Moral emotions have been assessed with correlational imaging techniques and framed as monolithic domains. Hernando et al. present a novel task indexing dimensions of schadenfreude and envy: deservingness, morality, and legality. An increase in both schadenfreude and envy accompanies atrophy of social cognition networks in patients with behavioural variant FTD.
Keywords: dementia, social cognition, brain atrophy, frontotemporal dementia
Abstract
The study of moral emotions (i.e. Schadenfreude and envy) is critical to understand the ecological complexity of everyday interactions between cognitive, affective, and social cognition processes. Most previous studies in this area have used correlational imaging techniques and framed Schadenfreude and envy as unified and monolithic emotional domains. Here, we profit from a relevant neurodegeneration model to disentangle the brain regions engaged in three dimensions of Schadenfreude and envy: deservingness, morality, and legality. We tested a group of patients with behavioural variant frontotemporal dementia (bvFTD), patients with Alzheimer’s disease, as a contrastive neurodegeneration model, and healthy controls on a novel task highlighting each of these dimensions in scenarios eliciting Schadenfreude and envy. Compared with the Alzheimer’s disease and control groups, patients with bvFTD obtained significantly higher scores on all dimensions for both emotions. Correlational analyses revealed an association between envy and Schadenfreude scores and greater deficits in social cognition, inhibitory control, and behaviour disturbances in bvFTD patients. Brain anatomy findings (restricted to bvFTD and controls) confirmed the partially dissociable nature of the moral emotions’ experiences and highlighted the importance of socio-moral brain areas in processing those emotions. In all subjects, an association emerged between Schadenfreude and the ventral striatum, and between envy and the anterior cingulate cortex. In addition, the results supported an association between scores for moral and legal transgression and the morphology of areas implicated in emotional appraisal, including the amygdala and the parahippocampus. By contrast, bvFTD patients exhibited a negative association between increased Schadenfreude and envy across dimensions and critical regions supporting social-value rewards and social-moral processes (dorsolateral prefrontal cortex, angular gyrus and precuneus). Together, this study provides lesion-based evidence for the multidimensional nature of the emotional experiences of envy and Schadenfreude. Our results offer new insights into the mechanisms subsuming complex emotions and moral cognition in neurodegeneration. Moreover, this study presents the exacerbation of envy and Schadenfreude as a new potential hallmark of bvFTD that could impact in diagnosis and progression.
Introduction
The emerging cognitive neuroscience of moral emotions, such as Schadenfreude (a German word implying pleasure for others’ misfortunes) and envy (Takahashi et al., 2009; Jankowski and Takahashi, 2014) have opened a window for comprehending ecological affective processes (Fontenelle et al., 2015). So far, this field has mainly relied on correlational imaging studies, largely neglecting the contributions of the lesion model approach, which helps to reveal direct links between brain regions and fine-grained cognitive functions. Active tasks in neuroimaging and electromagnetic studies provide only indirect evidence of brain-behaviour associations (Linden, 2012). The lesion model (Rorden and Karnath, 2004; Shahid et al., 2017) and, in particular, the neurodegenerative lesion model (Irish et al., 2012; García-Cordero et al., 2016; Melloni et al., 2016; O'Callaghan et al., 2016; Ibanez et al., 2017), reveals the critical areas, which directly relate to performance in a specific domain. Here we profit from this methodological strategy to shed new light on the neural correlates of both emotion types and their key dimensions.
Emotions constitute an adaptive mechanism supporting the regulation of one’s own acts and the assessment of others’ behaviours (Haidt, 2003; Adolfi et al., 2016). Moral emotions, in particular, seem to be mediated by culturally defined conventions that are critical for group organization and cohesiveness (Haidt, 2003; Tangney et al., 2007). Contrary to basic emotions, these are linked to the welfare of social groups (Moll et al., 2003; Moll and de Oliveira-Souza, 2007), as they encourage or inhibit behaviours depending on their social acceptability (Ortony et al., 1990). Moral emotions seem to modulate how humans assess which behaviours are social and morally acceptable. According to Haidt (2003), moral emotions are shaped under the influence of two factors: elicitors and action tendencies. Elicitors range from the behaviour of others to events affecting them and their evaluations of own actions. As regards social action tendencies, moral emotions are experienced when we are motivated to deploy other-targeted actions, modulating social order and welfare. For Haidt (2003), envy and Schadenfreude appear to integrate another category as they involve no pro-social action tendencies (Haidt, 2003). Similar to the self-conscious family of emotions, which arise from a discrepancy between one’s ideal self and current self (Ortony et al., 1990), Schadenfreude and envy imply a discrepancy between one’s current status and the status of another person (Ortony et al., 1990). Thus, social comparison is crucial for describing all types of moral emotions. New approaches in the study of moral emotions have described Schadenfreude and envy as counter-empathy (Cikara and Fiske, 2013) or fortune of others’ emotions (Shamay-Tsoory et al., 2014).
Schadenfreude (to gloat), refers to the perceiver’s experience of pleasure at another’s misfortune (van Dijk et al., 2006; Dvash et al., 2010b; Jankowski and Takahashi, 2014). Envy, on the other hand, is defined as displeasure associated with another’s success. These emotions have been shown to involve a critical interplay between social, moral, and affective processes (Takahashi et al., 2009; Dvash et al., 2010b; Jankowski and Takahashi, 2014), highlighting the complexity of their organization. At the neuroanatomical level, Schadenfreude has been associated with activity in ventral striatum, as assessed with functional MRI (Takahashi et al., 2009; Cikara and Fiske, 2013; Jankowski and Takahashi, 2014). By contrast, envy has been associated with activation of temporal regions as well as the anterior and medial cingulate cortices (ACC and MCC, respectively) (Takahashi et al., 2009; Cikara and Fiske, 2013; Jankowski and Takahashi, 2014). Despite the above advances, the literature on moral emotions still faces two caveats: the assumption of monolithic conceptions of the constructs and the lack of lesion studies to address the issue.
First, Schadenfreude and envy are most often treated as monolithic phenomena (van Dijk et al., 2006; Takahashi et al., 2009). However, research on various other emotion types have revealed partially dissociable dimensions (Portmann, 2000; Chester et al., 2013). Indeed, Schadenfreude or envy can be differently triggered by a broad spectrum of behaviours and affective scenarios (Portmann, 2000). Previous studies have shown that envy and Schadenfreude have different modulators, including (i) the inferred desirability of an outcome for a target (Haidt, 2003; Tangney et al., 2007; Takahashi et al., 2009; Jankowski and Takahashi, 2014); (ii) target likeability (Haidt, 2003; Tangney et al., 2007; Takahashi et al., 2009; Jankowski and Takahashi, 2014); (iii) inferred target deservedness (Feather and Sherman, 2002; van Dijk et al., 2005; Smith and Kim, 2007; Dvash et al., 2010a; Chester et al., 2013; Ben-Ze’ev, 2014; van Dijk and Ouwerkerk, 2014; Zaki et al., 2015); and (iv) subject’s perception of justice and fairness in others’ outcomes (Feather and Sherman, 2002; Smith, 2009; Dvash et al., 2010a; Jankowski and Takahashi, 2014; Shamay-Tsoory et al., 2014; van Dijk and Ouwerkerk, 2014; Yoder and Decety, 2014; Najle, 2015; Portmann, 2017). The situations used to trigger envy and Schadenfreude in our study are in part supported by some of these factors. In particular, deservingness situations trigger moral emotions with modulating factors such as target likeability and inferred target deservedness. In contrast, moral and legal situations evoke envy and Schadenfreude by modifying the subjects’ perception of justice and fairness in outcomes reached by a receptor.
Deservingness, morality, and legality concepts are all intertwined notions able to modulate experiences of Schadenfreude (van Dijk and Ouwerkerk, 2014) and envy (Smith and Kim, 2007; Chester et al., 2013). Considering that these emotions respond to the disagreement between one’s current situation and that of another person, different sources and modulators can be identified for these emotions, including deduced target deservedness (Feather and Sherman, 2002; van Dijk et al., 2005; Smith and Kim, 2007; Dvash et al., 2010a; Chester et al., 2013; Ben-Ze’ev, 2014; van Dijk and Ouwerkerk, 2014; Zaki et al., 2015) and the perception of fairness in others’ outcomes (Feather and Sherman, 2002; Smith, 2009; Dvash et al., 2010a; Jankowski and Takahashi, 2014; Shamay-Tsoory et al., 2014; van Dijk and Ouwerkerk, 2014; Yoder and Decety, 2014; Najle, 2015; Portmann, 2017). Along these lines, different approaches ranging from social psychology (Ortony et al., 1990; Portmann, 2002; Ben-Ze’ev, 2014), to sociology (Plutchik, 1980; Fiske, 1992) and philosophy (de Spinoza, 1883) and, more recently, research in the field of social cognitive neuroscience (van Dijk et al., 2005; Takahashi et al., 2009; Dvash et al., 2010a; Chester et al., 2013; Cikara and Fiske, 2013; Fiske and Taylor, 2013), indicate that Schadenfreude and envy are complex and non-unified emotional experiences modulated by different factors, including the interplay between high and low order affective and cognitive mechanisms (Chester et al., 2013; Jankowski and Takahashi, 2014).
Also, building on Schopenhauer’s (1788–1860) account of Schadenfreude as a most obscure expression of human emotionality, this emotion can be considered to possess a component of maliciousness, even if the outcome is deserved. However, Schadenfreude may also rely on notions of justice (van Dijk et al., 2006; Ben-Ze’ev, 2014), and moral or legal analyses can exonerate the pleasure experienced in presence of others’ misfortunes. Enjoying the misfortune of someone who has violated a legal code or moral norm may be considered as a well-intentioned emotion, as it would reflect a reaction to fairness (van Dijk et al., 2006; Ben-Ze’ev, 2014). Although both situations involve Schadenfreude, they clearly differ in their affective implications. Likewise, previous studies suggest that envy can be elicited either when someone gets an undeserved outcome or when someone is rewarded despite moral or legal transgressions (Portmann, 2000; Ben-Ze’ev, 2014; van Dijk and Ouwerkerk, 2014).
From a neurobiological perspective, abundant evidence indicates that morality is not a wholly unified faculty, but a collection of dissociable neurocognitive processes depending on the type of transgression being judged (Parkinson et al., 2011; Hayashi et al., 2014; Sinnott-Armstrong, 2016). Although different moral situations can share some neurocognitive mechanisms, they also exhibit dissociable neural pathways. For instance, processing of different moral situations, including disgust and moral transgressions, share neurocognitive processes as all of them are related to activity of the ACC, the medial prefrontal cortex, and the temporoparietal junction. However, these situations also have dissociable neural activations, as moral transgressions are more related to activity of the amygdala and the parahippocampus, whereas disgust is more associated to the activity of the posterior cingulate cortex and the dorsolateral prefrontal cortex (Parkinson et al., 2011; Hayashi et al., 2014; Sinnott-Armstrong, 2016). Our study aims to delve deeper into perspective by analysing the emotional responses associated with envy and Schadenfreude as dissociable experiences rather than as unified, monolithic constructs. We use the term ‘monolithic’ to refer to different situations than can elicit a same emotional response. For instance, although all dimensions of moral emotions can elicit pleasure or displeasure, each of them is typical of different situations (moral, legal or deservedness). This approach could offer new options in the study of envy and Schadenfreude by providing evidence on how they can be modulated by situations that differ in their nature.
