Abstract
Dipeptidyl peptidase IV (DPP-IV) is a serine protease best known for its role in inactivating glucagon-like peptide-1 (GLP-1), pituitary adenylate cyclase-activating polypeptide (PACAP) and glucose-dependent insulinotropic peptide (GIP), three stimulators of pancreatic insulin secretion with beneficial effects on glucose disposal. Owing to the relationship between DPP-IV and these peptides, inhibition of DPP-IV enzyme activity is considered as an attractive treatment option for diabetic patients. Nonetheless, increasing studies support the idea that DPP-IV might also be involved in the development of neurological disorders with a neuroinflammatory component, potentially through its non-incretin activities on immune cells. In this review article, we aim at highlighting recent literature describing the therapeutic value of DPP-IV inhibitors for the treatment of such neurological conditions. Finally, we will illustrate some of the promising results obtained using berberine, a plant extract with potent inhibitory activity on DPP-IV.
Keywords: neurodegeneration, inflammation, immune system, insulin, diabetes, alkaloids, berberine
Introduction
Dipeptidyl peptidase-IV (DPP-IV) (aka adenosine deaminase complexing protein 2 or T-cell activation antigen CD26) is a serine exopeptidase widely expressed throughout the body. DPP-IV is usually found tethered to the intravascular portion of vascular endothelial cells (where it is less active) but also exists in a soluble fully active circulating form (Mentlein et al., 1993; Mentlein, 1999; Omar and Ahrén, 2014). It belongs to the S9B protein family that acts on the N-terminus of the X-proline dipeptides, including chemokines, neuropeptides, and peptide hormones. Beside the intravascular compartment, DPP-IV is expressed on the surface of several types of cells in the form of a type II transmembrane glycoprotein.
One of the main known functions of the DPP-IV is to deactivate incretins. The enzyme is strongly implicated in the inhibition of the biological activity of glucose-regulating hormones, such as glucagon-like peptide-1 (GLP-1), pituitary adenylate cyclase-activating polypeptide (PACAP) and glucose-dependent insulinotropic polypeptide (GIP) (Zhu et al., 2003; Matteucci and Giampietro, 2009; Marzagalli et al., 2015). DPP-IV is also broadly distributed in numerous organs/tissues such as the liver, lungs, intestinal epithelium, placenta, kidney, renal proximal tubules and neurons, as well as in bodily fluids such as in seminal and synovial fluids, urine, plasma and the cerebrospinal fluid (Green et al., 2006a; Kosaraju et al., 2013a). Aside from its canonical role in glucose metabolism, DPP-IV is also largely involved in regulating various non-incretin related processes in the body, including inflammatory responses, neurophysiological and neuroendocrine functions (Aertgeerts et al., 2004). DPP-IV, through its enzymatic activity, causes the degradation of several cytokines, chemokines and neuropeptides regulating inflammation, immunity, and vascular function that are normally released in the blood stream (Fadini and Avogaro, 2011). In addition, DPP-IV is thought to take part in the control of the maturation and phenotypic differentiation of T-lymphocytes, since activated T-cells are DPP-IV+, making it a particularly attractive target for studies on immune system reactivity.
From a biochemical point of view, DPP-IV cleavage of substrates occurs at the N-terminus of X-proline dipeptides. An example of such cleavage is that observed with GLP-1. DPP-IV enzymatically cleaves GLP-1 at the amino acid ‘Alanine’ located in the second position, converting the intact GLP-1 (7–36) amide peptide into the GLP-1 (9–36) amide fragment (missing the first two amino acids), which is then biologically inactive (as depicted in Figure 1). Inactivation of GLP-1 by DPP-IV is rapid and extensive; for this reason DPP-IV inhibition has been estimated to significantly increase GLP-1 bioavailability and concentration, especially in the peripheral venous plasma and in portal blood (Hjøllund et al., 2011; Nagatsu, 2017). Interestingly, DPP-IV-mediated enzymatic inactivation has been described for at least two further relevant circulating bioactive peptides involved in glucose metabolisms, among other functions: PACAP and GIP.
Figure 1.

Amino acid structure of glucagon-like peptide-1 (GLP-1) and dipeptidyl peptidase IV (DPP-IV) cleavage site.
As illustrated by Green and co-workers, inactivation of circulating PACAP by DPP-IV occurs rapidly (Green et al., 2006b). Combined genetic and tandem mass spectrometry studies have demonstrated that the levels of PACAP clearance is significantly lower in mice deficient for the DPP-IV gene, due to abolished enzymatic activity at the N-terminal portion of the peptide (Zhu et al., 2003; Ma et al., 2015). In support of these findings, a research group has shown that the administration of DPP-IV inhibitors in mice improved the insulinotropic effects of PACAP and improved their long-term glycaemic profile (Ahrén and Hughes, 2005). Taken together, these findings suggest that the effects of DPP-IV inhibitors extend circulating PACAP half-life, hence its peripheral and central nervous system (CNS) bioavailability (Yada et al., 2000; Ahrén and Hughes, 2005; Omar and Ahrén, 2014).
Glucose-dependent insulinotropic polypeptide (GIP) is another incretin molecule functionally inactivated by the endogenous DPP-IV. Interestingly, studies addressing the function of DPP-IV inhibitors have demonstrated that GIP levels are increased much more than GLP-1 levels following inhibitor treatment, suggesting that GIP might be a preferential target of DPP-IV compared to GLP-1 and PACAP (Yabe et al., 2017; Yanagimachi et al., 2017).
DPP-IV Inhibition – Overview of the Direct and Indirect Effects
In this section, we will attempt to summarise the modes of action through which DPP-IV inhibitors elicit anti-inflammatory and neuroprotective functions, with specific emphasis on the benefits arising from their use in the treatment of chronic conditions such as diabetes mellitus. Of note, the indirect effects triggered by DPP-IV inhibition emerge as the predominant therapeutic mechanisms observed with respect to the CNS.
Direct effects
The positive effects of DPP-IV inhibition are believed to correlate, at least in part, with its direct modulatory activities on those molecular pathways driving the inflammatory response, generally defined as ‘the inflammasome’. In animal models of diabetes, there is evidence showing that treatment with DPP-IV inhibitors cause a reduction in retinopathy, with a decrease in the local release pro-inflammatory cytokines and lower interleukin-1β immunoreactivity, particularly in the ganglion cell layer and inner plexiform layer of the retina (Zhang et al., 2011). In clinical studies, DPP-IV inhibitors have been reported to diminish retinal thinning, lower the ratio of anti-inflammatory/pro-inflammatory cytokines and the apoptotic cascade in diabetic patients. These properties of DPP-IV inhibition have been corroborated by pre-clinical evidence in type 1 diabetic rats, where enzyme inhibition also prevented blood-retinal barrier breakdown, increased the survival rates of retinal neurons and reduced the overall inflammatory burden (Gonçalves et al., 2014). Sitagliptin, a FDA approved oral antihyperglycemic drug of the DPP-IV inhibitor class, has shown to reduce proliferative micro-angiopathy in the diabetic retina by preventing the increase of ICAM-1 protein (intercellular adhesion molecule-1, an endothelial adhesion molecule commonly induced in diabetes) and by reducing the expression of the pro-apoptotic BAX gene when intravitreally injected (Zhang et al., 2011). Additional studies investigating the role of DPP-IV inhibition in the context of immune regulation have strongly supported its anti-inflammatory function. Shirakawa and colleagues reported that DPP-IV inhibitors modulate immune cells activation in the adipose tissue (Shirakawa et al., 2011), while others suggested that blockade of DPP-IV activity may significantly reduce the expression of the macrophage marker F4/80 and their activation (Klein et al., 2014). These results confirm that blockade of DPP-IV may have direct immunosuppressive effects, providing interesting insights for the future therapeutic development of treatments neurological conditions with recognizable immune-related dysfunctions.
