Keywords: nerve regeneration, National Institutes of Health Stroke Scale, middle cerebral artery occlusion, collateral circulation, modified Rankin Scale score, cerebral ischemia, acute stroke, diffusion-weighted imaging, fluid-attenuated inversion recovery, neural regeneration
Abstract
Fluid-attenuated inversion recovery (FLAIR) vascular hyperintensity (FVH) is used to assess leptomeningeal collateral circulation, but clinical outcomes of patients with FVH can be very different. The aim of the present study was to assess a FVH score and explore its relationship with clinical outcomes. Patients with acute ischemic stroke due to middle cerebral artery M1 occlusion underwent magnetic resonance imaging and were followed up at 10 days (National Institutes of Health Stroke Scale) and 90 days (modified Rankin Scale) to determine short-term clinical outcomes. Effective collateral circulation indirectly improved recovery of neurological function and short-term clinical outcome by extending the size of the pial penumbra and reducing infarct lesions. FVH score showed no correlation with 90-day functional clinical outcome and was not sufficient as an independent predictor of short-term clinical outcome.
Introduction
Middle cerebral artery occlusion (MCAO) is one of the most severe ischemic strokes and is always associated with poor functional outcome (Smith et al., 2009; Gawlitza et al., 2015). Very early short-term outcome predictors are essential for planning therapeutic strategies during the first few days of stroke onset (Park et al., 2016).
The outcome of acute ischemic stroke (AIS) is influenced by several factors, both non-modifiable (such as gender, age, heredity and race) and modifiable (such as arterial hypertension, hyperlipidemia, diabetes, coronary heart disease, and atrial fibrillation) (Liu et al., 2015). Despite a large amount of clinical research into the factors determining AIS outcome, no consensus has been reached (Liang et al., 2015). Advances in medical imaging, including in magnetic resonance imaging (MRI), computed tomography angiography (CTA) and computed tomography perfusion (CTP), have enabled more accurate evaluations of AIS severity (Adams et al., 2005; Lee et al., 2009; Sanossian et al., 2009; Azizyan et al., 2011; Guo et al., 2012; Yang et al., 2013; Deng et al., 2016; Dengler et al., 2016; Harteveld et al., 2016; Hernandez-Perez et al., 2016; Husson et al., 2016; Kvistad et al., 2016; Wang et al., 2016b; Yi et al., 2016). Consequently, the outcomes of patients with AIS can be predicted from evaluations based on factors other than clinical symptoms, and considerable progress has been made in predicting AIS prognosis (Cheng et al., 2013).
The appearance of fluid-attenuated inversion recovery (FLAIR) vascular hyperintensity (FVH) in stroke patients was first described about 10 years ago (Cosnard et al., 1999; Tsushima and Endo, 2001). Slow collateral circulation may explain why FVH is consistent with beneficial collateral arterial flow beyond the arterial occlusion site in AIS patients (Sanossian et al., 2009; Förster et al., 2015; Legrand et al., 2015; Karadeli et al., 2016; Kim et al., 2016; Liu et al., 2016a, b). However, there is little consensus on the determination of prognostic information from FVH (Flacke et al., 2000; Schellinger et al., 2005; Lee et al., 2009). In previous similar studies, stroke populations were heterogeneous; patients with anterior and posterior circulation occlusions, and with or without thrombolytic treatment, were considered together, potentially confounding results. Furthermore, studies used a variety of prognostic evaluation criteria, including a 10-day National Institutes of Health Stroke Scale (NIHSS) score, the 90-day modified Rankin Scale (mRS) score, and recanalization (Flacke et al., 2000; Schellinger et al., 2005; Lee et al., 2009; Olindo et al., 2012). To determine the clinical implications of FVH, similar groups of patients should be compared, such as those with similar sites of arterial occlusion, and those receiving similar treatments. In addition, studies should adopt an accepted standard as a prognostic evaluation tool. Here, we use the 90-day mRS score.
The combination of clinical and neuroimaging parameters is of interest because they represent different aspects of stroke-related cerebral impairment. We hypothesized that clinical variables and FVH can provide additive predictive utility for AIS. The aim of the present prospective study was to evaluate the prognostic value of clinical parameters including FVH in AIS patients with M1-middle cerebral artery (MCA) occlusion with respect to 90-day clinical outcome.
Subjects and Methods
Subjects
A single-center cohort study was performed in consecutive AIS patients presenting to the Stroke Unit of Huashan Hospital, China, within 3 hours of onset. The stroke team, including neurophysicians and neuroradiologists, assessed all patients. The patients underwent a computed tomography (CT) + MRI-based stroke protocol, including non-contrast CT, CTP, CTA and MRI, within 72 hours of admission. The study protocol was approved by the institutional review board of Huashan Hospital (approval number: 2013-158) and was conducted in accordance with the Declaration of Helsinki. Close family members or legal guardians gave the informed consent. Study flow chart is shown in Figure 1.
Figure 1.
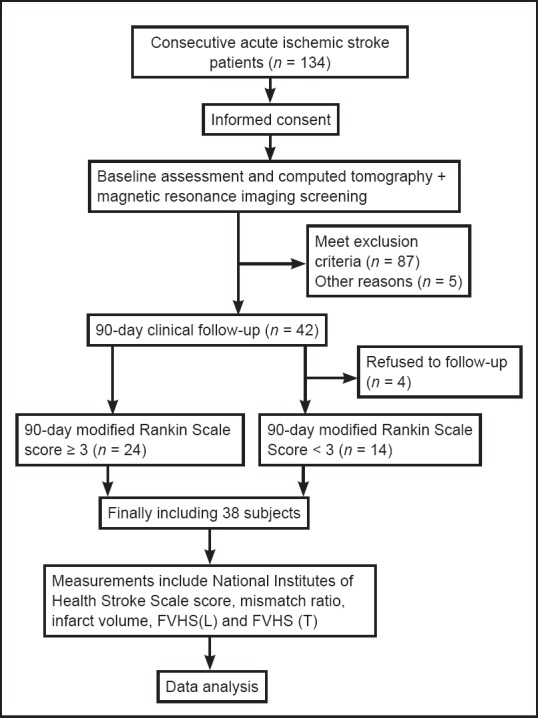
Study flow chart.
FVHS(L)/(T): Fluid-attenuated inversion recovery (FLAIR) vascular hyperintensity score (longitudinal/transverse directions).
Inclusion criteria
Patients presenting with all of the following criteria were considered for study inclusion: (1) AIS; (2) isolated M1-MCA occlusion; (3) not receiving thrombolytic therapy; (4) MRI, CTP and CTA obtained within 72 hours of stroke onset; (5) ability to complete 90-day clinical follow-up.
