Abstract
Background:
A period of starvation after colorectal anastomosis to permit for resolution of the clinical evidence of ileus has been an unchallenged surgical dogma until recent years. We intended to determine the safety and feasibility of an unconventional postoperative oral intake protocol in patients experiencing colorectal anastomosis.
Materials and Methods:
Between 2013 and 2015, sixty consecutive patients underwent colorectal anastomosis and they were randomized into two groups. The early feeding group began fluids on the first postoperative day while the regular feeding group was managed in the traditional way - nothing by mouth until the complete resolution of ileus.
Results:
The majority of patients (93%) tolerated the early feeding. The times to first passage of flatus (2.66 ± 0.71 days vs. 3.9 ± 0.071 days) and stool (3.9 ± 0.92 days vs. 5.4 ± 0.77 days) were significantly quicker in early feeding group. Hospital stay was also significantly shorter in the early feeding group (4 ± 0.64 days vs. 6.1 ± 0.84 days). Anastomosis leakage and abscess formation were not seen in early feeding group. The patient's satisfaction (visual analog scale) in the early feeding group was higher than delayed feeding group (8.56 ± 1.16 vs. 7.06 ± 1.59, P < 0.001).
Conclusions:
Early oral feeding after colorectal surgeries is safe and tolerated by the majority of patients.
Keywords: Colon, enteral nutrition, postoperative period, rectum, surgical anastomosis
Introduction
Proper and adequate nutrition has so far been one of the major concerns in postoperative care. Although there are still two fear-related common perceptions about ileus including the routine adoption of nasogastric tube and prevention of oral feeding,[1] more recent research emphasizes that routine adoption of nasogastric tube is unnecessary.[2,3,4,5,6,7] On the other hand, feeding through the mouth in colonic anastomosis has for many years been subject to prevention of flatus and establishing bowel movements, by which ileus is resolved and low-volume feeding is initiated. The diet gradually expands from filtrate liquids to normal diet. This procedure continues to be practiced in many medical centers. Normally, feeding is delayed 4–5 days.[8,9] The first comparison concerning the early onset of feeding with elemental diet in digestive tract surgery dates back to 1979. In their study, Sagar et al. carefully initiated the elemental diet on the first day after surgery. The patients had a shorter stay in hospital and showed better metabolic status.[10] The development and widespread adoption of laparoscopic surgery strengthened the idea of feeding patients early. In this way, the early onset of feeding in patients undergoing laparoscopic colectomy led to more appropriate metabolic status and adequate tolerance. Gradually, the idea made it to open surgery for colon including a variety of anastomoses. Numerous papers have been published on the subject, many of these which dealt with reduction of the length of hospital stay among patients and improvement of metabolic status.[11,12,13,14,15,16] The rest of relevant literature focused on additional benefits of early feeding such as lower septic complications after surgery and lower morbidity rate.[17,18,19,20,21,22,23,24,25] In their review paper, Ng and Neill explored and collected the studies on early feeding in patients who underwent elective open colorectal surgeries. In fact, a total of 15 studies including 1352 patients were reviewed, finding that early feeding was recommended in all cases. The overall incidence of side effects was 12.5% (ranging between 0% and 25%), where early feeding increases the risk of anastomotic leaks, pneumonia caused by aspiration and obstruction. The tolerability of early feeding is generally 86% (ranging between 73% and 100%). In a study it has been concluded that early oral feeding reduced the postoperative ileus and the duration of hospital ization This study intended to challenge the old idea of postponing the onset of diet.[26]
Despite extensive research carried out in this area, it is still discussed what standard method to employ in most medical centers. Moreover, oral feeding for colorectal anastomosis is subject to prevention of flatus and establishment of bowel movements in many centers, by which ileus is resolved and low-volume feeding is initiated. The diet gradually expands from filtrate liquids to normal diet. This procedure continues to be practiced in many medical centers. Normally, feeding is delayed 4–5 days.[8,9] More extensive research is needed in this area, particularly given the lack of randomized clinical trials in Iran and the high prevalence of colon surgery.
Given the patient comfort in early feeding after colorectal anastomosis and cost-saving for patients and hospital costs in case the prospective research proves the benefits and effectiveness of this method, there will be practical implementation in surgical wards, huge cost-saving, and improvement in patient satisfaction.
