Abstract
The aim of the present study was to investigate the role of Semaphorin 3A (Sema3A) in the osteogenic differentiation of human alveolar bone marrow mesenchymal stem cells (hABMMSCs). To investigate whether Sema3A affects hABMMSC proliferation and osteogenic differentiation, a stable Sema3A-overexpression cell line was generated by infection with the pAdCMV-SEMA3A-MCS-EGFP vector. Cell counting kit-8 and clone formation assays were performed to determine the proliferation ability of hABMMSCs, while cell osteogenic differentiation was assayed using Alizarin Red S staining. In addition, reverse transcription-quantitative polymerase chain reaction was employed to detect the mRNA expression level of osteogenesis-associated genes, Runt-related transcription factor 2 (Runx2), osteopontin (Opn) and osteocalcin (Ocn), during the osteogenic differentiation. The results revealed that, compared with the normal control group, the cell morphology of the infected cells was stable and no significant alterations were observed. Overexpression of Sema3A in hABMMSCs significantly increased the cell proliferation ability compared with the control group. Furthermore, the Alizarin Red S staining assay results indicated that the ossification process of hABMMSCs overexpressing Sema3A was evidently faster in comparison with that of the control group cells. Overexpression of Sema3A by pAdCMV-SEMA3A-MCS-EGFP infection also significantly increased the mRNA expression levels of the osteogenic marker genes Runx2, Opn and Ocn. In conclusion, Sema3A was observed to be a key positive regulator in hABMMSC osteogenic differentiation.
Keywords: semaphorin 3A, osteogenic differentiation, human alveolar bone marrow mesenchymal stem cells
Introduction
Mesenchymal stem cells (MSCs), which belong to the pluripotent stem cells, were initially identified in the bone marrow. Due to their various characteristics, including multidifferentiation potential, hematopoiesis support, stem cell implantation promotion, immune regulation and self-renewal (1,2), MSCs are currently a research focus. In addition, MSCs have become an attractive cell source for use in bone repair and tissue engineering due to their capacity for self-renewal and differentiation into osteoblasts (3).
Semaphorins (Semas) are a large family of conserved guidance proteins that regulate cellular shape and function (4). Semas were first identified as axon guidance factors during nervous system development, while they were found to be regulators of various developmental processes, including the heart, bone, kidney, lung and immune development, as well as angiogenesis (5–9). Previous studies have indicated that Semas serve important roles in osteoporosis, cardiovascular diseases, cancer and immune-mediated diseases (10–12). As a member of class 3 Semas, Sema3A serves a role in suppressing the progression of various types of cancer by inhibiting angiogenesis (13–16). More recently, Sema3A has been found to serve key roles in bone metabolism, at the same time, Sema3A could promote osteoblast differentiation and inhibit osteoblast activity, and is the hotspot in research of bone diseases (17,18). However, the role of Sema3A in the osteogenic differentiation of human alveolar bone marrow MSCs (hABMMSCs) remains unclear.
Therefore, in the present study, the fundamental functions of Sema3A in hABMMSC osteogenic differentiation were investigated and the underlying mechanism was analyzed.
Materials and methods
Materials
The α-minimum essential medium (MEM) culture medium, fetal bovine serum (FBS), streptomycin, penicillin and L-glutamine were supplied by Thermo Fisher Scientific, Inc. (Gibco; Waltham, MA, USA). Tryptase was obtained from Amresco, LLC (Solon, OH, USA). Dexamethasone, β-glycerin sodium phosphate, ascorbic acid, dimethyl sulfoxide and Alizarin Red S were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The flow cytometer was purchased from Beckman Coulter, Inc. (Brea, CA, USA), while the enzyme-linked immune detector was supplied by BioTek Instruments, Inc. (Winooski, VT, USA). The ultraviolet spectrophotometer instrument (BioSpectrometer) and the polymerase chain reaction (PCR) instrument (Mastercycler nexus) were supplied by Eppendorf (Hamburg, Germany).
