Keywords: nerve regeneration, neurodegeneration, Parkinson's disease, acupuncture, moxibustion, rotenone, alpha-synuclein, autophagy, phosphorylated mammalian target of rapamycin kinase, phosphorylated ribosomal protein S6 kinase, light chain 3-II, neural regeneration
Abstract
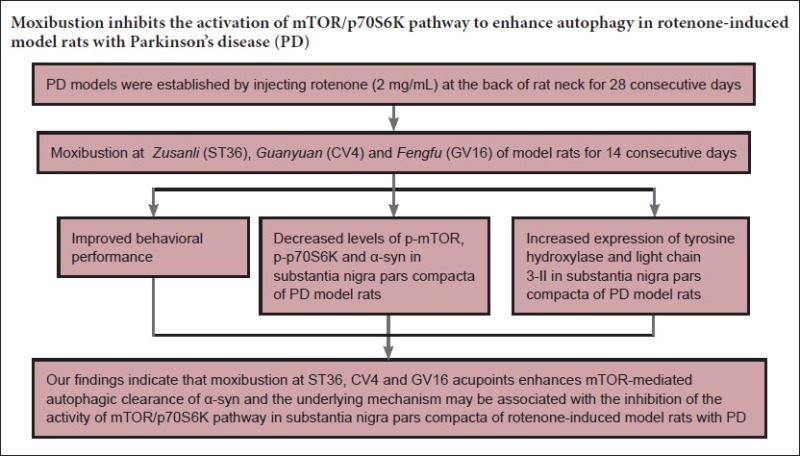
Defects in autophagy-mediated clearance of α-synuclein may be one of the key factors leading to progressive loss of dopaminergic neurons in the substantia nigra. Moxibustion therapy for Parkinson's disease has been shown to have a positive effect, but the underlying mechanism remains unknown. Based on this, we explored whether moxibustion could protect dopaminergic neurons by promoting autophagy mediated by mammalian target of rapamycin (mTOR), with subsequent elimination of α-syn. A Parkinson's disease model was induced in rats by subcutaneous injection of rotenone at the back of their necks, and they received moxibustion at Zusanli (ST36), Guanyuan (CV4) and Fengfu (GV16), for 10 minutes at every point, once per day, for 14 consecutive days. Model rats without any treatment were used as a sham control. Compared with the Parkinson's disease group, the moxibustion group showed significantly greater tyrosine hydroxylase immunoreactivity and expression of light chain 3-II protein in the substantia nigra, and their behavioral score, α-synuclein immunoreactivity, the expression of phosphorylated mTOR and phosphorylated ribosomal protein S6 kinase (p-p70S6K) in the substantia nigra were significantly lower. These results suggest that moxibustion can promote the autophagic clearance of α-syn and improve behavioral performance in Parkinson's disease model rats. The protective mechanism may be associated with suppression of the mTOR/p70S6K pathway.
Introduction
Parkinson's disease (PD) is one of the most common neurodegenerative diseases, and it is widely believed that the abnormal aggregation of alpha-synuclein (α-syn) in substantia nigra pars compacta (SNpc) is strongly associated with the pathophysiology of PD (Ross et al., 2004; Dehay et al., 2010; Bové et al., 2011). There is a positive correlation between the degree of aggregation of proteins and toxicity to neurons, and the disease symptoms tend to be relieved after the aggregates are eliminated (Neumann et al., 2006). Therefore, promoting the clearance of α-syn aggregates may be the key to preventing the degeneration and death of dopaminergic neurons in PD patients. Autophagy plays a key role in the degradation of aggregation-prone proteins, and it has been suggested that defects in autophagy-mediated clearance of α-syn contribute to the loss of nigral dopaminergic neurons (Decressac, 2013). Hence, promoting the clearance of aggregation-prone proteins through enhancing autophagy shows great promise in the treatment of PD and other neurodegenerative diseases (Wong et al., 2010).
Mammalian target of rapamycin (mTOR) kinase is a key regulator of autophagy in cells, and inhibition of mTOR activity can positively regulate autophagy (Wu et al., 2011). Excessive activation of mTOR may lead to dyskinesia in patients with PD (Guo et al., 2015). Crews et al. (2010) found that inhibiting the activation of mTOR enhanced autophagy in PD mouse models, and degraded toxic synuclein aggregates and proteins that had been denatured. Rapamycin has proven to be a potent inducer of autophagy and is used for the treatment of certain diseases in experimental models, including Alzheimer's disease, PD, Huntington's disease and spinocerebellar ataxia type 3 (Bové et al., 2011; Li et al., 2014). However, rapamycin is known to have many severe side effects. Therefore, there is a pressing need to find a safer treatment to inhibit the excessive activation of mTOR to enhance autophagy and promote the removal of aggregation-prone proteins.
