See Zhou and Rademakers (doi:10.1093/brain/awx318) for a scientific commentary on this article.
Hypomyelinating leukodystrophies are a heterogeneous group of disorders. Simons et al. identify four patients with brain hypomyelination and a remarkably benign clinical presentation, all with the same dominant missense mutation in TMEM106B encoding a protein involved in lysosomal trafficking. The findings emphasize the essential role that lysosomes play in myelination.
Keywords: hypomyelinating leukodystrophies, TMEM106B, myelin, MRI, lysosomes
Abstract
See Zhou and Rademakers (doi:10.1093/brain/awx318) for a scientific commentary on this article.
Hypomyelinating leukodystrophies are a heterogeneous group of disorders with a clinical presentation that often includes early-onset nystagmus, ataxia and spasticity and a wide range of severity. Using next-generation sequencing techniques and GeneMatcher, we identified four unrelated patients with brain hypomyelination, all with the same recurrent dominant mutation, c.754G>A p.(Asp252Asn), in TMEM106B. The mutation was confirmed as de novo in three of the cases, and the mildly affected father of the fourth affected individual was confirmed as mosaic for this variant. The protein encoded by TMEM106B is poorly characterized but is reported to have a role in regulation of lysosomal trafficking. Polymorphisms in TMEM106B are thought to modify disease onset in frontotemporal dementia, but its relation to myelination is not understood. Clinical presentation in three of the four patients is remarkably benign compared to other hypomyelinating disorders, with congenital nystagmus and mild motor delay. These findings add TMEM106B to the growing list of genes causing hypomyelinating disorders and emphasize the essential role lysosomes play in myelination.
Introduction
Hypomyelinating leukodystrophies account for ∼20% of all cases with inherited white matter disorders and are characterized by severe and permanent myelin deficit. Genetic causes for hypomyelination are numerous, and most modes of inheritance—X-linked, autosomal dominant (including de novo) and autosomal recessive—have been described. Subtle MRI abnormalities beyond hypomyelination, or clinical signs such as hypodontia or cataracts can sometimes facilitate the identification of a specific molecular diagnosis, but typically there are neither characteristic MRI anomalies nor indicative clinical features (Steenweg et al., 2010; Pouwels et al., 2014). With the advance of next-generation sequencing techniques, genetic confirmation of leukodystrophies has become much easier, and several novel genes for hypomyelination have recently been described (Kevelam et al., 2016).
Mutations in genes encoding structural oligodendrocyte proteins as PLP1 or TUBB4A cause hypomyelinating leukodystrophies, as well as mutations in genes coding for transcription factors controlling expression of structural genes, e.g. SOX10 and NKX6-2 (Pouwels et al., 2014; Chelban et al., 2017; Dorboz et al., 2017). Mutations in other genes with cellular roles less obviously linked with oligodendrocyte function have also been identified, including POLR3A, POLR3B and POLR1C, all coding for subunits of RNA polymerase 3, and the tRNA synthetases RARS and DARS (Kevelam et al., 2016). In this study, we report that a recurrent de novo mutation in the lysosomal protein TMEM106B also causes a hypomyelinating leukodystrophy.
Patients and methods
Patients
Family 1 was recruited into the Care4Rare Canada Consortium research project that investigates the causes and mechanisms of rare unexplained disease. Families 2 and 3 were investigated as part of an on-going study by the Amsterdam Database of Unclassified Leukoencephalopathies to unravel the genetic cause of unclassified leukodystrophies. Patient 4 underwent diagnostic whole-exome sequencing. Approval from the institutional ethical committees of the participating centres was obtained and written informed consent secured from the patients’ guardians. Affected individuals were examined by neurologists at their primary care centres. Clinical and imaging data were reviewed by N.I.W. Genomic DNA specimens were obtained from circulating leucocytes using standard procedures.