Separation and purification of hABMMCs
The present study was approved by the Ethics Committee of the Stomatological Hospital of Jiangsu Province (Nanjing, China), and written informed consent was obtained from each patient. hABMMSCs were isolated and expanded as described by Zhang et al (19). Between January 2014 and December 2015, a total of 15 patients (male 8, female 7; aged 18–22 years; mean age: 20.5 years), were admitted at the Department of Oral and Maxillofacial Surgery in Stomatological Hospital of Jiangsu Province. All patients with systemic or metabolic disease were excluded from the present study and received orthognathic surgery due to malocclusion. All the bone collected from these patients were fresh and healthy. Briefly, healthy jaw cancellous bone was collected from patients during the orthognathic surgery under sterile conditions. Subsequently, the samples were washed and centrifuged with 1 mol/l PBS at 800 × g for three or four times for 5 min each time at 4°C. The collected endothelial cells (5×104 cells per well) and bone fragments were then seeded into a 6-well plate and cultured in α-MEM medium supplemented with 10% FBS, 100 units/ml penicillin, 100 µg/ml streptomycin and 2 mM L-glutamine and incubated in a 5% CO2 incubator at 37°C. The culture medium was replaced every 3 days, and cells were passaged until 80% confluence was reached. Next, first generation log-phase cells were seeded into 6-well plates (300–450 cells per well) and cultured for 7–10 days prior to the observation of visible hABMMSC colonies. Subsequently, the cells were harvested with using 0.25% trypsin and then cultured in maintenance medium consisting of 10% FBS, 100 units/ml penicillin, 100 µg/ml streptomycin and 2 mM L-glutamine at 37°C in an atmosphere containing 5% CO2, and third to fifth generation cells were used in subsequent experiments.
Cell infection and morphology observation
The adenovirus expression vector pAdCMV-SEMA3A-MCS-EGFP, which overexpressed human Sema3A, and the control vector pCMV-MCS-EGFP were synthesized by Genechem (Shanghai Genechem Co., Ltd., Shanghai, China). The hABMMSCs were infected with the pAdCMV-SEMA3A-MCS-EGFP (Sema3A group) or pCMV-MCS-EGFP (control group) vector in 10 µg/ml hexadimethrine bromide and incubated for an additional 48 h at 37°C in an atmosphere containing 5% CO2. The efficiency of infection was observed under an inverted fluorescence microscope at 48 h after the infection, and the cell morphology was examined.
Clone formation assay
Log-phase hABMMSCs were harvested with trypsin, counted with a hemocytometer, and transferred to 75-cm2 cell culture flasks (3×104 cells/cm2; three replicates per sample). Subsequent to incubation for 10 days, the cells were carefully rinsed twice with PBS, followed by fixing with 4% paraformaldehyde for 20 min at room temperature and staining with 0.5% crystal violet for 20 min. Subsequently, the cells were washed with distilled water and dried naturally. The number of cell clones with >50 cells was counted under the microscope and the cloning efficiency was calculated according to the following formula: Cloning efficiency (%)=(number of clones/number of cells incubated) ×100% (20).
Cell proliferation assay by cell counting kit-8 (CCK-8)
Third generation log-phase hABMMSCs were infected with an empty vector (pCMV-MCS-EGFP) or pAdCMV-SEMA3A-MCS-EGFP, and then the cells were seeded into a 96-well plate with an initial density of 2×103 cells per well (three replicates per sample) and cultured in the osteogenesis-inducing media containing 10% FBS, 100 units/ml penicillin, 100 µg/ml streptomycin, 2 mol/l dexamethasone, 0.01 mol/l β-glycerin sodium phosphate and 50 µg/ml ascorbic acid at 37°C in an atmosphere containing 5% CO2. The viability of these cells was detected on days 1, 2, 3, 4, 5 and 6 according to the protocol of the CCK-8 assay (Dojindo, Molecular Technologies, Inc., Kumamoto, Japan), and the results were statistically analyzed.
Alizarin Red S staining
At 24 h after third generation log-phase hABMMSCs were infected with pCMV-MCS-EGFP or pAdCMV-SEMA3A-MCS-EGFP, the cells were seeded into 6-well plates (5×104 cells per well) and grown in osteogenesis-inducing media consisting of α-MEM medium supplemented with 10% FBS, 10 mmol/l β-glycerin liquid sodium phosphate, 0.3 mmol/l vitamin C and 1×10−5 mmol/l dexamethasone. Following incubation for 7, 14 and 21 days, Alizarin Red S staining was performed as described by Cai et al (21) with minor modification. Briefly, the cultured cells in the 6-well plates were rinsed twice with PBS and fixed with 4% paraformaldehyde for 20 min. The cells were then washed with PBS and exposed to Alizarin Red S (2% aqueous) for 5 min. Subsequently, they were washed again with PBS and observed under a microscope. Positive staining is represented as a red/purple color.