Moxibustion is a treatment of traditional Chinese medicine that stimulates certain acupoints with heat arising from burning and the pharmacological action of Ay Tsao (Yi et al., 2016; Li et al., 2017). A recent clinical meta-analysis has shown that moxibustion therapy has a beneficial effect in the improvement of motor function and myotonia in PD patients (Deng, 2010). Wen et al. (2008) applied abdominal acupuncture combined with moxibustion to treat PD and found that the symptoms were improved and the progress of the disease was delayed. However, little is known about the underlying mechanism. Zhao et al. (2010) found that the effect of electroacupuncture combined with moxibustion in PD model rats was better than that of electroacupuncture alone and suggested that the mechanism may be related to the inhibition of apoptosis in the SNpc. Based on this, we explored whether moxibustion intervention could play a role in the protection of dopaminergic neurons by improving mTOR-mediated autophagy to promote the elimination of α-syn.
Materials and Methods
Animals
Sixty healthy male Sprague-Dawley rats, weighing 180–220 g, were provided by Hubei Laboratory Animal Research Center (Wuhan, Hubei Province, China; license number: SCXK (E) 2015-0018). All rats were raised in cages in a climate-controlled room with a 12 hour light-dark cycle. Environmental temperature and humidity were maintained at 25 ± 2°C and 55 ± 5%, respectively. The rats were allowed free access to regular standard rat chow and water. The study was approved by the Ethics Committee of the Hubei University of Chinese Medicine of China (approval No. 00138319).
The 60 rats were randomly allocated to four groups (n = 15 each): a normal group, a sham model group (injection of sunflower oil that did not include rotenone), a PD group (injection of rotenone dissolved in sunflower oil at the back of the neck) and a moxibustion group (stimulation with moxibustion on the acupoints Zusanli (ST36), Guanyuan (CV4) and Fengfu (GV16) after the PD rat model had been established).
Establishment of the PD model
To induce the PD model, rats received a subcutaneous injection of 2 mg/mL rotenone (Sigma, St. Louis, MO, USA) dissolved in sunflower oil (2 mg/kg body weight) at the back of the neck (Betarbet et al., 2000; Chen et al., 2008a, b, 2009; Drolet et al., 2009). The sham group received an injection of sunflower oil (1 mL/kg body weight) that did not include rotenone. The injections were repeated daily, once per day, for 28 consecutive days. In the rotenone-treated groups, rat fur became yellow and dirty; the rats showed reduced and slow movement, rigor, tremor, and an unstable gait, which were identified as PD-like symptoms (Chang et al., 2011).
Moxibustion
Rats in the moxibustion group were given improvised clothing for fixation and were treated with a mild moxibustion at the acupoints with moxa sticks in hand after model establishment. The location of acupoints was chosen according to the Experimental Guidance of Experimental Acupuncture Science (Guo et al., 2012).
The current study selected three acupoints. ST36 is a point that is posterolateral to the knees and approximately 5 mm below the fibular head. CV4 is located approximately 25 mm under the navel. GV16 is located at the depression of the dorsal atlanto-occipital joint behind the occipital tuberosity. A moxa stick that was 10 cm long and 0.7 cm in diameter (Nanyang Bo Xi Trading Co., Ltd., Henan, China) was placed 2–3 cm above each point for 10 minutes, once per day, for 7 days as a course of treatment, with two courses. The other groups wore the improvised clothing for fixation but were not given any treatment.
Behavioral observations
The day after the rats in the moxibustion group received the last moxibustion, rats in all groups were scored using evaluation criteria created by Chen et al. (2008). They carried out detailed observation over the whole course of neurotoxicity induced by chronic long-term administration of rotenone. Their behavioral scoring system measures the close relationship between behavioral changes and pathological damage of the SNpc in rats administered rotenone; an increase of the behavioral score correlates with increased injury to the SNpc, such that the behavioral score reflects the extent of dopaminergic neuron damage. At the same time, the evaluation system includes assessment of appearance and morphological changes. Thus, we selected the behavioral scoring criteria formulated by Chen et al. (2008a, b) to observe the changes in appearance and behavior of the rats in each group. The criteria were as follows: Score 1: the ability of the rats to resist restraint was weakened, the rats were hunched, with piloerection, their fur turned yellow and dirty and active mobility decreased. Score 2: apart from the above indications, active mobility was reduced more obviously and was accompanied with tremor, slow motion and gait instability. Score 4: apart from the above indications, gait instability worsened and the rats could not walk straight, rotating towards one side. Score 6: apart from the above performance, they lay obliquely towards one side or showed unilateral forelimb or hindlimb paralysis and difficultly in walking and eating. Score 8: complete unilateral limb (forelimb or hindlimb) paralysis, limb spasms, rapid weight loss and inability to eat. Score 10: agonal stage or death. Rats with evaluation scores 2–8 were included in the study. Behavioral scoring in the moxibustion group was conducted after treatment and compared with the other groups.
