Mitochondrial dysfunction is an early pathological feature of the Alzheimer’s disease-affected brain. Du et al. demonstrate that restoring PINK1 function in mAPP/Pink1-/- mice reduces Aβ levels, amyloid-associated pathology, oxidative stress, and mitochondrial and synaptic dysfunction. Activation of PINK1 may represent a new therapeutic avenue for combating Alzheimer’s disease.
Keywords: PINK1, autophagy, Aβ, mitochondrial dysfunction, synaptic injury
Abstract
Mitochondrial dysfunction and synaptic damage are early pathological features of the Alzheimer’s disease-affected brain. Memory impairment in Alzheimer’s disease is a manifestation of brain pathologies such as accumulation of amyloid-β peptide and mitochondrial damage. The underlying pathogenic mechanisms and effective disease-modifying therapies for Alzheimer’s disease remain elusive. Here, we demonstrate for the first time that decreased PTEN-induced putative kinase 1 (PINK1) expression is associated with Alzheimer’s disease pathology. Restoring neuronal PINK1 function strikingly reduces amyloid-β levels, amyloid-associated pathology, oxidative stress, as well as mitochondrial and synaptic dysfunction. In contrast, PINK1-deficient mAPP mice augmented cerebral amyloid-β accumulation, mitochondrial abnormalities, impairments in learning and memory, as well as synaptic plasticity at an earlier age than mAPP mice. Notably, gene therapy-mediated PINK1 overexpression promotes the clearance of damaged mitochondria by augmenting autophagy signalling via activation of autophagy receptors (OPTN and NDP52), thereby alleviating amyloid-β-induced loss of synapses and cognitive decline in Alzheimer’s disease mice. Loss of PINK1 activity or blockade of PINK1-mediated signalling (OPTN or NDP52) fails to reverse amyloid-β-induced detrimental effects. Our findings highlight a novel mechanism by which PINK1-dependent signalling promotes the rescue of amyloid pathology and amyloid-β-mediated mitochondrial and synaptic dysfunctions in a manner requiring activation of autophagy receptor OPTN or NDP52. Thus, activation of PINK1 may represent a new therapeutic avenue for combating Alzheimer’s disease.
Introduction
Mitochondrial dysfunction and synaptic failure are prominent and early pathological features of Alzheimer’s disease (Selkoe, 2002; Lin and Beal, 2006; Du et al., 2010, 2012; Swerdlow, 2012; Fang et al., 2015; Yu et al., 2016). Perturbed bioenergetics function, aberrant mitochondrial dynamics, and increased reactive oxygen species (ROS) are observed in Alzheimer’s disease-affected brains. Amyloid-β peptide has deleterious effects on mitochondrial and synaptic function (Lustbader et al., 2004; Manczak et al., 2006, 2011; Reddy and Beal, 2008; Takuma et al., 2009; Chen and Yan, 2010; Reddy et al., 2012). Damaged mitochondria are progressively accumulated in neurons, particularly in synapses over the lifetime of Alzheimer’s disease-affected neurons (Du et al., 2010). The accumulation of damaged mitochondria in Alzheimer’s disease brains contributes to aberrant synaptic structure and function such as loss of synapses, dendritic spines, synaptic proteins, and disruption of synaptic transmission. The underlying mechanisms of amyloid-β-mediated progressive mitochondrial and synaptic degeneration remain elusive and strategies to rescue such injuries remain unavailable. However, it has become increasingly evident that defective mitochondria must be removed in order to maintain neuronal function and synaptic transmission.
PTEN-induced putative kinase 1 (PINK1) is critical to the maintenance of mitochondrial integrity and function by promoting the removal of damaged mitochondria via mitophagy (Geisler et al., 2010; Batlevi and La Spada, 2011; Ashrafi et al., 2014; Lazarou et al., 2015; Pickrell and Youle, 2015)—a selective form of autophagy whereby defective mitochondria are specifically engulfed by autophagosomes and targeted for degradation in the lysosomes. Upon membrane depolarization, damaged mitochondria stabilize and activate PINK1 leading to the recruitment of parkin (Narendra et al., 2008, 2010) from the cytosol to the mitochondria. Once recruited, parkin initiates mitophagy. However, a recent study has demonstrated that PINK1 is capable of activating mitophagy directly without parkin by recruiting the autophagy receptors optineurin (OPTN) and nuclear dot protein 52 kDa (NDP52, encoded by CALCOCO2) (Lazarou et al., 2015). Parkin can further amplify the mitophagy signal triggered by PINK1. This new pathway further strengthens the concept that targeting PINK1 is a novel therapeutic strategy. Consistent with its protective role, PINK1 downregulation causes mitochondrial dysfunction, increased oxidative stress, and neuronal dysfunction (Beilina et al., 2005; Clark et al., 2006; Yang et al., 2006; Gautier et al., 2008; Tufi et al., 2014). Several mutations of PINK1 have been found to be involved in the pathogenesis of Parkinson’s disease. However, the significant role of PINK1 in Alzheimer’s disease pathogenesis has yet to be elucidated.
Our present study offers new insights into PINK1-dependent amyloid pathology through autophagy signalling and mitochondrial quality, contributing to the synaptic and cognitive dysfunction in the pathogenesis of Alzheimer’s disease.
Materials and methods
Animal studies
Animal studies were carried out with the approval of the Institutional Animal Care and Use Committee of the University of Kansas Lawrence in accordance with the National Institutes of Health guidelines for animal care.
Human subjects for PINK1 expression
We obtained human brain tissues of hippocampus tissues from individuals with Alzheimer’s disease and age-matched, non-Alzheimer’s disease controls from the University of Arizona. Detailed information for each of the cases studied is shown in Supplementary Table 1. We obtained informed consent from all subjects.
Production of AAV2-PINK construct
Viral production and packaging using recombinant adeno-associated virus type 2 (rAAV2) encoding a protein of interest under control of the cytomegatovius (CMV) promoter has been described in our previous study (Rappold et al., 2014). Briefly, human wild-type PINK1 and mutant PINK1-L347P (Cui et al., 2010) were subcloned into the pBSFBrmcs shuttle vector and then the modified pFBGR plasmid backbone containing EGFP to monitor the expression of PINK1 proteins after rAAV injection. Finally, rAAV2 packaging was performed as described previously (Rappold et al., 2014). Control experiments were performed with an AAV2-GFP construct.
Intrahippocampal injections with AAV2-PINK1, AAV2-mPINK1 and AAV2-GFP
Non-transgenic mice and transgenic mAPP mice that overexpress a human mutant form of amyloid precursor protein (APP) bearing both the Swedish (K670N/M671L) and the Indiana (V717F) mutations (APPSwInd, J-20 line, obtained from Jackson Lab) were used in this study. Both male and female animals were used for the described experiments. The investigators were blind to the mouse genotype in performing all experiments. For the intrahippocampal stereotaxic injections, mice were deeply anaesthetized and placed in the stereotaxic frame. Two microlitres of each AAV2 (AAV2-PINK, AAV2-mPINK or AAV2-GFP, ∼5 × 1012 vg/ml) or vehicle were injected with a 10 μl Hamilton syringe at a rate of 0.25 μl/min by a nano-injector system. The needle was allowed to remain in the brain for an additional 5 min. Coordinates for stereotaxic injections were determined according to the Paxinos atlas of the mouse brain by using the following coordinates: ±3.2 mm medial/lateral, −2.7 mm anterior/posterior, and −2.7 mm dorsal/ventral from the bregma. Before waking, mice were allowed to recover in a heated pad. At 2 months post-injection, mice were subjected to a behavioural test and electrophysiological analysis. Then, assessment of amyloid-β levels, mitochondrial function and neuropathology were performed using mouse hippocampal samples.
Hippocampal neuronal cell culture, N2a-APPsw cell culture, and production of lentiviral constructs of Lenti-PINK1, Lenti-mPINK1, Lenti-triple-mPINK1 and Lenti-GFP are described in the Supplementary material.
Amyloid-β measurement, cytochrome c oxidase (CcO) activity assay, ATP levels, mitochondrial and the intracellular ROS, mitochondrial membrane potential measurement, immunoblotting, immunofluorescent staining and measurement of synaptic density were performed as previously described (Fang et al., 2015, 2016c) and are detailed in the Supplementary material.
Behavioural test and electrophysiological studies
We performed behavioural studies to assess spatial learning and memory using the Morris water maze as previously described (Fang et al., 2015) with modifications (Supplementary material).
Statistical analysis
All data were expressed as the mean ± standard error of the mean (SEM). Data were analysed by one-way ANOVA for repeated measure analysis using commercially available software (Statview, version 5.0.1, Berkeley, CA), followed by Fisher’s protected least significant difference for post hoc comparisons. P < 0.05 was considered significant.
Results
PINK1 reduces cerebral and mitochondrial amyloid-β accumulation
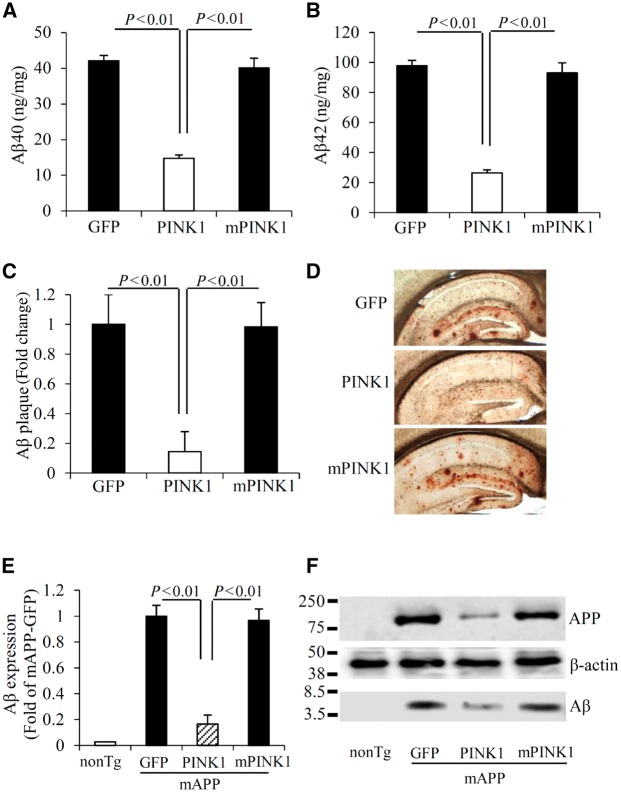
As PINK1 was downregulated in the brains of patients with Alzheimer’s disease and transgenic Alzheimer’s disease mice (Supplementary Fig. 1A–F), we sought to develop a model system in which neuronal expression of PINK1 would be restored or increased so that the consequences of PINK1-dependent signalling in an Alzheimer’s disease- and amyloid-β-rich environment could be analysed. We first explored whether increasing hippocampal PINK1 expression would affect amyloid-β accumulation. To this end, we performed stereotaxic injection to deliver human PINK1 to the hippocampus via rAAV2 encoding human PINK1. As a control for PINK1 kinase activity, we also delivered the loss-of-function form of PINK1 without kinase activity (L347P, mPINK1) (Beilina et al., 2005; Pridgeon et al., 2007). AAV-GFP was used as a viral vector control. PINK1 expression was increased in hippocampal neurons at 2 months post AAV-PINK1 injection (Supplementary Fig. 2). AAV-PINK1 transduction significantly reduced human amyloid-β levels by 65–70% in the hippocampus of mAPP/PINK1 mice compared to mAPP mice at 8 months of age (Fig. 1A and B), a time point when progressive accumulation of cerebral amyloid-β became evident. Similarly, human amyloid-β levels were reduced by 65–70% in the hippocampus (Supplementary Fig. 3A and B) of the 11–13-month-old mAPP mice, which were also transduced with AAV-PINK1 for 2 months. Transduction of AAV-mPINK1 did not affect cerebral amyloid-β levels in any amyloid-β measurements (Fig. 1A, B and Supplementary Fig. 3A and B). The percentage of amyloid-β immunoreactive plaque loads was also reduced in the hippocampus of mAPP/PINK1 mice receiving AAV-PINK1 but not AAV-mPINK1 (Fig. 1C and D).
