Abstract
This study was designed to investigate the protective effect and possible molecular mechanism of liquiritin on oxybuprocaine-induced apoptosis of human corneal endothelial cells (HCECs). In this study, the effect of oxybuprocaine on the proliferation of HCEC-12 was detected using cell counting kit-8 (CCK-8). The inductive effect of oxybuprocaine on HCEC-12 apoptosis and protective effect of liquiritin against oxybuprocaine-induced HCEC-12 apoptosis were tested by Annexin V/propidium iodide (PI) staining and flow cytometry. The production of reactive oxygen species (ROS) was analyzed by 2,7-dichlorodi-hydrofluorescein diacetate (DCFH-DA) staining and fluorescent-activated cell sorting (FACS), and the expression of nuclear factor-κB (NF-κB) p65 and apoptosis-related proteins, caspase-3 and Bax, was determined by western blot analysis. Our results show that liquiritin resisted the inhibitory effect of oxybuprocaine on the proliferation of HCEC-12, and cell activity had the most significant increase in pretreatment with liquiritin group in the concentration of 8 mg/ml; compared with that in oxybuprocaine group. Apoptosis in pretreatment with liquiritin was distinctly decreased and liquiritin resisted the production of ROS in HCEC-12 induced by oxybuprocaine. Investigation of molecular mechanism revealed that the pretreatment with liquiritin and pyrrolidinedithiocarbamic acid (PDTC) obviously blocked the expression of NF-κB p65 in nuclear protein increased by oxybuprocaine and the expression levels of total proteins, caspase-3 and Bax.Moreover, tumor necrosis factor-α (TNF-α) blocked the inhibitory effect of liquiritin on the expression of NF-κB p65 in nuclear protein and total proteins, caspase-3 and Bax, thus obstructing the protective effect of liquiritin on corneal epithelial cells. The results of this study indicated that liquiritin reduces the expression of apoptosis protein and increases the expression of anti-apoptotic protein through inhibiting NF-κB signal pathway, thus resisting HCEC-12 apoptosis induced by oxybuprocaine.
Keywords: liquiritin, oxybuprocaine, human corneal endothelial cells, apoptosis, nuclear factor-κB signal pathway
Introduction
Oxybuprocaine, as an ophthalmic topical anesthetic, is widely applied in a variety of eye surgery procedures. After drop administration with anesthetic, corneal sensation decreases or disappears and the epithelium loses its regularity and easily becomes dry, so it has certain toxic effects (1). One study (2)reported that allergic conjunctivitis and severe corneal damage have occurred after drop administration of oxybuprocaine after operation in clinical practice. Another study (3) revealed that 0.5–4 g/l oxybuprocaine has an obvious inductive effect on apoptosis of human corneal endothelial cells (HCECs), displaying a significant concentration- and time-dependent manner, but the concentration of oxybuprocaine used in clinical practice is 4 g/l, so oxybuprocaine with clinical concentration has a strong inductive effect on apoptosis of HCECs. Thus, there is a need to develop efficient new drugs for HCECs to inhibit oxybuprocaine toxicity.
Licorice, belonging to Leguminosae Glycyrrhiza, is derived from the dry roots and rhizomes of Glycyrrhiza uralensis, Glycyrrhiza glabra and Glycyrrhiza inflata Batal., which is commonly added into a variety of traditional Chinese medicine compounds as an adjuvant or messenger drug; it tastes sweet and is neutral in nature, with effects such as invigorating spleen and replenishing qi, clearing away heat and toxic materials, expelling phlegm and arresting coughing, relieving spasm and stopping pain and moderating the property of herbs (1–3). Liquiritin is one of the main flavonoids in Glycyrrhiza uralensis, which has antidepressant, neuroprotective and therapeutic effects on heart system diseases (3–7). In Chinese traditional medicine, licorice has been used to treat eye disease, for example, viral keratitis, ulcerative keratitis, and irritability of keratitis. Liquiritin can significantly reduce apoptosis of human umbilical vein endothelial cells (HUVECs) induced by AGEs (8,9) and play a strong protective effect on vascular endothelial cells in myocardial ischemia-reperfusion injury model (6,10). It can also protect smoking-induced lung epithelial cell injury (11). Our past studies (unpublished)showed liquiritin was worthy of further study by HPLC-MS analysis and biological experiments. However, whether it can resist corneal epithelial cell damage by oxybuprocaine has not been reported yet.
