Abstract
Hepatitis B cirrhosis is caused by liver cell necrosis, residual liver cell nodular regeneration, connective tissue hyperplasia and fiber formation, which frequently leads to adrenal insufficiency. Previous reports have demonstrated that human fibroblast growth factor (hFGF)-21 is a multifunctional protein that exhibits potential therapeutic value for metabolic diseases. The present study investigated the diagnostic value of hFGF-21 and analyzed the potential molecular mechanism in the progression of hepatitis B cirrhosis combined with adrenal insufficiency. Characteristics of cellular immunity and humoral immunity were analyzed in patients with hepatitis B cirrhosis combined with adrenal insufficiency (PhbA). Results demonstrated that expression levels of hFGF-21 were downregulated in plasma and liver cells isolated from clinical specimens. Plasma concentration levels of hFGF-21 were upregulated in prognostic PhbA. In vitro assays indicated that hFGF-21 treatment decreased the continuous deposition of extracellular matrix and reactive oxygen species in liver cells isolated from clinical specimens. Results also demonstrated that hFGF-21 treatment downregulated inflammatory cytokines. It was observed that hFGF-21 treatment downregulated nuclear factor (NF)-κB and Kruppel-like factor 6. Notably, transforming growth factor (TGF)-β, platelet-derived growth factor and epidermal growth factor levels were improved by hFGF-21 treatment. In conclusion, these results indicated that hFGF-21 inhibits inflammation by regulation of the NF-κB-mediated TGF-β signaling pathway, which may serve as a predictor and prognostic factor in PhbA.
Keywords: hepatitis B cirrhosis, adrenal insufficiency, human fibroblast growth factor-21, inflammation, nuclear factor-κB, transforming growth factor-β
Introduction
Liver cirrhosis is a kind of metabolic disease that is reversible and may be treated when it is identified at an early stage (1). Liver cirrhosis is divided into hepatitis B cirrhosis and hepatitis C cirrhosis according to pathogenesis in clinical research (2,3). Previous reports have indicated risks for alcoholic liver cirrhosis, and regression of fibrosis/cirrhosis by glycine propionyl-l-carnitine treatment has been also investigated in D-Galactosamine-induced chronic liver damage (4,5). The main pathogenesis of liver cirrhosis is progressive fibrosis (6). A comprehensive review has evaluated the management of patients with autoimmune hepatitis with decompensated cirrhosis (7). Furthermore, a study by Wang et al (7) suggested that chronic hepatitis B and hepatitis B virus-related cirrhosis contributes to other metabolic syndromes, which further influences renal function and increases the risk of renal damage, hypophosphatemia, and adrenal insufficiency (8–10).
Fibroblast growth factor (FGF)-21 is an atypical member of the FGF family, as well as a multifunctional protein predominantly secreted by adipose tissue, the pancreas and liver, which has been regarded as an efficient polypeptide for the treatment of metabolic disorders (11,12). Previous research has reported that metabolic hormone effects of FGF-21 on energy metabolism were essential for human vascular endothelial cells (13,14). A study by Wang et al (15) indicated that FGF-21 is positively associated with atrial fibrosis in patients with atrial fibrillation with rheumatic heart disease. FGF-21 has been reported as a novel liver safeguard (16), as well as being identified as a momentous controller and regulator of glucose and lipid metabolism, and long-term energy balance (17,18). Notably, transplantation of basic FGF-pretreated adipose tissue-derived stromal cells enhances regression of liver fibrosis in mice (19). However, the molecular mechanisms of liver fibrosis associated with FGF-21 are not well understood or clearly elaborated.
Chronic inflammation associated with hepatitis C virus infection contributes to hepatic transforming growth factor (TGF)-β signaling that promotes cirrhosis and hepatocellular carcinoma (20). Research has also indicated protective effects of allopurinol against acute liver damage and cirrhosis induced by carbon tetrachloride through modulation of nuclear factor (NF)-κB, cytokine production and oxidative stress (21). The present study analyzed the potential diagnostic value of human (h)FGF-21 and investigated the hFGF-21-mediated signaling pathway of hepatitis B cirrhosis combined with adrenal insufficiency in liver cells. The present data indicated that plasma concentration levels of hFGF-21 were downregulated in patients with hepatitis B cirrhosis combined with adrenal insufficiency (PhbA), which may be associated with the NF-κB-mediated TGF-β signaling pathway.
Patients and methods
Patients and healthy volunteers
A total of 186 PhbA (90 male and 96 female) and 68 healthy volunteers (35 male and 33 female) were recruited in the present clinical investigation following presentation to Beijing You'an Hospital, Capital Medical University (Beijing, China) between May 2014 and October 2015. The mean age was 38.5 (16.4–62.5 years) and 34.2 (22.5–46.2 years) in PhbA and healthy volunteers, respectively. A total of 10 patients [male/female, 5/5; 34.2 years old (22.5–46.2)] who had recovered from hepatitis B cirrhosis combined with adrenal insufficiency (PPhbA) were also recruited to the present study. Patients with diabetes mellitus and digestive tract diseases were excluded from the present study. Patients were diagnosed with PhbA as described previously (22). All participants were required to provide written informed consent prior to initiation of the study. The present study was approved by the Ethics Committee of Beijing You'an Hospital, Capital Medical University (Beijing, China).
