Abstract
The in vitro immune activities of Saccharum Alhagi polysaccharides (SAP) have been previously studied. The present study aimed to investigate the effects of SAP-1 and SAP-2 on the activity of RAW264.7 mouse macrophages. RAW264.7 cells were treated with 150, 300 and 600 mg/l concentrations of SAP-1 (a 50% alcohol precipitation) and SAP-2 (an 80% alcohol precipitation) or with 10 mg/l lipopolysaccharide. Untreated cells were used as a negative control. An MTT assay was used to detect the proliferation of the cells, and Hoechst 33528 staining was conducted in order to visualize the cell nuclei. Additionally, the Griess method was used to measure nitric oxide (NO) levels. A neutral red uptake assay was performed to determine the phagocytic activity of the macrophages, and ELISAs were performed to detect cytokine levels. Furthermore, reverse transcription-quantitative polymerase chain reaction was used to measure the mRNA expression of certain cytokines. The results demonstrated that SAP increased the proliferative activity and activated the immune function of RAW264.7 cells, and was lacking in cytotoxicity. In addition, SAP-1 exhibited a stronger effect in promoting RAW264.7 cell proliferation than did SAP-2. Furthermore, SAP-1 and SAP-2 significantly increased the level of NO, with the effect of SAP-1 being stronger than that of SAP-2. SAP-1 increased the phagocytic activity of RAW264.7 cells and promoted the secretion of the cytokines interleukin (IL)-1β, IL-2 and tumor necrosis factor (TNF)-α by RAW264.7 cells, with an effect that was stronger than that of SAP-2. Finally, different concentrations of SAP-1 or SAP-2 had distinct effects in upregulating the expression of TNF-α, IL-1β, nuclear factor-κB and inducible nitric oxide synthase mRNA. The results of the present study demonstrate that SAP is capable of enhancing the immune activity of mouse macrophages.
Keywords: Saccharum Alhagi polysaccharides, macrophage, immunocompetence, in vitro, cytokine, immune activity
Introduction
Saccharum Alhagi is a type of granulated sugar that is condensed from the secreted fluid of the leaves of Alhagi pseudalhagi Desv. Alhagi pseudalhagi Desv. is a deciduous shrub of leguminous plants and has prickly branches, oval shaped leaves, pink flowers and pods (1). Furthermore, it has honey glands both inside and outside the flowers. The glands outside the flowers secrete fluids that condense into granulated Saccharum Alhagi that has a round shape, light yellow color and sweet taste. Saccharum Alhagi is harvested from July to September every year and may be used as medicine immediately (1). In Xinjiang, it is often used as a Uyghur medicine for the treatment of pediatric cold (2). Polysaccharides are natural macromolecular compounds. In recent years, polysaccharides have been used as broad spectrum immune promoters for immune regulation (3). In addition, polysaccharides have exhibited a broad range of pharmacological activities against infection, hyperglycemia, hyperlipidemia, oxidization and tumors (4). Studies have demonstrated that polysaccharides are able to activate T and B lymphocytes, macrophages and natural killer cells, stimulate the generation of cytokines and antibodies, and regulate immune functions of the body (5,6).
Macrophages are immune effector cells with defensive and regulatory functions, which actively phagocytose foreign antigens or pathogenic microorganisms, and trigger an immune response (7,8). A previous study demonstrated that the main components of Saccharum Alhagi are polysaccharides with antioxidant activities and in vivo immune activities (9,10). Saccharum Alhagi polysaccharides (SAP) are products that are obtained by water extraction and alcohol precipitation, and are subjected to alcohol precipitation with different gradients (11). The product SAP-1 is obtained by 50% alcohol precipitation, and SAP-2 is obtained by 80% alcohol precipitation (11). Although in vitro immune activities of SAP have been studied, the specific effects of SAP-1 and SAP-2 have not been elucidated. Therefore, the present study investigated the in vitro immune activities of SAP-1 and SAP-2, and their effects on immune cells, immunocytokines and the regulation of the expression of associated genes.
Materials and methods
Cells
Mouse RAW264.7 mononuclear macrophages were purchased from Boster Biological Technology, Ltd. (Wuhan, China) and cultured in Dulbecco's modified Eagle's medium (NAE1388; GE Healthcare Life Sciences, Little Chalfont, UK) supplemented with 10% fetal bovine serum (NZJ1221; Thermo Fisher Scientific, Inc., Waltham, MA, USA). SAP were obtained by water extraction and alcohol precipitation, and subjected to alcohol precipitation with different gradients as described previously (11). SAP-1 was prepared using 50% alcohol precipitation, and SAP-2 was obtained using 80% alcohol precipitation as described previously (11). The cells were adjusted to a density of 5×104 cells/ml, and cultured in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum, 0.5% penicillin and 0.5% streptomycin (all from Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C for 5 days. Then, 150 mg/l (low), 300 mg/l (medium) or 600 mg/l (high) concentrations of SAP-1 or SAP-2, or 10 mg/l lipopolysaccharide (LPS; L2880; Biosharp, Inc., Hefei, China) were added. Cells that were not treated with SAP or LPS were used as negative control.
MTT assay
An MTT assay was performed to investigate the proliferation of the cells (cat. no. 150206; Shanghai Regal Biotechnology, Inc., Shanghai, China), and the cells were observed under a fluorescent inverted microscope (Zeiss Axio Observer Z1; Carl Zeiss AG, Jena, Germany) according to previous reports (12,13). Dimethyl sulfoxide was used to dissolve purple formazan and 490 nm was used to measure the levels of formazan. Each concentration was tested in triplicate. The stimulation index (SI) was calculated as follows: SI=(optical density of experimental group-optical density of non-cell blank control group)/(optical density of negative control group-optical density of non-cell blank control group). Cells that were not treated were used as negative control. The non-cell blank control group had medium but not any cells in the container.