This study investigated the protective effect of liquiritin on oxybuprocaine-induced apoptosis of HCECs, so as to provide some experimental foundation and theoretical basis for its application in clinical protection of corneal epithelial cells from injury.
Materials and methods
Cell culture
The HCEC-12 cells were purchased from Creative-Bioarray Co. (cat. no. CSC-C3457; New York, NY, USA) and placed in the RPMI-1640 medium containing 10% fetal bovine serum (FBS) (both from HyClone, Logan, UT, USA), followed by placement in a cell culture incubator (37°C, 5% CO2). Penicillin and streptomycin with each concentration of 1.0×105 µl were added into nutrient solution to resist bacterial contamination. Microscopic observation showed that cells were in the adherent growth in culture fluid, with multiplication every 26–48 h. The cell concentration was controlled at 106 cells/ml. The fluid was changed every two days, and cells were subcultured once every four days. Oxybuprocaine: 0.4 g oxybuprocaine powder was dissolved in 100 ml Dulbecco's modified Eagle's medium (DMEM)/F12 for preparation of 4 g/l solution, adding medium to the desired concentration. Cells were cultured and oxybuprocaine was added into the cells on the second day for the required time.
Cell counting kit-8 (CCK-8)
The HCEC-12 in logarithmic growth phase was inoculated to the wells of a 96-well plate, followed by adjustment of density to 2×103 in each well. Subsequently, 200 µl RPMI-1640 medium containing 10% FBS was added. Six duplicated wells were set in each group. After culture for 24 h, 10 µl CCK-8 solution was added into each well, and then the sample was incubated in an incubator containing CO2 for 4 h. The well with phosphate-buffered solution (PBS) was regarded as the control, and the absorbance A value at 450 nm was detected by the enzyme analyzer. The growth curve was drawn.
Detection of apoptosis by flow cytometry
The adherent cells were digested with trypsin without ethylene diamine tetraacetic acid (EDTA) and collected (the digestion time was shortened as much as possible to avoid false positive); cells were rinsed by PBS twice (centrifuged at 600 × g for 5 min), and then 1–5×105 cells were collected. Cell suspension (500 µl) with binding buffer was added. After 5 µl of Annexin V-family of intracellular (FITC) protein was added, followed by mixing well, then 5 µl propidium iodide (PI) was added. The fluid was mixed well, and reacted at room temperature avoiding light for 5–15 min. The sample was observed and determined by flow cytometer within 1 h with excitation wavelength Ex=488 nm and emission wavelength Em=530 nm. The green fluorescence of Annexin V was detected by FITC channel (FL1). The red fluorescence of PI was determined by PI channel using FL3. Statistical analysis was performed by GraphPad Software, Inc. (La Jolla, CA, USA).
Detection of reactive oxygen species (ROS)
The treated cells were digested by pancreatin, followed by collection. The cells were re-suspended using pre-cooling PBS. Subsequently, serum-free medium was used to prepare 10 µM probe dyeing working fluid. The pre-cooling and re-suspended cells were centrifuged and re-suspended in the probe dyeing working fluid, followed by mixing well to make the probe fully contact with cells. After incubation, cells were rinsed by serum-free medium three times, so as to fully remove 2,7-dichlorodi-hydrofluorescein diacetate (DCFH-DA) that did not enter the cells. The sample was detected by flow cytometry, followed by excitation with 480 nm wavelength and determination of emission light at 525 nm. ROS-positive cells showed strong green fluorescence correspondening to FL1 detection channel of BD Biosciences (Franklin Lakes, NJ, USA) flow cytometer.
Western blotting
Polyacrylamide gel electrophoresis (PAGE) was conducted. The loading amount of protein in each well was 150 µg. Eighty volts was changed to 100 V for electrophoresis when Marker began to separate. When Marker was completely separated and the target band could be obtained, the electrophoresis was stopped. The protein was electrically transferred onto polyvinylidene fluoride (PVDF) membrane with electric current of 350 mA for ~2 h. The membrane was sealed with 5% bovine serum albumin (BSA)/milk at room temperature for 1 h, followed by incubation with the diluted rabbit anti-human primary monoclonal antibodies [NF-κB p65 (cat. no. 4764), caspase-3 (cat. no. 9665), Bax (cat. no. 2774), B-cell lymphoma-2 (Bcl-2; cat. no. 2872) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; cat. no. 2118); (all 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA)] according to the instructions at 4°C overnight. On the second day, goat anti-rabbit secondary polyclpnal antibody (cat. no. 7074; 1:2,000; Cell Signaling Technology, Inc.) was added. Then, the sample was incubated at 37°C for 1 h and added with exposure liquid, followed by photographing using chemiluminescence apparatus.