Indirect effects
GLP-1 and GLP-2 are two main targets of DPP-IV. Both molecules are abundant in the brain, along with their related receptors GLP-1R and GLP-2R (Lovshin et al., 2001). Upon inhibition of DPP-IV, GLP-1 and -2 are spared from enzymatic cleavage and therefore can exert their biological functions. Active GLP-1 and GLP-2, bind to the G-protein coupled receptor GLP-1R to increase intracellular cyclic adenosine monophosphate (cAMP). This leads to activation of protein kinase A (PKA), which phosphorylates and activates the PI3K-Akt and MAPK pathways, two downstream signalling cascades involved in promoting protein synthesis, axonal growth, mitochondrial function, inhibition of apoptosis and attenuation of the neuroinflammatory response (Baggio and Drucker, 2007; Flock et al., 2007). To date, GLP-1 neurotrophic and anti-apoptotic activities have been demonstrated in different neuronal cell lineages. GLP-1 facilitates differentiation and induces neurite outgrowth in PC12 neuronal cells, and protects rat hippocampal neurons from apoptosis (Brubaker and Drucker, 2004). These findings have led to the idea that GLP-1 might be useful for the treatment of Alzheimer's and other neurodegenerative diseases (Brubaker and Drucker, 2004; Li et al., 2010). In line with GLP-1 studies, researchers have found that GLP-2, (another target of DPP-IV), is also capable to protect the CNS from excitotoxic insults. GLP-2 stimulates the proliferation of rat astrocytes in vitro (Velázquez et al., 2003) and reduces the extent of glutamate-induced cytotoxicity in cultured murine hippocampal cells (Lovshin et al., 2004), supporting an additional role of the DPP-IV inhibition in boosting GLP-2 protective activity.
In mice deficient for the GLP-1R, a study has demonstrated that these animals exhibit learning deficits and enhanced susceptibility to neural injury following kainate administration (During et al., 2003). GLP-1R agonist administration to wild-type but not to knockout animals prevented kainate-induced apoptosis, suggesting that direct targeting of GLP-1R or the use of drugs aimed at extending GLP-1 half-life could indeed be effective for the treatment of neurodegenerative diseases (During et al., 2003; Bae, 2016).
Further, some evidences propose that the effects of DPP-IV inhibition on the GLP-1/GLP-1R signalling cascade could also be beneficial in improving late complications of diabetes in animal models, including peripheral neuropathy (Jin et al., 2009). In uncontrolled diabetic patients, DPP-IV inhibition by sitagliptin reduced the levels of C-reactive proteins in the blood of diabetic patients, and significantly increased the amount of circulating CD34+ cells (i.e., a marker of endothelial progenitor cells), suggesting an additional ameliorative role in the vascular compartment (Matsubara et al., 2013; Nakamura et al., 2014).
DPP-IV Inhibition in Neurological Disorders
Since the discovery of neurotrophic and immune regulating functions of DPP-IV inhibitors in the CNS, more and more interest has been given to this class of drugs for the management of neuroinflammatory/neurodegenerative disorders; evidence from basic and pre-clinical research has now demonstrated that this has become more than just a forthright association. Below we will discuss current experimental evidences in support of the use of DPP-IV inhibitors in the context of the major chronic progressive neurodegenerative conditions where overt signs of neuroinflammation have been reported, such as Parkinson's disease (PD), Alzheimer's disease (AD) and multiple sclerosis (MS).
DPP-IV inhibition in PD
PD is a multifactorial neurodegenerative disease considered to be the result of both environmental and genetic factors (Trinh and Farrer, 2013). Despite the efforts, to date the exact pathogenesis of PD is not completely understood. The main pathological features of PD are the massive cell loss of dopaminergic neurons in the substantia nigra pars compacta (SnPc) (Davie, 2008) and the presence of Lewy bodies (accumulations of the protein alpha-synuclein) in the surrounding neurons. By the time neuronal loss becomes obvious, astrocytes' death and signs of chronic microgliosis are already established (Kim et al., 2013), suggesting that inflammation might precede or even initiate the condition.
Molecular genetics and cell biology have identified links between PD and type 2 diabetes mellitus (T2DM). A number of discoveries have highlighted the existence of common cellular pathways that correlate neurodegenerative processes with abnormal mitochondrial function and abnormal glucose metabolism. In this context, the relationship between DPP-IV inhibitors and PD has proven to be mostly indirect, but has significantly contributed to provide the scientific bases for the discovery of a new synthetic analogues of GLP-1. One such analogue, known as exenatide, exhibits potent GLP-1R agonist activities, and available data seems to point to both its robust anti-inflammatory functions (Huang et al., 2012; Gullo et al., 2017) and its role in stimulating neurogenesis (Hunter and Hölscher, 2012; Gumuslu et al., 2016). Moreover, a very recent clinical trial testing exenatide in PD patients has proven to be promising, with significant improvements in motor scores, which were sustained beyond the period of drug exposure (Athauda et al., 2017).
A growing number of studies show that GLP-1 mimetics can act as a neurotrophic factors (Perry et al., 2002), enhance mitochondrial biogenesis (An et al., 2015), inhibit apoptosis (Li et al., 2016), reduce the inflammatory response and oxidative stress (Jalewa et al., 2016), all elements that have contributed to establish the beneficial properties of GLP-1 agonists across a range of experimental models of PD (Athauda and Foltynie, 2016). In fact, due to the number of cellular processes GLP-1R modulates, it is perhaps not unsurprising that GLP-1 stimulation can rescue functions that become disrupted in PD (Baggio and Drucker, 2007).
Recently, a population-based case control study found a significantly reduced incidence of PD among individuals with a record of DPP-IV use. Svenningsson and colleagues conducted a nationwide case-control study in a Swedish cohort receiving DPP-IV inhibitors-based therapy. The authors suggested the beneficial effects might not only be a consequence of the increase in GLP-1 → GLP-1R interaction, but also to the positive contribution provided by other enzymatic substrates spared from DPP-IV cleavage, such as PACAP, substance P, neuropeptide Y and gastrin-releasing peptide (Matteucci and Giampietro, 2015), as well as their inhibitory activities on T cells proliferation and cytokine release (Yazbeck et al., 2009; Svenningsson et al., 2016).
