Abstract
Systematic inflammatory response after spinal cord injury (SCI) is one of the factors leading to lesion development and a profound degree of functional loss. Anti-inflammatory compounds, such as curcumin and epigallocatechin gallate (EGCG) are known for their neuroprotective effects. In this study, we investigated the effect of combined therapy of curcumin and EGCG in a rat model of acute SCI induced by balloon compression. Immediately after SCI, rats received curcumin, EGCG, curcumin + EGCG or saline [daily intraperitoneal doses (curcumin, 6 mg/kg; EGCG 17 mg/kg)] and weekly intramuscular doses (curcumin, 60 mg/kg; EGCG 17 mg/kg)] for 28 days. Rats were evaluated using behavioral tests (the Basso, Beattie, and Bresnahan (BBB) open-field locomotor test, flat beam test). Spinal cord tissue was analyzed using histological methods (Luxol Blue-cresyl violet staining) and immunohistochemistry (anti-glial fibrillary acidic protein, anti-growth associated protein 43). Cytokine levels (interleukin-1β, interleukin-4, interleukin-2, interleukin-6, macrophage inflammatory protein 1-alpha, and RANTES) were measured using Luminex assay. Quantitative polymerase chain reaction was performed to determine the relative expression of genes (Sort1, Fgf2, Irf5, Mrc1, Olig2, Casp3, Gap43, Gfap, Vegf, NfκB, Cntf) related to regenerative processes in injured spinal cord. We found that all treatments displayed significant behavioral recovery, with no obvious synergistic effect after combined therapy of curcumin and ECGC. Curcumin and EGCG alone or in combination increased axonal sprouting, decreased glial scar formation, and altered the levels of macrophage inflammatory protein 1-alpha, interleukin-1β, interleukin-4 and interleukin-6 cytokines. These results imply that although the expected synergistic response of this combined therapy was less obvious, aspects of tissue regeneration and immune responses in severe SCI were evident.
Keywords: spinal cord injury, epigallocatechin gallate, curcumin, inflammatory response, neural regeneration
Introduction
Spinal cord injury (SCI) is one of the most common causes of death and paralysis worldwide. The primary insult results in neuronal cell death, which is followed by a cascade of events. These include the release of excitotoxic glutamate, activation of microglia, death of oligodendrocytes and glial scar formation (Dubendorf, 1999; Witiw and Fehlings, 2015). One of the factors leading to worsening of the injury is the immune response after the initial insult, resulting in a release of pro-inflammatory cytokines, which trigger the secondary processes. Influencing the early immune response after SCI may decelerate the cavity formation processes, thus resulting in the preservation of spinal tracts and decreased locomotor deficits after SCI (Gal et al., 2009; Mietto et al., 2015).
Curcumin (1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6- heptadiene-3,5-dione) is a well-known anti-inflammatory agent that is isolated from curcuma longa. It is also known for its neuroprotective features and has been studied as an effective therapeutic agent in experimental models of SCI (Ormond et al., 2012; Sanli et al., 2012). Curcumin application after SCI or TBI results in a suppressed inflammatory response via the TLR4-MyD88-NFκB dependent pathway, which in turn attenuates the microglial activation and neuronal apoptosis after injury (Zhu et al., 2014; Ni et al., 2015). The nuclear factor kappaB (NF-κB) pathway shows a strong correlation with tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) levels after injury (Yuan et al., 2015). Additionally, the impact of curcumin on the Stat-3 and NF-κB pathway strongly influenced nitric oxide (NO) levels and the reduction of astrogliosis after SCI (Wang et al., 2014; Machova Urdzikova et al., 2015; Gokce et al., 2016; Sanivarapu et al., 2016). Following curcumin application, increased behavioral recovery after SCI was observed (Ormond et al., 2012; Zu et al., 2014; Machova Urdzikova et al., 2015). Moreover, curcumin displayed a role in decreased neuropathic pain via antagonizing TRPV1 channels (Lee et al., 2013). Epigallocatechin gallate (EGCG) is an active compound from green tea which is also known for its anti-inflammatory potential and neuroprotective features (Khalatbary and Ahmadvand, 2011). In a model of Alzheimer's disease, EGCG can elevate alpha secretase activity and enhance hydrolysis of the TNF-α-converting enzyme, and thus promote the cleavage of α-c terminal fragment of APP (Rezai-Zadech et al 2005). The application of EGCG after SCI decreased the level of TNF-α and IL-1, major players in inflammatory response after SCI, and consecutively also decreased levels of inducible nitric oxide synthase, cyclooxygenase-2, and myelin peroxidase. The application of EGCG showed an anti-apoptotic effect by decreasing the levels of Bax gene expression and immunohistochemical positivity on tunnel staining, and increasing expression levels of Bcl2, Bdnf and Gdnf genes (Khalatbary et al., 2010; Tian et al., 2013). We reported that EGCG decreases the nuclear translocation of subunit p65 (RelA) of the NF-κB dimer, and therefore attenuates the canonical NF-κB pathway (Urdzikova et al., 2017). In several studies, EGCG administration after neural damage led to a reduction of neuropathic pain (Kuang et al., 2012; Xifro et al., 2015). Furthermore, several studies have shown the effect of EGCG on edema, tissue protection, and functional recovery after SCI (Khalatbary et al., 2010; Ge et al., 2013). Despite that, all the mechanisms of action of these compounds are unknown. Curcumin (Aydin et al., 2014; Garcia-Nino et al., 2015; Liu et al., 2017) and EGCG (Dudka et al., 2005; Meng et al., 2007) are used for their anti-oxidative, chelating and immunomodulating properties in various diseases. In these therapies, TNF-α and IL-1 level reduction is one of the key factors in their pro-regenerative features after SCI (Khalatbary and Ahmadvand, 2011; Yuan et al., 2015) and may result in a synergistic effect of curcumin and EGCG. So far, a combination of both drugs is used as a complementary therapy in experimental cancer therapy (Yunos et al., 2011; Eom et al., 2015).
In this study, the effect of the combined therapy of curcumin with EGCG in a balloon compression model of acute SCI in rats was evaluated. White and grey matter tissue sparing, axonal sprouting, astrogliosis, the expression of genes related to regenerative processes, immunomodulation, and behavioral recovery after SCI were assessed.
Materials and Methods
Animals
Ten-week-old male Wistar rats (n = 131; AnLab, Prague, Czech Republic), weighing 300 ±15 g, were used in this study. The animals were housed in pairs in individually ventilated cage systems (Techniplast), with food and water ad libidum. Animals were separated prior to the injury into the two major groups: a behavioral group and a cytokine group. Immediately after SCI, all animals from the behavioral group were randomly divided into four subgroups for behavioral examinations: saline (n = 10), curcumin (n = 13), EGCG (n= 19) and curcumin + EGCG (n = 9). These animals were also used for histological, immunohistochemical (n = 5/group) and qPCR analysis after behavioral examinations (n = 4/group). Animals in the cytokine group consisted of 20 rats per subgroup (saline, curcumin, EGCG, and curcumin + EGCG), which were sacrificed at different time points (n = 5/group per time point: 1, 3, 7 and 10 days) after injury and were evaluated for the levels of selected cytokines. All experiments were performed in accordance with the European Communities Council Directive of 22 September 2010 (2010/63/EU) regarding the use of animals in research, and were approved by the Ethics Committee of the Institute of Experimental Medicine, Academy of Sciences of the Czech Republic (approval number: 277/2011 and 53/2014).
