Abstract
Recent incidence rates for Hashimoto's thyroiditis (HT) and hypothyroidism are higher than those of previous studies. Previous studies indicated that T helper cells may have a major role in the pathogenesis and development of HT, but there is no consensus in the literature. The aim of the present study was to explore the peripheral T helper cell response in the different stages of HT. In this cross-sectional study, we performed flow cytometry analysis to determine the various T cell subsets of 389 patients with HT (34 patients with HT who developed overt hypothyroidism, and 148 patients with HT who developed subclinical hypothyroidism), as well as 51 healthy controls. Anti-thyroid antibodies, and thyroid function were measured. The findings demonstrated that the proportion of peripheral Th1 cells was significantly lower in patients with HT than in healthy euthyroid controls (P<0.001), and the proportion of peripheral Th2, Treg cells was significantly higher in patients with HT than in healthy euthyroid controls (P<0.001). Therefore the Th1/Th2 ratio was significantly lower in HT patients than in healthy euthyroid controls (P<0.001). The Th17/Treg ratio in HT patients was significantly lower than that control subjects (P<0.001). Th1 proportions in patients with overt hypothyroidism HT were significantly higher than in subclinical hypothyroidism HT patients (P=0.031). In conclusion, the findings of the present study demonstrated that there is an increased immune deviation of Th1 lymphocytes and compensatory accelerating activity of Treg cells in HT, and the peripheral Th1 cells from the HT patients correlated to the developmental stage of hypothyroidism.
Keywords: T helper cell, Hashimoto's thyroiditis, hypothyroidism
Introduction
Autoimmune thyroid disease (AITD), which includes Graves' disease and Hashimoto's thyroiditis (HT), is characterized by a variable degree of lymphocytic infiltration of the thyroid gland and reactivity to self-thyroid antigens, due to a combination of genetic and environmental factors (1,2). Cluster of differentiation (CD)4+ T helper lymphocytes have a key role in the pathogenesis of autoimmune diseases (3). CD4+ helper T cells are subdivided into functionally distinct subsets, including T helper (Th)1, Th2, Th17 and Treg cells, which predominantly produce interferon (IFN)-γ as it has a critical role in the regulation of the cellular-mediated immune response, and interleukin (IL)-4, IL-5 and IL-10, which are involved in the modulation of antibody production and antigen-specific immunosuppression (4–6). A relatively newly discovered subset of T helper cells, Th17, that primarily produce IL-17 may be involved not only in the defense against certain pathogens but also in driving inflammation and autoimmunity (7). Another subset of CD4+ T cells, Th3 lymphocytes, predominantly synthesize tumor growth factor-β and are considered as regulatory cells (8). In animal models of autoimmune diseases, Th1 cells mediate tissue damage and disease progression; whereas Th2 cells are associated with disease remission and suppression of the immune response (9). While Th17 cells promote autoimmunity, Treg cells can control autoimmunity and have a critical role in autoimmune pathogenesis by maintaining self-tolerance and controlling the activation of autoreactive CD4+ T effector cells (10).
Hashimoto's disease is likely to exist at a higher frequency than is diagnosed clinically, and its frequency appears to be increasing (11). Positive thyroglobulin antibodies (TgAb) and thyroid peroxidase antibodies (TPOAb) are present in patients with HT, positive TPOAb are identified in 12–26% of the general population and TgAb in ~14% (12). High titers of thyroglobulin antibodies (TgAb) and thyroid peroxidase antibodies (TPOAb) are present in many patients with HT. Most patients with HT maintain a euthyroid state throughout their lifetime without any medical treatment, whereas others become hypothyroid. Approximately 20% of patients exhibit signs and symptoms of mild hypothyroidism when initially seen (13). Progression from subclinical hypothyroidism to overt hypothyroidism typically develops over a period of several years and occurs at a rate of ~5% a year; the presence of either subclinical hypothyroidism or raised thyroid antibodies indicates an increased risk of overt hypothyroidism and the risk is greater when these factors occur together (14). In Hashimoto's disease, both Th1 and Th2 responses are found (15), although with different reciprocal intensity in relationship with the clinical expression of the disease process (16–18). However, recent studies have demonstrated the existence of new subpopulations of T helper cells that are also important in immunoregulation and host defense, like Treg cells and Th17 cells (19–21). In recent years, discoveries pertaining to this newly identified T helper subset in humans and mice have accumulated with tremendous speed (22–24). There is a negative reciprocal regulatory relationship between Treg and Th17 lymphocytes; patients with AITD exhibit an expansion of Th17 cells and these cells are involved in the pathogenesis of AITD (25). Enhanced levels of Th17 cells and Th17 cytokines are found in patients with AITD, predominantly those with HT (26). Another previous study suggested that the proportion of activated cytotoxic T cells and the titer of TgAb are independently involved in the disease severity of HT (16). However, to our knowledge, the frequency of T helper cells has not been investigated in a large, community-based sample with different stages of HT, and the possible impact on the health of the patient remains unclear.