Specimen collection
Twelve rats in each group were included in the final analyses after rats that had died or that scored less than 2 or greater than 8 were excluded. Six rats from each group were randomly selected for immunohistochemistry and the remaining six rats were used for western blot assay.
Immunohistochemical staining
The day after the behavioral scoring was completed, rats were intraperitoneally anesthetized with 10 % chloral hydrate (0.4 mL/100 g) then fixed with paraformaldehyde via the left ventricle. The substantia nigra was dissected from the brain and placed in 4% paraformaldehyde overnight. After paraffin embedding, samples were sectioned. These sections were dewaxed, treated twice with xylene, twice with absolute alcohol, 95%, 90%, 80%, and 70% alcohol, and then immersed in distilled water for 2 minutes. Antigen retrieval was performed using a microwave. Afterwards, 3% H2O2 was added to block endogenous peroxidase. The sections were incubated with rabbit anti-tyrosine hydroxylase (TH) antibody and rabbit anti-α-syn antibody (TH, 1:100, α-syn, 1:100; Wuhan Sanying Biotechnology) overnight at 4°C. The sections were then incubated with biotinylated anti-rabbit IgG (Immunostain SP Kit, PV-9000; Beijing Zhongshan Biotechnology, Beijing, China) marked with horseradish peroxidase for 30 minutes at 37°C. The sections were incubated with 3,3′-diaminobenzidine (DAB) (Beijing Solarbio Science & Technology, Beijing, China) for 3 minutes. The sections were stained with Harris hematoxylin, generally for 30 seconds to 1 minute. Finally, the sections were dehydrated, coverslipped, and imaged using a bright-field microscope. Sections were selected at the same anatomical position from each sample and cut in series of five, and three high-power lens fields were selected for analysis. IPP 6.0 software (Media Cybernetics, Maryland, USA) was used to analyze the optical densities of immunohistochemical images. Five different visual fields of the positive expression area of each section were randomly selected. The integrated optical density of each image was measured by IPP 6.0 image analysis. The average integrated optical density of five images represented the mean integrated optical density value of a specimen.
Western blot assay
The day after the behavioral scoring was completed, rats were sacrificed. Rats were decapitated, the brain tissues were removed and the SNpc was quickly dissected on the ice. The substantia nigra was separated, wrapped in aluminum foil, frozen in liquid nitrogen, and placed in a freezer at −80°C. A small amount of tissue was cut into pieces and homogenized with 400 μL of single detergent lysate containing phenylmethyl sulfonylfluoride and then placed on ice. After a few minutes, the tissue was stroked and placed on ice again. After repeating several times, the samples were ground extensively, lysed in buffer containing phenylmethylsulfonylfluoride for 30 minutes, and then centrifuged in a 1.5 mL centrifuge tube at 12,000 r/min for 5 minutes at 4°C. The supernatant was placed in a 0.5 mL centrifuge tube and stored at −20°C. Protein concentrations were detected with a bicinchoninic acid protein assay kit (Beyotime, Wuhan, China). The extracted protein supernatant was mixed with 5 × protein sample buffer at 4:1 and boiled at 100°C for 10 minutes, then 40 μg total protein samples were electrophoresed in 10% sodium dodecyl sulfate polyacrylamide gels to separate proteins. Proteins were then transferred to polyvinylidene fluoride membranes using the wet transfer method. For phosphorylated mTOR (p-mTOR), the conditions were 200 mA, 120 minutes, 300 mA, 60 minutes; for phosphorylated ribosomal protein S6 kinase (p-p70S6k), 200 mA, 120 minutes; for light chain 3 (LC3) I/II, 200 mA, 50 minutes and for β-actin, 200 mA, 90 minutes. Membranes were first blocked for 2 hours in Tris buffered saline (TBS) with Tween 20 (TBS/Tween 20) containing 5% nonfat milk at room temperature then incubated overnight at 4°C with a primary antibody (rabbit anti-p-mTOR, 1:800, Bioworld, MN, USA; rabbit anti-p-p70S6K, 1:2,000, Wuhan Sanying Biotechnology Co., Ltd., Wuhan, China; rabbit anti-LC3-II, 1:800, CST, Boston, USA; rabbit anti-β-actin, 1:200, Wuhan Boster Bioengineering, Wuhan, China). The next day, membranes were washed with TBS/Tween 20 five to six times, 5 minutes each time, and then incubated with a horseradish peroxidase-conjugated anti-rabbit antibody (1:50,000, Wuhan Boster Bioengineering) at 37°C for 2 hours. After thorough washing in TBS/Tween 20, the membranes were incubated with an enhanced chemiluminescence reagent (Thermo, MA, USA) for several minutes. The internal control was β-actin. BandScan 4.50 software (Glyko, Canada) was used to analyze the gray level of the target bands and the ratios of p-mTOR/β-actin, p-p70S6K/β-actin and LC3-II/β-actin were obtained.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Data are presented as the mean ± SD. Measurement data between groups were compared using one-way analysis of variance followed by the Student-Newman-Keuls test. A value of P < 0.05 was considered statistically significant.