Materials and Methods
This randomized clinical trial involved patients admitted during 2013–2015 to Imam Hossein (AS) General Surgery Hospital (Shahid Beheshti University of Medical Sciences, Tehran) undergoing anastomosis surgery in the colon or rectum in a nonrandomized procedure. The patients were selected from all the participants who underwent anastomosis of colon or rectum regardless of whether it was an elective or emergency surgery. In this respects, the selection could be considered nonrandom. The sample size was calculated to be 28 patients taking into account α =5% and power of 95% in each group. For ease of analysis, thirty patients in each group were considered. The patients were randomly divided into two groups based on the random number table. The first group included patients with early feeding after surgery, where the diet initiated by filtrate liquids within 24 h after surgery. Over the next 24 h, the liquid diet was replaced by a normal diet in case tolerance was desirable. The diet continued in this group if there was no vomiting. In the second group, patients received the routine diet (late feeding) including filtrate liquids was only after the resolution of ileus, while the patients remained not per oral (NPO) until the resolution of ileus.
All patients in the current study were fully aware of the procedure and submitted their informed written consent after receiving sufficient information. Lack of patient consent to participate at any stage led to exclusion from the study. This study was not faced with serious ethical challenges since the early feeding after colon surgery had already been practiced in many previous studies.
All patients were under general anesthesia and could stand up independently at earliest possible time. In this respect, there was no difference between the two groups of patients.
The inclusion criteria in the current study were any history of surgery involving anastomosis in the colon or rectum, and age limit was not applied on the participants. All patients submitted their informed written consent to participate in the study.
The exclusion criteria were diabetic patients with fasting blood sugar >200 mg/dl, immunosuppressive patients taking corticosteroid, patients with untrustworthy psychiatric problems, hypothyroid patients, patients who had experienced anastomosis apart from those of the colon or rectum, patients who had undergone total colectomy, patients with a history of radiotherapy, colostomy, or protective ileostomy. In the current study, there was no limit imposed on elective or emergency surgery.
In all patients, nasogastric tube was removed immediately after surgery. The nasogastric tube was reembedded dependent on two episodes of vomiting >100 ml within 24 h in the absence of bowel movements.
In the group of patients with routine feeding, resolution of ileus was realized in the form of bowel movements in the absence of abdominal distention or vomiting. This was the main condition for starting the diet in this group of patients.
The criteria for discharging patients from the hospital were quite similar in the two groups, including tolerance of normal diet for at least 24 h.
The patients in both groups were compared during their stay in terms of clinical symptoms after surgery (such as nausea, vomiting and distention), onset of bowel sounds (BSs), resolution of ileus, febrile, need for NPO or reembedding nasogastric tube, complication of ulcer (such as infection or dehiscence), gas passing, and defecation, intra-abdominal abscess and anastomotic leaks, need for further surgery, overall satisfaction based on visual analog scale (VAS) criteria, total duration of hospital stay, and systemic effects (i.e., pneumonia, sepsis, myocardial infarction [MI], and mortality). The elective patients received residue-free diet 48 h before surgery. The day before surgery, laxative and oral antibiotics (1 g of erythromycin and 1 g of metronidazole at hour 13, 14, and 23) were given to patients.
The patients went fasting in case there were symptoms of intolerance including vomiting and abdominal pain and distention after starting the diet.
The data were recorded in a specific form by a person blind to the details of patients. The clinical and laboratory characteristics of the two groups were described through statistical measures such as central tendency and frequency distribution.
Qualitative variables were compared through Chi-square test or Fisher's exact test. The quantitative variables in the two groups involved t-test and Mann–Whitney test. In all calculations, P < 0.05 was considered the significance level. Data analysis involved SPSS Version 20.(SPSS, Inc., Chicago, IL, USA, version 20).
Results
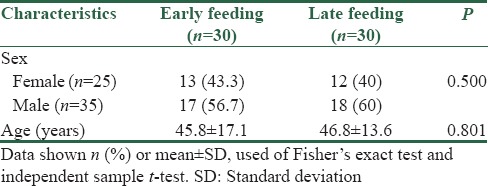
Of thirty patients in this study, there were 13 males (43.3%) and 17 females (56.7%) for early feeding with an average age of 45.8 ± 17.1 years. Moreover, there were 12 males (40%) and 18 females (60%) for late feeding with an average age of 46.8 ± 13.6 years. Statistically, the age and gender were not different between the two groups (P > 0.05). In other words, the two groups were matched in terms of age and gender [Table 1].