In this respect, the present study aimed to characterize the alteration of T helper cells, particularly Th17 and Treg cells, in patients with different stages of HT and healthy euthyroid controls in a large sample study. The aim being to further to clarify the role of the imbalance between T helper cells with the pathology or disease outcomes in HT and to analyze the association between the imbalance with thyroid-specific autoantibodies.
Subjects and methods
Subjects
This study was approved by the Ethics Committee of People's Hospital of Xinjiang Uygur Autonomous Region and informed consent was obtained from all participants prior to blood collection. A total of 596 individuals, aged 18–80 years, were recruited from two communities in Urumqi (Xinjiang, China) between May and June 2013. We excluded participants who had missing lab results (n=139) and those with hyperthyroidism (n=17). Finally, 389 patients with HT, who positive for anti-thyroid antibody including TPOAb and/or TgAb (318 females and 71 males, aged 20–80 years), and 51 healthy euthyroid controls without thyroid antibodies (31 females and 20 males, aged 19–79 years) were enrolled. The overt hypothyroidism HT group (34 patients) contained participants with thyrotropin >4.2 mIU/l (normal, 0.27–4.2 mIU/l), and free thyroxine (FT4) <11.5 pmol/l (normal, 11.5–22.7 pmol/l). The subclinical hypothyroidism HT group (148 patients) contained participants with thyrotropin >4.2 mIU/l, and FT4 within the normal range. The euthyroid HT group (207 patients) contained participants that had HT, as determined via TPOAb and/or TgAb positivity, but were euthyroid.
Thyroid function and autoantibodies
Venous blood samples were drawn after an overnight fast of at least 8 h and were centrifuged for 5 min at 4°C at 2,136 × g after collection. Blood samples were collected and stored at −80°C Thyroid parameters, serum TSH, levels of TgAb and TPOAb, and serum concentrations of FT4 and free triiodothyronine (FT3) were measured using an electro-chemiluminometric analyzer (E601; Roche Diagnostics GmbH, Mannheim, Germany), with an interassay variance of <10%. Serum levels of >35 IU/ml TPOAb and/or >116 IU/ml TgAb were considered autoantibody positivity. The normal range of TSH is 0.27–4.2 mIU/l, and the normal range of serum FT4 is 11.5–22.7 pmol/l.
Flow cytometry analyses
Peripheral blood mononuclear cells (PBMCs) were prepared from heparinized venous blood by density-gradient centrifugation within 8 h of blood collection. Stained cells were analyzed using a flow cytometer (BD LSRII; BD Biosciences, Franklin Lakes, NJ, USA). All reagents and fluorescent antibodies used in the present study were produced by BD Biosciences. PBMC were cultured in PMA + ionomysin + Brefeldin A (BFA) for 4 h at 37°C in a humidified atmosphere containing 5% CO2. Following incubation, the suspended cells were washed twice in cold PBS and were resuspended in cold PBS. According to the supplier's protocol, aliquots (200 µl) of PBMC were incubated with mouse anti-human CD3 (cat. no. 563798), CD4 (cat. no. 555346) and CD8 (cat. no. 557834) FITC antibodies (all BD Biosciences) for 20 min at 48°C. The working concentration of the antibodies was 1:100 dilution. Cells were then fixed with Human FoxP3 Buffer A fixation reagent. Following incubation for 10 min at room temperature in the dark, cells were rinsed twice in 2 ml of appropriate concentration reagent to cross membranes (Human FoxP3 Buffer C). Aliquots of suspended cells in permeabilization buffer were incubated for 30 min at room temperature in the dark with IFN-y, IL-4, IL-17A and Foxp3 antibodies, respectively. After rinsing in 2 ml of permeabilization buffer, PBMCs were resuspended in 500 µl PBS and analyzed using FCS express software, version 4 for the BD LSRII flow cytometer.
