Abstract
Spinal cord injury (SCI) is a highly debilitating neurological disease, which still lacks effective treatment strategies, causing significant financial burden and distress to the affected families. Nevertheless, nanotechnology and regenerative medicine strategies holding promise for the development of novel therapies that would reach from bench to bedside to serve the SCI patients. There has already been significant progress in the field of cell-based therapies, with the clinical application for SCI, currently in phase II of the clinical trial. Stem cells (e.g., induced pluripotent stem cells, fetal stem cells, human embryonic stem cells, and olfactory ensheathing cells) are certainly not to be considered the panacea for neural repair but, especially when combined with rehabilitation or other combinatorial approaches using the help of nanotechnology, they seem to be the source of some of the most promising and clinical translatable cell-based therapies that could help solving impactful problems on neural repair.
Keywords: spinal cord injury, stem cells, neuroregeneration, plasticity, repair
Introduction
Spinal cord injury (SCI) is a highly debilitating neurological disease, caused either by trauma or by a disease i.e. a tumour. SCI brings an unbearable burden, both to the patient and to his family, but also to the society as a whole, given the great psychological, physical, financial and social impact of the injury. The incidence of SCI around the world is on average 40–80 per million with 250,000 to 500,000 being injured globally each year (World Health Organization, 2013). For the traumatic SCI, depending on the region, there are differences in incidents; namely, within the United States of America (USA), such differences are estimated to 40 per million and within Western Europe to 15 per million (Lee et al., 2014). From the patient's perspective, after a SCI there is almost no system that is not affected due to the injury, but high priority is given in hand and arm function for tetraplegic patients and in bowel, bladder and mobility for paraplegic patients (Simpson et al., 2012). In terms of the societal burden, the lifetime cost varies, with an average, for a high-level tetraplegic patient, being $4,724,181 and $2,310,104 for a paraplegic one, when injured at the age of 25 in the USA (National Spinal Cord Injury Statistical Center, 2013).
It has been speculated for many years that cell therapies are the key approach for SCI in order to restore the function. Ever since the first peripheral transplantation in 1980 (Richardson et al., 1980) when central nervous system (CNS) axons were grown in peripheral nerve grafts, several other types of cells have been used. The cells try to target different stages of the injury, namely the acute, sub-acute or chronic stages, with the ultimate goal to create a permissive environment and/or replace the lost neurones so that new axons and synapses can be formed and function restoration can be achieved after SCI. In the rest of the review, we will include a small description of the pathophysiologic stages of SCI and describe the most promising cells that are either in clinical trials or they are very promising and may be approved in the near future. Finally, we will try to explore the obstacles for clinical translation and give future directions for catalysing the clinical applications.
Pathophysiology
The pathophysiology of SCI can be divided into three stages acute, sub-acute and chronic. It should be noted that the scientific community has not globally agreed in the exact duration of each of those stages so certain variations may be seen in the literature.
In the acute phase of SCI (seconds to minutes after the injury), the initial mechanical impact leads to direct damage of the tissue, meaning haemorrhage, local oedema, necrosis, and laceration of the tissue (Kakulas, 2004; Silva et al., 2014). During this phase, various systemic and local events emerge (Hulsebosch, 2002), such as systemic hypotension, spinal shock, vasospasm, plasma membrane compromise, ischemia, and neurotransmitter/ionic disturbances (Rowland et al., 2008; Silva et al., 2014).
Some of the acute phase events pass into the sub-acute phase (minutes to weeks after the injury), just like some sub-acute phase events continue into the chronic phase of SCI (months to years after injury).
In the sub-acute phase, a cascade of secondary events takes place, including further oedema, vasospasm, excitotoxicity, inflammation, free radical production, lipid peroxidation, ischemia, apoptosis, demyelination and neurotransmitter/electrolyte disturbances (Donnelly and Popovich, 2008; Silva et al., 2014).
In the sub-acute and chronic phases, the central part of the spinal cord contains a lentiform-shaped cyst filled with fluid, while hypertrophic astrocytes are found around that cyst, initiating a process called “cavitation” process (Rowland et al., 2008; Silva et al., 2014). Those astrocytes along with other cells secrete extracellular matrix and inhibitory molecules, thereby causing inhibitory factors, such as chondroitin sulfate proteoglycans (CSPGs), to get locally up-regulated. In turn, that leads to the glial scar formation, which sets both a physical and a chemical barrier to the process of neuroregeneration (Liu et al., 2013). Finally, retrograde degeneration occurs to the damaged neurones through apoptosis similarly to the neurodegenerative disorders (Crowe et al., 1997; Freund et al., 2013).
Having a good understanding of the pathophysiology of SCI along with a valid hypothesis and robust methodology, researchers can use different cell transplantation methods targeting any of those stages. The choice of the stage to aim for depends on the scientific rationale and prior experimental evidence.