Results
Moxibustion treatment improved behavioral performance of PD model rats
In the PD group, rats presented the following symptoms at 7–10 days after rotenone injection: their fur became rough and dirty, they trembled slightly and moved slowly and their ability to resist restraint was weakened. Compared with the normal and sham model groups, rats in the PD group had obvious abnormal behaviors (P < 0.01), indicating that subcutaneous injection of rotenone caused obvious PD-like behavioral changes. Rats in the moxibustion group showed significantly better behaviors after moxibustion and had lower behavior scores compared with the PD group (P < 0.01). There was no behavioral change in the sham model group or the normal group (P > 0.05; Figure 1).
Figure 1.

Effect of moxibustion on the behavioral performance of Parkinson's disease (PD) model rats according to behavioral scoring criteria.
Higher scores indicate greater impairment. Data are presented as the mean ± SD (n = 12 per group, one-way analysis of variance followed by the Student-Newman-Keuls test; **P < 0.01, vs. the normal or sham group; ##P < 0.01, vs. the PD group). The normal group did not receive any treatment. The sham model group (sham group) was injected with sunflower oil that did not include rotenone. The PD group was injected with rotenone dissolved in sunflower oil (2 mg/mL). The moxibustion group (moxa) was stimulated with moxibustion on the acupoints Zusanli (ST36), Guanyuan (CV4) and Fengfu (GV16) after establishment of the PD model.
Moxibustion increased the expression of TH and reduced the level of α-syn in the SNpc of PD model rats
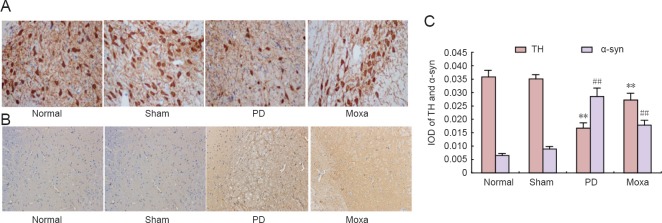
As shown in Figure 2, TH immunohistochemical staining of the SNpc was obvious. The numbers of TH-immunoreactive neurons in the SNpc of rats in the normal and sham model groups were greater than in the PD group (P < 0.01) and the cells were more intensely stained than in the PD group. After moxibustion, the number of TH-immunoreactive neurons was significantly greater compared with the PD group (P < 0.01), but remained lower than in the normal group. No significant difference was observed between the normal and sham model groups. Figure 2 shows α-syn immunohistochemical staining of SNpc. In the normal and sham model groups, no obvious α-syn-positive aggregates were observed but there were obvious α-syn-positive aggregates in the SNpc of rats in the PD group. After moxibustion, the number of α-syn-positive aggregates was significantly smaller in the moxibustion group compared with the PD group (P < 0.01).
Figure 2.
Moxibustion effects on alpha-synuclein (α-syn) and tyrosine hydroxylase (TH) levels in the substantia nigra of rotenone-induced Parkinson's disease (PD) model rats.