SCI induction and treatment
A lesion was created via a balloon-induced spinal cord compression as previously detailed (Vanicky et al., 2001; Urdzikova et al., 2006). This model was chosen for its relevance to closed injuries, with vertebrate body and arch preserved, leading to a higher validity of local immune response after SCI. Briefly, approximately one centimeter of a 2-French Fogarty catheter (Edwards, Irvine, CA, USA) was inserted into the epidural space through a laminectomy at T10, aligned with the midline of the spinal cord and inflated for 5 minutes. The volume of the inflated balloon was adjusted to 15 μL and the function was carefully checked in every animal. Immediately after SCI, rats received daily (for 28 days after SCI) intraperitoneal doses of curcumin (Sigma-Aldrich, St. Louis, MO, USA; 6 mg/kg, diluted in olive oil), EGCG (Sigma-Aldrich, 17 mg/kg, diluted in PBS), or their combination, and weekly intramuscular injection (1, 7, 14, 21 and 28 days post-SCI, surrounding lesion area) of curcumin (60 mg/kg in olive oil (Ormond et al. 2014), Sigma), EGCG (17 mg/kg, diluted in PBS (Tian et al., 2013; Renno et al., 2015), or a combination of both drugs.
Behavioral test
The Basso, Beattie, and Bresnahan (BBB) open-field locomotor test
Basic locomotor function was evaluated using the BBB open-field locomotor test (Basso et al., 1995). Rats were placed in an open field area for approximately four minutes every week starting seven days after SCI. Animals were scored by two independent examiners according to the BBB scale (0–21). The scale 0–4 points reflect no or only small movement of hindlimbs with no weight support; scale 5–8 points indicate larger extent of movement with no weight support; scale 9–14 points suggest increasing frequency of weight-supported steps and hindlimb coordination; scale 15–18 points reflect precise movements such as specific paw placement rotation; scale 18–20 points indicate tail balance and trunk stability; and scale 21 points indicate healthy animals. Hindlimbs scored separately, and then an average value per animal was calculated.
Flat beam test
To evaluate advanced locomotor skills of injured animals, such as weight support and hindlimb coordination, the flat beam test was used (Goldstein, 1997). The latency (in seconds) and trajectory of animals crossing the beam was recorded and evaluated. Additionally, the rats were scored according to the modified version of the Goldstein scale (0–7) (Goldstein, 1997). The flat beam test measures the ability to balance on the beam (scale points 0–2), attempts of crossing the beam (scale points 2–3) and the frequency of using hindlimbs for the task, technique of hindlimb coordination and the gait (scale points 4–7). Time score reflects the time that each rat needs to start performing the task (maximum is 60 seconds). After a pre-training session, animals were assessed twice a day for 3 consecutive days, and weekly from the third week after injury.
Plantar test
To evaluate the presence of hyperalgesia to thermal nociceptive stimulus, an Ugo Basile test apparatus (Ugo Basile, Comercio, Italy) was used as previously described (Carstens and Ansley, 1993). A radiant thermal stimulus was applied to the plantar surface of the paws, and the latency (seconds) of the paw withdrawal response was measured.
Tissue processing, histology and immunohistochemistry
To analyze the effect of curcumin, EGCG, or the combined therapy on injured spinal cords, fifteen cross-sections (5 μm thickness) per animal were selected at 1-mm intervals along the cranio-caudal axis, including the lesion center. Cresyl violet-Luxol fast blue staining was used to distinguish the white and grey matter (WM/GM) of the injured spinal cord. The total volume and distribution of spared white matter/grey matter and cavity size were analyzed using ImageJ software (NIH, Bethesda, MD, USA) (Additional Figure 1A (91.5KB, pdf) , data are expressed as a percentage to the saline group (100%)). For immunohistochemical analysis of axonal sprouting, the antibody against Gap43 (Millipore, Billerica, MA; 1:2000, overnight 4°C) was used. To visualize primary antibody reactivity, goat anti-mouse IgG conjugated with Alexa-Fluor 488 (Molecular Probes, Eugene, OR, USA; 1:200, 2 hours 4°C) was applied. The number of Gap43-positive fibers per slide was counted using Tissue FAXS software (Tissue Gnostics, Vienna, Austria) across the whole area of the section (Additional Figure 1B (91.5KB, pdf) ). The results are displayed as the average number of GAP43-positive fibers per slide in percentage, when compared to the saline group (set to 100%). To determine the effect of treatment on glial scar formation, antibody against glial fibrillary acidic protein (GFAP) (mouse anti-GFAP-Cy3; Sigma, St. Louis, MO, USA; 1:300, overnight 4°C) was used. The thickness of GFAP+ glial scar (the square area of GFAP-positive signal of high density, directly surrounding the main lesion cavity; data are expressed as a percentage per total section area) and the number of protoplasmic astrocytes were analyzed using ImageJ software (Additional Figure 1C (91.5KB, pdf) ). Histological and immunohistochemical images were analyzed and quantified using Aioskop 2 plus microscope (Zeiss, Oberkochen, Germany), LEICACTR6500 microscope (Leica Microsystems, Milton Keynes, UK) and TissueFAXS and ImageJ software.
Histological and immunohistochemical staining of injured spinal cords.
(A) Examples of analyzed images of cresyl violet luxol staining for white and grey matter sparing. (B, C) Gap 43 staining (green) for axonal sprouting (B) and GFAP-Cy3 staining (red)showing glial scar (C). Scale bars: 100 μm.
Quantitative reverse transcription-PCR (qRT-PCR)
To study the relative expression of rat genes related to regenerative processes after SCI, qRT-PCR was applied (Heid et al., 1996).
RNA was isolated from parafomadehyde-fixed spinal cord tissue sections using High Pure RNA Paraffin Kit (Roche, Penzberg, Germany) (Bibikova et al., 2004; Streubel et al., 2005). To quantify the amount of RNA, a spectrophotometer (NanoPhotometer™ P-Class, Munchen, Germany) was used. The isolated RNA was then reverse transcribed into cDNA, using Transcriptor Universal cDNA Master (Roche, Penzberg, Germany), and a thermal cycler [T100™ Thermal Cycler (Bio-Rad, Hercules, CA, USA)]. cDNA solution, FastStart Universal Probe Master (Roche, Penzberg, Germany) and TaqMan® Gene Expression Assays genes (Gapdh/Rn01775763_g1 (housekeeping gene), Sort1 (Nt3) /Rn01521847_m1, Olig2/Rn01767116_m1, Fgf2/Rn00570809_m1, Gfap/Rn00566603_m1, Gap43/Rn01474579_m1, Vegf/Rn01511601_m1, Casp3/Rn00563902_m1, Cntf/Rn00755092_m1,Irf5/Rn01500522_m1, Mrc1/Rn01487342_m1, and Life Technologies, Carlsbad, CA, USA) were used to perform PCR.
The final volume of 10 μL, containing 25 ng of extracted RNA was amplified by a real-time PCR cycler (StepOnePlus™, Life Technologies, Carlsbad, CA, USA). All amplifications were run under the same cycling conditions (2 minutes at 50°C, 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C). A negative control was included in each array. Tested samples were run in duplicates, and to determine the relative quantification of gene expression, the ΔΔCt method was applied (Pfaffl, 2001). Data were analyzed by StepOnePlus® software (Life Technologies, Carlsbad, CA, USA). The gene expression levels were normalized using Gapdh as a reference gene, and all data are related to the values of SCI rats treated with vehicle. A log2 scale was used to display the symmetric magnitude for up and down regulated genes.