Table 1.
Frequency distribution and descriptive statistics by gender and age of the patients under study
Evaluation of postoperative complications in terms of both groups receiving early and late feeding showed that the average prolonged days until the incidence of complications such as intestinal sounds, ileus, liquid diet, regular diet, discharge, gas passing, and defecation was significantly greater in the late feeding group compared to the early feeding group. In contrast, the pain scores (VAS) of patients in the late feeding group with an average of 7.1 ± 1.6 were significantly lower than those in the early feeding group with an average of 8.6 ± 1.2 (P < 0.0001). However, the other complications were not significantly different between the two groups (P > 0.05) [Table 2].
Table 2.
Comparison of the two groups in terms of postoperative complications
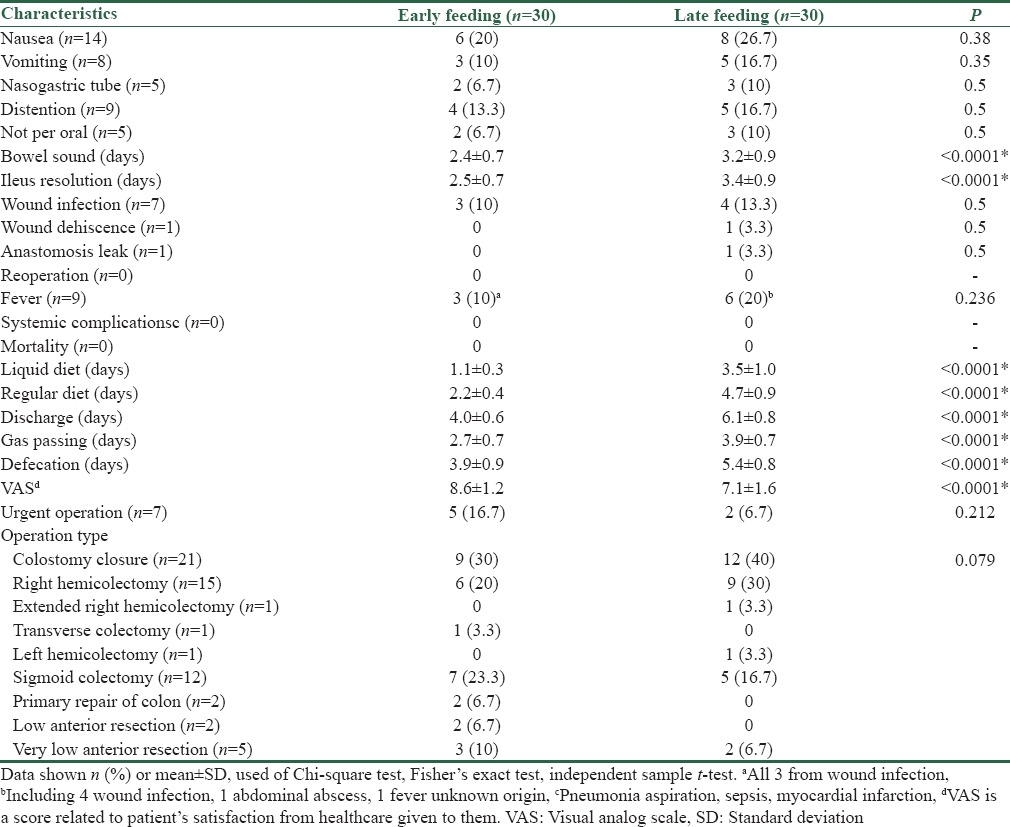
We achieved earlier start of BSs, ileus resolution, gas passing, and defecation, and lower period of regular diet intake and hospital stay, and higher satisfaction state (VAS) in patients; all the above findings reached significance level.
On the other hand, there is no increase in the outcomes of early oral feeding group such as nausea, vomiting, distension, and systemic complications.
We have found some other significant relations that are mentioned below separately.
Distention and vomiting related with P = 0.001 from Chi-square test.
Wound infection is significantly related to urgency in operation (P = 0.029 from Chi-square test)
Correlation between VAS and discharge is significant at the 0.01 level (2-tailed).
