Abstract
Background:
Nowadays, cartilage tissue engineering is the best candidate for regeneration of cartilage defects. This study evaluates the function of herbal extracts icariin (ICA), the major pharmacological constituent of herba Epimedium, compared with transforming growth factor β3 (TGFβ3) to prove its potential effect for cartilage tissue engineering.
Materials and Methods:
ICA, TGFβ3, and TGFβ3 + ICA were added fibrin-cell constructions derived from adipose tissue stem cells. After 14 days, cell viability analyzed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H- tetrazolium bromide assay and the expression of cartilage genes was evaluated with real-time polymerase chain reaction (RT-PCR).
Results:
The results showed ICA, TGFβ3, and TGFβ3 + ICA increased the rate of proliferation and viability of cells; but there were no significant differences between them (P > 0.05). Furthermore, quantitative RT-PCR analysis demonstrated that cooperation of ICA with TGFβ3 showed a better effect in expression of cartilaginous specific genes and increased Sox9, type II collagen, and aggrecan expression significantly. Furthermore, the results of the expression of type I and X collagens revealed that TGFβ3 increased the expression of them (P < 0.01); However, treatment with ICA + TGFβ3 down regulated the expression of these genes significantly.
Conclusion:
The results indicated ICA could be a potential factor for chondrogenesis and in cooperation with TGFβ3 could reduce its hypertrophic effects and it is a promising factor for cartilage tissue engineering.
Keywords: Adipose-derived stem cells, chondrocytes, chondrogenesis, icariin
Introduction
Osteoarthritis is a prevalent and chronic form of joint disease and one of the major causes of disability among old people.[1] The disease affects whole joint and destructs articular cartilage.[2] Due to the absence of nutritional vessels in articular cartilage and lack of self-healing capacity in this tissue,[3,4] its damages improve generally through scar tissue formation that is mostly fibrocartilage.[5] Fibrocartilage tissue in compared with normal cartilage has substandard biomechanical properties and progressively degrades with time, and finally leading to inability of the joint.[5,6]
Using stem cells in tissue engineering is a promising approach for the treatment of cartilage defects after injury[7,8] and is based on the fact that stem cells have the potential to differentiate into multilineage cells.[9,10] Growth factors play an important role for regulating stem cells differentiation and are considered as a powerful tool for tissue engineering.[11,12] Among these, transforming growth factor-β (TGFβ) superfamily widely used in cartilage tissue engineering. Different studies have demonstrated the effects of these factors on chondrogenic differentiation and formation of cartilage-like tissue in vivo and in vitro.[13,14] However, along with chondrogenic induction, they lead to hypertrophy of chondrocytes;[15,16,17] Hence, it is necessary to develop low cost and effective compounds without hypertrophic effects as a replacement for growth factors.[18,19]
Herba Epimedium (HEP) is one of 52 species of flowering herbaceous plants.[20] It has been broadly used as an anti-rheumatoid, tonic, and aphrodisiac for more than 2000 years in Japan, Korea, and China.[21,22,23] More than 260 different combinations have been identified from different Epimedium species. Among them, flavonoids and their derivatives are as the most important component.[24,25] Icariin (ICA; C33H40O15; molecular weight, 676.65), as a common flavonoid glycoside, is the most important pharmacologically component of HEP. This factor is selected as a medical marker for quality control of HEP in Chinese Pharmacopeia.[26] Different studies showed ICA is a safe anabolic agent for chondrogenesis.[9,18,19,27] However, previous studies were based on the use of bone marrow mesenchymal stem cells (BM-MSCs) and chondrocytes and there is no study to investigate the chondrogenic effect of ICA on adipose-derived stem cells (ADSCs). In the current research, for the first time, we investigated the effect of ICA on chondrogenic differentiation of adipose-derived stem cells in fibrin scaffold.