Statistical analysis
Continuous variables are presented as means ± standard deviation and median (interquartile range) for the non-normally distributed variables. Student's t-test or non-parametric Mann Whitney U test were used to determine whether differences between means were significant. Mann-Whitney U test was used to analyze the difference in the proportion of T helper cell subsets between the examined groups. Spearman rank correlation coefficient was also used to analyze the correlation between anti-thyroid antibody and the proportion of peripheral Th cell subsets. P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS v.16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Clinical characteristics of subjects
A total of 440 subjects were recruited into this study, including 51 normal controls (47.59±12.38 years; 20 males and 31 females) and 389 patients with HD (46.70±13.34 years; 71 males and 318 females). There was no significant difference among the four groups regarding age (P=0.654) and FT3 levels (P=0.424). Relevant clinical and biochemical data for all HT patients and healthy controls are presented in Table I. Subgroups of HT patients included 207 euthyroid HT patients (46.05±13.51 years; 46 males and 161 females), 148 subclinical hypothyroid HT patients (47.44±13.14 years; 23 males and 125 females), and 34 overt hypothyroid HT patients (47.50±13.30 years; 2 males and 32 females). Titers of TPOAb and TgAb were significantly higher in HT patients with overt hypothyroidism than in HT patients with subclinical hypothyroidism (P=0.035 and P=0.001, respectively). Clinical and biochemical data of the subgroups of HT patients are presented in Table II.
Table I.
Clinical characteristics of HT patients and healthy controls enrolled in the present study.
| Characteristics | Control | Hashimoto's thyroiditis |
|---|---|---|
| N (male/female) | 51 (20/31) | 389 (71/318) |
| Mean age | 47.59±12.38 | 46.70±13.34 |
| TPOAb, IU/ml (IQR) | 29.85 (27.35–31.73) | 89.13 (38.53–355.30)a |
| TgAb, IU/ml (IQR) | 27.01 (19.9–30.55) | 214.9 (48.62–540.00)a |
| TSH, mIU/l (IQR) | 2.06 (1.61–3.19) | 4.02 (2.40–5.98)a |
| FT4, pmol/l | 14.92±1.81 | 13.88±2.86 |
| FT3, pmol/l | 5.39±0.44 | 5.20±0.69 |
P≤0.001 vs. control subjects. TPOAb, anti-thyroid peroxidase antibody; TgAb, anti-thyroid globulin antibody; TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine; IQR, interquartile range.
Table II.
Clinical characteristics of the subgroups of Hashimoto's thyroiditis patients enrolled in the present study.
| Characteristics | HT-EU | HT-SH | HT-OH |
|---|---|---|---|
| N (male/female) | 207 (46/161) | 148 (23/125) | 34 (2/32) |
| Mean age | 46.05±13.51 | 47.44±13.14 | 47.50±13.30 |
| TPOAb, IU/ml (IQR) | 76.89 (38.84–257.70) | 95.43 (37.77–395.90) | 248.6 (66.10–537.90)a |
| TgAb, IU/ml (IQR) | 192.3 (45.47–538.50) | 196.8 (40.02–515.40) | 440.7 (260.8–690.40)a |
| TSH, mIU/l (IQR) | 2.51 (1.63–3.57) | 5.84 (4.85–7.74) | 15.94 (7.80–37.07)a |
| FT4, pmol/l | 15.29±1.90 | 14.42±2.06a | 9.10±2.38a |
| FT3, pmol/l | 5.39±0.58 | 5.26±0.60 | 4.57±0.88 |
P≤0.001 vs. control subjects. HT-EU, Hashimoto's thyroiditis with euthyroid; HT-SH, Hashimoto's thyroiditis with subclinical hypothyroidism; HT-OH, Hashimoto's thyroiditis with overt hypothyroidism; TPOAb, anti-thyroid peroxidase antibody; TgAb, anti-thyroid globulin antibody; TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine; IQR, interquartile range.