Cell culture
Liver and renal epithelial cells were obtained from PhbA using a biopsy needle as previously described (23). Cells were cultured in minimal essential medium supplemented with 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Liver and renal epithelial cells were incubated with hFGF-21 (1.0 mg/ml, Sigma-Aldrich; Merck KGaA) for 24 h to analyze purpose protein expression with non-treated cells used as controls. The cells were cultured in a humidified atmosphere containing 5% of CO2 at 37°C.
ELISA
Serum levels of hFGF-21 (cat. no. DF2100), tumor necrosis factor (TNF)-α (cat. no. DTA00C), interleukin (IL)-1β (cat. no. DLB50), IL-6 (cat. no. D6050) and IL-8 (cat. no. D8000C) were detected in PhbA and healthy volunteers using ELISA kits (IBL International GmbH, Hamburg, Germany), according to the manufacturer's protocol. The serum levels of hFGF-21 were also analyzed between PhbA and PPhbA on day 30 following treatments. The serum concentration levels of hFGF-21, TNF-α, IL-1β, IL-6 and IL-8 were measured by an enzyme microplate reader at 450 nm.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from liver and renal epithelial cells using an RNeasy Mini kit (Qiagen Sciences, Inc., Gaithersburg, MD, USA), according to the protocol provided by the manufacturer. RNA was reversed transcribed using a PrimeScript RT Master Mix kit (Takara Bio, Inc., Otsu, Japan). All forward and reverse primers were synthesized by Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA; Table I). For amplification diluted cDNA was combined with a reaction mixture containing SYBR-Green PCR Core Reagents (cat. no. 4304886; Applied Biosystems; Thermo Fisher Scientific, Inc.). Relative mRNA expression levels were calculated using the 2−ΔΔCq method (24). PCR cycling was performed under the following conditions: 94°C for 30 sec and 45 cycles of 95°C for 5 sec, 57°C for 10 sec and 72°C for 10 sec. The results were expressed as the n-fold of the control.
Table I.
Primer sequences used in the study for polymerase chain reaction.
| Sequences (5′-3′) | ||
|---|---|---|
| Gene name | Reverse | Forward |
| FGF-21 | CTGCTGGGGGTCTACCAAG | CTGCGCCTACCACTGTTCC |
| TNF-α | TCCAGACTTCCTTGAGACA | GGCGATTACAGACACAACT |
| IL-6 | CCACACAGACAGCCACTCA | CATCCATCTTTTTCAGCCATCT |
| IL-1β | GGCTGCTTCCAAACCTTTGA | GAAGACACGGATTCCATGGT |
| IL-8 | TACTCCAAACCTTTCCACCC | AACTTCTCCACAACCCTCTG |
| β-actin | CGGAGTCAACGGATTTGGTC | AGCCTTCTCCATGGTCGTGA |
FGF, fibroblast growth factor; TNF, tumor necrosis factor; IL, interleukin.
Western blot analysis
Liver and renal epithelial cells from PhbA were incubated with hFGF-21 (2 mg/ml) for 12 h at 37°C. Cells not treated with hFGF-21 were used as the controls. Cells were homogenized in a lysate buffer containing protease-inhibitor (P3480; Sigma-Aldrich; Merck KGaA) and were centrifuged at 4,000 × g at 4°C for 10 min. Western blot analysis was subsequently performed as previously described (25). Protein concentration was measured by a BCA protein assay kit (Thermo Fisher Scientific, Inc.). Protein samples (20 µg/lane) were resolved by 15% SDS-PAGE and then transferred to polyvinylidene fluoride membranes (Merck KGaA). Monoclonal rabbit anti-human epidermal growth factor (EGF), platelet-derived growth factor (PDGF; ab32570), hFGF-21 (ab64857), TNF-α (ab6671), IL-1β (ab2105), IL-6 (ab6672) and IL-8 (ab7747), TGF-β (ab31013), NF-κB (ab32360) and Kruppel-like factor 6 (KLF6; ab135783), extracellular matrix (ECM; ab28666), reactive oxygen species (ROS; ab5512) and β-actin (ab8227) antibodies (all 1:200; Abcam, Shanghai, China) were incubated with protein samples for 1 h at room temperature. After blocking with 1% bovine serum albumin (Sigma-Aldrich; Merck KGaA), followed by incubation with horseradish peroxidase-conjugated polyclonal anti-rabbit immunoglobulin G antibodies (1:10,000; PV-6001; OriGene Technologies, Inc., Beijing, China) for 1 h at room temperature. Signals were visualized by chemiluminescence detection (Z370398; Sigma-Aldrich; Merck KGaA). Densitometric quantification of the immunoblot data was performed using Quantity-One software (version 3.24; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Gene knockdown with small interfering RNA (siRNA)
Liver cells (1×104/well) were incubated with hFGF-21 (2 mg/ml) for 5 days at 37°C in a six-well plate. To silence NF-κB gene expression, liver cells were transfected with 100 pmol siRNA-NF-κB sense, 5-′CUUGGUCAAUCUCAAGAUAtt-3′ and antisense, 5′-UAUCUUGAGAUUGACCAAGca-3′; with siRNA-vector sense, 5′-CGGACAAACGGCUCACUUUtt-3′ and antisense, 5′-AAAGUGAGCCGUUUGUCCGgg-3′ as a control (Applied Biosystems; Thermo Fisher Scientific, Inc.) using a Cell Line Nucleofector kit L (Lonza Group, Ltd., Basel, Switzerland), according to the manufacturer's protocol (26). The cells were analyzed 48 h following transfection.