Molecular genetic analysis
Exome sequencing was performed on the affected individual and parents of Family 1 using Agilent SureSelect All Exon 50MB (V4) capture kit and sequenced on an Illumina HiSeq 2000 platform with 100 base pair paired-end reads. Data analysis and candidate variant identification was performed as previously described (Beaulieu et al., 2014; Hamilton et al., 2016). Trio whole genome sequencing (WGS) was performed on all members of Families 2 and 3 using 2 × 150-nucleotide paired-end reads on an Illumina X10 (Illumina Cambridge Ltd). Read alignment was performed using BWA-mem; variant calling was performed using GATK HaplotypeCaller v3.7. SnpEff v4.3m was used for variant annotation and a custom script for variant filtration and prioritization. Diagnostic trio-exome sequencing was performed on Family 4 (Wolf et al., 2014) and directly queried for the presence of TMEM106B variants.
Validation of mosaicism
A 919-nucleotide amplicon centred on the TMEM106B mutation was amplified using the PCR primers CTGGATGTCACAAGAGTTCAGGA and GGGAAGTATCAGCTTTTCCCTGT from the genomic DNA of Patient 3 and her parents. Each PCR product was prepared for sequencing with the Nextera XT Library Kit (Illumina Inc.) according to manufacturer’s recommendations. Pooled libraries were sequenced by the Institute for Molecular Bioscience Sequencing Facility (University of Queensland) on a MiSeq instrument using v2 chemistry to generate paired 150 bp reads. Reads were mapped to the human genome (GRCh37) using BWA-mem. A minimum of 5000 reads were analysed per DNA sample.
Expression data
We checked expression patterns of TMEM106B using the Brain RNA-Seq database (accessed June 2017): http://web.stanford.edu/group/barres_lab/brainseq2/brainseq2.html (Zhang et al., 2014, 2016; Bennett et al., 2016).
Results
Clinical characterization
All patients are from European, non-consanguineous families. Pregnancy and delivery were uneventful. They underwent diverse genetic and metabolic tests, which were unrevealing, including searching for alterations of PLP1 in all of them and of GJC2 in three.
Patient 1, now aged 19 years, is a male who presented with marked hypotonia and nystagmus shortly after birth. Brain MRI showed lack of myelination, and a clinical diagnosis of Pelizaeus-Merzbacher-like disease was given when PLP1 testing was negative. A gastrostomy tube was placed at 5 months of age for a failure to thrive. Around that time, he developed intermittent episodes of choreoathetosis, initially thought to be seizures, evolving to dystonic arm posturing in teen years. Otherwise, clinical course was stable. He was able to crawl during early-mid childhood years and walk unassisted at age 13 years. At age 19 years, he was non-verbal, but able to vocalize to express simple needs. Gait was wide-based with pronated feet. Muscle bulk, tone and strength were normal. Deep tendon reflexes were brisk with equivocal plantar reflexes. Family history was unremarkable for individuals with intellectual disability, although one maternal uncle had a diagnosis of cerebral palsy.
Patient 2, now 5 years old, is a male who presented at age 6 weeks with continuous horizontal and vertical nystagmus of both eyes and mild muscular hypotonia in combination with mildly elevated muscle tone in his legs with increased tendon reflexes. Global development was moderately delayed (walking without support at age 3.8 years), but at the age of 5 years, he could run and climb stairs without holding on to the banisters. His fine motor skills were normal. Muscle tendon reflexes remained brisk; horizontal nystagmus was still present. Receptive language skills were age-adequate; expressive language suffered from poor pronunciation and grammar. He could make four-to-five word sentences and had a large vocabulary.
Patient 3, now 38 years old, is a female who presented at age 3 weeks with a horizontal, pendular nystagmus. Her motor development was mildly delayed; she was able to sit at age 1.5 years and walk without support at age 3 years. She had several seizures at age 6 months and was treated with valproic acid for 1 year. Her IQ was 76 at age 11 years. Clinical course was stable. She is living on her own, with some support. Neurological examination at age 30 years revealed dysarthria, gait ataxia, intention tremor and dysmetria, and a pyramidal syndrome, all mild. She was ambidextrous. Eye movements were remarkable for saccadic pursuit and gaze-evoked nystagmus. Her vision was reduced (0.5). Her father, now aged 65 years, had a similar history of nystagmus and mild developmental delay in infancy, with normal cognition and no obvious neurological abnormalities besides the finding that he was also ambidextrous. Otherwise, family history was unremarkable.
