Sensory cell damage is a major cause of hearing loss: Sensorineural hearing loss (SNHL) is a common sensory deficit characterized by tissue damage and/or cell death in the cochlea. Moderate and severe SNHL causes deficits in communication, associated with increased isolation from human relationships, and negativly impacts employability. Many etiologies have been associated with SNHL: Noise overexposure, certain drugs, inner or middle ear infection or immune-induced inflammation being common. However, the most prevalent form of SNHL is presbycusis or age-related hearing loss, often aggravated by other factors including a history of noise exposure, diabetes or high blood pressure (Wong and Ryan, 2015). Though systemic steroids are used in the treatment of sudden SNHL, other drugs to prevent or cure hearing loss have not yet been developed. Cochlear sensory cells, known as hair cells (HCs) and neurons have very limited repair capacity. Moreover since these cells do not regenerate, making any loss permanent, prevention of cell damage is critical to protecting hearing.
Reactive oxygen species (ROS) contribute to HC damage: A major change associated with HC loss is the accumulation of intracellular free radicals. Generation of ROS and reactive nitrogen species (RNS) in cochlear cells is triggered by exposure to loud sound or ototoxic drugs, and often is followed by apoptosis (Henderson et al., 2006). ROS are normally produced in cells and have certain roles in metabolism, cell signaling and other processes. However, an excess of ROS can lead to cell damage and death. Excessive ROS and RNS cause damage by reacting with DNA, proteins, cytosolic molecules, cell surface receptors, and breaking down membrane lipids. ROS also lead to inflammation, and production of the pro-inflammatory cytokines such as interleukin-6 or tumor necrosis factor-α (TNF-α) (Kim et al., 2014). The presence of vasoactive lipid peroxidation products such as isoprostanes can reduce cochlear blood flow (Ohinata et al., 2000). All of these changes can contribute to HC death and, in turn, SNHL.
HCs have a natural capacity to resist ROS as they are equipped with antioxidant enzymes to sustain redox homeostasis. Studies in mice deficient in superoxide dismutase 1 (SOD1; cytosolic copper/zinc superoxide dismutase) or glutathione peroxidase 1 (GPx1; selenium-dependent glutathione peroxidase) found increased susceptibility to noise, further indicating that natural ROS defense mechanisms are important to HC survival under stress (McFadden et al., 2001). Only once these mechanisms have been exhausted can ROS cause significant harm to HCs. Thus, it is not surprising that exogenous antioxidants can protect HCs from damage (Wong and Ryan, 2015).
Different antioxidants exert their effects via distinct mechanisms and targets. This includes scavenging the radical species that initiate peroxidation, quenching singlet oxygen, chelating metals, interrupting free radical chain reactions, reducing the concentration of oxygen, preventing oxidation of proteins or DNA, and/or stimulating endogenous antioxidant enzymes (Lü et al., 2010). Because antioxidants may employ one or more of these mechanisms, differences in their effectiveness may vary with the cellular processes involved. Therefore, in order to effectively reduce HC loss, we are likely to need single drugs or drug combinations that block multiple aspects of ROS damage. Drug screening offers an efficient method by which large numbers of candidate protective drugs can be rapidly identified and evaluated.
Methods for screening potential HC protectants: There are several ways to test the effects of antioxidants on ROS-induced HC damage.
1) In vivo study using animal models is a traditional means of evaluating antioxidant protection. Aminoglycosides, cisplatin or noise are widely used for inducing oxidative stress. A candidate substance which has an antioxidant effect is applied to the cochlea before and after ROS induction. This method has many advantages. First, the intact adult cochlea has all cell types, including both inner and outer HCs as well as cochlear neurons. Second, the adult cochlea is fully functional and hearing can be assessed by auditory brain stem response or distortion product of acoustic emission recordings. Third, histopathological analysis allows for the identification of the exact site of cochlear damage and protection. Fourth, this method has the most direct applicability to the clinical setting. Disadvantages of in vivo testing include potential problems with delivering effective levels of antioxidants to the cochlea. Moreover, testing large numbers of drugs is impractical, time consuming and costly. For these reasons, in vivo testing is often employed to validate results obtained from primary screening.
2) Cochlear cell lines that have characteristics similar to HCs are another means of investigating antioxidant HC protection. HEI-OC1 is one such cell line. These cells express several molecular markers that are characteristic of HCs, are extremely sensitive to ototoxic drugs and grow well in general cell culture conditions (Kalinec et al., 2016). This in vitro method has the advantage that cell survival can be assessed readily using standard cell viability assays and flow cytometry. Because very large numbers of cells can be generated, many compounds can be evaluated in a high-throughput assay with rapid turnaround. A primary disadvantage of cell lines is that these partially differentiated cells only mimic HCs in part, and do not reflect inner versus outer HCs. Compounds identified in cell lines must be confirmed using in vivo mammalian models.