Exclusion criteria
Patients with one or more of the following conditions were excluded from this study: (1) History of brain tumor, cerebral hemorrhage, brain surgery, or other brain disease that might affect prognosis; (2) ischemic stroke in bilateral cerebral hemispheres; (3) posterior circulation infarct; (4) hemorrhagic stroke.
Clinical variables
Clinical data were collected by neuroradiologists and maintained in a prospective stroke database developed by Huashan Hospital. NIHSS was used to evaluate neurologic deficit at admission and 10 days after admission (Schellinger et al., 2005; Lee et al., 2009; Miteff et al., 2009; Tan et al., 2009; Cheng et al., 2012; Olindo et al., 2012). 90-day mRS was used to evaluate the final functional outcome, with an mRS score > 2 defining poor functional outcome (Miteff et al., 2009; Olindo et al., 2012). Categorical variables included sex, arterial hypertension, hyperlipidemia, diabetes, coronary heart disease, atrial fibrillation, C-reactive protein increase, history of myocardial infarction, family history of stroke, anticoagulant therapy and anti-platelet therapy. Arterial hypertension was defined as blood pressure > 140/90 mmHg on admission or by the use of an antihypertensive. Hyperlipidemia was defined as serum low-density lipoprotein cholesterol > 140 mg/dL, or serum triglycerides > 150 mg/dL on admission, or use of anti-hyperlipidemia agents. Diabetes was defined as fasting blood glucose > 126 mg/dL or random blood glucose > 200 mg/dL, and hemoglobin A1c > 6.4% on admission (National Glycohemoglobin Standardization Program), or by the use of anti-diabetic agents. Coronary heart disease was defined according to the nomenclature and criteria for diagnosis of ischemic heart disease. Atrial fibrillation was diagnosed by 24-hour ambulatory electrocardiographic monitoring performed on admission. C-reactive protein increase was defined as C-reactive protein > 0.5 mg/dL. Diagnostic criteria for myocardial infarction included documented typical rise and gradual fall (troponin T) or more rapid rise and fall (CK-MB) of biochemical markers of myocardial necrosis with at least one of the following: ischemic symptoms (such as chest pain), development of pathological Q waves on the electrocardiogram, or electrocardiographic changes indicative of ischemia (ST segment elevation or depression), either during admission or in medical reports from other hospitals. All parameters are defined in a study by Li et al. (2015). A family history of stroke was defined as an immediate family member having had a stroke. Anticoagulants included warfarin and direct oral anticoagulants. Antiplatelet drugs included aspirin, clopidogrel, and cilostazol. Continuous variables included age, admission NIHSS score, 10-day NIHSS score, and 90-day mRS score.
Imaging
All patients underwent non-contrast CT/CTP/CTA and MRI within 72 hours of stroke onset. The stroke protocol was performed on a 256-section CT scanner (Brilliance iCT, Phillips Medical Systems, Cleveland, OH, USA) and 3.0T super-conducting MRI scanner (Verio, Siemens AG, Erlangen, Germany).
Whole-brain no-contrast CT was performed at 120 kV, 150 mA, in contiguous 6 mm axial slices. This was followed by CTP, which included a 50-second scan reconstructed at 0.4-second intervals to produce a series of 312 sequential images for 13 sections, covering a total of 120 mm from the foramen magnum to the lateral ventricles. CTP scanning parameters were as follows: 120 kVp; 150 mAs; slice thickness, 5 mm; field of view (FOV) 220 mm; matrix, 512 × 512, intravenous administration of 50 mL non-ionic contrast (Ultravist Iodine 370 mgI/mL, Bayer Healthcare, Berlin, Germany) at a rate of 5 mL/s via a power injector (Stellate Injection System, Indianola, PA, USA) with a 5-second delay. CTA was performed 10 minutes later from the aortic arch to the vertex with the following parameters: 120 kVp; 150 mAs; slice thickness, 1 mm, pitch, 0.7; FOV, 220 mm, matrix, 512 × 512, helical scanning mode and intravenous administration of 50 mL non-ionic contrast (Ultravist) at 5 mL/s via a power injector (Stellate Injection System) with an 8-second delay. Time to CTP and CTA from stroke onset was 4.55 ± 2.80 hours.
MRI sequences comprised axial T2-weight (repetition time/echo time, 3,480/110 ms; FOV, 240 mm; matrix, 256 × 256; section thickness, 6 mm; gap, 2 mm), T1-FLAIR (repetition time/echo time/inversion time, 1,780/20/860 ms; FOV, 240 mm; matrix, 256 × 256; section thickness, 6 mm; gap, 2 mm), T2-FLAIR (repetition time/echo time/inversion time, 8,600/120/2,370 ms; FOV, 240 mm; matrix, 256 × 256; section thickness, 6 mm; 2-mm gap), diffusion-weighted imaging (DWI) (repetition time/echo time, 4,800/74 ms; FOV, 240 mm; matrix, 256 × 256; section thickness, 6 mm with no gap; b = 0 and 1,000 s/mm2). Axial MRI was performed from the vertex to the foramen magnum, with 16 images per sequence. Time to MRI from stroke onset was 26.14 ± 5.08 hours.
Imaging analysis
CTP data were analyzed with Phillips brain perfusion software (Vision 5.0.2) using an identical deconvolution algorithm to generate parametric maps of mean transit time (MTT) and cerebral blood volume (CBV). An MTT > 145 % of that of the contralateral hemisphere was used to calculate automated MTT lesion volume (Miteff et al., 2009). Within the MTT lesion, infarct core volume was determined from a CBV map using an automated threshold of < 2.0 mL/100 g (Miteff et al., 2009). Thus, penumbral volume was automatically determined from the difference between the MTT lesion and the CBV lesion, and the CTP penumbral/infarct core mismatch ratio was calculated using the formula (MTT lesion − CBV lesion)/CBV lesion.