Reverse transcription-quantitative PCR (RT-qPCR)
Following infection for 7 or 14 days, RT-qPCR was performed to detect the mRNA expression level of three osteogenesis-associated genes, namely Runt-related transcription factor 2 (Runx2), osteopontin (Opn) and osteocalcin (Ocn). Briefly, total RNA was extracted from the cell lines using TRIzol reagent (Takara Bio, Inc., Otsu, Japan) following the manufacturer's protocol. RNA concentration and quality were measured using a NanoDrop spectrophotometer (ND-1,000; NanoDrop Technologies, Wilmington, DE, USA). Next, cDNA was obtained from total RNA using a cDNA RT kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to manufacturer's instructions. qPCR was performed to analyze the synthesized cDNA using a PCR thermal cycler with the following amplification parameters: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec, and 60°C for 60 sec. All the primers used in qPCR were synthesized by Nanjing GenScript Co., Ltd. (Nanjing, China), and were as follows: Runx-2, 5′-TGGCAGCACGCTATTAAATC-3′ (forward) and 5′-TCTGCCGCTAGAATTCAAAA-3′ (reverse); Opn, 5′-ACGCCGACCAAGGAAAACTC-3′ (forward) and 5′-GTCCATAAACCACACTATCACCTCG-3′ (reverse); Ocn, 5′-CAGACACCATGAGGACCATC-3′ (forward) and 5′-GGACTGAGGCTCTGTGAGGT-3′ (reverse); GAPDH, 5′-GAAGGTGAAGGTCGGAGTC-3′ (forward) and 5′-GAAGATGGTGATGGGATTTC-3′ (reverse). GAPDH served as an internal control.
Statistical analysis
All data are displayed as the mean ± standard deviation. Statistical comparisons between two groups were conducted with the Student's t-test. Statistical analysis was performed using the SPSS version 18.0 statistical software package (SPSS, Inc., Chicago, IL, USA). Values of P<0.05 were considered to indicate a difference that was statistically significant.
Results
Cell morphology
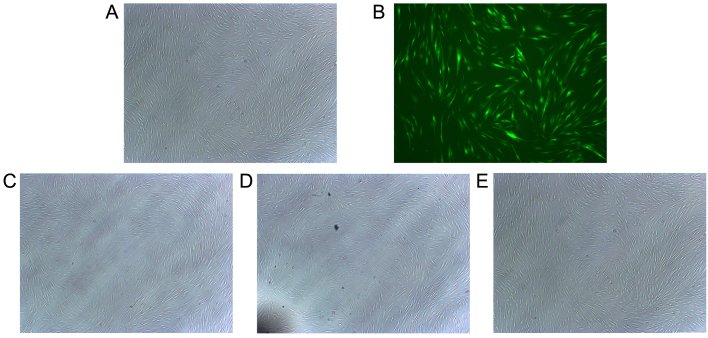
hABMMCs were separated and purified as described previously (22). The majority of the hABMMCs were spindle shaped, there was an abundant cytoplasm and only a few hABMMCs were oval-shaped. The efficiency of infection and cell morphology were examined under an inverted fluorescence microscope at 48 h after the infection. The results revealed that the cell transfection efficiency was >60%, while the cell morphology of the infected cells was stable and exhibited no significant alterations when compared with the normal control group (Fig. 1).
Figure 1.
Overexpression of Sema3A is successfully achieved by vector infection and the cell morphology is examined. Representative overlay images of (A) phase contrast and (B) fluorescence microscopy demonstrated the transduction efficiency of Sema3A for green fluorescent protein using adenoviral transduction. The cell morphology of the (C) untreated control cells, (D) cells infected with pCMV-MCS-EGFP (control vector) and (E) cells infected with pAdCMV-SEMA3A-MCS-EGFP (Sema3A overexpressing vector) was examined under an electron microscope. Experiments were performed in triplicate. Sema3A, semaphorin 3A.