Proportion of peripheral Th1 and Th2 cells
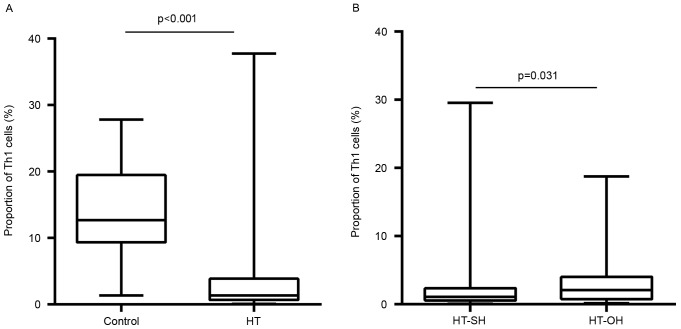
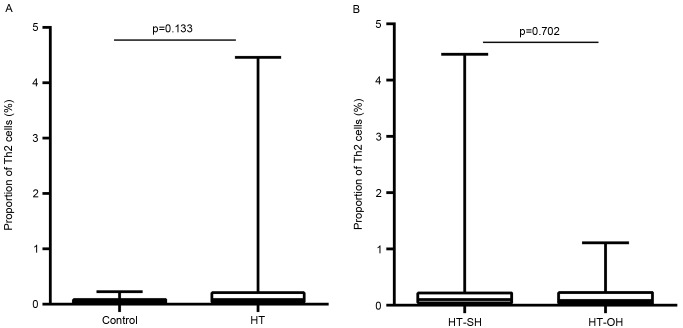
The proportions of peripheral Th1 cells in Th cells in the HT patients were significantly lower than those healthy euthyroid controls (3.9±6.0 vs. 14.2±6.3%; P<0.001; Fig. 1A). The Th1 proportions in patients with overt hypothyroidism HT were significantly higher than those in subclinical hypothyroidism HT patients (3.6±4.3 vs. 2.7±4.4%; P=0.031; Fig. 1B). In contrast, the proportions of Th2 cells in Th cells were significantly higher in patients with HT than those with euthyroid controls (0.2±0.3 vs. 0.06±0.05%; P<0.001; Fig. 2A). This proportion did not differ significantly between patients with overt hypothyroidism HT and subclinical hypothyroidism HT (0.2±0.4 vs. 0.2±0.3%; P=0.702; Fig. 2B). The Th1/Th2 ratio in HT patients was lower than that control subjects (P<0.001). However, no apparent differences were found between overt hypothyroid HT and subclinical hypothyroid HT patients (P=0.146).
Figure 1.
Differences in the proportions of Th1 cells among total CD4+ cells between (A) HT patients and control subjects, and (B) overt and subclinical hypothyroid HT patients. HT-SH, Hashimoto's thyroiditis with subclinical hypothyroidism; HT-OH, Hashimoto's thyroiditis with overt hypothyroidism.
Figure 2.
Differences in the proportions of Th2 cells among total CD4+ cells between (A) HT patients and control subjects, and (B) overt and subclinical hypothyroid HT patients. HT-SH, Hashimoto's thyroiditis with subclinical hypothyroidism; HT-OH, Hashimoto's thyroiditis with overt hypothyroidism.
Proportion of peripheral Th17 and Treg cells
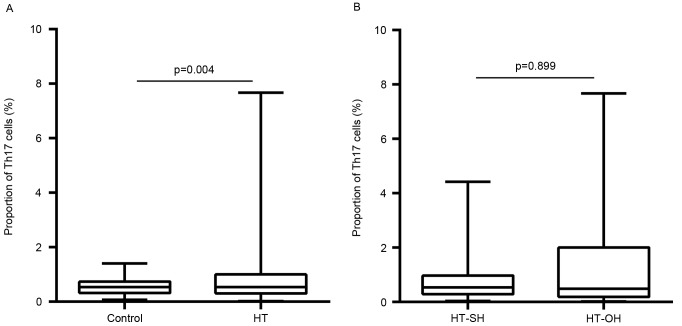
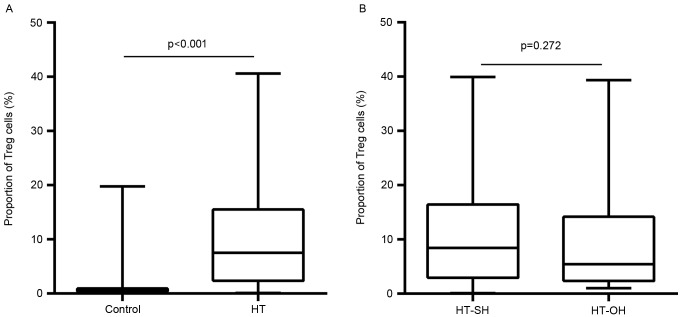
Peripheral proportions of Th17 and Treg cells are presented in Figs. 3 and 4, respectively. The proportions of peripheral Th17 cells were not significantly different between the patients with HT and control subjects (0.9±0.9 vs. 0.6±0.3%; P=0.566; Fig. 3A). The proportions of peripheral Treg cells in Th cells in HT patients were significantly higher than those in control subjects (10.2±9.4 vs. 1.2±2.7%; P<0.001; Fig. 4A). These proportions did not differ significantly between patients with overt hypothyroidism HT (Th17, 0.8±0.9%; Treg, 11.0±9.4%) and subclinical hypothyroidism HT (Th17, 1.2±1.6% P=0.899; Treg, 9.5±10.1% P=0.272; Figs. 3B and 4B). The Th17/Treg ratio in HT patients was lower than that control subjects (P<0.001). However, no apparent differences were found between overt and subclinical hypothyroid HT patients (P=0.053).