Promising Types of Cells and Stem Cells
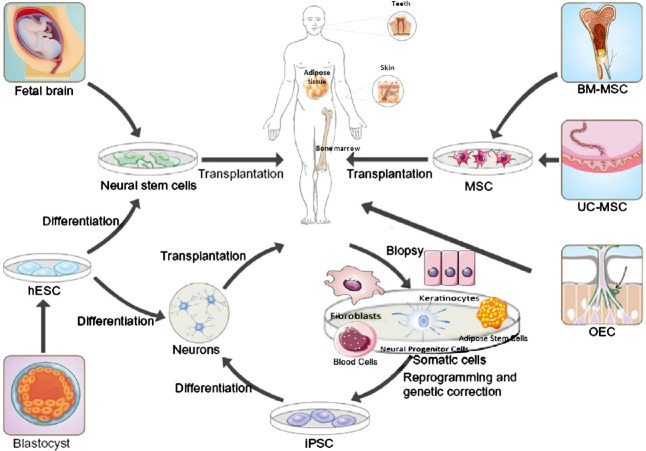
Different types of cells have been tried out for SCI (Figure 1). Regardless of whom the cells are taken from (either from the patient himself or from a donor), very few of the cells demonstrated adequately promising results, which could allow them to reach the clinical trial phases.
Figure 1.
Schematic illustration of the various adult and fetal sources for stem cells.
BM-MSC: Bone marrow-derived mesenchymal stem cells; MSC: mesenchymal stem cells; UC-MSC: umbilical cord-derived mesenchymal stem cells; OEC: olfactory ensheathing cells; iPSC: induced pluripotent stem cells; hESC: human embryonic stem cells.
In the next section, we will describe the rationale behind the use of each cell type in different phases of SCI, as well as the advantages and disadvantages linked to the most promising cells that are either on clinical trials or about to enter. In addition, a quick reference guide with the most promising cells used in SCI will be provided in the form of a table, aiming at helping researchers, clinicians and investors to quickly scan through all the possibly translatable cell-based novel interventions, given that the field is growing extremely fast to follow (Additional Table 1 (49KB, pdf) ).
Clinical potentials of cell-based therapies for SCI within the next decade.
Olfactory Ensheathing Cells (OECs) as a Possible Treatment for SCI
The olfactory system is comprised of peripheral nervous system (PNS) and CNS components. The olfactory mucosa is divided into the olfactory epithelium and lamina propria. The olfactory epithelium contains olfactory receptor neurons, which project cilia into the nasal cavity, and other supportive and mucosal cells. The lamina propria consists of loose connective tissue and OECs, which ensheathe bundles of olfactory receptor axons, extending from the olfactory epithelium (Barnett and Chang, 2004). These OECs are considered CNS glia and they display properties of both astrocytes and Schwann cells (SCs). In the adult olfactory system, OECs lie between the CNS and PNS, both anatomically and physiologically, and they stimulate and guide nerve regeneration. This is why it is believed that they encourage nerve regeneration after injury in the CNS (Choi and Gladwin, 2015).
Since the first transplantation of OECs in the spinal cord in 1994 (Ramón-Cueto and Nieto-Sampedro, 1994), several laboratory experiments, both in small and large animal models, have demonstrated great functional and structural outcomes. Even though, so far, it is not clear how the OECs achieved this outcome, i.e., nerve regeneration, trophic factors secretion, attraction of SCs etc., OECs demonstrated several promising results and they were easily accessible from the surgeons' point of view, thereafter moving to clinical trials (Li et al., 2003; López-Vales et al., 2006; Granger et al., 2012). Even though the pre-clinical results did not always come to an agreement, the controversy noted has been attributed to several factors, such as the different cell cultures used or the different techniques for transplantation, etc. (Richter et al., 2005; Novikova et al., 2011).
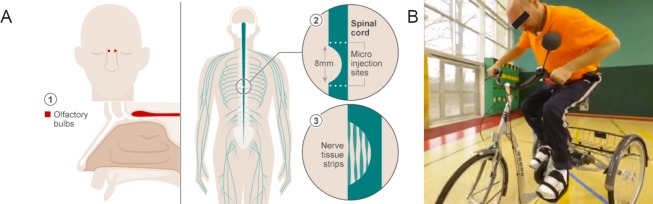
Ever since the first clinical trials of OECs, which took place in China, Australia and Spain in the years 2003, 2005 and 2006 respectively (Huang et al., 2003; Féron et al., 2005; Lima et al., 2006), a lot of progress has been made. Although the safety of the OECs transplantation in humans is now well-documented with many phase I clinical trials (Li et al., 2015), the need for a robust well-designed phase II clinical trials is still unmet. Another issue that needs to be taken into consideration before measuring the efficacy of OECs, is that certain technical parameters need to be optimized and pre-processing is essential to ensure sampling of the optimal OECs, i.e. via the study of the immunohistochemical profile of the cells and the sampling of the right type of cells among the various cells included in the mucosa or bulbs. In addition, the transplantation techniques need to be improved so that a bigger viable number of OECs can be delivered to the injury site through needles without harming the healthy tissue (Choi and Gladwin, 2015). Despite all those hurdles linked to the use of OECs, very promising results were released in 2014 by the Prof. Raisman's team in the University College of London (UCL) in London, United Kingdom, showing functional recovery below the level of injury in a patient, who was a recipient of transplanted OECs. This was a strong finding favouring the use of OECs as an efficient treatment for SCI (Tabakow et al., 2014) (Figure 2).