3) The zebrafish larva has numerous qualities that make it an excellent in vivo whole organism model for HC protection screening. The zebrafish's small size, high fecundity and its optically translucent body make it useful for examining large numbers of treatment conditions and for phenotypic evaluation of HCs. The lateral line HCs are functionally mature. While the lateral line detects the direction and flow rate of water, not auditory stimuli, they operate on the same principal as cochlear HCs and are sensitive to ototoxic drugs. Because large numbers of larvae can be generated, they are consistent with high-throughput screening. Disadvantages of this model are the many differences between lateral line HCs and mammalian cochlear HCs. HCs of the lateral line extend their stereocilia and kinocilia into the surrounding medium, with no separation of ionically distinct fluid spaces analogous to those of the mammalian inner ear. Lateral line HCs do not reflect the properties of inner and outer HCs, and could be considered more similar to vestibular HCs. Thus, discoveries made in zebrafish must be confirmed in vivo mammalian models prior to translational applications (Ou et al., 2012).
An in vitro assay based on neonatal micro-explants of the mammalian cochlear sensory epithelium: While no screening assay is ideal, it would be advantageous to be able to screen a larger number of compounds using mammalian HCs with higher throughput than can be accomplished in vivo. We therefore developed an assay based on micro-explants from the basal and middle turns of the neonatal mouse cochlea. To perform the assay, the sensory epithelium is dissected from the cochlea of postnatal day 3–5 pou4f3/eGFP mouse pups. These transgenic animals selectively express eGFP (enhanced green fluorescent protein) in HCs under the control of a pou4f3 promoter construct (Masuda et al., 2011). The apical region of each epithelium, which is relatively insensitive to aminoglycoside toxicity, is discarded. The basal and middle regions of the epithelium are divided, using a diamond scalpel, into micro-explants consisting of approximately 20 inner HCs and the 60 associated outer HCs (Figure 1). Micro-explants are individually plated in each well of flat-bottom 96-well plates in media consisting of DMEM/F-12 (Gibco, Gaithersburg, MD, USA) plus 30 U/mL Penicillin and 5% fetal bovine serum (FBS), maintained in a humidified tissue culture incubator (37°C, 5% CO2).
Figure 1.
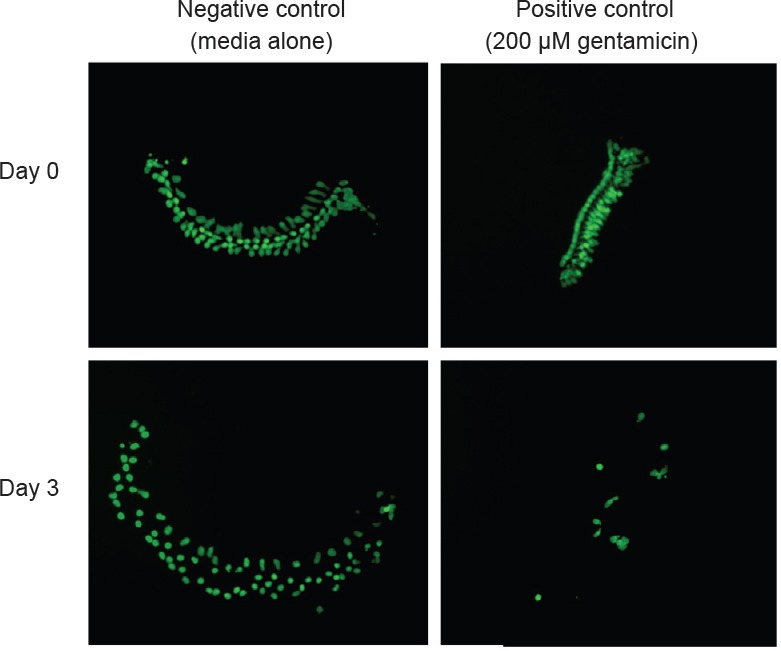
Micro-explants from the cochlear sensory epithelium of pou4f3/eGFP transgenic mice just before gentamicin treatment (day 0) and 3 days after gentamicin treatment.
Left column: Control explants maintained in culture media. Right column: explants treated with 200 μM of gentamicin.