Flow cytometry
The following antibodies were used: FITC-conjugated anti-CD11b (cat. no. 557686; clone M1/70; BD Biosciences, Franklin Lakes, NJ, USA), allophycocyanin-conjugated anti-Ly-6B.2 (cat. no. NBP2-13077APC; clone 7/4; Bio-Rad Laboratories, Inc.), FITC-conjugated anti-CD4 (cat. no. MCA2649; clone RM 4–5; eBioscience; Thermo Fisher Scientific, Inc.), PercP-conjugated anti-CD8a (cat. no. 555369; clone, 53–6.7; BD Biosciences), PE-conjugated anti-CD45R/B220 (cat. no. A15835; clone RA3-6B2; eBioscience; Thermo Fisher Scientific, Inc.) for 12 h at 4°C after blocking with 1% bovine serum albumin for 2 h at 37°C. All antibodies were used at a dilution of 1:100. B-lymphocytes were identified as CD11bhiLy6G-7/4hi/lo and macrophagocytes were identified as CD11chi. Cells were washed three times with 0.1% tris-buffered saline-Tween-20. Cells were analyzed using a flow cytometer (LSR II; BD Biosciences). Data was analyzed using BD FACSDiva™ software version 8.0.1 (BD Biosciences).
Statistical analysis
All data were presented as the mean ± standard deviation of triplicate independent trials in each experiment. All data were analyzed using SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA). Statistical differences between groups were assessed using analysis of variance with the post hoc Dunnett's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Analysis of expression levels of hFGF-21 in PhbA
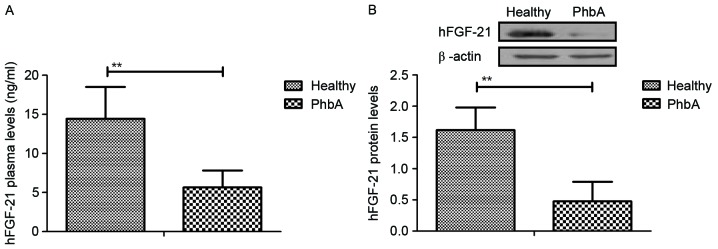
Expression levels of hFGF-21 were analyzed in serum and cellular units in PhbA. Characteristics of patients were summarized in Table II. Plasma concentration levels of hFGF-21 were significantly downregulated in PhbA compared with the those in healthy volunteers (P<0.01) (Fig. 1A). Western blotting demonstrated that hFGF-21 protein expression levels were significantly downregulated in liver cells isolated from PhbA compared to those from healthy volunteers (P<0.01) (Fig. 1B). These outcomes suggested that hFGF-21 is downregulated in PhbA. Healthy.
Table II.
Characteristics of patients and healthy volunteers.
| Characteristics | Patients | Healthy volunteers |
|---|---|---|
| Number | 186 | 68 |
| Age, years (range) | 16.4–62.5 | 22.5–46.2 |
| Sex, n | ||
| Male | 70 | 30 |
| Female | 116 | 38 |
Figure 1.
Analysis of changes to hFGF-21 levels in PhbA. (A) Plasma concentration levels of hFGF-21 in PhbA and healthy volunteers. (B) Expression levels of hFGF-21 in liver cells isolated from clinical patients and healthy volunteers. **P<0.01. hFGF-21, human fibroblast growth factor-21; PhbA, patients with hepatitis B cirrhosis combined with adrenal insufficiency.
Analysis of expression levels of hFGF-21 in PhbA and PPhbA
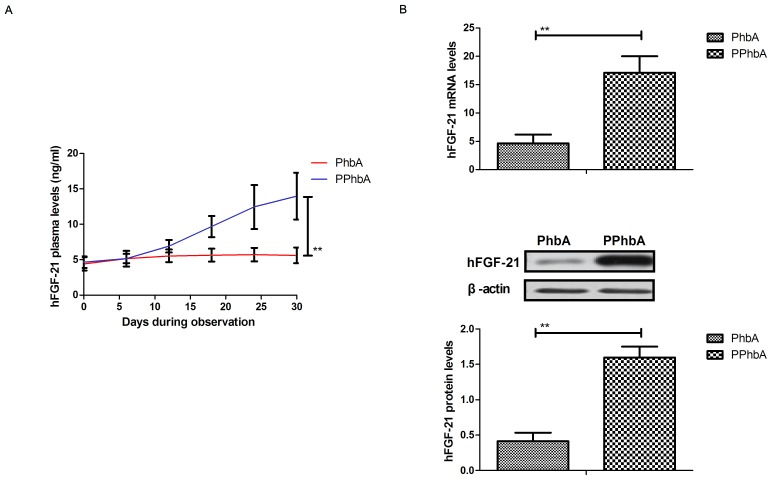
Expression levels of hFGF-21 were detected in PPhbA. As demonstrated in Fig. 2A, plasma concentration levels of hFGF-21 were significantly increased in PPhbA compared with those in PhbA on day 30 (P<0.01). Cellular hFGF-21 mRNA and protein expression levels in liver cells isolated from PPhbA were significantly upregulated compared with those isolated from PhbA (P<0.01) (Fig. 2B). These results indicated that hFGF-21 may be a prognostic indicator in PhbA.
Figure 2.