Figure 2.
The paralysed man who can ride a bike.
(A) Schematic illustration of the OECs transplantation technique and the rational behind it. After the removal of the olfactory bulb or biopsies from the olfactory mucosa, the SCI injury site is surgically exposed and the OECs are injected, after having been isolated, cultured, expanded and characterized, based on the hypothesis that the OECs transplantation will help the nerve tissue to regenerate across the spinal cord to repair the damage and lead to the patient's functional improvement. (B) This 38-year-old man is believed to be the first person in the world to recover from complete severing of the spinal nerves. He can now walk using a frame, ride an adapted tricycle and live an independent life thanks to the pioneering OECs transplantation, which was combined with a physiotherapy plan. The OEC transplantation technique was developed by the team of Prof. Raisman from the University College of London and the surgery was performed by a Polish team led by Dr. Tabakow from Wroclaw Medical University (BBC News, 2016). OECs: Olfactory ensheathing cells.
SCs at Clinical Trial
SCs are the supporting cells surrounding the peripheral nerves. It is widely known that peripheral nerves demonstrate the ability to regenerate and SCs seem to play an important role in guiding the axons in order to find their way back to the muscle (Son and Thompson, 1995). It is also known that after a SCI, SCs migrate to the site of the injury (Bruce et al., 2000; Guest et al., 2005). In addition, several lab results show that SCs have beneficial effects when transplanted into injured spinal cords of experimental models, although some reaction within the hostile CNS environment at the site of transplantation was observed during the first days of transplantation, leading to significant apoptosis of the SCs (Bunge and Wood, 2012; Wiliams and Bunge, 2012). The safety of the use of SCs has already been checked and so far it has been confirmed by two clinical trials (already completed) involving SCs' transplantation in human spinal cords (Saberi et al., 2008, 2011; Zhou et al., 2012). Two other clinical trials regarding the safety of transplantation are ongoing in Miami, USA for both the sub-acute and chronic phase of SCI. The chronic phase clinical trial is currently recruiting patients and is expected to be completed by 2018.
Cells-Based Immune Modulatory Therapies for SCI to Promote Neural Repair
For many years there was the notion that the immune responses in the CNS, contrary to what applies for other organ systems, were detrimental to wound healing and functional recovery (Allan and Rothwell, 2003). Even though this was established as a widely accepted dogma and was supported by many studies, there were many more recent studies that challenged this theory and provided evidence of the potentially beneficial effect of neuroinflammation (Kigerl et al., 2009; David and Kroner, 2011). Thus, it has been hypothesized by many researchers that there are certain aspects of neuroinflammation, which are indeed detrimental to the healing process, but there are other aspects, which are absolutely essential to the improvement of the functional outcome after CNS damage.
Myeloid-derived suppressor cells (MDSCs)
MDSCs are a heterogenous group of immune cells from the myeloid lineage, to which dendritic cells, macrophages and neutrophils also belong. Some novel myeloid subsets have recently been identified, i.e., monocytic MDSCs and M2 macrophages and they are considered to be beneficial after SCI. Even though the anti-inflammatory (M2) phenotype of macrophages is considered to facilitate neuroregeneration, neural plasticity and neuroprotection, it is found that the pro-inflammatory (M1) macrophages persist after SCI and result in protracted cell and tissue loss (Geng et al., 2015). It is also known that Gr-1+ immune cells, mainly neutrophils, also include other immune cells subtypes, being categorized according to their relative expression of Ly6-G and Ly6-C. Recent studies suggest that depleting Gr-1+ cells can be catastrophic for the process of functional recovery, but the same was not found for the subset of Ly6G+ cells, indicating a beneficial role of the Ly6-C+ cells, presumably of the monocytic MDSCs (Plemel et al., 2014).
In terms of the neutrophils presence, there are currently no studies of phenotypic analysis to demonstrate the presence of a particular subtype of neutrophils after SCI or other CNS traumatic lesions. It is known that neutrophils are classified in accordance to their pro- or anti-tumoral global actions as described in cancer studies, namely N1 (pro-tumour) and N2 (anti-tumour). Nevertheless, given that the neutrophil heterogeneity concept is relatively recent, further studies need to work on the subsets recruited after SCI to draw conclusions for the beneficial or detrimental effects of any particular neutrophils subset on neural repair mechanisms. It has been hypothesized though that the same duality may apply to the subsets of neutrophils, just like the one found for the macrophages, something that may justify the conflicting previous reports targeting these cells after SCI (Cuartero et al., 2013; Neirinckx et al., 2014).
In an attempt to reverse the loss of motor and sensory function, a clinical trial phase II with autologous skin co-incubated macrophages (ProCord) was initiated in 2013. The concept derived from the pioneering research of Prof. Michal Schwartz at the Weizmann Institute of Science but, unfortunately, the trial did not manage to demonstrate the anticipated efficiency and stopped (Knoller et al., 2005; Lammertse et al., 2012).
In the future, though, a better understanding of the complex immune system and polarization of certain MDSCs subsets (i.e., macrophages or neutrophils) towards the beneficial subset may finally make them part of potential SCI repair strategies.
