Keywords: nerve regeneration, electroacupuncture, focal cerebral ischemia/reperfusion injury, dynamin-related protein 1, death-associated protein kinases, mitochondrial dynamics, mitochondrial ultrastructure, apoptosis, cytochrome c, neural regeneration
Abstract
Electroacupuncture preconditioning at acupoint Baihui (GV20) can reduce focal cerebral ischemia/reperfusion injury. However, the precise protective mechanism remains unknown. Mitochondrial fission mediated by dynamin-related protein 1 (Drp1) can trigger neuronal apoptosis following cerebral ischemia/reperfusion injury. Herein, we examined the hypothesis that electroacupuncture pretreatment can regulate Drp1, and thus inhibit mitochondrial fission to provide cerebral protection. Rat models of focal cerebral ischemia/reperfusion injury were established by middle cerebral artery occlusion at 24 hours after 5 consecutive days of preconditioning with electroacupuncture at GV20 (depth 2 mm, intensity 1 mA, frequency 2/15 Hz, for 30 minutes, once a day). Neurological function was assessed using the Longa neurological deficit score. Pathological changes in the ischemic penumbra on the injury side were assessed by hematoxylin-eosin staining. Cellular apoptosis in the ischemic penumbra on the injury side was assessed by terminal deoxyribonucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling staining. Mitochondrial ultrastructure in the ischemic penumbra on the injury side was assessed by transmission electron microscopy. Drp1 and cytochrome c expression in the ischemic penumbra on the injury side were assessed by western blot assay. Results showed that electroacupuncture preconditioning decreased expression of total and mitochondrial Drp1, decreased expression of total and cytosolic cytochrome c, maintained mitochondrial morphology and reduced the proportion of apoptotic cells in the ischemic penumbra on the injury side, with associated improvements in neurological function. These data suggest that electroacupuncture preconditioning-induced neuronal protection involves inhibition of the expression and translocation of Drp1.
Introduction
Ischemic stroke is associated with a high morbidity, mortality and disability (Yamaguchi et al., 2016; Hameed et al., 2017; Patel and McMullen, 2017). Early reperfusion of the ischemic regions is an effective way to restore brain function, although this can actually increase brain damage, termed cerebral ischemia/reperfusion (IR) injury (Winquist and Kerr, 1997). In modern medicine conception, “prevention of disease” is one of important opinions. Thereby, reducing the incidence of stroke or the severity of the IR insult has clinical value. Electroacupuncture (EA) preconditioning, as a simple, safe, convenient and effective intervention, was widely studied (Wang et al., 2009). EA has been previously reported to reduce cerebral IR injury (Lan et al., 2017; Ting et al., 2017). Recent studies showed that EA preconditioning at Baihui (GV20) exerted a neuroprotective effect via multiple mechanisms, including increased expression of total and phosphorylated adenosine monophosphate activated protein kinase-α (Ran et al., 2015), and increased expression of excitatory amino acid transporters (Zhu et al., 2013), in the cerebral cortex and hippocampus. However, the precise molecular mechanism of cerebral protection induced by EA pretreatment remains elusive.
An increase of mitochondrial fission can lead to morphological and functional abnormalities of the mitochondria, which are key events in neuronal apoptosis following IR injury (Knott et al., 2008; Chin-Chan et al., 2015; Flippo and Strack, 2017; Vakifahmetoglu-Norberg et al., 2017). Dynamin-related protein 1 (Drp1) is an important protein induced during mitochondrial fission. Under physiological conditions, Drp1 is located predominantly in the cytosol, with only 3% assembled on the mitochondrial outer membrane (Graef, 2016). After being recruited by relative receptors (e.g., mitochondrial fission protein 1 and mitochondrial fission factor) on the mitochondrial outer membrane, Drp1 translocates to the mitochondria and aggregates at the potential fission site. The Drp1 polymer gradually contracts using energy from guanosine triphosphate hydrolysis until mitochondrial fission. Drp1 then returns to the cytoplasm, and the cycle is repeated (Losón et al., 2013; Lee et al., 2016; Michalska et al., 2016). Therefore, Drp1 is essential for mitochondrial division (Pradeep et al., 2014; Hu et al., 2017). Overexpression of Drp1 was also reported to accelerate mitochondrial division and produce marked mitochondrial fragmentation during neuronal apoptosis following cerebral IR injury (Wu et al., 2011). Interestingly, treatment with the mitochondrial division inhibitor (mdivi-1, a selective inhibitor of Drp1) or inhibition of endogenous Drp1 with small interfering RNA or gene knockout can reduce mitochondrial fission, inhibit or delay the release of cytochrome c and maintain mitochondrial membrane potential, and thus suppress neuronal apoptosis (Pradeep et al., 2014; Li et al., 2015).
In the present study, we examined the hypothesis that the regulation of Drp1 is involved in the mechanism of cerebral protection by EA preconditioning.
Materials and Methods
Animals
All the experimental procedures were conducted strictly according to the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. Experiments were authorized by the ethics committee of Qingdao Municipal Hospital of China (approval No. 201613). A total of 96 specific-pathogen-free male Sprague-Dawley rats, 8–12 weeks of age, and weighing 300 ± 20 g were purchased from Cavens Experimental Animal Center (Jiangsu, China) (license No. SCXK [Su] 20160007). Rats were randomly assigned to the sham group, EA group (I/R + EA) and IR (I/R only) group (n = 32 per group). Rats were housed in the Animal Facility at Qingdao University of China with free access to food and water on a 12-hour light/dark cycle at 24 ± 2°C with a humidity of 60–70%. Rats were fasted for 12 hours with free access to water before being anesthetized with 1% pentobarbital sodium (30 mg/kg intraperitoneally). During anesthesia, surgery and EA, the rectal temperature of rats was kept at 37.0 ± 0.3°C using a heating pad, and blood pressure, heart rate and respiratory rate were monitored.
