Abstract
Many therapies with potential for treatment of brain injury have been investigated. Few types of cells have spurred as much interest and excitement as stem cells over the past few decades. The multipotentiality and self-renewing characteristics of stem cells confer upon them the capability to regenerate lost tissue in ischemic or degenerative conditions as well as trauma. While stem cells have not yet proven to be clinically effective in many such conditions as was once hoped, they have demonstrated some effects that could be manipulated for clinical benefit. The various types of stem cells have similar characteristics, and largely differ in terms of origin; those that have differentiated to some extent may exhibit limited capability in differentiation potential. Stem cells can aid in decreasing lesion size and improving function following brain injury.
Keywords: brain injury, brain trauma, infarction, ischemia, neural stem cells, neuronal regeneration, stroke
Introduction
Brain injury is a major cause of injury and death in both the United States and around the world. Many therapies have been investigated for the treatment of brain injury and stroke, and many investigations continue. The properties and characteristics of neural stem cells have been researched, and are being applied in therapeutic measures that could improve patient condition following brain injury.
Neural stem cells (NSCs) are plastic and are involved in creating new connections in adaptation, and in response to injury (Kennea and Mehmet, 2002; Teng et al., 2002; Galli et al., 2003; Imitola et al., 2004). NSCs play a fundamental role in development, and in the ability to respond to stimuli in the environment and injury.
Brain Injury
There are many ways in which the brain can be compromised or injured. Congenital defects such as neural tube defects, and neurodegenerative conditions like Parkinson's disease have dramatic impacts on survival, function, and overall health. Other conditions such as multiple sclerosis and varied infections similarly can have debilitating effects. This section, however, will focus on more acute and vascular processes. Traumatic brain injuries (TBI) are suffered in large numbers annually. It is estimated that approximately 500,000–700,000 children and 2.5 million adults experience TBI each year (Wilde et al., 2012; Li and Liu, 2013; Centers for Disease Control and Prevention, 2015; Dewan et al., 2016; Doan et al., 2016). More than 795,000 people have strokes each year in the United States alone (Centers for Disease Control and Prevention (CDC), 2012). Stroke is one of the leading causes of death and impairment in industrialized countries (Taylor et al., 1996).
Ischemia/Infarction
The brain requires a continuous supply of glucose and oxygen which is provided through the cerebral vasculature. Even though the brain only accounts for about 2% of the total body weight, it consumes approximately 20% of the oxygen pumped through the body. There are many regulatory systems relating to baroreceptors and their pathways controlling vascular dilation, constriction, and overall resistance that maintain relatively constant blood flow to the brain despite changes in blood pressure and intracranial pressure through autoregulation. Being a highly vascular organ, reduction in blood flow to the brain causes interruption of oxidative metabolism and a consequent deprivation of adenosine triphosphate (ATP). The inability of the brain to effectively metabolize substrates other than glucose, and perform glycolysis heighten its dependence on constant blood flow. This loss of ATP and appropriate membrane potential in an ischemic event causes impairment of neuronal function, and eventual cell death. Calcium ions also play a cytotoxic role in ischemic injury. Cells experience an influx of calcium due to the activation of glutamate receptors such as N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), and kainate (KA), or through the opening of other channels, activation of transporters, or release of intracellular calcium stores. This final mechanism is mediated by physical damage to organelles such as the mitochondria or endoplasmic reticulum, or by malfunction of their associated receptors or channels (Szydlowska and Tymianski, 2010). This excessive cytoplasmic calcium leads to the activation of several enzymes and processes that lead to cell death and impair neuronal function (Dirnagl et al., 1999). While calcium ions are required by neurons for proper function and maintenance (Penn and Loewenstein, 1966), they can be induced to toxic levels by release of the excitatory neurotransmitter, glutamate, due to deficiency of high-energy carrier molecules and substrates needed for energy production (Lucas and Newhouse, 1957; Olney, 1969; Simon et al., 1984; Dirnagl et al., 1999; Orrenius et al., 2003).
It should also be noted that cells are presumed to die by apoptosis as an illustration of secondary injury. This is suggested by the concept of the penumbra, the area of at-risk tissue that can be saved in animal models through the administration of anti-apoptotic agents (Yepes et al., 2000; Nakase et al., 2003; Xu et al., 2003; Lei et al., 2004; Price et al., 2010; Kumar et al., 2015). Damage sustained in the penumbra in ischemic injury results from excitotoxicity as well as indirect mechanisms such as peri-infarct depolarization, inflammation, and/or apoptosis which spread from the point of injury in an expansive manner. Peri-infarct depolarization is the repetitive depolarization of cells in the penumbral region that consumes energy in the face of its deficiency. This type of depolarization has been shown to increase the size of infarcts in animals, and similar electrocorticographic depression in humans indicates that these depolarization events may cause tissue damage in ischemic injury (Mies et al., 1993; Dirnagl et al., 1999; Hartings et al., 2003; Fabricius et al., 2006; Strong et al., 2007).
Inflammation can cause substantial tissue damage throughout the body and the brain. Many pro-inflammatory factors are induced by second messenger systems activated by calcium ions, and oxygen radicals. Some of these include nuclear factor kappa beta (NF-κB), hypoxia-inducible factor 1 (HIF-1), interferon regulatory factor 1 (IRF1), and signal transducer and activator of transcription 3 (Stat3), which upregulate tumor necrosis factor alpha (TNFα), platelet-activating factor (PAF), and interleukin 1 beta (IL-1β) (Astrup et al., 1981; Iijima et al., 1992; Obrenovitch, 1995; Furlan et al., 1996; Read et al., 1998; Dirnagl et al., 1999). Adhesion molecules such as selectins and integrins recruit neutrophils in the inflammatory process, which are followed by macrophages and monocytes (Etzioni, 1996; Iadecola, 1997). In addition to blood-borne inflammatory agents, native neural cells such as astrocytes and microglia also contribute to inflammatory processes following ischemia (Planas et al., 1996; Dirnagl et al., 1999). It has been shown that neutrophils can worsen ischemia by blocking microvasculature, and that inducible toxins like nitric oxide, superoxide, and toxic prostanoids, produced by native cells, cause additional damage (Nogawa et al., 1997; Gong et al., 1998; Zhang et al., 1998).
Cells that are damaged during brain injury by excessive cytoplasmic calcium, reactive oxygen species (ROS), or other mechanisms can continue toward cell death by necrosis or apoptosis. A primary factor contributing to apoptosis is the degree of damage and associated signaling (Loddick and Rothwell, 1996). Necrosis tends to follow the more severe ischemic injuries, but in milder cases, apoptosis can contribute significantly to the overall damage induced in the penumbra (Dirnagl et al., 1999). Ischemia induces increased expression of caspase-encoding genes, which causes cleavage of proteins involved in homeostasis, and the consequent death of cells. Caspases 1 and 3 seem to be particularly involved in programmed cell death in ischemic events; it has been specifically noted that mice without Caspase 1 are resistant to apoptotic ischemic insult (Bruce et al., 1996; Chopp et al., 1996; Baird et al., 1997; Nogawa et al., 1997; Dirnagl et al., 1999). Attenuation of the effects of apoptosis following ischemic injury could potentially reduce damage, and should continue to be explored.
Hemorrhage
While the majority of strokes (87% in the United States) are ischemic, hemorrhagic strokes account for approximately 13% of the total insults, being divided into 10% intracerebral hemorrhage (ICH) and 3% subarachnoid hemorrhage (SAH) (Benjamin et al., 2017). Despite the greater occurrence of ischemic strokes, of the 6.5 million people who died of strokes in 2013, 3.3 million died of ischemic stroke, and 3.2 million died of hemorrhagic stroke (Benjamin et al., 2017). Cerebral hemorrhage includes intraparenchymal, subdural, and epidural bleeds, and has a mortality rate around 50% (Frizzell, 2005). These hemorrhages are typically the result of trauma, but can also occur due to vascular malformations (Table 1). It is common to see more rapid progression of symptoms in epidural hematomas, and less rapid progression in subdural hematomas. Also, cerebral hemorrhage events often result from ruptured cerebral arteries. The devastating results of these strokes are often due to their effect on the surrounding tissue. It is not uncommon for pressure to be exerted on sensitive structures, or for tissue adjacent to the initial clot to become necrotic even following its resolution and reduction in size (Messing, 2003). Surrounding vessels can be irritated upon contact with hemorrhagic blood, causing vasospasm. Vascular malformations are also a reported cause of intracranial hemorrhage (Malik et al., 1988; Brouillard and Vikkula, 2007; Sayama et al., 2009).
Table 1.