Mesenchymal stem cells (MSCs)
MSCs are a group of heterogeneous non-hematopoietic multipotent cells that demonstrate the ability to self-renew and differentiate into cells of mesodermal origin such as osteoblasts, chondrocytes, and adipocytes in different culture settings. In addition, it has been shown that MSCs have the potential to modulate the inflammatory response, as well as the ability to differentiate into tissue of non-mesoderm origin, such as nerve tissue (Kopen et al., 1999; Deans and Moseley, 2000). Several sources can be used to obtain MSCs, the most promising for clinical translation purposes being bone marrow, adipose tissue and umbilical cord (Pittenger et al., 1999; Zuk et al., 2001; Lee et al., 2004). Several experiments in small and big SCI animal models have demonstrated the beneficial effects of MSCs from different sources (Dasari et al., 2014), making the way to the clinic a reality.
Right now, on clinicaltrial.gov there are six clinical trials, which are actively recruiting, with one using umbilical cord MSCs, three using bone marrow MSCs, and two using MSCs of unreported origin. Several other clinical trials have already been completed though and others are about to begin now, holding out some hope with regard to the results to be reported in the future.
Embryonic stem cells (ESCs)
After the first successful isolation of human ESCs (hESCs) the idea of regenerative medicine started to come closer to the reality (Thomson et al., 1998). There are three main ways for ESCs to be obtained: i) by pre-implantation or blastocyst-stage embryos (from unused embryos produced during in vitro fertilization procedures), ii) by a somatic cell nuclear transfer (Wilmut et al., 2002), 3) by parthenogenetic activation of eggs (Cibelli et al., 2002; Vrana et al., 2003).
The major obstacles for the clinical translation of ESCs were ethical issues and chromosomal instability leading to tumor formation. After several experiments have demonstrated positive results in different SCI animal models (Iwanami et al., 2005; Keirstead et al., 2005) and after having created protocols that produce stable cell lines, ethical issues were bended and the pioneering company Geron attempted to transplant ESCs into the first patients in the world. Unfortunately, Geron stopped the trial due to financial issues and, eventually, Asterias Biotherapeutics Inc. Company relaunched the first clinical trial transplanting ESCs-derived oligodendrocyte progenitor cells into humans (Ilic et al., 2015). Asterias Biotherapeutics Inc. has been currently recruiting participants for a clinical trial phase I/IIa, named SCIStar Study (Asterias Biotherapeutics Inc., 2015).
Fetal stem cells (FSCs)
The neural FSCs are isolated from donated aborted embryos between 4 and 8 weeks. The fetal subtype of cells is multipotent, which means that those cells lack the differentiation potential of hESC, but they can still produce certain lineages, such as neural. For over 30 years, several studies have demonstrated beneficial results when transplanted in SCI models (Reier et al., 1986; Houlé and Reier, 1988). Perhaps the experiments with FSCs are some of the oldest types of experiments, which managed to demonstrate functional recovery in experimental models. The fetal grafts or the FSCs do not only induce the growth of new neuronal relays that integrate into the host tissue, but they also act as a bridge for the injured tracts to facilitate nerve fibers regrowth. The exact mechanisms are still not well understood, but there are several very promising attempts using other experimental models in order to potentially extend the results to human applications in the future (Kadoya et al., 2016).
Even though FSCs are considered to hold promise for the future of nerve regeneration in humans, there are still certain major drawbacks that need to be considered. 1) There is a need for immunosuppression following transplantation, 2) There are also ethical concerns linked to that method, 3) The risk of tumorigenesis from neural FSCs is present and constitutes a major concern that halts progress to clinical translation.
Finally, NeuralStem Inc. is currently in clinical trial phase I using human spinal cord stem cells (NSI-566), which are derived from the spinal cord of a single 8-week-old fetus and expanded serially by epigenetic means only. Even though there are no published data available, it is reported by the company that there had been no serious adverse events, that implantation of stem cells in chronic SCI patients is feasible and, that implantation of their NSI-566 FSCs in the SCI patients has been safe and well tolerated. The last surgery was completed in July of 2015 and the patients have recently completed a 6-month post-observation period (NeuralStem Inc., 2015). The company actually has already proceeded with a phase I/II clinical trial using those cells for motor deficits in stroke patients and with a phase II clinical trial with NSI-566 cells for the treatment of amyotrophic lateral sclerosis (ALS). Therefore, in the next decade there is a lot to be explored in terms of the clinical applications of FSCs.
StemCells Inc. is another company, which has already launched a clinical trial phase II for cervical SCI, named the Pathway Study (StemCells Inc., 2015a, b), while the results of a clinical trial phase I/II has already been completed in May 2014 for thoracic SCI with favourable safety profile and signs of biological activity and preliminary efficacy. According to the company, human fetal brains were obtained from Advance Bioscience Resources, in accordance with all state and federal guidelines. The company has been transplanting a human central nervous system stem cells (HuCNS-SC) product, which is a highly purified population of human neural stem cells that are grown in a suspension as clusters of cells called “neurospheres.” These cells can be expanded for a number of generations in culture while still retaining their potential to, at a single cell level, self-renew and differentiate into the three major cell types of the CNS – neurons, astrocytes and oligodendrocytes. The first cohort of the Pathway Study, which was completed in April 2015, was designed to assess the safety of cell administration into the cervical cord and select the dose level for the 40-patient second cohort, a randomized, controlled and single-blinded arm of the trial. Enrollment for the second cohort of the pathway study is currently underway.