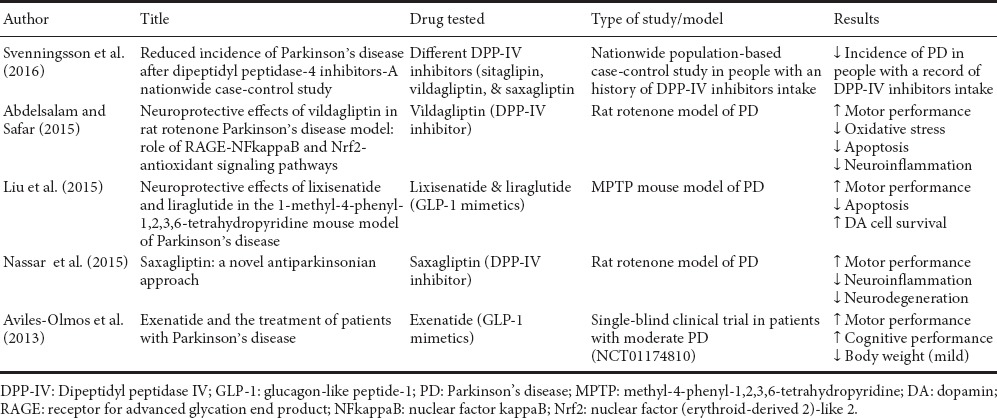
In a rotenone environmental model of PD, rats receiving pre-treatment with a DPP-IV inhibitor (vildagliptin) for a week exhibited resilience to dopaminergic cell loss in the SnPc and striatal terminals and increased dopamine synthesis compared to the untreated controls (Abdelsalam and Safar, 2015). Interestingly, similar results were described by Nassar and co-workers using another DPP-IV inhibitor (saxagliptin) (Nassar et al., 2015). These authors also found that saxagliptin treatment decreased the rotenone-induced nuclear factor-κκ, inducible nitric oxide synthase, tumor necrosis factor-α, ICAM-1 and myeloperoxidase levels, all signatures of ongoing inflammation. The antiapoptotic marker B-cell lymphoma-2 and brain derived neurotrophic factor levels were both enhanced by saxagliptin and reductions in caspase-3 and its intrinsic apoptotic activator cytochrome C were also observed (Nassar et al., 2015). In conclusion, there is ample data to support the idea that DPP-IV inhibitors and GLP-1 mimetics might exert beneficial roles in PD (summarized in Table 1), although more insights on the specific modes of action and targeted substrates are needed to address the safety profile of these drugs in PD and/or for the identification of unwanted off-targets. A good idea could be to test the most promising DPP-IV blocking compounds/GLP-1 agonists in additional well-established animal models of PD, in order to verify their exact impact on disease progression in the context of the complex pathological domains activated by this devastating disease.
Table 1.
Summary of recent literature addressing the beneficial effects of DPP-IV inhibitors/GLP-1 mimetics in PD
DPP-IV inhibition in AD
AD is a chronic neurodegenerative disease characterised by a slow and relentless pathological progression and is recognised as the cause of 60% to 70% of the total cases of dementia (Melnikova, 2007). The exact causes of AD are not fully established, and beside the several attempts to provide a viable explanation for the pivotal pathophysiological mechanisms leading to AD, the amyloid hypothesis remains the most accredited. But what initiates the amyloidogenic cascade? As reported by Heneka and collaborators, aberrant immune activation in the brain of AD patients might play an important role (Heneka et al., 2015), despite there is no sufficient evidence to claim whether immune activation is the real initiator or it is secondary to tissue damage. Instead, of particular interest have been the commonalities found between AD and T2DM. Indeed, both pathologies include impaired neuronal insulin signalling and endoplasmic reticulum (ER) stress, but also inflammation and mitochondrial dysfunction (Vieira et al., 2017). At the biochemical level, both T2DM and AD share features like amyloid beta (Aβ) aggregation, increased glycogen synthase kinase-3 (GSK-3) activity, deregulated protein phosphorylation, aging-related processes, high cholesterol levels, metabolic disorders, blood vessel abnormalities, increased oxidative stress, increased inflammatory response, correlation with apolipoprotein E ε4 allele and glyceraldehyde-derived advanced glycation end-products, but also impaired neurotrophic signalling (Ahrén and Hughes, 2005; Li and Hölscher, 2007). In regards to the latter point, the naturally-occurring peptide PACAP acts as a neurotrophic factor both in the pancreas and brain, and has shown partial efficacy both in T2DM and AD (Rat et al., 2011; Marzagalli et al., 2015), which unfortunately, is strongly limited by DPP-IV proteolytic activity. In addition, T2DM is considered a high risk factor for AD (Butterfield et al., 2014; Wijesekara et al., 2017). These similarities may justify the attempts currently being made to identify a common strategy for intervention for both conditions. Current efforts have been focused on tackling DPP-IV in the attempt to improve glycaemic control. Unfortunately, the mechanisms underlying the neuroprotective effects of DPP-IV inhibitors in AD are not entirely clear yet. Nonetheless, as a main target of DPP-IV inhibition, the indirect activation and potentiation of the GLP-1 receptor signalling are thought to be the leading actors in DPP-IV driven disease-modifying activities, along with some neurotrophic and immune regulatory activities triggered by direct enzyme inhibition (Lin and Huang, 2016) (Table 2).
Table 2.
Summary of recent literature addressing the beneficial effects of DPP-IV inhibitors/GLP-1 mimetics in AD
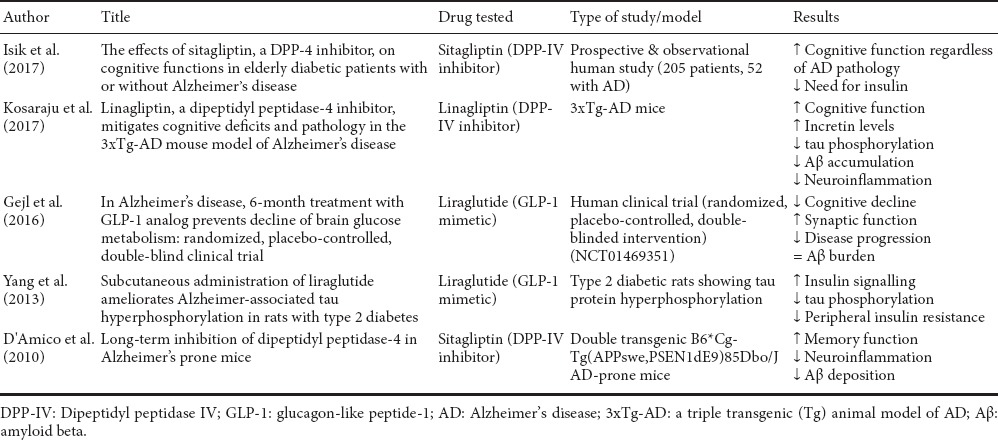
In human neuronal cells, the DPP-IV inhibitor linagliptin has demonstrated to protect neurons against amyloid beta (Aβ)-induced cytotoxicity and prevented the activation of glycogen synthase kinase 3β (GSK3β) and tau hyperphosphorylation by restoring insulin downstream signalling (disrupted in AD). Furthermore, linagliptin alleviated Aβ-induced mitochondrial dysfunction and intracellular reactive oxygen species (ROS) generation, probably by a mechanism involving the activation of 5′ AMP-activated protein kinase (AMPK)-Sirt1 signalling pathway (Kornelius et al., 2015). In vivo, the efficacy of these compounds has been investigated in streptozotocin-induced AD mice models. Researches have highlighted how treatment with saxagliptin elevated hippocampal GLP-1 levels, increased the Aβ and tau protein clearance rate, and improved the global neuroinflammatory profile (Kosaraju et al., 2013a, b). More recently, the same research group reported that linagliptin was effective to increase brain incretin levels in a triple transgenic (Tg) animal model of AD (3xTg-AD), whilst remarkably dampening amyloid burden, tau phosphorylation, and neuroinflammation (Kosaraju et al., 2017). Interestingly, similar outcomes had already been demonstrated in a Tg mouse model of AD administered with sitagliptin, another DPP-IV inhibitor with similar efficacy as saxagliptin (Gerrald et al., 2012). Chronic administration of sitagliptin in Tg AD mice was associated with increased levels of brain GLP-1 and dose dependent reductions in inflammatory biomarkers, amyloid precursor protein levels and Aβ deposition (D'Amico et al., 2010). Interestingly, in a very recent clinical study involving diabetic patients either diagnosed or not with cognitive impairment, administration of sitagliptin increased glycemic control and significantly improved cognitive performance (Isik et al., 2017).
