Abstract
Bone marrow-derived mesenchymal stem cells (BMSCs) have been shown to promote the regeneration of injured peripheral nerves. Pulsed electromagnetic field (PEMF) reportedly promotes the proliferation and neuronal differentiation of BMSCs. Low-frequency PEMF can induce the neuronal differentiation of BMSCs in the absence of nerve growth factors. This study was designed to investigate the effects of low-frequency PEMF pretreatment on the proliferation and function of BMSCs and the effects of low-frequency PEMF pre-treated BMSCs on the regeneration of injured peripheral nerve using in vitro and in vivo experiments. In in vitro experiments, quantitative DNA analysis was performed to determine the proliferation of BMSCs, and reverse transcription-polymerase chain reaction was performed to detect S100 (Schwann cell marker), glial fibrillary acidic protein (astrocyte marker), and brain-derived neurotrophic factor and nerve growth factor (neurotrophic factors) mRNA expression. In the in vivo experiments, rat models of crush-injured mental nerve established using clamp method were randomly injected with low-frequency PEMF pretreated BMSCs, unpretreated BMSCs or PBS at the injury site (1 × 106 cells). DiI-labeled BMSCs injected at the injury site were counted under the fluorescence microscope to determine cell survival. One or two weeks after cell injection, functional recovery of the injured nerve was assessed using the sensory test with von Frey filaments. Two weeks after cell injection, axonal regeneration was evaluated using histomorphometric analysis and retrograde labeling of trigeminal ganglion neurons. In vitro experiment results revealed that low-frequency PEMF pretreated BMSCs proliferated faster and had greater mRNA expression of growth factors than unpretreated BMSCs. In vivo experiment results revealed that compared with injection of unpretreated BMSCs, injection of low-frequency PEMF pretreated BMSCs led to higher myelinated axon count and axon density and more DiI-labeled neurons in the trigeminal ganglia, contributing to rapider functional recovery of injured mental nerve. These findings suggest that low-frequency PEMF pretreatment is a promising approach to enhance the efficacy of cell therapy for peripheral nerve injury repair.
Keywords: nerve regeneration, mesenchymal stem cells, low-frequency pulsed electromagnetic field, peripheral nerve injury, crush-injured mental nerve
Introduction
Peripheral nerve damage can result in severe dysesthesia, persistent paresthesia, and/or post-traumatic pain (Li et al., 2012a). Injured peripheral nerves may regenerate in time, but the regeneration is often not complete, and the process takes a long time. Patients may benefit from surgical interventions such as neuroplasty, neurorrhaphy, or nerve grafting, these are delicate procedures that have a risk of no improvement or worsening of the condition, particularly in case of severe injury (Lee and Wolfe, 2000). Benefits of cell therapy (Kilmer and Carlsen, 1987) and electromagnetic stimulation (Aebischer et al., 1987) on the recovery of injured peripheral nerve have been reported.
Schwann cells (SCs) produce a large variety of neurotrophic factors and cytokines, and also express cell adhesion molecules and extracellular molecules known to support axonal regeneration (Ide, 1996; Fu and Gordon, 1997; Chernousov and Carey, 2000; Goldberg and Barres, 2000; Lonze et al., 2002; Mimura et al., 2004). Following transection, SCs provide structural support as well as neurotrophic guidance to the regenerating axons (Bunge, 1994; Scherer, 1997). SCs were shown to assist in nerve regeneration when injected into an injured peripheral nerve (Mosahebi et al., 2001; Kobayashi et al., 2012). Despite these advantages, clinical application of SCs is limited due to their lengthy and complex culture process. Moreover, this technique requires sacrifice of another peripheral nerve for auto-transplantation (Mosahebi et al., 2002; Mimura et al., 2004; Dai et al., 2013). An alternate choice for cellular therapy is the mesenchymal stem cells (MSCs). MSCs are a well-established entity with wide application possibilities in regenerative medicine for their availability and capability of multi-lineage differentiation (Parekkadan and Milwid, 2010; Koh et al., 2012). Due to these positive attributes, MSCs have been used in the clinical trial as a treatment option for injured nerves (Tohill et al., 2004; Sung et al., 2012; Cooney et al., 2016). Numerous studies have shown that low-frequency electromagnetic field therapy has a positive effect on the recovery of damaged nerves (Alrashdan et al., 2010; Kim et al., 2015; Hei et al., 2016). Pulsed electromagnetic field (PEMF) has been reported to accelerate proliferation of BMSCs (Sun et al., 2009) and the differentiation of BMSCs into neuron-like cells under certain conditions (Kim et al., 2012). Low-frequency electromagnetic fields influences the actions of intracellular proteins and membrane proteins including ion channels (Lacy-Hulbert et al., 1998). Application of low-frequency PEMF has been shown to induce neural differentiation of BMSCs in an environment without nerve growth factors (Cho et al., 2012). Hence, nerve regeneration potential of MSCs may be further enhanced with application of PEMF.
The present study aimed to evaluate the effects of low-frequency PEMF on the proliferation and growth factor release of BMSCs, and to evaluate the effects of PEMF pre-treated BMSCs (PMSCs) on peripheral nerve regeneration.
Materials and Methods
In vitro experiment
Isolation and culture of BMSCs
BMSCs were isolated following a previously described method (Deng et al., 2003, 2004). Five 5-day-old Sprague-Dawley (SD) male rats, weighing 220–250 g, were scarified with carbon dioxide (CO2). Their hind limbs were harvested and washed in 70% ethanol and 1× phosphate-buffered saline (PBS). Using a 10-mL syringe with 26-gauge needle, the DMEM solution was injected into the spongy bone (cancellous bone), and the leakage was collected in a 50-mL conical tube. The collected medium was filtered using a 70-μm nylon mesh (Falcon, Franklin Lake, NJ, USA) to remove bone debris and muscle fibers. The filtered medium was centrifuged at 800 r/min for 5 minutes, and the supernatant was removed through aspiration. Cells collected through centrifugation were suspended in MSC growth medium consisting of a low glucose DMEM containing 10% MSC Qualified Fetal Bovine Serum (Thermo Fisher Scientific, Waltham, MA, USA), and 10 mg/mL of gentamicin (Thermo Fisher Scientific). The suspended cells were then seeded into a 100-mm cell culture dish and incubated at 37°C, 95% humidity, and 5% CO2 until adhesion. When cell confluency reached 85%, subculture was proceeded using 0.05% trypsin (assigned as passage 1), and the BMSCs were cultivated in this manner for the main experiment.