Each micro-explant is pretreated for 24 hours with one of the candidate compounds at various concentrations, performed in triplicate wells. The following day, the media are withdrawn, fresh media containing 200 μM gentamicin plus the candidate compound at the appropriate concentration is added, and the micro-explants are cultured for 72 hours. GFP-positive HCs are imaged by fluorescence microscopy before the application of gentamicin and then at 1, 2, and 3 days afterward to evaluate HC survival. HC counts, including both inner and outer HCs, are evaluated using ImageJ software (NIH, Bethesda, MD, USA), and normalized as percentages to the number of HCs present on day 1, prior to the start of gentamicin treatment. Any micro-explants that do not attach and flatten in the well by day 1 are excluded (≤ 3% per plate). Gentamicin is used, as opposed to a direct oxidant such as H2O2, since it is a clinically relevant HC toxin.
Compound “hits” are identified in the initial round of screening as deviating significantly from the control explants treated with gentamicin only. Following this initial identification, repeat plates are prepared in an identical manner for all hits and controls, for a total N of 6 micro-explants/hit compound.
The assay was used to screen a redox compound library (Screen-Well Redox Library, Enzo Life Sciences, Farmingdale, NY, USA) containing 81 antioxidants and 3 pro-oxidants (as controls to provide oxidative stress), as previously reported (Noack et al., 2017). The results of the screen are illustrated in Figure 2. It is clear from the figure that only a minority of compounds altered gentamicin-induced HC loss. A total of thirteen antioxidants proved to be protective: seratrodast, idebenone, resveratrol, BHA, BHT, α-lipoic acid, hinokitiol, dithiotreitol, MC186, procysteine, trolox, thiourea and thymoquinone. One pro-oxidant and five anti-oxidants increased HC death. Of note, an additional screen of 160 protein kinase inhibitors that spanned the kinome was also recently performed using this assay (Ryals et al., 2017).
Figure 2.
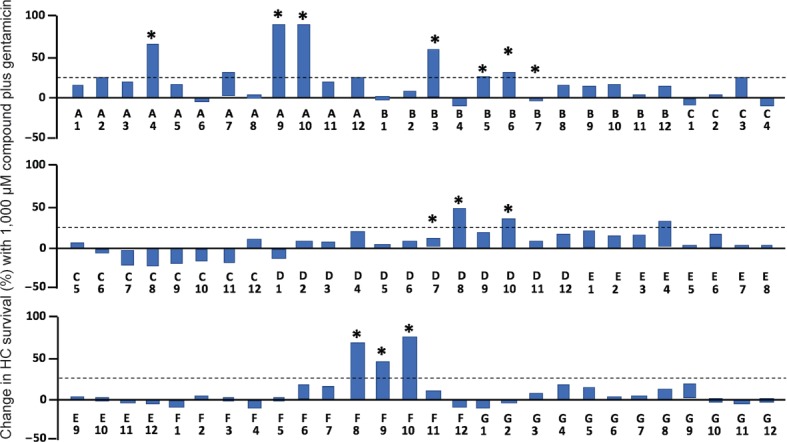
Assay results for gentamicin exposure plus a compound dose of 1,000 μM.
The percent of hair cells (HCs) initially present in micro-explants exposed to gentamicin alone has been subtracted from that for micro-explants exposed to the compound plus gentamicin. Because antioxidants showed peak protection on different days after the initiation of gentamicin exposure, the highest difference observed either on Day 1, Day 2 or Day 3 of culture is presented. Compounds that proved to provide statistically significant protection after validation are indicated by an asterisk. Most of these compounds showed a protective effect above 25% at 1,000 μM compound concentration. A few (B7 and F7) showed protective effects at lower compound dosages but not at the 1,000 μM concentration illustrated here. Compound identities can be found in the Supplementary Table 1 of Noack et al. (2017). Data were evaluated by analysis of variance and Fisher's post hoc test with Bonferonni correction for multiple conditions. *P < 0.05.
Advantages and disadvantages of the microexplant assay: This assay has the advantage that mammalian inner and outer HCs can be evaluated. The outer HC, the most vulnerable element in the mammalian cochlea, is a unique feature that is not present in other classes of animals, such as fish, nor is it modeled in cell lines. Mammalian cochlear HCs thus provide results that may be more applicable to humans. In addition, microexplants allow a much larger number of compounds to be evaluated than could be screened practically in vivo. While the assay is by no means high-throughput, it readily allows the screening of a few hundred compounds. In addition, HC-specific eGFP expression allows continuous monitoring of HC survival throughout the period of the screening assay, instead of a single timepoint snapshot. This eliminates the need for multiple samples to evaluate different times of treatment, as well as the need for HC staining. Finally, because the assay is uniform, it allows not only hit identification, but also information on relative effectiveness between different compounds.