A conceivable connection between AD and the ameliorative effects of these compounds (linagliptin, saxagliptin and sitagliptin, respectively) seems to be related to their ability to rescue the insulin cascade. In fact, as elegantly reported by De Felice et al. (2014), insulin resistance is believed to be the main pathophysiological link between AD and T2DM. Brain insulin signalling has been reported to decline with age (Cole and Frautschy, 2007), the major risk factor for AD, suggesting that restoring insulin signalling might be beneficial to patients with AD. Of interest is the finding that intranasal insulin administration, a preferential route for CNS delivery (Freiherr et al., 2013), improves memory in healthy adults without affecting circulating levels of insulin or glucose (Benedict et al., 2004; Hanson and Frey, 2008). Intranasal insulin also enhances verbal memory in memory-impaired subjects (Dhamoon et al., 2009) and improves cognitive performance in patients with early AD (Craft et al., 2012). All these evidences support the employment of new therapies aimed at increasing the insulin pathway in AD. Alternatively, it is conceivable that novel therapeutic options for AD will arise from efforts aimed at unravelling mechanisms accounting for brain insulin resistance, including the DPP-IV pathway.
DPP-IV inhibition in MS
MS is a chronic T-cell mediated autoimmune disease that is believed to be triggered by a dysfunctional and self-reactive immune system, probably determined by genetic vulnerability and environmental causes. Unfortunately, the exact aetiology still remains largely unknown. MS has for long been regarded as a chronic inflammatory disease of the white matter that leads to demyelination and eventually to neurodegeneration. Pathophysiological features of the disease include the formation of CNS lesions (referred to as plaques), inflammation and destruction of the myelin sheaths surrounding neurons (demyelination) (Compston and Coles, 2008). The sequelae of symptoms include loss of sensitivity, hypersensitivity or numbness, muscle weakness, blurred vision, hyperreflexia, muscle spasms, or difficulty in moving; difficulties with coordination and balance (ataxia); problems with speech or swallowing, visual problems (nystagmus, optic neuritis or double vision), fatigue, acute or chronic pain, and bladder and bowel difficulties (Pittock and Lucchinetti, 2007). The pathological mechanisms seems to be mainly mediated by Th1/Th17 cells, where self-reactive effector T cells induce demyelination of the brain and spinal cord white matter axons, with subsequent neuronal degeneration and cell loss (Table 3).
Table 3.
Summary of recent literature addressing the beneficial effects of DPP-IV inhibitors/GLP-1 mimetics in MS
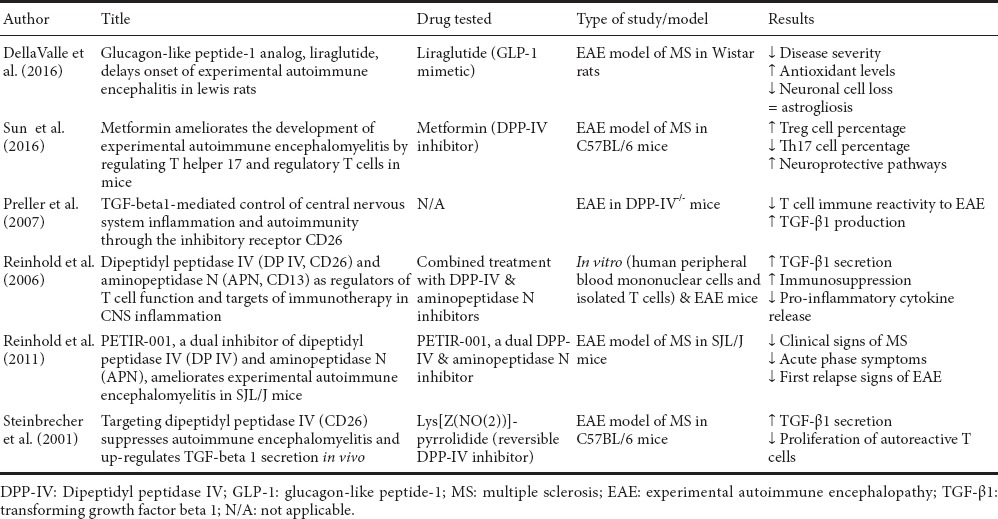
DPP-IV has attracted major interest as a potential target for the development of anti-inflammatory therapies in MS based on findings showing the co-localisation of DPP-IV with myelin-reactive T cell lines in these patients (Reinhold et al., 1998, 2008, 2009). A study has suggested that T cells exhibit higher levels of DPP-IV/IX compared with peripheral blood cells in mice with experimental autoimmune encephalopathy (EAE) (Reinhold et al., 2006; Yazbeck et al., 2009). Reinhold and co-workers subsequently observed that DPP-IV inhibitors stimulated T-cell clones, inhibited the production of IL-4, interferon (IFN)-γ and tumour necrosis factor (TNF)-α and caused a reduction in pro-inflammatory cytokines production in mice with EAE, along with an increase in the levels of the immune suppressive transforming growth factor beta 1 (TGF-β1) and reduced T-cell proliferation. Additional findings revealed that the combined inhibition of DPP-IV and aminopeptidase N (another enzyme exhibiting proteolytic activity) activity in vivo could suppress the inflammatory response associated with MS. The authors suggested that the achieved inhibition on pathogenic T cells could represent a novel and efficient remedy to manage the aberrant activation of the innate and adaptive immune system present in autoimmune diseases of the CNS, possibly through a mechanism involving TGF-β1 (Reinhold et al., 2008). However, the findings about TGF-β1 role remain somewhat controversial. In fact, another group has demonstrated that in actively induced EAE, there is strong expression of TGF-β1 in meningeal and perivascular mononuclear infiltrates at the onset of the disease, long lasting continued TGF-β1 in mononuclear cells at maximal disease severity, and expression in scattered parenchymal cells during recovery (Dobolyi et al., 2012). Nevertheless, it is important to notice that these data have been gathered from cellular and animal models of MS, which may be suitable to study specific features of the disease but, at the same time, may lack to reproduce with fidelity all of the pathogenic features of the disease. Further studies are warranted in order to gain a better understanding of the exact mechanisms underlying TGF-β1, which will hopefully shed more light into the protective roles of DPP-IV inhibitors in counteracting MS progression.
“Berberine” – An Herbal Extract with DPP-IV Inhibiting Properties
The implementation of medicinal plants and plant extracts to traditional medicine has been practiced for centuries in parallel to modern Western medicine. Among more than 300,000 seed plants, about 60% have been utilized for therapeutic interventions (Jiao et al., 2011), particularly in South America (Cercato et al., 2015), Africa (Olivier et al., 2015), and Asia (Lü et al., 2015). More recently, numerous phytochemicals have been valued for their ability to improve symptoms triggered by chronic metabolic conditions like type 1 and T2DM, as well as neurodegenerative diseases. Among these, extracts from plants such as Pterocarpus marsupium, Eugenia jambolana, Helichrysum stoechas and Rheum palmatum have collectively demonstrated positive effects in pathologies of the CNS, which were in part associated with their moderate DPP-IV inhibitory activity (Kosaraju et al., 2014; Les et al., 2017; Wang et al., 2017). However, one extract in particular, known as berberine, has revealed robust neuroprotective, anti-apoptotic, anti-inflammatory and anti-oxidative properties in various animal models of CNS disorders such as AD, PD, stroke, and even depression or anxiety (Kulkarni and Dhir, 2008; Ahmed et al., 2015; Liu et al., 2016; Shen et al., 2016; Maleki et al., 2017).