Patterns of vascular injury based on location, etiology and pathological features
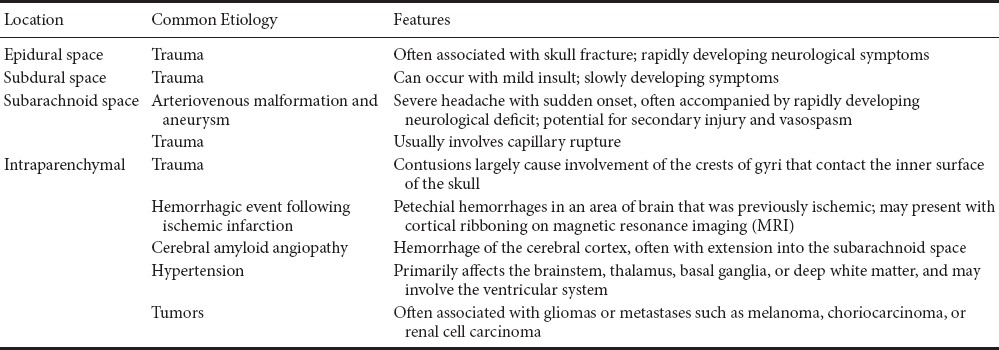
Upon cerebral artery rupture, blood can spread in the brain and subdural space, depending on its location (Table 1). If blood moves into the ventricles, there is an increased risk of mortality (Smith et al., 2005). However, hemorrhages of hypertensive origin typically present with a rapid onset of symptoms, whereas those caused or triggered by anticoagulant therapy often have a much slower onset (Frizzell, 2005). The blood from acute hemorrhage can exert pressure on the surrounding tissue which leads to ischemic injury and ensuing edema and necrosis if not managed. Around 48 hours following the initial injury, macrophages are onsite phagocytizing both blood and necrotic tissue. This action and the corresponding inflammation cause cavitation of the brain tissue. Astrocytes are recruited to reside in the cavity, and initiate angiogenesis (Frizzell, 2005; Kumar et al., 2015).
Brain trauma synopsis
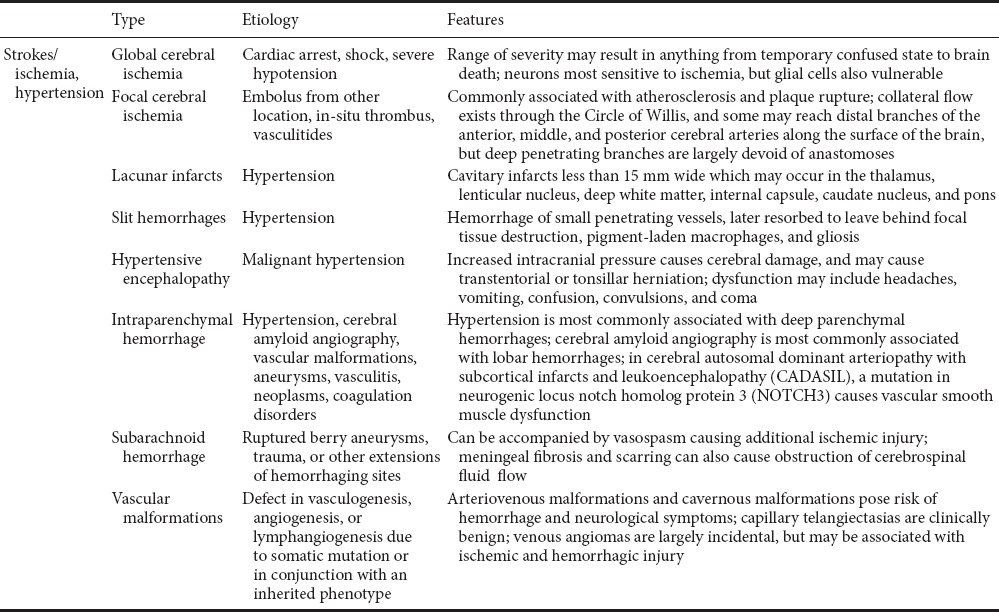
Beyond ischemic injury, there are varying gross and microscopic observations that can be made in different types of TBI (Table 2). Open injuries are those involving penetration of the scalp and skull by bullets or sharp objects, or skull fracture with associated skin laceration (Parikh et al., 2007). Closed injuries do not involve penetration, but rapid acceleration or deceleration of the brain, typically due to striking or shaking of the head. This can cause damage in coup/contrecoup patterns or diffusely, with the anterior tips of the frontal and temporal lobes being particularly susceptible. This often leads to shearing or tearing of axons or vasculature.
Table 2.
The types, etiology and features of strokes and ischemia
Concussion is a temporary and reversible alteration in mental status. Concussion results in temporary disability and deficit in aspects such as memory, and may cause nausea, dizziness, headache, and other symptoms (Parikh et al., 2007). While concussion alone has not been shown to cause permanent neurological damage, concussive injuries can be accompanied by axonal injury or progressive tauopathy which may have more lasting effects, particularly if multiple injuries are sustained (McCrea et al., 2003; Parikh et al., 2007; McKee et al., 2009).
Diffuse axonal injury (DAI) results from rapid deceleration of the head which causes generalized, damage to axons and their myelin sheaths. Petechial hemorrhages in the white matter are often observable on magnetic resonance imaging (MRI) following DAI, and may rarely be visualized using computed tomography (CT) imaging. DAI can be associated with transient alteration in mental status, and can have dangerous repercussions in cases in which increased intracranial pressure ensues (Gentry et al., 1988a, b; Blumbergs et al., 1989; Parikh et al., 2007).
Contusions of the brain are associated with both open and closed injuries, and can have varying effects depending on their size and location. The contusions that involve more tissue are more likely to cause significant edema and increased intracranial pressure, with increased likelihood of neurological deficit (Adams et al., 1985; Parikh et al., 2007).
Skull fractures are often classified as linear, depressed, or comminuted. They range in severity from posing great risk of mortality, to being relatively benign. Depressed fractures are often the most dangerous as they are associated with the greatest risk of dural tear and brain damage. In most cases, linear skull fractures do not pose a large risk unless there is underlying neurological damage or the affected individual is particularly susceptible, usually due to young age (Parikh et al., 2007).
The damage in TBI can occur from the direct tissue injury, or from secondary events following the initial insult (DeWitt and Prough, 2003; Nortje and Menon, 2004). As previously discussed, these secondary events largely result from mass effect due to edema or hemorrhage, increased intracranial pressure, or ischemia. Secondary injury following brain trauma has a particular impact on the hippocampus; hippocampal injuries are typically associated with memory and learning disabilities (Sun, 2014).
Regenerative Changes Associated with Brain Injury
Glial cells are involved in the formation of scars in neural tissue following damage. While astrogliosis, other forms of glial scarring, and various obstructive processes can complicate the healing process, or reduce neural function, much of the disability following brain injury results from a loss of tissue or cells. This is well illustrated by cavitation and other signs of neuronal and glial loss after injury. The progression from intraparenchymal hemorrhage, to necrosis, to cavitation has been observed in animal models, and has also been demonstrated in pathological studies of humans (Edward Dixon et al., 1991; Rosenfeld et al., 2013). Reactive astrogliosis is the response of astrocytes to CNS insult or injury; this could include anything from trauma or ischemia, to infection or degenerative conditions. In this process, alterations in the gene expression and morphology of astroctyes, and the formation of glial scar tissue is prevalent (Eddleston and Mucke, 1993; Pekny and Nilsson, 2005; Sofroniew, 2005, 2009; Maragakis and Rothstein, 2006; Correa-Cerro and Mandell, 2007). In reactive astrogliosis, it has been noted that astrocytes can suffer loss of function due to injury, but may also exhibit abnormal gain of function (Sofroniew, 2009). Both effects could contribute to deleterious disease processes or neural deficit following the initial injury.
The loss of neurons and glia in the process of brain injury can have devastating effects. It has been shown that NSCs or multipotent neural progenitor cells continue to be produced in the subventricular zone (SVZ), and the dentate gyrus of the hippocampus throughout the life of an animal (Lois and Alvarez-Buylla, 1993; Gage et al., 1998; Sun, 2014). Recently, there has been much interest in the therapeutic potential of NSCs or neuroepithelial cells. It has been noted that the brain can replenish or regenerate lost neurons through the division and differentiation of NSCs in response to neuronal damage or death; this cell proliferation and neurogenesis increases in response to trauma (Sun, 2014).
NSCs
Neuroepithelial cells are originally derived from the neural plate that forms along the midline of the embryo, and continues to fold into the neural tube (Clarke, 2003). Neuroepithelial cells then begin to differentiate, either into neurons or glial cells. Differentiation patterning is largely dependent on inductive organizing centers, such as those at the basal and alar plates, which stimulate different types of growth depending on the signaling gradients. Sonic hedgehog (SHH) induces ventral signaling patterns, and bone morphogenetic proteins (BMP) and growth differentiation factor (GDF) induce dorsal patterning (Altmann and Brivanlou, 2001). Fibroblast growth factor (FGF), Wingless-type MMTV integration site family member (Wnt), and retinoic acid signaling pathways have been shown to be involved in the caudalization of neural tissue. It is suggested that Hox gene patterning affects the antero-posterior axis of development. Activin has been implicated in left-right axis development (Altmann and Brivanlou, 2001). These signaling pathways interact with neural tissue in a concentration-dependent manner to effect the differentiation and development of neural tissue (Lumsden and Krumlauf, 1996; Kobayashi et al., 2002; Clarke, 2003). Migration of neuroepithelial cells occurs along the lattices or pathways formed by radial glia from the ventricular zone to the SVZ. The radial glia connects the ventricular surface of the neural tube with the pial surface. This connection enables neuronal cells to migrate to the SVZ where a secondary germinal center is established. It is thought that glial cells primarily originate from neuroepithelial cells that remain in the ventricular zone until differentiation into glioblasts, following which they migrate to the SVZ to proliferate and become astrocytes and oligodendrocytes (Price and Thurlow, 1988; Levison and Goldman, 1997; Rao and Mayer-Proschel, 1997; Barres and Barde, 2000; Clarke, 2003). Some SVZ cells can give rise to multiple lineages such as astrocytes and oligodendrocytes, and in some cases, both neurons and glial cells can differentiate from the same cell, though this is not universally accepted (Levison and Goldman, 1993; Price, 1994; Clarke, 2003).