Analysis of hFGF-21 expression levels in PPhbA and PhbA. (A) Plasma concentration levels of hFGF-21 between PPhbA and PhbA on day 30. (B) mRNA and protein expression levels of hFGF-21 in liver cells isolated from PPhbA and PhbA. **P<0.01. hFGF-21, human fibroblast growth factor-21; PPhbA, prognostic patients with hepatitis B cirrhosis combined with adrenal insufficiency; PhbA, patients with hepatitis B cirrhosis combined with adrenal insufficiency; PPhbA, patients who recovered form hepatitis B cirrhosis combined with adrenal insufficiency.
Association of hFGF-21 plasma concentration with cellular immunity and humoral immunity in PhbA
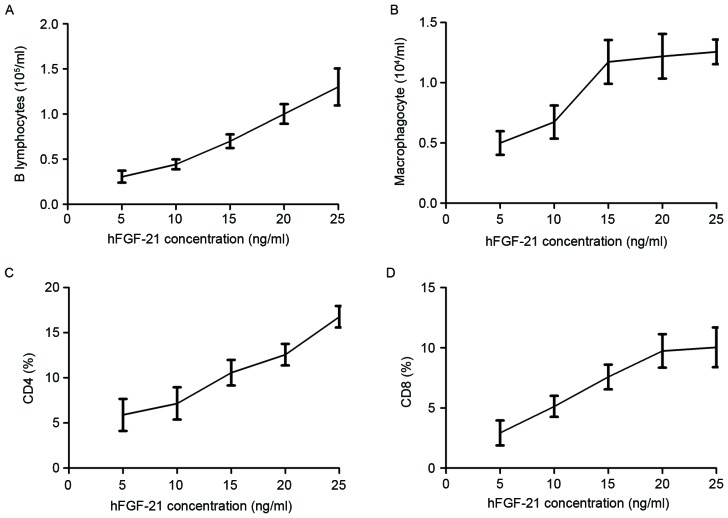
Characteristics of cellular immunity and humoral immunity were investigated in clinical PhbA prior and post treatments. Results demonstrated that the B lymphocyte level increased as the hFGF-21 plasma concentration increased during treatment (Fig. 3A). Macrophagocyte concentration levels were demonstrated to be positively associated with hFGF-21 plasma concentration during treatment (Fig. 3B). Results indicated that the percentage of cluster of differentiation (CD)4+ and CD8+ cells increased in serum as the hFGF-21 plasma concentration increased during treatment (Fig. 3C and D). These results indicated that hFGF-21 plasma concentration may be associated with cellular immunity and humoral immunity in PhbA during treatment.
Figure 3.
Association of hFGF-21 plasma concentration with cellular immunity and humoral immunity in clinical patients. Relationship between concentration levels of hFGF-21 and (A) B lymphocyte level and (B) macrophagocyte level in patients during treatment. Association of hFGF-21 plasma concentration with percentage of (C) CD4+ and (D) CD8+ cells in serum in patients during treatment. hFGF-21, human fibroblast growth factor-21; CD, cluster of differentiation.
Effects of hFGF-21 on inflammatory cytokine expression levels in liver cells isolated from clinical patients
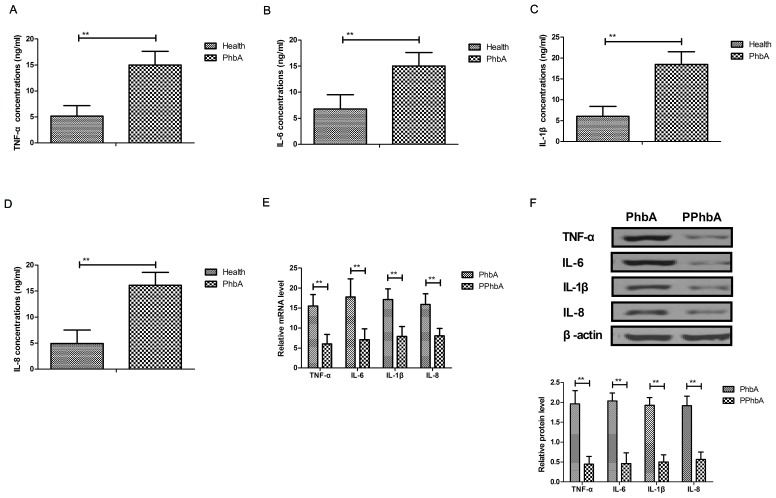
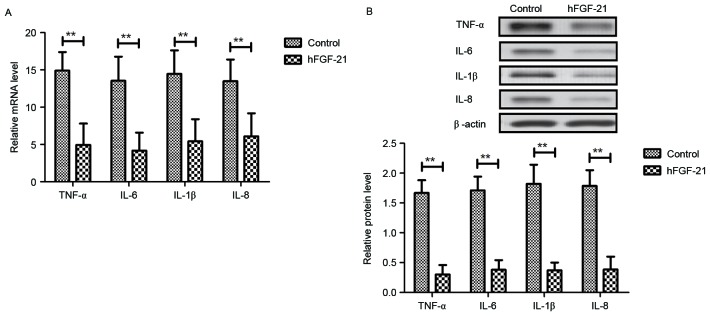
Inflammatory cytokine levels were investigated in PhbA. As demonstrated in Fig. 4A-D, plasma concentration levels of TNF-α, IL-6, IL-1β and IL-8 were significantly upregulated in PhbA compared with those in healthy volunteers (P<0.01). Western blot analysis and RT-qPCR indicated that protein and mRNA expression levels of TNF-α, IL-6, IL-1β and IL-8 were significantly downregulated in the PPhbA groups compared with PhbA groups (P<0.01) (Fig. 4E and F). These results indicated that hFGF-21 treatment decreases inflammatory cytokine expression levels in liver cells isolated from clinical patients.