CTA data were processed using the Phillips Brilliance Workspace portal software (Vision 5.0.2), including 10-mm axial and multi-planar maximum intensity projection reconstructions. MCA occlusion was carried out as described in a study by Lee et al. (2009). M1-MCA occlusion was defined as a main MCA trunk occlusion before the bifurcation, with or without ipsilateral internal carotid artery occlusion. FVH was defined as a linear or serpentine hyperintensity on the T2-FLAIR image corresponding to a typical arterial course. Absent FVH was defined as no FVH on any T2-FLAIR images. The presence of FVH was categorized as proximal or distal in relation to arterial branching of the MCA: proximal when it was near to or within the Sylvian fissure (i.e., corresponding to the MCA M1 or M2 segments) and distal when it was present at MCA M3 or distal segments. Absent and proximal FVH were grouped together as no distal FVH, because proximal FVH did not offer collateral arterial circulation information. This part of the study was carried out as described in a study from Lee et al. (2009). The fluid-attenuated inversion recovery (FLAIR) vascular hyperintensity score (longitudinal direction) [FVHS(L)] was based on a rostrocaudal extension of FVH (Olindo et al., 2012). Thus, all 16 axial T2-FLAIR images were analyzed. Slices with no FVH were scored as 0, and those with one or more FVH were scored as 1. As 16 images were analyzed, the resulting FVH score was 0–16. FLAIR vascular hyperintensity (transverse direction) [FVHS(T)] was scored on a scale of 0–3 (Tan et al., 2007, 2009), with 0 indicating absent FVH in the occluded MCA territory, 1 indicating FVH filling ≤ 50% but > 0% of the occluded MCA territory, 2 indicating FVH filling > 50% but < 100% of the occluded MCA territory, and 3 for 100 % FVH supply of the occluded MCA territory. Two investigators blinded to the other sequence quantified FVHS(L) and FVHS(T). In case of discrepancy between the scores of the two investigators, T2-FLAIR images were reviewed by the investigators until a consensus was established.
The DWI infarct volume was measured on MRI images using Siemens Functool 9.4.05 software. All CTP MTT and CBV lesion volumes and MRI DWI infarct volumes were measured by a third experienced reader who was blinded to the FVH score.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, Chicago, IL, USA). The Mann-Whitney U and Kruskal-Wallis H tests were used to compare radiological and clinical differences between the groups. Logistic regression analysis of significantly associated variables (P < 0.05) was used to identify factors predictive of a good outcome. Univariate and multivariate logistic regression analysis was performed using 90-day mRS score as an outcome variable. The Spearman nonparametric correlation was performed to assess the correlation between variables (NIHSS score, mismatch ratio, infarct volume, FVHS(L) and FVHS(T)) and 90-day mRS score. Inter-observer variability of FVH grading was evaluated with k statistics. A two-tailed value of P < 0.05 was considered significant.
Results
Demographic data
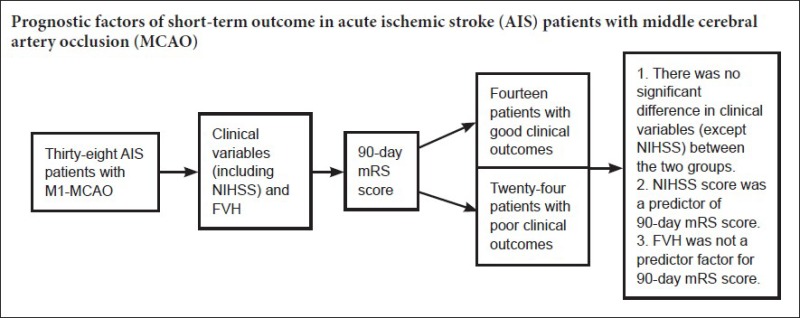
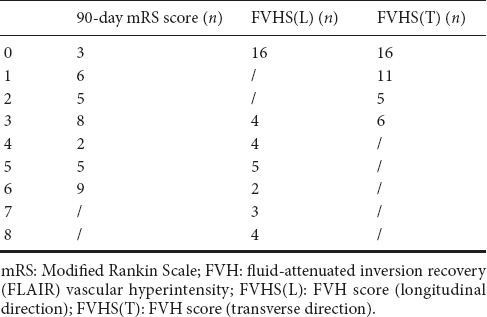
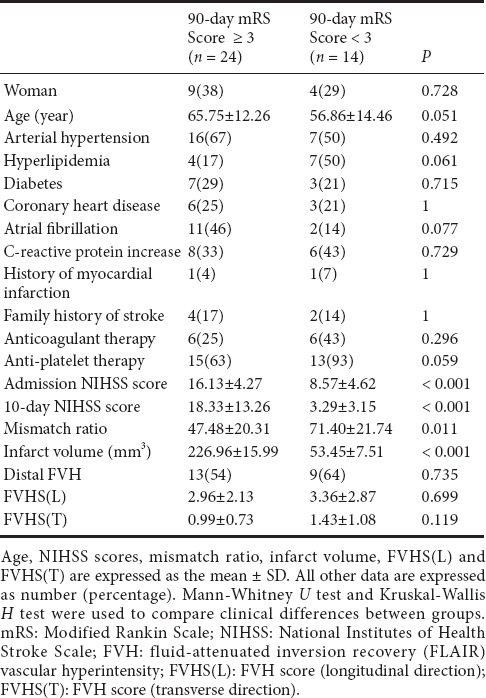
From 134 consecutive AIS patients who met inclusion criteria at admission, 87 were excluded for the following reasons: vertebra-basilar artery territory infarction or lacunar infarction (26), cerebral hemorrhage (23), brain tumor (20), stroke in bilateral cerebral hemispheres (9), brain surgery (7), unreadable FLAIR (5), parasite infections (2), and no follow-up (4). The final cohort comprised 38 AIS patients with M1-MCA occlusion (including 9 with ipsilateral internal carotid artery occlusion): mean age was 62.52 ± 13.61 years and 13 (34%) were women. The 90-day mRS scores are shown in Table 1. Poor clinical outcome was seen in 24 patients (63%).
Table 1.
Distribution of 90-day mRS score, FVHS(L) and FVHS(T)
Comparison of clinical variables between MCAO patients with poor and good clinical outcomes
There was no significant difference between the patients with poor and good clinical outcomes in terms of gender, age, arterial hypertension, hyperlipidemia, diabetes, coronary heart disease, atrial fibrillation, C-reactive protein increase, history of myocardial infarction, family history of stroke, anticoagulant therapy or anti-platelet therapy (Table 2).
Table 2.
Group comparison of poor (90-day mRS score ≥ 3) and good (90-day mRS score < 3) clinical outcomes
Relationship between NIHSS score and 90-day mRS score
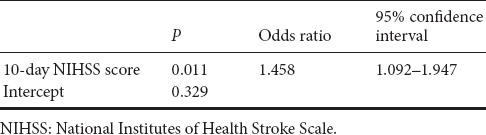
Mean admission NIHSS score was 13.29 ± 3.11. Mean 10-day NIHSS score was 12.12 ± 5.25. Patients with poor clinical outcomes had higher admission NIHSS score (P < 0.001), and higher 10-day NIHSS score (P < 0.001) (Table 2). There was a positive correlation between 90-day mRS score and both NIHSS scores (admission and 10-day) (P < 0.001 for all parameters) (Table 3). Regression analysis was performed using 90-day mRS score as an outcome variable. Univariate and multivariate logistic regression analysis demonstrated that 10-day NIHSS score was a predictor of 90-day mRS score [odds ratio (OR) = 1.458; 95 % confidence interval (CI), 1.092–1.947; P = 0.011] (Tables 4 and 5).
