV than other treatments at the storage period with no significant difference in 2-thiobarbituric acid (TBA) between N and N + P. The P group showed the highest TBA value (P < 0.01). TPC remained below maximal permissible limit recommended by ISIRI during 30 days of storage in all sausage formulations (6.9798 log CFU). There was not found any coliforms bacteria, Clostridium perfringens, and S. aureus. The sensory evaluation indicated that there is no significant difference between samples in texture, taste, and smell. The color and overall acceptability of N group were higher and N + P group was closer to N group.
Conclusion:
The results suggest that G. lucidum powder might be considered as a potential natural preservative for meat products.
Keywords: Antimicrobial, antioxidant, Ganoderma lucidum, sausage
Introduction
Sausage is one of the oldest forms of processed food which is usually made from minced meat, animal fat, salt, spices, and sometimes aromatic herbs.[1] It often forms a significant part of diet and its consumption is increasing because of several reasons (i) increase in young population, (ii) increase in women's employment, (iii) decrease in purchasing power of families, (iv) need for an appropriate substitute for meat, (v) a gradual tendency toward simple living, and (vi) simple, fast, and cheap industry of sausage production.[2]
Nitrate/nitrite is a common ingredient in sausage formulation with clear and distinct functions; it is a microbial preservative particularly against anaerobic ones such as Clostridiums also it acts in the development of specific pink color, taste, and smell of sausage.[3] However, its usage in sausage formulation is controversial as carcinogenic. Nitrosamines can be created as a consequence of acid nitrous with secondary amines. This reaction may take place in both foods or stomach.[4] Production of nitrosamines could be accelerated through the activity of intestine flora, acidic conditions, and high heat over the storage period.[5] Accordingly, minimizing the use of sodium nitrate as a preservative and replacing it with natural and less toxic alternatives is one of the interested areas of research in food safety.
Ganoderma lucidum is a mushroom in red, light yellow, or brownish-red colors and is used in Far East countries specially Japan, China, and Korea for pharmaceutic purposes.[6,7,8] It is known as “Reishi” in Japan, “Linzhi” in China, and “Yeongji” in Korea. Traditionally, it is used to treat stomach cancer, hepatitis A, B, and C, osteoarthritis, insomnia, bronchitis, nervous disorders, asthma, stomach ulcer, high blood pressure, and high cholesterol.[9] Its fruit body and mycelia contain different groups of compounds including sterols, steroids, peptides, lactones, alkaloids, polysaccharides, triterpenoids, and proteins with proven medicinal effects.[10,11,12] Polysaccharides are the most significant because of their anticancer features.[13] Sa-Ard et al. showed that G. lucidum protein extracts have antioxidant and antibacterial activities.[14] Wannasupchue et al. showed that application of G. lucidum in the production of smoked fish sausage delayed lipid oxidation during the storage period.[15] More lactic acid bacteria and polysaccharide with higher viscosity and acetaldehyde content were observed in Ganoderma yogurt than control sample (yogurt without G. lucidum).[16]
The present study evaluates the possible use of G. lucidum as a natural preservative in cooked sausage production. Furthermore, its antioxidant and antibacterial effect in emulsion type sausage is investigated for thefirst time.
Materials and Methods
Sample collection and identification
G. lucidum grown on Carpinus betulus L. (Corylaceae) was collected by hand from Behshahr and Neka forests in the north of Iran and transferred in plastic bags to the laboratory. They were identified using keys and morphological characters provided by Hallenberg.[17]
Sample preparation
G. lucidum fruiting bodies were oven-dried at 40°C for 2–3 days. Then, oven-dried mushrooms were grinded using a blender and stored in plastic bags in a freezer at −20°C for later analyses.[18]
Sausage preparation
Beef was purchased from a local store. It was grinded using a mincer (Pars Khazar Co., Iran) with 3 mm hole. Emulsion type sausage was made following this recipe: 600 g of minced meat mixed in a food maker (Moulinex Co., France) with ingredients including 200 g shattered ice, 25 g milk powder, 60 g flour, 25 g starch, 20 g gluten, 5 g soya, 50 g sunflower oil, 15 g salt, 20 g garlic, 3 g phosphate, 0.5 g ascorbic acid, 1 g coriander powder, 1 g red pepper, 1 g white pepper, 1.5 g nutmeg, and 1.5 g ginger. This mixture was used to make three different treatments of the study; (i) 1% w/w Ganoderma lucidum powder (GLP) without nitrite (P), (ii) 0.5% w/w GLP with 80 ppm nitrite (N + P), and (iii) sausage with 120 ppm nitrate (N). Then, the prepared sausages packed using a commercial artificial casing and cooked in a water bath at 72°C for 2.5 h. Sausages were stored in a fridge at 3°C–4°C for 45 days.