(A) Representative immunohistochemical staining images of TH in the substantia nigra pars compacta (SNpc) of rats (original magnification, 400 ×). TH immunohistochemical staining of SNpc was chocolate-brown. The numbers of TH-immunoreactive neurons in the SNpc of rats in the normal and sham groups were greater and they were more darkly stained than those in the PD group. After treatment, the number of TH-immunoreactive neurons was greater in the moxibustion group compared with the PD group but remained lower than that in the normal group. (B) Representative immunohistochemical staining images of α-syn in the SNpc (original magnification, 100 ×). The α-syn staining in SNpc was tan. In normal and sham groups, there were no obvious α-syn-positive aggregates in the SNpc. There were α-syn-positive aggregates in the SNpc of rats in the PD group. After treatment, the number of α-syn-positive aggregates was lower in the moxibustion group compared with the PD group. (C) The mean optical density of TH-immunoreactive neurons and α-syn-positive aggregates in the SNpc of rats in each group is shown. Data are presented as the mean ± SD (n = 6 per group; one-way analysis of variance followed by the Student-Newman-Keuls test; **P < 0.01, vs. the normal group and the sham group; ##P < 0.01, vs. the PD group). The normal group did not receive any treatment. The sham model group (sham group) was injected with sunflower oil that did not include rotenone. The PD group was injected with rotenone dissolved in sunflower oil (2 mg/mL). The moxibustion group (moxa) was stimulated with moxibustion on the acupoints Zusanli (ST36), Guanyuan (CV4) and Fengfu (GV16) after establishment of the PD model.
Moxibustion decreased the levels of p-mTOR and p-p70S6K and increased the level of LC3-II in the SNpc of PD model rats
As shown in Figure 3, levels of p-mTOR and p-p70S6K in the PD group were significantly higher than in the normal and sham model groups (P < 0.01). Moxibustion significantly reduced the levels of p-mTOR and p-p70S6K (P < 0.01) and no significant difference was observed between the normal and sham model groups. The level of LC3-II in the PD group increased compared with the normal and sham model groups (P < 0.05) and moxibustion treatment significantly enhanced LC3-II protein expression compared with the PD group (P < 0.01). No significant difference was observed between the normal and sham model groups.
Figure 3.
Moxibustion effects on p-mTOR, p-p70S6K and LC3-II protein expression in the substantia nigra of rotenone-induced Parkinson's disease (PD) rats.
(A) Representative western blot bands of p-mTOR, p-p70S6K and LC3-II protein expression in the SNpc. The LC3 protein has two bands: the upper band is LC3-I and the lower band is LC3-II. (B) Quantitation of p-mTOR, p-p70S6K and LC3-II protein expression in the SNpc of rats in each group. The vertical axis represents the ratio of protein to β-actin gray levels. Data are presented as the mean ± SD (n = 6 per group; one-way analysis of variance followed by the Student-Newman-Keuls test; *P < 0.05, **P < 0.01, vs. the normal and sham groups; ##P < 0.01, vs. the PD group). The normal group did not receive any treatment. The sham model group (sham group) was injected with sunflower oil that did not include rotenone. The PD group was injected with rotenone dissolved in sunflower oil (2 mg/mL). The moxibustion group (moxa) was stimulated with moxibustion on the acupoints Zusanli (ST36), Guanyuan (CV4) and Fengfu (GV16) after establishment of the PD model.
Discussion
In this study, we induced a model of PD by subcutaneous injection of rotenone over a long period of time. Rotenone has been widely used as an insecticide and epidemiological evidence suggests that long-term exposure to pesticides may increase the risk of PD (Deng et al., 2006). Rotenone was used to establish the PD rat model because this model causes selective degeneration and death of dopaminergic neurons in the SNpc, compared with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and other models. Further, the behavioral and pathological characteristics of PD are also better simulated (Betarbet et al., 2000; Sherer et al., 2003; Alam et al., 2004; Saravanan et al., 2005).
Previous studies demonstrated positive effects of moxibustion on PD, especially in improving the symptoms and reducing the side effects of drugs in PD patients (Nian, 2007; Niu, 2007; Zhang 2010, 2012, 2016). However, the underlying molecular mechanism remains poorly understood. In this study, a link between moxibustion and autophagy was established. The results showed that moxibustion improved the behavioral performance of the PD model rats, decreased the levels of p-mTOR, p-p70S6K and α-syn, and increased the levels of TH and LC3-II in the SNpc of PD model rats.