Model establishment of focal cerebral IR injury
Transient cerebral focal ischemia was produced by unilateral left middle cerebral artery occlusion according to a previously described method (Longa et al., 1989). In brief, the common carotid artery, external carotid artery and internal carotid artery on the left side were dissected under anesthesia. A monofilament nylon suture (3.0 cm in length) was inserted into the internal carotid artery 18.5–19.5 mm from the external carotid artery until the suture blocked the origin of the middle cerebral artery. The middle cerebral artery was reperfused after a 2-hour occlusion by withdrawing the nylon monofilament. To guarantee success of the model occlusion and reperfusion, laser Doppler flowmetry (PeriFlux 5000, Perimed, Sweden) was used to monitor regional cerebral blood flow. In the sham group, only the arteries were exposed without occlusion.
EA preconditioning
EA preconditioning was performed in accordance with a previously described method (Wang et al., 2005). In brief, after anesthesia, a 0.24 × 30 mm stainless steel acupuncture needle (Suzhou Huatuo Medical Equipment Co., Ltd., Suzhou, China) was twisted into GV20, which is located at the junction of the sagittal midline and the line linking both ears of the rats), at a depth of 2 mm. The needle handle was connected to an EA apparatus (G6805-1; XinSheng Co., Ltd., Qingdao, Shandong Province, China). The stimulation parameters were set as follows: intensity, 1 mA; frequency, 2/15 Hz; duration, 30 minutes. Rats in the EA group were stimulated once a day for 5 consecutive days. The middle cerebral artery occlusion model was established at 24 hours after the last stimulation.
Neurologic deficit scores
The behavioral scores of all rats at 6, 24 and 48 hours after reperfusion or sham surgery were evaluated using Longa neurological deficit scores (Longa et al., 1989). The scores were determined according to the following categories: 0, normal neurologic behavior; 1, flexion in the right forelimb; 2, failure to extend the right forelimb completely and strength to resist lateral push declined obviously; 3, forelimb flexion, rotation, and crawling toward the right side; 4, unable or difficult to ambulate spontaneously. The mean value of three scores was recorded at each timepoint (higher scores represented more severe neurological deficit).
Hematoxylin-eosin staining and terminal deoxyribonucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling staining
After anesthesia, four rats at from each group at each time point were transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde. Brain tissues were then collected, and the ischemic penumbras (peri-ischemic region, junction between the pale ischemic region and normal cortex tissue visible to the naked eye) were isolated. The tissues were then fixed in 10% formaldehyde and paraffin-embedded. The samples were sliced into 5 μm-thick coronal sections for hematoxylin-eosin staining 6, 24 and 48 hours after reperfusion and terminal deoxyribonucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling (TUNEL) staining at 24 hours after reperfusion.
Hematoxylin-eosin staining was performed as follows: hematoxylin staining for 10 minutes, discoloring by 75% hydrochloric acid alcohol solution for 30 seconds, eosin staining for 10 minutes and discoloring by 90% ethanol for 35 seconds. Six visual fields of each brain slice were randomly photographed and observed using a light microscope (400 × magnification; Olympus, Tokyo, Japan). Nuclear pyknosis and morphological abnormalities were evaluated by an experienced pathologist blinded to the group assignment.
Cell apoptosis was detected using TUNEL assay kits (Abcam, Cambridge, MA, USA) according to the manufacturer's instructions. The nuclei of the apoptotic cells presented as brown particles. The numbers of TUNEL-positive and total neurons were counted in four fields at 400× magnification (Olympus). Five sections of each animal were used. The mean values were calculated to determine the number of TUNEL-positive cells.
Transmission electron microscopy and multiple parameters for macro-quantitative analysis of mitochondrial morphology
Four rats of each group were sacrificed at 24 hours after reperfusion or sham surgery for transmission electron microscopy. The ischemic penumbra tissues of rats were removed after perfusion with 4% paraformaldehyde and 2.5% glutaraldehyde buffer (pH 7.4) under anesthesia. The ischemic penumbra was dissected and separated into brain blocks (1 × 1 × 1 mm3). Blocks were then fixed in 1% osmium tetroxide, dehydrated in graded ethyl alcohol and then embedded in epoxy resin. Blocks were sectioned into 50 nm ultrathin sections with an ultramicrotome (Leica, UC6, Wetzlar, Germany) and placed on 200-mesh copper grids. The sections were stained with saturated uranyl acetate and then visualized with an H-7650 transmission electron microscope (Hitachi, Hiyoda, Tokyo).
Four random fields were visualized from each slice at 100,000× magnification, from a total of five slices from each sample. The aspect ratio (major axes to minor axes of the analyzed mitochondria), vacuolation ratio and mean area density of vacuolated mitochondria were measured using ImageJ software (Image J, NIH, Bethesda, MD, USA) as morphological parameters. The vacuolation ratio was defined as the number of vacuolated mitochondria relative to the total number. The mean density was defined as the area sum of the vacuolated mitochondria relative to the area sum of all mitochondria (Wiemerslage and Lee, 2016).