Sensory evaluations
Sensory assessment including six attributes; color, flavor, taste, texture, appearance, and overall acceptability were evaluated by twenty volunteered panelists (staff and students) from the Department of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan. Panelists were asked to present their idea using a nine-point hedonic scales, in which 1 was “extremely dislike” and 9 was “extremely like.”[19]
Measurement of peroxide value
Peroxide value (PV) was determined according to the AOAC International 1991.[20] Five grams of each sample was weighed in a 100 ml glass stoppered Erlenmeyer flask and heated in a water bath at 60°C for 3 min to melt the fat. Then, samples were mixed with 30 ml acetic acid: chloroform solution (3:2 v/v) for 3 min to dissolve the fat. Samples were then filtered through a Whatman filter paper using vacuum. Saturated potassium iodide solution (0.5 ml) was added to the filtrate. The titration was allowed to run against a standard solution of sodium thiosulfate (25 g/l). PV was calculated and expressed as milliequivalent peroxide per kilogram of sample:[20]
PV (meq/kg) = (S × N)/W × 1000
Where S is the volume of titration (ml), N is the normality of sodium thiosulfate solution (n = 0:01), and W is the sample weight (kg).
Measurement of thiobarbituric acid value
2-thiobarbituric acid (TBA) assays were conducted following the procedure described by Buege and Aust.[21] A composition of 0.5 g of each sample was mixed with 2.5 ml of 0.375% TBA - 15% TCA - 0.25 N HCl stock solution. The mixture was heated for 10 min in a water bath at 95°C–100°C to develop a pink color, then was cooled with tap water and centrifuged at 5500 rpm for 25 min in a ultracentrifuge (Sigma 3K30). The absorbance was measured at 532 nm using a UV/visible spectrophotometer (Cecil Instruments Ltd., United Kingdom). TBA number was calculated as mg malondialdehyde (MDA)/kg sample and calculated based on the following formula:[21]
TBA value = Absorbance at 532 nm × 2.77
Microbiological analysis
The samples of three treatment sausages were analyzed for microbiological quality during storage using standard procedures of Iran.[22,23,24,25] Ten grams of each sausage sample was homogenized with 90 ml sterile Ringer's solution and after that serial dilutions were prepared. One milliliter of each dilution was transferred to the agar medium plates for bacterial total count (37°C, 48 h), YGC agar medium for yeast and molds (25°C, 5 d), VRBA medium for coliforms (30°C, 24 h), and SPS agar for Clostridium perfringens (37°C, 48 h).
A volume of 0.1 ml of each dilution was spread with a bent sterile glass rod on triplicate plates of prepoured and dried on Baird-Parker agar for Staphylococcus aureus (37°C, 48 h). Colonies were counted after incubation and results were expressed as CFU/g of sausage sample.
Statistical analyses
Each experiment was conducted in triplicate. The difference between treatments was assessed using analysis of variance (one-way ANOVA) followed by means comparison using either Tukey's HSD test, if variance was homogeneous, or Dunnett's T3 test, if variance was not homogeneous. The data were analyzed by analysis of variance (ANOVA) using IBM statistic SPSS for windows, version 22.0. Armonk, NY: IBM Corp. P < 0.05 was considered statistically significant.
Results
Sensory evaluations
The sensory panels collected for the different treatments are presented in Table 1. There was no statistically significant difference between N, P, and N + P treatments in texture, taste, smell, and color. The overall acceptability of N group was higher and N + P group was closer to N group. Sausage with 1% w/w GLP (P group) had the lowest score color.
Table 1.
Means of sensory evaluations of sausage with/without Ganoderma lucidum powder

Measurement of peroxide value
Changes occurring in the PV of different treatment groups during storage time were shown in Figure 1. In day 0, PV of P group was 0.833 ± 0.0577, and it was the highest (P < 0.01) among treatments analyzed. In days 14, 21, 30, and 45, there was no significant difference between P and N samples, but the P + N samples showed lower PV at the storage period. PVs of all samples were below 25 meq of active O2/kg, which is considered as acceptable limit for PV of fatty foods.