During development, the ventricular zone disappears, and remaining neuroepithelial cells largely develop into ependymal cells which line the ventricular system. The SVZ persists throughout adulthood, and is directly adjacent to the ependymal layer around most of the ventricular regions. As continued development and compartmentalization of the CNS occurs, NSCs are primarily concentrated in the regions of the olfactory bulb, the SVZ of the forebrain, the hippocampus, the cerebellum, the cerebral cortex, and the spinal cord, though these distributions vary with species (Clarke, 2003). Interestingly, the stem cells found in these locations appear to be distinct from one another, those cells in a particular region will give rise to progeny typical of the cells in that region (Gaiano and Fishell, 1998; Kalyani et al., 1998; Temple, 2001).
While in typical scenarios in vivo, NSCs differentiate into either neuronal or glial cells, it is thought that this preferential differentiation results from the environment and signaling factors to which the stem cells are exposed. It has been shown that NSCs can differentiate into hematopoietic cells and muscle cell types in vitro (Bader et al., 1982; Bjornson et al., 1999; Clarke et al., 2000). This plasticity appears to be multifaceted as bone marrow stem cells have likewise been demonstrated to develop into astroglia and microglia (Eglitis and Mezey, 1997; Price and Williams, 2001). Clark et al. (2000) found that a mouse blastocyst injected with lactose operon Z (lacZ)-expressing NSCs develops into a chimera, with the NSCs developing into all cell types. Nerve growth factor (NGF) has been shown to increase the differentiation of mouse embryonic stem cells into neurons (Antonov et al., 2017).
Cell markers
Rushing and Ihrie categorized the currently-known markers for neural cells and their progenitors (Rushing and Ihrie, 2016). Beginning with patterns of differentiation characteristic of embryonic development, notable markers for neuroepithelial cells include Nestin (neuroectodermal stem cell marker), sex determining region Y-box 2 (SOX2), Vimentin, and RC2 (type of radial glia antibody). Radial glia also express these markers, along with paired box gene 6 (Pax6), L-glutamate/L-aspartate transporter (GLAST), and brain lipid binding protein (BLBP). Neuronal-restricted precursors, arising from the neuroepithelium are marked by embryonic neural cell adhesion molecule (E-NCAM), and the mature neurons developing from them express neuronal-specific nuclear protein (NeuN), microtubule-associated protein 2 (MAP2), neuron-specific enolase (NSE), and beta III tubulin (TuJ1). It appears to be from the radial glia that all glial cells develop, though they are still capable of developing into neurons through the pathway of intermediate progenitor cells which are marked by T-brain factor 2 (Tbr2). The mature neurons that arise from radial glia express T-brain factor 1 (Tbr1), NeuN, MAP2, NSE, and TuJ1. Radial glia develops into oligodendrocyte precursor cells expressing neural/glial antigen 2 (NG2), ganglioside precursor disialohematoside (GD3), and platelet-derived growth factor receptor alpha (PDGFRα), with fully-differentiated oligodendrocytes being marked by myelin-associated glycoprotein (MAG), BMP, and O4 (common oligodendrocyte marker). Mature astrocytes express S100β (multifunctional protein abundant in astrocytes), GLAST, and glial fibrillary acidic protein (GFAP). It has been suggested that cluster of differentiation 44 (CD44) serves as a marker for astrocyte-restricted precursors, but the existence of such precursors remains largely unsubstantiated (Liu et al., 2004).
Adult neural cell populations express some of the same markers as those of embryonic populations, but also some that are different. Adult NSCs or B1 cells (subpopulation of adult neural stem cells with astroglial properties) are noted to express GFAP, integrin alpha 6 (ITGA6/CD49f), 3-fucosyl-N-acetyl-lactosamine (LeX/CD15/SSEA-1), SOX2, Vimentin, and GLAST; additionally, BLBP, epidermal growth factor receptor (EGFR), and mammalian achaete scute homolog-1 (Mash1) are expressed on activated B1 cells, vascular cell adhesion molecule 1 (VCAM-1) is expressed on quiescent B1 cells, prominin-1 (CD133) is present on the primary cilia, and Nestin is often found on adult NSCs, but depends on regulatory processes. Transit amplifying (TA) precursors differentiate from B1 cells, and express distal-less homeobox 2 (DLX2), ITGA6/CD49f, GLAST, and in some cases, EGFR and Mash1. TA precursors may then differentiate into immature neuroblasts which express Tuj1, DLX2, polysialylated-neural cell adhesion molecule (PSA-NCAM), doublecortin (DCX), and low levels of cluster of differentiation 24 (CD24). Mature neurons that differentiate postnatally still express Tuj1, NeuN, MAP2, and NSE. It was also noted that ependymal cells express Nestin, CD133, CD24, and Vimentin. The understanding of neural cell lineages and their markers and effective characterization continues to progress (Rushing and Ihrie, 2016).
Activation, migration, and differentiation of neural stem cells
In adults, NSCs have been identified in areas near blood vessels in the dentate gyrus, SVZ, and in the high vocal center of songbirds (Louissaint et al., 2002; Shen et al., 2004). Shen et al. (2014) demonstrated that endothelial cells release factors that are important for the self-renewal of NSCs, and also inhibit their differentiation and increase the production of neurons. It has been observed that co-culturing NSCs with endothelial cells promotes that growth of epithelial sheets with increased junctional contact, and this could play a role in increasing the self-renewing qualities of stem cells by influencing β-catenin pathways, modifying the mode of cell division, and affecting Notch expression through Hes1 upregulation (Nakamura et al., 2000; Lu et al., 2001; Ohtsuka et al., 2001; Chenn and Walsh, 2002; Hitoshi et al., 2002; Chojnacki et al., 2003; Shen et al., 2004). It was shown that despite the ability of fibroblast growth factor-2 (FGF2) to promote NSC proliferation, endothelial factors were required to perpetuate self-renewal (Shen et al., 2004). FGF2 has been shown to be a potent activator of latent NSCs from varying regions of the adult brain to promote proliferation and neurogenesis (Palmer et al., 1999).
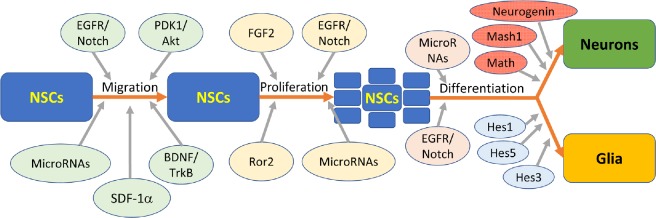
‘Niches’ for stem cell property modulation have been identified; these niches consist of various growth or signaling factors and the environments conducive to promoting certain characteristics or developmental steps in stem cells (Figure 1). The niches for NSCs in adults are those previously mentioned: namely the SVZ, and the dentate gyrus. Aguirre et al noted the delicate balance that must be maintained between NSCs and neural progenitor cells in order to continue providing the brain with the needed populations of neural cells (Aguirre et al., 2010). Moreover, the interplay between epidermal growth factor receptor (EGFR) and Notch signaling pathways is essential to maintenance of appropriate niches for self-renewal, differentiation, and proliferation of NSCs. In examination of the SVZ niche, it was observed that Notch influences NSC identity and self-renewal, while EGFR is primarily responsible for neural progenitor cell proliferation and migration (Lillien and Raphael, 2000; Hitoshi et al., 2002; Alexson et al., 2006; Breunig et al., 2007; Aguirre et al., 2010).
Figure 1.
The general process of neural stem cell (NSC) migration, proliferation, and differentiation involves many factors.
Many nuances that have been determined are not illustrated in this figure; for example, certain microRNAs induce proliferation of, and differentiation to specific cell types. It is also notable that the distinction between the specific functions or mechanisms of many factors is not yet well-defined in many cases; it has been observed that a certain factor results in an increase of neurons or astrocytes, but the mechanism, whether it acts on proliferation or differentiation, for example, is yet to be determined. EGFR: Epidermal growth factor receptor; PDK1: phosphoinositide dependent kinase 1; FGF2: fibroblast growth factor-2; Mash1: mammalian achaete scute homolog-1; Math: mammalian atonal homolog protein; SDF-1α: stromal cell-derived factor 1α; BDNF: brain derived neurotrophic factor; Ror2: receptor tyrosine kinase like orphan receptor 2.
The maintenance of NSC populations with multipotent abilities requires stromal cell-derived factor 1 (SDF-1) and its main receptor, C-X-C chemokine receptor type 4 (CXCR4) as their removal causes neurogenic differentiation (Ho et al., 2017). Ho et al. (2017) observed that in response to the elimination of SDF-1 in mice, expression of the proneural gene, achaete-scute homolog 1 (Ascl1), and the antiproliferative gene, p27, was increased significantly, which could account for the maintenance of the ‘stemness’ of the population.
NSC differentiation is largely dependent on the expression of basic helix-loop-helix (bHLH) genes. As was noted by Kageyama et al. (2005), NSC populations initially proliferate exclusively, and then differentiate first into neurons and later into glial cells. Repressor-type bHLH genes include Hes1, Hes3, and Hes5 (genes encoding hairy enhancer of split proteins), and appear to be crucial for the proliferation of glial cells such as astrocytes and ependymal cells. It was also observed that in the absence of repressor-type bHLH genes, proliferation was inadequate to give rise to significant glial populations. The activator-type bHLH genes include Mash1, Math, and Neurogenin, and promote the differentiation of NSCs to neurons. These genes are also involved in the induction of Notch ligands that regulate Hes1 and Hes5 expression. In this manner, the activator-type and the repressor-type bHLH genes inter-regulate each other in maintenance of appropriate signals for proliferation of both neurons and glia (Kageyama et al., 2005) (Figure 1).