Figure 1.
PINK1 reduces amyloid-β accumulation in mAPP mice. (A and B) Amyloid-β (Aβ) levels in the hippocampus of the indicated transgenic (Tg) mAPP mice, 2 months post-intrahippocampal injection of AAV2-PINK1 (PINK1), AAV2-mPINK1 (mPINK1), and AAV2-GFP (GFP) were measured by ELISA. n = 9–13 mice per group. (C) Quantification of amyloid-β plaque was performed with the hippocampi of the indicated mice and (D) representative images showed amyloid-β deposits. n = 6 mice per group. (E) The bar graph presents quantification of immunoreactive bands for amyloid-β normalized to β-actin. (F) The representative immunoblots show immunoreactive bands for APP and amyloid-β proteins from indicated hippocampal homogenates, and β-actin served as a loading control. n = 3 mice per group.
Given the involvement of PINK1 in the maintenance of mitochondrial integrity and function, we evaluated the effect of PINK1 on mitochondrial amyloid-β accumulation. Consistent with our previous studies (Du et al., 2008, 2010; Fang et al., 2015), amyloid-β is progressively accumulated in cortical mitochondria of mAPP mice. As expected, statistically significant reduction in mitochondrial pool of amyloid-β driven by PINK1 overexpression was evident in mAPP/PINK1 mice but not in mAPP/mPINK1 mice (Supplementary Fig. 3F and G). These data indicate that PINK1 efficiently eliminates amyloid-β from mitochondria.
Because increased PINK1 expression attenuated amyloid-β accumulation, we assessed the potential effect of PINK1 on amyloid-β production by detecting changes in protein expression levels of APP and amyloid-β. Strikingly decreased human APP and amyloid-β expressions were observed in hippocampal and mitochondrial fractions of mAPP/PINK1 mice, but not in mAPP/mPINK1 mice compared with mAPP mice (Fig. 1E, F and Supplementary Fig. 3), suggesting that PINK1 overexpression reduces both the cellular and mitochondrial pool of APP and amyloid-β.
PINK1 attenuates mitochondrial defects and oxidative stress in mAPP mice
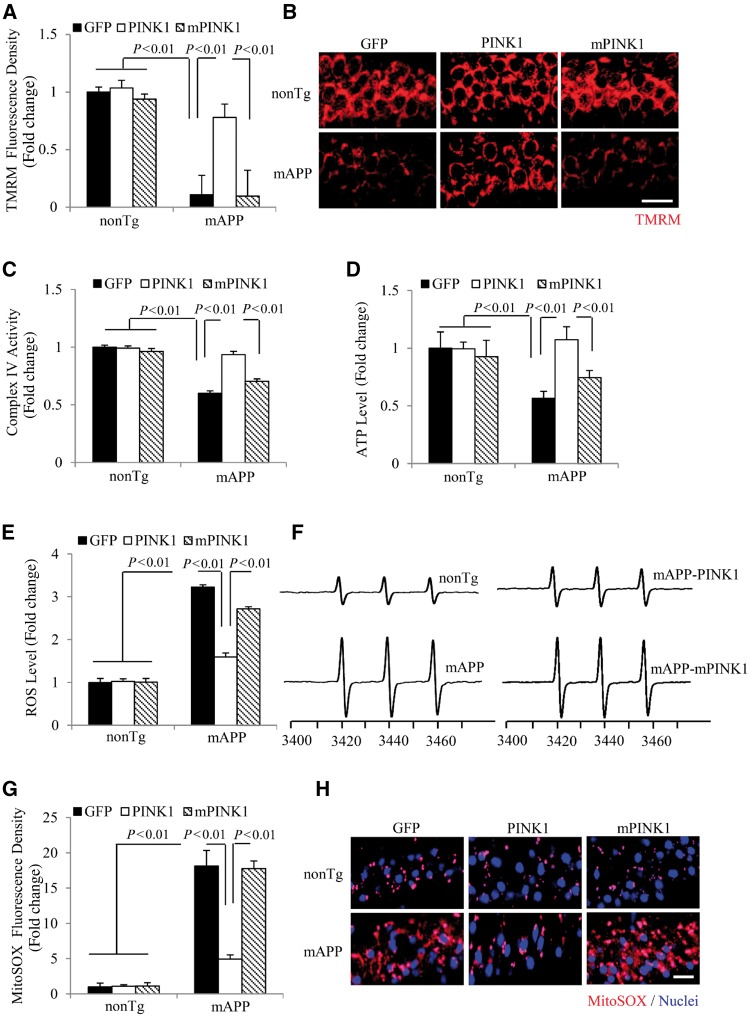
Next, we determined whether PINK1-enhanced amyloid-β clearance would improve mitochondrial function. Mitochondrial membrane potential (ΔΨm) is critical for maintaining the physiological function of the respiratory chain to generate ATP. Consistent with our previous studies (Du et al., 2008; Fang et al., 2015; Yu et al., 2016), mitochondrial membrane potential was reduced in mAPP hippocampal neurons as evidenced by decreased tetramethylrhodamine (TMRM) signals, whereas PINK1 rescued the loss of TMRM signals in mAPP/PINK1 hippocampal neurons (Fig. 2A and B). We then evaluated mitochondrial respiratory function by measuring CcO activity, a key enzyme in mitochondrial complex IV activity in response to amyloid-β toxicity (Caspersen et al., 2005; Du et al., 2008, 2011; Fang et al., 2015). CcO enzyme activity was significantly decreased in mAPP/GFP hippocampus compared to non-transgenic/GFP controls, whereas CcO activity in hippocampus of mAPP/PINK1 mice was restored to the levels similar to non-transgenic/GFP mice (Fig. 2C). In parallel, the decline in ATP level in hippocampus of mAPP mice was reversed in mAPP/PINK1 mice (Fig. 2D). In contrast, mAPP/mPINK1 mice did not show any protection against changes in mitochondrial membrane potential, CcO activity, and ATP levels (Fig. 2A–D).
Figure 2.
PINK1 rescues mitochondrial defects in mAPP mice. (A and B) Evaluation of mitochondrial membrane potential by measuring TMRM staining intensity (A) and the representative images of TMRM signal in hippocampi of the indicated mice (B). n = 3–5 mice per group. (C and D) CcO (complex IV, C) activity and ATP levels (D) in brain hippocampal tissues of the indicated mice. n = 6–10 mice per group. (E and F) Quantification of EPR spectra (E) and representative spectra of EPR (F) in the indicated mice. The peak height in the spectrum indicates levels of ROS. n = 3–5 mice per group. (G and H) Intensity of MitoSOX staining (G) and representative images of MitoSOX™ signals in the hippocampus of the indicated mice (H). n = 3–5 mice per group. Data are expressed as fold increase relative to non-transgenic mice received intrahippocampal injection of AAV2-GFP. Scale bars = 25 μm (B and H).
Given that mitochondria are the main source of ROS production, we next evaluated whether increased PINK1 expression would diminish ROS overproduction in amyloid-β-affected hippocampus of 8-month-old mAPP mice. We first quantitatively measured the intracellular ROS levels in the hippocampus by highly specific electron paramagnetic resonance (EPR) spectroscopy. The intracellular ROS levels indicated by EPR peaks were significantly elevated in mAPP/GFP mice compared to non-transgenic/GFP mice. This was largely abolished in mAPP/PINK1 mice but not in mAPP/mPINK1 mice (Fig. 2E and F). Furthermore, MitoSOX™ Red signals, an indicator of mitochondria-derived superoxide was significantly increased in mAPP hippocampi. In contrast, MitoSOX™ Red signals were significantly suppressed in mAPP/PINK1 hippocampi, but not in mAPP/mPINK1 hippocampi (Fig. 2G and H). Together, these data suggest that increased PINK1 expression/activity attenuates mitochondrial oxidative stress and mitochondrial dysfunction.