Of course, there are also disadvantages to this assay system. Since adult HCs do not survive in culture, the assay is based on neonatal HCs that are not yet functionally mature. As noted, the number of compounds that can be tested is limited compared to cell lines or zebrafish larvae. Similarly, evaluating a very large number of conditions, such as a large range of gentamicin dosages or additional compound concentrations, would be impractical. Finally, as with all screening assays, it does not provide definitive data, but rather identifies candidates that warrant further study. These limitations must be considered when interpreting the results of the assay.
Conclusions: Many experimental studies in animals have shown that the application of exogenous antioxidants can effectively protect HCs from various forms of damage in vitro and in vivo. In contrast, the results of clinical studies have been more modest or even negative (see Noack et al., 2017 for a review). This may be related to the difficulty of controlling damage to HCs in humans, or insufficient drug delivery to HCs. However, it is also possible that the antioxidants tested were not optimal for HC protection. In vitro screening assays can be used to target in vivo studies, by identifying lead compounds with an increased probability of success. We have developed an efficient and standardized in vitro test with which to screen compounds for their ability to modify the response of mammalian HCs to ototoxins. This can be used to screen larger numbers of antioxidants, as well as a variety of other compound classes suspected of having protective ability. The assay allows the evaluation of multiple compound doses and times of treatment, increasing the chance to detect hits. Given the results presented above, several select antioxidants appear to be attractive candidates for the protection of HCs from the oxidative stress associated with ototoxic drugs and potential noise or aging. These must, of couse, be validated by further study and in vivo testing. In vivo evaluation of the most protedtive antioxidants is planned. It may also be possible to combine these antioxidants with other proteicive compounds, such as those indeitified in our kinase inhibitor screen (Ryals et al., 2017).
This study was supported by NIH/NIDCD grant DC00139 and VA grant BX001205.
Footnotes
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: David Fisher, University of the Western Cape, South Africa.
Comments to authors: The authors sketch the background, the clinical importance and the anatomical basis for SNHL. Furthermore, the authors indicate the connection between the accumulations to ROS/RNS and their generation due to various causative events, and their possible connection to HC death and thus SNHL. Importantly, the paper crucially points out that antioxidants (AOs) utilize distinct and various mechanisms in neutralizing ROS/RNS. The paper challenges the idea that AOs are generic and are effective to neutralize all cases of ROS. The paper also comments on the pros and cons of three methodologies used to examine the effects of various substances on the HCs (inner and outer), viz., in vivo studies, in vitro cell culture and also the use of Zebrafish (and their lateral line HCs). Lastly, the authors report on the advantages and disadvantages of using a microexplant “high throughput” assay, to evaluate the effects of 81 antioxidants on their redox neutralizing ability. The reported results confirm that not all exogenous AOs are equal, with 13 AOs having a positive effect, while 5 actually resulting in increased HC death. The perspective is of scientific interest and value, and should be published.
References
- 1.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 2.Kalinec G, Thein P, Park C, Kalinec F. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear Res. 2016;335:105–117. doi: 10.1016/j.heares.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Kim SJ, Lim JY, Lee JN, Choe SK, Kim YI, Song SR, Cho M, So HS, Park R. Activation of beta-catenin by inhibitors of glycogen synthase kinase-3 ameliorates cisplatin-induced cytotoxicity and pro-inflammatory cytokine expression in HEI-OC1 cells. Toxicology. 2014;320:74–82. doi: 10.1016/j.tox.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuda M, Dulon D, Pak K, Mullen LM, Li Y, Erkman L, Ryan AF. Regulation of POU4F3 gene expression in hair cells by 5′ DNA in mice. Neuroscience. 2011;197:48–64. doi: 10.1016/j.neuroscience.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFadden SL, Ohlemiller KK, Ding D, Shero M, Salvi RJ. The influence of superoxide dismutase and glutathione peroxidase deficiencies on noise-induced hearing loss in mice. Noise Health. 2001;3:49–64. [PubMed] [Google Scholar]
- 7.Noack V, Pak K, Jalota R, Kurabi A, Ryan AF. An antioxidant screen identifies candidates for protection of cochlear hair cells from gentamicin toxicity. Front Cell Neurosci. 2017;11:242. doi: 10.3389/fncel.2017.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878:163–173. doi: 10.1016/s0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- 9.Ou H, Simon JA, Rubel EW, Raible DW. Screening for chemicals that affect hair cell death and survival in the zebrafish lateral line. Hear Res. 2012;288:58–66. doi: 10.1016/j.heares.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryals M, Pak K, Jalota R, Kurabi A, Ryan AF. A kinase inhibitor library screen identifies novel enzymes involved in ototoxic damage to the murine organ of Corti. PLoS One. 2017;12:e0186001. doi: 10.1371/journal.pone.0186001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong AC, Ryan AF. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front Aging Neurosci. 2015;7:58. doi: 10.3389/fnagi.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]