Characterization of BMSCs
The cultivated BMSCs were used in this experiment until passage 5 and were placed in culture slides. Immunocytochemical analysis was performed on untreated PC12 cells which were used as negative control. Markers for MSCs CD29 (Biolegend, San Diego, CA, USA) and CD105 (Abcam Inc., Cambridge, UK) primary antibodies (diluted 1:100) were used, and the cells were stored at 4°C overnight (Boxall and Jones, 2012). A secondary antibody, FITC (diluted 1:200) was applied for 30 minutes at room temperature. The culture slides were mounted with DAPI for investigation under a fluorescent microscope (CLSM, LSM700, Carl Zeiss, Oberkochen, Germany).
PEMF exposure on BMSCs
A PEMF device was constructed with help from Professor Soochan Kim of Signal Processing Applications Lab (Graduate School of Bio & Information Technology, Hankyong National University, Korea). The device consisted of a Helmholtz coils made of two enamel copper wire coils (AWG #25, 0.5 mm diameter) with 1,000 turns per coil. The inner diameter of the device was 30 cm, with a width of each coil was 7 cm, and the distance between the two coils was 15 cm. The PEMF conditions were 50 Hz, 1 mT, 1 hour/day (Sert et al., 2002; Feng et al., 2011; Li et al., 2012b; Bai et al., 2013; Hei et al., 2016). Cell culture dishes were placed in the center between the coils of the PEMF device under the condition of 50 Hz, 1 mT for 1 hour (Figure 1).
Figure 1.

A pulsed electromagnetic field (PEMF) device.
Two Helmholtz coils of 30-cm diameter, 7-cm width, and 15-cm distance apart. The PEMF device was placed in a CO2 incubator. Cell culture dishes were placed at the center between the coils for 5, 7, or 10 days.
Cell proliferation assay
To determine the effect of PEMF pre-treatment on cell proliferation, a quantitative DNA assay was performed (Lee et al., 2009). BMSCs and PMSCs were seeded into 96-well plates at a density of 1 × 103 cells/well. Culture medium was removed on the 5th, 7th, and 10th days. After PBS washes, 10 μL of EZ-Cytox solution (Daeil Lab Service Co. Std., Seoul, South Korea) was added to each well, and the plate was placed in a CO2 incubator (Thermo Fisher Scientific) for 4 hours. DNA was quantified with a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) with a light absorbance wavelength of 450 nm.
Detection of mRNA expression by RT-PCR
To decide the duration of PEMF, BMSCs were seeded and harvested on days 5, 7, and 10 to compare the gene expression (six dishes each group, a total of 18 dishes). On the day of the experiment, BMSCs were harvested into 1.5-mL centrifuge tubes. Next, 200 μL of chloroform was added, the solution was mixed by shaking, and the tubes were incubated for 12 minutes. The mixture was centrifuged for 10 minutes at 12,000 × g at 4°C. Following centrifugation, the top layer of the liquid was transferred to a new tube containing 500 μL of isopropyl alcohol and was incubated for 10 minutes. Then, the tube was centrifuged for another 10 minutes at 12,000 × g at 4°C. The supernatant was removed, and the mixture was washed with 1 mL of 75 % ethanol. The mixture was then centrifuged again for 5 minutes at 7,500 × g and then air-dried at room temperature. It was subsequently dissolved into diethylpyrocarbonate-treated water and incubated for 20 minutes at 50°C. RNA was quantified using an ultraviolet spectrophotometer at 260 nm and 280 nm. Reverse transcription (RT)-polymerase chain reaction (PCR) was performed as previously described (Kashani et al., 2011). Using an RT reagent kit (Thermo Fisher Scientific), total RNA was synthesized into cDNA. PCR was performed using primers for S100, glial fibrillary acidic protein (GFAP), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Kashani et al., 2011; Jiang et al., 2012; Bai et al., 2013). The PCR products were analyzed using 2% agarose gel electrophoresis using Quantity One software (version 4.3.1, BIO-RAD, Munich, Germany). The bands were graphically presented using ImageJ software (NIH, Bethesda, MD, USA). Additional experiments were performed in which BMSCs were pre-treated with PEMF for 10 days.
Effect of PEMF pre-treated BMSCs (PMSCs) on the regeneration of crush-injured mental nerve in rats (in vivo experiment)
Animal crush injury model
Sprague-Dawley (SD) rats, aged 5 weeks, weighing 200–250 g, were obtained from Orient Bio Inc. (Seongnam, Korea). Rats were randomly divided into four groups with six animals per group: sham surgery group, injury group (5 μL PBS was injected after mental nerve crush injury), BMSCs group (BMSCs were injected), and PMSCs group (BMSCs that were pre-treated with PEMF for 10 days were injected) (Figure 2A). After anesthesia with chloropent (1 mL/100 g), rat left mental nerve was exposed, and crush injury was induced using a needle holder with a beak width of 3 mm (Fine Science Tools Inc., North Vancouver, British Columbia, Canada, No. 12503-15) that was locked to the second ratchet (Figure 2B). All animal experiment procedures were performed in accordance with the guidance of the Laboratory of Animal Resources of Seoul National University, South Korea (SNU-130201-2).
Figure 2.
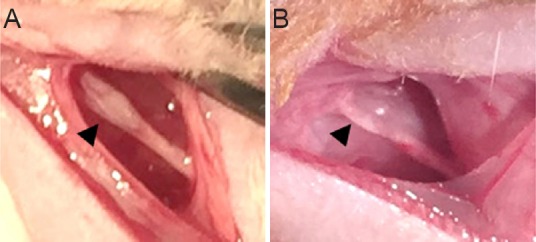
A rat model of mental nerve crush injury.
(A) PMSCs and BMSCs injection using Hamilton syringe (arrowhead) after mental nerve crush injury (round shape). (B) The mental nerve with a 3-mm-long crush injury (arrowhead) created by a needle holder (flat shape). BMSCs: Bone marrow-derived mesenchymal stem cells; PMSCs: pulsed electromagnetic field-pretreated BMSCs.
Tracking of DiI-labeled BMSCs and PMSCs
In order to evaluate cell viability in vivo after injection, BMSCs and PMSCs were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanineperchlorate (DiI solution, Molecular Probes Inc., Eugene, OR, USA) according to the manufacturer's instructions: BMSCs and PMSCs suspensions (1 × 106 cells) were mixed with 5 μL DiI solution by gentle pipetting. The mixtures were incubated for 15 minutes at 37°C in a CO2 incubator. The DiI-labeled BMSCs and PMSCs were injected into the injured nerve using a 26-gauge Hamilton syringe (Hamilton Company, Reno, NV, USA) 3 mm proximal to the injury site. The mental nerves were harvested at 1 and 2 weeks after injection, and 18 μm-thick nerve sections were obtained using a cryocut microtome (CM3050 S, Leica, Nussloch, Germany). Sections were observed under a fluorescence microscope (CLSM, LSM700, Carl Zeiss, Oberkochen, Germany).