Western blot assay
The mitochondrial and cytosolic fractions of the ischemic penumbra in the EA and IR groups or the same region in the sham group (6, 24 and 48 hours post-reperfusion or sham surgery) were separated using a cytosol/mitochondria fractionation kit (Beyotime Biotechnology, Beijing, China) according to the manufacturer's instructions. In brief, the brain ischemic penumbra tissues of rats were rapidly removed and washed with ice-cold phosphate-buffered saline, and then placed into ice-cold isolation medium with 1 mM phenylmethylsulfonyl fluoride. The suspension was homogenized 8–10 times by hand. The supernatant was sequentially centrifuged at 800 × g for 5 minutes, 11,000 × g for 10 minutes and 12,000 × g for 20 minutes, and the cytosolic fraction collected. The remaining pellets were dissolved with ice-cold isolation medium and then centrifuged at 12,000 × g for 15 minutes. The final pellets were collected as the mitochondrial fraction. All centrifugations were performed at 4°C. The purified mitochondria were lysed with the pre-cooling lysis buffer containing phenylmethyl sulfonylfluoride for western blot assay. The expression of specific markers (cyclooxygenase for mitochondria, Rho guanylic acid dissociation inhibitor [GDI] for cytosol) were analyzed to ensure the purity of each fraction.
The brain ischemic penumbra tissues were homogenized in lysis buffer (phenylmethyl sulfonylfluoride and radioimmunoprecipitation assay buffer) (Beyotime Biotechnology), and then centrifuged at 12,000 × g for 15 minutes at 4°C. The samples were used for total protein assays. The mitochondrial and cytosol proteins were obtained using the separation steps described above.
Protein concentrations were determined using a bicinchoninic acid protein assay (Beyotime Biotechnology). Proteins were mixed with 2× loading buffer and then heated at 99°C for 5 minutes. Equal amounts of proteins (30 μg) were loaded into each well and separated using 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis. The proteins were then transferred onto polyvinylidene fluoride membranes (Merck Millipore, Darmstadt, Germany). The membranes were blocked in tris-buffered saline tween-20 with 5% non-fat milk for 2 hours, and then incubated with primary antibodies overnight: rabbit anti-rat Drp1 monoclonal antibody (1:1,000), rabbit anti-rat cytochrome c monoclonal antibody (1:1,000), rabbit anti-rat cyclooxygenase-IV monoclonal antibody (1:2,000), rabbit anti-rat Rho GDP dissociation inhibitor GDI monoclonal antibody (1:2,000) (all from Abcam) or rabbit anti-rat β-actin monoclonal antibody (1:2,000; Zhongshan Goldenbridge Biotechnology, Beijing, China). The membranes were then washed with tris-buffered saline tween-20 and incubated with the goat anti-rabbit IgG (1:5,000; Jackson ImmunoResearch Laboratories, Baltimore, PA, USA). The blots were then developed using enhanced chemiluminescence (Beyotime Biotechnology), and then quantified using QuantiScan One Software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All data are presented as the mean ± SD, and analyzed with statistical software (SPSS 19.0; IBM Corporation, Armork, NY, USA). One-way analysis of variance followed by the post hoc Student-Newman-Keuls test was used for comparison between the groups. A value of P < 0.05 was considered statistically significant.
Results
EA preconditioning decreased neurological deficit scores in rats with focal cerebral IR injury
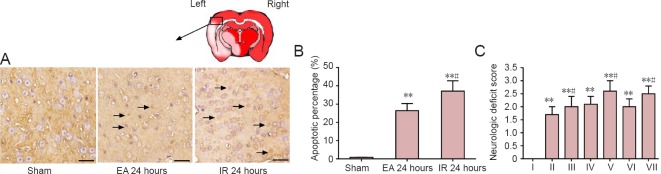
Neurological deficit scores at 6, 24 and 48 hours after IR insults were increased compared with sham group (P < 0.01; (Figure 1C). By contrast, neurological deficit scores in the EA group after reperfusion were lower than those in the IR group at each time point (6 hours, P = 0.038; 24 hours, P = 0.024; 48 hours, P = 0.029; Figure 1C).
Figure 1.
Effects of EA pretreatment on cellular apoptosis and neurological deficit scores in rats with focal cerebral ischemia/reperfusion (IR) injury.
(A) Terminal deoxyribonucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling (TUNEL) staining of cells (arrows) at 24 hours after IR or sham surgery. Scale bars: 50 μm. (B) Proportion of TUNEL-positive cells in the ischemic penumbra (n = 4 rats per group). (C) Neurological deficit score at 6, 24 and 48 hours after reperfusion or sham surgery (n = 8 per group at 6 and 48 hours after reperfusion; n = 16 per group at 24 hours after reperfusion). Data are presented as the mean ± SD, and analyzed by one-way analysis of variance followed by the post hoc Student-Newman-Keuls test. **P < 0.01, vs. sham group; #P < 0.05, vs. electroacupuncture (EA) group at the same time point. I: Sham; II: EA 6 hours; III: IR 6 hours; IV: EA 24 hours; V: IR 24 hours; VI: EA 48 hours; VII: IR 48 hours.
EA preconditioning decreased cell apoptosis in the ischemic penumbra of rats with focal cerebral IR injury
TUNEL staining was performed to examine cell apoptosis (Nie et al., 2016). The proportion of TUNEL-positive cells indicated a marked increase in neuronal apoptosis in the ischemic penumbra in the EA and IR groups at 24 hours after reperfusion compared with the sham group (P = 0.0008; Figure 1A, B). By contrast, the EA group showed a significant decrease in the proportion of TUNEL-positive neurons compared with the IR group (P = 0.028; Figure 1A, B).
EA preconditioning alleviated the histopathological changes of neurons in the ischemic penumbra of rats with focal cerebral IR injury
Hematoxylin-eosin staining showed the appearance of shrunken cell bodies and nuclear pyknosis in the ischemic penumbra of the EA and IR groups after reperfusion. By contrast, the pathological changes were milder in the EA group compared with the IR group (Figure 2).
Figure 2.