Thus, at least three distinct dimensions of Schadenfreude and envy can be identified as more or less prominent depending on the situation: deservingness (the degree to which an actor deserves the outcome he experienced), morality (the degree to which an actor gets a different outcome than he/she could expected involving a moral precept violation), and legality (the degree to which an actor gets a different outcome than he/she could expected involving a legal precept violation). While each of these dimensions involves different cognitive and affective foundations and may thus rely on partially different neural mechanisms, such fine-grained associations have not yet been assessed.
Against this background, we conducted the first lesion-model study on the correlates of these dimensions for both Schadenfreude and envy. Specifically, we focused on the behavioural variant of frontotemporal dementia (bvFTD), a relevant neurodegenerative lesion model featuring selective atrophy of the main pathways associated with envy (the ACC and temporal areas) and Schadenfreude (the fronto-striatal network). This complex clinical syndrome is characterized by social cognition deficits and marked behavioural changes that impair social interaction (Piguet et al., 2011; Ibañez and Manes, 2012; Seeley et al., 2012; Ibanez et al., 2014, 2017). More particularly, bvFTD have been associated with alterations in social and moral cognition, including reduced empathic concern for others’ suffering (Eslinger et al., 2011a; Baez et al., 2014c, 2016b; Melloni et al., 2014), diminished prosocial sentiments (Moll et al., 2011), and reduced long-term cooperative behaviours (Melloni et al., 2016; Ibanez et al., 2017). Furthermore, patients with bvFTD show altered moral judgements, displaying more utilitarian judgements in the face of moral dilemmas (Baez et al., 2014a, 2016a). A similar pattern has been also observed in extreme criminal terrorists (Baez et al., 2017b). Finally, these patients have been shown to display increased antisocial and criminal behaviour (de Oliveira-Souza et al., 2008; Liljegren et al., 2015), as well as a relatively high incidence of legal violations (Mendez, 2010). In sum, bvFTD offers a relevant lesion model to assess the specific neural correlates of the different dimensions operative in the experience of Schadenfreude and envy.
To this end, we created a novel task tapping the dimensions of deservingness, morality, and legality in moral emotions, and administered it to patients with bvFTD, matched healthy controls, and patients with early stage Alzheimer’s disease—another form of dementia offering a contrastive neurodegenerative model. Our task features situations whose characters are involved in fortunate and unfortunate events evoking envy and Schadenfreude, respectively. Crucially, each emotion type involved a subset of scenarios dominated by feelings of deservingness, morality, or legality. In addition, neutral situations were added to test task comprehension and attentional engagement.
We propose two sets of hypotheses at behavioural and neurocognitive levels. First, we expected a specific pattern of responses regarding the dimensions of moral emotions. Previous studies have shown that situations where justice notions are disrupted (and which feature severe violations of social codes) involve increased discomfort and heightened emotional responses (Tangney et al., 2007; Yoder and Decety, 2014). Thus, for Schadenfreude and envy, healthy controls can be expected to show more emotional responses in situations with prominent moral and legal components compared to deservingness situations. Second, given that patients with bvFTD present disruptions of social skills, moral cognition, disinhibited behaviours, and altered affective states, they were expected to experience exacerbated degrees of envy and Schadenfreude relative to the other two groups. Also, since these patients usually exhibit low sensitivity to follow moral and legal codes (Mendez et al., 2005; Mendez, 2010; Baez et al., 2014a, 2016a), and usually present counter-empathy behaviours (Moll et al., 2011), we expected bvFTD patients to exhibit higher scores across all dimensions (deservingness, moral or legal) in both emotion types. Thus, in those patients, a general (non-selective) increase of emotional responses can be predicted, irrespective of whether they are elicited by deservingness or moral-legal scenarios. Regarding neuroanatomy (assessed with voxel-based morphometry, VBM), we expected to find differential pathways implicated in Schadenfreude (striatum) and envy (ACC). In bvFTD patients, atrophy of frontotemporal regions subserving social cognition, cognitive control, and behavioural regulation (Ibañez and Manes, 2012; Seeley et al., 2012; Sedeno et al., 2017) should be associated with enhanced experiences of both emotion types. Furthermore, atrophy of regions involved in judging third-party and personal moral and legal situations (Buckholtz et al., 2008), such as the ACC and angular gyrus, which are impaired in bvFTD and associated with moral deficits and illegal behaviour in this condition (Mendez, 2010; Baez et al., 2016b), should also be associated with envy.
Materials and methods
Participants
We recruited 64 participants from an ongoing protocol (Couto et al., 2013; Baez et al., 2014c, 2016a, 2017c; García-Cordero et al., 2016; Melloni et al., 2016; Santamaria-Garcia et al., 2016; Sedeno et al., 2016), namely, 20 patients that met revised criteria for probable bvFTD (Rascovsky et al., 2011), 24 patients diagnosed with early onset Alzheimer’s disease (McKhann et al., 2011), and 20 healthy control subjects. All patients were assessed by a multidisciplinary group of experts, including two neurologists (A.L., C.H.C.), three psychiatrists (J.M.S., G.O., H.S.), and two geriatricians (J.F.M., S.H.). Groups did not differ significantly in terms of age, gender, or years of education (Table 1). Patients and controls were included if they had no history of major neurological or psychiatric illnesses (other than bvFTD or Alzheimer’s disease, in the case of patients) or alcohol/drug abuse. Patients and healthy controls were included in the study if they had no general language deficits, including semantic and comprehension alterations (see additional test below). All participants provided written informed consent in agreement with the Declaration of Helsinki. The study was approved by the Institution’s Ethics and Research Committee.
Table 1.
Demographic and neuropsychological data for all three groups
| Controls (n = 20) | BvFTD (n = 20) | Alzheimer’s disease (n = 24) | P-value | P-value | |
|---|---|---|---|---|---|
| Mean /SD | Mean /SD | Mean /SD | Controls versus bvFTD | BvFTD versus Alzheimer’s disease | |
| Demographics | |||||
| Age (years) | 61.1 (7.98) | 58.9 (6.35) | 63.1 (5.64) | N.S. | N.S. |
| Gender (F:M) | 11:9 | 9:11 | 13:11 | N.S. | N.S. |
| Education (years) | 13.32 (4.9) | 14.81 (4.3) | 13.88 (5.6) | N.S. | N.S. |
| Years since disease onset | NA | 3.1 (2.2) | 3.9 (1.9) | NA | N.S. |
| MMSE | 27.4 (2.1) | 21.6 (4.5)** | 20.7 (4.8)** | <0.01 | N.S. |
| General cognitive assessment | |||||
| MoCA | 26.2 (3.1) | 19.2 (6.7)** | 22.1 (6.1)** | <0.01 | N.S. |
| Total IFS score | 24.2 (2.9) | 16.9 (5.4) | 19.1 (5.9) | N.S | N.S. |
| Inhibitory cognitive control measure | |||||
| Hayling test | 9.4 (2.1) | 16.4 (2.8) | 14.8 (3.1) | <0.01 | <0.05 |
| Behavioural changes | |||||
| FrSBE (Frontal System Behavioural Scale) total* | NA | 48.4 (31.1) | 37.9 (27.7) | NA | <0.01 |
| Social cognitive measure | |||||
| RMET | 0.88(0.10) | 0.51 (0.16)** | 0.69 (0.12)** | <0.01 | <0.05 |
*To obtain an index of progression of Frontal System Behavioural Scale (FrSBE) scores, we subtracted the present score from the previous score.
**Differences compared to controls.
N.S = differences were not significant; NA = not assessed.
Neuropsychological tests
General cognitive state
Participants’ general cognitive state was assessed with the Montreal Cognitive Assessment (MOCA) (Nasreddine et al., 2005), and their executive skills were evaluated with a frontal battery including the INECO Frontal Screening (IFS) test (Torralva et al., 2009). The IFS is a 30-point scale that has been shown to successfully detect executive dysfunction in patients with neurological and psychiatric diseases (Torralva et al., 2009; Baez et al., 2014b). This test includes the following eight subtests: (i) motor programming (Luria series, ‘fist, edge, palm’); (ii) conflicting instructions (hitting the table once when the administrator hits it twice, or hitting it twice when the administrator hits it only once); (iii) motor inhibitory control; (iv) numerical working memory (backward digit span); (v) verbal working memory (months backwards); (vi) spatial working memory (modified Corsi tapping test); (vii) abstraction capacity (inferring the meaning of proverbs); and (viii) verbal inhibitory control (modified Hayling test).
As a complementary measure of inhibitory control, we used the complete version of the Hayling test (Perez-Perez et al., 2016). This scale tracks response initiation and response suppression (Hornberger et al., 2010; Perez-Perez et al., 2016) and has been largely used to assess disinhibition in patients with neurodegenerative diseases (Torralva et al., 2009; Hornberger et al., 2010). Higher scores on the Hayling test are thought to reflect difficulties in inhibitory control (Torralva et al., 2009; Hornberger et al., 2010).
Neuropsychiatric manifestations, including apathy and disinhibition, were assessed with the subjective subscales of apathy, executive functions, and disinhibition of the Frontal System Behavioural Scale, which tracks the degree of behavioural changes since disease onset (Carvalho et al., 2013) and is usually reported as the percentage of changes of behavioural disturbances between free stages of disease and current stages (Carvalho et al., 2013; Santamaria-Garcia et al., 2016).
Theory of mind skills were assessed with the Reading the Mind in the Eyes Test (RMET) (Baron-Cohen et al., 1997), which is also sensitive for the assessment of bvFTD and patients with Alzheimer’s disease (Gregory et al., 2002; Couto et al., 2013; Baez et al., 2014a). This is a computerized and validated test consisting of 36 pictures of the eye region of a face. Participants are asked to choose which of four words best describes what the person in each photograph is thinking or feeling.
Language assessment
Language subscale in the Montreal Cognitive Assessment
We analysed the scores in the language subscale from the MoCA to assess general language performance among the groups. This subscale has a maximum possible score of 3 points out of a total of 30 in the MoCA. Results of this task revealed non-significant differences between groups [F(2,82) = 1.76, P = 0.11]. Mean values of the ratings for this subscale were 2 [standard deviation (SD) = 0.8] for bvFTD patients, 2.3 (SD = 0.6) for patients with Alzheimer’s disease, and 2.6 (SD = 0.4) for healthy controls.
Picture naming task
To rule out semantic deficits, we administered a well-established picture-naming task (Snodgrass and Feenan, 1990; Sanfeliu and Fernandez, 1996) based on 60 black-and-white pictures depicting three categories of living things (animals, birds, and fruit) and three categories of artefacts (household items, tools, and vehicles). Subjects were required to name with a single spoken word a picture included in an array of other pictures within a same category. Results of this task revealed non-significant differences between groups [F(2,82) = 2.16, P = 0.09]. Mean values of the ratings for picture naming test in bvFTD group, Alzheimer’s disease group and healthy controls were 53.7 (SD = 5.6), 56.4 (SD = 4.7), and 58.8 (SD = 2.8), respectively.
Experimental task
For the current study we created a novel task based on previous studies from our own group and others (Takahashi et al., 2009; Baez et al., 2016c, 2017d). We designed 40 situations to evoke different types of pleasant (Schadenfreude, n = 15) or unpleasant (envy, n = 15) emotional experiences, as well as emotionally neutral scenarios (n = 10) for control purposes. The situations eliciting each emotion type included five scenarios dominated by deservingness, five dominated by morality, and five dominated by legality. For Schadenfreude, we used situations that evoke pleasure due to feelings of deservingness (e.g. a liar is excluded from his or her group of friends), morality (e.g. a subject is found guilty and punished for faking a physical disability), and legality (e.g. a subject is punished for not paying for public transportation). Moreover, we included five neutral situations (e.g. a person turns on the light in the house when it gets dark). Likewise, for envy, we created situations evoking displeasure related to feelings of deservingness (e.g. a young man got a better test score for being the son of a professor), morality (e.g. someone avoids waiting at the bank by simulating a physical disability), and legality (e.g. a politician takes a vacation using taxpayers’ money). As for the Schadenfreude situations, we also included five neutral situations for the envy condition.