Statistical analysis
Statistical results were analyzed by GraphPad Prism 5 software. The data are expressed as mean ± standard deviation. The independent samples t-test was used for comparison of difference between two groups, and analysis of variance was adopted for comparison of multivariate means. P<0.05 indicates that the difference was statistically significant.
Results
Oxybuprocaine inhibits the proliferation of HCEC-12, HCEC-H9C1, and HCEC-B4G12
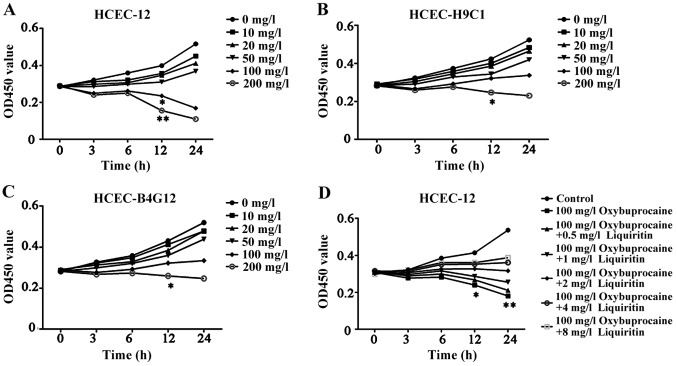
Oxybuprocaine is a common medicine for eye anesthesia. In order to study its influence on different human corneal endothelial cells, oxybuprocaine media with different concentrations were adopted to induce HCEC-12, HCEC-H9C1 and HCEC-B4G12 for 0, 3, 6, 12 and 24 h, and the effect of oxybuprocaine on activity of HCEC-12, HCEC-H9C1 and HCEC-B4G12 was detected by CCK-8. The results displayed that HCEC-12, HCEC-H9C1 and HCEC-B4G12 activity was inhibited and it was dependent on the concentration of oxybuprocaine; moreover, the cell activity of HCEC-12 was significantly inhibited after the reaction with concentration of oxybuprocaine over 100 mg/l for 12 h compared with those of HCEC-H9C1 and HCEC-B4G12 (P<0.05) (Fig. 1A-C). So we chose HCEC12 for the study.
Figure 1.
Detection of proliferation of HCEC-12, HCEC-H9C1, HCEC-B4G12 using CCK-8. (A-C) The effect of oxybuprocaine (0, 10, 20, 50, 100 and 200 mg/l) on the proliferation of HCEC-12, HCEC-H9C1 and HCEC-B4G12 is detected using CCK-8. (D) Liquiritin (0.5, 1, 2, 4 and 8 mg/ml) resisting the inhibitory effect of oxybuprocaine on the proliferation of HCEC-12 was detected using CCK-8. *P<0.05 and **P<0.01.
Liquiritin resists the proliferation of HCEC-12 inhibited by oxybuprocaine
The high concentration of oxybuprocaine can induce HCEC-12 thus significantly reducing the activity of HCEC-12. In order to investigate the effect of liquiritin on proliferation of HCEC-12 induced by oxybuprocaine, HCEC-12 was pretreated by liquiritin in different concentrations, followed by being induced by 100 mg/l oxybuprocaine for 0, 3, 6, 12 and 24 h. The activity of cells in each group was detected by CCK-8. The results showed that oxybuprocaine could significantly decrease cell activity compared with that in control group (P<0.05); the cell activity in pretreatment with liquiritin group was distinctly increased compared with that in oxybuprocaine group, and it showed the most significant increase in pretreatment with liquiritin group in the concentration of 8 mg/ml (Fig. 1D), indicating that liquiritin could resist the inhibitory effect of oxybuprocaine on the proliferation of HCEC-12.