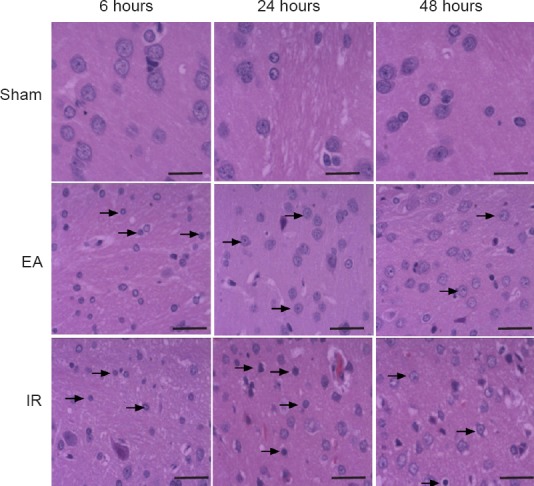
Effect of electroacupuncture (EA) preconditioning on neuronal histopathological structure in the ischemic penumbra of rats with focal cerebral ischemia/reperfusion (IR) injury (hematoxylin-eosin staining).
Representative stained images in the ischemic penumbra at 6, 24 and 48 hours post-reperfusion or sham surgery. Shrunken cell bodies and nuclear pyknosis (arrows) were observed in the EA and IR groups, but not in the sham group. The degree of histopathological changes in the IR group was more obvious than that in the EA group. Scale bars: 50 μm (n = 4 rats)
Effect of EA preconditioning on mitochondrial ultrastructure of neurons in rats with focal cerebral IR injury
The mitochondrial ultrastructure of neurons under transmission electron microscope can be used to assess the degree of mitochondrial fission (Kubli et al., 2013). Mitochondria were well arranged and exhibited an integrated double-membrane structure and normal cristae without any sign of swelling or injury in the sham group. Mitochondria presented degenerative signs, such as deficiency of typical tubular or elliptic morphology, disappearance of double-membrane structure, vacuolation and cristae swelling, in the IR and EA groups at 24 hours after reperfusion. By contrast, mitochondria in the EA group exhibited a less swollen and relatively integrated membrane compared with those in the IR group (Figure 3A).
Figure 3.
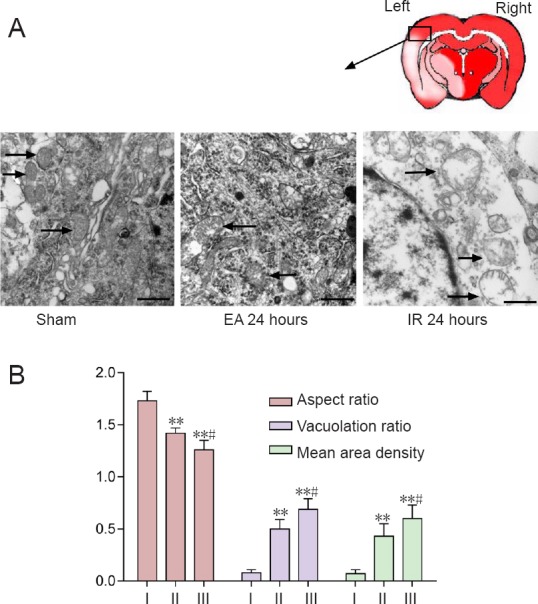
Effect of electroacupuncture (EA) preconditioning on mitochondrial ultrastructure in the brains of rats with focal cerebral ischemia/reperfusion (IR) injury.
Mitochondrial (arrows) morphological structure observed under the transmission electron microscope in the ischemic penumbra at 24 hours post-reperfusion or sham surgery. Scale bars: 0.5 μm. (B) Quantitative parameters for mitochondrial morphological changes, including aspect ratio, vacuolation ratio and mean area density of vacuolated mitochondria. Data are presented as the mean ± SD (n = 4 rats per group), and analyzed by one-way analysis of variance followed by the post hoc Student-Newman-Keuls test. **P < 0.01, vs. sham group; #P < 0.05, vs. EA 24 hour group. I: Sham; II: EA 24 hours; III: IR 24 hours.
The aspect ratio, vacuolation ratio and mean area density of vacuolated mitochondria were used to quantify changes in mitochondrial morphology. The aspect ratio in the sham group was significantly higher than that in the other two groups (EA 24 hours, P = 0.007; IR 24 hours, P = 0.005), while the aspect ratio in the EA group was significantly higher than that in the IR group (P = 0.020). The degree of swelling in the EA group was reduced compared with the IR group. Further, the IR group showed a markedly higher ratio of vacuolated mitochondria compared with the sham 24 hour (P = 0.001) and EA 24 hour (P = 0.030) groups, and a higher mean area density compared with the sham 24 hour (P = 0.001) and EA 24 hour (P = 0.035) groups (Figure 3B).
Effect of EA preconditioning on Drp1 expression in the focal cerebral IR injury rats
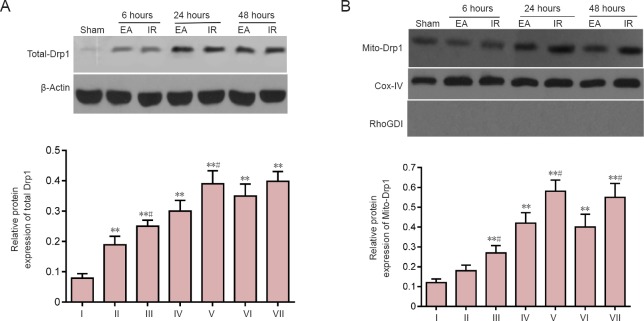
The expression of total Drp1 and mitochondrial Drp1 in the ischemic penumbra was measured by western blot assay. Compared with the sham group, total Drp1 was significantly increased at 6, 24 and 48 hours after reperfusion in the IR and EA groups (P < 0.01; Figure 4A). By contrast, total Drp1 in the EA group at 6 and 24 hours after reperfusion was significantly lower than that in the IR group (6 hours, P = 0.041; 24 hours, P = 0.024; Figure 4A).