Materials and Methods
Reagents
ICA (purity ≥94%) was purchased from Sigma-Aldrich Co., Stock solutions of ICA were dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich Co., USA) and stored at −20°C. Fetal bovine serum (FBS) was obtained from Bioidea (Iran). Penicillin/streptomycin and high glucose Dulbecco's modified Eagle medium (DMEM) were purchased from Gibco Co (USA). Majority of compounds including TGFβ3, phosphate-buffered saline (PBS), DMEM-low glucose, collagenase A, trypsin– ethylenediaminetetraacetic acid, dexamethasone, bovine serum albumin (BSA), insulin-transferrin-selenium (ITS), ascorbate-2-phosphate (ASP), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H -tetrazolium bromide (MTT), and linoleic acid, were purchased from Sigma-Aldrich Co (USA).
Isolation and culture of adipose-derived stem cells
Human subcutaneous adipose tissue was obtained from three healthy persons undergoing liposuction surgery. All procedures were carried out according to the Isfahan University of Medical Sciences, Medical Faculty Ethics Committee Approval. Fat tissue was cut into small pieces and washed thoroughly with PBS. Small pieces of fat were combined with 0.1% collagenase A and were incubated for 45 min at 37°C. Then, enzyme activity was neutralized with the same volume medium containing DMEM-low glucose, 10% FBS, and 1% penicillin/streptomycin. Following, the suspension was centrifuged for 10 min at 1400 rpm. The cell pellets were cultured in flasks containing fresh medium with DMEM-low glucose, 1% penicillin/streptomycin, and 10% FBS. The flasks were incubated at 37°C and 5% CO2 conditions.
Fibrin scaffold preparation and adipose-derived stem cells encapsulation
Fibrin formed cryoprecipitate, which is enriched in fibrinogen. Thrombin as a main component is required to convert fibrinogen to fibrin. For thrombin preparation, 16 ml fresh frozen plasma transferred to conical tubes. Then, 10 ml of calcium gluconate added to each tube and the suspension incubated at 37°C for 70–90 min. Following, the suspension centrifuged for 10 min at 2200 rpm. Finally, transparent supernatant obtained from centrifugation contains thrombin stored at −80°C.[28]
For preparation of cell-fibrin constructs, 106 cells at the third passage were resuspended in 500 μl fibrinogen in each well of 24-well plate. Then, 500 μl of thrombin was added to form the fibrin clot. After encapsulation, cell-fibrin constructs were classified into four groups: The first group was cultured with TGFβ3 (10 ng/ml); the second group was used with ICA (1 × 10-5 M);[18] the third group cultured with TGFβ3 and ICA together; and the fourth group without TGFβ3 and ICA as control group. In all groups, chondrogenic medium containing high glucose DMEM, 100 nM dexamethasone, 1% ITS, 1% BSA, 5 ng/ml linoleic acid, 50 μg/ml ASP, 1% penicillin-streptomycin was used. All groups were incubated for 14 days. The half of media refreshed every 3 days.
Cell viability evaluation
The effects of various agents on cell viability were analyzed by MTT assay. After 14 days, the cell-fibrin constructs were washed twice with PBS. The combination of 400 μl medium and 40 μl MTT solutions (5 mg/ml) was added to each well and incubated for 4 h. After the removal of the medium, the purple-blue formazan precipitates dissolved in 400 μl DMSO and plates placed for 2 h in the dark room. Then, 100 μl of each well solution was transferred to 96-well plate. The absorbance was measured at 540 nm by an ELISA reader (Spectra Max 340, Molecular Device Inc., CA, USA).
Real-time polymerase chain reaction analysis
To investigate the expression of chondrocyte-specific genes including type I, type II, type X collagens, aggrecan, and Sox9, the real-time polymerase chain reaction (RT-PCR) was performed. Total cellular RNA was extracted using RNA Extraction kit (Yekta Tajhiz Azma, Iran), according to the manufacturer's protocol. Using the RNA samples, cDNAs were synthesized by the PrimeScript™ RT reagent Kit (Fermentas, Canada). The RT-PCR reactions were performed using StepOnePlus™ RT-PCR System (Applied Biosystems, USA) with SYBR Green PCR Master Mix (Yekta Tajhiz Azma, Iran) under the implication condition including initial denaturation at 95°C followed by thirty cycles of denaturation at 95°C for 30 s, annealing at 56°C for 45 s and extension at 72°C for 45 s, and a final polymerization at 72°C for 10 min. RT-PCR was performed in triplicate. The primer sequences used for RT-PCR are summarized in Table 1. Relative cartilage gene expression was analyzed using the comparative Ct method (2−ΔΔCt).[29] All samples normalized to level of GAPDH, which used as the loading control.