At present, strong evidence exists suggesting neuronal toxicity caused by the increased expression and aggregation of α-syn in vivo, and the function of neurons may be disturbed through multiple pathways and targets (Uversky, 2008; Roberts et al., 2015). Autophagy is essential for removing toxic α-syn-aggregates and plays a central role in maintaining cellular homeostasis (Ebrahimifakhari et al., 2011), while mTOR is a sensor for cell nutritional status, stress and growth factor signaling that plays an important role in autophagy (Chang et al., 2010). When mTOR is activated, it stimulates the activation of downstream p70S6K and 4E binding protein 1 (4E-BP1) to form p-p70S6K and phosphorylated 4E-BP1, which then promote protein synthesis, proliferation and growth, accelerate cell metabolism, and inhibit cell autophagy (Ding et al., 2015). Related studies showed that the mTOR inhibitor rapamycin could reduce the toxicity of 6-hydroxydopamine in PC12 cells and increase the survival of dopaminergic neurons in the SNpc of PD mice induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (Hsuan et al., 2006; Malagelada et al., 2010). In this study, the levels of p-mTOR, p-p70S6K and α-syn were increased in the SNpc of PD model rats compared with normal rats, demonstrating that the mTOR/p70S6K pathway was excessively activated and autophagy was suppressed after injection of rotenone. When the upstream signal of the autophagy pathway, which is dependent on mTOR, is activated, activity of mTOR is inhibited and it loses its inhibitory effect on the serine/threonine kinase ULK1 complex, which is conducive to the formation of autophagic membranes (Li et al., 2015). Subsequently, with the Atg5/Atg12 complex, the autophagic membrane formed at the previous stage combines with LC3-II, which is the cleavage product of LC3-I/Atg8, to complete the extension of the autophagic membrane under the action of Atg9 and ULK, finally forming the autophagosome (Ghavami et al., 2012). LC3 and its family of proteins play an important role in the extension and closure of the isolation membrane, and these proteins, which bind to the surface of autophagic membranes, are important markers of autophagosomes in the cell (Schmeisser et al., 2014). At present, it is the only reliable marker protein related to complete autophagy; it exists in the inner and outer membranes at various stages of the formation of the autophagosome. The LC3-II/β-actin ratio can reflect changes in autophagy (Martinez-Vicente et al., 2010). TH is a rate-limiting enzyme in the process of dopamine synthesis. It is strongly associated with the dopaminergic neurons in the nigrostriatal system, so TH levels can reflect the activity of dopaminergic neurons (Zhang et al., 2016). After moxibustion, the levels of LC3-II and TH increased significantly and the levels of p-mTOR, p-p70S6K and α-syn decreased compared with the PD group, suggesting that the activity of autophagy was enhanced and promoted the clearance of α-syn, thereby exerting a neuroprotective effect.
PD is also known as a type of paralysis. Based on the clinical manifestations and the population at high risk of PD, traditional Chinese medicine believes that PD belongs to the “tremor syndrome, spasm syndrome” category. The lesion site is in the brain; kidney and spleen deficiencies, the blockage of phlegm congestion and blood stasis of brain collaterals are standard pathological properties (Xie et al., 2011; Wang et al., 2012, 2015; Chen et al., 2016). Based on the above-mentioned pathogenesis, this study selected acupoints ST36, CV4 and GV16. ST36 is the “He-sea” point and the “Lower He-sea” point of the Stomach Channel of Foot-Yangmin; it can regulate the spleen and stomach, nurse the root of after-birth and is the source of qi and blood. CV4 is the Conception Vessel point and the meeting point of the Spleen Channel of Foot-Taiyin, the Kidney Channel of Foot-Shaoyin, the Liver Channel of Foot-Jueyin and Conception Vessel; it can nourish the qi essence to help the congenital foundation. A key point was added to treat endogenous wind—GV16, which is the Governing Vessel point, and the three points work together to tone the kidney and spleen, regulate the collaterals of the brain, extinguish wind and stop tremors. Previous studies found that electroacupuncture at the four acupoints GV16, CV4, ST36, and Taichong (LR3) after PD model establishment increased the number of dopaminergic neurons, inhibited the number of activated microglia in substantia nigra and the levels of TNF-α and IL-1β in PD rats, which reduced the toxicity to neurons, regulated immune function, improved Bcl-2 mRNA expression and decrease the rate of apoptosis in the SNpc of PD rats (Wang et al., 2005; Gan, 2007).
In summary, moxibustion at ST36, CV4 and GV16 promoted the clearance of α-syn in the SNpc and improved the behavioral performance in PD model rats. The protective mechanism may be associated with suppression of the mTOR/p70S6K pathway, thereby enhancing the autophagic clearance of α-syn. However, the activity of mTOR depends on the input of various upstream signals, and it is not clear how moxibustion affects the upstream molecular mechanisms to regulate mTOR activity. Our results provide an experimental basis for the treatment of PD through clinical application of moxibustion.