Hoechst 33528 staining
The cells (5×104/ml; 100 µl) were cultured at 37°C for 24 h in an atmosphere containing 5% CO2, and different concentrations (150, 300, and 600 mg/l) of SAP-1 and SAP-2 were added. After 24 h of incubation, paraformaldehyde was added for fixation at 37°C for 15 min. Following staining using Hoechst 33528 fluorescent dye at 37°C for 15 min, the samples were washed with PBS and observed under a fluorescence inverted microscope (Leica DMI6000B; Leica Microsystems GmbH, Wetzlar, Germany).
Griess test
The cells were cultured at 37°C in an atmosphere containing 5% CO2, and different concentrations (150, 300, and 600 mg/l) of SAP-1 and SAP-2 were added. The Griess method was performed to determine the levels of nitric oxide (NO) according to the protocol described in a previous study (14), and the absorbance was read at 540 nm using a microplate reader (Benchmark Plus; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Neutral red uptake assay
The cells were cultured at 37°C in an atmosphere containing 5% CO2, and different concentrations (150, 300, and 600 mg/l) of SAP-1 and SAP-2 were added. Following incubation for 24 h, the cells were washed with PBS twice prior to the addition of neutral red saline solution. Following incubation at 37°C for 30 min, the supernatant was discarded and the cells were washed with PBS twice. Following the addition of 100 µl cell lysis solution (ethanol:glacial acetic acid, 1:1), the cells were incubated at 37°C for 4 h. Finally, the optical density was measured at 540 nm.
ELISA
The cells were cultured at 37°C in an atmosphere containing 5% CO2, and different concentrations (150, 300, and 600 mg/l) of SAP-1 and SAP-2 were added. After incubation for 24 h, the supernatants were collected for ELISAs of interleukin (IL)-2, IL-1β and tumor necrosis factor-α (TNF-α) using respective kits (cat nos. SBJ003780, SBJ003780 and SBJ003780, respectively; Nanjing Senbeijia Biological Technology Co., Ltd., Nanjing, China) as previously reported (15–17).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The cells were cultured at 37°C in an atmosphere containing 5% CO2, and different concentrations (150, 300, and 600 mg/l) of SAP-1 and SAP-2 were added prior to incubation for another 24 h. Next, the cells were trypsinized, centrifuged at 1,000 × g (25°C) for 20 min and lysed in 1 ml TRIzol (Thermo Fisher Scientific, Inc.). Following lysis, the supernatant was collected and mixed with chloroform. Following centrifugation at 5,000 × g (−4°C) for 15 min, the supernatant was mixed with isopropanol. The sample was centrifuged at 5,000 × g (−4°C) for 10 min, and the supernatant discarded. The residue was washed with 75% ethanol, and the total RNA was eluted with diethyl pyrocarbonate water (18–21). The purity of RNA was determined from the A260/A280 ratio using ultraviolet spectrophotometry (Nanodrop ND1000; Thermo Fisher Scientific, Inc.). Next, cDNA was obtained by reverse transcription (High-Capacity cDNA Reverse Transcription Kit, cat. no. 4368813; Thermo Fisher Scientific, Inc.) from 1 µg RNA and stored at −20°C. The reverse transcription protocol was 42°C for 60 min, followed by 70°C for 5 min. To measure the mRNA expression, qPCR was performed (DyNAmo Flash SYBR Green qPCR Kit, cat. no. F415S; Thermo Fisher Scientific, Inc.) using β-actin as an internal reference. The primer sequences are listed in Table I (Sangon Biotech Co., Ltd., Shanghai, China). The reaction system (20 µl) contained 2 µl cDNA, 10 µl mix, 1 µl upstream and 1 µl downstream primer and 6 µl ddH2O. The PCR protocol used was as follows: Initial denaturation at 95°C for 3 min; 40 cycles of denaturation at 95°C for 10 sec, followed by annealing at 57°C for interferon (IFN)-γ, TNF-α and inducible nitric oxide synthase (iNOS), 55°C for IL-1β, 58°C for nuclear factor-κB (NF-κB) and 56°C for β-actin for 30 sec, and elongation at 72°C for 10 sec (iQ5; Bio-Rad Laboratories, Inc.). The 2−ΔΔCq method (22) was used to calculate the relative expression of mRNA (21).
Table I.
Primer sequences of target genes and the internal reference gene.
| Genes | Primer sequences |
|---|---|
| IFN-γ | Forward: 5′-CACAGCCCTCTCCATCAACT-3′ |
| Reverse: 5′-GCATCTTCTCCGTCATCTCC-3′ | |
| TNF-α | Forward: 5′-AGGCACTCCCCCAAAAGAT-3′ |
| Reverse: 5′-CAGTAGACAGAAGAGCGTGGTG-3′ | |
| NF-κB | Forward: 5′-TGGTGGAGAACTTTGAGCCT-3′ |
| Reverse: 5′-GGAATTTCCAGCAGTTTGC-3′ | |
| IL-1β | Forward: 5′-TTGACAGTGATGAGAATGACCTG-3′ |
| Reverse: 5′-GCTCTTGTTGATGTGGTGCTGCT-3′ | |
| iNOS | Forward: 5′-AGGGAATCTTGGAGCGAGTT-3′ |
| Reverse: 5′-GCAGCCTCTTGTCTTTGACC-3′ | |
| β-actin | Forward: 5′-GCCGTCCTCTCTCTGTATGC-3′ |
| Reverse: 5′-GGGGACAGTGTGGCTGAC-3′ |
IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; NF-κB, nuclear factor-κB; IL-1β, interleukin-1β; iNOS, inducible nitric oxide synthase.