Table 1.
Primer sequences used in real-time polymerase chain reaction technique

Results
Adipose-derived stem cells morphology
Phase contrast microscopic morphology of ADSCs revealed homogenous fibroblast-like cells in the third passage in compared with multiform cells in primary culture (P0) [Figure 1].
Figure 1.
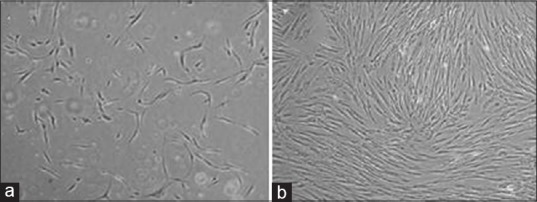
(a) Human subcutaneous adipose-derived stem cells in P0 passage after 3 days (×40); (b) Adipose-derived stem cells in third passage: cells reach 80% confluence and show spindle shape (×40)
Cell viability and proliferation
Our results indicated that the viability and proliferation of treatment groups increased in compared with the control group (P < 0.05) [Figure 2]. No significant difference was seen between treatment groups.
Figure 2.
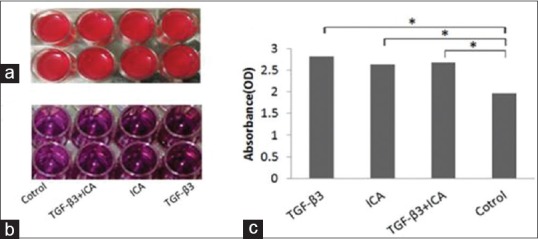
Cell-fibrin constructs in 24-well microplate. Cell viability was analyzed using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay after 14 days (a). Dissolved purple-blue formazan precipitate in dimethylsulfoxide (b). Comparison of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay results in different groups. There are no significant differences between groups treated with Icariin, TGFβ3, and TGFβ3-Icariin together; but compared with the control group the rate of proliferation and viability are increased in all of three groups (c) (P > 0.05). ICA, icariin; TGFβ3, transforming growth factor β3
Gene expression in different groups
The results of RT-PCR were revealed the expression of the cartilaginous-specific genes including type II collagen, aggrecan, and Sox9 in all groups.
As shown in Figure 3a and b, ICA and TGFβ3 increased the expression of type II collagen and Sox9 in compared with the control group although the difference was not significant. In the ICA + TGFβ3 group, expression of Sox9 and type II collagen increased in compared with the control group (P < 0.05). RT-PCR analysis of differentiated cells at day 14 showed that expression of aggrecan gene in ICA + TGFβ3 group was the highest. In this group, expression of aggrecan increased 2.25-fold in comparison of TGFβ3 group (P < 0.01) [Figure 3c].
Figure 3.
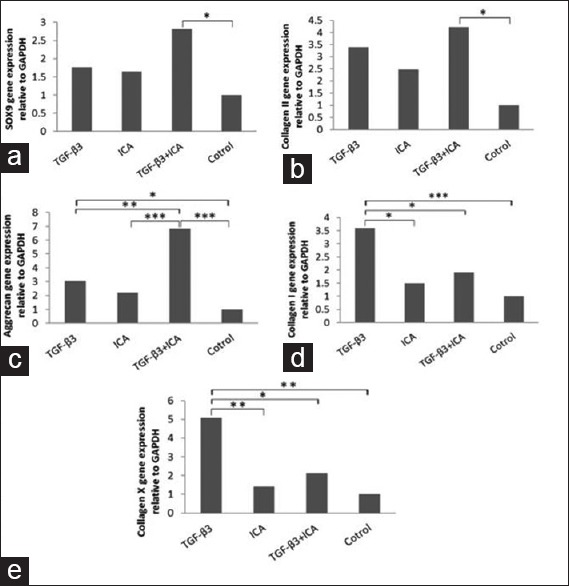
Effects of Icariin, TGFβ3, and TGFβ3 + icariin on gene expression of cartilage markers. Real-time polymerase chain reaction analysis was performed at day 14. Real-time polymerase chain reaction demonstrated that Sox9, type II collagen, and aggrecan significantly increased in icariin + TGFβ3 (a-c). Type I and X collagens decreased in icariin group (d and e). *P < 0.05; **P < 0.01; ***P < 0.001. ICA, icariin; TGFβ3, transforming growth factor β3
Quantitative RT-PCR results demonstrated that type I collagen (fibrocartilage marker) and type X collagen (hypertrophic marker) significantly upregulated in TGFβ3 group (P < 0.01). However, expression of these two markers downregulated in ICA group significantly. In addition, ICA alone decreased expression of type I and type X collagens 1.88- and 2.34-fold, respectively, in compared with TGFβ3 [Figure 3d and e].