Induced Pluripotent Stem Cells (iPSCs) is Promising
The ultimate goal of stem cell therapy for SCI are to find a source of neural progenitor cells (NPCs) or other neural lineage cell lines that are both ethical and easy to use in the clinic.
In 2006, Takahashi and Yamanaka (Takahashi and Yamanaka, 2006; Takahashi et al., 2007) found a way to reprogram somatic adult cells and bring them back to their pluripotent state, which is similar to the state of cells in embryos. They created the iPSCs by using human fibroblasts with only four genes. Several types of somatic cells have been used so far for the production of iPSCs, such as dermal fibroblasts, bone marrow CD34+ cells, human cord blood cells, peripheral blood cells, adipose-derived stromal cells, neural stem cells, and keratinocytes (Aasen et al., 2008; Haase et al., 2009; Kim et al., 2009; Sun et al., 2009; Loh et al., 2010; Takenaka et al., 2010).
Despite their seminal discovery for science, several hurdles need to be surpassed in order to see iPSCs in every-day clinic. First of all, the risk of genomic modification due to viral transgenes needs to be overcome by insertion-free or “footprint-free” iPSCs. Secondly, there is the risk of tumorigenicity, given that iPSCs are capable of being differentiated into any type of cell, forming any kind of tissue, even producing tumors. This is why it is of high importance to ensure the right differentiation of iPSCs before their transplantation. Finally, the way that iPSCs are cultured and grown also needs to be considered, because non-human pathogens or antigens, which were being triggered due to the co-culture with other cells, can make the iPSCs unsuitable for transplantation.
Several steps have been taken towards that direction in order to surpass these obstacles, like the fact that an adequate number of iPSCs was produced without co-cultures or lenti virus (Nakagawa et al., 2014). In addition, adult fibroblasts managed to directly grow into NPCs or neuronal cell lines without passing from the pluripotecy state, thereafter eliminating the risk of tumor formation (Pang et al., 2011; Han et al., 2012; Ring et al., 2012; Thier et al., 2012). An important step forward for iPSCs-related research is the fact that after iPSCs were used in SCI mouse and primate immunodeficient models, improved locomotor function was observed (Tsuji et al., 2010; Nori et al., 2011; Kobayashi et al., 2012). Last but not least, the first clinical trial with iPSCs started in 2014 for age-related macular degeneration (AMD). Thus, we hope that the following years will manage to ensure the safety of iPSCs for use in human patients, so that clinical trials for SCI could be initiated. Additional Table 2 (47.1KB, pdf) summarizes the cell-based clinical studies that have taken place for the treatment of SCI and have published their results.
Clinical application of cells for the treatment of SCI (published data).
Conclusion and Future Directions
In conclusion, although there has been a huge amount of research carried out at experimental and preclinical over decades on SCI, including stem cells therapy but there still no treatment available. However, currently, there are numbers of cell therapies at various stages of clinical trials.
A number of preclinical studies with embryonic tissue have shown that supraspinal pathways, such as the ones of reticulospinal, rubrospinal or even corticospinal tract can be reconnected, making hESCs and iPSCs a possible choice of stem cells. Other cells, such as the OECs exert their beneficial effects by helping local propriospinal interneurons to create new circuits for bypassing the lesion or by other unidentified mechanisms that can produce positive functional results. Currently, the most promising cell-based therapies have already reached or are approaching the phase I clinical safety trial studies. Nevertheless, despite the initial enthusiasm on cell-based therapies, it is now evident that such therapies cannot be panacea for SCI and other disorders and that no miraculous cure can occur without a deep understanding of the effect that cell transplantations have on the nerve tissue. A step-by-step systemic approach is required by tracking the lesion site at each stage.
One step forward could be the application of emerging technological advancements, such as the ones in the field of nanotechnology, given that those advancements could improve stem cells delivery, provide a “toolkit” for significant and impactful advances in the field of neural repair. The fast clearance or death of the cells within the CNS is a major problem that has been identified, which could be addressed in the future with the use of injectable matrices and other sustained-release bioengineered micro- or nano-carriers. Combinatorial approaches can further optimize such therapeutic strategies. For example, cells can be infected with vectors in order to manage to produce growth factors, Chondroitinase or other molecules, thereby creating a favourable environment that promotes survival. In addition to that, a mixture of different cell types for transplantation is thought to increase the effectiveness of cell-based interventions, as indicated by several in vivo animal studies.