BMSCs and PMSCs injection
The site of injury was tagged with single 9/0 nylon suture (Ethicon, Ethicon Inc., Somerville, NJ, USA) on the outer aspect of the nerve for recognition. BMSCs and PMSCs (1 × 106 cells/5 μL PBS) were injected immediately after injury using a 26-gauge Hamilton syringe 3 mm proximal to the injury site. Immediately after the injection, the surgical access was repositioned and sutured with a 4/0 nylon suture (Dafilon, B-BraunVetCare SA, Barcelona, Spain). Cell injection in crush-injured mental nerve was shown in Figure 2A.
Sensory function
A sensory test was performed using von Frey filaments (Semmes-Weinstein Monofilaments, North Coast Medical, Inc., Arcata, CA, USA), and scores were calculated using the method of Seino et al. (2009). To objectively assess sensory nerve function, a blind test was performed with the rats in all groups. A pre-test of sensory function was performed before crush injury, and the test was performed one and two weeks after surgery. When the ipsilateral lip area (a), ipsilateral mental area (b), and contralateral area (d) were stimulated with the filament, the act of only raising the forefoot was set as positive reaction, and this was recorded numerically. Statistical comparisons of differences in the mean scores at post-operative 1 week and 2 weeks within each group were recorded. The result was used to assess the behavioral response to mechanical stimulation. The difference score was defined as the difference between the mechanical touch thresholds (grams) of the ipsilateral and contralateral sides of the injury and was calculated as the value of the ipsilateral mental area (b) minus the value of the contralateral area (d). The gap score was defined as the difference between the mechanical touch thresholds of the proximal and distal parts of the mental nerve and was calculated as the value of the ipsilateral lip area (a) minus the value in the vicinity of the mental foramen (b). By definition, a higher score means poorer recovery. As the damaged nerve recovers functionally, the scores approach “score zero”.
Histomorphometric analysis
After the end of two-week experimental period, all six rats from each group were anesthetized, surgical access was reopened and left mental nerves were exposed. Then, 7 mm segments of mental nerve including the crush-injury site were harvested. To fix the specimens, harvested segments were immediately placed in 2.5% glutaraldehyde in PBS (pH 7.4) at 4°C and were allowed to sit for one day. The specimens were then post-fixed with 2% osmium tetroxide for two days. Following primary and secondary fixation, the specimens were washed with PBS (pH 7.4), routinely processed, and embedded in Epon812 (Nisshin EM, Tokyo, Japan). Serial transverse micro-thin sections of 0.45-μm thickness were cut with an ultra-microtome (RMC Boeckeler, Tucson, AZ, USA) and stained with 1% toluidine blue for light microscopy examination. Images were captured using SPOT RTTM-KE color mosaic system (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) and digitized by SPOT software (Ver.4.6). To estimate the number of axons, the total cross-sectional area of the nerve was measured at 40× magnification, and three sampling fields were then randomly selected at 200× magnification. Mean fiber density was calculated by dividing the total number of nerve fibers within the sampling field by its area (n/mm2). The counts of myelinated fibers were estimated by multiplying the mean fiber density by the entire cross-sectional area of the nerve, assuming a uniform distribution of nerve fibers across the whole section. The sections were analyzed using a transmission electron microscope (Olympus, BX41, TF, Japan).
Retrograde labeling of trigeminal ganglion (TG) neurons
TG neurons were retrograde-labeled with a fluorescent dye DiI (Molecular Probes, Eugene, OR, USA). At the end of the two-week experimental period, the surfaces of the exposed mental nerves were soaked in a generous amount of 5 μL DiI (Hei et al., 2016). Five days later, the animals were anesthetized and perfused with 4% paraformaldehyde (PFA) solution. Following craniotomy, TG were harvested and fixed overnight in 4% PFA. The fixed TG were immersed in a 20% sucrose solution for four days, embedded in Tissue Tek® specimen matrix (Sakura Finetek USA Inc., Torrance, CA, USA), and frozen in liquid nitrogen. Serial 18-μm longitudinal sections were made at –20°C in a cryostat microtome (Leica CM 3050, Milano, Italy). Sections were observed under a confocal fluorescence laser-scanning fluorescence microscope (CLSM, LSM700, Carl Zeiss) and the number of DiI-labeled axons was compared among specimens. Only DiI-labeled axons with rounded shape were counted.
Statistical analysis
Proliferation assay and mRNA expression data were analyzed using an independent samples t-test while analysis of variance (ANOVA) was done for the sensory test, histomorphometric analysis, and retrograde-labeled TG data using StatView software (version 5.0.1, SAS Institute, Cary, NC, USA). The Mann-Whitney U test was used for nonparametric analysis. Test results are expressed as the mean ± standard deviation, and statistical significance was given to values with P < 0.05.
Results
BMSCs characterization
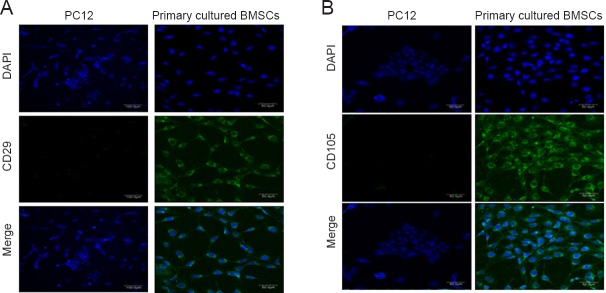
From the study with the fluorescent dye using CD29 and CD105 antibodies, expression was not observed in the negative control (PC12); however, in primary cultured passage 5 MSCs, CD29 and CD105 expression was observed (Figure 3).
Figure 3.
Immunostaining of primary cultured BMSCs with CD29 and CD105.
Primary cultured passage 5 BMSCs expressed positive (green) CD29 (A) and CD105 (B). However, PC12 cells showed no expression. Nuclei were stained with DAPI (blue). Scale bars: 50 μm. BMSCs: Bone marrow-derived mesenchymal stem cells; DAPI: 4′,6-diamidino-2-phenylindole.
Effect of PEMF on cell proliferation
The absorbance value at a wavelength of 450 nm indicates that PMSCs had a slightly, but not significantly, greater proliferation tendency than untreated BMSCs on days 5, 7 and 10 of culture (Figure 4).
Figure 4.