Table 3.
Spearman's rank correlations for 90-day mRS score
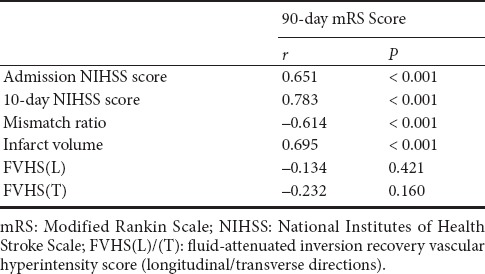
Table 4.
Univariate logistic regression analysis for good clinical outcomes
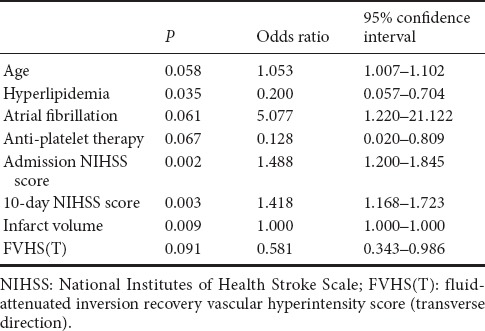
Table 5.
Multivariate logistic regression analysis for good clinical outcomes
Relationship between FVH score and 90-day mRS score
Mean mismatch ratio was 56.29 ± 20.59 and mean infarct volume was 163.03 ± 15.83 mL. Distal FVH was seen in 22 patients (58%). The FVHS(L) and FVHS(T) values are shown in Table 1. Sixteen had no distal FVH, meaning their corresponding FVHS(L) and FVHS(T) were 0. Patients with poor clinical outcomes had larger infarct volumes (P < 0.001) and smaller mismatch ratios (P = 0.011; Table 2). No differences in distal FVH, FVHS(L) or FVHS(T) were found between the poor and good clinical outcome groups (P > 0.05; Table 2), and no difference in 90-day mRS score was detectable among the FVHS(L) = 3–4, FVHS(L) = 5–6 or FVHS(L) = 7–8 groups. Similarly, no difference in 90-day mRS score was found between the FVHS(T) = 1 and FVHS(T) = 2–3 groups. The 90-day mRS score was positively correlated with infarct volume, and negatively with mismatch ratio (P < 0.001 for all parameters). The 90-day mRS score was not correlated with FVHS(L) and FVHS(T) (P = 0.421 and 0.160, respectively; Table 3). Logistic regression analysis demonstrated that neither infarct volume nor FVHS(T) were found to be independent variables associated with functional clinical outcome in the present model (Tables 4, 5, and Figure 2).
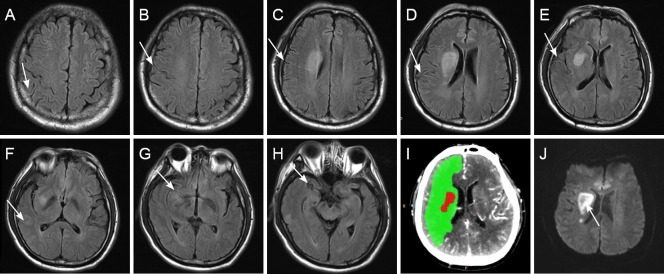
Figure 2.
T2-FLAIR, CTP and DWI images of a patient with acute ischemic stroke with right M1-MCA occlusion.
A 69-year-old patient with right M1-MCA occlusion. Admission NIHSS score = 8; 10-day NIHSS score = 6; FVHS(L) = 8 (A–H); FVHS(T) = 2 (E); CTP penumbral/infarct core mismatch ratio = 38.37 (I); DWI infarct volume (arrow) = 18.84 mL (J); 90-day mRS score = 2. Arrows, FVH. FLAIR. FLAIR: Fluid-attenuated inversion recovery; CTP: computed tomography perfusion; DWI: diffusion-weighted imaging; MCA: middle cerebral artery; NIHSS: National Institutes of Health Stroke Scale; FVHS(L)/(T): FLAIR vascular hyperintensity score (longitudinal/transverse directions); mRS: modified Rankin Scale.
Discussion
This study was conducted to find useful predictors for short-term functional outcome in AIS patients with M1-MCA occlusion. Our findings demonstrated that 10-day NIHSS score was positively correlated with 90-day mRS score and independently predicted 90-day functional outcome. In contrast, FVHS(L) and FVHS(T) had no correlation with the 90-day mRS score, and did not independently predict 90-day functional outcome in patients with AIS with M1-MCA occlusion.
Age is a powerful predictor of mortality and functional outcome after AIS (Weimar et al., 2004; König et al., 2008; Sato et al., 2008; Maruyama et al., 2017). However, we did not find a significant difference in age or other clinical variables between our two groups (poor and good clinical outcome). The small sample size and strict inclusion criteria in our study may explain this apparent discrepancy.
In the mid-1980s, the NIHSS was developed for the evaluation of AIS patients (Brott et al., 1989). Today, it is the standard instrument used for the assessment of patients with suspected ischemic stroke (Adams et al., 1999; Dancer et al., 2009; Lyden et al., 2009; Tan et al., 2009; Jauch et al., 2013). Many studies found that NIHSS score was the most powerful predictor of functional outcome and mortality after AIS (Woo et al., 1999; Fink et al., 2002; Wechsler et al., 2003; Weimar et al., 2004; Kimura et al., 2008; König et al., 2008; Sato et al., 2008; Nogueira et al., 2009; Olindo et al., 2012; Maruyama et al., 2017; Villalobos et al., 2017). Consistent with these reports, we found that NIHSS score was associated with 90-day functional outcome after AIS.
However, the NIHSS has been criticized for being biased towards language, meaning that cross-language or cross-cultural misinterpretations may occur (Woo et al., 1999; Fink et al., 2002). This has the potential to misguide treatment decisions (Villalobos et al., 2017). Therefore, a convenient and reproducible scoring system, to be used with NIHSS score, is necessary.