Figure 4.
Effects of hFGF-21 on inflammatory cytokine expression levels in liver cells isolated form clinical patients. Plasma concentration levels of (A) TNF-α, (B) IL-6, (C) IL-1β and (D) IL-8 in PhbA and healthy volunteers. (E) mRNA and (F) protein expression levels of inflammatory cytokines in cells treated with hFGF-21 isolated from patients and the PPhbA control group. **P<0.01. hFGF-21, human fibroblast growth factor-21; PhbA, patients with hepatitis B cirrhosis combined with adrenal insufficiency; PPhbA, patients who recovered form hepatitis B cirrhosis combined with adrenal insufficiency; TNF, tumor necrosis factor; IL, interleukin.
Effects of hFGF-21 on inflammatory cytokine expression levels in renal epithelial cells isolated from clinical patients
Inflammatory cytokines in renal epithelial cells isolated from clinical patients were analyzed following treatment with hFGF-21. As demonstrated in Fig. 5A and B, gene and protein expression levels of TNF-α, IL-6, IL-1β and IL-8 were significantly downregulated by hFGF-21 treatment in renal epithelial cells isolated from clinical patients compared to the levels in the control cells (P<0.01). These outcomes indicated that hFGF-21 suppresses inflammatory cytokine expression in renal epithelial cells isolated from clinical patients.
Figure 5.
Effects of hFGF-21 on inflammatory cytokine expression levels in renal epithelial cells isolated from clinical patients. (A) Gene expression levels of TNF-α, IL-6, IL-1β and IL-8 in renal epithelial cells isolated from clinical patients treated with hFGF-21 or not treated with hFGF-21. (B) Protein expression levels of TNF-α, IL-6, IL-1β and IL-8 in renal epithelial cells isolated from clinical patients treated with hFGF-21 or not treated with hFGF-21. **P<0.01. hFGF-21, human fibroblast growth factor-21; TNF, tumor necrosis factor; IL, interleukin.
hFGF-21 regulates inflammatory cytokines through downregulation of the NF-κB-mediated TGF-β signaling pathway
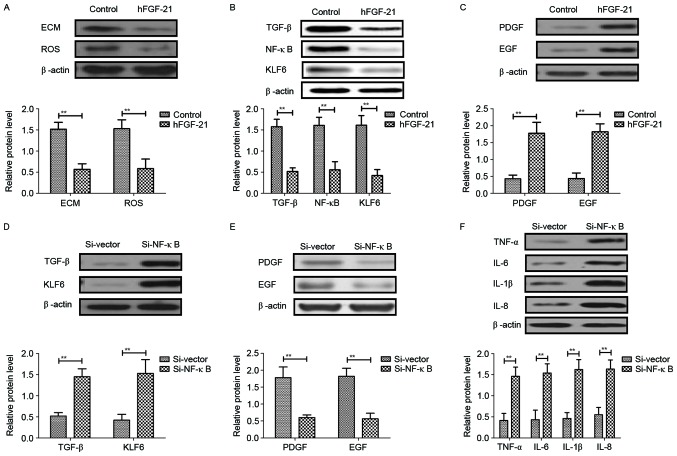
In order to analyze the potential mechanism mediated by hFGF-21, the NF-κB-mediated TGF-β signal pathway was investigated in liver cells isolated from clinical patients. Results demonstrated that hFGF-21 treatment significantly inhibited deposition of ECM and ROS expression levels in liver cells compared with the levels in control cells (P<0.01) (Fig. 6A). Western blotting indicated that expression levels of TGF-β, NF-κB and KLF6 were significantly downregulated and PDGF and EGF expression levels were significantly upregulated by hFGF-21 treatment in liver cells compared with the levels in the control cells (P<0.01) (Fig. 6B and C). Knockdown of NF-κB with siRNA-NF-κB significantly inhibited the hFGF-21-induced suppression of TGF-β and KLF6 expression and hFGF-21-promoted PDGF and EGF expression levels in liver cells compared with the levels in cells transfected with siRNA-vector (P<0.01; Fig. 6D and E). Findings also indicated that knockdown of NF-κB significantly inhibited the suppression of protein expression levels of TNF-α, IL-6, IL-1β and IL-8 in liver cells induced by hFGF-21 compared with the levels in cells transfected with siRNA-vector (P<0.01; Fig. 6F). These results indicated that hFGF-21 regulates inflammatory cytokines through downregulation of the NF-κB-mediated TGF-β signaling pathway.
Figure 6.
hFGF-21 regulates inflammatory cytokines through downregulation of the NF-κB-mediated TGF-β signaling pathway. (A) Effects of hFGF-21 on deposition of ECM and ROS expression levels in liver cells. (B) Effects of hFGF-21 on expression levels of TGF-β, NF-κB and KLF6 in liver cells. (C) Effects of hFGF-21 on expression levels of PDGF and EGF in liver cells. (D) Knockdown of NF-κB with Si-NF-κB increases TGF-β and KLF6 expression in liver cells. (E) Knockdown of NF-κB with Si-NF-κB suppresses PDGF and EGF expression in liver cells. (F) Effects of Si-NF-κB on protein expression levels of TNF-α, IL-6, IL-1β and IL-8 in liver cells. **P<0.01. hFGF-21, human fibroblast growth factor-21; NF, nuclear factor; TGF, transforming growth factor; ECM, extracellular matrix; ROS, reactive oxygen species; KLF6, Kruppel-like factor 6; PDGF, platelet-derived growth factor; EGF, epidermal growth factor; TNF, tumor necrosis factor; IL, interleukin; Si, small interfering RNA.