PINK1 activates autophagy signalling in amyloid-β-producing mAPP mice
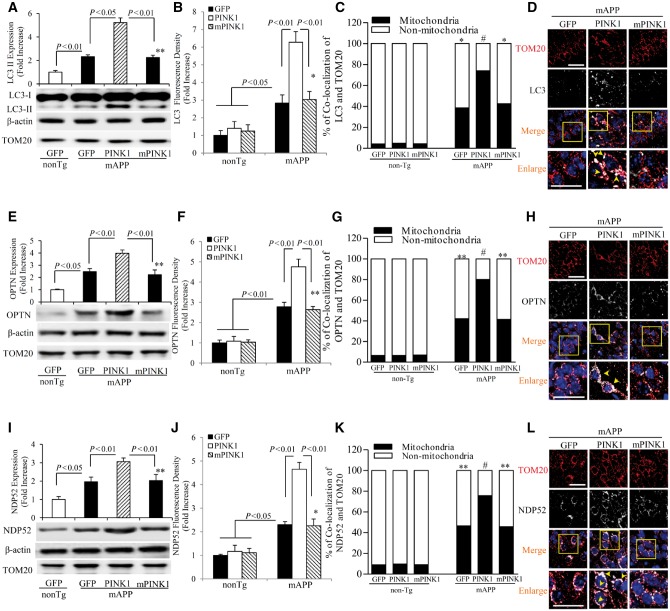
Dysfunctional mitochondria are progressively accumulated in Alzheimer’s disease-affected neurons (Caspersen et al., 2005; Du et al., 2010; Fang et al., 2015). This accumulation is likely attributable to inadequate mitophagy capacity to remove defective mitochondria (Hirai et al., 2001; Du et al., 2011; Hung et al., 2015; Weissmiller et al., 2015). In view of the significance of autophagy in mitochondrial clearance and the involvement of PINK1 in autophagy signalling by recruiting autophagy receptors of NDP52 and OPTN to damaged mitochondria to initiate autophagy (Lazarou et al., 2015), we assessed whether gain of PINK1 function would enhance autophagy signalling. Levels of the active form of LC3-II, an autophagosome marker, were significantly increased in mAPP hippocampus compared to non-transgenic hippocampus (Fig. 3A). The percentage of LC-3 positive neurons with puncta or aggregated morphology was significantly elevated in mAPP/PINK1 mice compared to mAPP/GFP mice (Fig. 3B–D). Importantly, PINK1 overexpression further elevated levels of autophagy receptor OPTN (Fig. 3E) and NDP52 (Fig. 3I) in mAPP/PINK1 mice compared to mAPP/GFP mice. Similarly, OPTN- and NDP52-positive neurons were elevated in mAPP/PINK1 hippocampi (Fig. 3F–H and J–L). Representative images show the co-localization of increased expression of autophagy proteins (LC3, OPTN, or NDP52) with mitochondrial protein TOM20 (Fig. 3D, H and L). Consistent with these observations, the mitochondrial pool of LC3, OPTN and NDP52 was elevated in mAPP/PINK1 mice (Supplementary Fig. 4A–C). The comparative graphs for the mitochondria and non-mitochondria showed increased percentage of co-localization of TOM20 with LC3II (Fig. 3C), OPTN (Fig. 3G), or NDP52 (Fig. 3K). However, mPINK1 overexpression failed to induce translocations of the above autophagy proteins into the mitochondria. In contrast, no changes in these autophagy molecules were found in mAPP/mPINK1 mice compared to mAPP/GFP mice, either in hippocampus tissues (Fig. 3) or in mitochondria (Supplementary Fig. 4A–C). No changes in LC3, OPTN and NDP52 expression were found in non-transgenic mice transduced by PINK1 or mPINK1 (Supplementary Fig. 4D–F). These results indicate that PINK1 activates autophagy signalling in an amyloid-β-rich environment.
Figure 3.
PINK1 activates autophagy signalling in mAPP mice. (A, E and I) Representative immunoblots show immunoreactive bands for LC3 (A), OPTN (E) and NDP52 (I) from indicated hippocampal homogenates, and β-actin served as a loading control. The bar graphs in A, E and I present quantification of immunoreactive bands for the corresponding proteins normalized to β-actin. n = 3 mice per group. (B, F and J) Quantification of LC3 (B), OPTN (F) and NDP52 (J) positive immunostaining intensity. Quantification of LC3, OPTN and NDP52 with TOM20 in mitochondria and non-mitochondria are shown in C, G and K, respectively. (D, H and L) Representative images of LC3 (D), OPTN (H) and NDP52 (L) in hippocampi of the indicated mice and the bottom panels show enlarged images of the interest area indicated by the box in the upper panels. n = 3–5 mice and 5–8 slides per group. *P < 0.05, **P < 0.01 versus non-transgenic (nonTg) group in A–C, E–G and I–K, #P < 0.01 versus mAPP/GFP or mAPP/mPINK1 mice in C, G and K. Scale bars = 25 μm (D, H and L).
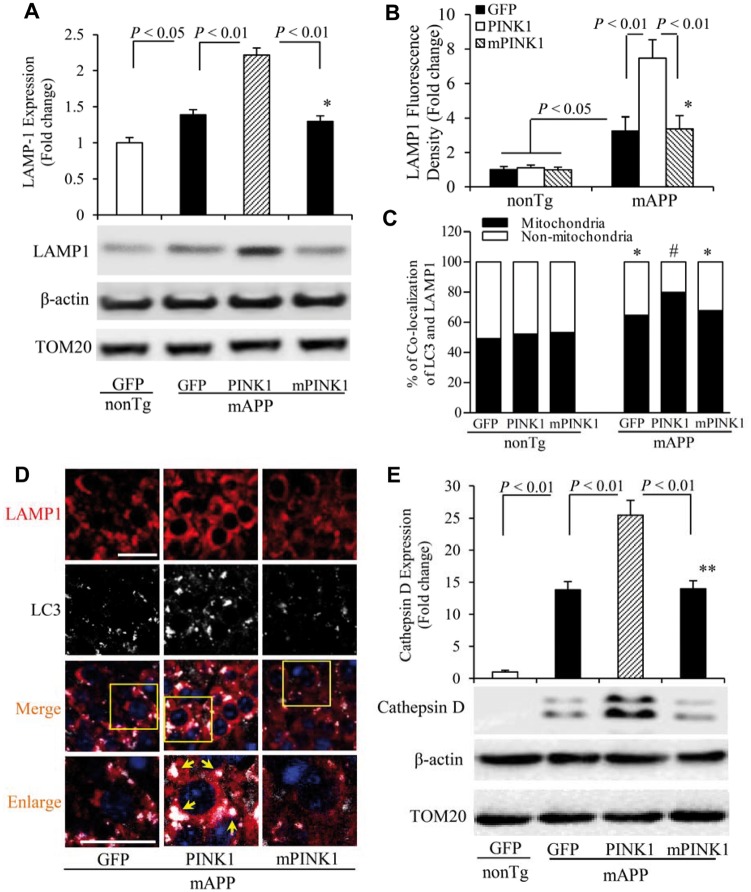
Lysosomes play a fundamental role in the autophagic pathway. Autophagy delivers cytoplasmic material, aggregated proteins, and organelles to lysosomes for degradation. Dysfunctional autophagy/lysosome pathway may also contribute to the accumulation of damaged organelles and proteins such as damaged mitochondria and amyloid-β (Barrachina et al., 2006; Qiao et al., 2008). In line with a previous observation of increased levels of lysosome-associated membrane protein 1 (LAMP1) in Alzheimer’s disease brains (Barrachina et al., 2006), LAMP1 expression was elevated in the hippocampi of mAPP/GFP mice. PINK1, but not mPINK1, overexpression induced more abundant LAMP1 expression in mAPP mice (Fig. 4A–C). Increased LAMP1-positive cells were co-localized with LC3 (Fig. 4D). There were no changes in LAMP1 expression or LC3 signalling in non-transgenic mice transduced with PINK1 or mPINK1 (Supplementary Fig. 4G). Cathepsin D, one of the lysosomal proteases, is important for the degradation of aggregated proteins including amyloid-β (Qiao et al., 2008). Levels of cathepsin D were higher in mAPP/GFP hippocampi than non-transgenic/GFP hippocampi. PINK1-overexpressed mAPP hippocampi exhibited a robust increase in expression of cathepsin D compared with mAPP/GFP hippocampi (Fig. 4E). These results indicate the involvement of PINK1 in amyloid-β-mediated autophagy/lysosome pathways including amyloid-β degradation and clearance.
Figure 4.
PINK1 promotes lysosomal recruitment in mAPP mice. (A) Representative immunoblots show immunoreactive bands for LAMP1 protein from hippocampal homogenates from indicated mice, and β-actin served as a loading control. The bar graph (A) presents quantification of immunoreactive bands for LAMP1 protein normalized to β-actin. n = 3 mice per group. (B–D) Quantification of LAMP1-positive immunostaining intensity is shown in B, and quantifications of LAMP1 in mitochondria and non-mitochondria are shown in C. Representative images of LAMP1 in hippocampi of indicated mice are shown in D, and the bottom panels show enlarged images of the field of interest indicated by the box in the top panels. (E) Representative immunoblots show immunoreactive bands for cathepsin D from hippocampal homogenates of indicated mice, and β-actin served as a loading control. The bar graph (E) presents quantification of immunoreactive bands for cathepsin D protein relative to β-actin. n = 3–5 mice and 5–8 slides per group. *P < 0.05, **P < 0.01 versus non Tg group in A–C and E. #P < 0.01 versus mAPP/GFP or mAPP/mPINK1 mice in C. Scale bars = 25 μm in D.
PINK1-mediated autophagy signalling contributes to the reduction in amyloid-β accumulation
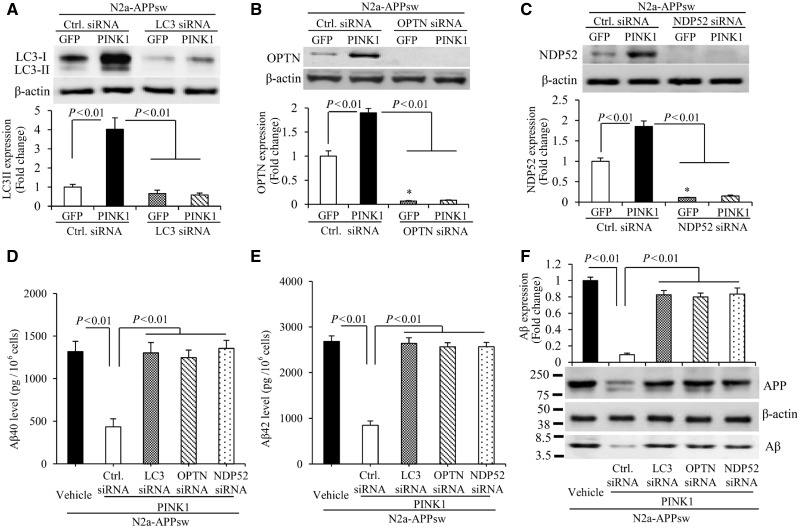
Given that PINK1 overexpression significantly elevated autophagosome marker LC3-II and autophagy receptors (OPTN and NDP52) in mAPP/PINK1 brains compared to mAPP brains (Fig. 3), we evaluated whether PINK1-mediated amyloid-β clearance via activation of autophagy signals. To this end, we examined the effect of PINK1-dependent autophagy activation on amyloid-β accumulation in amyloid-β-producing neurons. Neuro2a cells stably overexpressed APPsw (N2a-APPsw) were transduced with lentivirus-PINK1 or lentivirus-vector as a control in the absence or presence of siRNA against LC3, OPTN, or NPD52, respectively. Consistent with our observations of reduced PINK1 in patients with Alzheimer’s disease and mAPP mice (Supplementary Fig. 1), PINK1 level was lower in N2a-APPsw cells (Supplementary Fig. 5A and B). After PINK1 transduction, N2a-APPsw neurons revealed a significantly elevated expression of autophagy proteins, including the active form of LC3-II, OPTN and NDP52 (Supplementary Fig. 5A, C–E and 6A–C), as well as lysosomal protein LAMP1 (Supplementary Fig. 5A and F). Next, to determine the effects of suppressing these essential autophagy proteins, cells were treated with two independent sets of siRNA against LC3, OPTN, and NDP52 at different target sites, respectively. The scramble control siRNA-treated cells were used as negative controls (Fig. 5A–C and Supplementary Fig. 6A–C). PINK1-transduced N2a-APPsw cells displayed significant lower levels of amyloid-β and APP compared to vector-transduced N2a-APPsw cells (Fig. 5D–F and Supplementary Fig. 6D and E). Conversely, knockdown of LC3, OPTN or NDP52 by siRNA in PINK1-transduced N2a-APPsw cells abolished the protective effects of PINK1 on amyloid-β accumulation and APP levels (amyloid-β ELISA results are presented in Fig. 5D, E and Supplementary Fig. 6D and E, and immunoblotting results in Fig. 5F). These data suggest that increasing PINK1 expression enhances amyloid-β clearance via activation of OPTN and NDP52 signals in addition to LC3 autophagosome.