Berberine is an isoquinoline alkaloid, present in roots and stem-bark of Berberis-species and other plants. It is recognised for its insulinotropic effects, but also for its anti-inflammatory roles and ability to regulate T cell functions, possibly as a result of its potent DPP-IV inhibitory activities (Li and Hölscher, 2007; Durairajan et al., 2012; Yu et al., 2015; Imenshahidi and Hosseinzadeh, 2016; Kharkar, 2016; Liu et al., 2016; Huang et al., 2017). The alkaloid mimics the bioactivity of insulin and naturally preserves GLP-1 degradation (Singh and Mahajan, 2013; Kharkar, 2016). Berberis extracts have shown to inhibit acetylcholinesterase activity, increase the amyloid precursor protein processing towards the non-amyloidogenic pathway and increase Aβ clearance in animal and in vitro models of AD, hence corroborating the possible therapeutic role in AD pathology (Abd El-Wahab et al., 2013; Huang et al., 2017; Zhang et al., 2017). Recently, a study has also demonstrated that berberine suppresses demyelination and loss of neurophysiological function in the EAE model of MS, likely through a mechanism involving the sphingosine kinase 1/sphingosine 1 phosphate pathway (Luo et al., 2017). Interestingly, there has been previous evidence indicating that berberine administration elicited beneficial effects in the EAE model, but these were in great part attributed to its ability to preserve blood brain barrier integrity (Ma et al., 2010). Additional studies are required to clarify the exact mechanism of action exerted by berberine in MS and other autoimmune diseases.
At present, a major hurdle that has strongly limited the use of this extract in humans is the poor bioavailability (Liu et al., 2016). Several studies have shown that human plasma levels after oral administration of relatively high doses of berberine barely reach the therapeutic window, whilst potentially triggering adverse gastrointestinal effects (Zhang et al., 2008; Hu et al., 2012). In the attempt to address the issue, studies investigating alternative routes of administration through the use of spray dried mucoadhesive microparticle formulations (Godugu et al., 2014), delivery using liposomal technology (Liu et al., 2016) or development of solubility enhancers and permeations enhancers are still underway.
Concluding Remarks
Antidiabetic treatments such as DPP-IV inhibitors or GLP-1 agonists have shown promise in the treatment of PD, AD, MS and cognitive impairment in animals and humans. Notably, the study of the pathophysiology of neurodegeneration shared between diabetes and certain neurological disorders has given rise to a better understanding of disease pathogenesis and has opened new treatment possibilities. A common denominator that has been found for these neurological diseases seems to be neuroinflammation, which regardless of whether or not it represents the initiating event of the pathological cascades leading to disease appearance, it represents a viable option for the development of therapies.
DPP-IV inhibitors like linagliptin, saxagliptin and sitagliptin have been thoroughly investigated in vitro and in pre-clinical models of neurological disorders. Whilst more investigations are still warranted, these drugs exhibit a good safety profile in rodents and produced remarkable improvements through the antioxidant, antiapoptotic, neuroprotective, neurorestorative and especially, anti-inflammatory mechanisms. As such, DPP-IV inhibitors could be potentially introduced as a novel approaches for the management of neurological conditions such as PD, AD and MS, among others.
Unfortunately, no human clinical trials using DPP-IV inhibitors in PD, AD and/or MS have yet been undertaken. One of the strongest limitations before stepping forward to human studies is the dose regimen used in pre-clinical studies, which seems to be 10–20 times higher than the recommended one for the treatment of T2DM. As for berberine, most DPP-IV inhibitors display limited penetration of the blood brain barrier (Athauda and Foltynie, 2016), so new strategies are warranted before this class of oral hypoglycemic agents can be repurposed for the treatment of neurological disorders.
Footnotes
Conflicts of interest: None declared.
Financial support: None
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Manju Bhaskar, National Institute of Neurological Disorders and Stroke, USA.
Comments to authors: The paper highlights the significance of DPP-IV inhibitors in diabetes and neurological disorders. It gives an insight to the synthetic and natural DPP-IV inhibitors.
References
- 1.Abd El-Wahab AE, Ghareeb DA, Sarhan EE, Abu-Serie MM, El Demellawy MA. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement Altern Med. 2013;13:218. doi: 10.1186/1472-6882-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelsalam RM, Safar MM. Neuroprotective effects of vildagliptin in rat rotenone Parkinson's disease model: role of RAGE-NFkappaB and Nrf2-antioxidant signaling pathways. J Neurochem. 2015;133:700–707. doi: 10.1111/jnc.13087. [DOI] [PubMed] [Google Scholar]
- 3.Aertgeerts K, Ye S, Shi L, Prasad SG, Witmer D, Chi E, Sang BC, Wijnands RA, Webb DR, Swanson RV. N-linked glycosylation of dipeptidyl peptidase IV (CD26): effects on enzyme activity, homodimer formation, and adenosine deaminase binding. Protein Sci. 2004;13:145–154. doi: 10.1110/ps.03352504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed T, Gilani AU, Abdollahi M, Daglia M, Nabavi SF, Nabavi SM. Berberine and neurodegeneration: A review of literature. Pharmacol Rep. 2015;67:970–979. doi: 10.1016/j.pharep.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Ahrén B, Hughes TE. Inhibition of dipeptidyl peptidase-4 augments insulin secretion in response to exogenously administered glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, pituitary adenylate cyclase-activating polypeptide, and gastrin-releasing peptide in mice. Endocrinology. 2005;146:2055–2059. doi: 10.1210/en.2004-1174. [DOI] [PubMed] [Google Scholar]
- 6.An FM, Chen S, Xu Z, Yin L, Wang Y, Liu AR, Yao WB, Gao XD. Glucagon-like peptide-1 regulates mitochondrial biogenesis and tau phosphorylation against advanced glycation end product-induced neuronal insult: Studies in vivo and in vitro. Neuroscience. 2015;300:75–84. doi: 10.1016/j.neuroscience.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Athauda D, Foltynie T. Insulin resistance and Parkinson's disease: A new target for disease modification? Prog Neurobiol. 2016;145(146):98–120. doi: 10.1016/j.pneurobio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1664–1675. doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae EJ. DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control. Arch Pharm Res. 2016;39:1114–1128. doi: 10.1007/s12272-016-0813-x. [DOI] [PubMed] [Google Scholar]