Statistical analysis
The results were analyzed using SPSS v17.0 statistical software (IBM Corp., Armonk, NY, USA). Data are expressed as the mean ± standard deviation. Each test was performed in triplicate. One-way analysis of variance and Dunnett's T3 method were used to analyze the data. P<0.05 was considered to indicate a statistically significant result.
Results
SAP is not cytotoxic and promotes the proliferation of RAW264.7 cells
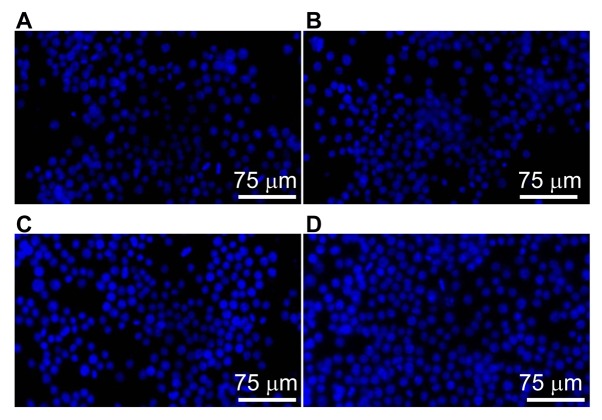
To visualize the nuclei of the RAW264.7 cells, Hoechst 33528 staining was performed. RAW264.7 cells in the control group exhibited evenly dispersed blue fluorescence, and the nuclei were not aggregated (Fig. 1A). Following treatment with low or medium concentrations of SAP mixture, the number of blue nuclei was increased (Fig. 1B and C). Following treatment with a high concentration of SAP, the nuclear aggregation became more evident, and most of the blue nuclei were intact (Fig. 1D). These observations indicate that SAP exhibited no cytotoxicity and promoted the proliferation of RAW264.7 cells.
Figure 1.
RAW264.7 cells stained with Hoechst 33528. (A) Control group, and (B) low (150 mg/l), (C) medium (300 mg/l) and (D) high (600 mg/l) concentration Saccharum Alhagi polysaccharide groups (magnification, ×200).
SAP increases the proliferative activity of RAW264.7 cells and activates their immune function
To observe the effect of SAP on the morphology of RAW264.7 cells, the cells were observed under a fluorescence inverted microscope. In the control group, the RAW264.7 cells were loosely distributed (Fig. 2A). Following treatment with a low concentration of SAP, the number of RAW264.7 cells was increased (Fig. 2B). Following treatment with medium or high concentrations of SAP, the number of RAW264.7 cells was further increased (Fig. 2C and D). Furthermore, when viewed under a microscope at a higher magnification (×100), the morphologies of the RAW264.7 cells following treatment with a high concentration of SAP were round, reniform or oval, and the cells were dispersed (Fig. 2E). Under an even higher magnification (×200), RAW264.7 cells following treatment with high concentration of SAP developed pseudopodia, where the cells became irregular ellipses, and aggregated together in certain dense areas (Fig. 2F). These observations indicate that SAP increased the proliferative activity of RAW264.7 cells, and activated their immune function.
Figure 2.
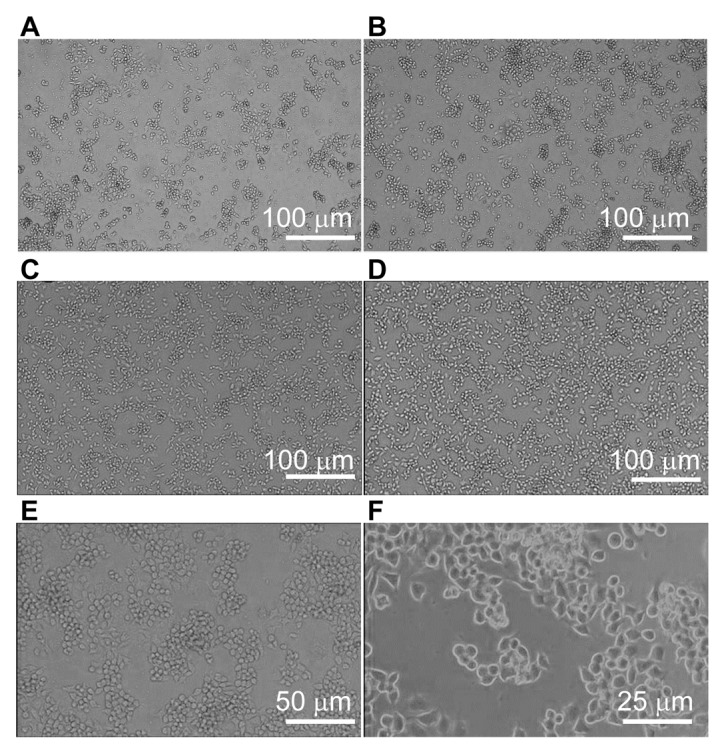
Images of RAW264.7 unstained cells under an inverted microscope in the (A) control group, and the (B) low (150 mg/l), (C) medium (300 mg/l) and (D) high (600 mg/l) concentration SAP groups (magnification, ×50). (E and F) Higher magnification images for the high concentration SAP group [(E) magnification, ×100; (F) magnification, ×200 (phase difference method)]. SAP, Saccharum Alhagi polysaccharides.
SAP-1 has a stronger effect than SAP-2 in promoting RAW264.7 cell proliferation
The number of macrophages was observed to increase following SAP intervention when examined using a microscope. To further study the effects of SAP-1 and SAP-2 on the proliferation of RAW264.7 cells, an MTT assay was performed. The data revealed that a high concentration of SAP-1 or SAP-2, or 10 mg/l LPS resulted in a significantly higher SI than the control (P<0.05). In addition, the SI in the presence of a medium or high concentration of SAP-1 was not significantly different from that in the LPS group (P>0.05), whereas the SI in all concentration groups of SAP-2 was significantly lower compared with that of the LPS group (P<0.05; Fig. 3). These results indicate that SAP-1 had a stronger effect in promoting RAW264.7 cell proliferation than did SAP-2.