MicroRNAs also play a role in bHLH regulation and neurogenesis with miR-1297 being an inhibitor of Hes1 expression (Zheng et al., 2017). Also, miR-199 and miR-214 have been shown to disrupt neurogenesis and neuron migration in mice (Mellios et al., 2017). It has been observed that miR-338-3p is highly expressed in the dentate gyrus, and is involved in neuronal maturation regulation; the influence of microRNAs on neurogenesis in the dentate gyrus is largely attributable to effects on gene expression (Howe et al., 2017). Many microRNAs are expressed in the dentate gyrus; miR-19 has also been shown to play a role in the migration of neurons in adults (Han and Gage, 2016). miR-410 appears to play a role in the differentiation of multipotent NSCs, as it has been noted to promote astrocyte differentiation while inhibiting neuron and oligodendrocyte differentiation (Tsan et al., 2016). It has been demonstrated that the miR-200 family is involved in regulation of neuronal maturation in normal, postnatal neurogenesis (Beclin et al., 2016). miR-193a has been observed to enable neurogenesis in F11 cells (Oh et al., 2017). miR-9 is involved in gene expression regulation, and is needed for neurogenesis, and sustaining and differentiation of neuronal progenitors (Radhakrishnan and Alwin Prem Anand, 2016). MicroRNA let-7a is reported to be involved in the regulation of NSC differentiation through its modulation of regulator expression (Song et al., 2016). Also, miR-17-92 has an effect on the regulation of neurogenesis in the adult hippocampus (Jin et al., 2016). This is not a comprehensive accounting of the functions of microRNAs in NSC activation, migration, and differentiation, but rather aims to convey that vast amounts of research continue to be done on the neuromodulatory effects of these molecules (Figure 1).
The methyl-CpG-binding domain protein, methyl-CpG-binding domain protein 1 (MBD1) has been shown to be necessary for effective neurogenesis (Lax and Sapozhnikov, 2017). The ability of MBD1 to enable neurogenesis may be facilitated primarily by methylated DNA promoter stabilization and gene expression silencing (Jørgensen et al., 2004; Lax and Sapozhnikov, 2017). It has also been suggested that MBD1 may perpetuate its effect through an indirect mechanism by inhibiting specific transcriptional repressors which causes an upregulation of transcription (Jobe et al., 2017; Lax and Sapozhnikov, 2017). Additional research will help to clarify the underlying mechanism of the effect of MBD1.
Cardiovascular exercise improves learning, memory, and executive function in humans, and has been shown to reduce the risk of developing neurodegenerative disorders; it is thought that this effect may be related to the increase in brain volume in regions prone to degeneration in old age (Cotman et al., 2007; Hillman et al., 2008; Blackmore et al., 2009). Neurogenesis declines significantly with age in mice, and it has been demonstrated that voluntary exercise and exposure to an enriched environment increases neurogenesis in the hippocampal and periventricular zones of mice (Kempermann et al., 1998, 2002; van Praag et al., 1999, 2005; Lindsey and Tropepe, 2006; Wu et al., 2008; Blackmore et al., 2009). Insulin-like growth factor-1 (IGF-1), brain derived neurotrophic factor (BDNF), and vascular endothelial-derived growth factor (VEGF) are important mediators of hippocampal neurogenesis augmentation due to exercise (Cotman et al., 2007). Blackmore et al. (2009) also observed that growth hormone and growth hormone receptor signaling (GH/GHR) was vital to the exercise-induced activation of neural progenitor cells in regions outside of the hippocampus. Interestingly, it has been noted that successful learning in the context of ‘mental training’ can improve neuronal survival in the wake of neurogenesis (Curlik 2nd and Shors, 2013).
The physical space and structural components in the environment of the NSCs appear to play a role in their differentiation. Christopherson et al. (2009) demonstrated that the diameter of fibers comprising laminin-coated electrospun polyethersulfone meshes had a significant impact on the NSC proliferation and differentiation. Cells were cultured for five days on tissue culture polystyrene (TCPS) plates, and 283 nm-, 749 nm-, and 1,452 nm-diameter fiber meshes. In the presence of FGF2 and serum-free medium, increasing fiber diameter correlated with decreased NSC migration and proliferation. In the presence of 1 μM retinoic acid and 1% fetal bovine serum (FBS), stem cells on the two-dimensional (2D) culture plate and the 283 nm fiber mesh were more prone to spread and differentiate into glia such as oligodendrocytes, whereas the stem cells cultured on the 749 nm and 1,452 nm fiber meshes were seen to elongate and differentiate in higher proportions to neuronal lineages. Ostensibly, this appears to indicate that the structural aspects in the stem cell environment influence migration, proliferation, and differentiation.
Historically, an efficient way of measuring the absence, occurrence, or level of neurogenesis has been lacking. Such measurements could aid researchers in examining the growth of neurons following injury in response to various treatments. The proteoglycan Glypican-2 (Gpc2) which can be measured in the cerebrospinal fluid, has been identified as being expressed in levels strongly correlative with adult hippocampal neurogenesis (Lugert et al., 2017). Gpc2 may prove to be an important factor in neurogenesis, or may serve as a useful measure of adult neurogenesis; however, more research is needed to illuminate the involvement of the glycoprotein in neural cell proliferation and differentiation.
There has been some recent interest in the potential difference in response between NSCs and differentiated cells to DNA damage, and the ways in which that may affect differentiation and proliferation (Barazzuol et al., 2017; Beckta et al., 2017; Shimura et al., 2017). However, the information reviewed does not readily prompt conclusion as to the existence, applicability, or influence of substantial difference in response. This underscores the need for further research in this area. There have also been some studies into the effects of melatonin on NSCs (Niles et al., 2004; Moriya et al., 2007; Kong et al., 2008; Sotthibundhu et al., 2010; Fu et al., 2011). Melatonin may enhance NSC differentiation into oligodendrocytes and neurons and engraftment following transplantation (Mendivil-Perez et al., 2017). It is thought that this may result from an increase in mitochondrial activity due to melatonin administration, and from protection of NSCs from the effects of inflammation (Song et al., 2015; Mendivil-Perez et al., 2017).
The role of sonic hedgehog (Shh) in neural progenitor cell migration and differentiation in development was mentioned previously. The effect of Shh pathway on NSCs following stroke was examined by Jin et al. (2017). They report that following administration of a sonic hedgehog agonist one month after ischemic stroke, there was improved survival of new NSCs in the subgranular and subventricular zones, as well as neuroblast cells and neurons. Continued investigation into the effects of the sonic hedgehog pathway on NSCs could be of benefit.
Endo and Minami (2018) reviewed the influence of Ror-family receptor tyrosine kinases (RTKs) on neural and glial cell development. They noted that receptor tyrosine kinase like orphan receptor 1 (Ror1) and receptor tyrosine kinase like orphan receptor 2 (Ror2) likely play an important role in nervous system development as they are found in relative abundance in neural progenitor cells during development, and in neurons in some regions. It has also been observed that the levels of Ror1 and Ror2 expressed decreases over the course of development. These particular RTKs have been found in extending neurites, and may be implicated in the extension process (Paganoni and Ferreira, 2003, 2005). Ror1, Ror2, and Wnt5a also play an important role in synaptogenesis, as their suppression significantly reduces the number and density of presynaptic clusters per neuron (Paganoni et al., 2010). Ror2 has been shown to be crucial to N-methyl-D-aspartate receptor (NMDAR)-mediated synaptic transmission as well as astrocyte function and proliferation during neural repair following injury (Cerpa et al., 2015; Endo and Minami, 2018). It is of interest that Ror2 expression is also increased in demyelinating disorders (Shimizu et al., 2016; Endo and Minami, 2018).
Stem cell-based therapy
The development of stem cell-based therapeutic treatments for brain injury is an emerging area of medical research. Among the promising therapies, mesenchymal stem cell (MSC) administration has been widely followed (Figure 2). MSCs alter the environment in brain injury and attenuate the inflammatory response through activation and polarization of macrophages and microglia (Xu et al., 2017). The polarization of microglia to M2 microglia has been shown to promote neurogenesis and oligodendrogenesis from NSCs, though there is a lack of consensus on the distinction between microglial populations in applicable in vivo models (Yuan et al., 2017). This regulation of neuroinflammation may facilitate reducing lesion size by prevention of secondary or inflammatory injury. Exosomes derived from MSCs, which are small, membranous vesicles containing various proteins, lipids, and RNAs, have been shown to be effective at altering neuroinflammation and neurogenesis, and have been proposed as a potential therapeutic agent in brain injury (Yang et al., 2017). Extracellular vesicles, such as exosomes, may be among the primary mediators of the positive effects of MSC administration to the site of brain injury.
Figure 2.