Figure 3.
Differences in the proportions of Th17 cells among total CD4+ cells between (A) HT patients and control subjects, and (B) overt and subclinical hypothyroid HT patients. HT-SH, Hashimoto's thyroiditis with subclinical hypothyroidism; HT-OH, Hashimoto's thyroiditis with overt hypothyroidism.
Figure 4.
Differences in the proportions of Treg cells among total CD4+ cells between (A) HT patients and control subjects, and (B) overt and subclinical hypothyroid HT patients. HT-SH, Hashimoto's thyroiditis with subclinical hypothyroidism; HT-OH, Hashimoto's thyroiditis with overt hypothyroidism.
Titers of thyroid-specific autoantibodies
Given the findings that patients with HT had higher serum TPOAb and TgAb levels and a lower proportion of Th1 cells, we evaluated the correlation using Spearman rank correlation analysis. A negative correlation (r=−0.134, P=0.008 and r=−0.114, P=0.024, respectively) was detected between TPOAb levels and Th1 cells, and TgAb levels and Th1 cells in patients with HT. Further analysis also revealed a negative correlation between Th1/Th2 ratio and Th17/Treg ratio with the two TPOAb and TgAb autoantibodies (r=−0.192, P<0.001; r=−0.126, P=0.008; r=−0.165, P=0.001; r=−0.211, P<0.001, respectively) (data not shown).
Discussion
The present study demonstrated that the proportions of peripheral Th1 cells were lower in patients with HT than in euthyroid control subjects, and a higher proportion of Th2 and Treg cells were found in the peripheral blood of patients with HT. Furthermore, the proportions of peripheral Th1 cells, but not Th17, Th2 and Treg, were elevated in HT patients with overt hypothyroidism when compared with HT patients with subclinical hypothyroidism. These data suggest that HT patients with a higher proportion of Th1 cells are more inclined to experience rapid destruction of thyroid follicular cells, resulting in hypothyroidism.
HT is an autoimmune disorder, characterized by the formation of tertiary lymphoid follicles within the thyroid (containing T cells, predominantly Th1, and B cells), in which the T-lymphocytes have an important role, with the diffuse process of thyroid follicules destruction generating hypofunction, and the presence of high titers of antiTPO and/or antiTg antibodies in the serum. The incidence of HT is 15–20 times more likely in women than in men (27). It is essentially the same process that the thyroid lymphocytic infiltrates when either of the thyroid autoantibodies are detected in serum, and this phenomenon presents in the ranges from focal thyroiditis to the lymphadenoid goitre of HT (1).
A prevalent Th1 cytokine profile is typically found in patients with organ-specific autoimmune disease, while a prevalent Th2 profile is usually associated with systemic autoimmunity (28). Our results showed a normal to low percentage of Th1 cells and an increased percentage of Th2 cells in all peripheral blood samples from patients with HT. This result confirmed the prevalent Th1 polarization in circulation. As reported previously, Th1 and Th2 cells are the extreme polarized forms of CD4+ Th cells, when it becomes dangerous, Th1 can be shifted to a less polarized profile Th0, or even Th2, via a process called immune deviation (29). Predominance of the immune response of Th1 accelerates the apoptosis of thyrocytes (30), which mediated by Fas and TRAIL, leading to HT (2,31). Whereas the predominance of a Th2 immune response induces antigen-specific B cells to produce antithyroid antibodies, just as stimulatory antiTSH receptor antibodies are responsible for Graves' disease and the blocking antiTSH receptor antibodies are responsible for atrophic thyroiditis; thus, the balance of Th1-Th2 directly affects the clinical expression of thyroid autoimmunity (32). However, in the HT patients with overt hypothyroidism investigated in the present study, a significant increase in the proportion of Th1 cells was noted when compared with that of subclinical hypothyroid HT patients. Hypothyroidism is not only the result of thyrocyte destruction, but also of thyroid function impairment in HT, which has been proposed to be induced by Th1 cytokines (29). Hence, we propose that Th1 cells may be present in different stages in patients with HT and depend on the severity of disease.