Figure 3.
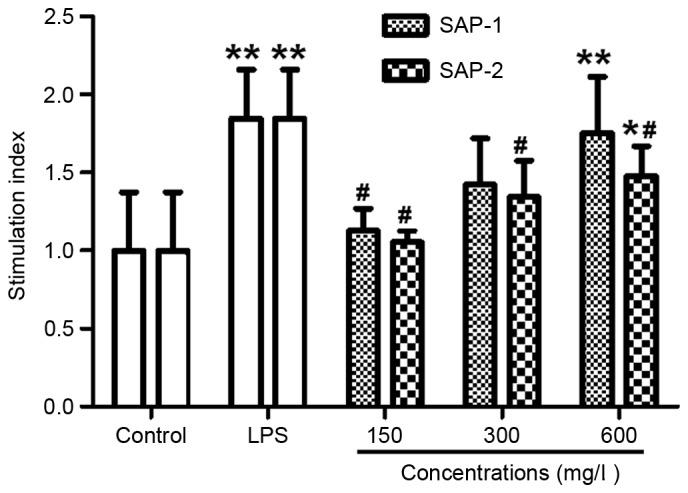
Effect of treatment with SAP-1 or SAP-2 on the proliferation of RAW264.7 cells. RAW264.7 cells (5×104 cells/ml) were treated with SAP-1, SAP-2 or 10 mg/l LPS and incubated at 37°C for 24 h. MTT assay was performed to investigate cell proliferation. *P<0.05 and **P<0.01 vs.≈the control group; #P<0.05 vs. the LPS group. SAP, Saccharum Alhagi polysaccharides; LPS, lipopolysaccharide.
SAP-1 and SAP-2 significantly increase the level of NO secreted by RAW264.7 cells, with SAP-1 having a stronger effect than SAP-2
NO produced by activated macrophages is able to kill or inhibit the growth of several types of microorganisms in the body (23). It is an important index with which to evaluate the immune activity of macrophages. To measure the level of NO secreted by RAW264.7 cells, the Griess method was used. Following treatment with different concentrations of SAP-1, the levels of NO were significantly increased compared with those in the control, in a dose-dependent manner (P<0.01). In addition, the effect of medium and high concentrations of SAP-1 was significantly stronger than that of LPS (P<0.05). As the concentrations of SAP-2 increased, the NO levels were significantly increased compared with those of the control (P<0.01), but the effect of SAP-2 appeared smaller than that of SAP-1. It is noteworthy that the effect of a low concentration of SAP-2 was significantly weaker than that of LPS (P<0.05; Fig. 4). These results indicate that SAP-1 and SAP-2 significantly increased the level of NO secreted by RAW264.7 cells, with the effect of SAP-1 being stronger than that of SAP-2.
Figure 4.
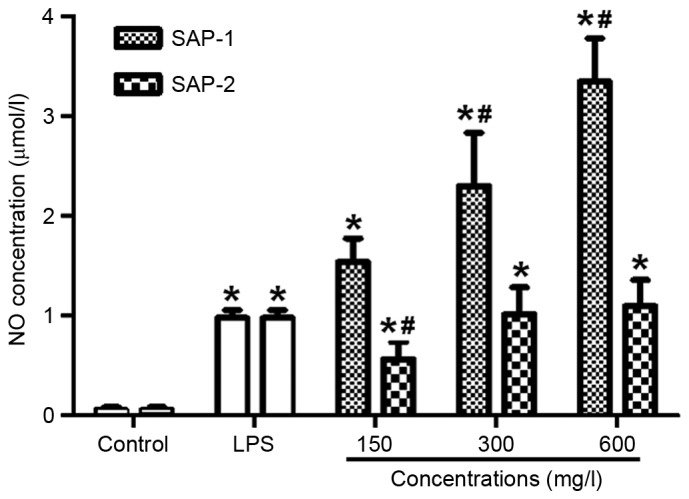
Effect of treatment with SAP-1 or SAP-2 on the secretion of NO by RAW264.7 cells. RAW264.7 cells (5×104 cells/ml) were treated with SAP-1, SAP-2 or 10 mg/l LPS and incubated at 37°C for 24 h. The Griess method was used to measure NO secretion. *P<0.01 vs. the control; #P<0.05 vs. the LPS group. SAP, Saccharum Alhagi polysaccharides; NO, nitric oxide; LPS, lipopolysaccharide.
SAP-1 increases the phagocytic activity of RAW264.7 cells
The main function of macrophages in the immune system is to serve a role in the phagocytosis of foreign materials (24). Examination of phagocytosis by RAW264.7 cells is an important means by which to investigate the immune activity of these cells. A neutral red uptake assay was employed to determine the phagocytic activity of the RAW264.7 cells. The data revealed that treatment with all concentrations of SAP-1 increased the optical density compared with the control (P<0.01). Furthermore, the optical density in the presence of a low or medium concentration of SAP-1 was not significantly different from that in the LPS group (P>0.05), but the optical density of the high concentration SAP-1 group was significantly higher compared with that of the LPS group (P<0.01). The effect of SAP-2 was not as strong as that of SAP-1. Additionally, the optical density in the presence of a low or medium concentration of SAP-2 was not significantly different from that in the control group (P>0.05), while the optical density in the high SAP-2 concentration group was significantly higher than that in the control (P<0.05), but not significantly different from that in the LPS group (P>0.05; Fig. 5). These results indicate that SAP-1 increased the phagocytic activity of RAW264.7 cells.