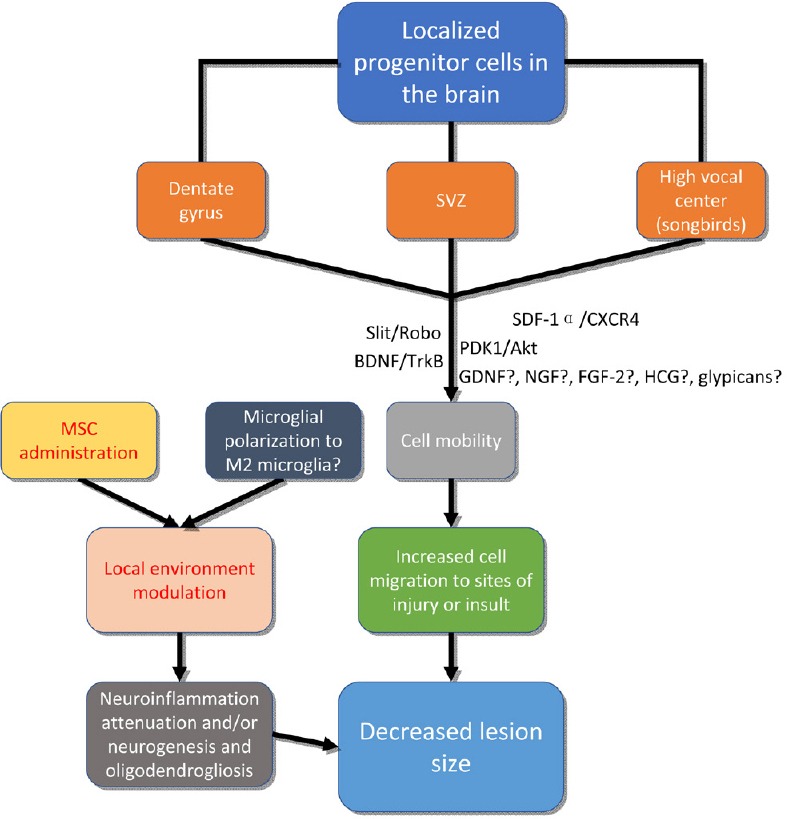
Therapeutic approaches to the treatment of brain injury may be adopted based on an understanding of factors involved in encouraging mobility of neural stem/progenitor cells, or local environment modulation.
Several factors such as GDNF, FGF-2, NGF and glypicans have roles in neural cell proliferation and development, and could be implicated in migratory processes by future studies. As noted, the concept of microglial polarization is somewhat ambiguous. SVZ: Subventricular zone; Robo: roundabout; BDNF: brain-derived neurotrophic factor; TrkB: tyrosine receptor kinase B; SDF-1α: stromal cell-derived factor 1α; PDK-1:phosphoinositide depend kinase1; HCG: human choionic gonadotophin; GDNF: glial cell line-derived neurotrophic factor; NGF: nerve growth factor; FGF2: fibroblast growth factor-2; CXCR4: C-X-C chemokine receptor type 4.
Some of the most promising novel approaches to brain injury treatment involve the therapeutic manipulation of endogenous precursor cells in the brain. Brain-derived neurotrophic factor (BDNF) has been shown to not only be important in development for the maintenance and growth of neurons, but also plays a vital role in axon remodeling and dendrite branching (Barde, 1994; Conner et al., 1997; Alsina et al., 2001; Ortiz-López et al., 2017b). BDNF has additionally been shown to influence cell migration (Borghesani et al., 2002; Chiaramello et al., 2007; Cao et al., 2012; Matsuda et al., 2012; Grade et al., 2013; Gasanov et al., 2015; Ortiz-López et al., 2017a). This action of BDNF on cell migration appears to be mediated by its action on the tyrosine receptor kinase B (TrkB) receptor, and the role of BDNF seems to be prevalent in normal development as well as response to ischemia or other insult. Ortiz-López et al. (2017b) observed that the migration rate of human NSCs to empty areas in vitro did not differ significantly between those treated with BDNF and controls after 2- and 6-hour-time periods, but that at 24-, 48-, 72-, and 96-hour periods, the stem cells treated with BDNF had migrated to the empty areas significantly more than the untreated cells (Figure 2).
Guerrero-Cazares et al. (2017) showed that Slit and roundabout (Robo) proteins and their interaction are important regulators of human fetal neural progenitor cell migration. It has also been noted that the PDK1-Akt pathway has a role in the regulation of the speed of neuronal migration through the mouse neocortical plate; it is likely that this influence is mediated through an interaction with the microtubule structural system (Itoh, 2016). Stromal cell-derived factor 1α (SDF-1α), which is an inflammatory chemoattractant, also plays a vital role in the migration of NSCs to the site of injury (Imitola et al., 2004). This provides the interesting perspective that inflammation may have not only a negative effect on the subject, but is also involved in the migration of regenerative cells to the site of deficit. NSCs are known to secrete neurotrophic factors such as nerve growth factor (NGF), BDNF, and glial cell line-derived neurotrophic factor (GDNF) that may increase resident axonal extension following spinal cord injury (Lu et al., 2003).
In investigating stem cell therapies for Huntington's disease (HD) in an experimental model induced through quinolinic acid administration, Song et al. (2007) found that transplantation of human embryonic stem cell (hESC)-derived NSCs rescued some motor function in the absence of medium-sized spiny projection neurons (MSNs). It is thought that this positive effect could be due to neuroprotective factors expressed by the transplanted NSCs (Connor, 2017). Drago et al. (2013) addressed the concepts of the paracrine hypothesis and the stem cell secretome (SCS) in explaining the therapeutic effect of stem cell transplantation in animal models of CNS disease. The SCS is comprised of cytokines, chemokines, and growth factors, and is involved in the repair or regeneration of injured tissue (Drago et al., 2013).
It should be noted that many of the studies that have been performed with NSCs have been conducted using embryonic or fetal cells. While it is reasonable to believe that the processes involved in adult subjects in response to injury or in standard repair mechanisms will be similar in many respects, there could be significant benefit derived from replication of studies using adult cells and microenvironments typically encountered.
Future Directions
There is extensive research being performed with stem cells and their potential therapeutic uses. Studies are shedding light on the markers, characteristics, and related factors of NSCs. There is still much to be learned to broaden and deepen our understanding of stem cells and the microenvironments that encourage their proliferation and migration. While our understanding of the niches and signaling, factors involved in NSC population are synergistic and proliferation is still developing, there could be potential for therapeutic benefit in exploring the ability to induce activation or migration of stem cells to sites of injury through administration of growth or signaling factors. However, it should be noted that care should be taken in examining the adverse effects of such therapies as interruption to the critical balance that normally exists can have highly detrimental consequences such as the development of gliomas due to increased EGFR exposure (though absence or mutation of other factors like Ink4a or Arf may contribute) (Holland et al., 1998; Bachoo et al., 2002). The role of microRNAs in modulating the activity and characteristics of NSCs is being aggressively investigated currently. These studies have, and will continue to enlighten our understanding of mechanisms involved in NSC maintenance, proliferation, and migration. Continuation in this area may prove to be of clinical benefit. The development of methods for transportation of stem cells or factors affecting their growth and development to sites of neural tissue damage could be helpful in treating brain injuries. Exosomes exhibit some promise as neuromodulatory agents, and continued research could be of benefit. As mentioned previously, the use of adult NSCs in studies could provide additional information as to the ways in which adult stem cells and embryonic stem cells differ, and the unique or similar characteristics between them.
Brain injury dramatically affects the lives of many people around the world, and the treatments available, while progressing, often produce unsatisfactory results. NSCs are fascinating populations that are being intensely investigated, and that offer potential to provide great therapeutic benefit in the future; this investigation should continue to expand our understanding of stem cell characteristics and their applications.
Acknowledgments
DKA is supported by research grants R01 HL112597, R01 HL116042, and R01 HL120659 from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Funding: This study is supported by research grants R01 HL112597, R01 HL116042, and R01 HL120659 (to DKA)..
Conflicts of interest: All authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest. The authors declared no conflict of interest with the content of the article.
Financial support: This study is supported by research grants R01 HL112597, R01 HL116042, and R01 HL120659 (to DKA).
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Masaaki Hori, Juntendo University School of Medicine, Japan.