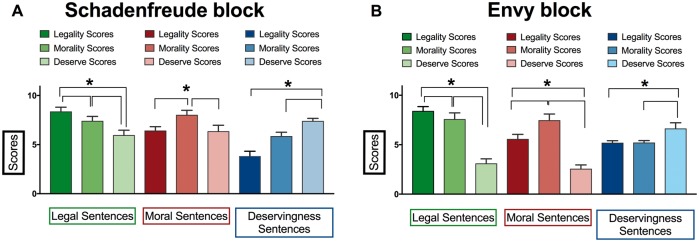
Situations associated with each dimension in each type of emotion were validated through a survey completed by 81 subjects (39 females), with a mean age of 39 years, (SD = 4), and an average of 12.6 years of education (SD = 2.2). In this validation study, participants scored a group of deservingness situations, a group of moral situations, and a group of legal situations according to their degree of deservedness, morality, and legality. Results confirmed that each group of situations effectively tracked the expected dimension for each moral emotion (Schadenfreude and envy). The deservingness situations obtained the highest deservingness scores, the moral situations obtained the highest moral content scores, and the legal situations obtained the highest legality scores (Fig. 1 and Supplementary material).
Figure 1.
Scores for deservingness, morality, and legality for each group of situations (deservingness, moral, and legal) of each moral emotion (envy and Schadenfreude). Situations dominated by deservingness, morality, and legality are identified in green, red, and blue, respectively. (A) Scores for the Schadenfreude block; (B) scores for the envy block.
In addition, an additional group of 39 extra subjects (details in the Supplementary material) validated the emotional profile of the situations of each dimension in each type of emotion (Schadenfreude and envy). Participants rated deservingness, moral, and legal situations according to their capacity to evoke different emotions. Mainly, we assessed the degree in which situations were able to evoke envy and Schadenfreude emotions, but also, as a control measure, we assessed to what extent the situations evoked other socio-moral emotions, such as pride and guilt. Thus, for Schadenfreude, the participants were required to score the situations according to how intensely they evoked (i) pleasure; (ii) Schadenfreude (as Spanish lacks a single word to name this experience, we translated it as: ‘La satisfacción por lo que le ha ocurrido al sujeto de la situación’; in English: ‘How much satisfaction do you feel for the protagonist’s outcome’); and (iii) pride. The participants also rated the envy situations in terms of how much these situations evoked (i) displeasure; (ii) envy; and (iii) guilt. Both pride and guilt emotions were used as control socio-moral emotions (Jankowski and Takahashi, 2014). Results confirmed that each group of situations effectively tracked the expected emotion (Schadenfreude/envy). Although the situations also evoked other socio-moral emotions, such as pride/guilt, the scores for those emotions were significantly lower than for envy/Schadenfreude and for more general emotional responses, namely displeasure/pleasure (Supplementary material and Supplementary Fig. 1).
Task procedure
First, each participant was shown a real-life photograph and a description of two target characters matched to the participant in age and gender (based on data obtained in a brief interview before the task). The situations for each type of emotion occurred to the character presented before each block. Based on the results from the validation study, situations were organized in two blocks. In the first block (envy), participants evaluated situations that evoked dimensions of envy and, in the second block, they evaluated situations that evoked dimensions of Schadenfreude. Envy situations were presented first, in line with previous evidence suggesting that the degree of envy predicts Schadenfreude responses (Takahashi et al., 2009; Jankowski and Takahashi, 2014). In each block, participants read five situations for each dimension. Within each block, we also included five situations describing five neutral events (e.g. a person washes clothes on weekends). Situations in each block were presented in pseudorandomized order.
Each situation was presented for 3 s. After reading each situation, participants reported the intensity of their ‘displeasure’ (for the envy block) or ‘pleasure’ (for the Schadenfreude block) regarding the character’s situation. This stage lasted ∼3 s (Fig. 2). All participants provided their responses using 9-point Likert scales, with 1 meaning ‘low emotional intensity’ and 9 meaning ‘high emotional intensity’. In line with previous research on empathy and moral processing (Baez et al., 2014a, c, 2016c; Patil and Silani, 2014; Carr et al., 2015), the scales were always accompanied by simple words indicating low and high pleasure/unpleasantness, thus minimizing memory-related confounds while reducing attentional demands and improving comprehension. This procedure has been reported in multiple neuropsychiatric conditions (Brown and Cohen, 2010; Ibanez et al., 2012; Baez et al., 2015, 2016a, c), including neurodegenerative diseases such as bvFTD (Zamboni et al., 2010; Eslinger et al., 2012; Cohen et al., 2016) and patients with Alzheimer’s disease (Lai et al., 2008; Tsoucalas et al., 2015).
Figure 2.
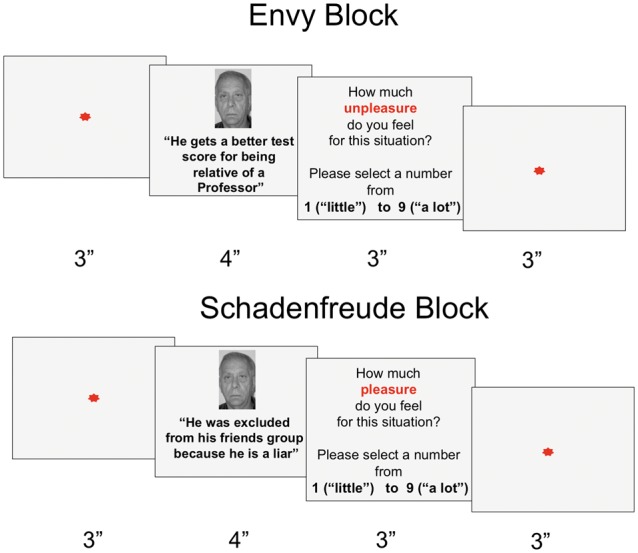
General task procedure. The upper panel shows a trial in the envy block and the bottom panel shows a trial in the Schadenfreude block. Participants first took part in the envy block and then performed the Schadenfreude block. In each block, participants read five situations (each lasting 3 s) in each dimension including a group of five neutral situations. In total, participants read 20 situations from each block, which were presented in randomized order. Next, participants reported the intensity of their ‘displeasure’ (for the envy block) or their ‘pleasure’ (for the Schadenfreude block) regarding the character’s situation using a 9-point Likert scale with 1 meaning low, and 9 meaning high intensities.
In our task, participants were asked to report their Schadenfreude and envy in terms of pleasure/displeasure given that these are the main overarching states elicited by each of those emotions. Previous studies have defined envy as: ‘an unpleasant, often painful emotion characterized by feelings of inferiority, hostility, and resentment caused by an awareness of a desired attribute by another person or group of persons’ (van Dijk et al., 2006; Smith and Kim, 2007; Smith, 2009; Takahashi et al., 2009; Dvash et al., 2010b; Jankowski and Takahashi, 2014). Also, Schadenfreude has been formally defined as the pleasure derived from the misfortune of others, involving ‘the expression of pleasure or self-satisfaction at another’s failure’ (van Dijk et al., 2006; Dvash et al., 2010b; Jankowski and Takahashi, 2014). Thus, we have used the terms ‘pleasure’ and ‘displeasure’ highlighting the critical emotional responses elicited by specific scenarios of envy and Schadenfreude. Also, explicit manifestations of envy and Schadenfreude are socially penalized (van Dijk et al., 2006; Dvash et al., 2010b; Jankowski and Takahashi, 2014) and, hence, subjects might report lower levels of these emotions due to social desirability. Thus, our procedure circumvents such biases by avoiding explicit questions to explore social situations and inquiring into pleasure/displeasure, in line with previous methodological recommendations for exploring social and affective cognitive processes (Berkman et al., 2014). In fact, asking about levels of pleasure/displeasure is the standard procedure reported in previous studies of envy and Schadenfreude (Takahashi et al., 2009; Baez et al., 2016c, 2017d). We have adopted this design to make our results comparable with those of previous relevant literature. Note, also, that the terms ‘pleasure’ and ‘displeasure’ are easier to understand and thus more reliable than ‘envy’ and ‘Schadenfreude’ as verbally cued measures. This is of critical relevance when assessing patients with such disorders as bvFTD and Alzheimer’s disease.
Task comprehension assessment
Before the emotion task, bvFTD and Alzheimer’s disease patients performed a pilot study involving situations with positive, negative, and neutral outcomes affecting a third person. Patients were asked to determine the type of valence (fortunate versus unfortunate) of each situation, which allowed us to assess general comprehension of situations while discarding potentially random response patterns. Relative to neutral situations, positive and negative situations were assigned higher and lower scores by both patient samples. These results suggest that patients discriminated the emotional valence of each situation type, showing that their performance was not biased by general comprehension difficulties (for details, see Supplementary material).
Data analysis
Demographic and cognitive state data were compared among groups using ANOVA and Tukey’s honest significant difference post hoc tests. Chi square tests were applied to analyse categorical variables. Differences between types of emotion (envy, Schadenfreude, neutral) in each group (bvFTD patients, Alzheimer’s disease patients, healthy controls) were first analysed using a 3 × 3 mixed repeated measures ANOVA. If any interaction among types of emotion and group was found, a second level of analysis was implemented for each emotion separately (Schadenfreude, envy, and neutral) and subsequently analysed with a one-way ANOVA using dimension (deservingness, morality, legality) as the within-subject factor and group (bvFTD patients, Alzheimer’s disease patients, healthy controls) as the between-subjects factor. To control for the effect of general cognitive status on the experimental tasks, we applied ANCOVA tests independently adjusted for total scores on the MoCA and the IFS batteries. In addition, we ran extra analyses controlling results on the experimental task for inhibitory control and theory of mind measures (Hayling test and RMET, respectively). As in other reports of neurodegenerative conditions from our group (Baez et al., 2014c; García-Cordero et al., 2016), we report only those effects that remained significant following covariation. Tukey’s HSD post hoc tests were used when appropriate. Effect sizes were calculated through the partial eta squared (η2) ratio.
Moreover, correlation analyses were used to explore the relationship between individual differences in disease presentation (regarding inhibitory control, theory of mind, and behavioural changes) and moral emotions (envy and Schadenfreude) in each group (bvFTD, Alzheimer’s disease, healthy controls). A global score of envy (mean of deservingness, morality, and legality dimensions) and a global score of Schadenfreude were created to facilitate the analysis of the relationship between each moral emotion and the cognitive-behavioural measures. We also conducted correlational analyses with the scores for each dimension of both moral emotions.