Increasing evidence supports the hypothesis that Th17 and Treg cells participate in the process of HT (26,33). Th17 lymphocytes are critical for the pathogenesis of different inflammatory and autoimmune conditions (7). Tregs are a subset of CD4+ T cells that suppress the excessive immune response, protect against tissue injury and prevent autoimmune diseases (10). The opposite role of these two kinds of CD4+ T cells can be seen in the development of autoimmune diseases, while Th17 cells promote autoimmunity, Treg cells control it (10,34). A recent study reported the decreased frequency and/or function of Treg cells in human autoimmune diseases (35). However, another study reported that, in autoimmune thyroiditis, Treg cell frequency and/or function is enhanced when compared with normal donors (36), and indicated that patients with HT exhibit enhanced levels of Th17 cells in their peripheral blood. In the present study, we observed an enhanced frequency of peripheral Th17 cells in patients with HT than in control subjects; however, the proportions of peripheral Treg cells in patients with HT were significantly higher than in control subjects. This suggests a compensatory attempt to overcome or reduce the autoimmunity by accelerating Treg cell activity (37). This is a notable finding as it has previously been hypothesized that the opposite roles of Th17, Treg cells and alteration of Th17/Treg may participate in the pathogenesis of HT (10,34). Although the Th17/Treg ratio in patients with HT was lower than in control subjects, no significant differences in the Th17/Treg ratio were found between our subgroups of overt hypothyroid HT patients and subclinical hypothyroid HT patients. These data suggest that the suppressive nature of Treg function is most likely a defect in autoimmune disease and patients are not able to reverse the clinical course of inflammation.
Predominance of the Th1 phenotype can induce cell-mediated apoptosis in thyrocytes with subsequent HT (29). Eventually, thyroid atrophy and myxedema may occur (38). Patients with AITD due to lymphocytic infiltration of the thyroid have a high incidence of TgAb and TPOAb in their serum (1). Bona et al (39) observed a direct correlation between anti-TPO antibody levels and the resistance of T cells to apoptosis in untreated patients with HT. A significant correlation between serum TPOAb/TgAb and Th1 cells supports the contention that antibodies are involved in the promotion of thyroid antibody-elicited immune responses. Antibody-mediated cytotoxicity leads to more damage to thyroid tissue in comparison to T cells and cytokine-mediated apoptosis (40).
There have been various studies addressing the issue of Th cells in HT patients; however, to our knowledge, the majority have been restricted by the amount of samples and animal experiments. As a results, the pathogenesis and progression of HT remains to be fully elucidated. The present study demonstrated that, in a large community-based sample, that an enhanced Th1, but not Th2 or Th17, immune response by Treg depletion is sufficient for the development of hypothyroidism in patients with HT. This finding appears to reflect a higher sustainability of the Th1 immune response. Although we do not have a commendable explanation for our partially contradictory results, it is feasible that the apparent discrepancies between previous studies (32,35) and our work may be due, at least in part, to the larger sample size of this study, different genetic background of the individuals studied, the presence or absence of HT, and the stage of the hypothyroidism in patients. Furthermore, only the peripheral T-cell subsets, not the intrathyroidal T cells, were studied and we did not exclude participants with other autoimmune conditions. In any case, we propose that performing a longitudinal study of Th cells in HT patients with or without hypothyroidism would be beneficial, predominantly because it has been suggested that a Th1-Th2 shift and Th17/Treg imbalance may occur during the evolution of HT.
In conclusion, our data suggest that patients with HT exhibit enhanced differentiation of Th1 lymphocytes, and that these cells may participate in the development of hypofunction in HT patients. Another important finding of the study was the reduced numerical proportion of Treg cells in patients with HT, indicating a compensatory attempt to overcome or reduce the autoimmunity, but this was not related to the severity of hypothyroidism in HT patients. This observation of thyroid autoimmunity from our study reflects the complexity of immune-thyroid interactions, clarifying the pathogenic roles of immune interaction. These data may support improved clinical management and earlier intervention in the progression of HT.
Acknowledgements
This study was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (grant no. 2013211A105) and the Fund for Less Developed Regions from the National Natural Science Foundation of China (grant no. 81260127).