Figure 1.
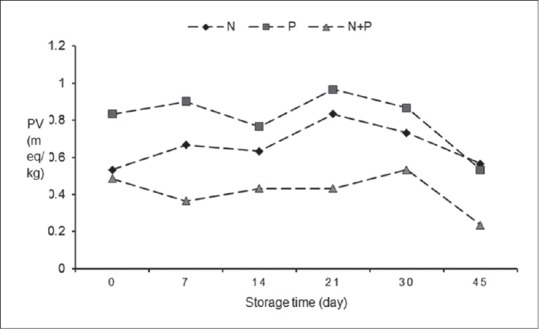
Peroxide value during storage at 4°C. N: Sausage with 120 ppm nitrite, P: Sausage with 1% Ganoderma lucidum powder, N + P: Sausage with 0.5% Ganoderma lucidum powder and 80 ppm nitrite
Measurement of 2-thiobarbituric acid value
The TBA values of sausage groups during 45 days of chilled storage are shown in Figure 2. There is no significant difference between N and N + P groups while P group shows the highest TBA value (P < 0.01).
Figure 2.
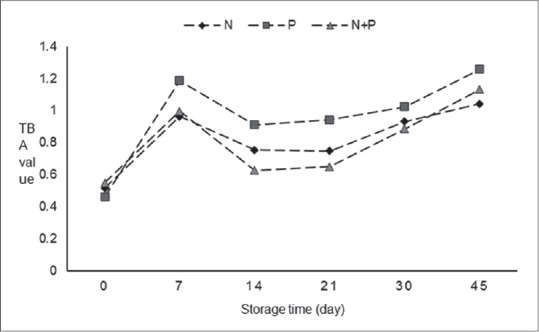
The 2-thiobarbituric acid during storage at 4°C. N: Sausage with 120 ppm nitrite, P: Sausage with 1% Ganoderma lucidum powder, N + P: Sausage with 0.5% Ganoderma lucidum powder and 80 ppm nitrite
Microbiological analysis
According to Figure 3, total bacterial count increased with storage time. There was no significant difference between N and N + P during the first 14 days and day 30. There was a significant difference between N and P just on day 7 (P < 0.05). Changes in microbial count during 30 days of storage in all sausage formulations remained below 6.98 Log CFU maximal permissible limit for total plate count recommended by ISIRI.
Figure 3.
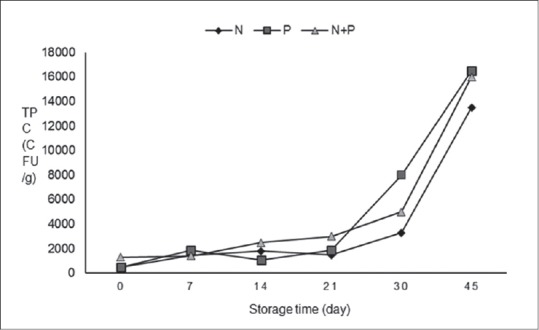
Total plate count during storage at 4°C, N: Sausage with 120 ppm nitrite, P: Sausage with 1% Ganoderma lucidum powder, N + P: Sausage with 0.5% Ganoderma lucidum powder and 80 ppm nitrite
Based on microbiological results, no growth of C. perfringens, Coliforms, and S. aureus was detected during 30 days of storage. In days 30 and 45, we observed 90 and 220 CFU/g of molds and yeasts, respectively, on the sausage samples in treatment P which was lower than which is recommended by ISIRI.
Discussion
Recently, substitution of natural preservatives for chemicals in food formulation has received special attention. Some studies have reported equal and/or even higher antioxidant effects from natural preservatives than chemical. The results of this study showed that GLP as a natural ingredient in sausage formulation could be used to reduce the amount of nitrite without significant adverse effects on sausage characters.