References
- 1.Adams JH, Doyle D, Graham DI, Lawrence AE, McLellan DR, Gennarelli TA, Pastuszko M, Sakamoto T. The contusion index: a reappraisal in human and experimental non-missile head injury. Neuropathol Appl Neurobiol. 1985;11:299–308. doi: 10.1111/j.1365-2990.1985.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexson TO, Hitoshi S, Coles BL, Bernstein A, van der Kooy D. Notch signaling is required to maintain all neural stem cell populations--irrespective of spatial or temporal niche. Dev Neurosci. 2006;28:34–48. doi: 10.1159/000090751. [DOI] [PubMed] [Google Scholar]
- 4.Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 5.Altmann CR, Brivanlou AH. Neural patterning in the vertebrate embryo. Int Rev Cytol. 2001;203:447–482. doi: 10.1016/s0074-7696(01)03013-3. [DOI] [PubMed] [Google Scholar]
- 6.Antonov SA, Manuilova ES, Dolotov OV, Kobylyansky AG, Safina DR, Grivennikov IA. Effect of nerve growth factor on neural differentiation of mouse embryonic stem cells. Bull Exp Biol Med. 2017;162:679–683. doi: 10.1007/s10517-017-3686-1. [DOI] [PubMed] [Google Scholar]
- 7.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 8.Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA. Epidermal growth factor receptor and Ink4a/Arf: Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 9.Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird AE, Benfield A, Schlaug G, Siewert B, Lövblad KO, Edelman RR, Warach S. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–589. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 11.Barazzuol L, Ju L, Jeggo PA. A coordinated DNA damage response promotes adult quiescent neural stem cell activation. PLoS Biol. 2017;15:e2001264. doi: 10.1371/journal.pbio.2001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- 13.Barres BA, Barde Y. Neuronal and glial cell biology. Curr Opin Neurobiol. 2000;10:642–648. doi: 10.1016/s0959-4388(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 14.Beckta JM, Adams BR, Valerie K. DNA damage response in human stem cells and neural descendants. Methods Mol Biol. 2017;1599:375–390. doi: 10.1007/978-1-4939-6955-5_27. [DOI] [PubMed] [Google Scholar]
- 15.Beclin C, Follert P, Stappers E, Barral S, Nathalie C, de Chevigny A, Magnone V, Lebrigand K, Bissels U, Huylebroeck D, Bosio A, Barbry P, Seuntjens E, Cremer H. miR-200 family controls late steps of postnatal forebrain neurogenesis via Zeb2 inhibition. Sci Rep. 2016;6:35729. doi: 10.1038/srep35729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornson CRR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 17.Blackmore DG, Golmohammadi MG, Large B, Waters MJ, Rietze RL. Exercise increases neural stem cell number in a growth hormone-dependent manner, augmenting the regenerative response in aged mice. Stem Cells. 2009;27:2044–2052. doi: 10.1002/stem.120. [DOI] [PubMed] [Google Scholar]
- 18.Blumbergs PC, Jones NR, North JB. Diffuse axonal injury in head trauma. J Neurol Neurosurg Psychiatry. 1989;52:838–841. doi: 10.1136/jnnp.52.7.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borghesani PR, Peyrin JM, Klein R, Rubin J, Carter AR, Schwartz PM, Luster A, Corfas G, Segal RA. BDNF stimulates migration of cerebellar granule cells. Dev Camb Engl. 2002;129:1435–1442. doi: 10.1242/dev.129.6.1435. [DOI] [PubMed] [Google Scholar]
- 20.Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouillard P, Vikkula M. Genetic causes of vascular malformations. Hum Mol Genet. 2007;16:R140–R149. doi: 10.1093/hmg/ddm211. [DOI] [PubMed] [Google Scholar]
- 23.Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 24.Cao L, Zhang L, Chen S, Yuan Z, Liu S, Shen X, Zheng X, Qi X, Lee KKH, Chan JYH, Cai D. BDNF-mediated migration of cardiac microvascular endothelial cells is impaired during ageing. J Cell Mol Med. 2012;16:3105–3115. doi: 10.1111/j.1582-4934.2012.01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Prevalence of stroke--United States, 2006-2010. MMWR Morb Mortal Wkly Rep. 2012;61:379–382. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Report to Congress on Traumatic Brain Injury in the United States. Epidemiology and Rehabilitation. 2015 doi: 10.1016/j.apmr.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Cerpa W, Latorre-Esteves E, Barria A. RoR2 functions as a noncanonical Wnt receptor that regulates NMDAR-mediated synaptic transmission. Proc Natl Acad Sci U S A. 2015;112:4797–4802. doi: 10.1073/pnas.1417053112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 29.Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, De Marchis S. BDNF/ TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur J Neurosci. 2007;26:1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- 30.Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S. Glycoprotein 130 signaling regulates notch1expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci. 2003;23:1730–1741. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chopp M, Li Y, Jiang N, Zhang RL, Prostak J. Antibodies against adhesion molecules reduce apoptosis after transient middle cerebral artery occlusion in rat brain. J Cereb Blood Flow Metab. 1996;16:578–584. doi: 10.1097/00004647-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Christopherson GT, Song H, Mao HQ. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials. 2009;30:556–564. doi: 10.1016/j.biomaterials.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Clarke DL. Neural stem cells. Bone Marrow Transplant. 2003;32:S13–S17. doi: 10.1038/sj.bmt.1703937. [DOI] [PubMed] [Google Scholar]
- 34.Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlström H, Lendahl U, Frisén J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- 35.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci Off J Soc Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connor B. Concise review: The use of stem cells for understanding and treating Huntington's disease. Stem Cells. 2017 doi: 10.1002/stem.2747. doi: 10.1002/stem.2747. [DOI] [PubMed] [Google Scholar]
- 37.Correa-Cerro LS, Mandell JW. Molecular mechanisms of astrogliosis: new approaches with mouse genetics. J Neuropathol Exp Neurol. 2007;66:169–176. doi: 10.1097/01.jnen.0000248555.53079.d5. [DOI] [PubMed] [Google Scholar]
- 38.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Curlik DM, 2nd, Shors TJ. Training your brain: Do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus? Neuropharmacology. 2013;64:506–514. doi: 10.1016/j.neuropharm.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dewan MC, Mummareddy N, Wellons JC, Bonfield CM. Epidemiology of global pediatric traumatic brain injury: qualitative review. World Neurosurg. 2016;91:497–509. doi: 10.1016/j.wneu.2016.03.045. e.1. [DOI] [PubMed] [Google Scholar]
- 41.DeWitt DS, Prough DS. Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J Neurotrauma. 2003;20:795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- 42.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 43.Doan N, Patel M, Doan H, Janich K. Traumatic brain injury. Int J Phys Med Rehabil. 2016;4:e120. [Google Scholar]
- 44.Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, Pluchino S. The stem cell secretome and its role in brain repair. Biochimie. 2013;95:2271–2285. doi: 10.1016/j.biochi.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eddleston M, Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edward Dixon C, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 47.Eglitis MA, Mezey É Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci U S A. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Endo M, Minami Y. Diverse roles for the ror-family receptor tyrosine kinases in neurons and glial cells during development and repair of the nervous system. Dev Dyn. 2018;247:24–32. doi: 10.1002/dvdy.24515. [DOI] [PubMed] [Google Scholar]
- 49.Etzioni A. Adhesion molecules-their role in health and disease. Pediatr Res. 1996;39:191–198. doi: 10.1203/00006450-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, Lauritzen M. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778–790. doi: 10.1093/brain/awh716. [DOI] [PubMed] [Google Scholar]
- 51.Frizzell JP. Acute stroke: pathophysiology, diagnosis, and treatment. AACN Clin Issues. 2005;16:421–440. doi: 10.1097/00044067-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Fu J, Zhao SD, Liu HJ, Yuan QH, Liu SM, Zhang YM, Ling EA, Hao AJ. Melatonin promotes proliferation and differentiation of neural stem cells subjected to hypoxia in vitro. J Pineal Res. 2011;51:104–112. doi: 10.1111/j.1600-079X.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 53.Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- 54.Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 55.Gaiano N, Fishell G. Transplantation as a tool to study progenitors within the vertebrate nervous system. J Neurobiol. 1998;36:152–161. [PubMed] [Google Scholar]
- 56.Galli R, Gritti A, Bonfanti L, Vescovi AL. Neural stem cells. Circ Res. 2003;92:598–608. doi: 10.1161/01.RES.0000065580.02404.F4. [DOI] [PubMed] [Google Scholar]
- 57.Gasanov EV, Rafieva LM, Korzh VP. BDNF-TrkB axis regulates migration of the lateral line primordium and modulates the maintenance of mechanoreceptor progenitors. PLoS One. 2015;10:e0119711. doi: 10.1371/journal.pone.0119711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol. 1988a;150:663–672. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- 59.Gentry LR, Godersky JC, Thompson B, Dunn VD. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol. 1988b;150:673–682. doi: 10.2214/ajr.150.3.673. [DOI] [PubMed] [Google Scholar]
- 60.Gong C, Qin Z, Betz AL, Liu XH, Yang GY. Cellular localization of tumor necrosis factor alpha following focal cerebral ischemia in mice. Brain Res. 1998;801:1–8. doi: 10.1016/s0006-8993(98)00489-2. [DOI] [PubMed] [Google Scholar]