References
- 1.Weetman AP. Autoimmune thyroid disease. Autoimmunity. 2004;37:337–340. doi: 10.1080/08916930410001705394. [DOI] [PubMed] [Google Scholar]
- 2.Stassi G, De Maria R. Autoimmune thyroid disease: New models of cell death in autoimmunity. Nat Rev Immunol. 2002;2:198–204. doi: 10.1038/nri750. [DOI] [PubMed] [Google Scholar]
- 3.Druet P, Sheela R, Pelletier L. Th1 and Th2 cells in autoimmunity. Chem Immunol. 1996;63:138–170. doi: 10.1159/000319483. [DOI] [PubMed] [Google Scholar]
- 4.Salgame P, Abrams JS, Clayberger C, Goldstein H, Convit J, Modlin RL, Bloom BR. Differing lymphokine profiles of functional subset of human CD4 and CD8 T cell clones. Science. 1991;254:279–282. doi: 10.1126/science.1681588. [DOI] [PubMed] [Google Scholar]
- 5.Carter LL, Dutton RW. Type 1 and Type 2: A fundamental dichotomy for a T-cell subsets. Curr Opin Immunol. 1991;8:336–342. doi: 10.1016/S0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 6.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 7.Torchinsky MB, Blander JM. T helper 17 cells: Discovery, function, and physiological trigger. Cell Mol Life Sci. 2010;67:1407–1421. doi: 10.1007/s00018-009-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shevach EM. Suppressor T cells: Rebirth, function and homeostasis. Curr Biol. 2000;10:R572–R575. doi: 10.1016/S0960-9822(00)00617-5. [DOI] [PubMed] [Google Scholar]
- 9.Liblau RL, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune disease. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Xu H, Wang Y, Ma J, Mao C, Shao Q, Ma B, Xu W, Yang S. Regulatory T cells induced by rAAV carrying the forkhead box P3 gene prevent autoimmune thyroiditis in mice. Int J Mol Med. 2006;18:1193–1199. [PubMed] [Google Scholar]
- 11.Staii A, Mirocha S, Todorova-Koteva K, Glinberg S, Jaume JC. Hashimoto thyroiditis is more frequent than expected when diagnosed by cytology which uncovers a pre-clinical state. Thyroid Res. 2010;3:11. doi: 10.1186/1756-6614-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42:252–265. doi: 10.1007/s12020-012-9703-2. [DOI] [PubMed] [Google Scholar]
- 13.Gordin A, Saarinen P, Pelkonen A, Lamberg BA. Serum thyroglobulin and the response to thyrotropin releasing hormone in symptomless autoimmune thyroiditis and in borderline and overt hypothyroidism. Acta Endocrinol (Copenh) 1974;75:274–285. doi: 10.1530/acta.0.0750274. [DOI] [PubMed] [Google Scholar]
- 14.Weetman AP. Hypothyroidism: Screening and subclinical disease. BMJ. 1997;314:1175–1178. doi: 10.1136/bmj.314.7088.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanba T, Watanabe M, Inoue N, Iwatani Y. Increases of the Th1/Th2 cell ratio in severe hashimoto's disease and in the proportion of Th17 cells in intractable graves' disease. Thyroid. 2009;19:495–501. doi: 10.1089/thy.2008.0423. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe M, Yamamoto N, Maruoka H, Tamai H, Matsuzuka F, Miyauchi A, Iwatani Y. Independent involvement of CD8+CD25+ cells and thyroid autoantibodies in disease severity of Hashimoto's disease. Thyroid. 2002;12:801–808. doi: 10.1089/105072502760339370. [DOI] [PubMed] [Google Scholar]
- 17.Ito C, Watanabe M, Okuda N, Watanabe C, Iwatani Y. Association between the severity of Hashimoto's disease and the functional +874A/T polymorphism in the interferon-gamma gene. Endocr J. 2006;53:473–478. doi: 10.1507/endocrj.K06-015. [DOI] [PubMed] [Google Scholar]
- 18.Nanba T, Watanabe M, Akamizu T, Iwatani Y. The 590CC genotype in the IL4 gene as a strong predictive factor for the development of hypothyroidism in Hashimoto disease. Clin Chem. 2008;54:621–623. doi: 10.1373/clinchem.2007.099739. [DOI] [PubMed] [Google Scholar]
- 19.Alunno A, Manetti M, Caterbi S, Ibba-Manneschi L, Bistoni O, Bartoloni E, Valentini V, Terenzi R, Gerli R. Altered immunoregulation in rheumatoid arthritis: The role of regulatory T cells and proinflammatory Th17 Cells and therapeutic implications. Mediators Inflamm. 2015;2015:751793. doi: 10.1155/2015/751793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148:13–21. doi: 10.1111/imm.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyzik A, Grywalska E, Matyjaszek-Matuszek B, Roliński J. Immune disorders in hashimoto's thyroiditis: What do we know so far? J Immunol Res 2015. 