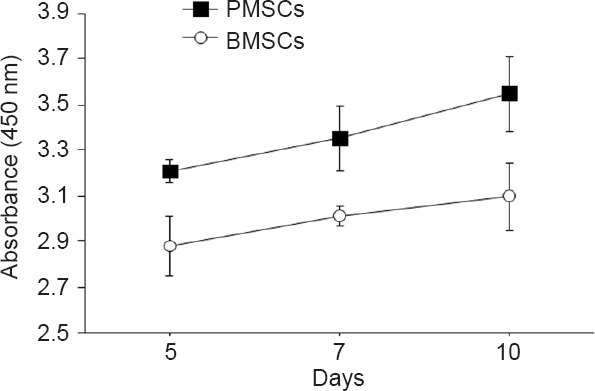
Effect of PEMF pre-treatment on BMSCs proliferation by quantitative DNA assay.
Cell proliferation was measured at 450 nm. PMSCs exhibited a slightly, but not significantly, greater proliferation tendency than untreated BMSCs on days 5, 7 and 10 of treatment. n = 6/group. Data are presented as the mean ± SEM. PEMF: Pulsed electromagnetic field; BMSCs: bone marrow-derived mesenchymal stem cells; PMSCs: BMSCs pretreated with PEMF.
Effect of PEMF on mRNA expression level
mRNA expression levels of BMSCs and PMSCs were compared on days 5, 7, and 10 of culture (Figure 5A), and the results were graphically presented (Figure 5B). S100, GFAP, and NGF mRNA expression levels were higher in PMSCs than in BMSCs on days 5, 7 and 10 of culture (P < 0.001). On day 10 of culture, BDNF expression level in PMSCs was significantly higher than in BMSCs (P < 0.001). Accordingly, an additional experiment was performed in which BMSCs were pre-treated with PEMF for 10 days without interruption.
Figure 5.
Effect of PEMF pre-treatment on mRNA expression in BMSCs as confirmed by reverse transcription-polymerase chain reaction.
(A) mRNA expression bands. mRNA levels of (a) S100, (b) GFAP, (c) NGF, and (d) BDNF were higher in PMSCs than in BMSCs on days 5, 7 and 10 of culture. (B) Graphs of numerical expression of each primer using ImageJ software. mRNA expression levels of (a) S100, (b) GFAP, and (c) NGF were higher in PMSCs than in BMSCs. mRNA expression level of (d) BDNF was significantly higher in PMSCs than in BMSCs (***P < 0.001). The expression level of PMSCs was highest on day 10 of culture (n = 6/group). mRNA expression data were analyzed using independent samples t-test. Data are presented as the mean ± SEM. BMSCs: Bone marrow-derived mesenchymal stem cells; PEMF: pulsed electromagnetic field; PMSCs: BMSCs that were pre-treated with PEMF.
Effect of PEMF on the regeneration of crush-injured mental nerve in rats
The viability of BMSCs in the mental nerve
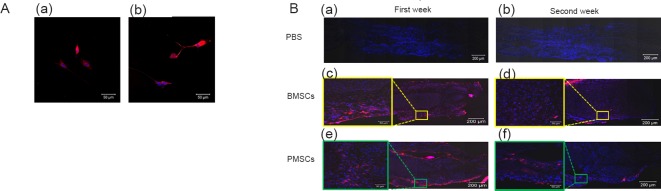
DiI-labeled BMSCs and PMSCs were visible under a fluorescence microscope (Figure 6A); however, they were not visible in PBS-injected negative control group. DiI-labeled BMSCs and PMSCs were observed at both weeks 1 and 2. Week 2 specimens showed decreased labeling in both BMSCs and PMSCs compared to week 1 specimen (Figure 6B).
Figure 6.
Tracking of DiI-labeled BMSCs and PMSCs at 1 and 2 weeks postoperatively.
(A) DiI-labeled (a) BMSCs and (b) PMSCs were observed. Scale bars: 50 μm. (B) DiI-labeled BMSCs (c, d) and PMSCs (e, f) in vivo with negative control PBS injection (a, b) harvested one week (a, c, e) and two weeks after injection (b, d, f). DiI labeling: red; DAPI: blue. Scale bars: 200 μm. Boxed area in the right image was enlarged. Scale bars: 50 μm. DiI: 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanineperchlorate; BMSCs: bone marrow-derived mesenchymal stem cells; PEMF: pulsed electromagnetic field; PMSCs: BMSCs that were pre-treated with PEMF; DAPI: 4′,6-diamidino-2-phenylindole.
Recovery of sensory function after PMSCs treatment
All of the groups exhibited decreased gap score at 1 week with the exception of the sham group. All groups showed some degree of sensory recovery at 2 weeks, and the recovery of sensory function in the PMSCs group was faster than in the BMSCs and PBS groups. The difference score result followed a similar trend as the gap score result. At 1 week, the least increased difference score was observed in the PMSCs group and the greatest increased difference score in the PBS group. At 2 weeks, greater recovery was observed in the PMSCs group than in the BMSCs and PBS groups. However, there was no significant difference in either gap or difference score (Figure 7).
Figure 7.
PMSCs on mental nerve sensory function of rats at 1 week and 2 weeks postoperatively.
The gap score values in all groups decreased in response to the crush injury at one week and began to recover at two weeks. (B) The difference scores of all groups increased due to the crush injury at one week and subsequently decreased at two weeks. The recovery rate was highest in the PMSCs group. But there was no statistical significance in recovery rate between groups (n = 6/group. Data are presented as the mean ± SEM). The difference score was defined as the difference between the mechanical touch thresholds (grams) of the ipsilateral and contralateral sides of the injury and was calculated as the value of the ipsilateral mental area minus the value of the contralateral area. The gap score was defined as the difference between the mechanical touch thresholds of the proximal and distal parts of the mental nerve and was calculated as the value of the ipsilateral lip area minus the value in the vicinity of the mental foramen. By definition, a higher score means poorer recovery. PBS: Phosphate buffered saline; BMSCs: bone marrow-derived mesenchymal stem cells; PMSCs: BMSCs that were pre-treated with pulsed electromagnetic field.
Axonal regeneration
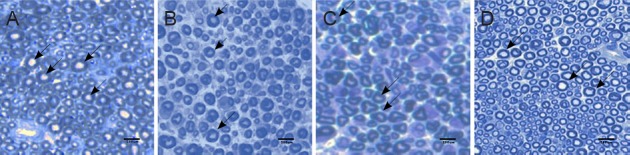
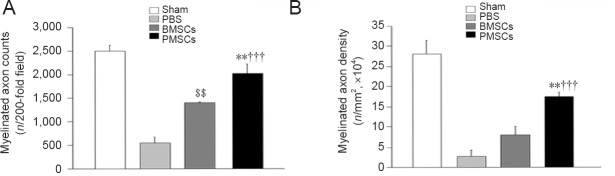
Histomorphological sections (Figure 8) were processed for myelinated axon counts and axon density, and the results are presented in Figure 9. In processing the number of myelinated axons, only axons with round cross-sections were included and counted. The PMSCs group showed a greater number of myelinated axon counts than the PBS group (P < 0.001) or the BMSCs group (P < 0.01). The BMSCs group showed a greater number of myelinated axon counts than the PBS group (P < 0.01). Axon density in the PMSCs group was also significantly higher than that in the PBS group or BMSCs group (P < 0.001 or P < 0.01).