Figure 5.
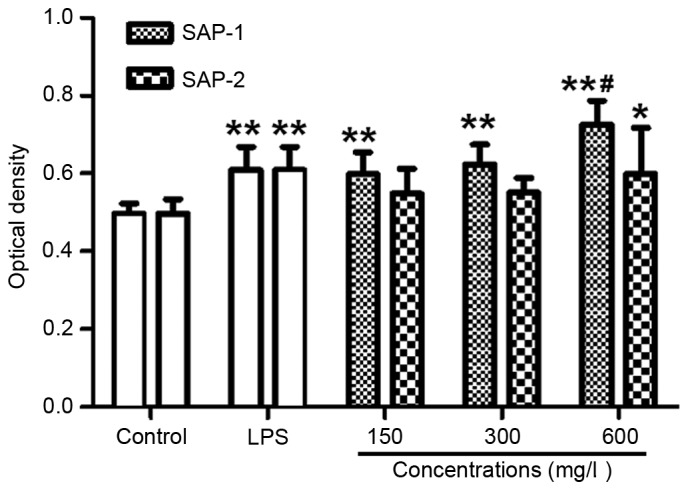
Effect of treatment with SAP-1 or SAP-2 on the phagocytic activity of RAW264.7 cells. RAW264.7 cells (5×104 cells/ml) were treated with SAP-1, SAP-2 or 10 mg/l LPS and incubated at 37°C for 24 h. Neutral red uptake assay was used to investigate the phagocytic activity. *P<0.05 and **P<0.01 vs. the control; #P<0.01 vs. the LPS group. SAP, Saccharum Alhagi polysaccharides; NO, nitric oxide; LPS, lipopolysaccharide.
SAP-1 promotes the secretion of IL-1β, IL-2 and TNF-α cytokines by RAW264.7 cells, with an effect that is stronger than SAP-2
IL-1β, IL-2 and TNF-α are mainly produced by activated macrophages, and can be involved in immune responses (25). Notably, IL-1β can significantly stimulate the secretion of antibodies by these cells (26). To test how SAP-1 and SAP-2 affect the secretion of cytokines by RAW264.7 cells, ELISAs were performed. The data demonstrated that the concentrations of IL-1β, IL-2 and TNF-α following treatment with all concentrations of SAP-1 were significantly higher compared with those in the control (P<0.05), with the effect of high concentration of SAP-1 being the strongest (Fig. 6). A low concentration of SAP-2 failed to significantly increase the concentrations of IL-1β, IL-2 and TNF-α (P>0.05), but medium and high concentrations of SAP-2 significantly elevated the concentrations of IL-1β, IL-2 and TNF-α compared with the control (P<0.05), to levels similar to those of the LPS group (P>0.05; Fig. 6). These results indicate that SAP-1 promoted the secretion of IL-1β, IL-2 and TNF-α by RAW264.7 cells, with an effect that was stronger than that of SAP-2.
Figure 6.
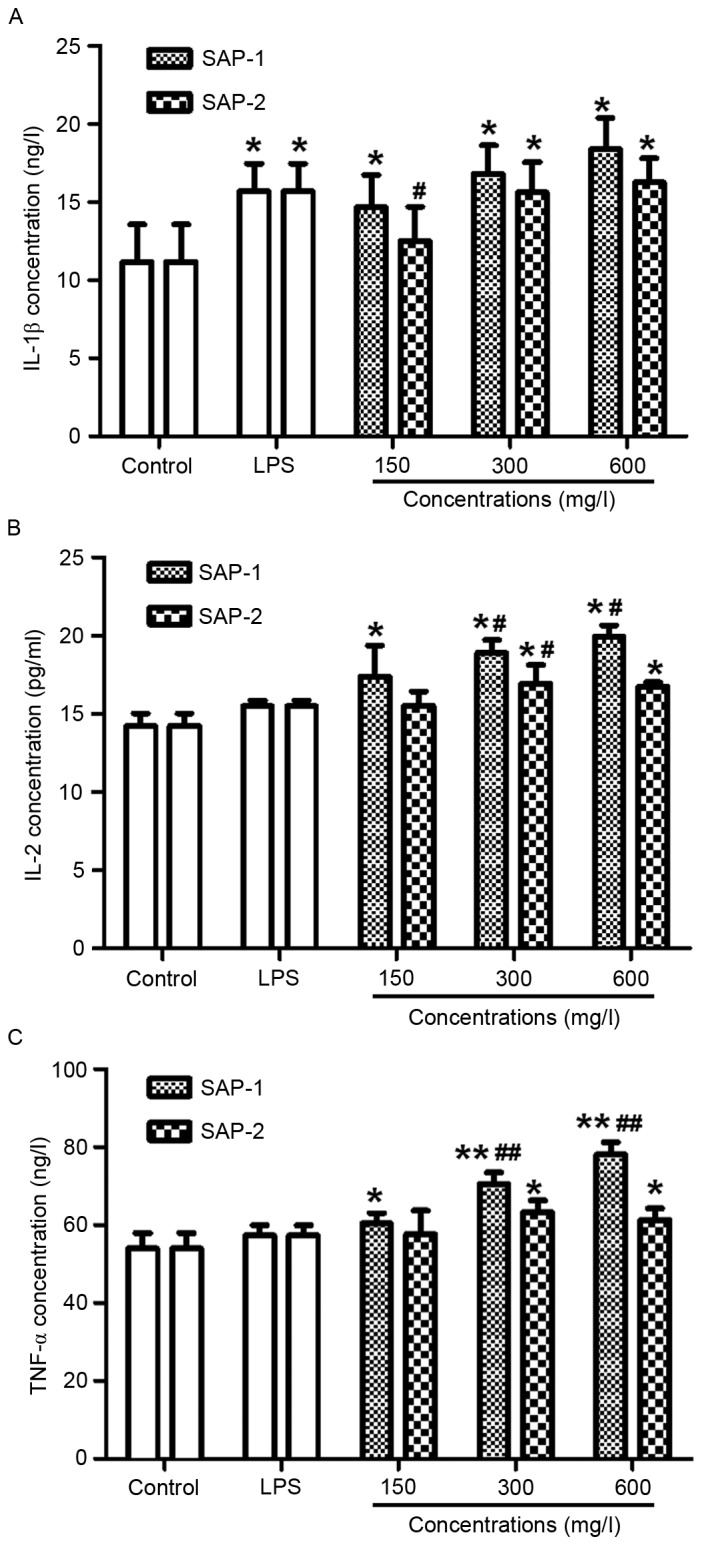
Effect of treatment with SAP-1 or SAP-2 on the secretion of cytokines by RAW264.7 cells. RAW264.7 cells (5×104 cells/ml) were treated with SAP-1, SAP-2 or 10 mg/l LPS and incubated at 37°C for 24 h. ELISAs were performed to determine the levels of secreted (A) IL-1β, (B) IL-2 and (C) TNF-α. *P<0.05 and **P<0.01 vs. the control; #P<0.05 and ##P<0.01 vs. the LPS group. SAP, Saccharum Alhagi polysaccharides; LPS, lipopolysaccharide; IL, interleukin; TNF-α, tumor necrosis factor-α.