Increasingly, MRI is being used to evaluate and manage AIS within the first hours of occurrence, and it might be more effective than CT (Chalela et al., 2007). Evaluation of collateral supply remains challenging due to the small size and complex routes of vessels (Liebeskind, 2003). Important information on early collateral circulation can be provided by FVH (Sanossian et al., 2009; Chan et al., 2016; Gerber et al., 2016; Liu et al., 2016a; van Seeters et al., 2016). In this study, we used a very simple points system to longitudinally quantify FVH by counting images on 16 standardized 6-mm-thick T2-FLAIR sequences to allow quantification from the proximal temporal territory to the distal parietal MCA territory. Furthermore, a modified collateral score was previously proposed in which a high-resolution multi-detector CTA would be used to grade the degree of leptomeningeal collateral supply in the MCA territory from 0 (no collateral supply) to 3 (complete collateral supply) (Tan et al., 2007, 2009). We call this FVHS(T), and physicians can obtain these scores on routine MRIs, considerably more easily than other scores requiring MRI perfusion analysis. In addition, the FVH inter-observer agreement was excellent and images can be scored easily. Although somewhat crude, FLAIR-derived FVHS(L) and FVHS(T) provide simple and reproducible assessment of collateral circulation.
Good collateral blood flow in AIS is known to influence prognosis (Bozzao et al., 1989; Roberts et al., 2002; Kucinski et al., 2003; Christoforidis et al., 2005; Lee et al., 2009; Maas et al., 2009; Lima et al., 2010). In our previous study, we showed that patients with lower FVH score were more likely to have a smaller CTP penumbral/infarct core mismatch ratio and larger infarct volume (Li et al., 2017). As a result, patients with a lower FVH score could represent poor collateral circulation, and our conclusion would be in accordance with studies that established the importance of collateral supply to predict infract volume (Bozzao et al., 1989; Christoforidis et al., 2005; Lee et al., 2009). However, although FVHS(L) and FVHS(T) were associated with 90-day mRS scores, they were not found to be independent variables in our multivariate regression analysis. The clinical significance of FVH may reflect a larger CTP penumbral/infarct core mismatch ratio and a smaller infarct volume; in addition, the small sample size of our study might explain this discrepant finding.
Collateral circulation prolongs tissue viability and maximizes the volume of salvageable tissue, and therefore has considerable clinical implications for treatment decisions after AIS (Lee et al., 2000; Qureshi, 2002; Higashida et al., 2003; Liebeskind, 2005; Ovbiagele et al., 2007; Hendrikse et al., 2008; Tan et al., 2009; Lima et al., 2010; Guo et al., 2012; Wang et al., 2013, 2016a; Pereira et al., 2015; Akiyama et al., 2016; Cai et al., 2016; Da Ros et al., 2016; Mao et al., 2016; Wen et al., 2016; Tso et al., 2017). Our findings and previous reported cases (Ovbiagele et al., 2007; McVerry et al., 2012; Chan et al., 2016) indicate that collateral blood flow is a potential therapeutic target. But for targeting collateral vessels in stroke therapy, there must be consistency in the examination of their extent at baseline, to permit further expansion in this field.
The study has some limitations. First, as a mono-center small-sample analysis of a homogeneous population, patient selection bias is possible. Second, FVH is difficult to observe in some new MRI developments, such as 3D FLAIR and synthetic techniques.
In summary, there is ongoing extensive research worldwide into clinical and radiologic predictors to help determine favorable clinical outcomes in AIS. No single parameter can predict outcome accurately, and it is clear that a predictive model must incorporate a number of easily derived and reliable factors. In this study, our findings showed that 10-day NIHSS scores correlated positively with 90-day mRS scores and independently predicted 90-day functional outcomes of AIS patients with MCAO. In addition, we demonstrated that neither FVHS(L) nor FVHS(T) were independent variables associated with 90-day mRS scores. Whether these results will aid therapeutic strategies remains to be determined.
Acknowledgments
We are very grateful to Le-kang Yin and Xiao-xue Zhang from Department of Radiology, Huashan Hospital, Fudan University, China for their support and selfless help.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81371521.
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81371521. The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of the funding organization.
Research ethics: The study protocol was approved by the institutional review board of Huashan hospital of China (approval number: 2013-158).
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form the patients' close family members or legal guardians have given their consent for patients' images and other clinical information to be reported in the journal. The patients' close family members or legal guardians understand that the patients' names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
(Copyedited by Slone-Murphy J, Haase R, Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Adams H, Adams R, Del Zoppo G, Goldstein LB Stroke Council of the American Heart Association; American Stroke Association. Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update a scientific statement from the Stroke Council of the American Heart Association/American Stroke Association. Stroke. 2005;36:916–923. doi: 10.1161/01.STR.0000163257.66207.2d. [DOI] [PubMed] [Google Scholar]
- 2.Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama T, Morioka T, Shimogawa T, Haga S, Sayama T, Kanazawa Y, Murao K, Arakawa S. Arterial spin-labeling magnetic resonance perfusion imaging with dual postlabeling delay in internal carotid artery steno-occlusion: validation with digital subtraction angiography. J Stroke Cerebrovasc Dis. 2016;25:2099–2108. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Azizyan A, Sanossian N, Mogensen MA, Liebeskind DS. Fluid-attenuated inversion recovery vascular hyperintensities: an important imaging marker for cerebrovascular disease. AJNR Am J Neuroradiol. 2011;32:1771–1775. doi: 10.3174/ajnr.A2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzao L, Fantozzi LM, Bastianello S, Bozzao A, Fieschi C. Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke. 1989;20:735–740. doi: 10.1161/01.str.20.6.735. [DOI] [PubMed] [Google Scholar]