Last but not least, the value of such cell-based interventions needs to be recognized despite the obstacles towards clinical translation, which could be tackled in the future with systemic combinatorial approaches. There is a significant danger that such cells-based approaches may be considered inefficient because they did not match the scientists' expectations, disregarding any minor improvements noted. Advanced technology should be utilized in order to detect the slightest post-intervention changes to the injury site, given that if cell therapies can accomplish even minimal repair within CNS, that could pave the way to the combinatorial approaches that are about to come, increasing the potentials for success. Advanced imaging modalities could be the key in order to monitor the outcomes. It is possible that the use of non-invasive imaging technology, like MRI/PET scanners, which are being introduced in clinical trials, will facilitate the screening of the cell-transplantations effect on the spinal cord, just like it has previously been done in regenerative medicine for other organ systems (Naumova et al., 2014). Even though such tools are still not ready to be applied to the clinical setting, they could possibly be utilized in clinical trials as an additional assessment tool (Martin et al., 2016), enabling the safe translation of promising cell-based therapies to the clinic.
Additional file:
Additional Table 1: (49KB, pdf) Clinical potentials of cell-based therapies for SCI within the next decade.
Additional Table 2: (47.1KB, pdf) Clinical application of cells for the treatment of SCI (published data).
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Joanna Czarzasta, Uniwersytet Warminsko-Mazurski, Poland.
Comments to authors: This is a review article on the use of stem cells. In this paper, authors describe pathophysiology of SCI and divide this disorder into three pathological stages acute, sub-acute and chronic. What is more, they also introduce the existing cell-based therapies in the treatment of SCI and their possible therapeutic potential in the neural repair.
References
- 1.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilić J, Pekarik V, Tiscornia G, Edel M, Boué S, Izpisúa Belmonte JC. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 2.Allan SM, Rothwell NJ. Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci. 2003;358:1669–1677. doi: 10.1098/rstb.2003.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asterias Biotherapeutics Inc. Spinal Cord Injury Research Study | SCiStar Study AST-OPC1. 2015. [Accessed April 12, 2016]. Available at: http://www.scistar-study.com/
- 4.Barnett SC, Chang L. Olfactory ensheathing cells and CNS repair: going solo or in need of a friend. Trends Neurosci. 2004;27:54–60. doi: 10.1016/j.tins.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 5.BBC News. The paralysed man who can ride a bike. 2016. [Accessed April 12 2016]. Available at: http://www.bbc.com/news/health-35660621 .
- 6.Bruce JH, Norenberg MD, Kraydieh S, Puckett W, Marcillo A, Dietrich D. Schwannosis: role of gliosis and proteoglycan in human spinal cord injury. J Neurotrauma. 2000;17:781–788. doi: 10.1089/neu.2000.17.781. [DOI] [PubMed] [Google Scholar]
- 7.Bunge MB, Wood PM. Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury. Handb Clin Neurol. 2012;109:523–540. doi: 10.1016/B978-0-444-52137-8.00032-2. [DOI] [PubMed] [Google Scholar]
- 8.Choi D, Gladwin K. Olfactory ensheathing cells: Part II--source of cells and application to patients. World Neurosurg. 2015;83:251–256. doi: 10.1016/j.wneu.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Cibelli JB, Grant KA, Chapman KB, Cunniff K, Worst T, Green HL, Walker SJ, Gutin PH, Vilner L, Tabar V, Dominko T, Kane J, Wettstein PJ, Lanza RP, Studer L, Vrana KE, West MD. Parthenogenetic stem cells in nonhuman primates. Science. 2002;295:819. doi: 10.1126/science.1065637. [DOI] [PubMed] [Google Scholar]
- 10.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Crowe MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 11.Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Corbí ÁL, Lizasoain I, Moro MA. N2 neutrophils, novel players in brain inflammation after stroke modulation by the PPARγ agonist rosiglitazone. Stroke. 2013;44:3498–3508. doi: 10.1161/STROKEAHA.113.002470. [DOI] [PubMed] [Google Scholar]
- 12.Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J Stem Cells. 2014;6:120–133. doi: 10.4252/wjsc.v6.i2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 14.Deans RJ, Moseley AB. Mesenchymal stem cells. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Féron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, Geraghty T, Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951–2960. doi: 10.1093/brain/awh657. [DOI] [PubMed] [Google Scholar]