- 10.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 11.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 13.Butterfield DA, Di Domenico F, Barone E. Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim Biophys Acta. 2014;1842:1693–1706. doi: 10.1016/j.bbadis.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cercato LM, White PA, Nampo FK, Santos MR, Camargo EA. A systematic review of medicinal plants used for weight loss in Brazil: Is there potential for obesity treatment? J Ethnopharmacol. 2015;176:286–296. doi: 10.1016/j.jep.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer's disease. Exp Gerontol. 2007;42:10–21. doi: 10.1016/j.exger.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 17.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Amico M, Di Filippo C, Marfella R, Abbatecola AM, Ferraraccio F, Rossi F, Paolisso G. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer's prone mice. Exp Gerontol. 2010;45:202–207. doi: 10.1016/j.exger.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Davie CA. A review of Parkinson's disease. Br Med Bull. 2008;86:109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- 20.De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer's disease? Alzheimers Dement. 2014;10:S26–32. doi: 10.1016/j.jalz.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 21.DellaValle B, Brix GS, Brock B, Gejl M, Landau AM, Moller A, Rungby J, Larsen A. Glucagon-like peptide-1 analog, liraglutide, delays onset of experimental autoimmune encephalitis in lewis rats. Front Pharmacol. 2016;7:433. doi: 10.3389/fphar.2016.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhamoon MS, Noble JM, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2009;72:292–293. doi: 10.1212/01.wnl.0000344246.91081.2c. author reply 293-294. [DOI] [PubMed] [Google Scholar]
- 23.Dobolyi A, Vincze C, Pal G, Lovas G. The neuroprotective functions of transforming growth factor beta proteins. Int J Mol Sci. 2012;13:8219–8258. doi: 10.3390/ijms13078219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durairajan SS, Liu LF, Lu JH, Chen LL, Yuan Q, Chung SK, Huang L, Li XS, Huang JD, Li M. Berberine ameliorates beta-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer's disease transgenic mouse model. Neurobiol Aging. 2012;33:2903–2919. doi: 10.1016/j.neurobiolaging.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 25.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 26.Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol. 2011;55:10–16. doi: 10.1016/j.vph.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007;56:3006–3013. doi: 10.2337/db07-0697. [DOI] [PubMed] [Google Scholar]
- 28.Freiherr J, Hallschmid M, Frey WH, 2nd, Brunner YF, Chapman CD, Holscher C, Craft S, De Felice FG, Benedict C. Intranasal insulin as a treatment for Alzheimer's disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27:505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gejl M, Gjedde A, Egefjord L, Moller A, Hansen SB, Vang K, Rodell A, Braendgaard H, Gottrup H, Schacht A, Moller N, Brock B, Rungby J. In Alzheimer's disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci. 2016;8:108. doi: 10.3389/fnagi.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerrald KR, Van Scoyoc E, Wines RC, Runge T, Jonas DE. Saxagliptin and sitagliptin in adult patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2012;14:481–492. doi: 10.1111/j.1463-1326.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- 31.Godugu C, Patel AR, Doddapaneni R, Somagoni J, Singh M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS One. 2014;9:e89919. doi: 10.1371/journal.pone.0089919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonçalves A, Marques C, Leal E, Ribeiro CF, Reis F, Ambrósio AF, Fernandes R. Dipeptidyl peptidase-IV inhibition prevents blood-retinal barrier breakdown, inflammation and neuronal cell death in the retina of type 1 diabetic rats. Biochim Biophys Acta. 2014;1842:1454–1463. doi: 10.1016/j.bbadis.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Green BD, Flatt PR, Bailey CJ. Dipeptidyl peptidase IV (DPP IV) inhibitors: A newly emerging drug class for the treatment of type 2 diabetes. Diab Vasc Dis Res. 2006a;3:159–165. doi: 10.3132/dvdr.2006.024. [DOI] [PubMed] [Google Scholar]
- 34.Green BD, Irwin N, Flatt PR. Pituitary adenylate cyclase-activating peptide (PACAP): assessment of dipeptidyl peptidase IV degradation, insulin-releasing activity and antidiabetic potential. Peptides. 2006b;27:1349–1358. doi: 10.1016/j.peptides.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Gullo F, Ceriani M, D'Aloia A, Wanke E, Constanti A, Costa B, Lecchi M. Plant polyphenols and exendin-4 prevent hyperactivity and TNF-alpha release in LPS-treated in vitro neuron/astrocyte/microglial networks. Front Neurosci. 2017;11:500. doi: 10.3389/fnins.2017.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gumuslu E, Mutlu O, Celikyurt IK, Ulak G, Akar F, Erden F, Ertan M. Exenatide enhances cognitive performance and upregulates neurotrophic factor gene expression levels in diabetic mice. Fundam Clin Pharmacol. 2016;30:376–384. doi: 10.1111/fcp.12192. [DOI] [PubMed] [Google Scholar]
- 37.Hanson LR, Frey WH., 2nd Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(Suppl 3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hjøllund KR, Deacon CF, Holst JJ. Dipeptidyl peptidase-4 inhibition increases portal concentrations of intact glucagon-like peptide-1 (GLP-1) to a greater extent than peripheral concentrations in anaesthetised pigs. Diabetologia. 2011;54:2206–2208. doi: 10.1007/s00125-011-2168-7. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y, Ehli EA, Kittelsrud J, Ronan PJ, Munger K, Downey T, Bohlen K, Callahan L, Munson V, Jahnke M, Marshall LL, Nelson K, Huizenga P, Hansen R, Soundy TJ, Davies GE. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine. 2012;19:861–867. doi: 10.1016/j.phymed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Huang HJ, Chen YH, Liang KC, Jheng YS, Jhao JJ, Su MT, Lee-Chen GJ, Hsieh-Li HM. Exendin-4 protected against cognitive dysfunction in hyperglycemic mice receiving an intrahippocampal lipopolysaccharide injection. PLoS One. 2012;7:e39656. doi: 10.1371/journal.pone.0039656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang M, Jiang X, Liang Y, Liu Q, Chen S, Guo Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of beta-amyloid in APP/tau/PS1 mouse model of Alzheimer's disease. Exp Gerontol. 2017;91:25–33. doi: 10.1016/j.exger.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imenshahidi M, Hosseinzadeh H. Berberis vulgaris and berberine: an update review. Phytother Res. 2016;30:1745–1764. doi: 10.1002/ptr.5693. [DOI] [PubMed] [Google Scholar]
- 45.Isik AT, Soysal P, Yay A, Usarel C. The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer's disease. Diabetes Res Clin Pract. 2017;123:192–198. doi: 10.1016/j.diabres.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Jalewa J, Sharma MK, Holscher C. Novel incretin analogues improve autophagy and protect from mitochondrial stress induced by rotenone in SH-SY5Y cells. J Neurochem. 2016;139:55–67. doi: 10.1111/jnc.13736. [DOI] [PubMed] [Google Scholar]
- 47.Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, dePamphilis CW. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473:97–100. doi: 10.1038/nature09916. [DOI] [PubMed] [Google Scholar]
- 48.Jin HY, Liu WJ, Park JH, Baek HS, Park TS. Effect of dipeptidyl peptidase-IV (DPP-IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats. Arch Med Res. 2009;40:536–544. doi: 10.1016/j.arcmed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Kharkar S. Drug Update: Berberine Old Alkaloid with wide spectrum of pharmacological activities with new anti - diabetics action. Vidarbha J Intern Med. 2016;20:46–49. [Google Scholar]
- 50.Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein T, Fujii M, Sandel J, Shibazaki Y, Wakamatsu K, Mark M, Yoneyama H. Linagliptin alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis. Med Mol Morphol. 2014;47:137–149. doi: 10.1007/s00795-013-0053-9. [DOI] [PubMed] [Google Scholar]
- 52.Kornelius E, Lin CL, Chang HH, Li HH, Huang WN, Yang YS, Lu YL, Peng CH, Huang CN. DPP-4 inhibitor linagliptin attenuates abeta-induced cytotoxicity through activation of AMPK in neuronal cells. CNS Neurosci Ther. 2015;21:549–557. doi: 10.1111/cns.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kosaraju J, Holsinger RMD, Guo L, Tam KY. Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3xTg-AD mouse model of Alzheimer's disease. Mol Neurobiol. 2017;54:6074–6084. doi: 10.1007/s12035-016-0125-7. [DOI] [PubMed] [Google Scholar]