- 61.Grade S, Weng YC, Snapyan M, Kriz J, Malva JO, Saghatelyan A. Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS One. 2013;8:e55039. doi: 10.1371/journal.pone.0055039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guerrero-Cazares H, Lavell E, Chen L, Schiapparelli P, Lara-Velazquez M, Capilla-Gonzalez V, Clements AC, Drummond G, Noiman L, Thaler K, Burke A, Quiñones-Hinojosa A. Brief report: robo1 regulates the migration of human subventricular zone neural progenitor cells during development. Stem Cells. 2017;35:1860–1865. doi: 10.1002/stem.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han J, Gage FH. A role for miR-19 in the migration of adult-born neurons and schizophrenia. Neurogenesis Austin Tex. 2016;3:e1251873. doi: 10.1080/23262133.2016.1251873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hartings JA, Rolli ML, Lu X, Tortella FC. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: relation to infarct growth and neuroprotection. J Neurosci. 2003;23:11602–11610. doi: 10.1523/JNEUROSCI.23-37-11602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 66.Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho SY, Ling TY, Lin HY, Liou JTJ, Liu FC, Chen IC, Lee SW, Hsu Y, Lai DM, Liou HH. SDF-1/CXCR4 signaling maintains stemness signature in mouse neural stem/progenitor cells. Stem Cells Int. 2017;2017:2493752. doi: 10.1155/2017/2493752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howe JR, Li ES, Streeter SE, Rahme GJ, Chipumuro E, Russo GB, Litzky JF, Hills LB, Rodgers KR, Skelton PD, Luikart BW. MiR-338-3p regulates neuronal maturation and suppresses glioblastoma proliferation. PLoS One. 2017;12:e0177661. doi: 10.1371/journal.pone.0177661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 71.Iijima T, Mies G, Hossmann KA. Repeated negative DC deflections in rat cortex following middle cerebral artery occlusion are abolished by MK-801: effect on volume of ischemic injury. J Cereb Blood Flow Metab. 1992;12:727–733. doi: 10.1038/jcbfm.1992.103. [DOI] [PubMed] [Google Scholar]
- 72.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Itoh Y. A balancing Akt: How to fine-tune neuronal migration speed. Neurogenesis Austin Tex. 2016;3:e1256854. doi: 10.1080/23262133.2016.1256854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin J, Kim SN, Liu X, Zhang H, Zhang C, Seo JS, Kim Y, Sun T. miR-17-92 cluster regulates adult hippocampal neurogenesis, anxiety, and depression. Cell Rep. 2016;16:1653–1663. doi: 10.1016/j.celrep.2016.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jin Y, Barnett A, Zhang Y, Yu X, Luo Y. Poststroke sonic hedgehog agonist treatment improves functional recovery by enhancing neurogenesis and angiogenesis. Stroke. 2017;48:1636–1645. doi: 10.1161/STROKEAHA.117.016650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jobe EM, Gao Y, Eisinger BE, Mladucky JK, Giuliani CC, Kelnhofer LE, Zhao X. Methyl-CpG-binding protein MBD1 regulates neuronal lineage commitment through maintaining adult neural stem cell identity. J Neurosci. 2017;37:523–536. doi: 10.1523/JNEUROSCI.1075-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jørgensen HF, Ben-Porath I, Bird AP. Mbd1 is recruited to both methylated and nonmethylated CpGs via distinct DNA binding domains. Mol Cell Biol. 2004;24:3387–3395. doi: 10.1128/MCB.24.8.3387-3395.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–348. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 79.Kalyani AJ, Piper D, Mujtaba T, Lucero MT, Rao MS. Spinal cord neuronal precursors generate multiple neuronal phenotypes in culture. J Neurosci. 1998;18:7856–7868. doi: 10.1523/JNEUROSCI.18-19-07856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 81.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kennea NL, Mehmet H. Neural stem cells. J Pathol. 2002;197:536–550. doi: 10.1002/path.1189. [DOI] [PubMed] [Google Scholar]
- 83.Kobayashi D, Kobayashi M, Matsumoto K, Ogura T, Nakafuku M, Shimamura K. Early subdivisions in the neural plate define distinct competence for inductive signals. Dev Camb Engl. 2002;129:83–93. doi: 10.1242/dev.129.1.83. [DOI] [PubMed] [Google Scholar]
- 84.Kong X, Li X, Cai Z, Yang N, Liu Y, Shu J, Pan L, Zuo P. Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cell Mol Neurobiol. 2008;28:569–579. doi: 10.1007/s10571-007-9212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2015. [Google Scholar]
- 86.Lax E, Sapozhnikov DM. Adult neural stem cell multipotency and differentiation are directed by the methyl-CpG-binding protein MBD1. J Neurosci. 2017;37:4228–4230. doi: 10.1523/JNEUROSCI.0411-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lei B, Popp S, Capuano-Waters C, Cottrell JE, Kass IS. Lidocaine attenuates apoptosis in the ischemic penumbra and reduces infarct size after transient focal cerebral ischemia in rats. Neuroscience. 2004;125:691–701. doi: 10.1016/j.neuroscience.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 88.Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 89.Levison SW, Goldman JE. Multipotential and lineage restricted precursors coexist in the mammalian perinatal subventricular zone. J Neurosci Res. 1997;48:83–94. [PubMed] [Google Scholar]
- 90.Li L, Liu J. The effect of pediatric traumatic brain injury on behavioral outcomes: a systematic review: Review. Dev Med Child Neurol. 2013;55:37–45. doi: 10.1111/j.1469-8749.2012.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lillien L, Raphael H. BMP and FGF regulate the development of EGF-responsive neural progenitor cells. Dev Camb Engl. 2000;127:4993–5005. doi: 10.1242/dev.127.22.4993. [DOI] [PubMed] [Google Scholar]
- 92.Lindsey BW, Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog Neurobiol. 2006;80:281–307. doi: 10.1016/j.pneurobio.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y, Han SSW, Wu Y, Tuohy TMF, Xue H, Cai J, Back SA, Sherman LS, Fischer I, Rao MS. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2004;276:31–46. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 94.Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 95.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Louissaint A, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 97.Lu B, Roegiers F, Jan LY, Jan YN. Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature. 2001;409:522–525. doi: 10.1038/35054077. [DOI] [PubMed] [Google Scholar]
- 98.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 99.Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- 100.Lugert S, Kremer T, Jagasia R, Herrmann A, Aigner S, Giachino C, Mendez-David I, Gardier AM, Carralot JP, Meistermann H, Augustin A, Saxe MD, Lamerz J, Duran-Pacheco G, Ducret A, Taylor V, David DJ, Czech C. Glypican-2 levels in cerebrospinal fluid predict the status of adult hippocampal neurogenesis. Sci Rep. 2017;7:46543. doi: 10.1038/srep46543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- 102.Malik GM, Morgan JK, Boulos RS, Ausman JI. Venous angiomas: An underestimated cause of intracranial hemorrhage. Surg Neurol. 1988;30:350–358. doi: 10.1016/0090-3019(88)90197-8. [DOI] [PubMed] [Google Scholar]
- 103.Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- 104.Matsuda S, Fujita T, Kajiya M, Takeda K, Shiba H, Kawaguchi H, Kurihara H. Brain-derived neurotrophic factor induces migration of endothelial cells through a TrkB-ERK-integrin αVβ3-FAK cascade. J Cell Physiol. 2012;227:2123–2129. doi: 10.1002/jcp.22942. [DOI] [PubMed] [Google Scholar]
- 105.McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion study. JAMA. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 106.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mellios N, Feldman DA, Sheridan SD, Ip JPK, Kwok S, Amoah SK, Rosen B, Rodriguez BA, Crawford B, Swaminathan R, Chou S, Li Y, Ziats M, Ernst C, Jaenisch R, Haggarty SJ, Sur M. MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.86. doi: 10.1038/mp.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mendivil-Perez M, Soto-Mercado V, Guerra-Librero A, Fernandez-Gil BI, Florido J, Shen YQ, Tejada MA, Capilla-Gonzalez V, Rusanova I, Garcia-Verdugo JM, Acuña-Castroviejo D, López LC, Velez-Pardo C, Jimenez-Del-Rio M, Ferrer JM, Escames G. Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function. J Pineal Res. 2017 doi: 10.1111/jpi.12415. doi: 10.1111/jpi.12415. [DOI] [PubMed] [Google Scholar]
- 109.Messing R. Nervous system disorders. In: McPhee S, Lingappa V, Ganong W, editors. In: Pathophysiology of Disease: An Introduction to Clinical Medicine. 4th ed. New York: Lange Medical Books/McGraw-Hill Medical Publishing Division; 2003. pp. 143–188. [Google Scholar]
- 110.Mies G, Iijima T, Hossmann KA. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport. 1993;4:709–711. doi: 10.1097/00001756-199306000-00027. [DOI] [PubMed] [Google Scholar]
- 111.Moriya T, Horie N, Mitome M, Shinohara K. Melatonin influences the proliferative and differentiative activity of neural stem cells. J Pineal Res. 2007;42:411–418. doi: 10.1111/j.1600-079X.2007.00435.x. [DOI] [PubMed] [Google Scholar]
- 112.Nakamura Y, Sakakibara S, Miyata T, Ogawa M, Shimazaki T, Weiss S, Kageyama R, Okano H. The bHLH gene Hes1 as a repressor of the neuronal commitment of CNS stem cells. J Neurosci. 2000;20:283–293. doi: 10.1523/JNEUROSCI.20-01-00283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakase T, Fushiki S, Naus CCG. Astrocytic gap junctions composed of connexin 43 reduce apoptotic neuronal damage in cerebral ischemia. Stroke. 2003;34:1987–1993. doi: 10.1161/01.STR.0000079814.72027.34. [DOI] [PubMed] [Google Scholar]
- 114.Niles LP, Armstrong KJ, Rincón Castro LM, Dao CV, Sharma R, McMillan CR, Doering LC, Kirkham DL. Neural stem cells express melatonin receptors and neurotrophic factors: colocalization of the MT1receptor with neuronal and glial markers. BMC Neurosci. 2004;5:41. doi: 10.1186/1471-2202-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nortje J, Menon DK. Traumatic brain injury: physiology, mechanisms, and outcome. Curr Opin Neurol. 2004;17:711–718. doi: 10.1097/00019052-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 117.Obrenovitch TP. The ischaemic penumbra: twenty years on. Cerebrovasc Brain Metab Rev. 1995;7:297–323. [PubMed] [Google Scholar]