- 17.Freund P, Weiskopf N, Ashburner J, Wolf K, Sutter R, Altmann DR, Friston K, Thompson A, Curt A. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 2013;12:873–881. doi: 10.1016/S1474-4422(13)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng CK, Cao HH, Ying X, Zhang HT, Yu HL. The effects of hyperbaric oxygen on macrophage polarization after rat spinal cord injury. Brain Res. 2015;1606:68–76. doi: 10.1016/j.brainres.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Granger N, Blamires H, Franklin RJM, Jeffery ND. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain. 2012;135:3227–3237. doi: 10.1093/brain/aws268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, Zweigerdt R, Gruh I, Meyer J, Wagner S, Maier LS, Han DW, Glage S, Miller K, Fischer P, Schöler HR, Martin U. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5:434–441. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, Greber B, Yang JH, Lee HT, Schwamborn JC, Storch A, Schöler HR. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Houlé JD, Reier PJ. Transplantation of fetal spinal cord tissue into the chronically injured adult rat spinal cord. J Comp Neurol. 1988;269:535–547. doi: 10.1002/cne.902690406. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Chen L, Wang H, Xiu B, Li B, Wang R, Zhang J, Zhang F, Gu Z, Li Y, Song Y, Hao W, Pang S, Sun J. Influence of patients' age on functional recovery after transplantation of olfactory ensheathing cells into injured spinal cord injury. Chin Med J (Engl) 2003;116:1488–1491. [PubMed] [Google Scholar]
- 25.Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- 26.Ilic D, Devito L, Miere C, Codognotto S. Human embryonic and induced pluripotent stem cells in clinical trials. Br Med Bull. 2015;116:19–27. doi: 10.1093/bmb/ldv045. [DOI] [PubMed] [Google Scholar]
- 27.Iwanami A, Kaneko S, Nakamura M, Kanemura Y, Mori H, Kobayashi S, Yamasaki M, Momoshima S, Ishii H, Ando K, Tanioka Y, Tamaoki N, Nomura T, Toyama Y, Okano H. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res. 2005;80:182–190. doi: 10.1002/jnr.20436. [DOI] [PubMed] [Google Scholar]
- 28.Kadoya K, Lu P, Nguyen K, Lee-Kubli C, Kumamaru H, Yao L, Knackert J, Poplawski G, Dulin JN, Strobl H, Takashima Y, Biane J, Conner J, Zhang SC, Tuszynski MH. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22:479–487. doi: 10.1038/nm.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42:549–563. doi: 10.1038/sj.sc.3101670. [DOI] [PubMed] [Google Scholar]
- 30.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JB, Greber B, Araúzo-Bravo MJ, Meyer J, Park KI, Zaehres H, Schöler HR. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–653. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 33.Knoller N, Auerbach G, Fulga V, Zelig G, Attias J, Bakimer R, Marder JB, Yoles E, Belkin M, Schwartz M, Hadani M. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine. 2005;3:173–181. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, Nori S, Hikishima K, Konomi T, Fujiyoshi K, Tsuji O, Toyama Y, Yamanaka S, Nakamura M, Okano H. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7:e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lammertse DP, Jones L a T, Charlifue SB, Kirshblum SC, Apple DF, Ragnarsson KT, Falci SP, Heary RF, Choudhri TF, Jenkins AL, Betz RR, Poonian D, Cuthbert JP, Jha A, Snyder DA, Knoller N. Autologous incubated macrophage therapy in acute, complete spinal cord injury: results of the phase 2 randomized controlled multicenter trial. Spinal Cord. 2012;50:661–671. doi: 10.1038/sc.2012.39. [DOI] [PubMed] [Google Scholar]
- 37.Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52:110–116. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 38.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Adnan H, Xu B, Wang J, Wang C, Li F, Tang K. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: a systematic review and meta-analysis. Eur Spine J. 2015;24:919–930. doi: 10.1007/s00586-014-3416-6. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Decherchi P, Raisman G. Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci. 2003;23:727–731. doi: 10.1523/JNEUROSCI.23-03-00727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med. 2006;29:191–203. doi: 10.1080/10790268.2006.11753874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Ye H, Satkunendrarajah K, Yao GS, Bayon Y, Fehlings MG. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013;9:8075–8088. doi: 10.1016/j.actbio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, Urbach A, Heffner GC, Grskovic M, Vigneault F, Lensch MW, Park I-H, Agarwal S, Church GM, Collins JJ, Irion S, Daley GQ. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.López-Vales R, Forés J, Verdú E, Navarro X. Acute and delayed transplantation of olfactory ensheathing cells promote partial recovery after complete transection of the spinal cord. Neurobiol Dis. 2006;21:57–68. doi: 10.1016/j.nbd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Martin AR, Aleksanderek I, Cohen-Adad J, Tarmohamed Z, Tetreault L, Smith N, Cadotte DW, Crawley A, Ginsberg H, Mikulis DJ, Fehlings MG. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. Neuroimage Clin. 2016;10:192–238. doi: 10.1016/j.nicl.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K, Morizane A, Doi D, Takahashi J, Nishizawa M, Yoshida Y, Toyoda T, Osafune K, Sekiguchi K, Yamanaka S. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep. 2014;4:3594. doi: 10.1038/srep03594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Spinal Cord Injury Statistical Center. Facts and Figures at a Glance. 2013. [Accessed March 7, 2016]. Available at: http://www.msktc.org/lib/docs/Data_Sheets_/MSKTC_SCIMS_Fact_Fig_2015.pdf .
- 48.Naumova AV, Modo M, Moore A, Murry CE, Frank JA. Clinical imaging in regenerative medicine. Nat Biotechnol. 2014;32:804–818. doi: 10.1038/nbt.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neirinckx V, Coste C, Franzen R, Gothot A, Rogister B, Wislet S. Neutrophil contribution to spinal cord injury and repair. J Neuroinflammation. 2014;11:150. doi: 10.1186/s12974-014-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.NeuralStem Inc. Neuralstem Reports Third Quarter 2015 Financial Results - Nov 9, 2015. 2015. [Accessed April 12, 2016]. Available at: http://investor.neuralstem. com/2015-11-09-Neuralstem-Reports-Third-Quarter-2015-Financial-Results .