Figure 5.
PINK1 decreases amyloid-β accumulation via activation of autophagy signalling in N2a-APPsw cells. (A–C) Immunoblotting of N2a-APPsw cell lysates for LC3 (A), OPTN (B) and NDP52 (C) in the indicated groups of cells. N2a-APPsw cells were transduced with lentivirus encoding GFP or PINK1 and co-transfected with siRNA against LC3 (A), OPTN (B), NDP52 (C) and control siRNA. The expression levels of LC3-II, OPTN, and NDP52 were eliminated in cells treated with corresponding siRNA compared to control siRNA. Representative immunoblots show the immunoreactive bands for LC3-II (A), OPTN (B), and NDP52 (C), and β-actin served as a loading control. The bar graphs present quantification of immunoreactive bands for LC3-II (A), OPTN (B), and NDP52 (C) normalized to β-actin. *P < 0.01 versus control siRNA treatment group in B and C. n = 3 independent experiments of each group. (D–F) The levels of amyloid-β40 (Aβ40) (D) and amyloid-β42 (Aβ42) (E) in the N2a-APPsw cells transduced with lentivirus encoding GFP or PINK1 and co-transfected with siRNA against LC3, OPTN, NDP52 or control siRNA were measured by amyloid-β ELISA (n = 4). (F) Representative immunoblots show immunoreactive bands for APP and amyloid-β proteins in N2a-APPsw cell lysates with above treatment, and β-actin served as a loading control. The bar graph (F) presents quantification of immunoreactive bands for amyloid-β relative to β-actin. n = 3 independent experiments of each group.
PINK1 improves synaptic function and learning memory in mAPP mice and alleviates synaptic loss
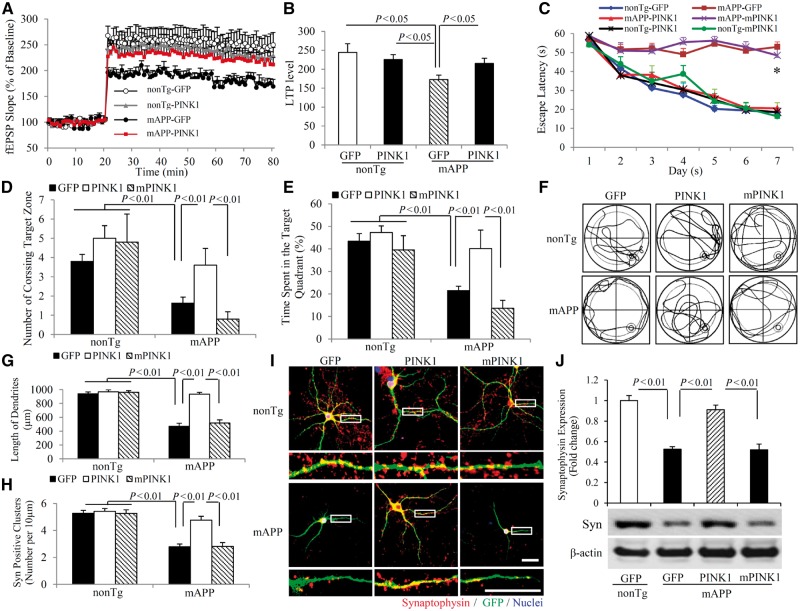
To investigate whether PINK1-mediated mitochondrial function links to synaptic network, we first assessed synaptic transmission under basal conditions and during long-term potentiation (LTP), a form of synaptic plasticity that is widely studied as a cellular model for learning and memory. LTP recorded in CA1 neurons of hippocampal slices was significantly declined in mAPP/GFP mice compared to non-transgenic/GFP or non-transgenic/PINK1 mice, whereas LTP was significantly restored in hippocampal slices from mAPP/PINK1 mice compared with those from mAPP/GFP mice (Fig. 6A and B). PINK1 expression also enhanced basal synaptic transmission [field-excitatory post-synaptic potential (fEPSPs), Supplementary Fig. 7A) in CA1 neurons of non-transgenic mice. These data demonstrate that PINK1 activation significantly alleviates amyloid-induced synaptic injury.
Figure 6.
PINK1 reduces LTP and learning impairments. (A and B) LTP was recorded in hippocampal CA1 neurons of the indicated mice at 12 months of age. Data are presented as mean ± standard error (SE). n = 3–5 mice per group. (C–F) Effect of PINK1 on learning and memory in mAPP mice. (C) Mean escape latency to the hidden platform during each day of the training session. (D) Mean number of crossings of the target during probe trials. (E) Time spent in the quadrant with the hidden platform in probe trials. (F) Pattern of representative searching traces for the indicated transgenic (Tg) mice in search of the target. n = 5–10 mice per group. *P < 0.05 compared to non-transgenic (nonTg)/GFP, non-transgenic/PINK1, non-transgenic mPINK1 and mAPP/PINK1 mice. (G–J) Effect of PINK1 on synaptic density. Hippocampal neurons cultured from non-transgenic and mAPP mice in vitro Day 5 (DIV 5) were transduced with lentivirus encoding PINK1, mPINK1 or GFP vector, respectively. Neurons at in vitro Day 14 were stained with Syn (red). Quantifications of the total length of dendrites (G) and the number of Syn-positive clusters (H) per micrometre of dendrite length are counted in the indicated groups of neurons. n = 16–21 neurons per group. (I) Representative images of immunofluorescence staining for Syn (red) and GFP (green) in the indicated groups of cells. The lower panels show enlarged images of the interest area indicated by the box in the upper panels. (J) The bar graph presents quantification of immunoreactive Syn bands normalized to β-actin from the hippocampal homogenates of the indicated transgenic mice. The representative immunoblots show the immunoreactive bands for Syn protein, and β-actin served as a loading control. n = 3 independent experiments of each group.
To determine whether the protective effect of PINK1 on synaptic function reflects improvement in cognitive function, we evaluated spatial reference memory and target searching strategy using a Morris water maze (Fig. 6C–F). Compared to mAPP/GFP mice and mAPP/mPINK1 mice, mAPP/PINK1 mice showed a shorter latency for locating the hidden platform during the training session (Fig. 6C). In the Probe test, mAPP/PINK1 mice increased both the number of times crossing the target and the time in the target quadrant (Fig. 6D–F). These results indicate that mAPP/PINK1 mice retained a better search strategy. There were no significant differences in latency, number of times crossing the target, and time in the target quadrant between non-transgenic/GFP and non-transgenic/PINK1 mice, indicating that PINK1 overexpression has no effect on baseline spatial reference memory. Mice among tested groups had similar swimming speed as measured by the visual swimming speed test (Supplementary Fig. 7B). Thus, the observed difference in spatial learning and memory is due to defects in cognition rather than differential motility or motivation. These data indicate that increased neuronal PINK1 expression and activity improve learning and memory in mAPP mice.
Given that synaptic loss is an early feature of Alzheimer’s disease, we assessed the morphological integrity of synapses in cultured Alzheimer’s disease neurons carrying mutant human APP (mAPP neurons) and neurons exposed to amyloid-β. Introduction of lentivirus encoding human PINK1 into mAPP neurons resulted in an increase in the length of dendrites, compared to mAPP neurons transduced by lentivirus control vector encoding GFP (Fig. 6G). Synaptic density was quantified by measuring synaptophysin (presynaptic marker)-positive clusters attaching to dendrites. PINK1-transduced mAPP neurons displayed increased synaptophysin density compared to vector-transduced mAPP neurons (Fig. 6H and I). Accordingly, loss of synaptophysin protein was completely restored in mAPP/PINK1 mice but not in mAPP/mPINK1 mice compared to mAPP/GFP mice (Fig. 6J).