- 54.Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, Basavan D. Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer's disease. J Pharm Pharmacol. 2013a;65:1773–1784. doi: 10.1111/jphp.12148. [DOI] [PubMed] [Google Scholar]
- 55.Kosaraju J, Madhunapantula SV, Chinni S, Khatwal RB, Dubala A, Muthureddy Nataraj SK, Basavan D. Dipeptidyl peptidase-4 inhibition by Pterocarpus marsupium and Eugenia jambolana ameliorates streptozotocin induced Alzheimer's disease. Behav Brain Res. 2014;267:55–65. doi: 10.1016/j.bbr.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Kosaraju J, Gali CC, Khatwal RB, Dubala A, Chinni S, Holsinger RM, Madhunapantula VS, Muthureddy Nataraj SK, Basavan D. Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer's disease. Neuropharmacology. 2013b;72:291–300. doi: 10.1016/j.neuropharm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Kulkarni SK, Dhir A. On the mechanism of antidepressant-like action of berberine chloride. Eur J Pharmacol. 2008;589:163–172. doi: 10.1016/j.ejphar.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 58.Lü S, Wang Q, Li G, Sun S, Guo Y, Kuang H. The treatment of rheumatoid arthritis using Chinese medicinal plants: From pharmacology to potential molecular mechanisms. J Ethnopharmacol. 2015;176:177–206. doi: 10.1016/j.jep.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Les F, Venditti A, Cásedas G, Frezza C, Guiso M, Sciubba F, Serafini M, Bianco A, Valero MS, López V. Everlasting flower (Helichrysum stoechas Moench) as a potential source of bioactive molecules with antiproliferative, antioxidant, antidiabetic and neuroprotective properties. Ind Crops Prod. 2017;108:295–302. [Google Scholar]
- 60.Li L, Hölscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev. 2007;56:384–402. doi: 10.1016/j.brainresrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Liu K, Zhao J, Holscher C, Li GL, Liu YZ. Neuroprotective role of (Val(8))GLP-1-Glu-PAL in an in vitro model of Parkinson's disease. Neural Regen Res. 2016;11:326–331. doi: 10.4103/1673-5374.177742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem. 2010;113:1621–1631. doi: 10.1111/j.1471-4159.2010.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin CL, Huang CN. The neuroprotective effects of the anti-diabetic drug linagliptin against Abeta-induced neurotoxicity. Neural Regen Res. 2016;11:236–237. doi: 10.4103/1673-5374.177724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu CS, Zheng YR, Zhang YF, Long XY. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia. 2016;109:274–282. doi: 10.1016/j.fitote.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Liu W, Jalewa J, Sharma M, Li G, Li L, Holscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson's disease. Neuroscience. 2015;303:42–50. doi: 10.1016/j.neuroscience.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 66.Lovshin J, Estall J, Yusta B, Brown TJ, Drucker DJ. Glucagon-like peptide (GLP)-2 action in the murine central nervous system is enhanced by elimination of GLP-1 receptor signaling. J Biol Chem. 2001;276:21489–21499. doi: 10.1074/jbc.M009382200. [DOI] [PubMed] [Google Scholar]
- 67.Lovshin JA, Huang Q, Seaberg R, Brubaker PL, Drucker DJ. Extrahypothalamic expression of the glucagon-like peptide-2 receptor is coupled to reduction of glutamate-induced cell death in cultured hippocampal cells. Endocrinology. 2004;145:3495–3506. doi: 10.1210/en.2004-0100. [DOI] [PubMed] [Google Scholar]
- 68.Luo J, Chen R, Zeng S, Yu J, Jiang G, Wang L, Qin X. The effects of berberine on a murine model of multiple sclerosis and the SPHK1/S1P signaling pathway. Biochem Biophys Res Commun. 2017;490:927–932. doi: 10.1016/j.bbrc.2017.06.142. [DOI] [PubMed] [Google Scholar]
- 69.Ma X, Jiang Y, Wu A, Chen X, Pi R, Liu M, Liu Y. Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS One. 2010;5:e13489. doi: 10.1371/journal.pone.0013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma Y, Fang S, Zhao S, Wang X, Wang D, Ma M, Luo T, Hong A. A recombinant slow-release PACAP-derived peptide alleviates diabetes by promoting both insulin secretion and actions. Biomaterials. 2015;51:80–90. doi: 10.1016/j.biomaterials.2015.01.064. [DOI] [PubMed] [Google Scholar]
- 71.Maleki SN, Aboutaleb N, Souri F. Berberine confers neuroprotection in coping with focal cerebral ischemia by targeting inflammatory cytokines. J Chem Neuroanat. 2017 doi: 10.1016/j.jchemneu.2017.04.008. doi: 10.1016/j.jchemneu.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marzagalli R, Scuderi S, Drago F, Waschek JA, Castorina A. Emerging role of PACAP as a new potential therapeutic target in major diabetes complications. Int J Endocrinol. 2015;2015:160928. doi: 10.1155/2015/160928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, Maeda H, Fujisue K, Yamamoto E, Kaikita K, Hokimoto S, Jinnouchi H, Ogawa H. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013;77:1337–1344. doi: 10.1253/circj.cj-12-1168. [DOI] [PubMed] [Google Scholar]
- 74.Matteucci E, Giampietro O. Dipeptidyl peptidase-4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem. 2009;16:2943–2951. doi: 10.2174/092986709788803114. [DOI] [PubMed] [Google Scholar]
- 75.Matteucci E, Giampietro O. Mechanisms of neurodegeration in type 2 diabetes and the neuroprotective potential of dipeptidyl peptidase 4 inhibitors. Curr Med Chem. 2015;22:1573–1581. doi: 10.2174/0929867322666150227153308. [DOI] [PubMed] [Google Scholar]
- 76.Melnikova I. Therapies for Alzheimer's disease. Nat Rev Drug Discov. 2007;6:341–342. doi: 10.1038/nrd2314. [DOI] [PubMed] [Google Scholar]
- 77.Mentlein R. Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 78.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 79.Nagatsu T. Prolyl oligopeptidase and dipeptidyl peptidase II/dipeptidyl peptidase IV ratio in the cerebrospinal fluid in Parkinson's disease: historical overview and future prospects. J Neural Transm (Vienna) 2017;124:739–744. doi: 10.1007/s00702-016-1604-8. [DOI] [PubMed] [Google Scholar]
- 80.Nakamura K, Oe H, Kihara H, Shimada K, Fukuda S, Watanabe K, Takagi T, Yunoki K, Miyoshi T, Hirata K, Yoshikawa J, Ito H. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110. doi: 10.1186/s12933-014-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nassar NN, Al-Shorbagy MY, Arab HH, Abdallah DM. Saxagliptin: a novel antiparkinsonian approach. Neuropharmacology. 2015;89:308–317. doi: 10.1016/j.neuropharm.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 82.Olivier DK, Van Vuuren SF, Moteetee AN. Annickia affinis and A. chlorantha (Enantia chlorantha)--A review of two closely related medicinal plants from tropical Africa. J Ethnopharmacol. 2015;176:438–462. doi: 10.1016/j.jep.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 83.Omar B, Ahrén B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63:2196–2202. doi: 10.2337/db14-0052. [DOI] [PubMed] [Google Scholar]
- 84.Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300:958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- 85.Pittock SJ, Lucchinetti CF. The pathology of MS: new insights and potential clinical applications. Neurologist. 2007;13:45–56. doi: 10.1097/01.nrl.0000253065.31662.37. [DOI] [PubMed] [Google Scholar]
- 86.Preller V, Gerber A, Wrenger S, Togni M, Marguet D, Tadje J, Lendeckel U, Rocken C, Faust J, Neubert K, Schraven B, Martin R, Ansorge S, Brocke S, Reinhold D. TGF-beta1-mediated control of central nervous system inflammation and autoimmunity through the inhibitory receptor CD26. J Immunol. 2007;178:4632–4640. doi: 10.4049/jimmunol.178.7.4632. [DOI] [PubMed] [Google Scholar]