Age, gender, type of operation, and urgency had no impact on the tolerability of early feeding.
Discussion
Usually, the bowel movements and gas passing are two clinical criteria for initiation of oral feeding after abdominal surgery. The postoperative ileus is resolved through gas passing during the first five days. The induction of NPO after intestinal anastomosis is a common method, which can prevent nausea and vomiting after surgery and help the recovery of anastomosis. Several studies have been conducted on the onset of early feeding in a variety of abdominal surgeries. Most of such studies obtained findings proving the usefulness of this method.
The current study involved 60 patients in the form of two early and late feeding groups. The number of emergency surgeries was 5 cases in patients with early feeding (16.66%) and 2 cases in patients with late feeding (6.66%), which indicated no statistically significant difference (P = 0.079). It should be noted that many previous studies merely focused on elective cases.[16,21,24,27,28,29,30,31] In a study by Lee et al.,[22] the gastrointestinal system was examined through early onset of diet on emergency anastomosis (no emphasis on colon). In the current study, there were no limits in terms of being elective or emergency surgery.
In their study, Reissman et al. found that 79% of patients tolerated early diet. In that study, the frequency of vomiting was 21% in early diet patients and 14% in later diet patients.[16] In a similar study, Ng and Neill reported oral diet tolerance in 86% of patients (ranging from 73 to 100%).[27] In their study, Ortiz et al. found that the frequency of vomiting was 21.5% in the early feeding group more than patients with later feeding.[21] In a study by Tavasolli et al., there were three cases of vomiting (4.7%) in both groups of early and late feeding, where there was no significant difference between the two groups of patients in terms of frequency of vomiting.[32] In another study, Stewart et al. found no statistically significant difference in the frequency of vomiting after starting the diet between the two groups of patients.[28] In their study, Seenu and Goel observed tolerance in patients with early feeding diet by 79%, while it was 86% in patients with late feeding. The relative frequency of vomiting in patients with late feeding was 14%. In none of these cases, there was a significant difference.[30]
In the current study, need for NPO and reembedding nasogastric tube (according to the protocol mentioned in the previous section) was observed in two patients (6.66%) in the early feeding group and three patients (10%) in the late feeding group, suggesting no statistically significant difference between the two groups of patients.
The data in the current study concerning need for NPO and reembedding nasogastric tube were consistent with previous studies (16,29,31 and 34) whereas, they were inconsistent with findings of Ortiz et al.[21] about higher need for NPO and reembedding nasogastric tube in the group of patients with early feeding.
The average first time BSs auscultation was 2.36 days in patients with early feeding and 3.20 days in patients with late feeding, indicating a significant difference between the two groups of patients.[26]
In the current study, resolution of ileus involved bowel movements or vomiting in the absence of distention. This parameter occurred on average 2.5 days after surgery in early feeding group and 3.4 days in the late feeding group.
According to the findings of Charoenkwan, the first hearing of BS in early feeding occurs on average half a day sooner in the patients.[32] Based on Ng and Neill's results, starting the early diet improved ileus faster.[27]
In their study, Ortiz et al. found that first bowel movements occurred averagely 4.3 days after surgery in patients with early feeding and 4.7 days after surgery in patients with late feeding.[21] The average time for BSs auscultation based on the findings of this study was longer in the two groups of patients compared to the current study. Based on the results of Sekhavat et al., early feeding in patients decreased the first BSs auscultation.[33] The average of 2.85 days versus 3.05 days in the early feeding group and late feeding group in the study by Seenu and Goel indicated no significant differences between the average onset of bowel movements between the two groups of patients with early and late feeding.[30] It seems that resolution time of ileus and onset of BSs auscultation occurred shorter in the current study compared to the previous studies.
In this study, the incidence of wound infection was in a statistically significant correlation with elective or emergency surgery. In fact, emergency surgery significantly increased the risk of wound infection.
In their study, Lee et al. reported wound complications in 18 patients (33%), which was significantly higher than wound complications in the current study (13%). This study included all patients under early diet, and therefore, there was no comparison between the two groups. In another study, Charoenkwan et al. examined the role of early oral feeding in gynecologic surgeries, finding that patients were less likely to develop wound complications with early feeding practices.[32]
In the current study, only 1 patient (3.3%) experienced anastomotic leaks and abscesses in the vicinity of the anastomotic zone, which was in the late feeding group. According to clinical evidences, this complication did not lead to reoperation, while embedding may happen under ultrasound-guided procedure.