PINK1-mediated autophagy signalling contributes to amyloid-β-induced mitochondrial and synaptic abnormalities
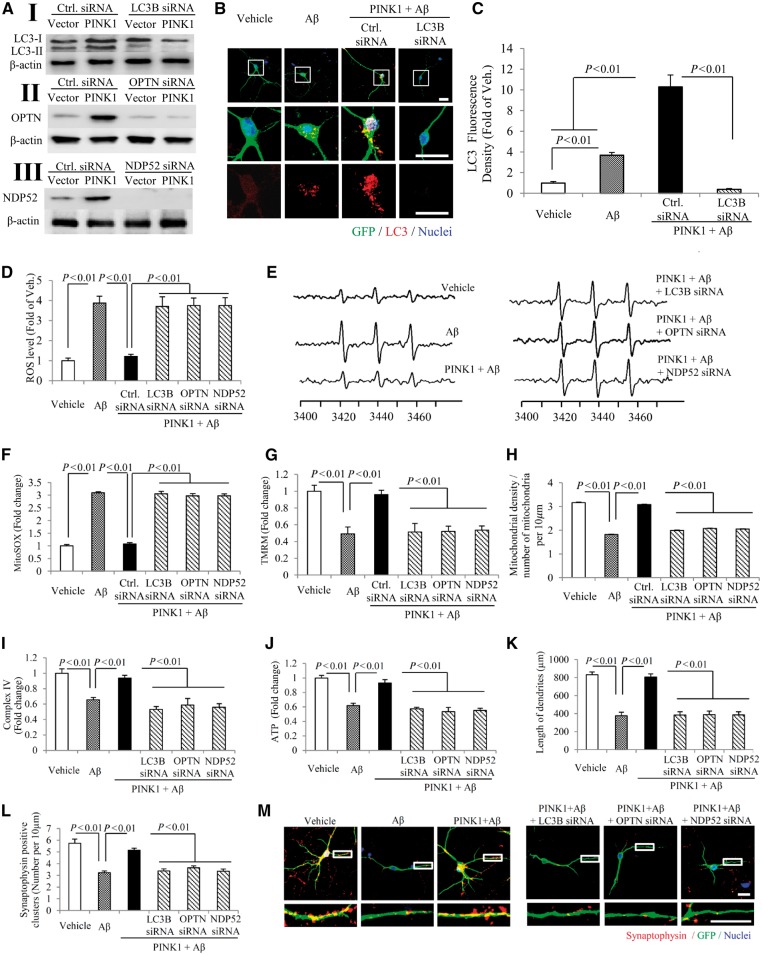
We then determined whether PINK1-mediated autophagy activity depends on OPTN or NDP52 autophagy signalling. Consistent with our observation obtained in mAPP/PINK1 mice (Fig. 3), PINK1-transduced primary neurons significantly elevated expression of autophagy proteins, including the active form of LC3-II, OPTN, and NDP52, compared to vector-induced neurons (Fig. 7A–C and Supplementary Fig. 8A). These effects were significantly diminished by siRNA knockdown of LC3-II, OPTN or NDP52 when the PINK1-transduced neurons were exposed to amyloid-β stimuli, compared to scramble control siRNA-treated neurons (Fig. 7A–C and Supplementary Fig. 8A). In addition, confirmation of such suppressive effects on activation of LC3, OPTN, or NDP52 proteins was achieved by the second set of siRNA knockdown of these genes (Fig. 7A–C and Supplementary Fig. 8A). Accordingly, loss of LC3, OPTN, or NDP52 in PINK1-expressed neurons failed to protect against amyloid-β-induced alterations in mitochondrial and synaptic function as evidenced by increased ROS levels (Fig. 7D–F, Supplementary Fig. 8B and C, and representative images of MitoSOX™ Red staining in Supplementary Fig. 9A), reduced mitochondrial membrane potential (Fig. 7G, and representative images of TMRM red staining in Supplementary Fig. 9B), decreased mitochondrial density (Fig. 7H, and representative images of SODII staining in Supplementary Fig. 9A), complex IV activity (Fig. 7I and Supplementary Fig. 8D), ATP levels (Fig. 7J and Supplementary Fig. 8E), neuronal process length, and synaptic density (Fig. 7K–M and Supplementary Fig. 8F–H). In parallel, knockdown by the second set of siRNA against LC3, OPTN, or NDP52 resulted in similar suppression of PINK1-induced protective effect on amyloid-β-triggered neuronal, mitochondrial and synaptic alterations (Supplementary Fig. 8). These data highlight the role of PINK1-mediated autophagy signalling via OPTN and NDP52 in protection against amyloid-β-insulted mitochondrial damage and synaptic injury.
Figure 7.
Effect of autophagy signalling on PINK1/amyloid-β-induced mitochondrial and synaptic alterations. (A) Immunoblotting of cell lysates for LC3 (I), OPTN (II) and NDP52 (III) in the indicated groups of cells. Hippocampal neurons at in vitro Day 10 (DIV 10) were transduced with PINK1 and co-transfected with siRNA against LC3, OPTN, NDP52 and control siRNA. The expression levels of LC3-II, OPTN, and NDP52 were eliminated in neurons treated with corresponding siRNA compared to control siRNA (I–III). n = 3 independent experiments. (B and C) Representative images for PINK1/GFP (green), LC3 (red) and nuclei (blue) in the indicated groups of neurons (B) and quantification of LC3-positive puncta (C). (D and E) Quantification of EPR spectra (D) and representative spectra of EPR (E) in the indicated neurons. (F–J) Quantifications of MitoSOX staining (F), TMRM staining (G), mitochondrial density (H), CcO activity (I), and ATP level (J) in the indicated neurons. (K–M) Quantifications of length of dendrites (K), synaptophysin-positive clusters (l), and representative images (M) for synaptophysin (red), GFP (green), and nuclei (blue). The lower panels show enlarged images of field of interest indicated by the box in the upper panels (M). n = 10 neurons for LC3-positive puncta counting, MitoSOX™ and TMRM staining quantifications, n = 10 for EPR, n = 3 for CcO activity and ATP level, and n = 20 neurons for quantifications of length of dendrites and synaptophysin-positive clusters. Scale bars = 25 μm in B.
Interestingly, upon a higher concentration of amyloid-β (1 µM) treatment, some mitochondria appeared as a doughnut-like shape in the cultured hippocampal non-transgenic neurons (Supplementary Fig. 10), whereas increasing PINK1 reduced the numbers and percentage of donut-shaped mitochondria (Supplementary Fig. 10). This result further validates that PINK1 rescues mitochondrial defects in amyloid-β-induced neurotoxicity.
Effects of PINK1 deficiency on amyloid-β accumulation, mitochondrial function, LTP and learning memory in mAPP mice
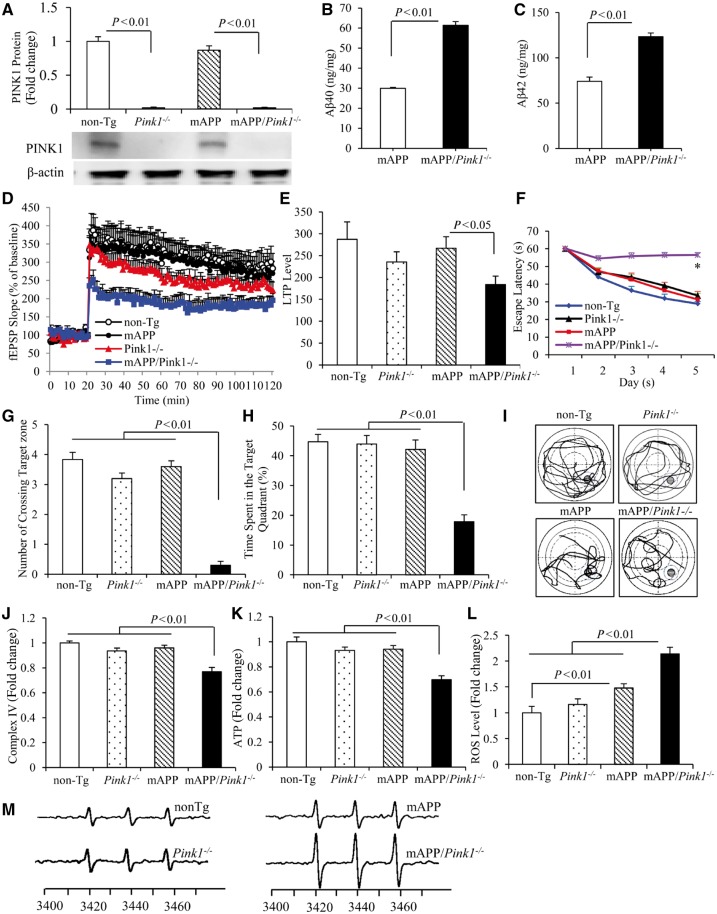
To validate the role of PINK1 in amyloid pathology and neuronal function further, PINK1 knockout (Pink1−/−) mice were crossed with mAPP mice to generate PINK1-deficient mAPP mice (mAPP/Pink1−/−). Next we evaluated the effects of PINK1 deficiency on amyloid-β level, LTP and learning impairments and mitochondrial defects in mAPP mice at 5–6 months of age, an early stage of amyloid pathology prior to mitochondrial defect. As shown in Fig. 8A, PINK1 protein was absent in the hippocampi from both Pink1−/− and mAPP/Pink1−/− mice. Notably, amyloid-β40 and amyloid-β42 levels were significantly elevated by 2-fold in mAPP/Pink1−/− hippocampi compared to mAPP mice at 5–6 months of age (Fig. 8B and C). The percentage of amyloid-β immunoreactive plaque loads was also 2-fold higher in mAPP/Pink1−/− hippocampi (Supplementary Fig. 11A and B). Similarly, amyloid-β levels and amyloid-β plaque load were further elevated in mAPP/Pink1−/− mice at 9 months of age (Supplementary Fig. 11C–F). These results demonstrated that lack of PINK1 promotes amyloid-β accumulation and amyloid pathology in mAPP mice. To validate the effect of PINK1 on amyloid-β accumulation further, we measured mouse endogenous amyloid-β in PINK1 null mice. Interestingly, amyloid-β42, a pathological toxic form of amyloid-β, was significantly elevated by 1.2-fold when compared to age-matched non-transgenic mice (Supplementary Fig. 11G and H) and implies the involvement of PINK1 in amyloid-β accumulation.
Figure 8.
PINK1 deficiency accelerates amyloid-β accumulation and exaggerates abnormalities in LTP, learning and memory, and mitochondrial function in mAPP mice. (A) Representative immunoblots show immunoreactive bands for PINK1 protein from hippocampal homogenates of non-transgenic (non-Tg) PINK1 knockout (Pink1−/−), mAPP, and mAPP/Pink1−/− mice at 5–6 months of age, and β-actin served as a loading control. The bar graph (A) presents quantification of immunoreactive bands for PINK1 protein normalized to β-actin. n = 3 mice per group. (B and C) Amyloid-β levels in hippocampi of mAPP and mAPP/Pink1−/− mice (5–6 months old) were measured by amyloid-β ELISA. n = 6 mice per group. (D and E) LTP was recorded in hippocampal CA1 neurons of the indicated mice. Data are presented as mean ± SE. n = 3–5 mice per group. (F–I) Effect of PINK1 knockout on learning and memory in mAPP mice. Learning and memory were tested using a Morris water maze (MWM) in the indicated groups of mice. (F) Mean escape latency to the hidden platform during each day of the training session. *P < 0.01 compared to non-transgenic, Pink1−/− and mAPP mice. (G) Mean number of crossings of the target during probe trials. (H) Time spent in the quadrant with the hidden platform in probe trials. (I) Pattern of representative searching traces for the indicated transgenic (Tg) mice in search of the target. n = 5–10 mice per group. (J and K) CcO (complex IV, J) activity and ATP levels (K) in brain hippocampal tissues of the indicated mice. n = 6 mice per group. Quantification of EPR spectra (L) in the indicated transgenic mice. Data are expressed as fold increase relative to non-transgenic mice. (M) Representative spectra of EPR. The peak height in the spectrum indicates levels of ROS. n = 5–6 mice per group.
Furthermore, mAPP/Pink1−/− mice displayed early deficit in synaptic function as shown by the reduction of LTP in CA1 neurons of hippocampus compared to mAPP mice and non-transgenic mice (Fig. 8D and E). There were no effects of PINK1 deficiency on the basal synaptic transmission (fEPSPs, Supplementary Fig. 12A) either in Pink1−/−or mAPP/Pink1−/− mice compared to non-transgenic mice. These data demonstrate that loss of PINK1 exacerbates/promotes synaptic injury prior to cerebral amyloid pathology/deposits.