G. lucidum previously showed antioxidant activity in vitro and in vivo studies.[26,27] Tao et al. showed that two polysaccharides from G. lucidum (GLP1 from fermentation broth and GLP2 from fruiting body) have strong antioxidant activities.[28]
Measurement of MDA that produced in the process of lipid peroxidation is used to evaluate lipid peroxidation to assess oxidative stress. TBA value was increased in the first stage of storage because of the MDA decomposition and polymerization. The gradual decrease in thiobarbituric acid reactive species between days 7 and 21 could be caused by microbial-mediated decay of MDA and its oxidation to other products such as alcohol and acid.[29,30] Wannasupchue et al. showed that increasing of TBA value can be controlled using water extract and spore of G. lucidum in smoked fish sausages.[15]
The data are in agreement with what reported earlier by Quereshi et al. and Yoon et al. who claimed that the different G. lucidum extracts can prevent bacterial growth.[31,32] Keypour et al. showed that chloroform extract of G. lucidum has antibacterial effect against Bacillus subtilis and S. aureus in all concentrations.[33] After pasteurization (at 72°C), no sign of Coliforms bacteria was observed in any samples mainly owing to their heat sensitivity. Moreover, the lack of oxygen in packed sausage further inhibited their growth. This confirms the appropriate hygiene quality of the product and production environment. To date, although the antimicrobial activity of G. lucidum compounds was widely reported in culture media, few reports are available on its effect in processed foods.
Conclusion
Based on the results obtained in the present study, sausage samples with 0.5% w/w GLP which reduced in nitrite were quite acceptable. Addition of GLP provides antioxidant and antimicrobial benefits during the storage, but GLP at 1% w/w did not result in high acceptance of color, so the use of nitrite was required. Therefore, it is suggested that GLP as a natural medical herb, could be used to reduce the usage of synthetic additives.
Financial support and sponsorship
This study was supported by a grant from Isfahan University of Medical Sciences as research project number 394641.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors gratefully acknowledge of Mr. Said Ali Moosazade, Dr. Fourutan, and Mr. Ziyaaldin Keshavarzpour, for their assistance.
References
- 1.Essien E. Sausage Manufacture: Principles and Practice. Cambridge, England: Wood Head Publishing Limited; 2003. pp. 5–15. [Google Scholar]
- 2.Pearson AM, Gillett TA. Processed Meats. United States: Springer; 2012. pp. 211–41. [Google Scholar]
- 3.Polic M. Technological and health aspects of using nitrite in meat industry. Tehnologija Mesa (Yugoslavia) 1994;35:25–28. [Google Scholar]
- 4.Lijinsky W. Chemistry and Biology of N-Nitroso Compounds. Cambridge: University Press; 1992. [Google Scholar]
- 5.Honikel KO. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008;78:68–76. doi: 10.1016/j.meatsci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Ulbricht C, Abrams TR, Bent S, Boon H, Costa D, Dacey C, et al. Reishi mushroom (Ganoderma lucidum): Systematic review by the Natural Standard Research Collaboration. J Soc Integr Oncol. 2010;8:148–159. [Google Scholar]
- 7.Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest. 2003;32:201–15. doi: 10.1081/imm-120022979. [DOI] [PubMed] [Google Scholar]
- 8.Luo J, Lin ZB. Advances of pharmacological effects of triterpenes from Ganoderma lucidum. Yao Xue Xue Bao. 2002;37:574–8. [PubMed] [Google Scholar]
- 9.Lindequist U, Niedermeyer TH, Jülich WD. The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2005;2:285–99. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao JJ, Min BS, Ahn EM, Nakamura N, Lee HK, Hattori M. New triterpene aldehydes, lucialdehydes A-C, from Ganoderma lucidum and their cytotoxicity against murine and human tumor cells. Chem Pharm Bull (Tokyo) 2002;50:837–40. doi: 10.1248/cpb.50.837. [DOI] [PubMed] [Google Scholar]
- 11.Bao XF, Wang XS, Dong Q, Fang JN, Li XY. Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry. 2002;59:175–81. doi: 10.1016/s0031-9422(01)00450-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhou H, Liu G, Huang F, Wu X, Yang H. Improved production, purification and bioactivity of a polysaccharide from submerged cultured Ganoderma lucidum. Arch Pharm Res. 2014;37:1530–7. doi: 10.1007/s12272-014-0391-8. [DOI] [PubMed] [Google Scholar]