Fever was another parameter studied in the two groups of patients. There were 3 cases of fever among patients with early feeding, all of which suffered wound infection. There were positive cases of fever in the patients with late feeding, one patient had intra-abdominal abscess and one patient had fever of unknown origin that was eventually resolved. In the current study, there was no case of systemic complications (aspiration pneumonia, sepsis, and MI). According to data from the current study, early feeding did not increase aspiration pneumonia.
In the study by Lee et al., one patient (1.8%) experienced aspiration pneumonia and subsequently sepsis by early feeding.[22] Based on the study by Charoenkwan et al., early feeding did not result in a higher incidence of fever and pneumonia after surgery.[32] Based on the conclusions made by Ng and Neill, early feeding in elective surgeries did not increase the risk of fever and aspiration pneumonia.[27]
According to the study by Seenu and Goel, early onset of diet in colorectal elective surgeries did not lead to increased risk of aspiration pneumonia and other systemic complications.[31] The findings of these studies were consistent with the findings of the current study.
In a study on 161 patients who were undergoing elective colorectal surgery, Reissman et al. found that early diet tolerance was an average of 2.6 ± 0.1 days in the early feeding group, while it was 5 ± 0.1 days in the late feeding group, suggesting a statistically significant difference.[16] The findings were quite consistent with the results of the current study.
In a study on ordinary diet tolerance among patients undergoing elective surgery of the colon and rectum, Ortiz et al. found that tolerance was about 80% over the first 4 days in the early diet group, which was significantly higher than that in the late diet group. Then, this difference disappeared after 4 days.[21] In their study, Stewart et al. examined eighty patients under open resection in colorectal and anastomosis in two groups. The tolerance was 80% in early oral feeding group during 48 h.[34] The findings were consistent with the current research.
In their study on eighty patients with early feeding and 81 patients with late feeding under elective colorectal surgery, Seenu and Goel obtained results quite similar with the findings of the current investigation. In fact, tolerance of normal diet was 2.6 ± 0.1 days in the early feeding group while it was 5 ± 0.1 days in the late feeding group, indicating a significant difference.[31] It should be noted that these figures are also consistent with the results of Reissman et al.[16]
The overall duration of hospital stay had a remarkable effect on patient satisfaction with the treatment procedure and costs. The criteria of discharge from hospital in the two groups of patients were identical in the current study, including tolerance of normal diet for at least 24 h in patients with early feeding. The average duration of hospital stay after surgery was 4 days with a standard deviation of 0.64, whereas average duration of hospital stay after surgery in late feeding patients was 6.1 days with a standard deviation of 0.84, which indicated a significant difference between the two groups.
This time was dramatically shorter in the early feeding group. On the other hand, there was a significant relationship between the duration of hospital stay and overall satisfaction with the treatment process.
According to Ng and Neill that reviewed and summarized 15 studies, the early onset of oral feeding decreased hospitalization in patients who underwent elective colorectal surgery. Such decrease was dramatically and statistically significant.[27]
In the study by Tavasolli et al., the duration of hospitalization was 6.3 days in patients with early feeding and 9.8 days in the control group which was statistically significant.[32] That paper made no comment on whether the figures were related to the total period of stay or merely related to the period after surgery. Although the average length of stay in the two groups of patients in this study was significantly higher than that in the current study, there was consistency concerning the significant difference between the two groups regarding the average hospital.
In the current study, the patients with early feeding expressed higher satisfaction with treatment process on the VAS compared to the patients with late feeding. This scale was rarely explored by previous studies. Another advantage of this study was no restriction on elective or emergency surgery that was not applicable in previous studies.
Conclusions
Based on the results of the current research, the early onset of diet in patients undergoing surgery involving anastomosis in the colon or rectum can lead to clinical efficacy such as shorter BSs auscultation after surgery and shorter time for resolution of ileus after surgery. Reduction of the first flatus and feces, shorter overall stay, and greater patient satisfaction with the treatment process were all significant.