Figure 8.
Photomicrographs of histological features in semi-thin sections of the mental nerve at 2 weeks postoperatively.
The mental nerves of the (A) sham, (B) PBS, (C) BMSCs, and (D) PMSCs groups were harvested at 2 weeks after injury. The myelinated axon (arrows) number and density in the PMSCs group were higher than in the PBS and BMSCs groups. Scale bars: 200 μm. PBS: Phosphate buffered saline; BMSCs: bone marrow-derived mesenchymal stem cells; PMSCs: BMSCs that were pre-treated with pulsed electromagnetic field.
Figure 9.
Effects of PMSCs on axon regeneration in the mental nerve of rats at 2 weeks postoperatively.
(A) Myelinated axon counts and (B) axon density (†††P < 0.001, vs. PBS group; **P < 0.01, vs. BMSCs group; $$P < 0.01, vs. PBS group) were significantly higher in the PMSCs group than in the BMSCs and PBS groups (n = 6/group). Axon count and density were analyzed using analysis of variance. Data are presented as the mean ± SEM. PBS: Phosphate buffered saline; BMSCs: bone marrow-derived mesenchymal stem cells; PMSCs: BMSCs that were pre-treated with pulsed electromagnetic field.
Retrograde labeling of trigeminal ganglion
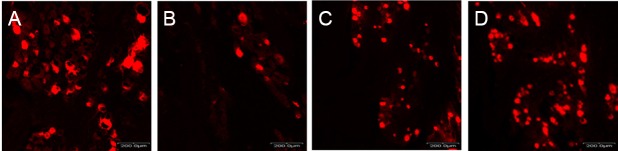
Following retrograde DiI-labeling of TG, only the neurons with round cross-sections were counted. DiI-labeled sections in all groups (Figure 10) were processed, and the results are presented in Figure 11. The PMSCs group showed a significantly greater number of stained TG neurons than the PBS and BMSC groups (P < 0.01 or P < 0.05).
Figure 10.
Retrograde trigeminal ganglion labeling with DiI at 2 weeks postoperatively.
(A) Sham, (B) PBS, (C) BMSCs, and (D) PMSCs groups. The PMSCs group showed more positive trigeminal ganglion neurons (red) than the BMSCs and PBS groups. Scale bars: 200 μm. DiI: 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanineperchlorate; PBS: phosphate buffered saline; BMSCs: bone marrow-derived mesenchymal stem cells; PMSCs: BMSCs that were pre-treated with pulsed electromagnetic field.
Figure 11.
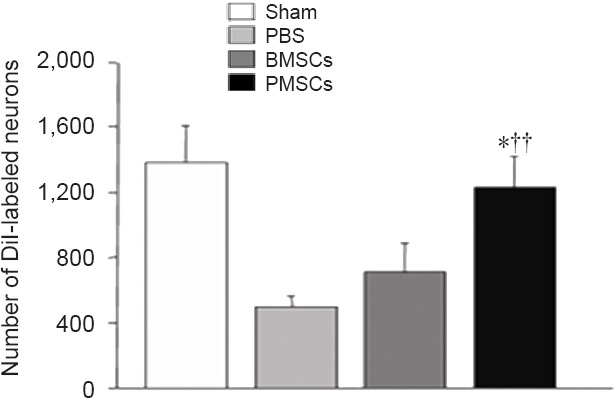
Effects of PMSCs on DiI-labeled neurons in trigeminal ganglion of rats at 2 weeks postoperatively.
There were significantly more DiI-labeled neurons in the PMSCs group than in the BMSCs and PBS groups (††P < 0.01, vs. PBS group; *P < 0.05, vs. BMSCs group) (n = 6/group). The number of retrograde-labeled trigeminal ganglion neurons was analyzed using analysis of variance. Data are presented as the mean ± SEM. DiI: 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanineperchlorate; PBS: phosphate buffered saline; BMSCs: bone marrow-derived mesenchymal stem cells; PMSCs: BMSCs that were pre-treated with pulsed electromagnetic field.
Discussion
MSCs have been shown to enhance injury recovery and improve tissue regeneration through the release of growth factors, cytokines, and adhesion molecules (Pittenger et al., 1999; Bhagavati and Xu, 2004). Although statistically significant difference in sensory recovery was not observed in the present study, the groups injected with MSCs, no matter pretreated with PEMF or not, exhibited faster recovery than the PBS group after mental nerve crush injury.
According to histomorphometric assay, the BMSCs group exhibited a significantly higher myelinated axon count than the PBS group. Regarding axon density, although statistical significance was not observed, a tendency for higher axon density was observed in the BMSCs group than in the PBS group. These in vivo results support that BMSCs promoted the regeneration of injured mental nerves.
PEMF pretreatment of cells reportedly leads to a significant increase in cell number (Sun et al., 2009), induces secretion of RA (Pirozzoli et al., 2003), FSK (Cain et al., 1987), bFGF (Hopper et al., 2009), and PDGF (Dimitriou et al., 2011), increases NGF secretion (Longo et al., 1999), enhances the proliferation of MSCs (Sun et al., 2009; Luo et al., 2012), and promotes neurite outgrowth (Zhang et al., 2006; Lekhraj et al., 2014).