Discussion
Due to cartilage's poor potential for natural repair, regeneration of cartilage defects is challenging to orthopedic surgeons. Using autologous adult stem cells such as BM-MSCs and ADSCs to repair defective cartilage may become a viable clinical option. Nowadays, two cell sources were widely used in cartilage tissue engineering: BM-MSCs and adipose-derived stem cells. Huang et al. in their study compared these two cells and showed that the chondrogenesis potential of bone marrow stem cells is higher than adipose-derived stem cells.[30] However, Zhu et al. reported that adipose-derived stem cells are better than them.[31] Other studies revealed that the similar differentiation potential for these two sources.[32,33,34] Adipose-derived stem cells in contrast with BM-MSCs obtained in large amounts and achieved easily with noninvasive techniques.[35,36,37] These advantages for adipose-derived stem cells were considered them for cartilage defects repair; hence, in this study, these cells were used.
Many investigators indicated that the cartilage harvested during chondrogenic differentiation of stem cells with TGFβs are not the same as normal hyaline cartilage because of having many type I and X collagens and not enough type II collagen.[38] Therefore, it is necessary to develop safe compounds that can substitute growth factors or cooperate with them. Therefore, it is necessary to develop a safe combination that can substitute growth factors or cooperate with them.
It has been revealed that ICA is an effective compound in the treatment of osteoporosis, the restoration of bone defects, and the bone tissue engineering.[9,19,39,40] Therefore, it is possible that ICA to be a potential promoting compound for cartilage tissue engineering. The present study investigated the effects of ICA, TGFβ3, and ICA + TGFβ3 on the ADSCs viability, proliferation, and expression some of cartilage-specific genes, in vitro. It proved that in treatment group cell viability and proliferation increased in compared with the control group. Furthermore, our findings indicated that ICA significantly downregulated the expression of type I and type X collagens in compared with TGFβ3. Although the expression of type II collagen, aggrecan, and Sox9 was superior in ICA + TGFβ3 group.
Zhang et al. reported that ICA affected on proliferation of chondrocytes in dose-dependent manner.[18] In contrast with Zhang’ study, we indicated that ICA in 10-5 M increased cell proliferation which might be related to the different kind of cell.
Some researchers have proven that ICA is an anabolic agent, which can enhance chondrocyte proliferation and reduce extracellular matrix degradation.[27,41] It was reported that ICA promotes the secretion of various growth factors, including BMP2 and TGFβ1 in osteoblasts, in vitro.[42,43] Different studies have shown that BMP signals upregulated the expression of Sox9.[44] Sox9, a key gene in chondrogenesis and differentiation, promotes the expression of type II collagen and aggrecan.[45] Li has demonstrated that the expression of Sox9 significantly increased by ICA.[19] Similarly, our results indicated that ICA with TGFβ3 enhances the expression of Sox9 considerable.
Furthermore, in contrast with other studies, our finding indicate that ICA in combination with TGFβ3 upregulated cartilage-specific gene.[18,19]
Type X and type I collagen are hypertrophic and fibrocartilage markers, respectively. Different reports have shown use of TGFβ3 lead to cell hypertrophy and fibrocartilage formation.[15,16,17] In the current study, it was revealed that TGFβ3 increased expression of type I and type X collagens. However, ICA reduced these two genes.
Conclusion
All of these findings suggest that ICA may be a potential promoting factor for cartilage tissue engineering and it could reduce cell hypertrophy
Financial support and sponsorship
This study was founded by the research council of Isfahan University of Medical Sciences, Isfahan, Iran (No. 394590).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We want to thanks the vice chancellor of Isfahan university of medical sciences and Mrs. Aliakbari who helps us to collect the samples.