Oxybuprocaine induces HCEC-12 apoptosis and ROS production
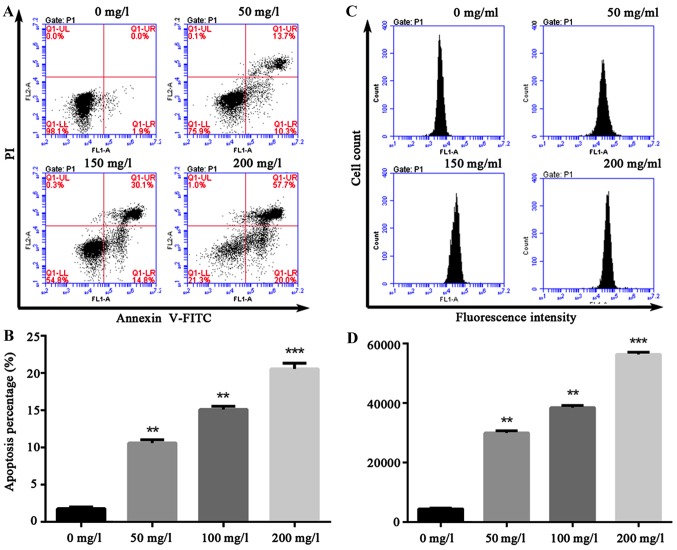
A variety of pro-apoptotic signals (such as unfavorable environmental factors, injury, radiation, chemotherapeutic agents, excitatory amino acid and death ligand) can cause increased cell endogenous or exogenous ROS or altered redox equilibrium. The production of ROS can serve as a signal triggering apoptosis in transduction pathway. Thus, HCEC-12 was intervened by oxybuprocaine media in different concentrations for 12 h in this study, and apoptosis was assessed by flow cytometry, revealing that compared with that in control group (1.9%), 200 mg/l oxybuprocaine can significantly induce HCEC-12 apoptosis (20%) (Fig. 2A and B). Additionally, DCFH-DA staining and fluorescent-activated cell sorting (FACS) were used to analyze the production of ROS after HCEC-12 was stimulated by oxybuprocaine, suggesting that different concentrations of oxybuprocaine could significantly induce the production of ROS in HCEC-12, which was concentration-dependent (Fig. 2C and D).
Figure 2.
Effect of oxybuprocaine on HCEC-12 apoptosis and ROS production. (A and B) The effect of oxybuprocaine (0, 50, 100 and 200 mg/l) on HCEC-12 apoptosis detected by flow cytometry. (B) Apoptosis percentage of early stage. (C and D) The effect of oxybuprocaine (0, 50, 100 and 200 mg/l) on ROS production in HCEC-12 cells analyzed by DCFH-DA staining and FACS. **P<0.05 and ***P<0.01.
Liquiritin resists proliferation of HCEC-12 apoptosis and ROS production is induced by oxybuprocaine
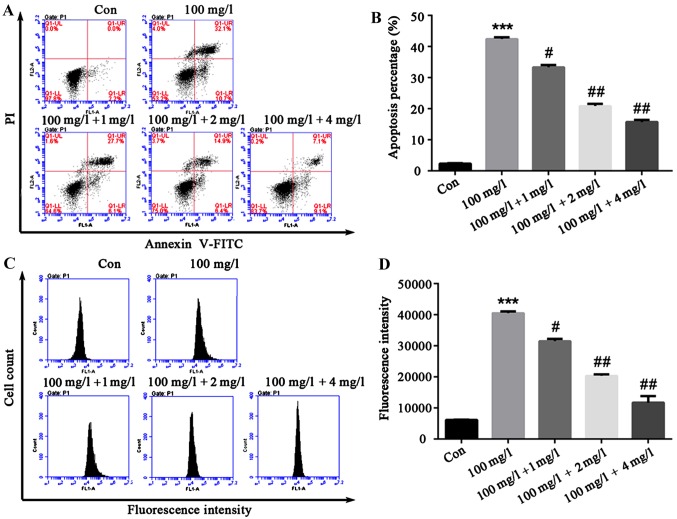
In order to explore the effect of liquiritin on HCEC-12 apoptosis and ROS production induced by oxybuprocaine, HCEC-12 was pretreated by liquiritin in different concentrations for 1h, and then induced by 100 mg/l oxybuprocaine for 12 h in this study, and apoptosis and ROS production were detected by flow cytometry. The results revealed that compared with that in control group, apoptosis in oxybuprocaine group was distinctly increased, and it was remarkably reduced in pretreatment with liquiritin group compared with that in oxybuprocaine group (Fig. 3A and B). The production of ROS analyzed by DCFH-DA staining and FACS obtained results that were consistent with that of apoptosis, suggesting that liquiritin could resist the production of ROS in HCEC-12 induced by oxybuprocaine (Fig. 3C and D).
Figure 3.