S100 is a calcium-binding protein that stimulates cell proliferation. PEMF affects calcium ion channels in cell membranes (Zienowicz et al., 1991; Grant et al., 1994; Shah et al., 2001) and enhances BDNF expression through an L-type voltage-gated calcium channel and Erk-dependent signaling pathways in neonatal rat DRG neurons (Li et al., 2014). The present study was designed to investigate the effects of PEMF on BMSC proliferation and growth factor release, and evaluate the effects of PMSCs on nerve regeneration of injured mental nerve. Hei et al. (2016) reported that PEMF treatment significantly increased cell proliferation and S100 and BDNF expression. A large number of studies regarding MSCs reported that cell proliferation and growth factor release increased after application of 50 Hz PEMF to cells (Sert et al., 2002; Feng et al., 2011; Li et al., 2012b; Bai et al., 2013). In the present study, PEMF was applied for 5, 7, or 10 days, and highest increases in BMSC proliferation and mRNA expression were observed in the 10-day group, so 10-day PEMF application was chosen. The in vitro results of the present study showed that PMSCs not only promoted cell proliferation and increased S100 and GFAP expression, but also increased the release of growth factors such as NGF and BDNF compared to untreated BMSCs. Studies have reported that the magnitude of effects of PEMF on cell survival, propagation, and peripheral nerve regeneration depend on intensity, time interval, and frequency of PEMF (Sun et al., 2009; Kim et al., 2012). The PEMF device used in the present study could be set at various frequencies (50, 60, 100, or 150 Hz) and binary time settings of 1 or 12 hours. However, the intensity was constant at 1 mT. In order to further optimize PEMF settings for peripheral nerve regeneration, a device with different intensity modes and time settings may help. To further confirm the positive effect of 10-day PEMF treatment on BMSCs, additional evaluations such as western blotting or neurite length measurement may be considered in future studies. After injecting PMSCs into injured mental nerve in rats, a sensory function test and an axonal regeneration assessment were performed. A mental nerve sensory test was performed according to the method reported by Seino et al. (2009). Numerous studies have reported that injured nerves recover between 1 and 2 weeks after injury (Hildebrand et al., 1995; Naftel et al., 1999; Kim et al., 2015; Hei et al., 2016). All of the experimental groups except the sham group showed significantly diminished sensory function of the mental nerve at 1 week post-injury and some degree of recovery at 2 weeks post-injury. The PMSCs group showed faster sensory function recovery than the BMSCs or PBS group; however, the difference was not statistically significant. In the sensory function test using filaments, experimenter bias could not be entirely eliminated as the reaction of the animal is determined by the experimenter's subjective judgment. Moreover, as the test was performed while the experimenter was holding the filaments with their hands, there could have been variations in the uniformity of the pressure that was delivered. In order to minimize bias and error, alternative methods to test sensory nerve function such as electronic von Frey measurements, should be considered. According to histological analysis of other studies, increases in total axon number and axon density are suggestive of axonal nerve regeneration (Hubbard, 1972; Vasconcelos and Gay-Escoda, 2000).
Retrograde labeling with a dye is a well-established method by which sensory neurons can be distinguished from motor neurons. Retrograde nerve labeling also explores the relationship between the spinal cord and peripheral nerves (Yu et al., 2015). To obtain functional recovery after injury, injured peripheral nerve fibers must grow into the correct target organ (Hayashi et al., 2007; Yu et al., 2015). Myelinated axon count and axon density in all experimental groups were lower than those in the sham group. Both PMSCs and BMSC groups showed significantly higher myelinated axon count and axon density compared to PBS group, and the PMSCs group showed significantly higher values than the BMSCs group. Some studies have shown that nerve fiber diameter is the only most reliable parameter for assessing sensory nerve recovery (Fernand and Young, 1951; Donovan, 1967), axonal diameter and the width and length of the myelin sheath are the most relevant histological findings regarding nerve recovery (May and Schaitkin, 2000). Hence, measurement of axon diameter, width, and length of the myelin sheath may be incorporated in future studies for a more thorough evaluation of sensory nerve recovery. In the retrograde DiI labeling of TG, the PMSCs group exhibited significantly higher retrograde tracing compared to the BMSCs and PBS groups.
To conclude, PEMF application in vitro increased not only the growth rate of BMSCs, but also the expression of nerve growth factors. Moreover, when these PMSCs were injected into an injured mental nerve, they were more effective at promoting nerve regeneration than unpretreated BMSCs. This suggests that PEMF pretreatment of BMSCs can be used as an enhanced strategic tool in cell therapy for recovery of injured mental nerves.
Footnotes
Funding: This study was supported by a grant of the Korea Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C1535).
Conflicts of interest: The authors declare no competing financial interests.
Financial support: This study was supported by a grant of the Korea Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HI15C1535). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Research ethics: All animal experiment procedures were performed in accordance with the guidance of the Institute of Laboratory of Animal Resources of Seoul National University, South Korea (SNU-130201-2).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
(Copyedited by Li CH, Song LP, Zhao M)
References
- 1.Aebischer P, Valentini RF, Dario P, Domenici C, Galletti PM. Piezoelectric guidance channels enhance regeneration in the mouse sciatic nerve after axotomy. Brain Res. 1987;436:165–168. doi: 10.1016/0006-8993(87)91570-8. [DOI] [PubMed] [Google Scholar]
- 2.Alrashdan MS, Park JC, Sung MA, Yoo SB, Jahng JW, Lee TH, Kim SJ, Lee JH. Thirty minutes of low intensity electrical stimulation promotes nerve regeneration after sciatic nerve crush injury in a rat model. Acta Neurol Belg. 2010;110:168–179. [PubMed] [Google Scholar]