Acknowledgments
We sincerely thank staffs from Acupuncture-Moxibustion Institute of Hubei University of Chinese Medicine of China for offering experimental areas and instruments.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81403456, 81473788; a grant from the Hubei Provincial Collaborative Innovation Center of Preventive Treatment by Acupuncture and Moxibustion, No. HBPCIC-2016-003.
Conflicts of interest: The authors declare no competing financial interests.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81403456, 81473788; a grant from the Hubei Provincial Collaborative Innovation Center of Preventive Treatment by Acupuncture and Moxibustion, No. HBPCIC-2016-003. None of the funding bodies play any role in the study other than to provide funding.
Research ethics: All procedures were approved by the ethics committee of Hubei University of Chinese Medicine of China (approval No. 00138319). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate..
Peer review: Externally peer reviewed.
(Copyedited by Turnley A, Haase R, Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Alam M, Mayerhofer A, Schmidt WJ. The neurobehavioral changes induced by bilateral rotenone lesion in medial forebrain bundle of rats are reversed by L-DOPA. Behav Brain Res. 2004;151:117–124. doi: 10.1016/j.bbr.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 3.Bové J, Martínez-vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12:437–452. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- 4.Chang HJ, Ro SH, Jing C, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang YT, Luo XG, Ren Y. Behavior alteration and damage of dopaminergic neurons of substantia nigra caused by rotenone in rats. Jiepouxue Yanjiu Jingzhan. 2011;7:60–62. [Google Scholar]
- 6.Chen X, Zhang N, Li C. Changes of mitochondrial respiratory chain complex i and complex iv activity in rotenone-induced rat model of Parkinson's disease. Zhongguo Shiyan Dongwu Xuebao. 2009;17:135–137. [Google Scholar]
- 7.Chen X, Zhang N, Zhao H, Zou HY, Mu Y, Xue B, Song YW, Wang XC, Jiang HM. The protect effect of Baicalin on the substantial nigra dopaminergic neuron in Parkinson's rats induced by rotenone. Zhongfeng yu Shenjing Jibing Zazhi. 2008a;25:174–177. [Google Scholar]
- 8.Chen X, Zhang N, Zhao H, Mu Y. The relationship between the behavior and the pathological damage of substantia nigra in rotenone induced Parkinson's disease. Zhongguo Jingshen yu Shenjing Jibing Zazhi. 2008b;34:232–234. [Google Scholar]
- 9.Chen XD, Zhang LP. Advances in treatment of Parkinson's disease with traditional Chinese Medicine. Changchun Zhongyiyao Daxue Xuebao. 2016;32:213–215. [Google Scholar]
- 10.Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, Hansen L, Adame A, Galasko D, Masliah E. Selective molecular alterations in the autophagy pathway in patients with lewy body disease and in models of α-synucleinopathy. PLoS One. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Björklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synucleintoxicity. Proc Natl Acad Sci U S A. 2013;110:E1817–1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehay B, Bové J, Rodríguez-muela N, Perier C, Recasens A, Boya P, Vila M. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng B, Wang YY, Wang MW. Rotenone and Parkinson's disease animal models. Nao yu Shenjing Jibing Zazhi. 2006;14:152–154. [Google Scholar]
- 14.Deng XB. Observations of the clinical curative effect of moxibustion treatment for Parkinson's disease myotonia. Guangzhou: Guangzhou University of Chinese Medicine; 2010. [Google Scholar]
- 15.Ding YH, Li YF. The relationship between mTOR signaling pathway and autophagy and apoptosis. Xiandai Yixue. 2015;43:801–804. [Google Scholar]
- 16.Drolet RE, Cannon JR, Montero L, Greenamyre JT. Chronic rotenone exposure reproduces Parkinson's disease gastrointestinal neuropathology. Neurobiol Dis. 2009;36:96–102. doi: 10.1016/j.nbd.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Ebrahimifakhari D, Cantuticastelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of α-synuclein. J Neurosci. 2011;31:14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gan . Study on the regulation of immune function of the Parkinsonian rats by the acupuncture therapy of “Shuanggu Yitong”. Wuhan: Hubei University of Chinese Medicine; 2007. [Google Scholar]