Figure 4.
Effects of electroacupuncture (EA) preconditioning on dynamin-related protein 1 (Drp1) expression in focal cerebral ischemia/reperfusion (IR) injury rats.
Total-Drp1 (A) and Mitochondrial Drp1 (Mito-Drp1) (B) at 6, 24 and 48 hours post-reperfusion or sham surgery, as measured by western blot assay and densitometric analysis, and quantified relative to β-actin (Total-Drp1) or cyclooxygenase (Cox)-IV (Mito-Drp1). The lack of expression of the Rho GDP dissociation inhibitor (RhoGDI; a specific cytosolic [but not mitochondrial] biomarker (Francis et al., 2014)) verified the purity of the mitochondrial fraction. Data are presented as the mean ± SD (n = 4 rats per group at each time point), and analyzed by one-way analysis of variance followed by the post hoc Student-Newman-Keuls test. Data are representative of three independent tests. *P < 0.05, **P < 0.01, vs. sham group; #P < 0.05, vs. EA group at the same time point. I: Sham; II: EA 6 hours; III: IR 6 hours; IV: EA 24 hours; V: IR 24 hours; VI: EA 48 hours; VII: IR 48 hours.
The mitochondria fraction was isolated using hypothermal differential centrifugation, and the Drp1 attached to mitochondria (Mito-Drp1) was measured, as Mito-Drp1 rather than cytosolic Drp1 directly mediates mitochondrial fission (Michalska et al., 2016). Higher expression of Mito-Drp1 was measured in the EA and IR groups at 6, 24 and 48 hours after reperfusion compared with the sham group (P < 0.05 or P < 0.01). By contrast, rats in the EA group showed reduced Mito-Drp1 levels at 6, 24 and 48 hours after reperfusion compared with the IR group (6 hours, P = 0.025; 24 hours, P = 0.030; 48 hours, P = 0.028; Figure 4B).
Effect of EA preconditioning on cytochrome c expression in the focal cerebral IR injury rats
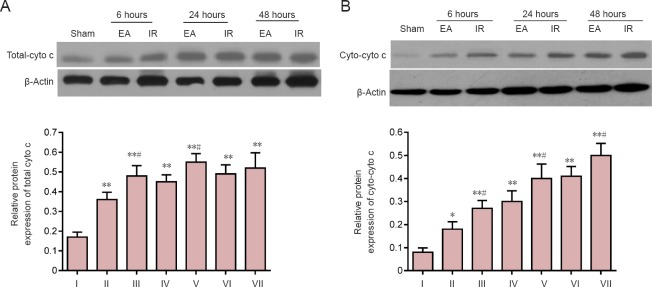
During mitochondrial fission, cytochrome c is released to the cytosol from mitochondria, which can trigger neuronal apoptosis (Kim et al., 2015; Atkins et al., 2016). Therefore, the expression of total cytochrome c (total-cyto c) and cytosolic cytochrome c (cyto-cyto c) were measured by western blot assay to represent the changes in mitochondrial function. As shown in Figure 5A, total-cyto c expression in the IR and EA groups at 6, 24 and 48 hours after reperfusion was markedly higher than that in the sham group (P < 0.01). By contrast, rats in the EA group showed decreased levels of total-cyto c at 6 and 24 hours after reperfusion compared with the IR group (6 hours, P = 0.020; 24 hours, P = 0.038). cyto-cyto c was detected after separating the cytosolic and mitochondrial fractions. Compared with the sham group, the cyto-cyto c levels significantly increased in the IR and EA groups at 6, 24 and 48 hours after reperfusion (P < 0.01). By contrast, there was a significant decrease in cyto-cyto c levels in the EA group compared with the IR group at 6, 24 and 48 hours after reperfusion (6 hours, P = 0.026; 24 hours, P = 0.030; 48 hours, P = 0.035; Figure 5B).
Figure 5.
Effects of electroacupuncture (EA) preconditioning on cytochrome (cyto) expression in focal cerebral ischemia/reperfusion (IR) injury rats.
Total-cyto c (A) and cytosolic cytochrome c (cyto-cyto c) (B) at 6, 24 and 48 hours post-reperfusion or sham surgery. Data were determined by western blot assay, and are presented as the densitometric ratio relative to β-actin. Data are shown as the mean ± SD (n = 4 rats per group at each time point), and analyzed by one-way analysis of variance followed by the post hoc Student-Newman-Keuls test. Data are representative of three independent tests. **P < 0.01, vs. sham group; #P < 0.05, vs. EA group at the same time point. I: Sham; II: EA 6 hours; III: IR 6 hours; IV: EA 24 hours; V: IR 24 hours; VI: EA 48 hours; VII: IR 48 hours.
Discussion
The occurrence and development of IR injury involve a range of complex pathophysiological processes, including calcium overload, oxidative stress and inflammation (Prentice et al., 2015; Jin et al., 2016; Wu et al., 2016). Importantly, mitochondria are involved in all these processes, and mitochondria can release multiple essential apoptosis-inducing factors to induce neuronal apoptosis. Thus, mitochondria are considered a ‘gatekeeper’ during cell death (Perez-Pinzon et al., 2012; Bhola and Letai, 2016). Under physiological conditions, the mitochondria remain in a highly dynamic state with frequent fission and fusion. The balance between fusion and fission is important for maintaining the mitochondrial reticulum, regulating cellular energy metabolism and controlling cellular development and death. Nevertheless, during cerebral IR injury, mitochondrial fission predominates, with various degrees of mitochondrial fragmentation which can lead to neuronal apoptosis (Suen et al., 2008; Westermann, 2010; Balog et al., 2016; Lee and Yoon, 2016).