Effect of Sema3A on hABMMSC cell proliferation activity
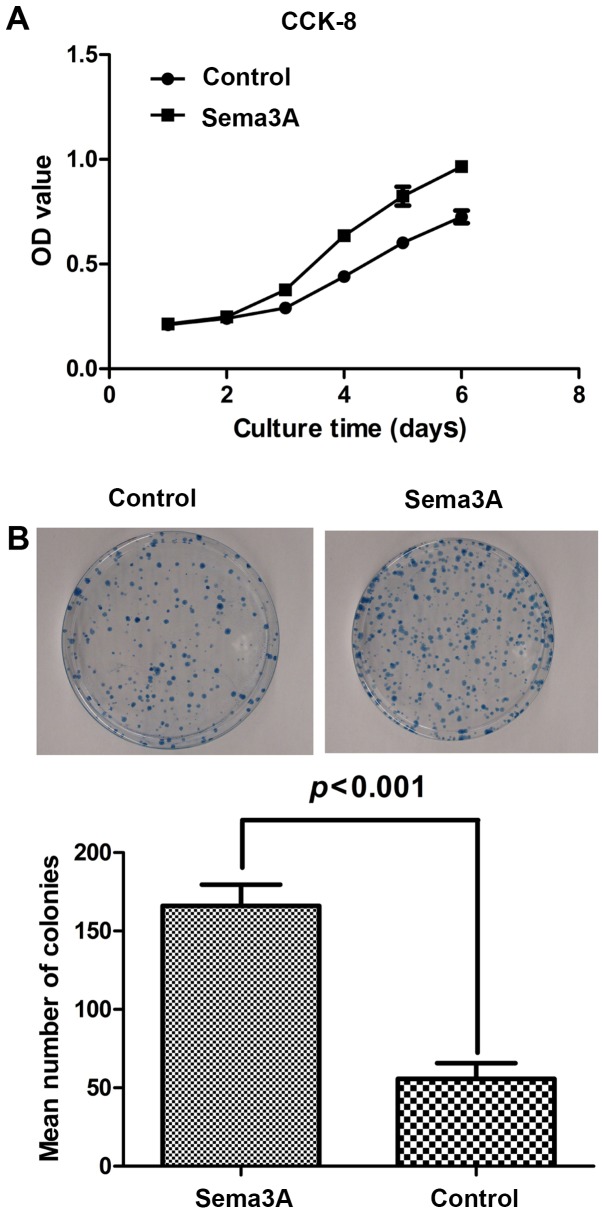
To investigate the effect of Sema3A on the hABMMSC cell proliferation activity, clone formation assay and cell proliferation (CCK-8) assays were performed. As shown in Fig. 2A, compared with the control group, infection of hABMMSCs with the pAdCMV-SEMA3A-MCS-EGFP vector significantly affected the cell proliferation, while the cell viability was significantly enhanced. As shown in Fig. 2B, the results of the clone formation assay suggested that the clone formation ability of pAdCMV-SEMA3A-MCS-EGFP-infected cells was significantly increased as compared with that of the pCMV-MCS-EGFP-infected cells. All these data indicated that Sema3A overexpression significantly increased the hABMMSC proliferation.
Figure 2.
Sema3A promotes the cell proliferation ability of human alveolar bone marrow mesenchymal stem cells. (A) CCK-8 proliferation assay was conducted, and the OD was measured at 490 nm to determine the cell proliferation. (B) Clone formation assay results. The number of cell clones with >50 cells was counted under a microscope and the cloning efficiency was calculated. Tests were performed in triplicate, and data are represented as the mean ± standard deviation. Sema3A, semaphorin 3A; CCK-8, Cell Counting Kit-8; OD, optical density.