Patient 4, now aged 26 years, is a male who presented with rotatory nystagmus several days after birth. His motor development was delayed, he learnt to walk without support at age 5 years. His IQ was around 50. He was diagnosed with epilepsy at age 4 months and treated with valproic acid for 3 years. At age 16 years, he had one episode of psychosis, successfully managed with haloperidol. Recent neurological examination showed mild gait ataxia and minimal intention tremor, a shuffling gait with stooped posture and mild pyramidal signs. He had a rapid, pendular nystagmus with small amplitude and pale optic disks.
Brain MRI findings: hypomyelination
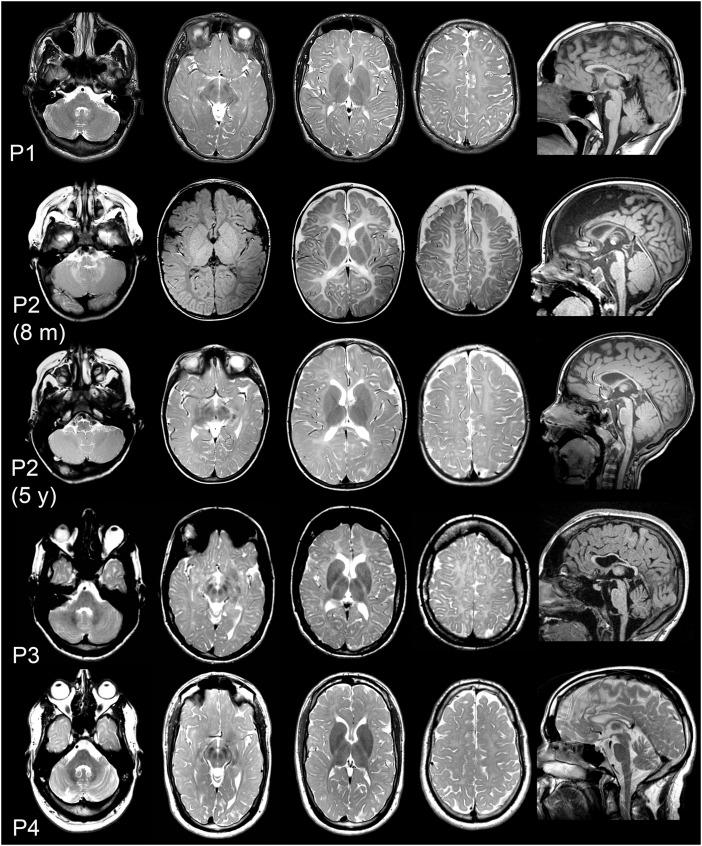
Patient 1 underwent his last brain MRI at age 17 years, with hypomyelination as the prominent finding similar to the MRI performed in the first year of life. MRIs in Patient 2 were performed at age 5 weeks, 8 months and 5 years, the last one confirming the diagnosis of hypomyelination already suspected in infancy. The latest MRI in Patient 3 was performed at age 30 years and showed hypomyelination. Her father underwent an MRI at age 65 years without obvious abnormalities. Patient 4 had a diagnosis of myelin deficit in the first year of life, confirmed by several other MRIs. Prominent cerebellar atrophy was not identified in any of the four patients. The three older patients had a thin corpus callosum, a feature often present in older patients with hypomyelination (Fig. 1).
Figure 1.
TMEM106B patient brain MRIs show hypomyelination. Brain MRIs of Patient 1 (top row; age 17 years), Patient 2 (second row: age 8 months; third row: age 5 years), Patient 3 (fourth row: age 30 years) and Patient 4 (bottom row: age 26 years). Columns 1–4 axial T2-weighted images (other than Patient 2 at age 8 months where the second image is a T1-weighted image). Column 5 depicts sagittal T1-weighted images (T2-weighted for Patient 4). Supratentorial white matter signal is diffusively mildly elevated in the patients, indicating hypomyelination. At age 8 months, Patient 2 is too young to formally diagnose hypomyelination, but a previous MRI at age 5 weeks was identical with the one depicted here, and myelination is clearly deficient also on the T1-weighted image with uniform mildly hypointense supratentorial white matter signal. The MRI obtained at age 5 years confirms hypomyelination, with the internal capsule being better myelinated. The sagittal images demonstrate thin corpus callosum in all patients and only mild cerebellar atrophy in Patient 4.