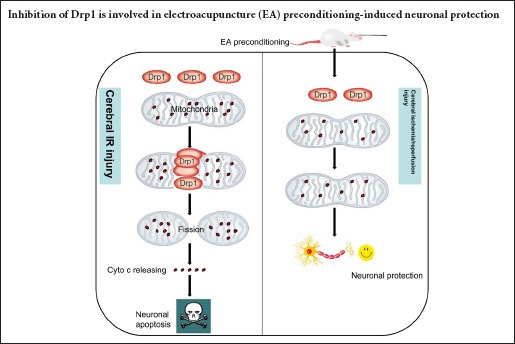
Drp1, a key component of the mitochondrial fission system, plays a crucial role in regulating the homeostasis between fission and fusion, and in maintaining mitochondrial morphology (Song et al., 2015). Under conditions of stress such as cerebral IR injury, Drp1 expression and binding to the mitochondrial outer membrane are increased, which leads to mitochondrial fission and mitochondria-dependent cell apoptosis (Atkins et al., 2016). Therefore, total mitochondrial and mitochondrial Drp1 were used to assess Drp1 activation during cerebral IR injury. Based on our findings, we suggest that Drp1 activation increased mitochondrial fission (ultrastructural abnormalities and Cyto c release) and activated downstream apoptotic pathways, resulting in cerebral IR injury (hematoxylin-eosin staining, TUNEL staining and neurologic deficit scores).
Previous studies have confirmed that diversified preconditioning, including EA, remote ischemia, hypoxia and anesthesia, can attenuate cerebral IR insults (Ma et al., 2016; Thushara Vijayakumar et al., 2016; Li et al., 2017). EA preconditioning is safe, effective, simple and convenient, with good controllability, and has thus been widely studied for the prevention and treatment of ischemic cerebral disease. Recently, stimulating the acupoint GV20 was also shown to induce ischemic tolerance and attenuate IR injury (Zhang et al., 2009; Jung et al., 2016). Consistent with these results, in the present study there was a decrease in the proportion of TUNEL-positive cells, pathological changes and neurologic deficit scores in the EA group compared with IR group, indicating cerebral protection with EA pretreatment.
Multiple signaling pathways have been reported in EA-induced cerebral protection (Kim et al., 2013; Zhan et al., 2016). However, the precise mechanisms remain unclear. Interestingly, EA was reported to attenuate cerebral injury by improving mitochondrial energy metabolism (Tian et al., 2015). In the present study, we found that EA preconditioning altered Drp1-mediated mitochondrial fission, with downregulation of Drp1 expression, inhibition of translocation of cytosolic Drp1 to the mitochondrial outer membrane and reduced restrained mitochondrial fission, resulting in reduced cyto c release and pathological mitochondrial morphological changes.
There are several limitations of this study.First, further studies using Mdivi-1 (a selective inhibitor of Drp1) or Drp1 knockout mice are required to confirm our findings. Second, mitochondrial membrane potential and reactive oxygen species should be examined to assess changes in mitochondrial function. Finally, further studies using a sham EA group (EA at non-acupoint points) are required. Nevertheless, previous studies and our preliminary experiments suggest that EA preconditioning at non-acupoint points have limited effects on cerebral protection (Cheng et al., 2014a, b; Zhu et al., 2017). Thus, the sham EA group was not designed in our present study.
In summary, EA preconditioning at acupoint GV20 alleviated focal cerebral IR injury, which was associated with the suppression of Drp1 expression and translocation, and inhibition of Drp1-mediated mitochondrial fission, in a rat model of middle cerebral artery occlusion. This study suggests a new mechanism of EA preconditioning-induced cerebral protection, and lays the theoretical basis for clinical application of acupuncture in the prevention of stroke.
Acknowledgments
We would like to thank Dr. Bing Luo from Qingdao University in China for generously providing the laboratory.
Footnotes
Funding: This study was supported by the Natural Science Foundation of Shandong Province of China, No. ZR2015HM023; a grant from the Science and Technology Plan Project of Shinan District of Qingdao City of China, No. 2016-3-029-YY.
Conflicts of interest: None declared.
Financial support: This study was supported by the Natural Science Foundation of Shandong Province of China, No. ZR2015HM023; the Science and Technology Plan Project of Shinan District of Qingdao City of China, No. 2016-3-029-YY. None of the funding bodies played any role in the study other than to provide funding.
Research ethics: The study protocol was approved by the Animal Ethics Committee of Qingdao Municipal Hospital of China (approval No. 201613). The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1985).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Nagendran Muthusamy, North Carolina State University, USA.
Comments to authors: In this study, the authors tested if any association exists between DRP1, molecule that mediates mitochondrial fission following cerebral ischemia reperfusion injury, and the neuroprotective effects of electroacupuncture. Using correlative data obtained with imaging and molecular techniques, the authors conclude that the protective effects of electroacupuncture is mediated by (1) decreasing the levels of Drp1, (2) decreasing cytochrome c levels, and (3) decreasing apoptosis when compared to ischemia and reperfusion group. Lower Drp1 levels mediated by electroacupuncture are found to be protective as decreased Drp1 is correlated with decreased mitochondrial fission and reduced reactive oxygen species generation following ischemia/reperfusion injury.
(Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Atkins K, Dasgupta A, Chen KH, Mewburn J, Archer SL. The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease. Clin Sci (Lond) 2016;130:1861–1874. doi: 10.1042/CS20160030. [DOI] [PubMed] [Google Scholar]
- 2.Balog J, Mehta SL, Vemuganti R. Mitochondrial fission and fusion in secondary brain damage after CNS insults. J Cereb Blood Flow Metab. 2016;36:2022–2033. doi: 10.1177/0271678X16671528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhola PD, Letai A. Mitochondria-judges and executioners of cell death sentences. Mol Cell. 2016;61:695–704. doi: 10.1016/j.molcel.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng CY, Lin JG, Tang NY, Kao ST, Hsieh CL. Electroacupuncture-like stimulation at the Baihui (GV20) and Dazhui (GV14) acupoints protects rats against subacute-phase cerebral ischemia-reperfusion injuries by reducing S100B-mediated neurotoxicity. PLoS One. 2014a;9:e91426. doi: 10.1371/journal.pone.0091426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng CY, Lin JG, Su SY, Tang NY, Kao ST, Hsieh CL. Electroacupuncture-like stimulation at Baihui and Dazhui acupoints exerts neuroprotective effects through activation of the brain-derived neurotrophic factor-mediated MEK1/2/ERK1/2/p90RSK/bad signaling pathway in mild transient focal cerebral ischemia in rats. BMC Complement Altern Med. 2014b;14:92. doi: 10.1186/1472-6882-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci. 2015;9:124. doi: 10.3389/fncel.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flippo KH, Strack S. Mitochondrial dynamics in neuronal injury, development and plasticity. J Cell Sci. 2017;130:671–681. doi: 10.1242/jcs.171017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis C, Natarajan S, Lee MT, Khaladkar M, Buckley PT, Sul JY, Eberwine J, Kim J. Divergence of RNA localization between rat and mouse neurons reveals the potential for rapid brain evolution. BMC Genomics. 2014;15:883. doi: 10.1186/1471-2164-15-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graef M. A dividing matter: Drp1/Dnm1-independent mitophagy. J Cell Biol. 2016;215:599–601. doi: 10.1083/jcb.201611079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hameed A, Zafar H, Mylotte D, Sharif F. Recent trends in clot retrieval devices: a review. Cardiol Ther. 2017 doi: 10.1007/s40119-017-0098-2. doi: 10.1007/s40119-017-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu C, Huang Y, Li L. DRP1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin XL, Li PF, Zhang CB, Wu JP, Feng XL, Zhang Y, Shen MH. Electroacupuncture alleviates cerebral ischemia and reperfusion injury via modulation of the ERK1/2 signaling pathway. Neural Regen Res. 2016;11:1090–1098. doi: 10.4103/1673-5374.187041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung YS, Lee SW, Park JH, Seo HB, Choi BT, Shin HK. Electroacupuncture preconditioning reduces ROS generation with NOX4 down-regulation and ameliorates blood-brain barrier disruption after ischemic stroke. J Biomed Sci. 2016;23:32. doi: 10.1186/s12929-016-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HJ, Shaker MR, Cho B, Cho HM, Kim H, Kim JY, Sun W. Dynamin-related protein 1 controls the migration and neuronal differentiation of subventricular zone-derived neural progenitor cells. Sci Rep. 2015;5:15962. doi: 10.1038/srep15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JH, Choi KH, Jang YJ, Kim HN, Bae SS, Choi BT, Shin HK. Electroacupuncture preconditioning reduces cerebral ischemic injury via BDNF and SDF-1alpha in mice. BMC Complement Altern Med. 2013;13:22. doi: 10.1186/1472-6882-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, Jimenez R, Petrosyan S, Murphy AN, Gustafsson AB. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013;288:915–926. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan X, Zhang X, Zhou GP, Wu CX, Li C, Xu XH. Electroacupuncture reduces apoptotic index and inhibits p38 mitogen-activated protein kinase signaling pathway in the hippocampus of rats with cerebral ischemia/reperfusion injury. Neural Regen Res. 2017;12:409–416. doi: 10.4103/1673-5374.202944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Yoon Y. Mitochondrial fission and fusion. Biochem Soc Trans. 2016;44:1725–1735. doi: 10.1042/BST20160129. [DOI] [PubMed] [Google Scholar]
- 20.Lee JE, Westrate LM, Wu H, Page C, Voeltz GK. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016;540:139–143. doi: 10.1038/nature20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Saiyin H, Xie J, Ma L, Xue L, Wang W, Liang W, Yu Q. Sevoflurane preconditioning induced endogenous neurogenesis against ischemic brain injury by promoting microglial activation. Oncotarget. 2017;8:28544–28557. doi: 10.18632/oncotarget.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Wang P, Wei J, Fan R, Zuo Y, Shi M, Wu H, Zhou M, Lin J, Wu M, Fang X, Huang Z. Inhibition of Drp1 by Mdivi-1 attenuates cerebral ischemic injury via inhibition of the mitochondria-dependent apoptotic pathway after cardiac arrest. Neuroscience. 2015;311:67–74. doi: 10.1016/j.neuroscience.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma XM, Liu M, Liu YY, Ma LL, Jiang Y, Chen XH. Ischemic preconditioning protects against ischemic brain injury. Neural Regen Res. 2016;11:765–770. doi: 10.4103/1673-5374.182703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalska B, Duszyński J, Szymański J. Mechanism of mitochondrial fission - structure and function of Drp1 protein. Postepy Biochem. 2016;62:127–137. [PubMed] [Google Scholar]