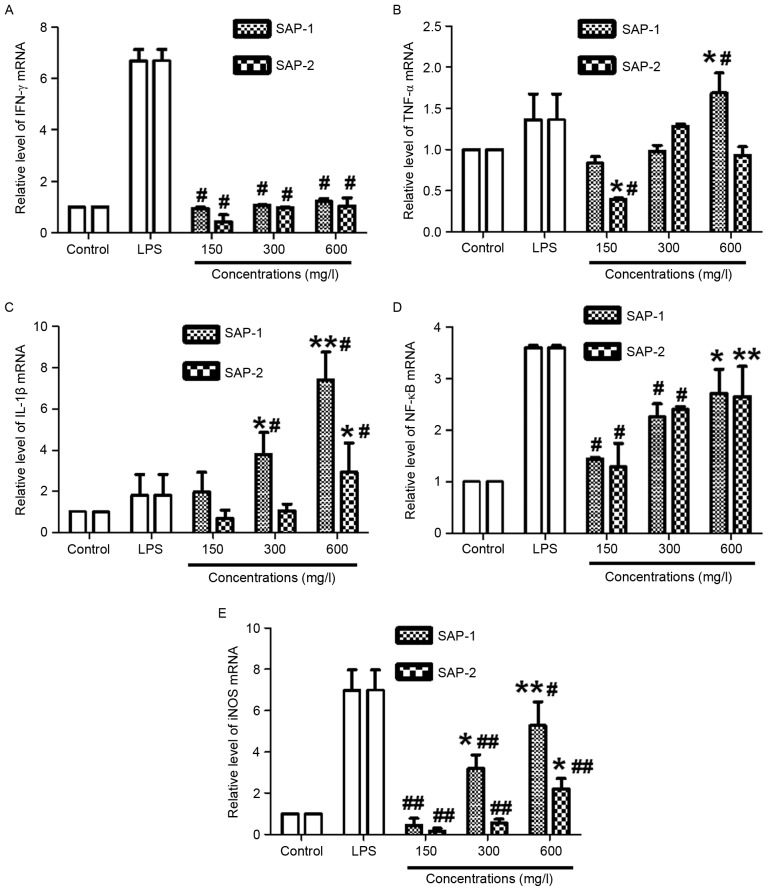
Different concentrations of SAP-1 or SAP-2 possess distinct effects in upregulating the expression of TNF-α, IL-1β, NF-κB and iNOS mRNA
RT-qPCR was employed to measure the mRNA expression of IFN-γ, TNF-α, IL-1β, NF-κB and iNOS. The data revealed that treatment with all concentrations of SAP-1 or SAP-2 had no effect on the IFN-γ mRNA level compared with the control (P>0.05), but IFN-γ mRNA levels in all SAP-1 or SAP-2 concentration groups were significantly lower than those in the LPS group (P<0.05; Fig. 7A). The level of TNF-α mRNA in the high SAP-1 concentration group was significantly higher compared with those in the control and LPS groups (both P<0.05). In addition, the level of TNF-α mRNA in the low SAP-2 concentration group was significantly lower than those in the control and LPS groups (both P<0.05). For other concentrations of SAP-1 or SAP-2, the levels of TNF-α mRNA were not significantly different from either control or LPS values (P>0.05; Fig. 7B).
Figure 7.
Effect of treatment with SAP-1 or SAP-2 on the mRNA expression of (A) IFN-γ, (B) TNF-α, (C) IL-1β, (D) NF-κB and (E) iNOS in RAW264.7 cells. RAW264.7 cells (5×104 cells/ml) were treated with SAP-1, SAP-2 or 10 mg/l LPS and incubated at 37°C for 24 h. Expression of mRNA was measured using reverse transcription-quantitative polymerase chain reaction. *P<0.05 and **P<0.01 vs. the control; #P<0.05 and ##P<0.01 vs. the LPS group. SAP, Saccharum Alhagi polysaccharides; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; NF-κB, nuclear factor-κB; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide.