Imaging recordings and voxel-based morphometry
Recordings were restricted to bvFTD and healthy control groups. Structural brain images were obtained using a Philips Achieva 3 T scanner with a 16-channel SENSE antenna. The anatomical and 3D T1-weighted images had the following parameters: repetition time = 7.9 ms, echo time = 3.8 ms, ACQ matrix 220 × 220 pixels, voxel size 0.5 × 0.5 × 0.5 mm, 310 sections. Neuroanatomical correlates were analysed using VBM (Ashburner and Friston, 2000). Data processing and analysis were performed with VBM8 on the Statistical Parametric Mapping 8 package (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm), running under Matlab 2012 (MATLAB and Statistics Toolbox Release 2012b, The MathWorks, Inc., Natick, MA, USA). All imaging analysis steps were conducted as described in the VBM pipeline (http://www.fil.ion.ucl.ac.uk/∼john/misc/VBM), as follows. The T1-weighted images were normalized to the same stereotaxic space generated from the complete dataset using the DARTEL algorithm, which significantly reduces the imprecision of inter-subject recordings. The images were then segmented into white matter, and grey matter, and non-brain voxels (CSF). Subsequently, all images were modulated to correct volume changes by Jacobian determinants. Finally, images were smoothed by convolution with an isotropic 8-mm full-width Gaussian kernel at half maximum for statistical analyses.
First, we performed a whole-brain analysis using VBM to assess differences in brain atrophy between the group of patients with bvFTD and healthy controls (controlling for global intracranial volume, age, gender, and length of disease duration) (Supplementary Fig. 2). The statistical threshold for whole-brain analysis was defined as P < 0.001 (extent threshold = 50 voxels). In addition, to investigate potential, the association between brain areas and moral emotions, we performed a more restrictive analysis using a mask including the brain regions significantly associated with socio-moral cognitive processes and moral emotions (Schadenfreude and envy) (Supplementary Fig. 3). Then, using this mask, we performed multiple regression analyses for each group using topological false discovery rate (FDR) for correction, at a threshold of P < 0.05 (Figs 5 and 6). The topological FDR was used following previous studies that suggest that this procedure is more sensitive than voxel-wise FDR (Chumbley et al., 2010).
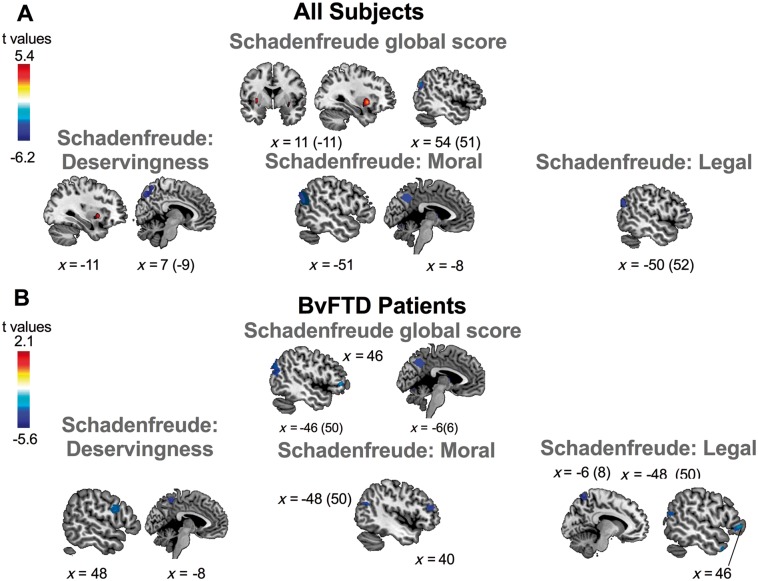
Figure 5.
VBM analyses and Schadenfreude dimensions. VBM regression analyses within the brain areas associated with the social cognition network and moral emotion areas and the scores for each sub-dimension of Schadenfreude. (A) VBM analysis in all subjects. (B) VBM analysis in bvFTD patients only (in both cases, P < 0.05, FDR corrected).
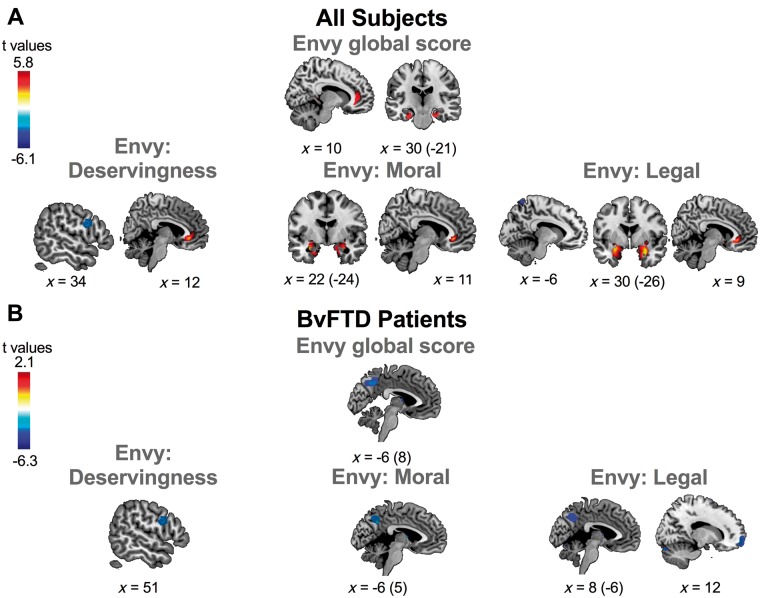
Figure 6.
VBM analyses and envy. VBM regression analyses within the brain areas associated with the social cognition network and moral emotion areas and the scores for each sub-dimension of envy. (A) VBM analysis in all subjects. (B) VBM analysis in bvFTD patients only (in both cases, P < 0.05, FDR corrected).
In assessing regions of atrophy as predictors of group performance in the experimental task, and following previous studies (Couto et al., 2013; Baez et al., 2016a), we restricted the data’s multidimensionality using an extended mask that included the brain areas reported to be involved in social-moral cognition and moral emotions. The group of brain areas composing the mask was corroborated using the Neurosynth database (http://www.neurosynth.org). This meta-analytic database aggregates activation from thousands of previous functional MRI studies (Yarkoni et al., 2011). This technique has been used to define main brain areas related to cognitive processes even in studies using imaging methods, including VBM (Vendetti and Bunge, 2014; Fermin et al., 2016; Allen et al., 2017). In Neurosynth online system we introduced as keywords: ‘moral’ and ‘social cognition’. Afterwards, we downloaded the mask for both terms and integrated the brain areas selected in a single unified mask. This mask, following the Neurosynth database, includes a default correction for brain multiple comparisons using a FDR criterion of P = 0.01. In addition, we included basal ganglia in this mask considering its role in processing moral emotions, in particular Shadenfreude (Takahashi et al., 2009). Furthermore, we also included bilateral frontal lobe as these brain areas are involved in inhibitory control (Collette et al., 2001; Nathaniel-James and Frith, 2002; Roca et al., 2010; Volle et al., 2011) and are related to regulation of moral emotions (Koechlin, 2011; Rudebeck et al., 2013). Therefore, we used an extended and unified mask including brain areas derived of two Neurosynth keywords (moral and social cognition), the basal ganglia, and frontal lobes. The final unified mask was smoothed with a standard σ = 4 mm isotropic Gaussian kernel (corresponding to full-width at half-maximum = 9.4 mm) following a procedure used in previous studies (Radua et al., 2014; Scarpazza et al., 2015).
In this study we used a mask that includes the ventromedial prefrontal cortex (VMPFC), the dorsolateral prefrontal cortex (DLPFC), the cingulate cortex (the anterior and posterior portions), the bilateral insula, the temporal lobes, bilateral angular gyrus, the superior and medial temporal gyri, the precuneus, the amygdala, the hippocampus, and the parahippocampus (socio-moral areas) (Moll et al., 2008; Pievani et al., 2011; Bzdok et al., 2012; Ibañez and Manes, 2012; Chiong et al., 2013; Baez et al., 2016a; Santamaria-Garcia et al., 2016; Sedeno et al., 2016), areas involved in inhibitory control (in particular the frontal lobe) (Collette et al., 2001; Nathaniel-James and Frith, 2002; Roca et al., 2010; Eslinger et al., 2011b; Volle et al., 2011), and regions involved in moral emotions, including the anterior cingulate cortex and the ventral and dorsal striatum (Takahashi et al., 2009; Dvash et al., 2010a; Jankowski and Takahashi, 2014; Shamay-Tsoory et al., 2014; van Dijk and Ouwerkerk, 2014; Baez et al., 2016c, 2017d) (Supplementary Fig. 3). Considering that the different dimensions of Schadenfreude and envy (deservingness, moral and legal) could encompass different complex cognitive-affective and social constructs, we also analysed the brain-behaviour correlations using a whole-brain analysis to reveal other brain areas involved in processing specific dimensions of moral emotions (Supplementary Fig. 4).
Brain–behaviour associations
To detect distinctive neurocognitive correlates of moral emotions, we performed a two-step analysis based on regression models between moral emotion measures and brain volume. The VBM regression models were run for of each dimension of moral emotions independently. This procedure was applied to analyse which brain areas were exclusively associated to each dimension and which areas exhibited overlap between dimensions. We did not run all variables together in order to avoid collinearity.
First, both bvFTD patients and controls were included in a single set (all subjects) to increase behavioural variance and statistical power (Sollberger et al., 2009; Irish et al., 2014a; O'Callaghan et al., 2016). This procedure has been also previously reported in studies exploring the structural correlates of social cognition in bvFTD (Melloni et al., 2016; Sedeno et al., 2016). In a second stage, aimed to assess the specific association between brain atrophy and potentially abnormal moral emotion performance, we conducted the same analysis only on bvFTD patients. Thus, we compared VBM findings first in a grouped set (all subjects) and later in the bvFTD group only. This two-step procedure addresses two critical requisites of the present study. First, as shown by previous studies (Irish et al., 2012, 2014b; Kumfor et al., 2013; Melloni et al., 2016), the report of combined results between patients and healthy controls aims to tackle individual differences in VBM analyses while improving statistical power by increasing sample size, stability of VBM results, and consistency of the anatomical correlates of the cognitive measure analysed. Second, the study of a cognitive measure exclusively in bvFTD allows exploring which areas are critical for a particular cognitive process (Rorden and Karnath, 2004; García-Cordero et al., 2016; Melloni et al., 2016; Shahid et al., 2017).
Results
General cognitive state and performance on cognitive control, behavioural, and social cognition measures
As expected, bvFTD patients were outperformed by controls on the IFS and the MoCA tests, but they did not differ significantly from patients with Alzheimer’s disease (Table 1). BvFTD patients also obtained significantly lower scores than both other groups on the inhibitory control measure (Hayling B, Table 1). Furthermore, the Frontal System Behavioural Scale revealed that patients with bvFTD had significantly greater behavioural impairments than patients with Alzheimer’s disease (Table 1). Finally, patients with bvFTD were also significantly more impaired than the other two groups on a social cognition measure (RMET), while patients with Alzheimer’s disease performed worse than healthy controls (Table 1).
Moral emotions
An ANOVA between type of emotion (envy, Schadenfreude, neutral) and group (bvFTD, Alzheimer’s disease, healthy controls) revealed a main effect of type of emotion [F(2,82) = 43.51, P < 0.0001, η2 = 0.43]. In all groups, Schadenfreude (P < 0.01) and envy (P < 0.01) situations were given higher scores than neutral situations. Also, we observed an interaction of group × type of emotion [F(4,82) = 13.51, P < 0.001, η2 = 0.19]. This result allowed us to perform additional ANOVAs over each emotion type.