Effect of liquiritin on HCEC-12 apoptosis and ROS production induced by oxybuprocaine. (A and B) The effect of liquiritin (1, 2 and 4 mg/ml) on HCEC-12 apoptosis induced by oxybuprocaine was detected by flow cytometry. (B) Apoptosis percentage of early and late stage. (C and D) The effect of liquiritin (1, 2 and 4 mg/ml) on ROS production in HCEC-12 cells induced by oxybuprocaine was analyzed by DCFH-DA staining and FACS. ***P<0.05 compared with control group; #P<0.05 compared with oxybuprocaine group; ##P<0.01 compared with oxybuprocaine group.
Liquiritin resists the NF-κB signal pathway activated by oxybuprocaine
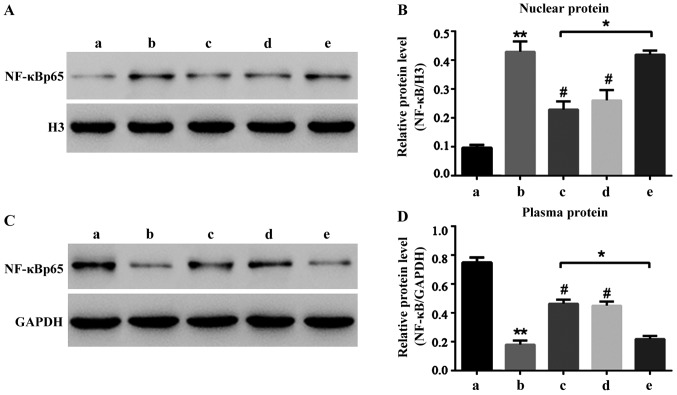
Liquiritin can significantly reduce HCEC-12 apoptosis induced by oxybuprocaine thus protecting HCEC-12. It is well known that NF-κB signal pathway is widely involved in the process of apoptosis in many cells (12–15). Hence, this study aimed to investigate whether HCEC-12 apoptosis induced by oxybuprocaine is dependent on the NF-κB signal pathway, and whether liquiritin resists the induction of HCEC-12 apoptosis by oxybuprocaine through inhibiting the NF-κB signal pathway. The results revealed that 50 mg/l oxybuprocaine could significantly increase the expression of NF-κB p65 in nuclear protein (Fig. 4A) and decrease the expression of NF-κB p65 in plasmosin (Fig. 4B), and the pretreatment with 2 mg/ml liquiritin and 50 µmol/l pyrrolidinedithiocarbamic acid (PDTC) obviously blocked the expression of NF-κB p65 in nuclear protein increased by oxybuprocaine (Fig. 4A). Additionally, 10 ng/ml tumor necrosis factor-α (TNF-α) blocked the inhibitory effect of liquiritin on the expression of NF-κB p65 in nuclear protein (Fig. 4A), thus obstructing the protective effect of liquiritin, indicating that liquiritin resists HCEC-12 apoptosis induced by oxybuprocaine through inhibiting the NF-κB signal pathway.
Figure 4.
Liquiritin resistance of NF-κB signal pathway activated by oxybuprocaine. (A and B) The expression of NF-κB p65 in nuclear protein of HCEC-12 cells is determined by western blotting. (C and D) The expression of NF-κB p65 in plasmosin in HCEC-12 cells is determined by western blotting. a, Control group. b, 50 mg/l oxybuprocaine group; c, oxybuprocaine + 2 mg/l liquiritin group; d, oxybuprocaine + 50 µmol/l PTDC group; e, oxybuprocaine + 2 mg/l liquiritin + 10 ng/ml TNF-α group. *P<0.05 compared with oxybuprocaine + 2 mg/l liquiritin group; **P<0.05 compared with 50 mg/l oxybuprocaine group; #P<0.05 compared with control group.