- 27.Nie J, Tian Y, Zhang Y, Lu YL, Li LS, Shi JS. Dendrobium alkaloids prevent Abeta25-35-induced neuronal and synaptic loss via promoting neurotrophic factors expression in mice. PeerJ. 2016;4:e2739. doi: 10.7717/peerj.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel RAG, McMullen PW. Neuroprotection in the treatment of acute ischemic stroke. Prog Cardiovasc Dis. 2017;59:542–548. doi: 10.1016/j.pcad.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Pinzon MA, Stetler RA, Fiskum G. Novel mitochondrial targets for neuroprotection. J Cereb Blood Flow Metab. 2012;32:1362–1376. doi: 10.1038/jcbfm.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pradeep H, Sharma B, Rajanikant GK. Drp1 in ischemic neuronal death: an unusual suspect. Curr Med Chem. 2014;21:2183–2189. doi: 10.2174/0929867321666131228203513. [DOI] [PubMed] [Google Scholar]
- 31.Prentice H, Modi JP, Wu JY. Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev. 2015;2015:964518. doi: 10.1155/2015/964518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ran QQ, Chen HL, Liu YL, Yu HX, Shi F, Wang MS. Electroacupuncture preconditioning attenuates ischemic brain injury by activation of the adenosine monophosphate-activated protein kinase signaling pathway. Neural Regen Res. 2015;10:1069–1075. doi: 10.4103/1673-5374.160095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., 2nd Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–285. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thushara Vijayakumar N, Sangwan A, Sharma B, Majid A, Rajanikant GK. Cerebral ischemic preconditioning: the road so far. Mol Neurobiol. 2016;53:2579–2593. doi: 10.1007/s12035-015-9278-z. [DOI] [PubMed] [Google Scholar]
- 36.Tian WQ, Peng YG, Cui SY, Yao FZ, Li BG. Effects of electroacupuncture of different intensities on energy metabolism of mitochondria of brain cells in rats with cerebral ischemia-reperfusion injury. Chin J Integr Med. 2015;21:618–623. doi: 10.1007/s11655-013-1512-9. [DOI] [PubMed] [Google Scholar]
- 37.Ting Z, Jianbin Z, Luqi H. Protective effect of electroacupuncture on neurons autophagy in perfusion period of cerebral ischemia. Neurosci Lett. 2017;661:41–45. doi: 10.1016/j.neulet.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 38.Vakifahmetoglu-Norberg H, Ouchida AT, Norberg E. The role of mitochondria in metabolism and cell death. Biochem Biophys Res Commun. 2017;482:426–431. doi: 10.1016/j.bbrc.2016.11.088. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Peng Y, Chen S, Gou X, Hu B, Du J, Lu Y, Xiong L. Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke. 2009;40:2157–2164. doi: 10.1161/STROKEAHA.108.541490. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, Xiong L, Chen S, Liu Y, Zhu X. Rapid tolerance to focal cerebral ischemia in rats is induced by preconditioning with electroacupuncture: window of protection and the role of adenosine. Neurosci Lett. 2005;381:158–162. doi: 10.1016/j.neulet.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 42.Wiemerslage L, Lee D. Quantification of mitochondrial morphology in neurites of dopaminergic neurons using multiple parameters. J Neurosci Methods. 2016;262:56–65. doi: 10.1016/j.jneumeth.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winquist RJ, Kerr S. Cerebral ischemia-reperfusion injury and adhesion. Neurology. 1997;49:S23–26. doi: 10.1212/wnl.49.5_suppl_4.s23. [DOI] [PubMed] [Google Scholar]
- 44.Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15:1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- 45.Wu S, Zhou F, Zhang Z, Xing D. Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. FEBS J. 2011;278:941–954. doi: 10.1111/j.1742-4658.2011.08010.x. [DOI] [PubMed] [Google Scholar]
- 46.Wu XG, Qiu ZF, Meng J, Zu BX, Li MM, Miao H. Effects of Buyanghuanwu decoction on the protein expression of PI3K, Akt, Bcl-2 and BAX in brain tissue of a rat model of cerebral hemorrhage. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:5933–5938. [Google Scholar]
- 47.Yamaguchi T, Fujiwara T, Tsai YA, Tang SC, Kawakami M, Mizuno K, Kodama M, Masakado Y, Liu M. The effects of anodal transcranial direct current stimulation and patterned electrical stimulation on spinal inhibitory interneurons and motor function in patients with spinal cord injury. Exp Brain Res. 2016;234:1469–1478. doi: 10.1007/s00221-016-4561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhan J, Qin W, Zhang Y, Jiang J, Ma H, Li Q, Luo Y. Upregulation of neuronal zinc finger protein A20 expression is required for electroacupuncture to attenuate the cerebral inflammatory injury mediated by the nuclear factor-kB signaling pathway in cerebral ischemia/reperfusion rats. J Neuroinflammation. 2016;13:258. doi: 10.1186/s12974-016-0731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S, Li N, Liu M. Use of acupuncture for stroke in China. Acupunct Med. 2009;27:146. doi: 10.1136/aim.2009.001669. [DOI] [PubMed] [Google Scholar]
- 50.Zhu X, Yin J, Li L, Ma L, Tan H, Deng J, Chen S, Zuo Z. Electroacupuncture preconditioning-induced neuroprotection may be mediated by glutamate transporter type 2. Neurochem Int. 2013;63:302–308. doi: 10.1016/j.neuint.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y, Deng L, Tang H, Gao X, Wang Y, Guo K, Kong J, Yang C. Electroacupuncture improves neurobehavioral function and brain injury in rat model of intracerebral hemorrhage. Brain Res Bull. 2017;131:123–132. doi: 10.1016/j.brainresbull.2017.04.003. [DOI] [PubMed] [Google Scholar]