Schadenfreude
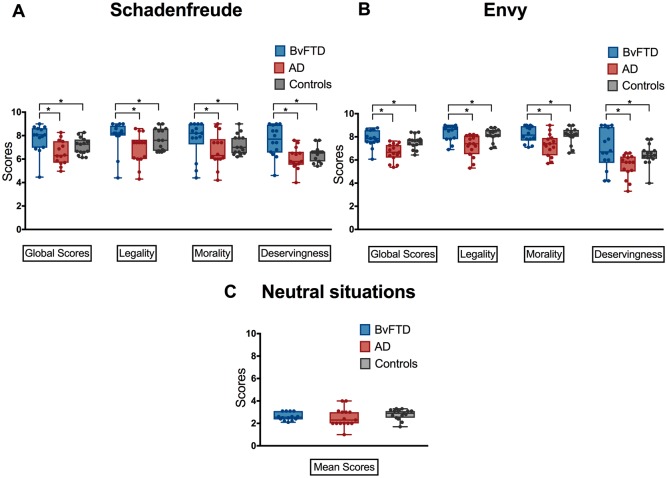
The ANOVA showed a main effect of dimension [F(2,82) = 14.53, P < 0.001, η2 = 0.13], as deservingness showed lower scores than morality and legality. In addition analyses showed an interaction between dimension and group [F(4,82) = 12.28, P < 0.01, η2 = 0.22]. Post hoc analyses (Tukey HSD, mean square = 12.5, df = 2) revealed higher scores across all three dimensions of Schadenfreude in bvFTD patients (Fig. 3A) than in patients with Alzheimer’s disease (deservingness, P = 0.001; morality, P = 0.03; legality, P = 0.004) and healthy controls (deservingness, P = 0.04; morality, P = 0.04; legality, P = 0.05). No differences were observed between patients with Alzheimer’s disease and healthy controls (deservingness, P = 0.31; morality, P = 0.24; legality, P = 0.12).
Figure 3.
Dimensions of moral emotions in each group. (A) Schadenfreude results by group. (B) Envy results for each dimension by group. (C) Results for the neutral condition in each group. The whisker box plot depicts scores of each dimension in each group and the range of responses (depicted by dots) in each group. Each group was represented by different colours: bvFTD in blue; Alzheimer’s disease (AD) in red, controls in grey. Asterisks depict significant differences at P < 0.01.
Envy
Envy scores were analysed using the same factors reported above for Schadenfreude. The analyses showed a main effect of dimension [F(2,82) = 23.53, P < 0.0001, η2 = 0.39], as deservingness situations had lower scores than morality and legality; and an interaction between dimension and group [F(4,82) = 22.38, P < 0.0001, η2 = 0.52]. Post hoc analyses of this last interaction (Tukey HSD, mean square = 12.5, df = 2) showed that bvFTD patients assigned higher scores for envy in the legal, moral and deservingness dimensions (Fig. 3B) than patients with Alzheimer’s disease (deservingness, P = 0.001; morality, P = 0.03; legality, P = 0.011) and healthy controls (deservingness, P = 0.05; morality, P = 0.043; legality, P = 0.031). No differences were observed on any dimension between patients with Alzheimer’s disease and healthy controls (deservingness, P = 0.17; morality, P = 0.31; legality, P = 0.27).
Neutral situations
No significant between-group differences emerged in the ratings for neutral situations [F(2,82) = 0.89, P = 0.41, η2 = 0.001] (Fig. 3C).
Consistency of behavioural results among groups
Relative to controls, bvFTD and Alzheimer’s disease patients evinced less consistent (i.e. more varied) scores across situations in both moral emotions (Supplementary material). Nevertheless, no difference emerged between both patient groups.
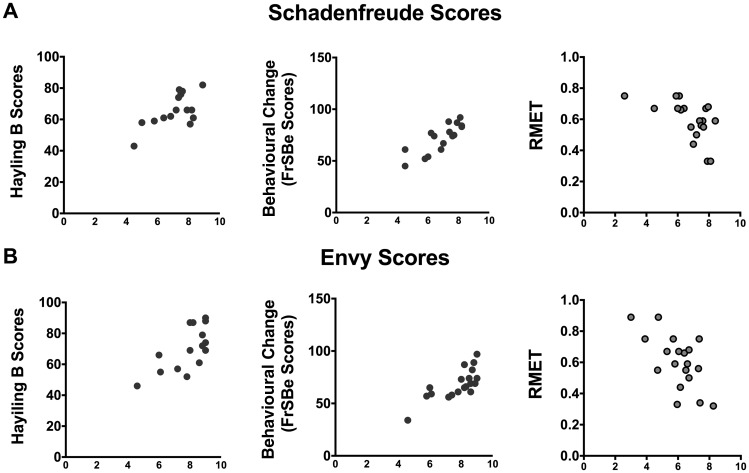
Correlation analyses between moral emotions and cognitive-behavioural measures
Correlational analyses revealed that in bvFTD patients, the stronger experience of Schadenfreude (Fig. 4A), the least inhibitory control (Hayling test, r2 = 0.39, P < 0.05), the greatest behavioural changes (Frontal System Behavioural Scale, r2 = 0.44, P < 0.01), and the lowest theory of mind skills (RMET, r2 = −0.27, P < 0.05). Similarly, higher envy was associated with reduced inhibitory control (Frontal System Behavioural Scale, r2 = 0.39, P < 0.05; Fig. 4B) and theory of mind skills (RMET, r2 = −0.31, P < 0.05). Correlational analyses for each specific dimension of Schadenfreude and envy did not reach significance (all P-values > 0.32). No associations were found in patients with Alzheimer’s disease (all P-values > 0.24) or healthy controls (all P-values > 0.31).
Figure 4.
Correlations between moral emotions and individual bvFTD profiles. (A) Positive correlations between Schadenfreude scores and behavioural measures (behavioural changes tracked by FrSBE, inhibitory control tracked by the Hayling B test and social cognition tracked by RMET). (B) The same results are depicted for these behavioural scores and envy. FrSBe = Frontal System Behavioural Scale.
Reanalysis of scores on the Schadenfreude, envy, and neutral situations with cognitive measures as covariates
Group differences in Schadenfreude [F(2,82) = 5.34, P < 0.001, η2 = 0.11] and envy [F(2,82) = 2.28, P < 0.05, η2 = 0.07] remained significant after adjusting for MoCA. Similarly, differences in Schadenfreude [F(2,102) = 2.86, P < .05, η2 = 0.06], and envy [F(2,102) = 3.18, P < 0.05, η2 = 0.07] were preserved after covarying for Hayling scores. Finally, differences between groups in Schadenfreude [F(1,59) < 7.1, P < 0.01, η2 = 0.11] and envy [F(1,59) = 3.46, P < 0.05, η2 = 0.04] were also preserved after covarying for RMET scores.
Atrophy in bvFTD patients: VBM results
Whole-brain analyses comparing the bvFTD group with healthy controls showed widespread bilateral atrophy predominantly involving the medial frontal cortex (MFC), the orbitofrontal cortex (OFC), the anteromedial temporal areas, the ACC, the bilateral insula, the basal ganglia, the right DLPFC, and bilateral medial frontal regions (P = 0.001, uncorrected) (Supplementary Fig. 1). Atrophy distribution was consistent with that reported in previous VBM studies (Rosen et al., 2002; Kipps et al., 2009; Seeley et al., 2009; Whitwell et al., 2009; Santamaria-Garcia et al., 2016; Baez et al., 2017c).
Structural neuroimaging of Schadenfreude and envy
Here we report the regression analyses (P < 0.05, FDR corrected) between each emotion (envy and Schadenfreude) for each dimension (deservingness, morality, and legality) and grey matter volume in all subjects (first step) and in bvFTD patients only (second step).
The global score for Schadenfreude was positively associated with grey matter volume in the bilateral ventral striatum and negatively associated with the volume of the bilateral angular gyrus. Schadenfreude scores for the deservingness dimension were positively associated with grey matter in the dorsal striatum (left caudate and putamen) and negatively correlated to bilateral precuneus. Scores for moral Schadenfreude were negatively associated with the left angular gyrus and left precuneus. Legal Schadenfreude scores were negatively associated with bilateral angular gyrus (Fig. 5A and Table 2).
Table 2.
Association between grey matter volume and scores for each dimension of Schadenfreude and envy (FDR corrected at P < 0.05)
| Moral emotion by group analyses | Brain regions (+) (−) | Coordinates x, y, z {mm} | Cluster size | Peak T |
|---|---|---|---|---|
|
Bilateral angular gyrus (−) | 51 (−54)* 62 23 | 332 | 5.65 |
| Right − left putamen (+) | 28 (−27)* −6 −15 | 281 | 5.69 | |
| Right − left Caudate (+) | 10 −12 14 4 | 133 | 5.43 | |
|
Bilateral precuneus | 7 (−) 48 9 | 223 | 5.49 |
| Right putamen (+) | 28 −6 −15 | 281 | 5.39 | |
| Right caudate (+) | 10 14 4 | 133 | 5.43 | |
| Bilateral angular gyrus (−) | −54 −64 24 | 382 | 5.43 | |
|
Bilateral precuneus (−) | 10 (−12)* 14 4 | 133 | 5.43 |
| Bilateral angular gyrus (−) | 52 (−54)* −64 24 | 822 | 6.16 | |
|
Right frontal pole (−) | 46 47 25 | 236 | 5.32 |
|
Bilateral precuneus (−) | 6(−6) −63 47 | 510 | 5.13 |
| Bilateral angular gyrus (−) | −46 (50)* −64 24 | 232 | 5.44 | |
| Right DLPFC (−) | 46 39 1 | 242 | 5.11 | |
|
Bilateral precuneus (−) | 10 (−12) 14 4 | 222 | 4.38 |
|
Right frontal pole (−) | 40 47 25 | 433 | 5.76 |
| Bilateral angular gyrus (−) | 50 (−48)* −64 24 | 394 | 5.23 | |
| Right frontal pole (−) | 46 42 2 | 638 | 5.33 | |
|
Bilateral precuneus (−) | 8(−6) −48 9 | 523 | 5.44 |
| Bilateral angular gyrus (−) | 50 (−48)* −64 24 | 682 | 5.56 | |
| Right ACC (+) | 2 3 45 | 457 | 5.42 | |
|
Bilateral amygdala (+) | 30 (−21)* −6 −15 | 134 | 5.43 |
| Bilateral hippocampus (+) | 16 (−19) −33 1 | 101 | 4.48 | |
| Right ACC (+) | 12 6 45 | 503 | 5.77 | |
|
Right DLPFC (−) | −51 39 1 | 346 | 5.66 |
| Right ACC (+) | 11 3 45 | 468 | 5.44 | |
|
Bilateral amygdala (+) | 22 (−24)*−6 −15 | 381 | 5.88 |
| Bilateral hippocampus (+) | 16 (−19) −33 1 | 103 | 5.89 | |
| Right ACC (+) | 2 3 45 | 378 | 5.24 | |
|
Precuneus | −6 53 −30 | 328 | 4.32 |
| Bilateral amygdala (+) | 16 (−14)*−6 −15 | 317 | 4.12 | |
| Bilateral hippocampus (+) | 18 (−21) −33 1 | 564 | 4.09 | |
|
Bilateral precuneus (−) | 8 (−6) −56 39 | 127 | 4.57 |
|
Right DLPFC (−) | −51 39 1 | 206 | 4.16 |
|
Bilateral precuneus (−) | −5 (6) −48 9 | 323 | 3.94 |
| Bilateral precuneus (−) | 8 −6 −48 9 | 447 | 8.66 | |
| BvFTD envy (legal dimension) | Right ventromedial prefrontal cortex (−) | 12 (−17)*−6 −15 | 210 | 4.43 |
*Left x-axis coordinates (MNI space).
(+) Positive associations between moral emotion scores and grey matter volume.