Luminex assay
In order to evaluate the effect of anti-inflammatory drugs (curcumin, EGCG and combined therapy) on levels of cytokines after SCI, Luminex assay was applied (Urdzikova et al., 2014). On the 1st, 3rd, 7th and 10th days after SCI, the samples of injured spinal cord were taken, and incubated in cell culture media (24 hours, DMEM (Sigma), supplemented with 10% fetal bovine serum and 0.2% primocin). The levels of cytokines (six-plexbead assay for interleukin-1β (IL-1β), interleukin-4 (IL-4), interleukin-2 (IL-2), interleukin-6 (IL-6), macrophage inflammatory protein 1-alpha (MIP-1α), and regulated on activation, normal T cell expressed and secreted (RANTES); Millipore, Billerica, MA, USA) in the media were detected using a customized Milliplex inflammatory cytokine kit and Magpix instrumentation software. The raw data, consisting of mean fluorescence intensity, using a five-parameter logistic fit curve (standard curve) were recalculated to the concentration of each cytokine (ng/μL). Results are displayed as a percentage change from non-lesioned tissue (100%) for each time point.
Statistical analysis
Statistical evaluation was performed by SigmaStat software (Sistat Software Inc., San Jose, CA, USA). To evaluate the differences between treatment groups in behavioral study, white/grey matter sparing, and glial scar formation, two-way repeated measures analysis of variance (ANOVA) was used. For the evaluation of qPCR and luminex results, two-way ANOVA was applied. Axonal sprouting was evaluated by one-way ANOVA. As a pair-to-pair post-hoc test, the Student-Newman-Keuls (SNK) test was chosen. Data in graphs are represented as the mean ± SEM. Differences were considered statistically significant when P < 0.05. F values (ANOVA) or q values (SNK test) are presented when P < 0.05.
Results
Behavioral recovery
BBB open-field locomotor test
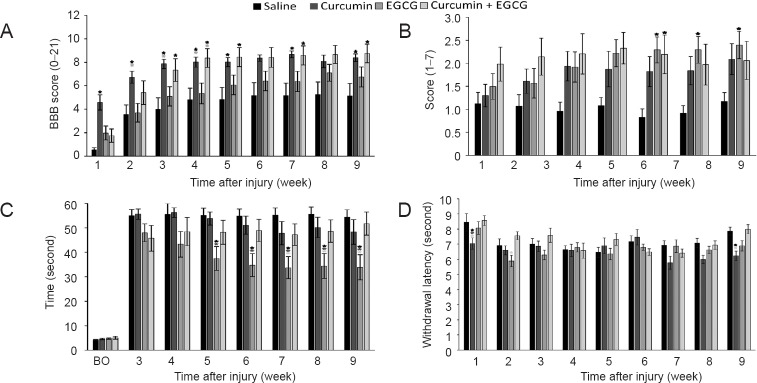
Behavioral recovery after SCI was assessed by the BBB open-field locomotor test. Results showed that behavioral recovery of rats treated with saline only reached a plateau four weeks after injury, with an average BBB score less than 6. Application of EGCG showed only mild improvement in locomotor recovery observed in the BBB open-field locomotor test. However, both curcumin and curcumin + EGCG treated animals showed significantly higher recovery in comparison with saline treated animals (Figure 1A) (two-way repeated measures ANOVA F = 4.039, P < 0.05; SNK test: curcumin vs. saline, q = 4.252, P < 0.05; curcumin + EGCG vs. saline, q = 3.509, P < 0.05). Furthermore, the combined therapy did not achieve a higher score than with application of curcumin only (SNK test: curcumin vs. saline: 1 week, q = 4.74, P < 0.01; 2 weeks, q = 3.744, P < 0.05; 3 weeks, q= 4.549, P < 0.05; 4 weeks, q = 3.789, P < 0.05; 5 weeks, q = 3.760, P < 0.05; 7 weeks, q = 4.147, P < 0.05; 9 weeks, q = 3.836, P < 0.05; curcumin vs. EGCG: 2 weeks, q = 4.172, P < 0.05; 3 weeks, q = 3.814, P < 0.05; 4 weeks, q = 3.682, P < 0.05; EGCG + curcumin vs. saline: 3 weeks, q = 3.592, P < 0.05; 4 weeks, q = 3.837, P < 0.05; 5 weeks, q = 3.900, P < 0.05; 7 weeks, q = 3.699, P < 0.05; 9 weeks, q = 3.906, P < 0.05; EGCG + curcumin vs. EGCG: 4 weeks, q = 3.694, P < 0.05).
Figure 1.
Locomotor and sensory recovery in rats following curcumin and epigallocatechin gallate (EGCG) application after spinal cord injury.
(A–C) The effect of curcumin, EGCG, and their combination on locomotor recovery after spinal cord injury was evaluated using the Basso, Beattie, and Bresnahan (BBB) open-field locomotor test (A), flat beam test (B), and flat beam time score (C). Animals treated with combined therapy of curcumin and EGCG, or curcumin alone, performed significantly better in the BBB open-field locomotor test than animals treated with saline. In the flat beam test, EGCG treated animals showed the best recovery, followed by curcumin + EGCG treated animals. (D) The thermal nociception was evaluated using the plantar test. No additional hyperalgesia was found after application of any of the used drugs. Two-way repeated measures analysis of variance with the Student-Newman-Keuls post hoc test was used to determine statistical significance. *P < 0.05 (The color of asterisks indicates the statistically different group(s)).
Flat beam test
Advanced locomotor skills after SCI were evaluated by the flat beam test. In this more physically demanding test, saline treated animals reached an average value of 1, which represents the ability to balance on the beam for at least 30 seconds consecutively without being able to cross it. All treated groups reached values above two, which represents the ability to cross at least half of the measured distance. Animals treated with EGCG showed a significantly higher locomotor score than saline treated animals (SNK test: EGCG vs. saline, q = 3.594, P < 0.05). In the beginning, the combined curcumin and EGCG group showed higher scores than curcumin or EGCG treated and saline treated animals. However, both groups containing curcumin showed only a trend toward the significance when compared to saline treated animals (SNK test: curcumin vs. saline: q = 2.529, P = 0.08; curcumin + EGCG vs. saline: q = 3.361, P = 0.096) (Figure 1B).
Simultaneously, the latency of flat beam crossing was measured (flat beam time score). The lowest latency in crossing the beam was observed in the EGCG group (SNK test: saline vs. EGCG: q = 4.343, P < 0.05; curcumin vs. EGCG, q = 3.828, P < 0.05). The latency in crossing the beam in the curcumin + EGCG group was lower than that in the EGCG group, however, there was no synergistic effect of the combined therapy (Figure 1C) (SNK test: EGCG vs. saline: 7 weeks, q = 4.364, P < 0.01; 8 weeks, q = 4.352, P < 0.05; 9 weeks, q = 3.874, P < 0.05; curcumin vs. saline, 7 weeks, q = 2.922, P < 0.05; EGCG + curcumin vs. saline, 7 weeks, q = 3.679, P < 0.05).
Plantar test
To estimate the effect of curcumin, EGCG and their combination on latency of response to nociceptive thermal stimulus, a plantar test apparatus was used. No increased hyperalgesia in response to nociceptive thermal stimulus was observed in any of the treated animals (SNK test: saline vs. curcumin, 1 week, q = 4.74, P < 0.05; 9 weeks, q = 4.088, P < 0.05; EGCG vs. curcumin, 1 week, q = 2.983, P < 0.05; EGCG + curcumin vs. curcumin, 1 week, q = 3.685, P < 0.05, 9 weeks, q = 4.282, P < 0.05; EGCG + curcumin vs. EGCG, 2 weeks, q = 4.345, P < 0.05) (Figure 1D).