- 6.Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 7.Cai J, Wu D, Mo Y, Wang A, Hu S, Ren L. Comparison of extracranial artery stenosis and cerebral blood flow, assessed by quantitative magnetic resonance, using digital subtraction angiography as the reference standard. Medicine (Baltimore) 2016;95:e5370. doi: 10.1097/MD.0000000000005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, Hill MD, Patronas N, Latour L, Warach S. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–298. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan SL, Sweet JG, Bishop N, Cipolla MJ. Pial collateral reactivity during hypertension and aging: understanding the function of collaterals for stroke therapy. Stroke. 2016;47:1618–1625. doi: 10.1161/STROKEAHA.116.013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng B, Ebinger M, Kufner A, Kohrmann M, Wu O, Kang DW, Liebeskind D, Tourdias T, Singer OC, Christensen S, Warach S, Luby M, Fiebach JB, Fiehler J, Gerloff C, Thomalla G Stroke Imaging Repository (STIR) Investigators. Hyperintense vessels on acute stroke fluid-attenuated inversion recovery imaging: associations with clinical and other MRI findings. Stroke. 2012;43:2957–2961. doi: 10.1161/STROKEAHA.112.658906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng SC, Lin CH, Chang YJ, Lee TH, Ryu SJ, Chen CH, Chang HK, Chang CJ, Hu WL, Hung YC. Fire-heat and Qi deficiency syndromes as predictors of short-term prognosis of acute ischemic stroke. J Altern Complement Med. 2013;19:721–728. doi: 10.1089/acm.2012.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 13.Cosnard G, Duprez T, Grandin C, Smith AM, Munier T, Peeters A. Fast FLAIR sequence for detecting major vascular abnormalities during the hyperacute phase of stroke: a comparison with MR angiography. Neuroradiology. 1999;41:342–346. doi: 10.1007/s002340050761. [DOI] [PubMed] [Google Scholar]
- 14.Da Ros V, Meschini A, Gandini R, Del Giudice C, Garaci F, Stanzione P, Rizzato B, Diomedi M, Simonetti G, Floris R, Sallustio F. Proposal for a vascular computed tomography-based grading system in posterior circulation stroke: a single-center experience. J Stroke Cerebrovasc Dis. 2016;25:368–377. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Dancer S, Brown AJ, Yanase LR. National Institutes of Health Stroke Scale reliable and valid in plain English. J Neurosci Nurs. 2009;41:2–5. doi: 10.1097/jnn.0b013e31819345bf. [DOI] [PubMed] [Google Scholar]
- 16.Deng X, Zhang Z, Zhang Y, Zhang D, Wang R, Ye X, Xu L, Wang B, Wang K, Zhao J. Comparison of 7.0- and 3.0-T MRI and MRA in ischemic-type moyamoya disease: preliminary experience. J Neurosurg. 2016;124:1716–1725. doi: 10.3171/2015.5.JNS15767. [DOI] [PubMed] [Google Scholar]
- 17.Dengler NF, Madai VI, Wuerfel J, von Samson-Himmelstjerna FC, Dusek P, Niendorf T, Sobesky J, Vajkoczy P. Moyamoya vessel pathology imaged by ultra-high-field magnetic resonance imaging at 7.0 T. J Stroke Cerebrovasc Dis. 2016;25:1544–1551. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 18.Förster A, Kerl HU, Wenz H, Murle B, Habich S, Groden C. Fluid attenuated inversion recovery vascular hyperintensities possibly indicate slow arterial blood flow in vertebrobasilar dolichoectasia. J Neuroimaging. 2015;25:608–613. doi: 10.1111/jon.12177. [DOI] [PubMed] [Google Scholar]
- 19.Fink JN, Selim MH, Kumar S, Silver B, Linfante I, Caplan LR, Schlaug G. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke. 2002;33:954–958. doi: 10.1161/01.str.0000013069.24300.1d. [DOI] [PubMed] [Google Scholar]
- 20.Flacke S, Urbach H, Keller E, Traber F, Hartmann A, Textor J, Gieseke J, Block W, Folkers PJ, Schild HH. Middle cerebral artery (MCA) susceptibility sign at susceptibility-based perfusion MR imaging: clinical importance and comparison with hyperdense MCA sign at CT. Radiology. 2000;215:476–482. doi: 10.1148/radiology.215.2.r00ma09476. [DOI] [PubMed] [Google Scholar]
- 21.Gawlitza M, Friedrich B, Quaschling U, Schob S, Schaudinn A, Hobohm C, Hoffmann KT, Lobsien D. Distance to thrombus on MR angiography predicts outcome of middle cerebral artery occlusion treated with IV thrombolysis. Neuroradiology. 2015;57:991–997. doi: 10.1007/s00234-015-1558-9. [DOI] [PubMed] [Google Scholar]
- 22.Gerber JC, Petrova M, Krukowski P, Kuhn M, Abramyuk A, Bodechtel U, Dzialowski I, Engellandt K, Kitzler H, Pallesen LP, Schneider H, von Kummer R, Puetz V, Linn J. Collateral state and the effect of endovascular reperfusion therapy on clinical outcome in ischemic stroke patients. Brain Behav. 2016;6:e00513. doi: 10.1002/brb3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo G, Yang YG, Yang WQ. Validation of hyperintense middle cerebral artery sign in acute ischemic stroke: Comparison between magnetic resonance imaging and angiography. Neural Regen Res. 2012;7:229–234. doi: 10.3969/j.issn.1673-5374.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harteveld AA, van der Kolk AG, Zwanenburg JJ, Luijten PR, Hendrikse J. 7-T MRI in cerebrovascular diseases: challenges to overcome and initial results. Top Magn Reson Imaging. 2016;25:89–100. doi: 10.1097/RMR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 25.Hendrikse J, Klijn CJ, van Huffelen AC, Kappelle LJ, van der Grond J. Diagnosing cerebral collateral flow patterns: accuracy of non-invasive testing. Cerebrovasc Dis. 2008;25:430–437. doi: 10.1159/000121344. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Perez M, Puig J, Blasco G, Perez de la Ossa N, Dorado L, Davalos A, Munuera J. Dynamic magnetic resonance angiography provides collateral circulation and hemodynamic information in acute ischemic stroke. Stroke. 2016;47:531–534. doi: 10.1161/STROKEAHA.115.010748. [DOI] [PubMed] [Google Scholar]
- 27.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology; Technology Assessment Committee of the Society of Interventional Radiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 28.Husson B, Hertz-Pannier L, Adamsbaum C, Renaud C, Presles E, Dinomais M, Kossorotoff M, Landrieu P, Chabrier S. MR angiography findings in infants with neonatal arterial ischemic stroke in the middle cerebral artery territory: A prospective study using circle of Willis MR angiography. Eur J Radiol. 2016;85:1329–1335. doi: 10.1016/j.ejrad.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H American Heart Association Stroke Council; Council on Cardiovascular Nursing, Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 30.Karadeli HH, Giurgiutiu DV, Cloonan L, Fitzpatrick K, Kanakis A, Ozcan ME, Schwamm LH, Rost NS. FLAIR vascular hyperintensity is a surrogate of collateral flow and leukoaraiosis in patients with acute stroke due to proximal artery occlusion. J Neuroimaging. 2016;26:219–223. doi: 10.1111/jon.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SE, Lee BI, Kim SE, Shin KJ, Park J, Park KM, Kim HC, Lee J, Baek HJ, Jin SC, Ha SY. Clinical significance of fluid-attenuated inversion recovery vascular hyperintensities in borderzone infarcts. Stroke. 2016;47:1548–1554. doi: 10.1161/STROKEAHA.115.012285. [DOI] [PubMed] [Google Scholar]