Genetic analysis identifies TMEM106B as candidate gene
Trio exome analysis in Family 1 identified a de novo mutation c.754G>A p.(Asp252Asn) in exon 8 of TMEM106B (NM_001134232); in addition, a second de novo missense variant was identified in USP7 [exon14, c.1406T>G p.(Val469Gly); NM_001286457]. Several frameshift-causing deletions and a nonsense mutation in USP7 have been reported in individuals with autism/intellectual disability/epilepsy. However, hypomyelination has not been described in individuals with USP7 loss-of-function mutations (Hao et al., 2015). Thus the USP7 variant was not considered an obvious explanation for the hypomyelination, and TMEM106B was submitted to GeneMatcher (Sobreira et al., 2015).
Trio exome analysis in Family 2 identified the same de novo c.754G>A mutation in TMEM106B, which was submitted to GeneMatcher independently. Additionally, review of TMEM106B variants in Trio-WGS data from a cohort of 10 undiagnosed families with hypomyelinating leukodystrophy resulted in the identification of Family 3, where both the index patient and her father carried the same c.754G>A variant. Inspection of the reads supporting each variant call revealed only 13 of 47 supported the c.754G>A variant in the father suggesting he may be mosaic for the variant. To confirm mosaicism, high depth sequencing of a PCR amplicon of this locus from each member of Family 3 was performed using an Illumina MiSeq giving a read depth >5000× per sample. The results showed that the mutant allele was present in 52% of reads from Patient 3, 0% of reads from her mother and 26% of reads from her father suggesting that approximately half of his (leucocyte) cells had the c.754G>A mutation. In Patient 4, the c.754G>A TMEM106B variant, occurring de novo in the proband, was identified directly from the results of trio exome sequencing; no further analyses were carried out.
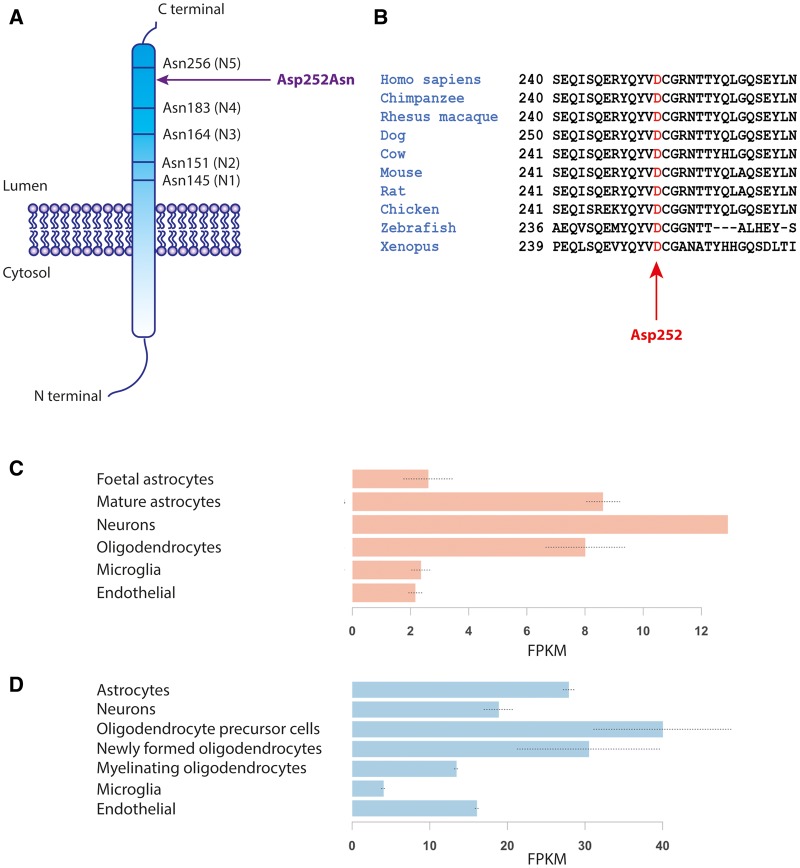
The TMEM106B variant c.754G>A p.(Asp252Asn) is not present in the dbSNP, ExAC or gnomAD databases. The mutation is predicted to be damaging by PolyPhen-2 (score: 0.999), MutationTaster (score 1.0) and CADD (score: 32). Asp252, located in the intraluminal part of the protein, is highly conserved among vertebrate species (Fig. 2A and B) and lies in close proximity to an asparagine residue (Asn256), which is a glycosylation site and essential for correct protein trafficking (Lang et al. 2012). TMEM106B is expressed in the CNS, including oligodendrocytes (Fig. 2C and D). In mice, expression is highest in oligodendrocyte precursors and early oligodendrocytes (Fig. 2D) compared to neurons and astrocytes.