References
- 1.Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451:716–9. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- 2.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–35. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 3.Ansar MM, Esfandiariy E, Mardani M, Hashemibeni B, Zarkesh-Esfahani SH, Hatef M, et al. A comparative study of aggrecan synthesis between natural articular chondrocytes and differentiated chondrocytes from adipose derived stem cells in 3D culture. Adv Biomed Res. 2012;1:24. doi: 10.4103/2277-9175.98145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadeghi F, Esfandiari E, Hashemibeni B, Atef F, Salehi H, Shabani F. The effect of estrogen on the expression of cartilage-specific genes in the chondrogenesis process of adipose-derived stem cells. Adv Biomed Res. 2015;4:43. doi: 10.4103/2277-9175.151252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16:305–29. doi: 10.1089/ten.TEB.2009.0590. [DOI] [PubMed] [Google Scholar]
- 6.Aigner T, Stöve J. Collagens – Major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv Drug Deliv Rev. 2003;55:1569–93. doi: 10.1016/j.addr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed TA, Hincke MT. Stem Cells and Cancer Stem Cells. Vol. 10. New York: Springer; 2013. Fibrin for encapsulation of human mesenchymal stem cells for chondrogenic differentiation; pp. 59–69. [Google Scholar]
- 8.Mardani M, Kabiri A, Esfandiari E, Esmaeili A, Pourazar A, Ansar M, et al. The effect of platelet rich plasma on chondrogenic differentiation of human adipose derived stem cells in transwell culture. Iran J Basic Med Sci. 2013;16:1163–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ZC, Sun HJ, Li KH, Fu C, Liu MZ. Icariin promotes directed chondrogenic differentiation of bone marrow mesenchymal stem cells but not hypertrophy in vitro . Exp Ther Med. 2014;8:1528–34. doi: 10.3892/etm.2014.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: Induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010;16:523–33. doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vacanti JP, Langer R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(Suppl 1):SI32–4. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 12.Reddi AH. Symbiosis of biotechnology and biomaterials: Applications in tissue engineering of bone and cartilage. J Cell Biochem. 1994;56:192–5. doi: 10.1002/jcb.240560213. [DOI] [PubMed] [Google Scholar]
- 13.Zamani S, Hashemibeni B, Esfandiari E, Kabiri A, Rabbani H, Abutorabi R. Assessment of TGF-ß3 on production of aggrecan by human articular chondrocytes in pellet culture system. Adv Biomed Res. 2014;3:54. doi: 10.4103/2277-9175.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung MR, Shim IK, Chung HJ, Lee HR, Park YJ, Lee MC, et al. Local BMP-7 release from a PLGA scaffolding-matrix for the repair of osteochondral defects in rabbits. J Control Release. 2012;162:485–91. doi: 10.1016/j.jconrel.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Giovannini S, Diaz-Romero J, Aigner T, Heini P, Mainil-Varlet P, Nesic D. Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro . Eur Cell Mater. 2010;20:245–59. doi: 10.22203/ecm.v020a20. [DOI] [PubMed] [Google Scholar]
- 16.Aung A, Gupta G, Majid G, Varghese S. Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum. 2011;63:148–58. doi: 10.1002/art.30086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Kujat R, et al. Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. Int Orthop. 2013;37:945–51. doi: 10.1007/s00264-013-1800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Zhang X, Li KF, Li DX, Xiao YM, Fan YJ, et al. Icariin promotes extracellular matrix synthesis and gene expression of chondrocytes in vitro . Phytother Res. 2012;26:1385–92. doi: 10.1002/ptr.3733. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Yuan T, Zhang X, Xiao Y, Wang R, Fan Y, et al. Icariin: A potential promoting compound for cartilage tissue engineering. Osteoarthritis Cartilage. 2012;20:1647–56. doi: 10.1016/j.joca.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Stearn WT, Shaw J, Green PS, Mathew B. Genus Epimedium and other Herbaceous Berberidaceae. Portland, OR, USA: Timber Press, Inc; 2002. [Google Scholar]
- 21.Li S, Ying Y. Ben Cao Gang Mu. Beijing, China: Xue Yuan Publishing House; 1991. [Google Scholar]