- 32.Kimura K, Iguchi Y, Shibazaki K, Terasawa Y, Inoue T, Uemura J, Aoki J. Large ischemic lesions on diffusion-weighted imaging done before intravenous tissue plasminogen activator thrombolysis predicts a poor outcome in patients with acute stroke. Stroke. 2008;39:2388–2391. doi: 10.1161/STROKEAHA.107.510917. [DOI] [PubMed] [Google Scholar]
- 33.König IR, Ziegler A, Bluhmki E, Hacke W, Bath PMW, Sacco RL, Diener HC, Weimar C. Predicting long-term outcome after acute ischemic stroke. A simple index works in patients from controlled clinical trials. 2008;39:1821–1826. doi: 10.1161/STROKEAHA.107.505867. [DOI] [PubMed] [Google Scholar]
- 34.Kucinski T, Koch C, Eckert B, Becker V, Kromer H, Heesen C, Grzyska U, Freitag HJ, Rother J, Zeumer H. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology. 2003;45:11–18. doi: 10.1007/s00234-002-0881-0. [DOI] [PubMed] [Google Scholar]
- 35.Kvistad CE, Oygarden H, Logallo N, Thomassen L, Waje-Andreassen U, Moen G, Naess H. A stress-related explanation to the increased blood pressure and its course following ischemic stroke. Vasc Health Risk Manag. 2016;12:435–442. doi: 10.2147/VHRM.S109032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee KH, Cho SJ, Byun HS, Na DG, Choi NC, Lee SJ, Jin IS, Lee TG, Chung CS. Triphasic perfusion computed tomography in acute middle cerebral artery stroke: a correlation with angiographic findings. Arch Neurol. 2000;57:990–999. doi: 10.1001/archneur.57.7.990. [DOI] [PubMed] [Google Scholar]
- 37.Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S. Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke. Neurology. 2009;72:1134–1139. doi: 10.1212/01.wnl.0000345360.80382.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legrand L, Tisserand M, Turc G, Naggara O, Edjlali M, Mellerio C, Mas JL, Meder JF, Baron JC, Oppenheim C. Do FLAIR vascular hyperintensities beyond the DWI lesion represent the ischemic penumbra. AJNR Am J Neuroradiol. 2015;36:269–274. doi: 10.3174/ajnr.A4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li CC, Tong T, Yang YM, Yao ZW, Feng XY. Prognostic value of the ABCD2 score on long-term follow-up of transient ischemic attack using the new tissue-based definition. Neurol Asia. 2015;20:15–21. [Google Scholar]
- 40.Li CC, Yin LK, Zhang XX, Hao XZ, Tian JQ, Yao ZW, Feng XY, Yang YM. Fluid-attention inversion recovery vascular hyper-intensity: Correlation with other radiologic findings in acute ischemic stroke with middle cerebral occlusion. Neurol Asia. 2017;22:193–202. [Google Scholar]
- 41.Liang J, Liu W, Sun J, Gu X, Ma Q, Tong W. Analysis of the risk factors for the short-term prognosis of acute ischemic stroke. Int J Clin Exp Med. 2015;8:21915–21924. [PMC free article] [PubMed] [Google Scholar]
- 42.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 43.Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. doi: 10.1016/j.nic.2005.08.012. x. [DOI] [PubMed] [Google Scholar]
- 44.Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, Harris GJ, Halpern EF, Koroshetz WJ, Smith WS, Yoo AJ, Nogueira RG. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010;41:2316–2322. doi: 10.1161/STROKEAHA.110.592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu D, Scalzo F, Rao NM, Hinman JD, Kim D, Ali LK, Saver JL, Sun W, Dai Q, Liu X, Liebeskind DS. Fluid-attenuated inversion recovery vascular hyperintensity topography, novel imaging marker for revascularization in middle cerebral artery occlusion. Stroke. 2016a;47:2763–2769. doi: 10.1161/STROKEAHA.116.013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Zhan L, Wang Y, Bai C, Guo J, Lin Q, Liang D, Xu E. Metabolic syndrome and the short-term prognosis of acute ischemic stroke: a hospital-based retrospective study. Lipids Health Dis. 2015;14:76. doi: 10.1186/s12944-015-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Pu Y, Pan Y, Zhang Y, Dou X, Tan Y, Liu L, Wang Y. Multi-mode CT in the evaluation of leptomeningeal collateral flow and the related factors: comparing with digital subtraction angiography. Neurol Res. 2016b;38:504–509. doi: 10.1080/01616412.2016.1187828. [DOI] [PubMed] [Google Scholar]
- 48.Lyden P, Raman R, Liu L, Emr M, Warren M, Marler J. National Institutes of Health Stroke Scale certification is reliable across multiple venues. Stroke. 2009;40:2507–2511. doi: 10.1161/STROKEAHA.108.532069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, Harris GJ, Halpern E, Kemmling A, Koroshetz WJ, Furie KL. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009;40:3001–3005. doi: 10.1161/STROKEAHA.109.552513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao YT, Mitchell P, Churilov L, Dowling R, Dong Q, Yan B. Early recanalization postintravenous thrombolysis in ischemic stroke with large vessel occlusion: a digital subtraction angiography study. CNS Neurosci Ther. 2016;22:643–647. doi: 10.1111/cns.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maruyama K, Uchiyama S, Shiga T, Iijima M, Ishizuka K, Hoshino T, Kitagawa K. Brain natriuretic peptide is a powerful predictor of outcome in stroke patients with atrial fibrillation. Cerebrovasc Dis Extra. 2017;7:35–43. doi: 10.1159/000457808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol. 2012;33:576–582. doi: 10.3174/ajnr.A2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 54.Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS, MERCI, Multi MERCI Writing Committee Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke. 2009;40:3777–3783. doi: 10.1161/STROKEAHA.109.561431. [DOI] [PubMed] [Google Scholar]
- 55.Olindo S, Chausson N, Joux J, Saint-Vil M, Signate A, Edimonana-Kapute M, Jeannine S, Mejdoubi M, Aveillan M, Cabre P, Smadja D. Fluid-attenuated inversion recovery vascular hyperintensity: an early predictor of clinical outcome in proximal middle cerebral artery occlusion. Arch Neurol. 2012;69:1462–1468. doi: 10.1001/archneurol.2012.1310. [DOI] [PubMed] [Google Scholar]