NF-κB signal pathway participates in liquiritin resistance of HCEC-12 apoptosis induced by oxybuprocaine
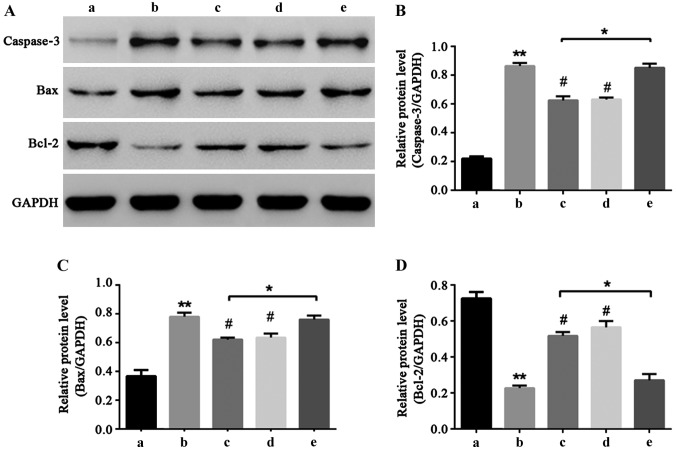
Caspase-3, Bax and Bcl-2 are important proteins that can directly reflect the extent of apoptosis (16–18). In order to explore the molecular mechanism of liquiritin resistance of HCEC-12 induced by oxybuprocaine, western blot was utilized to determine the expressions of caspase-3, Bax and Bcl-2 proteins. The results showed that oxybuprocaine increased the expression levels of caspase-3 and Bax and reduced the expression levels of anti-apoptotic protein Bcl-2, and pretreatment with liquiritin and PDTC inhibited the increasing effect of oxybuprocaine on expression levels of caspase-3 and Bax proteins and reduced the inhibitory effect of oxybuprocaine on Bcl-2 expression level; pretreatment with TNF-α blocked the inhibitory effect of liquiritin on the expression levels of caspase-3 and Bax proteins and reduced the increasing effect of liquiritin on Bcl-2 expression (Fig. 5), revealing that liquiritin reduced the expression of apoptosis proteins and increased the expression of anti-apoptosis proteins through inhibiting the NF-κB signal pathway, thus resisting HCEC-12 apoptosis induced by oxybuprocaine.
Figure 5.
NF-κB signal pathway participates in liquiritin resistance of HCEC-12 apoptosis induced by oxybuprocaine. The expressions of caspase-3, Bax and Bcl-2 proteins related to HCEC-12 apoptosis were determined by western blotting. a, Control group; b, 100 mg/l oxybuprocaine group; c, oxybuprocaine + 2 mg/l liquiritin group; d, oxybuprocaine + 50 µmol/l PTDC group; e, oxybuprocaine + 2 mg/l liquiritin + 10 ng/ml TNF-α group. *P<0.05 compared with oxybuprocaine + 2 mg/l liquiritin group; **P<0.05 compared with 50 mg/l oxybuprocaine group; #P<0.05 compared with control group.
Discussion
With the development of ophthalmic surgery techniques and equipment, ophthalmic topical anesthetics have been widely applied, and its side effects on cornea are getting increasing attention. The most commonly used topical anesthetic is oxybuprocaine (8–10). Cornea is mainly composed of endothelium, stroma, epithelium and its derivatives, and corneal endothelial cells in mammals (except for rabbits) lose their regenerative ability in adulthood. Therefore, the damage of human corneal endothelial cells cannot be repaired, and the study on HCEC is of uppermost priority. Previous studies show that oxybuprocaine can induce apoptosis of corneal epithelial cells, which causes certain damage on corneal epithelial cells, so it is urgent to develop drugs that can resist toxicity of oxybuprocaine (15–18).
Liquiritin is derived from Glycyrrhiza uralensis which belongs to leguminous plants. It has good anti-inflammatory and antioxidant activity (18). However, its protective effect on corneal epithelial cell injury has not been reported. The experimental results showed that oxybuprocaine inhibited the proliferation of human corneal epithelial cells and induced its apoptosis, which was concentration-dependent. The results of pretreatment with liquiritin revealed that oxybuprocaine significantly decreased cell activity compared with that in control group (P<0.05); the cell activity in pretreatment with liquiritin group was distinctly increased compared with that in oxybuprocaine group, and it showed the most significant increase in pretreatment with liquiritin group in the concentration of 8 mg/ml, indicating that liquiritin could resist the inhibitory effect of oxybuprocaine on the proliferation of HCEC-12. The results of apoptosis experiment showed that compared with that in the control group, apoptosis in oxybuprocaine group was distinctly increased, and it was remarkably reduced in pretreatment with liquiritin group compared with that in oxybuprocaine group. The production of ROS analyzed by DCFH-DA staining and FACS obtained results which were consistent with that of apoptosis, suggesting that liquiritin can resist the production of ROS in HCEC-12 induced by oxybuprocaine.