Sema3A facilitates hABMMSC osteogenic differentiation
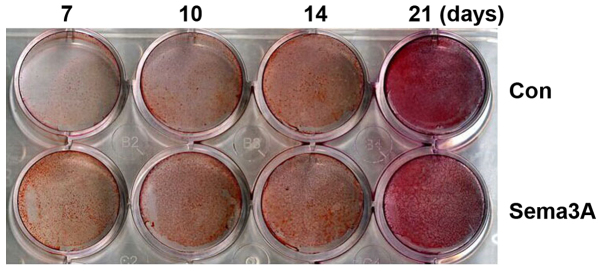
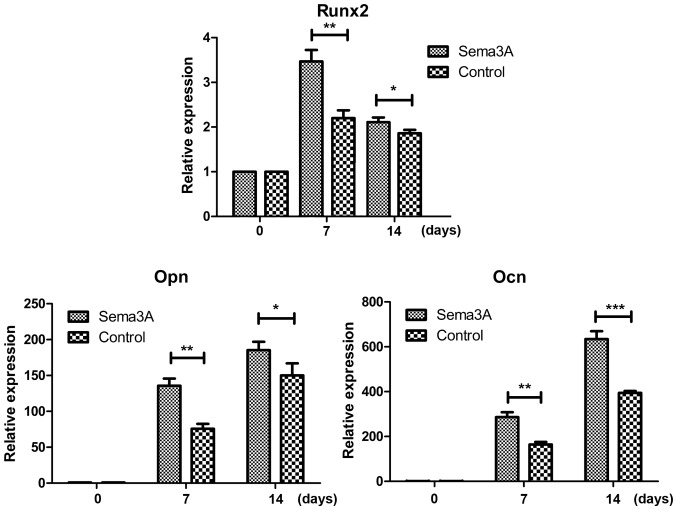
In order to investigate whether Sema3A exhibited an effect on osteogenic differentiation, pAdCMV-SEMA3A-MCS-EGFP or pCMV-MCS-EGFP vector was transfected into hABMMSCs. Alizarin Red S staining was then performed after 7, 10, 14 and 21 days of culturing in the osteogenesis-inducing media. hABMMSCs transfected with pAdCMV-SEMA3A-MCS-EGFP demonstrated matrix mineralization with more intense Alizarin Red S staining when compared with the pCMV-MCS-EGFP-transfected hABMMSCs (Fig. 3). Notably, the staining was more intense at earlier time points in Sema3A overexpressed hABMMSCs compared with the control. The mRNA expression levels of osteogenesis-associated genes (Runx2, Opn and Ocn) was also detected on days 0, 7 and 14 during the osteogenic differentiation using RT-qPCR. The results demonstrated that the mRNA expression levels of Runx2, Opn and Ocn were all significantly increased in the Sema3A overexpression hABMMSCs compared with the control hABMMSCs on days 7 and 14 (Fig. 4). These findings indicated that Sema3A serves an important role in promoting hABMMSC osteogenic differentiation.
Figure 3.
Alizarin Red S staining of hABMMSCs. At 24 h after third generation log-phase hABMMSCs were infected with the pCMV-MCS-EGFP (Con) or pAdCMV-SEMA3A-MCS-EGFP (Sema3A) vector, the cells were seeded in 6-well plates and grown in osteogenesis-inducing media. Following incubation for 7, 10, 14 and 21 days, Alizarin Red S staining was performed (n=3 per experiment). Sema3A, semaphorin 3A; Con, control; hABMMSCs, human alveolar bone marrow mesenchymal stem cells.
Figure 4.
Relative expression levels of the osteogenesis-associated genes. After infection for 7 and 14 days, reverse transcription-quantitative polymerase chain reaction was performed to detect the mRNA expression levels of osteogenesis-associated genes (Runx2, Opn and Ocn). GAPDH served as an internal control. Experiments were performed in triplicate, and data are represented as the mean ± standard deviation. ***P<0.001, **P<0.01, *P<0.05 vs. control as indicated. Sema3A, semaphorin 3A; Runx2, runt-related transcription factor 2; Opn, osteopontin; Ocn, osteocalcin.
Discussion
Sema3A has been reported to serve various important roles in the peripheral nerve, blood vessel and skeletal tissue development (23–25). In addition, a previous study has indicated that Sema3A-loaded chitosan intensely improved the osteogenic differentiation of osteoblasts and may be applied onto the Ti implant surface (26). In the present study, the aim was to investigate the role of Sema3A in hABMMSC osteogenic differentiation.