Furthermore, the early onset of diet based on the results of this study did not lead to an increase in the incidence of gastrointestinal complications such as nausea, vomiting, and systemic complications such as pneumonia destination and aspiration fever, sepsis, MI. The wound complications and mortality in the two groups of patients indicated no statistically significant difference. In the current study, the patients with early feeding expressed greater overall satisfaction with treatment process based on VAS criteria compared to patients with late feeding.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Levine M. A new gastroduodenal catheter. JAMA. 1981;76:1007. [Google Scholar]
- 2.Bauer JJ, Gelernt IM, Salky BA, Kreel I. Is routine postoperative nasogastric decompression really necessary? Ann Surg. 1985;201:233–6. doi: 10.1097/00000658-198502000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argov S, Goldstein I, Barzilai A. Is routine use of the nasogastric tube justified in upper abdominal surgery? Am J Surg. 1980;139:849–50. doi: 10.1016/0002-9610(80)90395-5. [DOI] [PubMed] [Google Scholar]
- 4.Nathan BN, Pain JA. Nasogastric suction after elective abdominal surgery: A randomised study. Ann R Coll Surg Engl. 1991;73:291–4. [PMC free article] [PubMed] [Google Scholar]
- 5.Meltvedt R, Jr, Knecht B, Gibbons G, Stahler C, Stojowski A, Johansen K. Is nasogastric suction necessary after elective colon resection? Am J Surg. 1985;149:620–2. doi: 10.1016/s0002-9610(85)80140-9. [DOI] [PubMed] [Google Scholar]
- 6.Wolff BG, Pemberton JH, Van Heerden JA. Elective colon and rectal surgery without nasogastric decompression. Ann Surg. 1987;154:640–2. doi: 10.1097/00000658-198906000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrelli NJ, Stulc JP, Rodriquez-Bigas M. Nasogastric decompression following elective colorectal surgery. Am Surg. 1993;59:632–5. [PubMed] [Google Scholar]
- 8.de Aguilar-Nascimento JE, Göelzer J. Early feeding after intestinal anastomoses: Risks or benefits? Rev Assoc Med Bras. 2002;48:348–52. [PubMed] [Google Scholar]
- 9.Petrini JL. Diet and drugs in colorectal surgery. In: Corman ML, editor. Colon and Rectal Surgery. 5th ed. New York: Lippincott Williams & Wilkins; 2005. p. 50. [Google Scholar]
- 10.Sagar S, Harland P, Shields R. Early postoperative feeding with elemental diet. Br Med J. 1979;1:293–5. doi: 10.1136/bmj.1.6159.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binderow SR, Cohen SM, Wexner SD, Nogueras JJ. Must early postoperative oral intake be limited to laparoscopy? Dis Colon Rectum. 1994;37:584–9. doi: 10.1007/BF02050994. [DOI] [PubMed] [Google Scholar]
- 12.Bufo AJ, Feldman S, Daniels GA, Lieberman RC. Early postoperative feeding. Dis Colon Rectum. 1994;37:1260–5. doi: 10.1007/BF02257793. [DOI] [PubMed] [Google Scholar]
- 13.Choi J, O'Connell TX. Safe and effective early postoperative feeding and hospital discharge after open colon resection. Am Surg. 1996;62:853–6. [PubMed] [Google Scholar]
- 14.Hartsell PA, Frazee RC, Harrison JB, Smith RW. Early postoperative feeding after elective colorectal surgery. Arch Surg. 1997;132:518–20. doi: 10.1001/archsurg.1997.01430290064011. [DOI] [PubMed] [Google Scholar]
- 15.Jeffery KM, Harkins B, Cresci GA, Martindale RG. The clear liquid diet is no longer a necessity in the routine postoperative management of surgical patients. Am Surg. 1996;62:167–70. [PubMed] [Google Scholar]
- 16.Reissman P, Teoh TA, Cohen SM, Weiss EG, Nogueras JJ, Wexner SD. Is early oral feeding safe after elective colorectal surgery? A prospective randomized trial. Ann Surg. 1995;222:73–7. doi: 10.1097/00000658-199507000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoover HC, Jr, Ryan JA, Anderson EJ, Fischer JE. Nutritional benefits of immediate postoperative jejunal feeding of an elemental diet. Am J Surg. 1980;139:153–9. doi: 10.1016/0002-9610(80)90245-7. [DOI] [PubMed] [Google Scholar]