The results of molecular mechanism investigation revealed that oxybuprocaine significantly increased the expression of NF-κB p65 in nuclear protein and decreased NF-κB p65 in plasmosin, and the pretreatment with liquiritin and PDTC obviously blocked the expression of NF-κB p65 in nuclear protein increased by oxybuprocaine. Additionally, TNF-α blocked the inhibitory effect of liquiritin on the expression of NF-κB p65 in nuclear protein, thus obstructing the protective effect of liquiritin. The results of western blotting showed that oxybuprocaine increased the expression levels of caspase-3 and Bax and reduced the expression levels of anti-apoptotic protein Bcl-2, and pretreatment with liquiritin and PDTC inhibited the increasing effect of oxybuprocaine on expression levels of caspase-3 and Bax proteins and reduced the inhibitory effect of oxybuprocaine on Bcl-2 expression level; pretreatment with TNF-α blocked the inhibitory effect of liquiritin on the expression levels of caspase-3 and Bax proteins. NF-κB has been reported to inhibit apoptosis or promote apoptosis depending on the contexts (19). The downregulation of Bcl-2, upregulation of Bax, and activation of caspase-3 are widely known in the occurrence of apoptosis. We found that oxybuprocaine induced changes of protein levels of NF-κB, Bcl-2, Bax and caspase-3, but did not further analyze the relationship between NF-κB and Bcl-2, Bax, or caspase-3. Regarding the relationship between NF-κB and Bcl-2, Bax, or caspase-3, Wier et al (20) showed that despite the cleavage of NF-κB p65 by caspase-3, the cleavage-generated p65 N-terminal fragment interferes with the RPS3/NF-κB-confering gene transcription. Cao et al (21) also found that inhibition of NF-κB lead to increase of Bcl-2 expression and attenuates caspase-3 activation. Therefore, there were interactions between NF-κB and Bcl-2, Bax or caspase-3, but the detailed relationships in the context of liquiritin against oxybuprocaine-induced apoptosis need to be analyzed in further studies.
In conclusion, the results of this study indicated that liquiritin reduces the expression of apoptosis proteins and increases the expression of anti-apoptosis proteins through inhibiting NF-κB signal pathway, thus resisting HCEC-12 apoptosis induced by oxybuprocaine. The protective effect of liquiritin on corneal epithelial cells is expected to be used in the clinical practice to inhibit the toxicity of oxybuprocaine on corneal epithelial cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors contributions
DL collected, analyzed and interpreted the patient data, and drafted the manuscript. PZ conceived and designed the study, and revised the manuscript for important intellectual content. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hatano T, Yasuhara T, Miyamoto K, Okuda T. Anti-human immunodeficiency virus phenolics from licorice. Chem Pharm Bull (Tokyo) 1988;36:2286–2288. doi: 10.1248/cpb.36.2286. [DOI] [PubMed] [Google Scholar]
- 2.Kelly-Pieper K, Patil SP, Busse P, Yang N, Sampson H, Li XM, Wisnivesky JP, Kattan M. Safety and tolerability of an antiasthma herbal Formula (ASHMI) in adult subjects with asthma: A randomized, double-blinded, placebo-controlled, dose-escalation phase I study. J Altern Complement Med. 2009;15:735–743. doi: 10.1089/acm.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whorwood CB, Sheppard MC, Stewart PM. Licorice inhibits 11 beta-hydroxysteroid dehydrogenase messenger ribonucleic acid levels and potentiates glucocorticoid hormone action. Endocrinology. 1993;132:2287–2292. doi: 10.1210/endo.132.6.8504732. [DOI] [PubMed] [Google Scholar]
- 4.Tamir S, Eizenberg M, Somjen D, Stern N, Shelach R, Kaye A, Vaya J. Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res. 2000;60:5704–5709. [PubMed] [Google Scholar]
- 5.Hatano T, Shintani Y, Aga Y, Shiota S, Tsuchiya T, Yoshida T. Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem Pharm Bull (Tokyo) 2000;48:1286–1292. doi: 10.1248/cpb.48.1286. [DOI] [PubMed] [Google Scholar]