The levels of IL-1β mRNA in the medium and high SAP-1 concentration groups were significantly higher compared with those in the control and LPS groups (all P<0.05). In addition, the level of IL-1β mRNA in the high SAP-2 concentration group was significantly higher than that in the control or LPS group (both P<0.05). However, for the other concentrations of SAP-1 or SAP-2, the levels of IL-1β mRNA were not significantly different from those in the control and LPS groups (P>0.05; Fig. 7C). The level of NF-κB mRNA in the high SAP-1 concentration group was significantly higher than that in the control (P<0.05), while the levels of NF-κB mRNA in the low and medium SAP-1 concentration groups were significantly lower than that in the LPS group (P<0.05). In addition, the level of NF-κB mRNA in the high SAP-2 concentration group was significantly higher than that in the control (P<0.01), while the levels of NF-κB mRNA in the low and medium SAP-2 concentration groups were significantly lower than that in the LPS group (P<0.05; Fig. 7D). The level of iNOS mRNA in the medium or high SAP-1 concentration group was significantly higher than that in the control (P<0.05), while the levels of iNOS mRNA in the low, medium or high SAP-1 concentration group was significantly lower than that in the LPS group (P<0.05). In addition, the level of iNOS mRNA in the high SAP-2 concentration group was significantly higher than that in the control (P<0.01), while the levels of iNOS mRNA in the low, medium or high SAP-2 concentration group was significantly lower than that in the LPS group (P<0.01; Fig. 7E). These results indicate that the different concentrations of SAP-1 or SAP-2 exhibited distinct effects in upregulating the expression of TNF-α, IL-1β, NF-κB and iNOS mRNA.
Discussion
Macrophages serve important roles in immunity, protecting the body from pathogen invasion (16). The present study investigated the effect of SAP on the activity of RAW264.7 macrophages. Hoechst 33528 staining and morphological examination revealed that SAP was not toxic to RAW264.7 cells, and stimulated the proliferation activity of RAW264.7 cells. SAP-1 and SAP-2 promoted the proliferation of RAW264.7 cells in a dose-dependent manner, with the effect of SAP-1 being stronger than that of SAP-2. Furthermore, macrophages have strong phagocytic activity, which serves to defend the body and to remove waste (24). The present study demonstrated that SAP-1 enhanced the phagocytic activity of RAW264.7 cells.
NO is an important macrophage immune effector molecule that is involved in immune regulation and the defense response, and its production is regulated by iNOS (23). When stimulated, Raw264.7 cells produce abundant quantities of NO, which assists macrophages in the immune response against pathogens (20). The results of the present study demonstrate that SAP-1 increased the levels of iNOS gene expression in RAW264.7 cells, and increased the production of NO by RAW264.7 cells. This indicates that SAP increases the immune activity of RAW264.7 cells by enhancing the level of NO produced by iNOS.
Cytokines serve important roles in cellular immune responses, and a variety of immune cell interactions are mediated by cytokines (8). The present study investigated the effects of SAP-1 and SAP-2 on the secretion and expression of cytokines by RAW264.7 cells. Following treatment with a high concentration of SAP-1, RAW264.7 cells strongly secreted IL-1β, IL-2 and TNF-α, and the results of mRNA expression analysis were consistent with this. By contrast, the effect of SAP-2 was weaker. NF-κB is a key transcription factor in the regulation of immune response (27). It promotes the expression of genes encoding numerous cytokines, leading to increased secretion of these cytokines; for example, NF-κB activates TNF-α and IL-1β, and regulates the relevant immune responses (12,21). The present study revealed that SAP-1 and SAP-2 upregulated the mRNA expression of NF-κB.
The molecular weight of SAP-1 is >14.5 kDa, and that of SAP-2 is <8.4 kDa (11). In the present study, SAP-1 exhibited a stronger effect on cytokines than did SAP-2, indicating that SAP with the higher molecular weight has a stronger immune activity on RAW264.7 cells. In addition, the present study indicated that SAP-1 may activate RAW264.7 cells by promoting the expression and secretion of TNF-α, IL-1β and iNOS via the NF-κB signaling pathway.
In conclusion, the present study indicates that SAP increases the immune activity of RAW264.7 macrophages, and promotes the immune function of the body by increasing the expression of cytokines and related genes. However, the exact pathway and mechanism by which SAP exerts immune effects on immune cells requires investigation in further studies.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81460633).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rukeyamu Shadike. Commonly used herbs in Uygur medicine (Written in Uygur) Xinjiang Science and Technology Press; Urumqi: 1993. p. 284. [Google Scholar]
- 2.Wu J, Li GR, Chang JM. Study on the extraction technology of polysaccharides from Saccharum Alhagi. Chin Traditional Patent Med. 2011;33:903. (In Chinese) [Google Scholar]
- 3.Gong Y, Wu J, Li ST. Immuno-enhancement effects of Lycium ruthenicum Murr. Polysaccharide on cyclophamide-induced immunosuppression in mice. Int J Clin Exp Med. 2015;8:20631–20637. [PMC free article] [PubMed] [Google Scholar]