- 22.Meng FH, Li YB, Xiong ZL, Jiang ZM, Li FM. Osteoblastic proliferative activity of Epimedium brevicornum Maxim. Phytomedicine. 2005;12:189–93. doi: 10.1016/j.phymed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Xie F, Wu CF, Lai WP, Yang XJ, Cheung PY, Yao XS, et al. The osteoprotective effect of herba epimedii (HEP) extract in vivo and in vitro . Evid Based Complement Alternat Med. 2005;2:353–61. doi: 10.1093/ecam/neh101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H, Lien EJ, Lien LL. Chemical and pharmacological investigations of Epimedium species: A survey. Prog Drug Res. 2003;60:1–57. doi: 10.1007/978-3-0348-8012-1_1. [DOI] [PubMed] [Google Scholar]
- 25.Ma H, He X, Yang Y, Li M, Hao D, Jia Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J Ethnopharmacol. 2011;134:519–41. doi: 10.1016/j.jep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Li Q, Mei Q, Lu T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in herba epimedii. Life Sci. 2015;126:57–68. doi: 10.1016/j.lfs.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Liu MH, Sun JS, Tsai SW, Sheu SY, Chen MH. Icariin protects murine chondrocytes from lipopolysaccharide-induced inflammatory responses and extracellular matrix degradation. Nutr Res. 2010;30:57–65. doi: 10.1016/j.nutres.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Yang SH, Wu CC, Shih TT, Chen PQ, Lin FH. Three-dimensional culture of human nucleus pulposus cells in fibrin clot: Comparisons on cellular proliferation and matrix synthesis with cells in alginate. Artif Organs. 2008;32:70–3. doi: 10.1111/j.1525-1594.2007.00458.x. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-Delta Delta CT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: A patient-matched comparison. J Orthop Res. 2005;23:1383–9. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–75. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 32.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helder MN, Knippenberg M, Klein-Nulend J, Wuisman PI. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng. 2007;13:1799–808. doi: 10.1089/ten.2006.0165. [DOI] [PubMed] [Google Scholar]
- 34.Schäffler A, Büchler C. Concise review: Adipose tissue-derived stromal cells – Basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–27. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 35.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 36.Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–7. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 37.Kim HJ, Im GI. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: Greater doses of growth factor are necessary. J Orthop Res. 2009;27:612–9. doi: 10.1002/jor.20766. [DOI] [PubMed] [Google Scholar]
- 38.Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243–62. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen KM, Ge BF, Ma HP, Liu XY, Bai MH, Wang Y. Icariin, a flavonoid from the herb Epimedium enhances the osteogenic differentiation of rat primary bone marrow stromal cells. Pharmazie. 2005;60:939–42. [PubMed] [Google Scholar]
- 40.Zheng D, Peng S, Yang SH, Shao ZW, Yang C, Feng Y, et al. The beneficial effect of icariin on bone is diminished in osteoprotegerin-deficient mice. Bone. 2012;51:85–92. doi: 10.1016/j.bone.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Sun P, Liu Y, Deng X, Yu C, Dai N, Yuan X, et al. An inhibitor of Cathepsin K, icariin suppresses cartilage and bone degradation in mice of collagen-induced arthritis. Phytomedicine. 2013;20:975–9. doi: 10.1016/j.phymed.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh TP, Sheu SY, Sun JS, Chen MH, Liu MH. Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine. 2010;17:414–23. doi: 10.1016/j.phymed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Liang W, Lin M, Li X, Li C, Gao B, Gan H, et al. Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int J Mol Med. 2012;30:889–95. doi: 10.3892/ijmm.2012.1079. [DOI] [PubMed] [Google Scholar]
- 44.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189:275–84. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 45.Tew SR, Li Y, Pothacharoen P, Tweats LM, Hawkins RE, Hardingham TE. Retroviral transduction with SOX9 enhances re-expression of the chondrocyte phenotype in passaged osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage. 2005;13:80–9. doi: 10.1016/j.joca.2004.10.011. [DOI] [PubMed] [Google Scholar]