Histology and immunohistochemistry
White and grey matter
The effect of the combined therapy of curcumin and EGCG on the preservation of spinal cord white (Figure 2A) and grey matter (Figure 2B) was evaluated on a series of transverse sections two months after SCI. None of the applied treatments showed a statistically significant effect on the preservation of injured spinal cord white and grey matter. Volume per 15 mm long analyzed spinal cord tissue was 23.1, 26.18, 25.98, and 21.4 mm3 in the saline, EGCG, curcumin, and curcumin + EGCG groups, respectively (Figure 2G). Either curcumin or EGCG application led to minor preservation of spinal cord tissue after SCI. However, their combination had no positive effect on tissue sparing (Figure 2A, B, G). On the contrary, the combined therapy of curcumin and EGCG showed a strong trend toward decreasing cavity size when compared to the control saline treated animals (Figure 2F) (SNK test: gray matter: EGCG vs. EGCG + curcumin, 3 cranial, q = 4.013, P < 0.05; 2 cranial, q = 6.461, P < 0.001; 1 cranial, q = 4.865, P < 0.01; EGCG vs. saline, 2 cranial, q = 3.679, P < 0.05; 1 cranial, q = 3.221, P < 0.05; EGCG vs. curcumin, 2 cranial, q = 2.967, P < 0.05; 1 cranial, q = 4.556, P < 0.05; white matter: saline vs. EGCG + curcumin, 2 cranial, q = 3.454, P < 0.05; EGCG vs. EGCG + curcumin, 1 cranial, q = 4.302, P < 0.05; 2 cranial, q = 5.372, P < 0.01; curcumin vs. EGCG + curcumin, 2 cranial, q = 3.753, P < 0.05).
Figure 2.
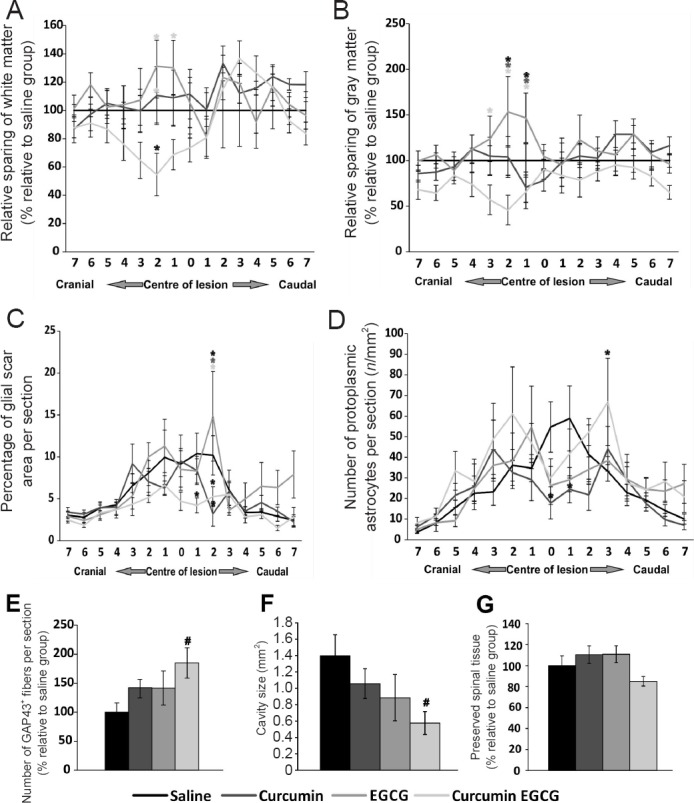
Histological and immunohistochemical analysis results of injured spinal cords.
The amount of spared white (A) and grey matter (B) was determined 9 weeks after spinal cord injury (SCI) in rats treated with saline, curcumin, EGCG, or their combination. No statistically significant effect of applied drugs on tissue preservation was observed. In addition, the volume of white matter and gray matter across the measured regions revealed no significant difference (G). However, a strong trend toward smaller cavity size was found (F). The effect of the application of curcumin, EGCG, or their combination on glial scar formation (C), and the average number of protoplasmic astrocytes per section (D) around the central lesion cavity was measured 9 weeks after SCI. The combined application of curcumin and EGCG had a suppressive effect on glial scar formation at the area of the lesion epicenter. In the central region of the injury, all treatments showed a positive effect on decreasing the number of protoplasmic astrocytes. The distribution of the effect on X axis is measured as distance in mm from the lesion center (set as 0) (A–D). The effect of applied curcumin, EGCG, or their combination on axonal sprouting. (E) The combination of curcumin and EGCG had a synergistic effect on axonal sprouting, when compared to saline treated animals. Two-way repeated measures analysis of variance (ANOVA) with Student-Newman-Keuls test was used to determine statistical significance (A–D). One-way ANOVA with Student-Newman-Keuls test was used to determine the level of statistical significance (E–G). *P < 0.05 (the color of asterisk indicates the statistically different group(s)). Symbol of # is used when trend toward significance has reached statistical value P = 0.05–0.06. n = 5 per group.
Astrogliosis
To detect the effect of the combined therapy of curcumin and EGCG on glial scar formation, the area of GFAP+ scar tissue (in%) on a series of transversal spinal cord sections was measured two months after SCI. None of the single treatment significantly reduced astrogliosis, however, the number of protoplasmic astrocytes was reduced in both curcumin and EGCG treated animals (Figure 2C, D). Combined therapy showed a partial decrease of GFAP+ scar tissue area in the central region of the damaged spinal cord, when compared to the saline group (two-way ANOVA; SNK test: 1 caudal, P < 0.05, 2 caudal, P < 0.001). The number of protoplasmic astrocytes was not changed in the curcumin + EGCG group (SNK test: glial scar: saline vs. curcumin, 2 caudal, q = 4.746, P < 0.01; saline vs. EGCG: 2 caudal, q = 2.967, P < 0.05; saline vs. EGCG + curcumin, 1 caudal, q = 4.206, P < 0.05, 2 caudal, q = 3.352, P < 0.05; EGCG vs. curcumin, 2 caudal, q = 7.139, P < 0.001; EGCG vs. EGCG + curcumin, 2 caudal, q = 5.881, P < 0.001) (SNK test: saline vs. curcumin, central, q = 4.230, P < 0.05, 1 caudal, q = 3.949, P < 0.05; EGCG + curcumin vs. saline, 2 caudal, q = 3.867, P < 0.05) (Figure 2C, D).
Axonal sprouting
The number of sprouting axons was measured using GAP43+ staining on a series of transversal spinal cord sections, two months after injury. Both curcumin and EGCG compounds increased the number of GAP43+ fibers compared to saline treated animals. This effect was enhanced in the combined therapy, however, remained only as a strong trend toward significance (Figure 2E) (one-way ANOVA: F = 2.723, P = 0.087; SNK test: q = 3.978, P = 0.057).
Endogenous gene response
The relative gene expression of several factors (Sort1, Fgf2, Irf5, Mrc1, Olig2, Casp3, Gap43, Gfap, Vegf, NfκB, Cntf) related to regenerative processes was measured from injured spinal cord tissue two months after injury. Both curcumin and EGCG showed a similar pattern in expression of targeted genes. The expression of most of the genes was upregulated or unchanged in comparison with saline treated animals, with only Gfap downregulated. The combined therapy showed opposite effects than each single treatment in all targeted genes, except for Olig2 and Gfap. This pattern was statistically significant in most cases when compared with each single treatment (SNK test: EGCG + curcumin vs. curcumin, Sort 1: q = 5.151, P < 0.01, FGF2 q = 4.1, P < 0.05, Irf5: q = 3.71, P < 0.05; Mrc1: q = 4.238, P < 0.05; Casp3: q = 6.373, P < 0.01; Gap43: q = 4.965, P < 0.01; Cntf: q = 5.664, P < 0.01; EGCG + curcumin vs. EGCG, Irf5: q = 5.386, P < 0.01; Casp3: q = 4.023, P < 0.05; NFκB: q = 4.212, P < 0.05; Cntf: q = 4.085, P < 0.05; saline vs. EGCG, Irf5: q = 4.059, P < 0.05; saline vs. curcumin, Casp3, q = 4.160, P < 0.05) (Figure 3).
Figure 3.