- 4.Sun H, Zhang J, Chen F, Chen X, Zhou Z, Wang H. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr Polym. 2015;121:388–402. doi: 10.1016/j.carbpol.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Brynjolfsson SF, Henneken M, Bjarnarson SP, Mori E, Del Giudice G, Jonsdottir I. Hyporesponsiveness following booster immunization with bacterial polysaccharides is caused by apoptosis of memory B cells. J Infect Dis. 2012;205:422–430. doi: 10.1093/infdis/jir750. [DOI] [PubMed] [Google Scholar]
- 6.Yao L, Wang Z, Zhao H, Cheng C, Fu X, Liu J, Yang X. Protective effects of polysaccharides from soybean meal against X-ray radiation induced damage in mouse spllen lymphocytes. Int J Mol Sci. 2011;12:8096–8104. doi: 10.3390/ijms12118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Y, Tachibana I, Takeda Y, He P, Kang S, Suzuki M, Kuhara H, Tetsumoto S, Tsujino K, Minami T, et al. Statins decrease lung inflammation in mice by upregulating tetraspanin CD9 in macrophages. PLoS One. 2013;8:e73706. doi: 10.1371/journal.pone.0073706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu M, Luo X, Xu X, Wei W, Yu M, Jiang N, Ye L, Yang Z, Fei X. Antioxidant and immunomodulatory activities of a polysaccharide from Flammulina velutipes. J Tradit Chin Med. 2014;34:733–740. doi: 10.1016/S0254-6272(15)30089-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Li GR, Zheng J. Screening of polysaccharides with antioxidant activities from Saccharum Alhagi. J Xinjiang Med Univ. 2015;38:1479–1481. (In Chinese) [Google Scholar]
- 10.Han Z, He J, Chang J. The influence of polysaccharides from Saccharum Alhagi on the immune activity of macrophage cell line RAW264.7. J Xinjiang Med Univ. 2017;40:361–365. (In Chinese) [Google Scholar]
- 11.Jian L, Li G, Chang J. Determination of monosaccharide composition in polysaccharide of alhagi-honey by pre-column derivatization-high performance capillary electrophoresis. Chin J N Drugs. 2012;21:78–81. [Google Scholar]
- 12.Su KY, Yu CY, Chen YP, Hua KF, Chen YL. 3,4-dihydroxytoluene, a metabolite of rutin, inhibits inflammatory responses in lipopolysaccharide-activated macrophages by reducing the activation of NF-κB signaling. BMC Complement Altern Med. 2014;14:21. doi: 10.1186/1472-6882-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KS, Cui X, Lee DS, Sohn JH, Yim JH, Kim YC, Oh H. Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp.SF-5989 through the Suppression of NF-кB and p38 MAPK pathways in lipopolysaccharide stimulated RAW264.7 macrophages. Molecules. 2013;18:13245–13259. doi: 10.3390/molecules181113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etoh T, Kim YP, Ohsaki A, Komiyama K, Hayashi M. Inhibitory effect of erythraline on Toll-like receptor signaling pathway in RAW264.7 cells. Biol Pharm Bull. 2013;36:1363–1369. doi: 10.1248/bpb.b12-00910. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Liu F, Fang L, Cai R, Zong C, Qi Y. Genkwanin inhibits proinflammatory mediators mainly through the regulation of miR-101/MKP-1/MAPK pathway in LPS-activated macrophages. PLoS One. 2014;9:e96741. doi: 10.1371/journal.pone.0096741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q, Qian Y, Li R, Tan B, Han H, Liu M, Qian M, Du B. Norcantharidin facilitates LPS-mediated immune responses by up-regulation of AKT/NF-kB signaling in macrophages. PLoS One. 2012;7:e44956. doi: 10.1371/journal.pone.0044956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JL, Zhong WJ, Tang GH, Li J, Zhao ZM, Yang DP, Jiang L. Norditerpenoids from Flickingeria fimbriata and their inhibitory activities on nitric oxide and tumor necrosis factor-α production in mouse macrophages. Molecules. 2014;19:5863–5875. doi: 10.3390/molecules19055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Mahony C, Scully P, O'Mahony D, Murphy S, O'Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F, O'Mahony L. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishitani Y, Zhang L, Yoshida M, Azuma T, Kanazawa K, Hashimoto T, Mizuno M. Intestinal anti-inflammatory activity of lentinan: Influence on IL-8 and TNFR1 expression in intestinal epithelial cells. PLoS One. 2013;8:e62441. doi: 10.1371/journal.pone.0062441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poormasjedi-Meibod MS, Jalili RB, Hosseini-Tabatabaei A, Hartwell R, Ghahary A. Immuno-regulatory function of indoleamine 2,3 dioxygenase through modulation of innate immune responses. PLoS One. 2013;8:e71044. doi: 10.1371/journal.pone.0071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohnishi T, Bandow K, Kakimoto K, Kusuyama J, Matsuguchi T. Long-Time treatment by low-dose N-Acetyl-L-Cysteine enhances proinflammatory cytokine expressions in LPS-stimulated macrophages. PLoS One. 2014;9:e87229. doi: 10.1371/journal.pone.0087229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Liang Z, Zeng Y, Huang X, Yang Z. The effect of Apigenin on proliferation and No secretion and phagocytosis of RAW264.7 cells. J Jinan Univ (Natural Sci) 2008;29:95–98. (In Chinese) [Google Scholar]
- 24.Xie Y, Chen Q, Luo D, Zhong Z. Anti-fatigue and immunoregulatory functions of high-purity rubusoside. Lishizhen Med Materia Med Res. 2010;21:1421–1422. (In Chinese) [Google Scholar]
- 25.Huang F, Guo Y, Zhang R, Yi Y, Deng Y, Su D, Zhang M. Effects of drying methods on physicochemical and immunomodulatory properties of polysaccharide-protein complexes from litchi pulp. Molecules. 2014;19:12760–12776. doi: 10.3390/molecules190812760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo J, Zhu R, Yi L Dong Y, Wang PX. Effect of sinomenine on mouse RAW264.7 macrophage cells line polarization induced by LPS or IL-4. Chin J Immunol. 2015;31:56–60. [Google Scholar]
- 27.Byeon SE, Lee J, Kim JH, Yang WS, Kwak YS, Kim SY, Choung ES, Rhee MH, Cho JY. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012;2012:1–7. doi: 10.1155/2012/512926. [DOI] [PMC free article] [PubMed] [Google Scholar]