(−) Negative associations between moral emotion scores and grey matter volume.
The global score for envy was positively associated with grey matter volume in the right ACC and with the volume of the bilateral amygdala and parahippocampus. Deservingness envy scores were positively associated with grey matter volume in the right ACC and negatively associated with the right DLPFC. In addition, moral and legal envy were positively associated with grey matter in the right ACC and with the volume of the bilateral amygdala and parahippocampus (Fig. 6A and Table 2).
BvFTD patients
An exacerbated experience of Schadenfreude was associated with atrophy of the left angular gyrus and and precuneus. Increased Schadenfreude for the deservingness dimension was negatively associated with atrophy of the right DLPFC and the left precuneus. Enhanced moral Schadenfreude was negatively associated with the bilateral angular gyrus, and right frontal pole. Legal Schadenfreude scores were negatively associated with the bilateral angular gyrus, bilateral precuneus, and right frontal pole (Fig. 5B and Table 2).
Increased envy was correlated with reduced grey matter volume in the bilateral precuneus. Scores for the deservingness dimension of envy were negatively associated with grey matter volume of the right DLPFC. Moral envy scores were negatively associated with the volume of the right precuneus. Legal envy was negatively associated with the volume of the right DLPFC and the right precuneus (Fig. 6B and Table 2).
The brain–behaviour association in patients with bvFTD remained similar even using a more general volumetric analysis (whole-brain analysis, Supplementary Fig. 4). This analysis revealed negative correlations between Schadenfreude and envy dimensions and a set of areas including the precuneus, the angular gyrus, the dorsolateral prefrontal cortex, and the frontal pole. In addition, using this analysis we observed an additional association between legal scores of Schadenfreude and the left superior parietal lobe (Brodmann area 7). Furthermore, regarding envy scores, (global score, moral, and legal dimensions), a positive association was observed with the bilateral amygdala and the parahippocampus. Also, other associations were observed beyond the regions included in the mask. Schadenfreude was associated with bilateral sensory association areas (deservingness, moral, and legal dimensions), the superior temporal lobe (global score), and the posterior cingulate cortex (legal dimension). In the same line, envy was associated with the superior parietal lobe (global score), the left frontal area (deservingness dimension), sensory association areas (moral and legal dimensions), and the right supplementary motor area (legal dimension) (Supplementary Fig. 4 and Supplementary Table 1.
Analysis of envy and Schadenfreude dimensions controlling by inhibitory control and theory of mind
For mean scores of Schadenfreude, only the association between the mean scores and atrophy of the precuneus remain significant after covarying for Hayling test and RMET scores. The negative association between Schadenfreude for the deservingness dimension and atrophy of the precuneus and DLPFC was preserved after controlling for such two measures. For moral Schadenfreude, only the negative association with atrophy of the angular gyrus survived the correction analyses, as the negative association with dorsolateral prefrontal cortex disappeared. Legal Schadenfreude scores remained associated with atrophy of the precuneus and the angular gyrus. As in the case of moral Schadenfreude, the negative association with frontal areas disappeared after covarying for RMET and Hayling scores (Supplementary Fig. 5 and Supplementary Table 2).
Increased envy remained negatively associated with reduced grey matter volume in the precuneus after correction for Hayling and RMET measures. The negative association between DLPFC and deservingness envy was preserved after covarying with Hayling and RMET scores. Moral envy scores remained negatively associated with the volume of the right precuneus after controlling for Hayling and RMET scores, too. The same was true for legal envy, although the negative association between this dimension and the ventromedial prefrontal cortex disappeared after covariation (Supplementary Fig. 5 and Supplementary Table 2).
Discussion
This study relied on a neurodegenerative lesion model (bvFTD) to offer the first examination of the neuroanatomical signatures of three key dimensions of Schadenfreude and envy (deservingness, legality, and morality).
Results supported our behavioural hypothesis. Healthy controls showed more emotional responses in situations with prominent moral and legal components compared to deservingness situations. Similarly, bvFTD patients experienced exacerbated degrees of envy and Schadenfreude relative to the other two groups. Results also provide support for the partially differentiated but overlapped dimensions of moral emotions (Hamann, 2012; Lindquist et al., 2012, 2013). These contextual differences in emotional responses between domains (deservingness, moral and legal) were supported by several findings. First, although the type of situations seem to elicit different degrees of emotional responses, all of them elicit pleasure in the context of Schadenfreude and displeasure in the context of envy situations. Second, those situations showed only partially dissociable brain patterns. Third, they seem to be related to different cognitive processes, as shown by correlations between moral emotion scores and scores in the theory of mind task and the Hayling test.
Regarding the neuroanatomical hypothesis, present results confirmed the critical role of the striatum in Schadenfreude and of the ACC in envy, while showing additional involvement of temporo-parietal regions related to social and moral cognition processes. Our results also provide evidence of a convergent pathway for the legal and moral dimensions, but not for deservingness, which involves parietal regions in the case of Schadenfreude and anterior temporal regions for envy.
Moral dimensions across groups
Relative to patients with Alzheimer’s disease and healthy controls, patients with bvFTD had increased scores for all dimensions of envy and Schadenfreude. Moreover, an increased experience of Schadenfreude and envy in bvFTD was associated with alterations in social cognition, executive functions, and behavioural disturbances reported in those patients. Specific analyses revealed that the legal and moral dimensions elicited higher scores than the deservingness dimension in all groups. These results suggest that envy and Schadenfreude are multi-dimensional emotions, in agreement with the notion that they can be elicited by a broad spectrum of social situations, dominated by feelings of deservingness but also (and especially) by notions of justice linked to moral and legal conventions (Shamay-Tsoory et al., 2007; Kipps et al., 2009; Jankowski and Takahashi, 2014). Judgements of moral and legal rightness recruit high emotional resources and require more pacing between cognitive and emotional processes (Moll et al., 2003; Moll and de Oliveira-Souza, 2007). Such processes are highly rooted in our cognitive systems and require special moral assessment, punishment evaluation, and semantic knowledge of a particular action (Moll et al., 2003; Moll and de Oliveira-Souza, 2007).
Our results are consistent with these previous findings, as they show enhanced emotional experience for fortunate or unfortunate situations that occur to other individual in both the moral and legal dimensions. Interestingly, our results also show that even in bvFTD patients, who exhibit various executive, emotional, and socio-cognitive alterations, judgements over morality and legality invoke higher emotional responses compared to deservingness (Supplementary material). Given the social and moral transgression in bvFTD (de Oliveira-Souza et al., 2008; Mendez, 2010; Baez et al., 2014a, 2016a; Liljegren et al., 2015), exacerbated emotional responses can be expected in these patients irrespective of the type of situations presented to elicit displeasure/envy.
The source of displeasure and envy facing moral and legal situations seems to be different from the source of displeasure for the purer deservingness situations. For moral and legal situations, displeasure and envy are probably associated to implicit inequity produced by an apparent success that the receptor achieves by avoiding sanctions in punishable situations. By contrast, for deservingness situations, the source of displeasure and envy are directly related to the receptor’s success in obtaining a desirable outcome. Thus, by exploring those differential dimensions of moral emotions, we have shown that scenarios featuring moral, legal, and deservingness situations can trigger different processes related to envy. Our design does not allow clarifying whether the displeasure reported in all envy scenarios (including deservingness, moral, and legal situations), is generated by the degree of desirability of each situation a priori, or by the third-party outcome described in each situation. Future studies should explore these questions and assess to what extent an undesirable situation modulates moral emotions and evokes other emotional responses.
Structural brain signatures of experiencing moral emotions in all subjects
First, through a combined analysis between bvFTD patients and healthy controls, we aimed to tackle individual differences in VBM analyses. The VBM analyses in all subjects showed that global Schadenfreude scores were positively associated with the volume of the striatum, confirming functional MRI evidence (Takahashi et al., 2009; Jankowski and Takahashi, 2014). In addition, our analyses showed that brain signatures of Schadenfreude go beyond the striatum, as reduced grey matter volume of the bilateral angular gyrus was negatively associated with the moral and legal Schadenfreude dimensions. By contrast deservingness was related with the right caudate and putamen but also with bilateral precuneus. The angular gyrus has been reported as a crucial area involved in moral judgements (Moll et al., 2005) and in the processing of third-party moral and legal judgements (Raine and Yang, 2006). In addition, regarding to precuneus, previous studies have associated this area to mentalizing abilities and socially valued decision-making processes (Bzdok et al., 2012; Schlaffke et al., 2015; Niemi et al., 2017). The precuneus has been reported as impaired in bvFTD patients (Ibañez and Manes, 2012; Devenney et al., 2014; Baez et al., 2017c). Thus, Schadenfreude would not only involve reward-related regions, but also more extended networks indexing moral and legal dimensions of this emotion.
Regarding envy, we provide the first lesion-based evidence of a positive association with the volume of the right ACC in all subjects, as previously reported with functional MRI (Takahashi et al., 2009; Cikara and Fiske, 2013; Jankowski and Takahashi, 2014). This region is modulated by threat of self-concept, similar to cognitive dissonance (Moll et al., 2005), or social pain (similar to social exclusion) (van Veen et al., 2009). In addition, the grouped analysis showed an association between bilateral the parahippocampus and amygdala with the moral and legal dimensions (deservingness was negatively associated with the right DLPFC). Both regions (parahippocampus and amygdala) are engaged in emotional appraisal (Levenson et al., 2014) and during unfair social comparisons (Jankowski and Takahashi, 2014; O'Callaghan et al., 2016). The amygdala seems to be a critical region for the early detection of the basic mosaic of moral evaluation [i.e. intention to harm (Hesse et al., 2016)]. Furthermore, previous studies have reported abnormal amygdala volume in subjects who engage in antisocial behaviours (Raine and Yang, 2006; Yang et al., 2009). In this report, envy scores were obtained by asking subjects about the level of displeasure they feel in deservingness, moral, and legal situations. The positive correlation between the volume of the amygdala/parahippocampus and scores of envy facing moral and legal situations fits well with previous studies reporting associations between the volume of the amygdala/paralimbic cortex and self-reported displeasure in emotion tasks (Vicario et al., 2017).
Neurocognitive correlates of moral dimensions in bvFTD
We also analysed results from the bvFTD group alone, which afforded a lesion model to explore which areas are critical for the cognitive process targeted in our task. Most of the brain-behaviour correlations for envy and Schadenfreude in bvFTD patients remain significant after covarying for Hayling and RMET scores (Supplementary Fig. 5 and Supplementary material). These results suggest that exacerbation in moral emotions in bvFTD is not a simple manifestation of social and behavioural disturbances, but rather a new expression of the social and affective deregulation in bvFTD. Moreover, the whole brain analyses in bvFTD confirmed a similar pattern of associations plus additional relevant regions (Supplementary material).
Schadenfreude
In bvFTD patients, the increased experience of Schadenfreude was associated with reduced volume in the bilateral angular gyrus and precuneus, and in the right frontal pole. Furthermore, as in the grouped analyses, we found a dissociable pattern between Schadenfreude dimensions. Deservingness showed a negative association with volume of the right DLPFC and the precuneus. These regions subserve social decision-making, moral cognition and mentalizing skills (Lieberman, 2007) and are disrupted in bvFTD (Kipps et al., 2009). Thus, higher experience of Schadenfreude in situations of deservingness would be related to disrupted social decision-making and moral-mediated behaviours.