Figure 2.
Protein characteristics. In A, TMEM106B is depicted with the luminal five glycosylated Asn residues. (B) High conservation of the mutated Asp residue in 10 different species. (C) TMEM106B expression in human oligodendrocytes and (D) in mouse oligodendrocytes including precursors (http://web.stanford.edu/group/barres_lab/).
Discussion
TMEM106B encodes a type 2 integral membrane glycoprotein that is predominantly located in the membranes of endosomes and lysosomes (Nicholson and Rademakers, 2016). Its exact function is not yet understood, and there are no structural models available. TMEM106B possesses five glycosylation sites, the two most distal of which (Asn183 and Asn256) are modified with complex glycans; both are essential for the correct intracellular trafficking to endosomes/lysosomes (Lang et al., 2012). The cytoplasmic N-terminal domain has been reported to interact with several proteins including CHMP2B, involved in recycling and degradation of receptor proteins, and microtubule-associated protein 6. To date, no proteins have been identified that interact with the luminal C-terminus of TMEM106B that contains the mutated residue Asp252 (Nicholson and Rademakers, 2016).
There is evidence that TMEM106B protein levels affect lysosomal function: high levels lead to larger and fewer lysosomes that are not able to produce normal intralysosomal acidity, leading to delayed endolysosomal-dependent degradation and increased lysosomal stress (Nicholson and Rademakers, 2016). Low TMEM106B levels cause clustering of small lysosomes near the nucleus and, in neurons, augmented retrograde lysosomal mobility and abridged dendritic branching, possibly due to disturbed lysosomal trafficking (Schwenk et al., 2014). Recent data from a knock-out mouse model show impaired lysosomal acidification and consequently reduced activity of selected lysosomal enzymes (Klein et al., 2017).
Several polymorphisms in TMEM106B, only one of which leads to an amino acid change, modify disease onset in patients with frontotemporal dementia (Nicholson and Rademakers, 2016), but at least one of these, rs1990622, only in combination with certain frontotemporal dementia mutations and low cognitive reserve (Premi et al. 2017). Levels of TMEM106B appear to be tightly regulated, and it has been reported that TMEM106B mRNA levels are elevated in the cortex of frontotemporal dementia with GRN mutations. The majority of these patients have neuronal intranuclear and/or cytoplasmic accumulations of ubiquitinated proteins, primarily TDP-43, consistent with impaired lysosomal protein degradation (Nicholson and Rademakers, 2016).
How mutated TMEM106B leads to hypomyelination, remains elusive. Our four patients all share the same missense mutation, and all have the classical clinical presentation of hypomyelination, with early-onset nystagmus and delayed motor development, albeit they are mildly affected compared to the largest hypomyelinating entity, Pelizaeus-Merzbacher disease. Patient 1 is the most severely affected individual in our small cohort; he was found to also have a potentially damaging mutation in USP7 (implicated in an intellectual disability syndrome) that may confound his clinical presentation. The clinical course in our patients is stable, even including the oldest patient now aged 37 years, suggesting that axonal degeneration, which is thought to underlie the slow decline at later ages in hypomyelination (Garbern et al., 2002) does not have a significant impact in TMEM106B-related hypomyelination. Remarkably, in another hypomyelinating leukodystrophy, hypomyelination with atrophy of basal ganglia and cerebellum (HABC), one single Asp to Asn missense mutation in TUBB4A was identified in the first patient group solved genetically (Simons et al., 2013) and later shown to cause the majority of HABC cases (Hamilton et al., 2014). A tight genotype–phenotype relationship is emerging for this disease, with cell-specific mutation effects (Duncan et al., 2017)—some patients do not have basal ganglia atrophy, others do not display hypomyelination (Hamilton et al., 2015a, b).