Discussion
Hepatitis B-induced liver cirrhosis poses a great threat to health and frequently leads to adrenal insufficiency that further affects the endocrine system and disturbs liver metabolism (27,28). Pathophysiologic and clinical evidences have suggested that inflammation is associated with hepatitis B-induced liver cirrhosis and inflammatory cytokines, including TNF and IL-1, which may be potential target agents in decompensated cirrhosis (29). Research has also indicated that FGF is altered and molecular signaling pathways are regulated by attenuating the expression of TGF-β (30). The present study detected hFGF-21 serum concentration and expression levels in PhbA. Outcomes indicated that hFGF-21 suppressed inflammatory cytokine levels in liver cells isolated from clinical specimens through regulation of the NF-κB-mediated TGF-β signaling pathway. These findings suggested that hFGF-21 may serve as a predictor and prognostic factor in PhbA.
Beneficial effects of inhibition of oxidative stress and inflammation have been reported in hepatitis C virus-positive patients with liver cirrhosis and findings indicate that inflammation inhibition influences microinflammation and the metabolism of iron in hepatitis C virus-positive patients with liver cirrhosis, which subsequently appeared to reduce the production of oxidative stress, possibly leading to a decrease in the occurrence of hepatocellular carcinoma (31). A study by Prystupa et al (32) indicated that proinflammatory cytokines (IL-1β and IL-6) and hepatocyte growth factor were upregulated in patients with alcoholic liver cirrhosis. Additionally, the levels of ghrelin, leptin, TNF-α and IL-8 in liver cirrhosis were increased following hepatitis B and hepatitis D virus infection (33,34). The present findings suggested that hFGF-21 treatment inhibits mRNA and protein expression levels of TNF-α, IL-6, IL-1β and IL-8 in liver cells. Inhibitory effects of hFGF-21 were demonstrated in the present study, indicating that hFGF-21 regulates inflammatory cytokines by downregulation of the NF-κB-mediated TGF-β signaling pathway.
Target-specific systemic delivery of siRNA for TGF-β has been proposed for the treatment of liver cirrhosis and has demonstrated feasible therapeutic effects on liver cirrhosis by reduction of nodule formation, collagen content and hepatic stellate cell numbers (35). A study by Chávez et al (36) suggested that Sulfasalazine prevents the increase in TGF-β, cyclooxygenase-2 and NF-κB translocation and fibrosis in carbon tetrachloride-induced liver cirrhosis in rats. A study by Aldaba-Muruato et al (21) indicated that modulation of NF-κB, cytokine production and oxidative stress may protect the liver against allopurinol-induced acute liver damage and cirrhosis induced by carbon tetrachloride. Therefore, we assumed that the regulation of inflammatory cytokines by hFGF-21 may be associated with the NF-κB signaling pathway. The present results supported this hypothesis and the findings suggested that hFGF-21 treatment suppresses ECM and ROS expression levels and downregulates TGF-β, NF-κB and KLF6 expression levels in liver cells.
Previous research has demonstrated that FGF-21 resulted in insulin resistance by inhibiting the activation of NF-κB (37). FGF-21 also served an endocrine hormone role in blocking somatic growth, leading to growth hormone resistance (38). Furthermore, FGF-21 has been reported to be associated with lipid metabolism and the incidence of cardiovascular disease (39), as well as various human diseases and metabolic syndromes, including geriatric obesity, type 2 diabetes mellitus and congenital hypothyroidism (40–42). In the present study, changes of hFGF-21 plasma concentration levels in PhbA were analyzed. Outcomes suggested that hFGF-21 is downregulated in clinical patients suffering with hepatitis B cirrhosis combined with adrenal insufficiency. Therefore, hFGF-21 may serve as a predictor and prognostic factor for hepatitis B cirrhosis combined with adrenal insufficiency.
In conclusion, the present study indicated that hFGF-21 improved inflammatory cytokine expression levels in renal epithelial cells and liver cells isolated from clinical patients. The results demonstrated the potential molecular mechanism mediated by hFGF-21 in liver cells in the progression of hepatitis B cirrhosis combined with adrenal insufficiency. The present study suggested that hFGF-21 administration downregulates inflammatory cytokine levels through the NF-κB-mediated TGF-β signaling pathway. Changes in hFGF-21 plasma concentration prior and post treatment were observed for PhbA, suggesting that hFGF-21 possesses the potential to act as an alternative predictor and prognostic indicator for the evaluation of prognosis of hepatitis B cirrhosis combined with adrenal insufficiency.