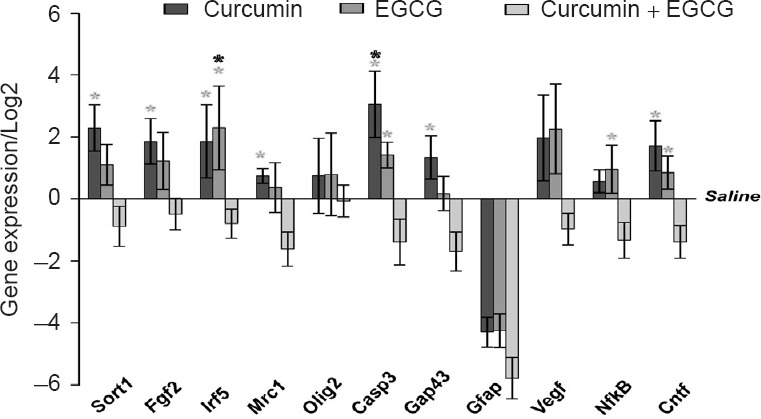
Gene expression changes in injured spinal cords after application of curcumin, epigallocatechin gallate (EGCG) or their combination.
Relative gene expression of factors related to the recovery process in response to application of curcumin, EGCG or their combination 9 weeks after spinal cord injury. The expression level of saline treated animals was set as zero value. One-way analysis of variance with Student-Newman-Keuls post hoc test was used to determine the level of statistical significance. *P < 0.05(the color of asterisk indicates the statistically different group(s)). n = 4 per group.
Inflammatory response
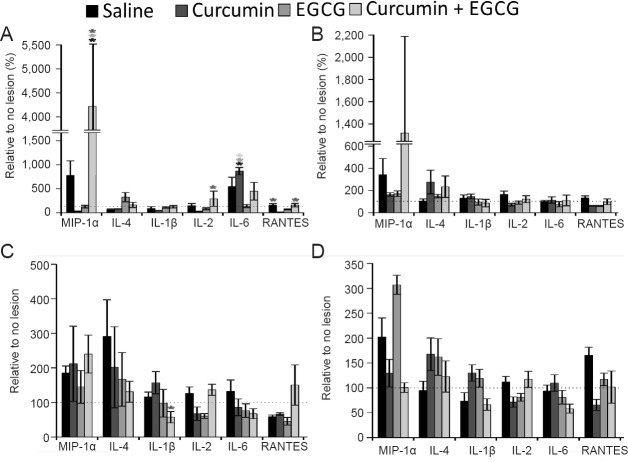
Changes in levels of cytokines (IL-1β, IL-4, IL-2, IL-6, MIP-1α, and RANTES,) after SCI were measured. At specific time points (1, 3, 7 and 10 days after injury), the effect of curcumin, EGCG or their combination was evaluated. In the curcumin + EGCG group, MIP-1α level was elevated for the first three days compared to that in the saline, curcumin or EGCG groups (1 day: two-way ANOVA, SNK test: P < 0.001, details at the bottom of the paragraph). No other changes were observed during this period. Seven days after injury, in the treated animals, we observed decreased levels of IL-4, IL-1β and IL-6 (two-way ANOVA, SNK test; curcumin vs. curcumin + EGCG, P < 0.05), and the decreased level of IL-6 persisted until day ten after injury. In the single treatment, curcumin initially decreased levels of IL-2 (two-way ANOVA, SNK test: curcumin vs. curcumin + EGCG, P < 0.05), and RANTES (two-way ANOVA, SNK test; curcumin + EGCG vs. curcumin, P < 0.05; saline vs. curcumin, P < 0.05) and increased the level of IL-6 (two-way ANOVA, SNK test: curcumin vs. curcumin + EGCG, P < 0.05; curcumin vs. EGCG, P < 0.05; curcumin vs. saline, P < 0.05; Figure 4). It is important to note that there was a surge in MIP-1α level at one day after injury in the curcumin + EGCG group, which persisted through days three and seven after injury. MIP-1α levels in the curcumin + EGCG group were reduced by day 10 after injury to levels in the curcumin or EGCG groups (SNK test: EGCG + curcumin vs. saline, MIP-1α: day 1, q = 5.615, P < 0.001; RANTES: day 1, q = 3.930, P < 0.05; EGCG + curcumin vs. curcumin, MIP-1α, day 1, q = 7.750, P < 0.001; IL-1β, day 1, q = 3.892, P < 0.05; IL-2, day 1, q = 4.505, P < 0.05; RANTES, day 1, q = 4.756, P < 0.05; EGCG + curcumin vs. EGCG, MIP-1α, day 1, q = 6.039, P < 0.001).
Figure 4.
Effect of curcumin, EGCG, or their combination on cytokine levels in injured spinal cords.
The levels of cytokines and chemokines are presented after application of curcumin, EGCG or their combination at 1 (A), 3 (B), 7 (C) and 10 days after spinal cord injury (D). The levels measured in animals with no lesion were set as 100%. Two-way analysis of variance with Student-Newman-Keuls post hoc test was used to determine statistical significance. *P < 0.05 (The color of asterisks indicates the statistically different group(s)). n = 5/group per time point. MIP-1α: Macrophage inflammatory protein 1-alpha; IL-4: interleukin-4; IL-1β: interleukin-1beta; IL-2: interleukin-2; IL-6: interleukin-6; RANTES: regulated on activation, normal T cell expressed and secreted; EGCG: epigallocatechin gallate.
Discussion
In our study, the combined therapy for the treatment of experimental SCI, using two anti-inflammatory compounds curcumin and EGCG, had some positive effects on behavioral recovery, decreased glial scar formation, and supported axonal sprouting. No spared white and grey matter was observed. In addition, the combined therapy influenced inflammatory response via regulating cytokine levels and altered the gene expression of regenerative process-related markers. The combined therapy did not exhibit synergistic effects compared to application of curcumin or EGCG alone. No studies have been reported on the detailed information regarding the combined therapy of curcumin and EGCG for the treatment of experimental SCI. An increasing number of publications have been reported on the positive effect of curcumin on an experimental model of SCI. An indirect impact of attenuated immune response may decrease apoptosis, enhance axonal sprouting or modulate glial scar formation, leading to behavioral recovery after SCI (Ormond et al., 2012; Zu et al., 2014) or reducing neuropathic pain (Lee et al., 2013). Curcumin influenced the regenerative processes, in which the most commonly described is the immunomodulatory effects, through the Jak/Stat3 and the TLR4-MyD88-NFκB pathways (Wang et al., 2014; Machova Urdzikova et al., 2015; Gokce et al., 2016). Immunomodulation by curcumin or EGCG inhibited the Jak/stat signaling pathway, leading to reduction of glial scar formation (You et al., 2017). The Jak/stat signaling pathway was also influenced by the combined therapy of curcumin and EGCG (Figures 3, 4). Decreased NF-κB activity, along with reduced levels of TNF-α and IL-1 and decreased expression of GFAP after SCI was also reported after curcumin application (Yuan et al., 2015). Application of EGCG after SCI not only promoted axonal sprouting (GAP-43 staining), but also increased GFAP immunoreactivity (Renno et al., 2014; Renno et al., 2015). In this study, we observed in vivo a trend towards an increase in GAP-43 levels after combined therapy of curcumin and EGCG (Figure 2E). In our in vitro studies, we tested the growth of neurites from adult dorsal root ganglia (DRGs) in the presence of EGCG. We did not observe any growth facilitation. However, EGCG reduced neuronal death after application of H2O2 in hippocampal neuronal culture, indicating a neuroprotective and anti-oxidative effect (data not shown/unpublished data). The concentrations of EGCG used in SCI studies vary from 10 to 50 mg/kg (Tian et al., 2013; Renno et al., 2015; Urdzikova et al., 2017). In our previous study, we used a concentration within the lower range to prevent a possible cytotoxic effect resulting from a high concentration of EGCG, since it was reported that a high concentration of EGCG (> 10 μM) can lead to increased apoptosis and mitochondrial dysfunction in hippocampal culture in vitro (Yin et al., 2009). Similarly to EGCG, curcumin can affect cell proliferation and survival and neurite outgrowth in a dose dependent manner. Curcumin at high concentration (above 10 μM) inhibited the proliferation and survival of various types of cancer cells (Yang et al., 2017). In PC12 cells, however, the in vitro application of curcuminoids at concentrations from 100 nM up to 20 μM has led to a significant increase of neurite outgrowth through stimulating MAPK/ERK and PKC dependent pathways (Liao et al., 2012; Dikmen, 2017). In addition, curcumin can act through the Akt/GSK-3β dependent pathway and regulate brain-derived neurotrophic factor level (Hoppe et al., 2013). Curcumin also has a dose dependent effect on the neural stem cells isolated from the subventricular zone of adult rats. Curcumin at 500 nM supported neurosphere proliferation, but at a higher concentration (μM), it resulted in widespread apoptosis (Ormond et al., 2014). In this study, we also observed downregulated Gfap expression and decreased GFAP staining intensity (Figures 2C and 3). However, the neuroprotective and antiapoptotic roles of curcumin and EGCG lead to partial tissue sparing after SCI, which were not observed in the combined therapy of curcumin and EGCG.