- 118.Oh HJ, Shin Y, Chung S, Hwang DW, Lee DS. Convective exosome-tracing microfluidics for analysis of cell-non-autonomous neurogenesis. Biomaterials. 2017;112:82–94. doi: 10.1016/j.biomaterials.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 119.Ohtsuka T, Sakamoto M, Guillemot F, Kageyama R. Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. J Biol Chem. 2001;276:30467–30474. doi: 10.1074/jbc.M102420200. [DOI] [PubMed] [Google Scholar]
- 120.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 121.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 122.Ortiz-López L, González-Olvera JJ, Vega-Rivera NM, García-Anaya M, Carapia-Hernández AK, César Velázquez-Escobar J, Ramírez-Rodríguez GB. Human neural stem/progenitor cells derived from the olfactory epithelium express the TrkB receptor and migrate in response to BDNF. Neuroscience. 2017b;35:84–100. doi: 10.1016/j.neuroscience.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 123.Ortiz-López L, Vega-Rivera NM, Babu H, Ramírez-Rodríguez GB. Brain-derived neurotrophic factor induces cell survival and the migration of murine adult hippocampal precursor cells during differentiation in vitro. Neurotox Res. 2017a;31:122–135. doi: 10.1007/s12640-016-9673-x. [DOI] [PubMed] [Google Scholar]
- 124.Paganoni S, Bernstein J, Ferreira A. Ror1-Ror2 complexes modulate synapse formation in hippocampal neurons. Neuroscience. 2010;165:1261–1274. doi: 10.1016/j.neuroscience.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paganoni S, Ferreira A. Expression and subcellular localization of Ror tyrosine kinase receptors are developmentally regulated in cultured hippocampal neurons. J Neurosci Res. 2003;73:429–440. doi: 10.1002/jnr.10674. [DOI] [PubMed] [Google Scholar]
- 126.Paganoni S, Ferreira A. Neurite extension in central neurons: a novel role for the receptor tyrosine kinases Ror1 and Ror2. J Cell Sci. 2005;118:433–446. doi: 10.1242/jcs.01622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Parikh S, Koch M, Narayan RK. Traumatic brain injury. Int Anesthesiol Clin. 2007;45:119–135. doi: 10.1097/AIA.0b013e318078cfe7. [DOI] [PubMed] [Google Scholar]
- 129.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 130.Penn RD, Loewenstein WR. Uncoupling of a nerve cell membrane junction by calcium-ion removal. Science. 1966;151:88–89. doi: 10.1126/science.151.3706.88. [DOI] [PubMed] [Google Scholar]
- 131.Planas AM, Soriano MA, Berruezo M, Justicia C, Estrada A, Pitarch S, Ferrer I. Induction of Stat3, a signal transducer and transcription factor, in reactive microglia following transient focal cerebral ischaemia. Eur J Neurosci. 1996;8:2612–2618. doi: 10.1111/j.1460-9568.1996.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 132.Price CD, Yang Z, Karlnoski R, Kumar D, Chaparro R, Camporesi EM. Effect of continuous infusion of asialoerythropoietin on short-term changes in infarct volume, penumbra apoptosis and behaviour following middle cerebral artery occlusion in rats. Clin Exp Pharmacol Physiol. 2010;37:185–192. doi: 10.1111/j.1440-1681.2009.05257.x. [DOI] [PubMed] [Google Scholar]
- 133.Price J. Glial cell lineage and development. Curr Opin Neurobiol. 1994;4:680–686. doi: 10.1016/0959-4388(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 134.Price J, Thurlow L. Cell lineage in the rat cerebral cortex: a study using retroviral-mediated gene transfer. Dev Camb Engl. 1988;104:473–482. doi: 10.1242/dev.104.3.473. [DOI] [PubMed] [Google Scholar]
- 135.Price J, Williams BP. Neural stem cells. Curr Opin Neurobiol. 2001;11:564–567. doi: 10.1016/s0959-4388(00)00250-6. [DOI] [PubMed] [Google Scholar]
- 136.Radhakrishnan B, Alwin Prem Anand A. Role of miRNA-9 in brain development. J Exp Neurosci. 2016;10:101–120. doi: 10.4137/JEN.S32843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev Biol. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- 138.Read SJ, Hirano T, Abbott DF, Sachinidis JI, Tochon-Danguy HJ, Chan JG, Egan GF, Scott AM, Bladin CF, McKay WJ, Donnan GA. Identifying hypoxic tissue after acute ischemic stroke using PET and 18F-fluoromisonidazole. Neurology. 1998;51:1617–1621. doi: 10.1212/wnl.51.6.1617. [DOI] [PubMed] [Google Scholar]
- 139.Rosenfeld JV, McFarlane AC, Bragge P, Armonda RA, Grimes JB, Ling GS. Blast-related traumatic brain injury. Lancet Neurol. 2013;12:882–893. doi: 10.1016/S1474-4422(13)70161-3. [DOI] [PubMed] [Google Scholar]
- 140.Rushing G, Ihrie RA. Neural stem cell heterogeneity through time and space in the ventricular-subventricular zone. Front Biol. 2016;11:261–284. doi: 10.1007/s11515-016-1407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sayama CM, Osborn AG, Chin SS, Couldwell WT. Capillary telangiectasias: clinical, radiographic, and histopathological features. J Neurosurg. 2009;113:709–714. doi: 10.3171/2009.9.JNS09282. [DOI] [PubMed] [Google Scholar]
- 142.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 143.Shimizu T, Smits R, Ikenaka K. Microglia-induced activation of noncanonical wnt signaling aggravates neurodegeneration in demyelinating disorders. Mol Cell Biol. 2016;36:2728–2741. doi: 10.1128/MCB.00139-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shimura T, Sasatani M, Kawai H, Kamiya K, Kobayashi J, Komatsu K, Kunugita N. A comparison of radiation-induced mitochondrial damage between neural progenitor stem cells and differentiated cells. Cell Cycle. 2017;16:565–573. doi: 10.1080/15384101.2017.1284716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Simon RP, Griffiths T, Evans MC, Swan JH, Meldrum BS. Calcium overload in selectively vulnerable neurons of the hippocampus during and after ischemia: an electron microscopy study in the rat. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1984;4:350–361. doi: 10.1038/jcbfm.1984.52. [DOI] [PubMed] [Google Scholar]
- 146.Smith W, Johnston S, Easton J. Cerebrovascular diseases. In: Kasper D, Fauci A, Longo D, Braunwald E, Hauser S, Jameson J, editors. In: Harrison's Principles of Internal Medicine. 16th ed. New York: McGraw-Hill Medical Publishing Division; 2005. pp. 2372–2393. [Google Scholar]
- 147.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 148.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Song J, Kang SM, Lee KM, Lee JE. The protective effect of melatonin on neural stem cell against LPS-induced inflammation. BioMed Res Int. 2015;2015:e854359. doi: 10.1155/2015/854359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Song J, Lee ST, Kang W, Park JE, Chu K, Lee SE, Hwang T, Chung H, Kim M. Human embryonic stem cell-derived neural precursor transplants attenuate apomorphine-induced rotational behavior in rats with unilateral quinolinic acid lesions. Neurosci Lett. 2007;423:58–61. doi: 10.1016/j.neulet.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 151.Song J, Oh Y, Kim JY, Cho KJ, Lee JE. Suppression of microRNA let-7a expression by agmatine regulates neural stem cell differentiation. Yonsei Med J. 2016;57:1461–1467. doi: 10.3349/ymj.2016.57.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sotthibundhu A, Phansuwan-Pujito P, Govitrapong P. Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J Pineal Res. 2010;49:291–300. doi: 10.1111/j.1600-079X.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- 153.Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, Nagafuji T, Ninomiya M, Nakamura H, Dunn AK, Graf R. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- 154.Sun D. The potential of endogenous neurogenesis for brain repair and regeneration following traumatic brain injury. Neural Regen Res. 2014;9:688–692. doi: 10.4103/1673-5374.131567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 156.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 157.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 158.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tsan YC, Morell MH, O'Shea KS. miR-410 controls adult SVZ neurogenesis by targeting neurogenic genes. Stem Cell Res. 2016;17:238–247. doi: 10.1016/j.scr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 160.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 161.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci Off J Soc Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wilde EA, Hunter, Bigler ED. Pediatric traumatic brain injury: Neuroimaging and neurorehabilitation outcome. Neurorehabilitation. 2012;31:245–260. doi: 10.3233/NRE-2012-0794. [DOI] [PubMed] [Google Scholar]
- 163.Wu CW, Chang YT, Yu L, Chen H, Jen CJ, Wu SY, Lo CP, Kuo YM. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. 2008;105:1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- 164.Xu C, Fu F, Li X, Zhang S. Mesenchymal stem cells maintain the microenvironment of central nervous system by regulating the polarization of macrophages/microglia after traumatic brain injury. Int J Neurosci. 2017;127:1124–1135. doi: 10.1080/00207454.2017.1325884. [DOI] [PubMed] [Google Scholar]
- 165.Xu J, Culman J, Blume A, Brecht S, Gohlke P. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- 166.Yang Y, Ye Y, Su X, He J, Bai W, He X. MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front Cell Neurosci. 2017;11:55. doi: 10.3389/fncel.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yepes M, Sandkvist M, Wong MKK, Coleman TA, Smith E, Cohan SL, Lawrence DA. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–576. [PubMed] [Google Scholar]
- 168.Yuan J, Ge H, Liu W, Zhu H, Chen Y, Zhang X, Yang Y, Yin Y, Chen W, Wu W, Yang Y, Lin J. M2 microglia promotes neurogenesis and oligodendrogenesis from neural stem/progenitor cells via the PPARγ signaling pathway. Oncotarget. 2017;8:19855–19865. doi: 10.18632/oncotarget.15774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Z, Chopp M, Goussev A, Powers C. Cerebral vessels express interleukin 1β after focal cerebral ischemia. Brain Res. 1998;784:210–217. doi: 10.1016/s0006-8993(97)01317-6. [DOI] [PubMed] [Google Scholar]
- 170.Zheng J, Yi D, Shi X, Shi H. miR-1297 regulates neural stem cell differentiation and viability through controlling Hes1 expression. Cell Prolif. 2017 doi: 10.1111/cpr.12347. doi: 10.1111/cpr.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]