- 56.Ovbiagele B, Saver JL, Starkman S, Kim D, Ali LK, Jahan R, Duckwiler GR, Vinuela F, Pineda S, Liebeskind DS. Statin enhancement of collateralization in acute stroke. Neurology. 2007;68:2129–2131. doi: 10.1212/01.wnl.0000264931.34941.f0. [DOI] [PubMed] [Google Scholar]
- 57.Park M, Kim KE, Shin NY, Lee SK, Lim SM, Song D, Heo JH, Kim JW, Oh SW. Thrombus length discrepancy on dual-phase CT can predict clinical outcome in acute ischemic stroke. Eur Radiol. 2016;26:2215–2222. doi: 10.1007/s00330-015-4018-3. [DOI] [PubMed] [Google Scholar]
- 58.Pereira BJ, Holanda VM, Giudicissi-Filho M, Borba LA, de Holanda CV, de Oliveira JG. Assessment of cerebral blood flow with micro-doppler vascular reduces the risk of ischemic stroke during the clipping of intracranial aneurysms. World Neurosurg. 2015;84:1747–1751. doi: 10.1016/j.wneu.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 59.Qureshi AI. New grading system for angiographic evaluation of arterial occlusions and recanalization response to intra-arterial thrombolysis in acute ischemic stroke. Neurosurgery. 2002;50:1405–1414. doi: 10.1097/00006123-200206000-00049. discussion 1414-1415. [DOI] [PubMed] [Google Scholar]
- 60.Roberts HC, Dillon WP, Furlan AJ, Wechsler LR, Rowley HA, Fischbein NJ, Higashida RT, Kase C, Schulz GA, Lu Y, Firszt CM. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: results from the PROACT II trial. Stroke. 2002;33:1557–1565. doi: 10.1161/01.str.0000018011.66817.41. [DOI] [PubMed] [Google Scholar]
- 61.Sanossian N, Saver JL, Alger JR, Kim D, Duckwiler GR, Jahan R, Vinuela F, Ovbiagele B, Liebeskind DS. Angiography reveals that fluid-attenuated inversion recovery vascular hyperintensities are due to slow flow, not thrombus. AJNR Am J Neuroradiol. 2009;30:564–568. doi: 10.3174/ajnr.A1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato S, Toyoda K, Uehara T, Toratani N, Yokota C, Moriwaki H, Naritomi H, Minematsu K. Baseline NIH Stroke Scale Score predicting outcome in anterior and posterior circulation strokes. Neurology. 2008;70:2371–2377. doi: 10.1212/01.wnl.0000304346.14354.0b. [DOI] [PubMed] [Google Scholar]
- 63.Schellinger PD, Chalela JA, Kang DW, Latour LL, Warach S. Diagnostic and prognostic value of early MR Imaging vessel signs in hyperacute stroke patients imaged < 3 hours and treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 2005;26:618–624. [PMC free article] [PubMed] [Google Scholar]
- 64.Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, Gonzalez G, Schaefer PW, Dillon WP, Koroshetz WJ, Furie KL. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40:3834–3840. doi: 10.1161/STROKEAHA.109.561787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, Martin M, Symons SP, Fox AJ, Aviv RI. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–531. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. 2007;61:533–543. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- 67.Tso MK, Lee MM, Ball CG, Morrish WF, Mitha AP, Kirkpatrick AW, Wong JH. Clinical utility of a screening protocol for blunt cerebrovascular injury using computed tomography angiography. J Neurosurg. 2017;126:1033–1041. doi: 10.3171/2016.1.JNS151545. [DOI] [PubMed] [Google Scholar]
- 68.Tsushima Y, Endo K. Significance of hyperintense vessels on FLAIR MRI in acute stroke. Neurology. 2001;56:1248–1249. doi: 10.1212/wnl.56.9.1248. [DOI] [PubMed] [Google Scholar]
- 69.van Seeters T, Biessels GJ, Kappelle LJ, van der Graaf Y, Velthuis BK. Determinants of leptomeningeal collateral flow in stroke patients with a middle cerebral artery occlusion. Neuroradiology. 2016;58:969–977. doi: 10.1007/s00234-016-1727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villalobos E, Barnes SR, Qureshi IA, Cruz-Flores S, Maud A, Rodriguez GJ. Spanish version of the national institutes of health stroke scale: awareness and use in united states. A survey study. J Vasc Interv Neurol. 2017;9:1–6. [PMC free article] [PubMed] [Google Scholar]
- 71.Wang G, Cheng X, Zhang X. Use of various CT imaging methods for diagnosis of acute ischemic cerebrovascular disease. Neural Regen Res. 2013;8:655–661. doi: 10.3969/j.issn.1673-5374.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang HK, Wang YX, Xue CB, Li ZM, Huang J, Zhao YH, Yang YM, Gu XS. Angiogenesis in tissue-engineered nerves evaluated objectively using MICROFIL perfusion and micro-CT scanning. Neural Regen Res. 2016a;11:168–173. doi: 10.4103/1673-5374.175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Zhang H, Ni D, Fan W, Qu J, Liu Y, Jin Y, Cui Z, Xu T, Wu Y, Bu W, Yao Z. High-performance upconversion nanoprobes for multimodal MR imaging of acute ischemic stroke. Small. 2016b;12:3591–3600. doi: 10.1002/smll.201601144. [DOI] [PubMed] [Google Scholar]
- 74.Wechsler LR, Roberts R, Furlan AJ, Higashida RT, Dillon W, Roberts H, Rowley HA, Pettigrew LC, Callahan AS, 3rd, Bruno A, Fayad P, Smith WS, Firszt CM, Schulz GA. Factors influencing outcome and treatment effect in PROACT II. Stroke. 2003;34:1224–1229. doi: 10.1161/01.STR.0000068782.15297.28. [DOI] [PubMed] [Google Scholar]
- 75.Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004;35:158–162. doi: 10.1161/01.STR.0000106761.94985.8B. [DOI] [PubMed] [Google Scholar]
- 76.Wen WL, Fang YB, Yang PF, Zhang YW, Wu YN, Shen H, Ge JJ, Xu Y, Hong B, Huang QH, Liu JM. Parametric digital subtraction angiography imaging for the objective grading of collateral flow in acute middle cerebral artery occlusion. World Neurosurg. 2016;88:119–125. doi: 10.1016/j.wneu.2015.12.084. [DOI] [PubMed] [Google Scholar]
- 77.Woo D, Broderick JP, Kothari RU, Lu M, Brott T, Lyden PD, Marler JR, Grotta JC. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30:2355–2359. doi: 10.1161/01.str.30.11.2355. [DOI] [PubMed] [Google Scholar]
- 78.Yang YJ, Gao LY, Fu J, Zhang J, Li YX, Yin B, Chen WJ, Geng DY. Apparent diffusion coefficient evaluation for secondary changes in the cerebellum of rats after middle cerebral artery occlusion. Neural Regen Res. 2013;8:2942–2950. doi: 10.3969/j.issn.1673-5374.2013.31.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yi TY, Chen WH, Zhang MF, Chen YH, Cai RW, Wu ZZ, Wu YM, Shi YC, Chen BL, Guo TH, Wu CX, Yang MX, Chen XJ. Diagnostic ability of 3-dimensional contrast-enhanced MR angiography in identifying vertebral basilar artery stenosis. J Neurol Sci. 2016;363:121–125. doi: 10.1016/j.jns.2016.02.013. [DOI] [PubMed] [Google Scholar]