One important finding of this study is that the levels of the chemokine MIP-1α increased dramatically one day after injury, which persisted to days three and seven after injury. MIP-1α has been implicated in cell adhesion and migration, which might be required in the early phase after injury to mobilize stem cells and astrocytes to the site of injury. Furthermore, recent observations have suggested that MIP-1α is not only a chemokine, but also has a pivotal role in the growth and survival of many cell types (Maurer and von Stebut, 2004; Terpos et al., 2005).
Moreover, the anti-oxidative effect of curcumin leads to an increased level of super oxide dismutase (Sahin Kavakli et al., 2011). Additionally, a dose dependent effect of curcumin on stem cell proliferation has been reported (Son et al., 2014). It is important to note that the combined therapy of curcumin and neural stem cells has been successfully used in experimental SCI, showing that curcumin has the potential to support the paracrine effect of stem cells (Ormond et al., 2014).
EGCG, on the contrary, is studied much less in SCI. Studies have demonstrated that EGCG can affect neuropathic pain (Xifro et al., 2015), tactile allodynia (Kuang et al., 2012) and thermal hyperalgesia (Alvarez-Perez et al., 2015). In addition, EGCG has been shown to decrease pro-inflammatory molecules, such as RhoA, FASN and TNF-α after SCI (Alvarez-Perez et al., 2015). EGCG has caused an improvement in locomotor recovery after SCI, which was described due to the positive impact on lipid peroxidation, neuronal apoptosis, and overall spinal cord tissue sparing (Khalatbary et al., 2010; Tian et al., 2013). In this study, only mild improvement was observed on thermal hyperalgesia after application of EGCG and curcumin (Figure 1D). EGCG exhibited an anti-edema effect after SCI through downregulating AQP-4 and GFAP protein levels, suggesting a neuroprotective effect (Ge et al., 2013). Functional recovery after EGCG application could be attributed to the upregulation of GAP-43 protein level (Renno et al., 2015), and increased intrinsic levels of BDNF and GDNF (Tian et al., 2013) after SCI.
Some of these effects that were observed in the combined therapy of curcumin and EGCG contributed to the recovery of SCI. The behavioral recovery in the combined therapy of curcumin and EGCG was similar to application of curcumin alone; however, no synergistic effect was evident. In more advanced tests, such as the flat beam test, the scores were closer to the EGCG results. According to the publications (Khalatbary et al., 2010; Renno et al., 2014), the EGCG therapy was more sensitive in mild to moderate injuries affecting advanced locomotor settings. The advanced setting of the flat beam test shows the ability of focusing on the task and balancing without proper usage of the hindlimbs. To be able to perform such a task, the animals must score from 8 to 10 in the BBB open-field locomotor test. A score of 1 indicates that to be able to balance on the flat, animals must use forelimbs for movement. To reach scores higher than 3, animals must be able to support their weight (above score 10 in the BBB open-field locomotor test) and at least partially coordinate hindlimb movements (scores 11-14 in the open-field locomotor test). However, the ability to cross the beam (score 3) does not directly correlate with the BBB open-field locomotor test score. Animals able to hold the beam with their hindlimbs from the sides can reach the target zone, even though in the BBB open-field locomotor test they would not perform better than animals trying to step on the top of the beam. Therefore, the flat beam test also reflects the motivation of the animal to reach the target zone, even without stepping properly. The sensory function and thermal hyperalgesia of the rats were assessed by the plantar test, which measures withdrawal latency of the hindlimbs to the thermal stimulus. The combined therapy of curcumin and EGCG did not negatively affect thermal allodynia. To report more about the effect of treatment on allodynia, studies on mechanical stimulus need to be added. The combined therapy of curcumin and EGCG does affect the immune response in a more discrete manner than monotherapy. We observed decreased levels of IL-1β and IL-6, which are in consistent with described anti-inflammatory properties of both compounds (Khalatbary and Ahmadvand, 2011; Wang et al., 2014; Gokce et al., 2016). In addition, with the exception of the 7th day after SCI, IL-4 levels were above the values measured in saline treated animals at other time points. The elevated level of MIP-1α in the early phase of SCI may function not only as a pro-inflammatory chemokine, but also as a molecule to promote migration, growth and survival.
In conclusion, the combined therapy of curcumin and EGCG for treatment of experimental SCI led to behavioral recovery, even though not significantly superior to monotherapy. The combined therapy of curcumin and EGCG did not influence growth factor response and spinal cord tissue sparing, but it displayed a strong immunomodulatory response after SCI, and supported axonal sprouting and glial scar reduction. Although the expected synergistic response of this combined therapy was not achieved, different aspects of tissue regeneration in severe SCI were understood.
Additional files:
Additional file 1: (7.8KB, pdf) Open peer review report 1.
Additional Figure 1: (91.5KB, pdf) Histological and immunohistochemical staining of injured spinal cords.
Footnotes
Funding: This study was supported by the grant GAČR (Grant Agency of the Czech Republic) P304/12/G069, from the Ministry of Education, Youth and Sports under the project “Centre of Reconstructive Neuroscience”, registration number CZ.02.1.01/0.0./0.0/15_003/0000419 and project InterAction LTAUSA17120.
Conflicts of interest: The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support: This study was supported by the grant GAČR (Grant Agency of the Czech Republic) P304/12/G069, from the Ministry of Education, Youth and Sports under the project “Centre of Reconstructive Neuroscience”, registration number CZ.02.1.01/0.0./0.0/15_003/0000419 and project InterAction LTAUSA17120. None of the funding bodies play any role in the study other than to provide funding.
Research ethics: All experiments were performed in accordance with the European Communities Council Directive of 22nd of September 2010 (2010/63/EU) regarding the use of animals in research, and were approved by the Ethics Committee of the Institute of Experimental Medicine, Academy of Sciences of the Czech Republic, approval No. 277/2011 and 53/2014.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Mitsuhiro Enomoto, Tokyo Medical and Dental University, Japan; Hızır Ufuk Akdemir, Ondokuz Mayis University, Faculty of Medicine, Turkey.