Hypomyelination is a heterogeneous group of disorders, with a multitude of different disease mechanisms, some still not fully understood. Mutations in structural myelin proteins such as PLP1 (Hobson and Garbern, 2012), the most abundant myelin protein, or oligodendrocyte differentiation factors as NKX6-2 (Chelban et al., 2017; Dorboz et al., 2017) are obvious causative candidates for hypomyelinating leukodystrophies. The role lysosomes play in hypomyelination, is not yet as clear. Interestingly, mutations in VPS11, which is involved in early to late endosome transport, have recently been shown to cause hypomyelinating leukodystrophy 12 (HLD12) (Edvardson et al., 2015), with subsequent studies presenting evidence of additional lysosomal storage dysfunction (Hörtnagel et al., 2016) although the hitherto described VPS11 patients are severely affected including microcephaly and epilepsy, unusual for primary hypomyelination disorders. We speculate that abnormal lysosomal/endosomal function might interfere with PLP1 trafficking through the late endosome/lysosome complex to the oligodendrocyte surface, which is essential during early myelination with its rapid increase of cell surface and incorporation of myelin proteins and lipids, especially PLP1, in myelin membranes (Saher and Stumpf, 2015), and that the TMEM106B mutation identified in our patients may impair this process. However, this remains hypothetical as functional data on neither wild-type nor mutated protein are available yet.
In conclusion, we provide evidence that an Asp to Asn substitution in TMEM106B leads to CNS hypomyelination with mild clinical course, which appears to affect oligodendrocytes more than neurons. This finding is essential for counselling of families, regarding both recurrence risk and prognosis. Additional research is needed to unravel the pathomechanism of this syndrome, which is likely to provide another link between lysosomes and myelination.
Acknowledgements
We are grateful to patients and families for their cooperation. We thank Carola van Berkel and the IMB sequencing facility for excellent technical assistance. Care4Rare Canada Consortium was funded by Genome Canada, the Canadian Institutes of Health Research, the Ontario Genomics Institute, Ontario Research Fund, Genome Quebec, and Children’s Hospital of Eastern Ontario Foundation.
Funding
This study was in part financed by the Australian National Health and Medical Research Council (NHMRC 1068278).
References
- Beaulieu C, Majewski J, Schwartzentruber J, Samuels M, Fernandez B, Bernier F, et al. FORGE Canada Consortium: outcomes of a 2-Year National Rare-Disease Gene-Discovery Project. Am J Hum Genet 2014; 94: 809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA 2016; 113: E1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelban V, Patel N, Vandrovcova J, Zanetti N, Lynch D, Ryten M, et al. Mutations in NKX6-2 cause progressive spastic ataxia and hypomyelination. Am J Hum Genet 2017; 100: 969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorboz I, Aiello C, Simons C, Stone RT, Niceta M, Elmaleh M, et al. Biallelic mutations in the homeodomain of NKX6-2 underlie a severe hypomyelinating leukodystrophy. Brain 2017; 140: 2550–6. [DOI] [PubMed] [Google Scholar]
- Duncan I, Bugiani M, Radcliff A, Moran J, Lopez-Anido C, Duong P, et al. A mutation in the Tubb4a gene leads to microtubule accumulation with hypomyelination and demyelination. Ann Neurol 2017; 81: 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardson S, Gerhard F, Jalas C, Lachmann J, Golan D, Saada A, et al. Hypomyelination and developmental delay associated with VPS11 mutation in Ashkenazi-Jewish patients. J Med Genet 2015; 52: 749–53. [DOI] [PubMed] [Google Scholar]