The process of osteogenic differentiation can be divided into three parts, including the proliferation, extracellular matrix (ECM) maturation and mineralization (27). To investigate whether Sema3A affects hABMMSC proliferation and osteogenic differentiation, hABMMSCs were initially isolated and expanded, and then a stable Sema3A-overexpression cell line was generated by infection with a pAdCMV-SEMA3A-MCS-EGFP vector, while cells infected with a control vector (pCMV-MCS-EGFP) were used as the negative control. The cell morphology of the infected cells was observed under a microscope, and no significant differences were detected between the Sema3A-overexpression and normal control groups. Subsequently, the cell proliferation ability of hABMMSCs was investigated using CCK-8 and clone formation assays, and the data suggested that Sema3A overexpression was able to significantly promote the proliferation ability of the hABMMSCs. Furthermore, Alizarin Red S staining was performed to analyze the cell osteogenic differentiation. As compared with the control group cells, the ossification process of hABMMSCs overexpressing Sema3A was evidently accelerated.
The current study also attempted to evaluate the expression levels of three osteogenic markers, Runx 2, Opn and Ocn. As an important transcription factor, Runx2 is essential for the initiation of osteoblast differentiation and bone formation (28). In the present study results, the relative expression level of Runx2 in hABMMSCs overexpressing Sema3A was markedly increased compared with that in the control cells. In addition, the osteoblast-associated proteins Ocn, which binds to calcium and promotes bone matrix calcification (29), and Opn, which is associated with cell attachment (30), were also investigated. These proteins are the main osteogenic genes that support proliferation, matrix formation and mineralization. The data of the current study revealed that these two proteins were increased in hABMMSCs overexpressing Sema3A when compared with the control cells. This observation confirmed the osteogenetic capacity of hABMMSCs demonstrated by the highest total protein content and the increased mRNA expression levels of osteogenic markers.
In conclusion, to the best of our knowledge, the present study demonstrated for the first time that Sema3A is a key positive regulator in hABMMSC osteogenic differentiation. These findings suggested that Sema3A may be a potentially novel therapeutic agent in bone diseases.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 81500823), the Priority Academic Program Development of Jiangsu Higher Education Institutions (grant no. 2014-37), a grant supported by Shanghai Stomatological Hospital (grant no. SSDC-2014-07), and the Natural Science Foundation of Jiangsu Province of China (grant no. BK20171057).
References
- 1.Tocci A, Forte L. Mesenchymal stem cell: Use and perspectives. Hematol J. 2003;4:92–96. doi: 10.1038/sj.thj.6200232. [DOI] [PubMed] [Google Scholar]
- 2.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone effects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 3.Mauney JR, Volloch V, Kaplan DL. Role of adult mesenchymal stem cells in bone tissue engineering applications: Current status and future prospects. Tissue Eng. 2005;11:787–802. doi: 10.1089/ten.2005.11.787. [DOI] [PubMed] [Google Scholar]
- 4.Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 5.Hinck L. The versatile roles of ‘axon guidance’ cues in tissue morphogenesis. Dev Cell. 2004;7:783–793. doi: 10.1016/j.devcel.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Singh MK, Degenhardt KR, Lu MM, Bennett J, Yoshida Y, Epstein JA. Tie2Cre-mediated inactivation of plexinD1 results in congenital heart, vascular and skeletal defects. Dev Biol. 2009;325:82–93. doi: 10.1016/j.ydbio.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reidy KJ, Villegas G, Teichman J, Veron D, Shen W, Jimenez J, Thomas D, Tufro A. Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development. 2009;136:3979–3989. doi: 10.1242/dev.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neufeld G, Sabag AD, Rabinovicz N, Kessler O. Semaphorins in angiogenesis and tumor progression. Cold Spring Harb Perspect Med. 2012;2:a006718. doi: 10.1101/cshperspect.a006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hota PK, Buck M. Plexin structures are coming: Opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci. 2012;69:3765–3805. doi: 10.1007/s00018-012-1019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maione F, Capano S, Regano D, Zentilin L, Giacca M, Casanovas O, Bussolino F, Serini G, Giraudo E. Semaphorin3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J Clin Invest. 