- 51.Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Yamanaka S, Nakamura M, Okano H. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novikova LN, Lobov S, Wiberg M, Novikov LN. Efficacy of olfactory ensheathing cells to support regeneration after spinal cord injury is influenced by method of culture preparation. Exp Neurol. 2011;229:132–142. doi: 10.1016/j.expneurol.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 53.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 55.Plemel JR, Wee Yong V, Stirling DP. Immune modulatory therapies for spinal cord injury - Past, present and future. Exp Neurol. 2014;258:91–104. doi: 10.1016/j.expneurol.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 56.Ramón-Cueto A, Nieto-Sampedro M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp Neurol. 1994;127:232–244. doi: 10.1006/exnr.1994.1099. [DOI] [PubMed] [Google Scholar]
- 57.Reier PJ, Bregman BS, Wujek JR. Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J Comp Neurol. 1986;247:275–296. doi: 10.1002/cne.902470302. [DOI] [PubMed] [Google Scholar]
- 58.Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurones regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- 59.Richter MW, Fletcher PA, Liu J, Tetzlaff W, Roskams AJ. Lamina propria and olfactory bulb ensheathing cells exhibit differential integration and migration and promote differential axon sprouting in the lesioned spinal cord. J Neurosci. 2005;25:10700–10711. doi: 10.1523/JNEUROSCI.3632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rowland JW, Hawryluk GWJ, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 62.Saberi H, Moshayedi P, Aghayan HR, Arjmand B, Hosseini SK, Emami-Razavi SH, Rahimi-Movaghar V, Raza M, Firouzi M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: an interim report on safety considerations and possible outcomes. Neurosci Lett. 2008;443:46–50. doi: 10.1016/j.neulet.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 63.Saberi H, Firouzi M, Habibi Z, Moshayedi P, Aghayan HR, Arjmand B, Hosseini K, Razavi HE, Yekaninejad MS. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J Neurosurg Spine. 2011;15:515–525. doi: 10.3171/2011.6.SPINE10917. [DOI] [PubMed] [Google Scholar]
- 64.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Simpson LA, Eng JJ, Hsieh JTC, Wolfe DL; Spinal Cord Injury Rehabilitation Evidence Scire Research Team The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma. 2012;29:1548–1555. doi: 10.1089/neu.2011.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Son YJ, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- 67.StemCells Inc. Pathway Study. 2015a. [Accessed April 12, 2016]. Available at: https://www.sciresearchstudy.com/Home/#page1 .
- 68.StemCells Inc. Phase II Trial in Cervical Spinal Cord Injury (SCI) 2015b. [Accessed April 12, 2016]. Available at: http://www.stemcellsinc.com/Clinical-Programs/SCI .
- 69.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, Wu JC. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci. 2009;106:15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tabakow P, Raisman G, Fortuna W, Czyz M, Huber J, Li D, Szewczyk P, Okurowski S, Miedzybrodzki R, Czapiga B, Salomon B, Halon A, Li Y, Lipiec J, Kulczyk A, Jarmundowicz W. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014;23:1631–1655. doi: 10.3727/096368914X685131. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 73.Takenaka C, Nishishita N, Takada N, Jakt LM, Kawamata S. Effective generation of iPS cells from CD34+ cord blood cells by inhibition of p53. Exp Hematol. 2010;38:154–162. doi: 10.1016/j.exphem.2009.11.003. e2. [DOI] [PubMed] [Google Scholar]
- 74.Thier M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, Brüstle O, Edenhofer F. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 76.Tsuji O, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vrana KE, Hipp JD, Goss AM, McCool BA, Riddle DR, Walker SJ, Wettstein PJ, Studer LP, Tabar V, Cunniff K, Chapman K, Vilner L, West MD, Grant KA, Cibelli JB. Nonhuman primate parthenogenetic stem cells. Proc Natl Acad Sci U S A. 2003;100:11911–11916. doi: 10.1073/pnas.2034195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.World Health Organization. Spinal cord injury. 2013. [Accessed March 7, 2016]. WHO Available at: http://www.who.int/mediacentre/factsheets/fs384/en/
- 79.Wiliams RR, Bunge MB. Schwann cell transplantation: a repair strategy for spinal cord injury? Prog Brain Res. 2012;201:295–312. doi: 10.1016/B978-0-444-59544-7.00014-7. [DOI] [PubMed] [Google Scholar]
- 80.Wilmut I, Beaujean N, de Sousa PA, Dinnyes A, King TJ, Paterson LA, Wells DN, Young LE. Somatic cell nuclear transfer. Nature. 2002;419:583–587. doi: 10.1038/nature01079. [DOI] [PubMed] [Google Scholar]
- 81.Zhou XH, Ning GZ, Feng SQ, Kong XH, Chen JT, Zheng YF, Ban DX, Liu T, Li H, Wang P. Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: six cases, more than five years of follow-up. Cell Transplant. 2012;21(Suppl 1):S39–47. doi: 10.3727/096368912X633752. [DOI] [PubMed] [Google Scholar]
- 82.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical potentials of cell-based therapies for SCI within the next decade.
Clinical application of cells for the treatment of SCI (published data).