Similarly, mAPP/Pink1−/− mice displayed a much longer latency for locating the hidden platform during the training session (Fig. 8F) compared with Pink1−/−, mAPP mice and non-transgenic littermates at 5–5.5 months of age (Fig. 8F–I). In the Probe test, mAPP/Pink1−/− mice revealed the reduction in both the number of times crossing the target (Fig. 8G and I) and time in the target quadrant (Fig. 8H and I). These results demonstrate that mAPP/Pink1−/− mice retained a poorer search strategy. Mice among tested groups had similar swimming speed as measured by the visual swimming speed test (Supplementary Fig. 12B), indicating that the observed difference in spatial learning and memory is due to defects in cognition. These results suggest an early perturbation of learning and memory, consequent to loss of PINK1 in mAPP mice.
We evaluated the effect of PINK1 deficiency on mitochondrial function and oxidative stress further. At the early stage of 5–6 months of age, no significant changes in CcO activity, a key complex IV enzyme, and ATP level were observed in mAPP or Pink1−/− mice compared to non-transgenic controls, while complex IV activity and ATP levels were significantly reduced only in mAPP/Pink1−/− mice (Fig. 8J and K). The ROS levels were significantly increased in mAPP/Pink1−/− mice compared to mAPP mice (Fig. 8L and M). Thus, lack of PINK1 exaggerates mitochondrial perturbation and oxidative stress relevant to amyloid-β pathogenesis.
PINK1 kinase activity is essential for reducing amyloid-β accumulation and rescuing amyloid-β-induced mitochondrial defects and synaptic abnormalities
Finally, to validate the role of PINK1 kinase activity on amyloid-β accumulation and synaptic and mitochondrial alterations, we examined the effects of a triple kinase dead mutants of PINK1 (K219A, D362A and D384A, triple-mPINK1) as a classical kinase dead mutant form (Beilina et al., 2005; Pridgeon et al., 2007) in mAPP neurons. Compared to wild-type PINK1 transduced-mAPP neurons, triple-mPINK1 transduced mAPP neurons failed to attenuate amyloid-β accumulation and had no protective effect on synaptic loss (Supplementary Fig. 13D–G) and mitochondrial dysfunction (Supplementary Fig. 13H–I). These results were similar to those from the mutant PINK1-L347P used in this study. Therefore, PINK1 kinase activity is required for the protection from amyloid pathology and amyloid-β-mediated mitochondrial and synaptic perturbation.
Discussion
Alzheimer’s disease is a neurodegenerative disorder clinically characterized by progressive dementia and pathologically characterized by early mitochondrial and synaptic degeneration associated with cerebral and mitochondrial amyloid-β accumulation and intra-neuronal fibrillary tangles with hyperphosphorylated tau (Selkoe, 2002; Lin and Beal, 2006; Du et al., 2010, 2012; Yu et al., 2016). The pathogenesis of Alzheimer’s disease involves multiple pathways, which may explain the limited clinical benefits of simply removing amyloid-β from patients. For example, early synaptic mitochondrial damage may cause damage to the brain for many years before an Alzheimer's patient experiences memory loss (Du et al., 2010, 2012). In the present study, we have provided substantial evidence of multiple PINK1-dependent pathways relevant to amyloid-β clearance, Alzheimer’s disease-specific mitochondrial and synaptic properties, and cognitive function in the Alzheimer’s disease mouse model in vivo and in vitro. Importantly, we also demonstrate that PINK1 kinase activity is crucially involved in amyloid pathology and Alzheimer’s disease- and amyloid-β-mediated mitochondrial and synaptic perturbation. Finally, lack of PINK1 promotes and exacerbates amyloid accumulation accompanied by abnormalities in synaptic and cognitive function as well as mitochondrial function, before such alterations were observed in mAPP mice.
First, we observed that expression of PINK1 is associated with amyloid pathology. PINK1 expression is significantly decreased in Alzheimer’s disease-affected human brains and Alzheimer’s disease mice overexpressing human amyloid-β. Given that amyloid pathology and oxidative stress are age-related, we examined the direct effect of amyloid-β or oxidative stress on PINK1 expression. PINK1 expression level was significantly increased in neurons treated with amyloid-β for 12 h or with H2O2 for 1.5–6 h, and then declined after 24 h of amyloid-β treatment or 9–12 h of H2O2 treatment. These data suggest that prolonged stimulation with amyloid-β or oxidative stress results in a significant reduction of PINK1 expression (Supplementary Figure. S14A and B). Consistent with these results in vitro, PINK1 expression levels correlate with amyloid pathology as shown by age-associated changes in PINK1 levels (Supplementary Fig. 14C). Compared to 3-month-old mAPP mice, PINK1 was first elevated in 6-month-old and then declined in 12–20-month-old mAPP mice with severe amyloid pathology and mitochondrial defects. These data suggest that amyloid-β and oxidative stress activate the induction of PINK1 at the early stage of these toxic stimuli to protect cells from stress-induced perturbation. However, sustained toxic stimuli eventually suppress PINK1 expression, which may explain the reduction in PINK1 in Alzheimer’s disease brain and amyloid-β-rich mAPP mice. Compromised PINK1 function may cause the observed amyloid-β- and Alzheimer’s disease-related impairment in mitochondrial function and ability of clearance and degradation of amyloid-β. Indeed, restoring/increasing PINK1 function in amyloid-β-enriched brains improves mitochondrial and synaptic function and eliminates amyloid pathology in the Alzheimer’s disease mouse model in vivo and in vitro. Conversely, genetic deletion or knockdown of PINK1 exacerbates amyloid-β-mediated detrimental effects. Previous studies reported that PINK1 accumulates on depolarized mitochondria in cells cultured under acute induction of pathological condition, such as depolarized mitochondria by exposing cells to CCCP (Narendra et al., 2010; Meissner et al., 2011). These results are consistent with our observations in in vitro cultured neurons exposed to amyloid-β and oxidative stress insults (Supplementary Fig. 14A and B), and in vivo mAPP mice at early stages of amyloid pathology (Supplementary Fig. 14C).
Second, we have provided substantial evidence of the effects of PINK1 on amyloid pathology from in vitro and in vivo not only in PINK1 overexpression and but also in PINK1 deficiency of mAPP mice. There are several potential mechanisms underlying PINK1-involved amyloid-β accumulation and amyloid pathology through amyloid-β production and clearance/degradation. We have demonstrated that PINK1 overexpression or depletion suppressed or enhanced ROS production in mAPP mice (Fig. 2E–H), together with improvement in early deficits in mitochondrial function (Fig. 2A–D). These results suggest that PINK1-mediated mitochondrial ROS production links to amyloid pathology in both PINK1/mAPP (Figs 1 and 2) and PINK1KO/mAPP mice (Fig. 8J–M and Supplementary Fig. 11). Indeed, scavenging mitochondria-derived ROS by mito-TEMPO not only blunt ROS production but also largely reduced cellular amyloid-β and APP levels in human amyloid-β-producing N2a-APP cells carrying human mutant APP gene in a dose-dependent manner (data not shown). Furthermore, mito-TEMPO robustly inhibited amyloid-β-induced activation of NF-κB by suppressing phosphorylation of NF-κB subunits p50 and p65 together with reduced amyloid-β and APP levels (data not shown). Oxidative stress decreases the activity of alpha-secretase while promoting the expression and activation of β- and γ-secretase, enzymes critical to the generation of amyloid-β from APP (Tamagno et al., 2002, 2008; Chen et al., 2008; Shen et al., 2008; Quiroz-Baez et al., 2009; Oda et al., 2010; Yoo et al., 2010). Oxidative stress mediated the activation of transcription factors including the redox-sensitive activation protein (AP1) and NF-κB enhances β-site APP cleaving enzyme 1 (BACE1) (Sambamurti et al., 2004; Chami et al., 2012; Chen et al., 2012). In fact, the promoter and 5′ untranslated region of the BACE gene contain binding sites for multiple transcription factors, including NF-κB, thereby activation of NF-κB by oxidative stress may in turn promote BACE expression (Sambamurti et al., 2004). PINK1 has been shown to protect against oxidative stress by targeting mitochondrial chaperone (Pridgeon et al., 2007). Thus, the PINK1/ROS/NF-κB pathway could contribute to alter APP/amyloid-β levels, possibly through APP transcription or α-, β-, or γ-secretases. Additional evidence of the impact of PINK1 signalling on NF-κB activation is demonstrated by the activation of the autophagy receptor OPTN. It has been reported that OPTN knockdown results in NF-κB activation (Akizuki et al., 2013). Therefore, activation of NF-κB due to a loss of PINK1/OPTN function could also be responsible for increased APP and amyloid-β. We demonstrated that knockdown of OPTN resulted in increased APP and amyloid-β in PINK1-expressing mAPP/amyloid-β-producing neurons (Fig. 5).
Furthermore, PINK1-mediated autophagy signalling to enhance mitochondrial clearance and lysosome activity may also contribute to amyloid-β degradation and clearance. Activation of PINK1 upregulates expression levels of LC3 and autophagy receptors (OPTN and NDP52) in amyloid-β-enriched mAPP brain. Blockade of these autophagy signals abolishes the protective effects of PINK1 on amyloid-β accumulation, suggesting that PINK1-activated autophagy pathways via OPTN and NDP52 are responsible for reducing amyloid pathology. We also observed that augmenting PINK1 expression activates autophagy/lysosome pathway by upregulation of lysosome-related protein such as LAMP1 and cathepsin D. The PINK1-involved autophagosome–lysosome pathway (Nguyen et al., 2016) could be another possible mechanism underlying amyloid-β metabolism and clearance in mAPP mice.