- 6.Sun YX, Tang Y, Wu AL, Liu T, Dai XL, Zheng QS, Wang ZB. Neuroprotective effect of liquiritin against focal cerebral ischemia/reperfusion in mice via its antioxidant and antiapoptosis properties. J Asian Nat Prod Res. 2010;12:1051–1060. doi: 10.1080/10286020.2010.535520. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Takasuka N, Iigo M, Baba M, Nishino H, Tsuda H, Okuyama T. Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci development. Cancer Sci. 2004;95:448–453. doi: 10.1111/j.1349-7006.2004.tb03230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Song Y, Han X, Feng L, Wang R, Zhang M, Zhu M, Jia X, Hu S. Liquiritin attenuates advanced glycation end products-induced endothelial dysfunction via RAGE/NF-κB pathway in human umbilical vein endothelial cells. Mol Cell Biochem. 2013;374:191–201. doi: 10.1007/s11010-012-1519-0. [DOI] [PubMed] [Google Scholar]
- 9.Feng L, Zhu MM, Zhang MH, Wang RS, Tan XB, Song J, Ding SM, Jia XB, Hu SY. Protection of glycyrrhizic acid against AGEs-induced endothelial dysfunction through inhibiting RAGE/NF-κB pathway activation in human umbilical vein endothelial cells. J Ethnopharmacol. 2013;148:27–36. doi: 10.1016/j.jep.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Guoqiang Q, Guoping Z. Optimal proportion of four effective components of Danggui Decoction on vascular endothelial cell protection in rats with myocardial ischemia reperfusion injury. Tradit Chin Med Mater. 2011;34:580–584. (In Chinese) [Google Scholar]
- 11.Guan Y, Li FF, Hong L, Yan XF, Tan GL, He JS, Dong XW, Bao MJ, Xie QM. Protective effects of liquiritin apioside on cigarette smoke-induced lung epithelial cell injury. Fundam Clin Pharmacol. 2012;26:473–483. doi: 10.1111/j.1472-8206.2011.00956.x. [DOI] [PubMed] [Google Scholar]
- 12.Koshimizu JY, Beltrame FL, de Pizzol JP, Jr, Cerri PS, Caneguim BH, Sasso-Cerri E. NF-κB overexpression and decreased immunoexpression of AR in the muscular layer is related to structural damages and apoptosis in cimetidine-treated rat vas deferens. Reprod Biol Endocrinol. 2013;11:29. doi: 10.1186/1477-7827-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora R, Yates C, Gary BD, McClellan S, Tan M, Xi Y, Reed E, Piazza GA, Owen LB, Dean-Colomb W. Panepoxydone targets NF-κB and FOXM1 to inhibit proliferation, induce apoptosis and reverse epithelial to mesenchymal transition in breast cancer. PLoS One. 2014;9:e98370. doi: 10.1371/journal.pone.0098370. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Decean H, Fischer-Fodor E, Tatomir C, Perde-Schrepler M, Somfelean L, Burz C, Hodor T, Orasan R, Virag P. Vitis vinifera seeds extract for the modulation of cytosolic factors BAX-α and NF-κB involved in UVB-induced oxidative stress and apoptosis of human skin cells. Clujul Med. 2016;89:72–81. doi: 10.15386/cjmed-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuo N, Zheng X, Liu H, Ma X. Fenofibrate, a PPARα agonist, protect proximal tubular cells from albumin-bound fatty acids induced apoptosis via the activation of NF-κB. Int J Clin Exp Pathol. 2015;8:10653–10661. [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson DW, Thornberry NA. Caspases: Killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/S0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 17.Shi L, Teng H, Zhu M, Li C, Huang K, Chen BI, Dai Y, Wang J. Paeoniflorin inhibits nucleus pulposus cell apoptosis by regulating the expression of Bcl-2 family proteins and caspase-9 in a rabbit model of intervertebral disc degeneration. Exp Ther Med. 2015;10:257–262. doi: 10.3892/etm.2015.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Ding Y, Ye N, Wild C, Chen H, Zhou J. Direct activation of bax protein for cancer therapy. Med Res Rev. 2016;36:313–341. doi: 10.1002/med.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing H, Lee S. NF-κB in cellular senescence and cancer treatment. Mol Cells. 2014;37:189–195. doi: 10.14348/molcells.2014.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wier EM, Fu K, Hodgson A, Sun X, Wan F. Caspase-3 cleaved p65 fragment dampens NF-κB-mediated anti-apoptotic transcription by interfering with the p65/RPS3 interaction. FEBS Lett. 2015;589:3581–3587. doi: 10.1016/j.febslet.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao ZH, Yin WD, Zheng QY, Feng SL, Xu GL, Zhang KQ. Caspase-3 is involved in IFN-γ- and TNF-α-mediated MIN6 cells apoptosis via NF-κB/Bcl-2 pathway. Cell Biochem Biophys. 2013;67:1239–1248. doi: 10.1007/s12013-013-9642-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.