References
- 1.Acharya UR, Raghavendra U, Fujita H, Hagiwara Y, Koh JE, Jen Hong T, Sudarshan VK, Vijayananthan A, Yeong CH, Gudigar A, Ng KH. Automated characterization of fatty liver disease and cirrhosis using curvelet transform and entropy features extracted from ultrasound images. Comput Biol Med. 2016;79:250–258. doi: 10.1016/j.compbiomed.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Dezső K, Rókusz A, Bugyik E, Szücs A, Szuák A, Dorogi B, Kiss M, Nemeskéri Á, Nagy P, Paku S. Human liver regeneration in advanced cirrhosis is organized by the portal tree. J Hepatol. 2017;66:778–786. doi: 10.1016/j.jhep.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Aguirre Valadez JM, Rivera-Espinosa L, Méndez-Guerrero O, Chávez-Pacheco JL, García Juárez I, Torre A. Intestinal permeability in a patient with liver cirrhosis. Ther Clin Risk Manag. 2016;12:1729–1748. doi: 10.2147/TCRM.S115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganai AA, Ganaie IA, Verma N, Farooqi H. Regression of fibrosis/cirrhosis by Glycine propionyl-l-carnitine treatment in d-Galactosamine induced chronic liver damage. Chem Biol Interact. 2016;260:117–128. doi: 10.1016/j.cbi.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Askgaard G, Leon DA, Kjaer MS, Deleuran T, Gerds TA, Tolstrup JS. Risk for alcoholic liver cirrhosis after an initial hospital contact with alcohol problems: A nationwide prospective cohort study. Hepatology. 2017;65:929–937. doi: 10.1002/hep.28943. [DOI] [PubMed] [Google Scholar]
- 6.Chiriac S, Stanciu C, Trifan A. Corticosteroid treatment in the setting of decompensated liver cirrhosis with relative adrenal insufficiency: A case report and a brief review of the literature. Rev Med Chir Soc Med Nat Iasi. 2016;120:288–292. [PubMed] [Google Scholar]
- 7.Wang Z, Sheng L, Yang Y, Yang F, Xiao X, Hua J, Guo C, Wei Y, Tang R, Miao Q, et al. The management of autoimmune hepatitis patients with decompensated cirrhosis: Real-world experience and a comprehensive review. Clin Rev Allergy Immunol. 2017;52:424–435. doi: 10.1007/s12016-016-8583-2. [DOI] [PubMed] [Google Scholar]
- 8.Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168–175. doi: 10.1016/j.intimp.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Fialla AD, Israelsen M, Hamberg O, Krag A, Gluud LL. Nutritional therapy in cirrhosis or alcoholic hepatitis: A systematic review and meta-analysis. Liver Int. 2015;35:2072–2078. doi: 10.1111/liv.12798. [DOI] [PubMed] [Google Scholar]
- 10.Manne V, Akhtar E, Saab S. Cirrhosis regression in patients with viral hepatitis B and C: A systematic review. J Clin Gastroenterol. 2014;48:e76–e84. doi: 10.1097/MCG.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 11.Eto K. FGF-21, a newcomer in the field of hypertension research. J Hum Hypertens. 2013;27:343–344. doi: 10.1038/jhh.2012.68. [DOI] [PubMed] [Google Scholar]
- 12.Reinehr T, Woelfle J, Wunsch R, Roth CL. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: A longitudinal analysis. J Clin Endocrinol Metab. 2012;97:2143–2150. doi: 10.1210/jc.2012-1221. [DOI] [PubMed] [Google Scholar]
- 13.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML, Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150:4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Yi X, Li X, Jiang X. Fibroblast growth factor-21 is positively associated with atrial fibrosis in atrial fibrillation patients with rheumatic heart disease. Int J Clin Exp Pathol. 2015;8:14901–14908. [PMC free article] [PubMed] [Google Scholar]
- 16.Cariello M, Moschetta A. Fibroblast growth factor 21: A new liver safeguard. Hepatology. 2014;60:792–794. doi: 10.1002/hep.27147. [DOI] [PubMed] [Google Scholar]
- 17.Suomalainen A, Elo JM, Pietiläinen KH, Hakonen AH, Sevastianova K, Korpela M, Isohanni P, Marjavaara SK, Tyni T, Kiuru-Enari S, et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: A diagnostic study. Lancet Neurol. 2011;10:806–818. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, Xiao J, Wang X, Feng W, Li X. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One. 2010;5:e15534. doi: 10.1371/journal.pone.0015534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamada Y, Yoshida Y, Saji Y, Fukushima J, Tamura S, Kiso S, Hayashi N. Transplantation of basic fibroblast growth factor-pretreated adipose tissue-derived stromal cells enhances regression of liver fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G157–G167. doi: 10.1152/ajpgi.90463.2008. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M, Fujisawa J, et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46:48–57. doi: 10.1002/hep.21672. [DOI] [PubMed] [Google Scholar]