- Garbern JY, Yool DA, Moore GJ, Wilds IB, Faulk MW, Klugmann M, et al. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 2002; 125: 551–61. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Tétreault M, Dyment DA, Zou R, Kernohan K, Geraghty MT, et al. Concordance between whole-exome sequencing and clinical Sanger sequencing: implications for patient care. Mol Genet Genomic Med 2016; 4: 504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton EM, Polder E, Vanderver A, Naidu S, Schiffmann R, Fisher K, et al. Hypomyelination with atrophy of the basal ganglia and cerebellum: further delineation of the phenotype and genotype-phenotype correlation. Brain 2014; 137: 1921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton EM, Wolf NI, van der Knaap MS. Reply: TUBB4A novel mutation reinforces the genotype-phenotype correlation of hypomyelination with atrophy of the basal ganglia and cerebellum. Brain 2015a; 138: e328. [DOI] [PubMed] [Google Scholar]
- Hamilton EM, Wolf NI, van der Knaap MS. Reply: a novel TUBB4A mutation suggests that genotype-phenotype correlation of H-ABC syndrome needs to be revisited. Brain 2015b; 138: e371. [DOI] [PubMed] [Google Scholar]
- Hao YH, Fountain M, Fon Tacer K, Xia F, Bi W, Kang SH, et al. USP7 acts as a molecular rheostat to promote WASH-dependent endosomal protein recycling and is mutated in a human neurodevelopmental disorder. Mol Cell 2015; 59: 956–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson G, Garbern J. Pelizaeus-Merzbacher disease, Pelizaeus-Merzbacher-like disease 1, and related hypomyelinating disorders. Semin Neurol 2012; 32: 62–7. [DOI] [PubMed] [Google Scholar]
- Hörtnagel K, Krägeloh-Mann I, Bornemann A, Döcker M, Biskup S, Mayrhofer H, et al. The second report of a new hypomyelinating disease due to a defect in the VPS11 gene discloses a massive lysosomal involvement. J Inherit Metab Dis 2016; 39: 849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevelam SH, Steenweg ME, Srivastava S, Helman G, Naidu S, Schiffmann R, et al. Update on leukodystrophies: a historical perspective and adapted definition. Neuropediatrics 2016; 47: 349–54. [DOI] [PubMed] [Google Scholar]
- Klein Z, Takahashi H, Ma M, Stagi M, Zhou M, Lam T, et al. Loss of TMEM106B ameliorates lysosomal and frontotemporal dementia-related phenotypes in progranulin-deficient mice. Neuron 2017; 95: 281–96.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Fellerer K, Schwenk B, Kuhn PH, Kremmer E, Edbauer D, et al. Membrane orientation and subcellular localization of transmembrane protein 106B (TMEM106B), a major risk factor for frontotemporal lobar degeneration. J Biol Chem 2012; 287: 19355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Rademakers R. What we know about TMEM106B in neurodegeneration. Acta Neuropathol 2016; 132: 639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels PJ, Vanderver A, Bernard G, Wolf NI, Dreha-Kulczewksi SF, Deoni SC, et al. Hypomyelinating leukodystrophies: translational research progress and prospects. Ann Neurol 2014; 76: 5–19. [DOI] [PubMed] [Google Scholar]
- Premi E, Grassi M, van Swieten J, Galimberti D, Graff C, Masellis M, et al. Cognitive reserve and TMEM106B genotype modulate brain damage in presymptomatic frontotemporal dementia: a GENFI study. Brain 2017; 140: 1784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Stumpf S. Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim Biophys Acta 2015; 1851: 1083–94. [DOI] [PubMed] [Google Scholar]
- Schwenk B, Lang C, Hogl S, Tahirovic S, Orozco D, Rentzsch K, et al. The FTLD risk factor TMEM106B and MAP6 control dendritic trafficking of lysosomes. EMBO J 2014; 33: 450–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons C, Wolf NI, McNeil N, Caldovic L, Devaney JM, Takanohashi A, et al. A de novo mutation in the β-tubulin gene TUBB4A results in the leukoencephalopathy hypomyelination with atrophy of the basal ganglia and cerebellum. Am J Hum Genet 2013; 92: 767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat 2015; 36: 928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenweg ME, Vanderver A, Blaser S, Bizzi A, de Koning TJ, Mancini GM, et al. Magnetic resonance imaging pattern recognition in hypomyelinating disorders. Brain 2010; 133: 2971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf NI, Salomons GS, Rodenburg RJ, Pouwels PJ, Schieving JH, Derks TG, et al. Mutations in RARS cause hypomyelination. Ann Neurol 2014; 76: 134–9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014; 34: 11929–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 2016; 89: 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]