- 87.Rat D, Schmitt U, Tippmann F, Dewachter I, Theunis C, Wieczerzak E, Postina R, van Leuven F, Fahrenholz F, Kojro E. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer's disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011;25:3208–3218. doi: 10.1096/fj.10-180133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reinhold D, Hemmer B, Gran B, Born I, Faust J, Neubert K, McFarland HF, Martin R, Ansorge S. Inhibitors of dipeptidyl peptidase IV/CD26 suppress activation of human MBP-specific CD4+ T cell clones. J Neuroimmunol. 1998;87:203–209. doi: 10.1016/s0165-5728(98)00100-3. [DOI] [PubMed] [Google Scholar]
- 89.Reinhold D, Biton A, Pieper S, Lendeckel U, Faust J, Neubert K, Bank U, Tager M, Ansorge S, Brocke S. Dipeptidyl peptidase IV (DP IV, CD26) and aminopeptidase N (APN, CD13) as regulators of T cell function and targets of immunotherapy in CNS inflammation. Int Immunopharmacol. 2006;6:1935–1942. doi: 10.1016/j.intimp.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 90.Reinhold D, Bank U, Tager M, Ansorge S, Wrenger S, Thielitz A, Lendeckel U, Faust J, Neubert K, Brocke S. DP IV/CD26, APN/CD13 and related enzymes as regulators of T cell immunity: implications for experimental encephalomyelitis and multiple sclerosis. Front Biosci. 2008;13:2356–2363. doi: 10.2741/2849. [DOI] [PubMed] [Google Scholar]
- 91.Reinhold D, Goihl A, Wrenger S, Reinhold A, Kuhlmann UC, Faust J, Neubert K, Thielitz A, Brocke S, Tager M, Ansorge S, Bank U. Role of dipeptidyl peptidase IV (DP IV)-like enzymes in T lymphocyte activation: investigations in DP IV/CD26-knockout mice. Clin Chem Lab Med. 2009;47:268–274. doi: 10.1515/cclm.2009.062. [DOI] [PubMed] [Google Scholar]
- 92.Reinhold D, Bank U, Entz D, Goihl A, Stoye D, Wrenger S, Brocke S, Thielitz A, Stefin S, Nordhoff K, Heimburg A, Tager M, Ansorge S. PETIR-001, a dual inhibitor of dipeptidyl peptidase IV (DP IV) and aminopeptidase N (APN), ameliorates experimental autoimmune encephalomyelitis in SJL/J mice. Biol Chem. 2011;392:233–237. doi: 10.1515/BC.2011.024. [DOI] [PubMed] [Google Scholar]
- 93.Shen JD, Ma LG, Hu CY, Pei YY, Jin SL, Fang XY, Li YC. Berberine up-regulates the BDNF expression in hippocampus and attenuates corticosterone-induced depressive-like behavior in mice. Neurosci Lett. 2016;614:77–82. doi: 10.1016/j.neulet.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 94.Shirakawa J, Amo K, Ohminami H, Orime K, Togashi Y, Ito Y, Tajima K, Koganei M, Sasaki H, Takeda E, Terauchi Y. Protective effects of dipeptidyl peptidase-4 (DPP-4) inhibitor against increased beta cell apoptosis induced by dietary sucrose and linoleic acid in mice with diabetes. J Biol Chem. 2011;286:25467–25476. doi: 10.1074/jbc.M110.217216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh IP, Mahajan S. Berberine and its derivatives: a patent review (2009 - 2012) Expert Opin Ther Pat. 2013;23:215–231. doi: 10.1517/13543776.2013.746314. [DOI] [PubMed] [Google Scholar]
- 96.Steinbrecher A, Reinhold D, Quigley L, Gado A, Tresser N, Izikson L, Born I, Faust J, Neubert K, Martin R, Ansorge S, Brocke S. Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up-regulates TGF-beta 1 secretion in vivo. J Immunol. 2001;166:2041–2048. doi: 10.4049/jimmunol.166.3.2041. [DOI] [PubMed] [Google Scholar]
- 97.Sun Y, Tian T, Gao J, Liu X, Hou H, Cao R, Li B, Quan M, Guo L. Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J Neuroimmunol. 2016;292:58–67. doi: 10.1016/j.jneuroim.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 98.Svenningsson P, Wirdefeldt K, Yin L, Fang F, Markaki I, Efendic S, Ludvigsson JF. Reduced incidence of Parkinson's disease after dipeptidyl peptidase-4 inhibitors-A nationwide case-control study. Mov Disord. 2016;31:1422–1423. doi: 10.1002/mds.26734. [DOI] [PubMed] [Google Scholar]
- 99.Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat Rev Neurol. 2013;9:445–454. doi: 10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- 100.Velázquez E, Ruiz-Albusac JM, Blázquez E. Glucagon-like peptide-2 stimulates the proliferation of cultured rat astrocytes. Eur J Biochem. 2003;270:3001–3009. doi: 10.1046/j.1432-1033.2003.03677.x. [DOI] [PubMed] [Google Scholar]
- 101.Vieira MNN, Lima-Filho RAS, De Felice FG. Connecting Alzheimer's disease to diabetes: Underlying mechanisms and potential therapeutic targets. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.11.014. doi: 10.1016/j.neuropharm.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 102.Wang Z, Yang L, Fan H, Wu P, Zhang F, Zhang C, Liu W, Li M. Screening of a natural compound library identifies emodin, a natural compound from Rheum palmatum Linn that inhibits DPP4. PeerJ. 2017;5:e3283. doi: 10.7717/peerj.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wijesekara N, Gonçalves da Silva RA, De Felice FG, Fraser PE. Impaired peripheral glucose homeostasis and Alzheimer's disease. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.11.027. doi: 10.1016/j.neuropharm.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 104.Yabe D, Eto T, Shiramoto M, Irie S, Murotani K, Seino Y, Kuwata H, Kurose T, Seino S, Ahrén B, Seino Y. Effects of DPP-4 inhibitor linagliptin and GLP-1 receptor agonist liraglutide on physiological response to hypoglycaemia in Japanese subjects with type 2 diabetes: A randomized, open-label, 2-arm parallel comparative, exploratory trial. Diabetes Obes Metab. 2017;19:442–447. doi: 10.1111/dom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yada T, Sakurada M, Filipsson K, Kikuchi M, Ahren B. Intraperitoneal PACAP administration decreases blood glucose in GK rats, and in normal and high fat diet mice. Ann N Y Acad Sci. 2000;921:259–263. doi: 10.1111/j.1749-6632.2000.tb06974.x. [DOI] [PubMed] [Google Scholar]
- 106.Yanagimachi T, Fujita Y, Takeda Y, Honjo J, Sakagami H, Kitsunai H, Takiyama Y, Abiko A, Makino Y, Kieffer TJ, Haneda M. Dipeptidyl peptidase-4 inhibitor treatment induces a greater increase in plasma levels of bioactive GIP than GLP-1 in non-diabetic subjects. Molecular metabolism. 2017;6:226–231. doi: 10.1016/j.molmet.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang Y, Zhang J, Ma D, Zhang M, Hu S, Shao S, Gong CX. Subcutaneous administration of liraglutide ameliorates Alzheimer-associated tau hyperphosphorylation in rats with type 2 diabetes. J Alzheimers Dis. 2013;37:637–648. doi: 10.3233/JAD-130491. [DOI] [PubMed] [Google Scholar]
- 108.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease. Trends Pharmacol Sci. 2009;30:600–607. doi: 10.1016/j.tips.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 109.Yu D, Tao BB, Yang YY, Du LS, Yang SS, He XJ, Zhu YW, Yan JK, Yang Q. The IDO inhibitor coptisine ameliorates cognitive impairment in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2015;43:291–302. doi: 10.3233/JAD-140414. [DOI] [PubMed] [Google Scholar]
- 110.Zhang H, Zhao C, Cao G, Guo L, Zhang S, Liang Y, Qin C, Su P, Li H, Zhang W. Berberine modulates amyloid-beta peptide generation by activating AMP-activated protein kinase. Neuropharmacology. 2017;125:408–417. doi: 10.1016/j.neuropharm.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Y, Zhang J, Wang Q, Lei X, Chu Q, Xu GT, Ye W. Intravitreal injection of exendin-4 analogue protects retinal cells in early diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:278–285. doi: 10.1167/iovs.09-4727. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J, Wu P, Ren G, Ning G. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 113.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, Schmidt K, Bagchi A, Griffin PR, Thornberry NA, Sinha Roy R. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1-38) J Biol Chem. 2003;278:22418–22423. doi: 10.1074/jbc.M212355200. [DOI] [PubMed] [Google Scholar]