It has been shown that amyloid-β localizes in the extracellular and intracellular compartments, including cell surfaces, endosome, lysosome, and outer mitochondrial membrane (Choy et al., 2012; Sannerud et al., 2016), which plays a role in cellular amyloid-β accumulation and neuronal perturbation. Emerging evidence has highlighted the role of mitochondrial amyloid-β in Alzheimer’s disease pathogenesis. Amyloid-β progressively accumulates in the mitochondria of the Alzheimer’s disease brain and several transgenic Alzheimer’s disease mouse models overexpressing amyloid-β (Hirai et al., 2001; Fernandez-Vizarra et al., 2004; Lustbader et al., 2004; Caspersen et al., 2005; Crouch et al., 2005; Devi et al., 2006; Manczak et al., 2006; Gillardon et al., 2007; Du et al., 2008, 2012; Hansson Petersen et al., 2008; Yao et al., 2009; Abramowski et al., 2012), including transgenic Alzheimer’s disease mice expressing human APPswe. Increased degradation of clearance of mitochondrial pool of amyloid-β by activating presequence protease (PreP), a novel mitochondrial amyloid-β degrading enzyme, not only reduced mitochondrial amyloid-β accumulation but also significantly affects total cerebral amyloid-β levels (Fang et al., 2015), suggesting that mitochondrial amyloid-β is not just a ‘spilling over’ from cellular aggregation and that PreP/amyloid-β in mitochondria also has an important regulating effect on total brain amyloid-β levels (Fang et al., 2015). Given that mitochondria function as guardians in the cytosol pathway (Ruan et al., 2017), and that exogenous or intracellular amyloid-β is capable of direct transport into mitochondria via mitochondrial channel proteins such as TOMM40 (Hansson Petersen et al., 2008), endoplasmic reticulum/mitochondria contact (Caspersen et al., 2005; Hedskog et al., 2013), the receptor for advanced glycation end product (RAGE), endoplasmic reticulum/mitochondrial contact (Caspersen et al., 2005; Hedskog et al., 2013), or by an unknown mechanism, the mitochondrial pool of amyloid-β may undergo dynamic changes in different intracellular compartments, contributing to the balance of intracellular/extracellular amyloid-β accumulation.
In summary, possible mechanisms underlying PINK1-altered amyloid-β accumulation are: (i) activation of βAPP transcription via NF-κB/oxidative stress induced by amyloid-β and mitochondrial damages; (ii) modulating APP processing by altering α-, β-, or γ-secretase activities via NF-κB/oxidative; and (iii) activation of PINK1-mediated autophagy signal promoting amyloid-β degradation and clearance, which could have an impact on crosstalk of mitochondria with cerebral and mitochondrial amyloid-β metabolism. Detailed mechanisms of how PINK1 affects APP/amyloid-β metabolism and production or processing require further investigations.
Third, PINK1 is an important regulator of mitochondrial quality control, which is essential for maintaining proper mitochondrial function and integrity by regulating the balance between the elimination of damaged mitochondria through autophagy, and augmentation of mitochondrial bioenergy (Lazarou et al., 2015). Alzheimer’s disease-affected brains reveal a decrease in healthy mitochondria and an increase in dysfunctional and damaged mitochondria, leading to disruption of mitochondrial homeostasis (Du et al., 2010; Gan et al., 2014a; Fang et al., 2015; Yu et al., 2016). We demonstrate that restoring PINK1 expression/activity protects against amyloid-β-induced aberrant mitochondrial function as shown by increased membrane potential, key respiratory enzyme activity and ATP levels. Increased PINK1 function significantly suppresses mitochondrial oxidative stress and ROS generation. Blockade of autophagosome LC3-II activation and autophagy receptor (OPTN or NPD52)-activated signalling failed to rescue amyloid-β-mediated aberrant mitochondrial and synaptic function through PINK1, suggesting that PINK1-dependent autophagy signalling is responsible for suppression of amyloid-β-induced perturbation. Lysosomes play a fundamental role in the autophagic pathway by degrading damaged organelles and aggregated protein such as damaged mitochondria and amyloid-β (Saido and Leissring, 2012; Orr and Oddo, 2013). Elevated levels of both LAMP1 and cathepsin D in mAPP/PINK1 mice suggest that PINK1 enhances autophagy-lysosome activity, promoting clearance and degradation of damaged mitochondria and amyloid-β.
Finally, PINK1-mediated mitochondrial function has been linked to synaptic plasticity, as we observed that PINK1 overexpression significantly improved synaptic and cognitive function in a mouse model of Alzheimer’s disease. Our previous studies showed that the addition of ROS-scavenging enzymes alleviated amyloid-β-induced decline in LTP (Du et al., 2008; Ma et al., 2011). Given that PINK1 protects against ROS accumulation and generation, the loss of antioxidant effects of PINK1 may also be a mechanism underlying the impairments in synaptic plasticity and memory in Alzheimer’s disease (Liu et al., 2003; Serrano and Klann, 2004; Esposito et al., 2006; Du et al., 2008). Mitochondria produce most of the ATP and control the local Ca2+ homeostasis required for maintaining synaptic function, phosphorylation, and synaptic plasticity (Rizzuto et al., 2012). The active concentration of mitochondria in specific neuronal regions, such as growth cones and synapses (Li et al., 2004; Chang et al., 2006; Fang et al., 2016a, b), is important for neuronal function. Mitochondria become dysfunctional through the permeability transition induced by the synergistic effects of oxidative stress and dysregulation of cytosolic free Ca2+ (Xie, 2004). Indeed, PINK1 overexpression suppressed cyclophilin D expression in mAPP/PINK1 brains (data not shown); therefore, PINK1 may be involved in other mechanisms such as impaired mitochondrial transition pore (Du et al., 2008, 2011), Ca2+-regulated signalling pathways, oxidative stress-mediated kinase systems and activation of transcription factors (Vitolo et al., 2002; Xie, 2004; Origlia et al., 2008; Du et al., 2014).
Notably, to address whether PINK1 kinase activity is required for amyloid pathology and amyloid-β-induced defects, we examined the effect of inactive mutant forms of PINK1, mPINK1 (L347P, defined as a loss-of-function form of PINK1) or triple-mPINK1 (PINK1 kinase dead mutant, K219A, D362A and D384A) as a classical kinase dead mutant form that is stable but lacking PINK1 kinase activity (Beilina et al., 2005; Pridgeon et al., 2007) on Alzheimer’s disease pathology, mitochondrial function, synaptic density and learning and memory abilities. PINK1 overexpression significantly reduced amyloid-β accumulation, alleviated mitochondrial and synaptic alterations, and improved learning and memory. The mutant PINK1 without kinase activity had no protective effects on these parameters. Thus, PINK1 activity is essential for the maintenance of mitochondrial and cognitive function as well as amyloid-β levels reflecting the balance between rates of production and clearance.
Using genetically manipulated PINK1 expression (gain and loss of PINK1 function) and inactive mutant PINK1 without kinase activity as a control to validate the role of PINK1 kinase activity, we comprehensively evaluated the effects of PINK1 on mitochondrial function. Our results clearly demonstrated the mitochondrial improvements dependent on PINK1 kinase activity. Furthermore, mitochondrial improvements disappeared by genetically deletion or knockdown of PINK1.
Mitochondrial dynamics are important for maintaining mitochondrial morphology, distribution and function. Amyloid-β or Alzheimer’s disease-derived mitochondria contributes importantly to the impaired mitochondrial dynamics by increased mitochondrial fission protein Drp1 or fusion MFN2 (Wang et al., 2008, 2009a, b; Gan et al., 2014a, b). Blocking excessive mitochondrial fission Drp1 or recruiting fusion MFN2 improves mitochondrial function (Gan et al., 2014a, b; Huang et al., 2015). This suggests that modulating mitochondrial dynamics genes that control mitochondrial fission or fusion to maintain normal balance of mitochondrial dynamics have protective effects on mitochondrial defects relevant to amyloid-β toxicity. In view of the involvement of PINK1 in mitochondrial dynamics showing overexpression of PINK1 reduces Drp1-induced mitochondrial fission (Cui et al., 2010), PINK1 could be directly or indirectly involved in amyloid-β-mediated imbalance between mitochondrial fission and fusion rates. The direct link of PINK1 to amyloid-β-induced abnormal mitochondrial dynamics requires further investigation.
Parkin serves as a downstream substrate for PINK1. Damaged mitochondria are removed by autophagy after activation of PINK1 kinase and the E3 ubiquitin ligase parkin (Narendra et al., 2008, 2010). Parkin amplifies the mitophagy signal triggered by PINK1. It is possible to have the similar protective effect on mitochondrial function by enhancing parkin-involved mitochondrial clearance. To clarify, the present study is to elucidate PINK1 signalling independent on parkin in amyloid-β-induced mitochondrial perturbation by recruiting the autophagy receptors OPTN and NDP52 (Lazarou et al., 2015).
Taken together, we have provided substantial evidence showing PINK1-dependent amyloid-β pathology and mitochondrial alterations from in vitro neuronal and in vivo transgenic mouse model. Our studies have clearly demonstrated that downregulation of PINK1 is associated with Alzheimer’s disease pathology and oxidative stress. Restoring the expression and activity of neuronal PINK1 significantly reduced amyloid-β accumulation and improved mitochondrial function and synaptic plasticity. As a result, cognitive function decline in Alzheimer’s disease mice was substantially reversed by PINK1 overexpression. Importantly, non-functional mutant human PINK1 lacking kinase activity has no such protective effects against Alzheimer’s disease-related mitochondrial defects or behaviour changes in both in vitro and in vivo settings. Lack of PINK1 accelerates amyloid-β accumulation and exaggerates mitochondrial and synaptic damages. Notably, our findings highlight a novel mechanism by which PINK1 kinase activity-dependent signalling promotes rescue of amyloid pathology and amyloid-β-mediated mitochondrial and synaptic dysfunction in a manner requiring activation of autophagy receptor OPTN or NDP52. We propose that compromised PINK1 function induced by ROS or amyloid-β in the Alzheimer’s disease milieu perturbs mitochondrial clearance, leading to imbalanced mitochondrial homeostasis via increased accumulation of damaged mitochondria and impaired degradation of proteins including amyloid-β, which contributes importantly to amyloid and mitochondrial pathology and synaptic degeneration in Alzheimer’s disease. Thus, activation of PINK1 may present a new therapeutic approach for halting Alzheimer’s disease progression at the early stage through mitochondrial quality control combined with eliminating amyloid pathology.
Supplementary Material
Acknowledgements
We thank Doris Chen for editing the paper and Justin T. Douglas for assistance using the EPR instrument. We thank Drs Mei and William Bowers for rAAV2 packaging.