- 3.Bai WF, Xu WC, Feng Y, Huang H, Li XP, Deng CY, Zhang MS. Fifty-Hertz electromagnetic fields facilitate the induction of rat bone mesenchymal stromal cells to differentiate into functional neurons. Cytotherapy. 2013;15:961–970. doi: 10.1016/j.jcyt.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Bhagavati S, Xu W. Isolation and enrichment of skeletal muscle progenitor cells from mouse bone marrow. Biochem Biophys Res Commun. 2004;318:119–124. doi: 10.1016/j.bbrc.2004.03.192. [DOI] [PubMed] [Google Scholar]
- 5.Boxall SA, Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012;2012:975871. doi: 10.1155/2012/975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunge RP. The role of the Schwann cell in trophic support and regeneration. J Neurol. 1994;242:S19–21. doi: 10.1007/BF00939235. [DOI] [PubMed] [Google Scholar]
- 7.Cain CD, Adey WR, Luben RA. Evidence that pulsed electromagnetic fields inhibit coupling of adenylate cyclase by parathyroid hormone in bone cells. J Bone Miner Res. 1987;2:437–441. doi: 10.1002/jbmr.5650020511. [DOI] [PubMed] [Google Scholar]
- 8.Chernousov MA, Carey DJ. Schwann cell extracellular matrix molecules and their receptors. Histol Histopathol. 2000;15:593–601. doi: 10.14670/HH-15.593. [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Seo YK, Yoon HH, Kim SC, Kim SM, Song KY, Park JK. Neural stimulation on human bone marrow-derived mesenchymal stem cells by extremely low frequency electromagnetic fields. Biotechnol Prog. 2012;28:1329–1335. doi: 10.1002/btpr.1607. [DOI] [PubMed] [Google Scholar]
- 10.Cooney DS, Wimmers EG, Ibrahim Z, Grahammer J, Christensen JM, Brat GA, Wu LW, Sarhane KA, Lopez J, Wallner C, Furtmuller GJ, Yuan N, Pang J, Sarkar K, Lee WP, Brandacher G. Mesenchymal stem cells enhance nerve regeneration in a rat sciatic nerve repair and hindlimb transplant model. Sci Rep. 2016;6:31306. doi: 10.1038/srep31306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai LG, Huang GS, Hsu SH. Sciatic nerve regeneration by cocultured Schwann cells and stem cells on microporous nerve conduits. Cell Transplant. 2013;22:2029–2039. doi: 10.3727/096368912X658953. [DOI] [PubMed] [Google Scholar]
- 12.Deng W, Bivalacqua TJ, Chattergoon NN, Jeter JR, Jr, Kadowitz PJ. Engineering ex vivo-expanded marrow stromal cells to secrete calcitonin gene-related peptide using adenoviral vector. Stem Cells. 2004;22:1279–1291. doi: 10.1634/stemcells.2004-0032. [DOI] [PubMed] [Google Scholar]
- 13.Deng W, Bivalacqua TJ, Chattergoon NN, Hyman AL, Jeter JR, Jr, Kadowitz PJ. Adenoviral gene transfer of eNOS: high-level expression in ex vivo expanded marrow stromal cells. Am J Physiol Cell Physiol. 2003;285:C1322–1329. doi: 10.1152/ajpcell.00141.2003. [DOI] [PubMed] [Google Scholar]
- 14.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donovan A. The nerve fibre composition of the cat optic nerve. J Anat. 1967;101:1–11. [PMC free article] [PubMed] [Google Scholar]
- 16.Feng X, He X, Li K, Wu W, Liu X, Li L. The effects of pulsed electromagnetic fields on the induction of rat bone marrow mesenchymal stem cells to differentiate into cardiomyocytes-like cells in vitro. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2011;28:676–682. [PubMed] [Google Scholar]
- 17.Fernand V, Young J. The sizes of the nerve fibres of muscle nerves. Proc R Soc Lond B Biol Sci. 1951;139:38–58. doi: 10.1098/rspb.1951.0045. [DOI] [PubMed] [Google Scholar]
- 18.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg JL, Barres BA. The relationship between neuronal survival and regeneration. Annu Rev Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- 20.Grant G, Cadossi R, Steinberg G. Protection against focal cerebral ischemia following exposure to a pulsed electromagnetic field. Bioelectromagnetics. 1994;15:205–216. doi: 10.1002/bem.2250150305. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi A, Moradzadeh A, Hunter DA, Kawamura DH, Puppala VK, Tung TH, Mackinnon SE, Myckatyn TM. Retrograde labeling in peripheral nerve research: it is not all black and white. J Reconstr Microsurg. 2007;23:381–389. doi: 10.1055/s-2007-992344. [DOI] [PubMed] [Google Scholar]
- 22.Hei WH, Byun SH, Kim JS, Kim S, Seo YK, Park JC, Kim SM, Jahng JW, Lee JH. Effects of electromagnetic field (PEMF) exposure at different frequency and duration on the peripheral nerve regeneration: in vitro and in vivo study. Int J Neurosci. 2016;126:739–748. doi: 10.3109/00207454.2015.1054032. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrand C, Fried K, Tuisku F, Johansson CS. Teeth and tooth nerves. Prog Neurobiol. 1995;45:165–222. doi: 10.1016/0301-0082(94)00045-j. [DOI] [PubMed] [Google Scholar]
- 24.Hopper RA, VerHalen JP, Tepper O, Mehrara BJ, Detch R, Chang EI, Baharestani S, Simon BJ, Gurtner GC. Osteoblasts stimulated with pulsed electromagnetic fields increase HUVEC proliferation via a VEGF-A independent mechanism. Bioelectromagnetics. 2009;30:189–197. doi: 10.1002/bem.20459. [DOI] [PubMed] [Google Scholar]
- 25.Hubbard JH. The quality of nerve regeneration. Factors independent of the most skillful repair. Surg Clin North Am. 1972;52:1099–1108. doi: 10.1016/s0039-6109(16)39829-2. [DOI] [PubMed] [Google Scholar]
- 26.Ide C. Peripheral nerve regeneration. Neurosci Res. 1996;25:101–121. doi: 10.1016/0168-0102(96)01042-5. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H, Qu W, Han F, Liu D, Zhang W. Establishment of immortalized Schwann cells derived from rat embryo dorsal root ganglia. Int J Mol Med. 2012;30:480–486. doi: 10.3892/ijmm.2012.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashani IR, Golipoor Z, Akbari M, Mahmoudi R, Azari S, Shirazi R, Bayat M, Ghasemi S. Schwann-like cell differentiation from rat bone marrow stem cells. Arch Med Sci. 2011;7:45–52. doi: 10.5114/aoms.2011.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilmer SL, Carlsen RC. Chronic infusion of agents that increase cyclic AMP concentration enhances the regeneration of mammalian peripheral nerves in vivo. Exp Neurol. 1987;95:357–367. doi: 10.1016/0014-4886(87)90144-0. [DOI] [PubMed] [Google Scholar]
- 30.Kim BC, Bae H, Kwon IK, Lee EJ, Park JH, Khademhosseini A, Hwang YS. Osteoblastic/cementoblastic and neural differentiation of dental stem cells and their applications to tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2012;18:235–244. doi: 10.1089/ten.teb.2011.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YT, Hei WH, Kim S, Seo YK, Kim SM, Jahng JW, Lee JH. Co-treatment effect of pulsed electromagnetic field (PEMF) with human dental pulp stromal cells and FK506 on the regeneration of crush injured rat sciatic nerve. Int J Neurosci. 2015;125:774–783. doi: 10.3109/00207454.2014.971121. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi M, Ishibashi S, Tomimitsu H, Yokota T, Mizusawa H. Proliferating immature Schwann cells contribute to nerve regeneration after ischemic peripheral nerve injury. J Neuropathol Exp Neurol. 2012;71:511–519. doi: 10.1097/NEN.0b013e318257fe7b. [DOI] [PubMed] [Google Scholar]