Higher scores for the moral dimension were associated with a volume reduction of the bilateral angular gyrus, and the right frontal pole, while increased legal scores were associated with reduced volume of the bilateral angular gyrus, the right precuneus and the right frontal pole. These structures are activated during processing of emotionally salient stimuli and moral judgements (Moll et al., 2005), and are related to social cognition impairments in bvFTD (Bertoux et al., 2012; Ibañez and Manes, 2012; Couto et al., 2013; Bertoux et al., 2014; Baez et al., 2016a). In fact Schadenfreude implies implicit self-other comparison of mental states (Shamay-Tsoory et al., 2007). In bvFTD, an impaired long-term self-other’s perspective integration is observed (Melloni et al., 2016). Accordingly, higher Schadenfreude scores in patients with atrophy in those social cognition regions support the gap between Schadenfreude and mentalizing skills (this interpretation is also supported by the negative correlation found between Schadenfreude and theory of mind).
Envy
BvFTD patients presented a negative association between envy and bilateral precuneus. In addition, a dissociable brain pattern was found between envy dimensions in this group. Scores for envy situations dominated by deservingness score (as in the case of Schadenfreude) were negatively associated with the DLPFC, further supporting the converging role of this area in high level social cognition in bvFTD. The envy elicited in the moral dimension in bvFTD patients was associated with volume reduction the precuneus, a region related to the interplay between mentalizing abilities and social decision-making (Bzdok et al., 2012), and affected in bvFTD (Ibañez and Manes, 2012). Feelings of envy associated with mentalizing involve precuneus engagement for immoral and illegal scenarios (Yamada et al., 2012; Baez et al., 2016a). Finally, scores for the legal dimension of envy were negatively associated with volume in the right VMPFC and the right precuneus. The VMPFC is engaged in scenarios with possible negative social consequences (Moll et al., 2005; Shamay-Tsoory et al., 2007). Arguably, reduced volume of this area in bvFTD may be associated to high scores for legal contexts, as these kinds of situations arguably recruit more emotional and cognitive resources.
The relevance of studying moral dimensions in bvFTD
Most of the reported anatomical pathways of Schadenfreude (ventral striatum, DLPFC, and frontal pole), and envy (ACC, prefrontal cortex, the parahippocampus, and the amygdala) correspond to targets of atrophy in bvFTD, namely, fronto-temporal (Piguet et al., 2011; Tosun et al., 2012; Sedeno et al., 2016) and striatal regions (Pan et al., 2012; Bertoux et al., 2015). Also, other relevant structures, such as the posterior cingulate cortex, medial temporal lobe, and parietal regions, has been associated with this condition (Supplementary material).
Our results pave the way for comprehending the interplay between more complex and ecological emotions and other cognitive processes affected in bvFTD. First, the increase in moral emotions, also considered as counter-empathy emotions, aligns with previous studies showing disruptions in social and morally determined behaviours, such as impairments in affective sharing (Eslinger et al., 2011a; Kumfor et al., 2013; Baez et al., 2014c, 2016b; Melloni et al., 2014; Kamminga et al., 2015; Sturm et al., 2015; Van den Stock et al., 2015; Hutchings et al., 2017), alterations in perspective taking and the more cognitive sides of empathy (Baez et al., 2014c, 2016b; Dermody et al., 2016; Ibanez et al., 2016; O'Callaghan et al., 2016; Ibanez et al., 2017; O'Callaghan and Hornberger, 2017), and diminished motivation for prosocial behaviours (Moll et al., 2011; O'Callaghan et al., 2016), alongside of a disruption in processing moral judgements (Baez et al., 2014a, 2016a) and an increase in immoral and illegal behaviours (de Oliveira-Souza et al., 2008; Mendez, 2010; Liljegren et al., 2015). The impaired moral emotions in bvFTD observed in the present results can be related to affective deregulation; to the relationship between basic emotion impairments and moral cognition; or to behavioural disturbances in these patients (Supplementary material).
Moreover, recent studies on bvFTD have shown that patients exhibit deficits in integrating self-perspectives with those of others and rewarding benefits (Melloni et al., 2016; O'Callaghan et al., 2016; Ibanez et al., 2017). These impairments are associated with impairments in frontotemporal structures. The integration of self-preferences with the outcomes of another person seems to be a crucial aspect of the Schadenfreude and envy (Jankowski and Takahashi, 2014; Fontenelle et al., 2015). Deficits in assessing self and other perspectives in bvFTD might abnormally enhance moral emotions irrespective of their positive/pleasant valence (as in the case of Schadenfreude) or negative displeasing valence (as in the case of envy).
Previous authors have suggested that although compliance with basic social norms can be maintained in bvFTD, more complex normative behaviours (prosociality or behaviour modulation in function of social information) that require integration of social contextual information are usually disrupted in this condition (O'Callaghan et al., 2016; Baez et al., 2017a). Thus, higher emotional scores for both Schadenfreude and envy seen in bvFTD patients could be related to impulsive behaviours, such that patients fail to integrate the implicit social information present in emotional situations with different social normative challenges.
Nonetheless, our results suggest that the exacerbation in moral emotions in all categories in bvFTD is not fully explained by social cognitive and executive deficits. In fact, responses in moral emotions in bvFTD patients remain preserved after controlling for inhibitory (Hayling test) and social cognitive measures (RMET). The exacerbation of moral emotions in bvFTD seems to be a partially independent and new hallmark of the disruption of social, behavioural, and affective processes in bvFTD.
Taken together, the above alterations seem related to deficits in the integration of social contextual information seen in bvFTD, arguably due to deficits in the frontal-temporo-insular network (Ibañez and Manes, 2012; Baez et al., 2017a). The bvFTD emerges as a relevant lesion model tapping into the ‘mystery of frontal lobes’ (Burgess et al., 2009): patients provide accurate responses to abstract, isolated and decontextualized cognitive tasks, but they typically fail in more ecological paradigms demanding a combination of cognitive, affective, and social processes (Ibañez and Manes, 2012; Melloni et al., 2014; Baez et al., 2017a). Such social contextual deficits would lie at the core of the behavioural, socio-moral, and emotional impairments observed in bvFTD. Future studies should further explore the links between specific compromise of the social context network and moral emotions.
Limitations and further assessments
Our study has some limitations. First, diagnosis in our patient sample was based on established clinical assessments, but it lacked pathological confirmation. However, such confirmation is not required for diagnosis at the probable level, as shown in several studies (Rascovsky et al., 2011; Chiong et al., 2016; García-Cordero et al., 2016; Melloni et al., 2016; Sedeno et al., 2016; Ahmed et al., 2017). Moreover, we have followed international standards (Knopman and Roberts, 2011; Piguet et al., 2011; Rascovsky et al., 2011), as diagnosis was performed in a memory clinic after deliberative consensus by a highly experienced group composed of geriatricians, neurologists, neuropsychologists, and psychiatrists (Baez et al., 2014a, 2016b; García-Cordero et al., 2016; Melloni et al., 2016; Sedeno et al., 2017). In particular, all diagnoses were performed following criteria by (Rascovsky et al., 2011), for probable bvFTD patients, and international criteria (McKhann et al., 2011), for patients with Alzheimer’s disease.
Second, our approach did not allow us to assess the relationship between envy and Schadenfreude dimensions with other cognitive processes, including reward processing, and other social cognitive processes such as emotional sharing (e.g. empathy). Further studies should explore the particular relationship between dimensions of moral emotions and their relationship with cognitive processes including social reward and socio-cognitive skills (including perspective taking and affective sharing), moral judgement, and cognitive control among other processes.
Third, we compared the behavioural responses of bvFTD patients to moral emotions relative to those of a contrastive lesion model (Alzheimer’s disease), to control for task comprehension problems associated with neurodegeneration. Future studies should explore the brain correlates of processing those emotions in Alzheimer’s disease (as well as other neurodegenerative conditions) to fully comprehend the role of other brain areas usually impaired across neurodegenerative conditions. Although our study did not include VBM analysis of patients with Alzheimer’s disease, several reasons attest to the validity of the neurocognitive pattern observed in bvFTD (Supplementary material). Despite these considerations, future studies should evaluate the degree of atrophy specificity by comparing bvFTD with other conditions, such as Alzheimer’s disease, other forms of FTD, and Huntington’s disease (another neurodegenerative condition associated with moral emotion impairments (Baez et al., 2017d).
Finally, we only found correlations between global scores of moral emotions and measures tracking social, cognitive, and behavioural impairments in bvFTD. Since this relationship was only reported with global scores, we do not know to what extent performance on each sub-dimension of envy and Schadenfreude is related to other cognitive and behavioural dimensions in bvFTD. Further studies should explore the extent to which the deservingness and moral-legal dimensions are related to specific cognitive and behavioural processes. The relationship between recognition and third-party evaluation of moral and legal situations and social cognitive processes (including empathy, emotion perception, and social decision-making) represents a promising avenue for future research.
Conclusions
Classical theoretical approaches have hinted at the multidimensional nature to the moral emotions of envy and Schadenfreude. Those earlier theories suggested that moral emotions are complex cognitive and affective states that, depending on the circumstances, may be considered the worst evil emotions (Schopenhauer 1788–1860) or, alternatively, good-natured emotions based on principles of fairness (Nietzsche 1844–1900). Together, our results provide unprecedented evidence of an exacerbated experience of moral emotions in bvFTD, while reinforcing the multidimensional nature of envy and Schadenfreude. Our results highlight the importance of analysing new and more ecological moral emotions in this population, with a view to examining relevant interactions between cognitive, moral, and emotional processes. In addition, our study reveals a new behavioural profile in bvFTD, which may help clinicians identify patients with neurocognitive disorders with mixed cognitive and behavioural alterations.