Funding
This study was supported by NIH/NIA (R37AG037319, R01AG044793, and R01AG053041), and NIH/NINDS (R01NS089116) and Howard Mossberg distinguished professorship endowment from University of Kansas to S.S.Y., and by NIEHS (R01 ES22274) to K.T. The EPR instrumentation was provided by NSF Chemical Instrumentation Grant (# 0946883).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- CcO
cytochrome c oxidase
- EPR
electron paramagnetic resonance
- LTP
long-term potentiation
- ROS
reactive oxygen species
References
- Abramowski D, Rabe S, Upadhaya AR, Reichwald J, Danner S, Staab D, et al. Transgenic expression of intraneuronal Abeta42 but not Abeta40 leads to cellular Abeta lesions, degeneration, and functional impairment without typical Alzheimer's disease pathology. J Neurosci 2012; 32: 1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akizuki M, Yamashita H, Uemura K, Maruyama H, Kawakami H, Ito H, et al. Optineurin suppression causes neuronal cell death via NF-kappaB pathway. J Neurochem 2013; 126: 699–704. [DOI] [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol 2014; 206: 655–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrachina M, Maes T, Buesa C, Ferrer I. Lysosome-associated membrane protein 1 (LAMP-1) in Alzheimer's disease. Neuropathol Appl Neurobiol 2006; 32: 505–16. [DOI] [PubMed] [Google Scholar]
- Batlevi Y, La Spada AR. Mitochondrial autophagy in neural function, neurodegenerative disease, neuron cell death, and aging. Neurobiol Dis 2011; 43: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, et al. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci USA 2005; 102: 5703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, et al. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J 2005; 19: 2040–1. [DOI] [PubMed] [Google Scholar]
- Chami L, Buggia-Prevot V, Duplan E, Del Prete D, Chami M, Peyron JF, et al. Nuclear factor-kappaB regulates betaAPP and beta- and gamma-secretases differently at physiological and supraphysiological Abeta concentrations. J Biol Chem 2012; 287: 24573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci 2006; 26: 7035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Zhou W, Liu S, Deng Y, Cai F, Tone M, et al. Increased NF-kappaB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer's disease. Int J Neuropsychopharmacol 2012; 15: 77–90. [DOI] [PubMed] [Google Scholar]
- Chen JX, Yan SS. Role of mitochondrial amyloid-beta in Alzheimer's disease. J Alzheimers Dis 2010; 20 (Suppl 2): S569–78. [DOI] [PubMed] [Google Scholar]
- Chen L, Na R, Gu M, Richardson A, Ran Q. Lipid peroxidation up-regulates BACE1 expression in vivo: a possible early event of amyloidogenesis in Alzheimer's disease. J Neurochem 2008; 107: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy RW, Cheng Z, Schekman R. Amyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid beta (Abeta) production in the trans-Golgi network. Proc Natl Acad Sci USA 2012; 109: E2077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 2006; 441: 1162–6. [DOI] [PubMed] [Google Scholar]
- Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, et al. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J Neurosci 2005; 25: 672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Tang X, Christian WV, Yoon Y, Tieu K. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J Biol Chem 2010; 285: 11740–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci 2006; 26: 9057–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med 2008; 14: 1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Wu X, Sosunov AA, McKhann GM, Chen JX, et al. Cyclophilin D deficiency rescues Abeta-impaired PKA/CREB signaling and alleviates synaptic degeneration. Biochim Biophys Acta 2014; 1842 (12 Pt A): 2517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci USA 2010; 107: 18670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Yan SS. Synaptic mitochondrial pathology in Alzheimer's disease. Antioxid Redox Signal 2012; 16: 1467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging 2011; 32: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, et al. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci 2006; 26: 5167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Qing Y, Yan S, Chen D, Yan SS. Development and dynamic regulation of mitochondrial network in human midbrain dopaminergic neurons differentiated from iPSCs. Stem Cell Rep 2016a; 7: 678–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Wang Y, Zhang Z, Du H, Yan S, Sun Q, et al. Increased neuronal PreP activity reduces Abeta accumulation, attenuates neuroinflammation and improves mitochondrial and synaptic function in Alzheimer disease's mouse model. Hum Mol Genet 2015; 24: 5198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Yan S, Yu Q, Chen D, Yan SS. Mfn2 is required for mitochondrial development and synapse formation in human induced pluripotent stem cells/hiPSC derived cortical neurons. Sci Rep 2016b; 6: 31462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Zhang Z, Li H, Yu Q, Douglas JT, Bratasz A, et al. Increased electron paramagnetic resonance signal correlates with mitochondrial dysfunction and oxidative stress in an Alzheimer's disease mouse brain. J Alzheimers Dis 2016c; 51: 571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S, Serrano J, Bentura ML, Martinez-Murillo R, et al. Intra- and extracellular Abeta and PHF in clinically evaluated cases of Alzheimer's disease. Histol Histopathol 2004; 19: 823–44. [DOI] [PubMed] [Google Scholar]
- Gan X, Huang S, Wu L, Wang Y, Hu G, Li G, et al. Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer's disease cybrid cell. Biochim Biophys Acta 2014a; 1842: 220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X, Wu L, Huang S, Zhong C, Shi H, Li G, et al. Oxidative stress-mediated activation of extracellular signal-regulated kinase contributes to mild cognitive impairment-related mitochondrial dysfunction. Free Radic Biol Med 2014b; 75: 230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA 2008; 105: 11364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 2010; 12: 119–31. [DOI] [PubMed] [Google Scholar]
- Gillardon F, Rist W, Kussmaul L, Vogel J, Berg M, Danzer K, et al. Proteomic and functional alterations in brain mitochondria from Tg2576 mice occur before amyloid plaque deposition. Proteomics 2007; 7: 605–16. [DOI] [PubMed] [Google Scholar]
- Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci USA 2008; 105: 13145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedskog L, Pinho CM, Filadi R, Ronnback A, Hertwig L, Wiehager B, et al. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer's disease and related models. Proc Natl Acad Sci USA 2013; 110: 7916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci 2001; 21: 3017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Wang Y, Gan X, Fang D, Zhong C, Wu L, et al. Drp1-mediated mitochondrial abnormalities link to synaptic injury in diabetes model. Diabetes 2015; 64: 1728–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SY, Huang WP, Liou HC, Fu WM. LC3 overexpression reduces Abeta neurotoxicity through increasing alpha7nAchR expression and autophagic activity in neurons and mice. Neuropharmacology 2015; 93: 243–51. [DOI] [PubMed] [Google Scholar]
- Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 2015; 524: 309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004; 119: 873–87. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Alzheimer's APP mangles mitochondria. Nat Med 2006; 12: 1241–3. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci USA 2003; 100: 8526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science 2004; 304: 448–52. [DOI] [PubMed] [Google Scholar]
- Ma T, Hoeffer CA, Wong H, Massaad CA, Zhou P, Iadecola C, et al. Amyloid beta-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J Neurosci 2011; 31: 5589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 2006; 15: 1437–49. [DOI] [PubMed] [Google Scholar]
- Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet 2011; 20: 2495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK. The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem 2011; 117: 856–67. [DOI] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 2008; 183: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 2010; 8: e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TN, Padman BS, Usher J, Oorschot V, Ramm G, Lazarou M. Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J Cell Biol 2016; 215: 857–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda A, Tamaoka A, Araki W. Oxidative stress up-regulates presenilin 1 in lipid rafts in neuronal cells. J Neurosci Res 2010; 88: 1137–45. [DOI] [PubMed] [Google Scholar]
- Origlia N, Righi M, Capsoni S, Cattaneo A, Fang F, Stern DM, et al. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J Neurosci 2008; 28: 3521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr ME, Oddo S. Autophagic/lysosomal dysfunction in Alzheimer's disease. Alzheimers Res Ther 2013; 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 2015; 85: 257–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol 2007; 5: e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Hamamichi S, Caldwell KA, Caldwell GA, Yacoubian TA, Wilson S, et al. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol Brain 2008; 1: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz-Baez R, Rojas E, Arias C. Oxidative stress promotes JNK-dependent amyloidogenic processing of normally expressed human APP by differential modification of alpha-, beta- and gamma-secretase expression. Neurochem Int 2009; 55: 662–70. [DOI] [PubMed] [Google Scholar]
- Rappold PM, Cui M, Grima JC, Fan RZ, de Mesy-Bentley KL, Chen L, et al. Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat Commun 2014; 5: 5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med 2008; 14: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer's disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta 2012; 1822: 639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 2012; 13: 566–78. [DOI] [PubMed] [Google Scholar]
- Ruan L, Zhou C, Jin E, Kucharavy A, Zhang Y, Wen Z, et al. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 2017; 543: 443–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saido T, Leissring MA. Proteolytic degradation of amyloid beta-protein. Cold Spring Harbor Perspect Med 2012; 2: a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambamurti K, Kinsey R, Maloney B, Ge YW, Lahiri DK. Gene structure and organization of the human beta-secretase (BACE) promoter. FASEB J 2004; 18: 1034–6. [DOI] [PubMed] [Google Scholar]
- Sannerud R, Esselens C, Ejsmont P, Mattera R, Rochin L, Tharkeshwar AK, et al. Restricted location of PSEN2/gamma-secretase determines substrate specificity and generates an intracellular abeta pool. Cell 2016; 166: 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science 2002; 298: 789–91. [DOI] [PubMed] [Google Scholar]
- Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev 2004; 3: 431–43. [DOI] [PubMed] [Google Scholar]
- Shen C, Chen Y, Liu H, Zhang K, Zhang T, Lin A, et al. Hydrogen peroxide promotes Abeta production through JNK-dependent activation of gamma-secretase. J Biol Chem 2008; 283: 17721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria and cell bioenergetics: increasingly recognized components and a possible etiologic cause of Alzheimer's disease. Antioxid Redox Signal 2012; 16: 1434–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci USA 2009; 106: 20021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis 2002; 10: 279–88. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, et al. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem 2008; 104: 683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufi R, Gandhi S, de Castro IP, Lehmann S, Angelova PR, Dinsdale D, et al. Enhancing nucleotide metabolism protects against mitochondrial dysfunction and neurodegeneration in a PINK1 model of Parkinson's disease. Nat Cell Biol 2014; 16: 157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo OV, Sant'Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci USA 2002; 99: 13217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 2009a; 29: 9090–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA 2008; 105: 19318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Neurochem 2009b; 109 (Suppl 1): 153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmiller AM, Natera-Naranjo O, Reyna SM, Pearn ML, Zhao X, Nguyen P, et al. A gamma-secretase inhibitor, but not a gamma-secretase modulator, induced defects in BDNF axonal trafficking and signaling: evidence for a role for APP. PLoS One 2015; 10: e0118379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie CW. Calcium-regulated signaling pathways: role in amyloid beta-induced synaptic dysfunction. Neuromol Med 2004; 6: 53–64. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA 2006; 103: 10793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 2009; 106: 14670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo MH, Gu X, Xu XM, Kim JY, Carlson BA, Patterson AD, et al. Delineating the role of glutathione peroxidase 4 in protecting cells against lipid hydroperoxide damage and in Alzheimer's disease. Antioxid Redox Signal 2010; 12: 819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Fang D, Swerdlow RH, Yu H, Chen JX, Yan SS. Antioxidants rescue mitochondrial transport in differentiated Alzheimer's disease trans-mitochondrial cybrid cells. J Alzheimers Dis 2016; 54: 679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.