(Copyedited by Li CH, Song LP, Zhao M)
References
- 1.Alvarez-Perez B, Homs J, Bosch-Mola M, Puig T, Reina F, Verdu E, Boadas-Vaello P. Epigallocatechin-3-gallate treatment reduces thermal hyperalgesia after spinal cord injury by down-regulating RhoA expression in mice. Eur J Pain. 2015;20:341–352. doi: 10.1002/ejp.722. [DOI] [PubMed] [Google Scholar]
- 2.Aydin MS, Caliskan A, Kocarslan A, Kocarslan S, Yildiz A, Gunay S, Savik E, Hazar A, Yalcin F. Intraperitoneal curcumin decreased lung, renal and heart injury in abdominal aorta ischemia/reperfusion model in rat. Int J Surg. 2014;12:601–605. doi: 10.1016/j.ijsu.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 4.Bibikova M, Yeakley JM, Chudin E, Chen J, Wickham E, Wang-Rodriguez J, Fan JB. Gene expression profiles in formalin-fixed, paraffin-embedded tissues obtained with a novel assay for microarray analysis. Clin Chem. 2004;50:2384–2386. doi: 10.1373/clinchem.2004.037432. [DOI] [PubMed] [Google Scholar]
- 5.Carstens E, Ansley D. Hindlimb flexion withdrawal evoked by noxious heat in conscious rats: magnitude measurement of stimulus-response function, suppression by morphine and habituation. J Neurophysiol. 1993;70:621–629. doi: 10.1152/jn.1993.70.2.621. [DOI] [PubMed] [Google Scholar]
- 6.Dikmen M. Comparison of the effects of curcumin and RG108 on NGF-induced PC-12 Adh cell differentiation and neurite outgrowth. J Med Food. 2017;20:376–384. doi: 10.1089/jmf.2016.3889. [DOI] [PubMed] [Google Scholar]
- 7.Dubendorf P. Spinal cord injury pathophysiology. Crit Care Nurs Q. 1999;22:31–35. doi: 10.1097/00002727-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Dudka J, Jodynis-Liebert J, Korobowicz E, Burdan F, Korobowicz A, Szumilo J, Tokarska E, Klepacz R, Murias M. Activity of NADPH-cytochrome P-450 reductase of the human heart, liver and lungs in the presence of (-)-epigallocatechin gallate, quercetin and resveratrol: an in vitro study. Basic Clin Pharmacol Toxicol. 2005;97:74–79. doi: 10.1111/j.1742-7843.2005.pto_98.x. [DOI] [PubMed] [Google Scholar]
- 9.Eom DW, Lee JH, Kim YJ, Hwang GS, Kim SN, Kwak JH, Cheon GJ, Kim KH, Jang HJ, Ham J, Kang KS, Yamabe N. Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep. 2015;48:461–466. doi: 10.5483/BMBRep.2015.48.8.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gal P, Kravcukova P, Mokry M, Kluchova D. Chemokines as possible targets in modulation of the secondary damage after acute spinal cord injury: a review. Cell Mol Neurobiol. 2009;29(6-7):1025–1035. doi: 10.1007/s10571-009-9392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Nino WR, Zatarain-Barron ZL, Hernandez-Pando R, Vega-Garcia CC, Tapia E, Pedraza-Chaverri J. Oxidative stress markers and histological analysis in diverse organs from rats treated with a hepatotoxic dose of Cr(VI): Effect of curcumin. Biol Trace Elem Res. 2015;167:130–145. doi: 10.1007/s12011-015-0283-x. [DOI] [PubMed] [Google Scholar]
- 12.Ge R, Zhu Y, Diao Y, Tao L, Yuan W, Xiong XC. Anti-edema effect of epigallocatechin gallate on spinal cord injury in rats. Brain Res. 2013;1527:40–46. doi: 10.1016/j.brainres.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Gokce EC, Kahveci R, Gokce A, Sargon MF, Kisa U, Aksoy N, Cemil B, Erdogan B. Curcumin attenuates inflammation, oxidative stress, and ultrastructural damage induced by spinal cord ischemia-reperfusion injury in rats. J Stroke Cerebrovasc Dis. 2016;25:1196–1207. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein LB. Effects of bilateral and unilateral locus coeruleus lesions on beam-walking recovery after subsequent unilateral sensorimotor cortex suction-ablation in the rat. Restor Neurol Neurosci. 1997;11:55–63. doi: 10.3233/RNN-1997-111206. [DOI] [PubMed] [Google Scholar]
- 15.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 16.Hoppe JB, Coradini K, Frozza RL, Oliveira CM, Meneghetti AB, Bernardi A, Pires ES, Beck RC, Salbego CG. Free and nanoencapsulated curcumin suppress β-amyloid-induced cognitive impairments in rats: involvement of BDNF and Akt/GSK-3β signaling pathway. Neurobiol Learn Mem. 2013;106:134–144. doi: 10.1016/j.nlm.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Khalatbary AR, Ahmadvand H. Anti-inflammatory effect of the epigallocatechin gallate following spinal cord trauma in rat. Iran Biomed J. 2011;15:31–37. [PMC free article] [PubMed] [Google Scholar]
- 18.Khalatbary AR, Tiraihi T, Boroujeni MB, Ahmadvand H, Tavafi M, Tamjidipoor A. Effects of epigallocatechin gallate on tissue protection and functional recovery after contusive spinal cord injury in rats. Brain Res. 2010;1306:168–175. doi: 10.1016/j.brainres.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 19.Kuang X, Huang Y, Gu HF, Zu XY, Zou WY, Song ZB, Guo QL. Effects of intrathecal epigallocatechin gallate, an inhibitor of Toll-like receptor 4, on chronic neuropathic pain in rats. Eur J Pharmacol. 2012;676:51–56. doi: 10.1016/j.ejphar.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Shin TJ, Choi JM, Seo KS, Kim HJ, Yoon TG, Lee YS, Han H, Chung HJ, Oh Y, Jung SJ, Shin KJ. Antinociceptivecurcuminoid, KMS4034, effects on inflammatory and neuropathic pain likely via modulating TRPV1 in mice. Br J Anaesth. 2013;111:667–672. doi: 10.1093/bja/aet176. [DOI] [PubMed] [Google Scholar]
- 21.Liao KK, Wu MJ, Chen PY, Huang SW, Chiu SJ, Ho CT, Yen JH. Curcuminoids promote neurite outgrowth in PC12 cells through MAPK/ERK- and PKC-dependentpathways. J Agric Food Chem. 2012;60:433–443. doi: 10.1021/jf203290r. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Xu Z, Li H, Guo M, Yang T, Feng S, Xu B, Deng Y. Protective effects of curcumin against mercury-induced hepatic injuries in rats, involvement of oxidative stress antagonism, and Nrf2-ARE pathway activation. Hum Exp Toxicol. 2017;36:949–966. doi: 10.1177/0960327116677355. [DOI] [PubMed] [Google Scholar]
- 23.Machova Urdzikova L, Karova K, Ruzicka J, Kloudova A, Shannon C, Dubisova J, Murali R, Kubinova S, Sykova E, Jhanwar-Uniyal M, Jendelova P. The anti-inflammatory compound curcumin enhances locomotor and sensory recovery after spinal cord injury in rats by immunomodulation. Int J Mol Sci. 2015;17:pii: E49. doi: 10.3390/ijms17010049. doi: 10.3390/ijms17010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machova Urdzikova L, Ruzicka J, Karova K, Kloudova A, Svobodova B, Amin A, Dubisova J, Schmidt M, Kubinova S, Jhanwar-Uniyal M, Jendelova P. A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines. Neuropharmacology. 2017;126:213–223. doi: 10.1016/j.neuropharm.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Meng M, Li YQ, Yan MX, Kou Y, Ren HB. Effects of epigallocatechin gallate on diethyldithiocarbamate-induced pancreatic fibrosis in rats. Biol Pharm Bull. 2007;30:1091–1096. doi: 10.1248/bpb.30.1091. [DOI] [PubMed] [Google Scholar]