2015:979167. doi: 10.1155/2015/979167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safdari V, Alijani E, Nemati M, Jafarzadeh A. Imbalances in T cell-related transcription factors among patients with hashimoto's thyroiditis. Sultan Qaboos Univ Med J. 2017;17:e174–e180. doi: 10.18295/squmj.2016.17.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirahara K, Nakayama T. CD4+ T-cell subsets in inflammatory diseases: Beyond the Th1/Th2 paradigm. Int Immunol. 2016;28:163–171. doi: 10.1093/intimm/dxw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sehrawat S, Rouse BT. Interplay of regulatory T cell and Th17 cells during Infectious diseases in humans and animals. Front Immunol. 2017;8:341. doi: 10.3389/fimmu.2017.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marazuela M, García-López MA, Figueroa-Vega N, de la Fuente H, Alvarado-Sánchez B, Monsiváis-Urenda A, Sánchez-Madrid F, González-Amaro R. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91:3639–3646. doi: 10.1210/jc.2005-2337. [DOI] [PubMed] [Google Scholar]
- 26.Figueroa-Vega N, Alfonso-Pérez M, Benedicto I, Sánchez-Madrid F, González-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in hashimoto's thyroiditis. J Clin Endocrinol Metab. 2010;95:953–962. doi: 10.1210/jc.2009-1719. [DOI] [PubMed] [Google Scholar]
- 27.Cipolla C, Sandonato L, Graceffa G, Fricano S, Torcivia A, Vieni S, Latteri S, Latteri MA. Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. Am Surg. 2005;71:874–878. [PubMed] [Google Scholar]
- 28.Romagnani S. Regulation of T cell response. Clin Exp Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 29.Lichiardopol C, Moţa M. The thyroid and autoimmunity. Rom J Intern Med. 2009;47:207–215. [PubMed] [Google Scholar]
- 30.Corona G, Biagini C, Rotondi M, Bonamano A, Cremonini N, Petrone L, Conforti B, Forti G, Serio M. Correlation between, clinical, biochemical, color Doppler ultrasound thyroid parameters, and CXCL-10 in autoimmune thyroid diseases. Endocr J. 2008;55:345–350. doi: 10.1507/endocrj.K07E-052. [DOI] [PubMed] [Google Scholar]
- 31.Fountoulakis S, Vartholomatos G, Kolaitis N, Frillingos S, Philippou G, Tsatsoulis A. Differential expression of Fas system apoptotic molecules in peripheral lymphocytes from patients with Graves' disease and Hashimoto's thyroiditis. Eur J Endocrinol. 2008;158:853–859. doi: 10.1530/EJE-08-0092. [DOI] [PubMed] [Google Scholar]
- 32.Tsatsoulis A. The role of stress in the clinical expression of thyroid autoimmunity. Ann N Y Acad Sci. 2006;1088:382–395. doi: 10.1196/annals.1366.015. [DOI] [PubMed] [Google Scholar]
- 33.Glick AB, Wodzinski A, Fu P, Levine AD, Wald DN. Impairment of regulatory T-cell function in autoimmune thyroid disease. Thyroid. 2013;23:871–878. doi: 10.1089/thy.2012.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettelli E, Carrire Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Tang X, Tian J, Zhu C, Peng H, Rui K, Wang Y, Mao C, Ma J, Lu L, et al. Th17/Treg cells imbalance and GITRL profile in patients with Hashimoto's thyroiditis. Int J Mol Sci. 2014;15:21674–21686. doi: 10.3390/ijms151221674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: Role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 37.Kahaly GJ, Shimony O, Gellman YN, Lytton SD, Eshkar-Sebban L, Rosenblum N, Refaeli E, Kassem S, Ilany J, Naor D. Regulatory T-cells in Graves' orbitopathy: Baseline findings and immunomodulation by anti-T lymphocyte globulin. J Clin Endocrinol Metab. 2011;96:422–429. doi: 10.1210/jc.2010-1424. [DOI] [PubMed] [Google Scholar]
- 38.Buchanan WW, Harden RM. Primary hypothyroidism and Hashimoto's thyroiditis A continuous spectrum. Arch Intern Med. 1965;115:411–417. doi: 10.1001/archinte.1965.03860160037006. [DOI] [PubMed] [Google Scholar]
- 39.Bona G, Defranco S, Chiocchetti A, Indelicato M, Biava A, Difranco D, Dianzani I, Ramenghi U, Corrias A, Weber G, et al. Defective function of Fas in T cells from paediatric patients with autoimmune thyroid diseases. Clin Exp Immunol. 2003;133:430–437. doi: 10.1046/j.1365-2249.2003.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiovato L, Bassi P, Santini F, Mammoli C, Lapi P, Carayon P, Pinchera A. Antibodies producing complement-mediated thyroid cytotoxicity in patients with atrophic or goitrous autoimmune thyroiditis. J Clil Endocrinol Metab. 1993;77:1700–1705. doi: 10.1210/jcem.77.6.7903315. [DOI] [PubMed] [Google Scholar]