- 21.Aldaba-Muruato LR, Moreno MG, Shibayama M, Tsutsumi V, Muriel P. Protective effects of allopurinol against acute liver damage and cirrhosis induced by carbon tetrachloride: Modulation of NF-κB, cytokine production and oxidative stress. Biochim Biophys Acta. 2012;1820:65–75. doi: 10.1016/j.bbagen.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 22.de Lédinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, Dhumeaux D, Beaugrand M. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–179. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 23.Iguchi T, Hiraki T, Matsui Y, Fujiwara H, Sakurai J, Masaoka Y, Gobara H, Kanazawa S. CT fluoroscopy-guided renal tumour cutting needle biopsy: Retrospective evaluation of diagnostic yield, safety, and risk factors for diagnostic failure. Eur Radiol. 2018;28:283–290. doi: 10.1007/s00330-017-4969-7. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Almeida Mde A, Pizzini CV, Damasceno LS, Muniz Mde M, Almeida-Paes R, Peralta RH, Peralta JM, Oliveira Rde V, Vizzoni AG, de Andrade CL, Zancopé-Oliveira RM. Validation of western blot for Histoplasma capsulatum antibody detection assay. BMC Infect Dis. 2016;16:87. doi: 10.1186/s12879-016-1427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattheolabakis G, Ling D, Ahmad G, Amiji M. Enhanced anti-tumor efficacy of lipid-modified platinum derivatives in combination with survivin silencing siRNA in resistant non-small cell lung cancer. Pharm Res. 2016;33:2943–2953. doi: 10.1007/s11095-016-2016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao S, Chen H, Wang Y, Zhu J, Tan J, Gao J. Splenectomy versus partial splenic embolization for massive splenomegaly secondary to hepatitis B-related liver cirrhosis: A case-control study. Gastroenterol Res Pract. 2016;2016:3471626. doi: 10.1155/2016/3471626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maan R, van Tilborg M, Deterding K, Ramji A, van der Meer AJ, Wong F, Fung S, Sherman M, Manns MP, Cornberg M, et al. Safety and effectiveness of direct-acting antiviral agents for treatment of patients with chronic hepatitis C virus infection and cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1821–1830.e6. doi: 10.1016/j.cgh.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Artigas A, Wernerman J, Arroyo V, Vincent JL, Levy M. Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care. 2016;33:62–70. doi: 10.1016/j.jcrc.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Shi C, Meng X, Zhang K, Li X, Wang C, Xiang Z, Hu K, Han X. Inhibition of Wnt/β-catenin signaling suppresses bleomycin-induced pulmonary fibrosis by attenuating the expression of TGF-β1 and FGF-2. Exp Mol Pathol. 2016;101:22–30. doi: 10.1016/j.yexmp.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohno T, Tanaka Y, Sugauchi F, Orito E, Hasegawa I, Nukaya H, Kato A, Matunaga S, Endo M, Tanaka Y, et al. Suppressive effect of oral administration of branched-chain amino acid granules on oxidative stress and inflammation in HCV-positive patients with liver cirrhosis. Hepatol Res. 2008;38:683–688. doi: 10.1111/j.1872-034X.2008.00319.x. [DOI] [PubMed] [Google Scholar]
- 32.Prystupa A, Kiciński P, Sak J, Boguszewska-Czubara A, Toruń-Jurkowska A, Załuska W. Proinflammatory cytokines (IL-1α, IL-6) and hepatocyte growth factor in patients with alcoholic liver cirrhosis. Gastroenterol Res Pract. 2015;2015:532615. doi: 10.1155/2015/532615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganji SH, Kashyap ML, Kamanna VS. Niacin inhibits fat accumulation, oxidative stress, and inflammatory cytokine IL-8 in cultured hepatocytes: Impact on non-alcoholic fatty liver disease. Metabolism. 2015;64:982–990. doi: 10.1016/j.metabol.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Cesaratto L, Codarin E, Vascotto C, Leonardi A, Kelley MR, Tiribelli C, Tell G. Specific inhibition of the redox activity of ape1/ref-1 by e3330 blocks tnf-α-induced activation of IL-8 production in liver cancer cell lines. PLoS One. 2013;8:e70909. doi: 10.1371/journal.pone.0070909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park K, Hong SW, Hur W, Lee MY, Yang JA, Kim SW, Yoon SK, Hahn SK. Target specific systemic delivery of TGF-β siRNA/(PEI-SS)-g-HA complex for the treatment of liver cirrhosis. Biomaterials. 2011;32:4951–4958. doi: 10.1016/j.biomaterials.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Chávez E, Castro-Sánchez L, Shibayama M, Tsutsumi V, Moreno MG, Muriel P. Sulfasalazine prevents the increase in TGF-β, COX-2, nuclear NFκB translocation and fibrosis in CCl4-induced liver cirrhosis in the rat. Hum Exp Toxicol. 2012;31:913–920. doi: 10.1177/0960327112438928. [DOI] [PubMed] [Google Scholar]
- 37.Salehi MH, Kamalidehghan B, Houshmand M, Aryani O, Sadeghizadeh M, Mossalaeie MM. Association of fibroblast growth factor (FGF-21) as a biomarker with primary mitochondrial disorders, but not with secondary mitochondrial disorders (Friedreich Ataxia) Mol Biol Rep. 2013;40:6495–6499. doi: 10.1007/s11033-013-2767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gahete MD, Córdoba-Chacón J, Luque RM, Kineman RD. The rise in growth hormone during starvation does not serve to maintain glucose levels or lean mass but is required for appropriate adipose tissue response in female mice. Endocrinology. 2013;154:263–269. doi: 10.1210/en.2012-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu D, Sun CY, Sun GP, Ren GP, Ye XL, Zhu SL, Wang WF, Xu PF, Li SJ, Wu Q, et al. The synergistic effect of FGF-21 and insulin on regulating glucose metabolism and its mechanism. Yao Xue Xue Bao. 2014;49:977–984. (In Chinese) [PubMed] [Google Scholar]
- 40.Ren G, Yin J, Wang W, Li L, Li D. Fibroblast growth factor (FGF)-21 signals through both FGF receptor-1 and 2. Sci China Life Sci. 2010;53:1000–1008. doi: 10.1007/s11427-010-4035-z. [DOI] [PubMed] [Google Scholar]
- 41.Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol. 2008;215:1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 42.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]