- 27.Mietto BS, Mostacada K, Martinez AM. Neurotrauma and inflammation: CNS and PNS responses. Mediators Inflamm. 2015;2015:251204. doi: 10.1155/2015/251204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni H, Jin W, Zhu T, Wang J, Yuan B, Jiang J, Liang W, Ma Z. Curcumin modulates TLR4/NF-kappaB inflammatory signaling pathway following traumatic spinal cord injury in rats. J Spinal Cord Med. 2015;38:199–206. doi: 10.1179/2045772313Y.0000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ormond DR, Peng H, Zeman R, Das K, Murali R, Jhanwar-Uniyal M. Recovery from spinal cord injury using naturally occurring antiinflammatory compound curcumin: laboratory investigation. J Neurosurg Spine. 2012;16:497–503. doi: 10.3171/2012.1.SPINE11769. [DOI] [PubMed] [Google Scholar]
- 30.Ormond DR, Shannon C, Oppenheim J, Zeman R, Das K, Murali R, Jhanwar-Uniyal M. Stem cell therapy and curcumin synergistically enhance recovery from spinal cord injury. PLoS One. 2014;9:e88916. doi: 10.1371/journal.pone.0088916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renno WM, Al-Maghrebi M, Rao MS, Khraishah H. (-)-Epigallocatechin-3-gallate modulates spinal cord neuronal degeneration by enhancing growth-associated protein 43, B-cell lymphoma 2, and decreasing B-cell lymphoma 2-associated x protein expression after sciatic nerve crush injury. J Neurotrauma. 2015;32:170–184. doi: 10.1089/neu.2014.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renno WM, Al-Khaledi G, Mousa A, Karam SM, Abul H, Asfar S. (-)-Epigallocatechin-3-gallate (EGCG) modulates neurological function when intravenously infused in acute and, chronically injured spinal cord of adult rats. Neuropharmacology. 2014;77:100–119. doi: 10.1016/j.neuropharm.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahin Kavaklı H, Koca C, Alıcı O. Antioxidant effects of curcumin in spinal cord injury in rats. Ulus Travma Acil Cerrahi Derg. 2011;17:14–18. [PubMed] [Google Scholar]
- 36.Sanivarapu R, Vallabhaneni V, Verma V. The potential of curcumin in treatment of spinal cord injury. Neurol Res Int. 2016;2016:9468193. doi: 10.1155/2016/9468193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanli AM, Turkoglu E, Serbes G, Sargon MF, Besalti O, Kilinc K, Irak A, Sekerci Z. Effect of curcumin on lipid peroxidation, early ultrastructural findings and neurological recovery after experimental spinal cord contusion injury in rats. Turk Neurosurg. 2012;22:189–195. doi: 10.5137/1019-5149.JTN.5193-11.1. [DOI] [PubMed] [Google Scholar]
- 38.Son S, Kim KT, Cho DC, Kim HJ, Sung JK, Bae JS. Curcumin stimulates proliferation of spinal cord neural progenitor cells via a mitogen-activated protein kinase signaling pathway. J Korean Neurosurg Soc. 2014;56:1–4. doi: 10.3340/jkns.2014.56.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A. T(3;14) (p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19:652–658. doi: 10.1038/sj.leu.2403644. [DOI] [PubMed] [Google Scholar]
- 40.Terpos E, Politou M, Viniou N, Rahemtulla A. Significance of macrophage inflammatory protein-1 alpha (MIP-1alpha) in multiple myeloma. Leuk Lymphoma. 2005;46:1699–1707. doi: 10.1080/10428190500175049. [DOI] [PubMed] [Google Scholar]
- 41.Tian W, Han XG, Liu YJ, Tang GQ, Liu B, Wang YQ, Xiao B, Xu YF. Intrathecal epigallocatechin gallate treatment improves functional recovery after spinal cord injury by upregulating the expression of BDNF and GDNF. Neurochem Res. 2013;38:772–779. doi: 10.1007/s11064-013-0976-5. [DOI] [PubMed] [Google Scholar]
- 42.Urdzikova L, Jendelova P, Glogarova K, Burian M, Hajek M, Sykova E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23:1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- 43.Urdzikova LM, Ruzicka J, LaBagnara M, Karova K, Kubinova S, Jirakova K, Murali R, Sykova E, Jhanwar-Uniyal M, Jendelova P. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int J Mol Sci. 2014;15:11275–11293. doi: 10.3390/ijms150711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanicky I, Urdzikova L, Saganova K, Cizkova D, Galik J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma. 2001;18:1399–1407. doi: 10.1089/08977150152725687. [DOI] [PubMed] [Google Scholar]
- 45.Wang YF, Zu JN, Li J, Chen C, Xi CY, Yan JL. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci Lett. 2014;560:51–56. doi: 10.1016/j.neulet.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 46.Wittiw CD, Fehlings MG. Acute spinal cord injury. J Spinal Disord Tech. 2015;28:202–210. doi: 10.1097/BSD.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 47.Xifro X, Vidal-Sancho L, Boadas-Vaello P, Turrado C, Alberch J, Puig T, Verdu E. Novel epigallocatechin-3-gallate (EGCG) derivative as a new therapeutic strategy for reducing neuropathic pain after chronic constriction nerve injury in mice. PLoS One. 2015;10:e0123122. doi: 10.1371/journal.pone.0123122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang J, Wang C, Zhang Z, Chen X, Jia Y, Wang B, Kong T. Curcumin inhibits the survival and metastasis of prostate cancer cells via the Notch-1 signaling pathway. APMIS. 2017;125:134–140. doi: 10.1111/apm.12650. [DOI] [PubMed] [Google Scholar]
- 49.Yin ST, Tang ML, Deng HM, Xing TR, Chen JT, Wang HL, Ruan DY. Epigallocatechin-3-gallate induced primary cultures of rat hippocampal neurons death linked to calcium overload and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:551–564. doi: 10.1007/s00210-009-0401-4. [DOI] [PubMed] [Google Scholar]
- 50.You T, Bi Y, Li J, Zhang M, Chen X, Zhang K. IL-17 induces reactive astrocytes and up-regulation of vascular endothelial growth factor (VEGF) through JAK/STAT signaling. Sci Rep. 2017;7:41779. doi: 10.1038/srep41779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan J, Zou M, Xiang X, Zhu H, Chu W, Liu W, Chen F, Lin J. Curcumin improves neural function after spinal cord injury by the joint inhibition of the intracellular and extracellular components of glial scar. J Surg Res. 2015;195:235–245. doi: 10.1016/j.jss.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 52.Yunos NM, Beale P, Yu JQ, Huq F. Synergism from sequenced combinations of curcumin and epigallocatechin-3-gallate with cisplatin in the killing of human ovarian cancer cells. Anticancer Res. 2011;31:1131–1140. [PubMed] [Google Scholar]
- 53.Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X, Chen F, Wang CS, Feng H, Lin JK. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-kappaB signaling pathway in experimental traumatic brain injury. J Neuroinflammation. 2014;11:59. doi: 10.1186/1742-2094-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zu J, Wang Y, Xu G, Zhuang J, Gong H, Yan J. Curcumin improves the recovery of motor function and reduces spinal cord edema in a rat acute spinal cord injury model by inhibiting the JAK/STAT signaling pathway. Acta Histochem. 2014;116:1331–1336. doi: 10.1016/j.acthis.2014.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histological and immunohistochemical staining of injured spinal cords.
(A) Examples of analyzed images of cresyl violet luxol staining for white and grey matter sparing. (B, C) Gap 43 staining (green) for axonal sprouting (B) and GFAP-Cy3 staining (red)showing glial scar (C). Scale bars: 100 μm.