- 33.Koh KS, Oh TS, Kim H, Chung IW, Lee KW, Lee HB, Park EJ, Jung JS, Shin IS, Ra JC, Choi JW. Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann Plast Surg. 2012;69:331–337. doi: 10.1097/SAP.0b013e31826239f0. [DOI] [PubMed] [Google Scholar]
- 34.Lacy-Hulbert A, Metcalfe JC, Hesketh R. Biological responses to electromagnetic fields. FASEB J. 1998;12:395–420. doi: 10.1096/fasebj.12.6.395. [DOI] [PubMed] [Google Scholar]
- 35.Lee DS, Park JT, Kim HM, Ko JS, Son HH, Gronostajski RM, Cho MI, Choung PH, Park JC. Nuclear factor I-C is essential for odontogenic cell proliferation and odontoblast differentiation during tooth root development. J Biol Chem. 2009;284:17293–17303. doi: 10.1074/jbc.M109.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Lekhraj R, Cynamon DE, DeLuca SE, Taub ES, Pilla AA, Casper D. Pulsed electromagnetic fields potentiate neurite outgrowth in the dopaminergic MN9D cell line. J Neurosci Res. 2014;92:761–771. doi: 10.1002/jnr.23361. [DOI] [PubMed] [Google Scholar]
- 38.Li BH, Kim SM, Yoo SB, Kim MJ, Jahng JW, Lee JH. Recombinant human nerve growth factor (rhNGF-beta) gene transfer promotes regeneration of crush-injured mental nerve in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012a;113:e26–34. doi: 10.1016/j.tripleo.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Zhang M, Bai L, Bai W, Xu W, Zhu H. Effects of 50 Hz pulsed electromagnetic fields on the growth and cell cycle arrest of mesenchymal stem cells: an in vitro study. Electromagn Biol Med. 2012b;31:356–364. doi: 10.3109/15368378.2012.662194. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Yan X, Liu J, Li L, Hu X, Sun H, Tian J. Pulsed electromagnetic field enhances brain-derived neurotrophic factor expression through L-type voltage-gated calcium channel-and Erk-dependent signaling pathways in neonatal rat dorsal root ganglion neurons. Neurochem Int. 2014;75:96–104. doi: 10.1016/j.neuint.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Longo FM, Yang T, Hamilton S, Hyde JF, Walker J, Jennes L, Stach R, Sisken BF. Electromagnetic fields influence NGF activity and levels following sciatic nerve transection. J Neurosci Res. 1999;55:230–237. doi: 10.1002/(SICI)1097-4547(19990115)55:2<230::AID-JNR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 42.Lonze BE, Riccio A, Cohen S, Ginty DD. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- 43.Luo F, Hou T, Zhang Z, Xie Z, Wu X, Xu J. Effects of pulsed electromagnetic field frequencies on the osteogenic differentiation of human mesenchymal stem cells. Orthopedics. 2012;35:e526–531. doi: 10.3928/01477447-20120327-11. [DOI] [PubMed] [Google Scholar]
- 44.May M, Schaitkin BM. second edition. George: Thieme Verlag; The facial nerve: May's. [Google Scholar]
- 45.Mimura T, Dezawa M, Kanno H, Sawada H, Yamamoto I. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J Neurosurg. 2004;101:806–812. doi: 10.3171/jns.2004.101.5.0806. [DOI] [PubMed] [Google Scholar]
- 46.Mosahebi A, Fuller P, Wiberg M, Terenghi G. Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol. 2002;173:213–223. doi: 10.1006/exnr.2001.7846. [DOI] [PubMed] [Google Scholar]
- 47.Mosahebi A, Woodward B, Wiberg M, Martin R, Terenghi G. Retroviral labeling of Schwann cells: in vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia. 2001;34:8–17. doi: 10.1002/glia.1035. [DOI] [PubMed] [Google Scholar]
- 48.Naftel JP, Richards LP, Pan M, Bernanke JM. Course and composition of the nerves that supply the mandibular teeth of the rat. Anat Rec. 1999;256:433–447. doi: 10.1002/(SICI)1097-0185(19991201)256:4<433::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 49.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pirozzoli MC, Marino C, Lovisolo GA, Laconi C, Mosiello L, Negroni A. Effects of 50 Hz electromagnetic field exposure on apoptosis and differentiation in a neuroblastoma cell line. Bioelectromagnetics. 2003;24:510–516. doi: 10.1002/bem.10130. [DOI] [PubMed] [Google Scholar]
- 51.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 52.Scherer SS. The biology and pathobiology of Schwann cells. Curr Opin Neurol. 1997;10:386–397. doi: 10.1097/00019052-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Seino H, Seo K, Maeda T, Someya G. Behavioural and histological observations of sensory impairment caused by tight ligation of the trigeminal nerve in mice. J Neurosci Methods. 2009;181:67–72. doi: 10.1016/j.jneumeth.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 54.Sert C, Mustafa D, Duz MZ, Aksen F, Kaya A. The preventive effect on bone loss of 50-Hz, 1-mT electromagnetic field in ovariectomized rats. J Bone Miner Metab. 2002;20:345–349. doi: 10.1007/s007740200050. [DOI] [PubMed] [Google Scholar]
- 55.Shah J, Midkiff P, Brandt P, Sisken B. Growth and differentiation of PC6 cells: the effects of pulsed electromagnetic fields (PEMF) Bioelectromagnetics. 2001;22:267–271. doi: 10.1002/bem.49. [DOI] [PubMed] [Google Scholar]
- 56.Sun LY, Hsieh DK, Yu TC, Chiu HT, Lu SF, Luo GH, Kuo TK, Lee OK, Chiou TW. Effect of pulsed electromagnetic field on the proliferation and differentiation potential of human bone marrow mesenchymal stem cells. Bioelectromagnetics. 2009;30:251–260. doi: 10.1002/bem.20472. [DOI] [PubMed] [Google Scholar]
- 57.Sung MA, Jung HJ, Lee JW, Lee JY, Pang KM, Yoo SB, Alrashdan MS, Kim SM, Jahng JW, Lee JH. Human umbilical cord blood-derived mesenchymal stem cells promote regeneration of crush-injured rat sciatic nerves. Neural Regen Res. 2012;7:2018–2027. doi: 10.3969/j.issn.1673-5374.2012.26.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362:200–203. doi: 10.1016/j.neulet.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 59.Vasconcelos BC, Gay-Escoda C. Facial nerve repair with expanded polytetrafluoroethylene and collagen conduits: an experimental study in the rabbit. J Oral Maxillofac Surg. 2000;58:1257–1262. doi: 10.1053/joms.2000.16626. [DOI] [PubMed] [Google Scholar]
- 60.Yu YL, Li HY, Zhang PX, Yin XF, Han N, Kou YH, Jiang BG. Comparison of commonly used retrograde tracers in rat spinal motor neurons. Neural Regen Res. 2015;10:1700–1705. doi: 10.4103/1673-5374.167772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Ding J, Duan W. A study of the effects of flux density and frequency of pulsed electromagnetic field on neurite outgrowth in PC12 cells. J Biol Phys. 2006;32:1–9. doi: 10.1007/s10867-006-6901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zienowicz RJ, Thomas BA, Kurtz WH, Orgel MG. A multivariate approach to the treatment of peripheral nerve transection injury: the role of electromagnetic field therapy. Plast Reconstr Surg. 1991;87:122–129. doi: 10.1097/00006534-199101000-00019. [DOI] [PubMed] [Google Scholar]