- 13.Smith JE, Rowan N, Sullivan R. Medicinal Mushrooms: Their Therapeutic Properties and Current Medical Usage with Special Emphasis on Cancer Treatments. Cancer Research UK. 2002 [Google Scholar]
- 14.Sa-Ard P, Sarnthima R, Khammuang S, Kanchanarach W. Antioxidant, antibacterial and DNA protective activities of protein extracts from Ganoderma lucidum. J Food Sci Technol. 2015;52:2966–73. doi: 10.1007/s13197-014-1343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wannasupchue W, Siriamornpun S, Huaisan K, Huaisan J, Meeso N. Effect of adding Ling-zhi (Ganoderma lucidum) on oxidative stability, textural and sensory properties of smoked fish sausage. Thai J Agric Sci. 2011;5:505–12. [Google Scholar]
- 16.Li J, Chen W, Li XY, Cheng F. Ganoderma yogurt and changes in colonies, physical and chemical properties during storage. China Dairy Ind. 2011;5:18. [Google Scholar]
- 17.Hallenberg N. Wood fungi (Polyporaceae, Ganodermataceae, Hymenochaetaceae, Cyphellaceae, Clavariaceae, Auriculariaceae, Tremellaceae, Dacrymycetaceae) in N Iran. II. Iran J Plant Pathol. 1979;15:11–31. [Google Scholar]
- 18.Mau JL, Tsai SY, Tseng YH, Huang SJ. Antioxidant properties of hot water extracts from Ganoderma tsugae Murrill. LWT Food Sci Technol. 2005;38:589–97. [Google Scholar]
- 19.Lawless HT, Heymann H. Sensory Evaluation of Food: Principles and Practices. Germany: Springer Science and Business Media; 2010. [Google Scholar]
- 20.AOAC International. Official Methods of Analysis. 16th ed. Washington, DC, US: Association of Official Analytical Chemist; 1991. [Google Scholar]
- 21.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 22.Iran IoSaIRo. Microbiology of Food and Animal Feeding Stuffs – Enumeration of Coagulase – Positive staphylococci (Staphylococcus aureus and other species) – Test Method Part 1: Technique Using Baird-Parker Agar Medium. Iran, Tehran: ISIRI; 2010. [Google Scholar]
- 23.Iran IoSaIRo. Microbiology of Food and Animal Feeding Stuffs – Horizontal Method for the Enumeration of Coliforms – Colony-Count Technique. Iran, Tehran: ISIRI; 2007. [Google Scholar]
- 24.Iran IoSaIRo. Microbiology of Food and Animal Feeding Stuffs – Horizontal Method for the Enumeration of Yeasts and Moulds – Part 1: Colony Count Technique in Products with Water Activity Greater Than 0.95. Iran, Tehran: ISIRI; 2008. [Google Scholar]
- 25.Iran IoSaIRo. Microbiology of the Food Chain – Horizontal Method for the Enumeration of Microorganisms – Part 1: Colony Count at 30°C by the Pour Plate Technique. Iran, Tehran: ISIRI; 2015. [Google Scholar]
- 26.Heleno SA, Barros L, Martins A, Queiroz MJ, Santos-Buelga C, Ferreira IC. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res Int. 2012;46:135–40. [Google Scholar]
- 27.Smina TP, Mathew J, Janardhanan KK, Devasagayam TP. Antioxidant activity and toxicity profile of total triterpenes isolated from Ganoderma lucidum (Fr.) P Karst occurring in South India. Environ Toxicol Pharmacol. 2011;32:438–46. doi: 10.1016/j.etap.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Tao R, Hao L, Jia S, Zheng X, Yu J, Jiang Q. A comparative study on the antioxidant activity of two polysaccharides from Ganoderma lucidum. Adv Appl Biotechnol. 2015;46:441–50. [Google Scholar]
- 29.Moerck KE, Ball H. Lipid autoxidation in mechanically deboned chicken meat. J Food Sci. 1974;39:876–9. [Google Scholar]
- 30.Fernández J, Pérez-Álvarez JA, Fernández-López JA. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997;59:345–53. [Google Scholar]
- 31.Quereshi S, Pandey A, Sandhu S. Evaluation of antibacterial activity of different Ganoderma lucidum extracts. J Sci Res. 2010;3:9–13. [Google Scholar]
- 32.Yoon SY, Eo SK, Kim YS, Lee CK, Han SS. Antimicrobial activity of Ganoderma lucidum extract alone and in combination with some antibiotics. Arch Pharm Res. 1994;17:438–42. doi: 10.1007/BF02979122. [DOI] [PubMed] [Google Scholar]
- 33.Keypour S, Riahi H, Moradali MF, Rafati H. Investigation of the antibacterial activity of a chloroform extract of Ling Zhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Aphyllophoromycetideae), from Iran. Int J Med Mushrooms. 2008;10:345–349. [Google Scholar]