2012;122:1832–1848. doi: 10.1172/JCI58976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takamatsu H, Kumanogoh A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends Immunol. 2012;33:127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty G, Kumar S, Mishra R, Patil TV, Kundu GC. Semaphorin 3A suppresses tumor growth and metastasis in mice melanoma model. PLoS One. 2012;7:e33633. doi: 10.1371/journal.pone.0033633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serini G, Maione F, Giraudo E, Bussolino F. Semaphorins and tumor angiogenesis. Angiogenesis. 2009;12:187–193. doi: 10.1007/s10456-009-9138-4. [DOI] [PubMed] [Google Scholar]
- 15.Maione F, Capano S, Regano D, Zentilin L, Giacca M, Casanovas O, Bussolino F, Serini G, Giraudo E. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J Clin Invest. 2012;122:1832–1848. doi: 10.1172/JCI58976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaur P, Bielenberg DR, Samuel S, Bose D, Zhou Y, Gray MJ, Dallas NA, Fan F, Xia L, Lu J, Ellis LM. Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin Cancer Res. 2009;15:6763–6770. doi: 10.1158/1078-0432.CCR-09-1810. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S, Sato T, Shibata S, Yoshida Y, Gu Z, Kimura A, et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497:490–493. doi: 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Cheng JQ, Huang Q, Yang J, Shen B, Zhou ZK, Kang PD, Lian YY, Pei FX. Increasing alcohol-induced osteogenesis of human bone marrow-derived mesenchymal cells using siRNA transient suppression of peroxisome proliferator activated receptor gamma: An in vitro experiment study. Zhonghua Yi Xue Za Zhi 88: 2603–2608, 2008. Zhonghua Yi Xue Za Zhi 88: 2603–2608, 2008. 2008;88: 2603–2608, 2008:2603-2608, 2008–2608, 2008. (In Chinese) [PubMed] [Google Scholar]
- 20.Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 21.Cai Y, Xu MJ, Teng X, Zhou YB, Chen L, Zhu Y, Wang X, Tang CS, Qi YF. Intermedin inhibits vascular calcification by increasing the level of matrix gamma-carboxyglutamic acid protein. Cardiovasc Res. 2010;85:864–873. doi: 10.1093/cvr/cvp366. [DOI] [PubMed] [Google Scholar]
- 22.Kim BS, Kim YC, Zadeh H, Park YJ, Pi SH, Shin HS, You HK. Effects of the dichloromethane fraction of Dipsaci Radix on the osteoblastic differentiation of human alveolar bone marrow-derived mesenchymal stem cells. Biosci Biotechnol Biochem. 2011;75:13–19. doi: 10.1271/bbb.100379. [DOI] [PubMed] [Google Scholar]
- 23.Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, Watson N, Klagsbrun M, Mamluk R, Newgreen DF. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev Biol. 2003;255:77–98. doi: 10.1016/S0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 24.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 25.Gomez C, Burt-Pichat B, Mallein-Gerin F, Merle B, Delmas PD, Skerry TM, Vico L, Malaval L, Chenu C. Expression of Semaphorin-3A and its receptors in endochondral ossification: Potential role in skeletal development and innervation. Dev Dyn. 2005;234:393–403. doi: 10.1002/dvdy.20512. [DOI] [PubMed] [Google Scholar]
- 26.Fang K, Song W, Wang L, Jia S, Wei H, Ren S, Xu X, Song Y. Immobilization of chitosan film containing semaphorin 3A onto a microarc oxidized titanium implant surface via silane reaction to improve MG63 osteogenic differentiation. Int J Nanomedicine. 2014;9:4649–4657. doi: 10.2147/IJN.S68895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: Reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 28.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/S0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 29.Lian JB, Stein GS, Stein JL, van Wijnen AJ. Osteocalcin gene promoter: Unlocking the secrets for regulation of osteoblast growth and differentiation. J Cell Biochem Suppl 30–31. 1998:1–72. [PubMed] [Google Scholar]
- 30.Donzelli E, Salvadè A, Mimo P, Viganò M, Morrone M, Papagna R, Carini F, Zaopo A, Miloso M, Baldoni M, Tredici G. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch Oral Biol. 2007;52:64–73. doi: 10.1016/j.archoralbio.2006.07.007. [DOI] [PubMed] [Google Scholar]