- 19.Ghavami S, Cunnington RH, Yeganeh B, Davies JJ, Rattan SG, Bathe K, Kavosh M, Los MJ, Freed DH, Klonisch T, Pierce GN, Halayko AJ, Dixon IM. Autophagy regulates trans fatty acid-mediated apoptosis in primary cardiac myofibroblasts. Biochim Biophys Acta. 2012;1823:2274–2286. doi: 10.1016/j.bbamcr.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Fang JQ. Experimental guidance of experimental acupuncture science. Beijing: China Press of Traditional Chinese Medicine; 2012. [Google Scholar]
- 21.Hsuan SL, Klintworth H, Xia Z. Basic fibroblast growth factor protects against rotenone-induced dopaminergic cell death through activation of extracellular signal-regulated kinases 1/2 and phos-phatidylinositol-3 kinase pathways. J Neurosci. 2006;26:4481–4491. doi: 10.1523/JNEUROSCI.4922-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Wang XY, Wang JJ, Zhang MM, Yu XJ, Li CY. Study on the abnormal function of autophagy in neurodegenerative diseases. Nao yu Shenjing Jibing Zazhi. 2015;23:140–143. [Google Scholar]
- 24.Li ZY, Yang YT, Hong J, Zhang D, Huang XF, Wu LJ, Wu HG, Shi Z, Liu J, Zhu Y, Ma XP. Extracellular signal-regulated kinase, substance P and neurokinin-1 are involved in the analgesic mechanism of herb-partitioned moxibustion. Neural Regen Res. 2017;12:1472–1478. doi: 10.4103/1673-5374.215259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malagelada C, Jin ZH, Jackson-lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease. J Neurosci. 2010;30:1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, Vries Rd, Arias E, Harris S, Sulzer D, Cuervo AM. Cargo recognition failure is responsible fatty acid-mediated apoptosis in primary cardiac myofibroblasts. Biochim Biophys Acta. 2010;1823:2274–2286. [Google Scholar]
- 27.Neumann M, Sampathu DM, Kwong LK, Truax AC Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 28.Roberts HL, Brown DR. Seeking a mechanism for the toxicity of oligomeric α-synuclein. Biomolecules. 2015;5:282–305. doi: 10.3390/biom5020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10:S10-17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 30.Saravanan KS, Sindhu KM, Mohanakumar KP. Acute intranigral infusion of rotenone in rats causes progressive biochemical lesions in the striatum similar to Parkinson's disease. Brain Res. 2005;1049:147–155. doi: 10.1016/j.brainres.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 31.Schmeisser H, Bekisz J, Zoon KC. New function of type IIFN: introduction of autophagy. Interferon Cytokine Res. 2014;34:71–78. doi: 10.1089/jir.2013.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- 33.Uversky VN. Alpha-synuclein misfolding and neurodegenerative diseases. Curr Protein Pept Sci. 2008;9:507–540. doi: 10.2174/138920308785915218. [DOI] [PubMed] [Google Scholar]
- 34.Wang HX. Chinese medicine treatment of Parkinson's disease. Jilin Zhongyiyao. 2015;35:791–793. [Google Scholar]
- 35.Wang WW, Li RK. The experience of diagnosis and treatment of Parkinson disease by Li RuKui. Jiangsu Zhongyiyao. 2012;44:9–10. [Google Scholar]
- 36.Wang YC, Ma J, Wang H. Experimental study of the protective effect on dopaminergic neurons in substance nigra of the Parkinsonian rats by the acupuncture therapy of “Shuanggu Yitong”. Hubei Zhongyiyao Daxue Xuebao. 2005;7:25–26. [Google Scholar]
- 37.Wen X, Li YW, Duan Q. Abnormal acupincture plus moxibustion for treatment of Parkinson disease rigidity in 30 patients. Guangzhou Yixueyuan Xuebao. 2008;36:59–61. [Google Scholar]
- 38.Wong E, Cuervo AM. Autophagy gone awry in neurodegerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu MM, Yuan YH, Chen NH. Research progress of mTOR signaling pathway and neurodegenerative diseases. Zhongguo Yaolixue Tongbao. 2011;27:1481–1483. [Google Scholar]
- 40.Xie K, Wang HB, Ye CL. Etiology and pathogenesis and treatment progress of Parkinson's disease. Jiangxi Zhongyi Xueyuan Xuebao. 2011;23:93–95. [Google Scholar]
- 41.Yi T, Qi L, Li J, Le JJ, Shao L, Du X, Dong JC. Moxibustion upregulates hippocampal progranulin expression. Neural Regen Res. 2016;11:610–616. doi: 10.4103/1673-5374.180746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang ZM, Bai J. Research progress of tyrosine hydroxylase and Parkinson's disease. Zhongguo Laonianxue Zazhi. 2016;36:2280–2282. [Google Scholar]
- 43.Zhao YH, Sun ZR, Huang L. Effect of electroacupuncture combined with moxibustion on behavior and apoptosis of rats with trembling palsy. Shijie Zhongyiyao. 2010;5:269–270. [Google Scholar]