- 18.Ryan JA, Jr, Page CP, Babcock L. Early postoperative jejunal feeding of elemental diet in gastrointestinal surgery. Am Surg. 1981;47:393–403. [PubMed] [Google Scholar]
- 19.Meguid MM, Campos AC, Hammond WG. Nutritional support in surgical practice: Part II. Am J Surg. 1990;159:427–43. doi: 10.1016/s0002-9610(05)81290-5. [DOI] [PubMed] [Google Scholar]
- 20.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications.The results of a meta-analysis. Ann Surg. 1992;216:172–83. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz H, Armendariz P, Yarnoz C. Is early postoperative feeding feasible in elective colon and rectal surgery? Int J Colorectal Dis. 1996;11:119–21. doi: 10.1007/s003840050032. [DOI] [PubMed] [Google Scholar]
- 22.Lee HS, Shim HJ, Lee HS, Lee JG, Kim KS. The safety of early enteral feeding after emergency gastrointestinal surgery. Korean J Gastroenterol. 2011;58:318–22. doi: 10.4166/kjg.2011.58.6.318. [DOI] [PubMed] [Google Scholar]
- 23.Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications.Cochrane Database Syst Rev. 2006;4 doi: 10.1002/14651858.CD004080.pub2. CD004080. [DOI] [PubMed] [Google Scholar]
- 24.Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: A systematic review and meta-analysis. J Gastrointest Surg. 2009;13:569–75. doi: 10.1007/s11605-008-0592-x. [DOI] [PubMed] [Google Scholar]
- 25.Lobato Dias Consoli M, Maciel Fonseca L, Gomes da Silva R, Toulson Davisson Correia MI. Early postoperative oral feeding impacts positively in patients undergoing colonic resection: Results of a pilot study. Nutr Hosp. 2010;25:806–9. [PubMed] [Google Scholar]
- 26.Ng WQ, Neill J. Evidence for early oral feeding of patients after elective open colorectal surgery: A literature review. J Clin Nurs. 2006;15:696–709. doi: 10.1111/j.1365-2702.2006.01389.x. [DOI] [PubMed] [Google Scholar]
- 27.Aihara H, Kawamura YJ, Konishi F. Reduced medical costs achieved after elective oncological colorectal surgery by early feeding and fewer scheduled examinations. J Gastroenterol. 2003;38:747–50. doi: 10.1007/s00535-002-1140-1. [DOI] [PubMed] [Google Scholar]
- 28.Stewart BT, Woods RJ, Collopy BT, Fink RJ, Mackay JR, Keck JO. Early feeding after elective open colorectal resections: A prospective randomized trial. Aust N Z J Surg. 1998;68:125–8. doi: 10.1111/j.1445-2197.1998.tb04721.x. [DOI] [PubMed] [Google Scholar]
- 29.Seenu V, Goel AK. Early oral feeding after elective colorectal surgery: Is it safe. Trop Gastroenterol. 1995;16:72–3. [PubMed] [Google Scholar]
- 30.El Nakeeb A, Fikry A, El Metwally T, Fouda E, Youssef M, Ghazy H, et al. Early oral feeding in patients undergoing elective colonic anastomosis. Int J Surg. 2009;7:206–9. doi: 10.1016/j.ijsu.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Charoenkwan K, Phillipson G, Vutyavanich T. Early versus delayed (traditional) oral fluids and food for reducing complications after major abdominal gynaecologic surgery. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD004508.pub3. CD004508. [DOI] [PubMed] [Google Scholar]
- 32.Tavasolli A, Abdollahi A, Darkhord A. Early versus delayed post operative oral feeding in patients undergoing colonic anastomosis. Med J Mashhad Univ Med Sci. 2010;53:104–9. [Google Scholar]
- 33.Sekhavat L, Karimi Zarchi M, Tabatabaii A. Early oral feeding effect on gastrointestinal symptoms and patients satisfaction after cesarean delivery under general anaesthesia. J Babol Univ Med Sci. 2009;10:67–72. [Google Scholar]
- 34.Kawamura YJ, Uchida H, Watanabe T, Nagawa H. Early feeding after oncological colorectal surgery in Japanese patients. J Gastroenterol. 2000;35:524–7. doi: 10.1007/s005350070075. [DOI] [PubMed] [Google Scholar]