Funding
This work was supported by grants from CONICYT/FONDECYT Regular (1170010), PICT 2012-0412, PICT 2012-1309, CONICET, CONICYT/FONDAP 15150012, and the INECO Foundation. This work also received support from Colciencias and APS Project 697 -2014. Finally, the Frontal Lobe Project from Universidad Javeriana and Hospital Universitario San Ignacio, Centro de Memoria y Cognición Intellectus also provided support for this study.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviations
- ACC
anterior cingulate cortex
- bvFTD
behavioural variant frontotemporal dementia
- DLPFC
dorsolateral prefrontal cortex
- IFS
INECO Frontal Screening
- MoCA
Montreal Cognitive Assessment
- RMET
Reading the Mind in the Eyes Test
- VBM
voxel-based morphometry
References
- Adolfi F, Couto B, Richter F, Decety J, Lopez J, Sigman M, et al. Convergence of interoception, emotion, and social cognition: a twofold fMRI meta-analysis and lesion approach. Cortex 2016; 88: 124–42. [DOI] [PubMed] [Google Scholar]
- Ahmed RM, Landin-Romero R, Collet TH, van der Klaauw AA, Devenney E, Henning E, et al. Energy expenditure in frontotemporal dementia: a behavioural and imaging study. Brain 2017; 140 (Pt 1): 171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M, Frank D, Glen JC, Fardo F, Callaghan MF, Rees G. Insula and somatosensory cortical myelination and iron markers underlie individual differences in empathy. Sci Rep 2017; 7: 43316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage 2000; 11 (6 Pt 1): 805–21. [DOI] [PubMed] [Google Scholar]
- Baez S, Couto B, Torralva T, Sposato LA, Huepe D, Montanes P, et al. Comparing moral judgments of patients with frontotemporal dementia and frontal stroke. JAMA Neurol 2014a; 71: 1172–6. [DOI] [PubMed] [Google Scholar]
- Baez S, Garcia AM, Ibanez A. The social context network model in psychiatric and neurological diseases. Curr Top Behav Neurosci 2017a; 30: 379–96. [DOI] [PubMed] [Google Scholar]
- Baez S, Herrera E, Garcia A, Manes F, Young L, Ibanez A. Outcome-oriented moral evaluation in terrorists. Nature Human Behavior 2017b; 1: 0118. [Google Scholar]
- Baez S, Herrera E, Gershanik O, Garcia AM, Bocanegra Y, Kargieman L, et al. Impairments in negative emotion recognition and empathy for pain in Huntington's disease families. Neuropsychologia 2015; 68: 158–67. [DOI] [PubMed] [Google Scholar]
- Baez S, Ibanez A, Gleichgerrcht E, Perez A, Roca M, Manes F, et al. The utility of IFS (INECO Frontal Screening) for the detection of executive dysfunction in adults with bipolar disorder and ADHD. Psychiatry Res 2014b; 216: 269–76. [DOI] [PubMed] [Google Scholar]
- Baez S, Kanske P, Matallana D, Montañes P, Reyes P, Slachevsky A, et al. Integration of intention and outcome for moral judgment in frontotemporal dementia: brain structural signatures. Neurodegener Dis 2016a; 16: 206–17. [DOI] [PubMed] [Google Scholar]
- Baez S, Manes F, Huepe D, Torralva T, Fiorentino N, Richter F, et al. Primary empathy deficits in frontotemporal dementia. Front Aging Neurosci 2014c; 6: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez S, Morales JP, Slachevsky A, Torralva T, Matus C, Manes F, et al. Orbitofrontal and limbic signatures of empathic concern and intentional harm in the behavioral variant frontotemporal dementia. Cortex 2016b; 75: 20–32. [DOI] [PubMed] [Google Scholar]
- Baez S, Pinasco C, Roca M, Ferrari J, Couto B, Garcia-Cordero I, et al. Brain structural correlates of executive and social cognition profiles in behavioral variant frontotemporal dementia and elderly bipolar disorder. Neuropsychologia 2017c. [DOI] [PubMed] [Google Scholar]
- Baez S, Pino M, Berrio M, Santamaria-Garcia H, Sedeno L, Garcia AM, et al. Corticostriatal signatures of schadenfreude: evidence from Huntington's disease. J Neurol Neurosurg Psychiatry 2017d, in press. http://dx.doi.org/10.1136/jnnp-2017-316055. [DOI] [PubMed] [Google Scholar]
- Baez S, Santamaria-Garcia H, Orozco J, Fittipaldi S, Garcia AM, Pino M, et al. Your misery is no longer my pleasure: reduced schadenfreude in Huntington's disease families. Cortex 2016c; 83: 78–85. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: evidence from very high functioning adults with autism or asperger syndrome. J Child Psychol Psychiatry 1997; 38: 813–22. [DOI] [PubMed] [Google Scholar]
- Ben-Ze’ev A. The personal comparative concern in schadenfreude. In: van Dijk W, Ouwerkerk J, editors. Schadenfreude: understanding pleasure at the misfortune of others. Cambridge: Cambridge University Press; 2014. p. 77–90. [Google Scholar]
- Berkman ET, Cunningham WA, Lieberman MD. Research methods in social and affective neuroscience. In: Handbook of research methods in social and personality psychology. 2014. p. 123–58. [Google Scholar]
- Bertoux M, O'Callaghan C, Flanagan E, Hodges JR, Hornberger M. Fronto-striatal atrophy in behavioral variant frontotemporal dementia and Alzheimer's disease. Front Neurol 2015; 6: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoux M, Volle E, de Souza LC, Funkiewiez A, Dubois B, Habert MO. Neural correlates of the mini-SEA (Social cognition and Emotional Assessment) in behavioral variant frontotemporal dementia. Brain Imaging Behav 2014; 8: 1–6. [DOI] [PubMed] [Google Scholar]
- Bertoux M, Volle E, Funkiewiez A, de Souza LC, Leclercq D, Dubois B. Social Cognition and Emotional Assessment (SEA) is a marker of medial and orbital frontal functions: a voxel-based morphometry study in behavioral variant of frontotemporal degeneration. J Int Neuropsychol Soc 2012; 18: 972–85. [DOI] [PubMed] [Google Scholar]
- Brown LA, Cohen AS. Facial emotion recognition in schizotypy: the role of accuracy and social cognitive bias. J Int Neuropsychol Soc 2010; 16: 474–83. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Asplund CL, Dux PE, Zald DH, Gore JC, Jones OD, et al. The neural correlates of third-party punishment. Neuron 2008; 60: 930–40. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Volle E, Benoit RG, Gilbert SJ. Mesulam's frontal lobe mystery re-examined. Restor Neurol Neurosci 2009; 27: 493–506. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, et al. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct 2012; 217: 783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AR, Paholpak P, Daianu M, Fong SS, Mather M, Jimenez EE, et al. An investigation of care-based vs. rule-based morality in frontotemporal dementia, Alzheimer’s disease, and healthy controls. Neuropsychologia 2015; 78: 73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho JO, Ready RE, Malloy P, Grace J. Confirmatory factor analysis of the Frontal Systems Behavior Scale (FrSBe). Assessment 2013; 20: 632–41. [DOI] [PubMed] [Google Scholar]
- Chester DS, Powell CA, Smith RH, Joseph JE, Kedia G, Combs DJ, et al. Justice for the average Joe: the role of envy and the mentalizing network in the deservingness of others' misfortunes. Soc Neurosci 2013; 8: 640–9. [DOI] [PubMed] [Google Scholar]
- Chiong W, Wilson SM, D'Esposito M, Kayser AS, Grossman SN, Poorzand P, et al. The salience network causally influences default mode network activity during moral reasoning. Brain 2013; 136 (Pt 6): 1929–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong W, Wood KA, Beagle AJ, Hsu M, Kayser AS, Miller BL, et al. Neuroeconomic dissociation of semantic dementia and behavioural variant frontotemporal dementia. Brain 2016; 139 (Pt 2): 578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K. Topological FDR for neuroimaging. Neuroimage 2010; 49: 3057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikara M, Fiske ST. Their pain, our pleasure: stereotype content and schadenfreude. Ann N Y Acad Sci 2013; 1299: 52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MH, Carton AM, Hardy CJ, Golden HL, Clark CN, Fletcher PD, et al. Processing emotion from abstract art in frontotemporal lobar degeneration. Neuropsychologia 2016; 81: 245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Delfiore G, Degueldre C, Luxen A, Salmon E. The functional anatomy of inhibition processes investigated with the Hayling task. Neuroimage 2001; 14: 258–67. [DOI] [PubMed] [Google Scholar]
- Couto B, Manes F, Montanes P, Matallana D, Reyes P, Velasquez M, et al. Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front Hum Neurosci 2013; 7: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Azevedo Ignacio F, Tovar-Moll F, et al. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage 2008; 40: 1202–13. [DOI] [PubMed] [Google Scholar]
- de Spinoza B. Ethics Part III. On the origin and nature of the emotions, Vol. 1. Library of Alexandria. Google Books, 1883. [Google Scholar]
- Dermody N, Wong S, Ahmed R, Piguet O, Hodges JR, Irish M. Uncovering the neural bases of cognitive and affective empathy deficits in Alzheimer's disease and the behavioral-variant of frontotemporal dementia. J Alzheimers Dis 2016; 53: 801–16. [DOI] [PubMed] [Google Scholar]
- Devenney E, Hornberger M, Irish M, Mioshi E, Burrell J, Tan R, et al. Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurol 2014; 71: 331–9. [DOI] [PubMed] [Google Scholar]
- Dvash J, Gilam G, Ben-Ze'ev A, Hendler T, Shamay-Tsoory SG. The envious brain: the neural basis of social comparison. Hum Brain Mapp 2010a; 31: 1741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvash J, Gilam G, Ben-Ze'ev A, Hendler T, Shamay-Tsoory SG. The envious brain: the neural basis of social comparison. Hum Brain Mapp 2010b; 31: 1741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. J Neuropsychiatry Clin Neurosci 2011a; 23: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. J Neuropsychiatry Clin Neurosci 2011b; 23: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Antani S, Anderson C, Grossman M. Apathy in Frontotemporal dementia: behavioral and neuroimaging correlates. Behav Neurol 2012; 25: 127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feather NT, Sherman R. Envy, resentment, schadenfreude, and sympathy: reactions to deserved and undeserved achievement and subsequent failure. Pers Soc Psychol Bull 2002; 28: 953–61. [Google Scholar]
- Fermin AS, Sakagami M, Kiyonari T, Li Y, Matsumoto Y, Yamagishi T. Representation of economic preferences in the structure and function of the amygdala and prefrontal cortex. Sci Rep 2016; 6: 20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske AP. The four elementary forms of sociality: framework for a unified theory of social relations. Psychol Rev 1992; 99: 689. [DOI] [PubMed] [Google Scholar]
- Fiske ST, Taylor SE. Social cognition: From brains to culture. Sage, 2013. [Google Scholar]
- Fontenelle LF, de Oliveira-Souza R, Moll J. The rise of moral emotions in neuropsychiatry. Dialogues Clin Neurosci 2015; 17: 411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cordero I, Sedeno L, de la Fuente L, Slachevsky A, Forno G, Klein F, et al. Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philos Trans R Soc Lond B Biol Sci 2016; 371: 1708 doi: 10.1098/rstb.2016.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer's disease: theoretical and practical implications. Brain 2002; 125 (Pt 4): 752–64. [DOI] [PubMed] [Google Scholar]
- Haidt J. The moral emotions. In: Davidson RJ, Scherer R, Goldsmith H, editors. Handbook of affective sciences. Oxford: Oxford University Press; 2003. p. 852–70. [Google Scholar]
- Hamann S. Mapping discrete and dimensional emotions onto the brain: controversies and consensus. Trends Cogn Sci 2012; 16: 458–66. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Abe N, Fujii T, Ito A, Ueno A, Koseki Y, et al. Dissociable neural systems for moral judgment of anti- and pro-social lying. Brain Res 2014; 1556: 46–56. [DOI] [PubMed] [Google Scholar]
- Hesse E, Mikulan E, Decety J, Sigman M, Garcia Mdel C, Silva W, et al. Early detection of intentional harm in the human amygdala. Brain 2016; 139 (Pt 1): 54–61. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Savage S, Hsieh S, Mioshi E, Piguet O, Hodges JR. Orbitofrontal dysfunction discriminates behavioral variant frontotemporal dementia from Alzheimer's disease. Dement Geriatr Cogn Disord 2010; 30: 547–52. [DOI] [PubMed] [Google Scholar]
- Hutchings R, Palermo R, Piguet O, Kumfor F. Disrupted face processing in frontotemporal dementia: a review of the clinical and neuroanatomical evidence. Neuropsychol Rev 2017; 27: 18–30. [DOI] [PubMed] [Google Scholar]
- Ibanez A, Billeke P, de la Fuente L, Salamone P, Garcia AM, Melloni M. Reply: towards a neurocomputational account of social dysfunction in neurodegenerative disease. Brain 2017; 140: e15. [DOI] [PubMed] [Google Scholar]
- Ibanez A, Garcia AM, Esteves S, Yoris A, Munoz E, Reynaldo L, et al. Social neuroscience: undoing the schism between neurology and psychiatry. Soc Neurosci 2016; 1: 1–39. doi: 10.1080/17470919.2016.1245214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez A, Kuljis RO, Matallana D, Manes F. Bridging psychiatry and neurology through social neuroscience. World Psychiatry 2014; 13: 148–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez A, Manes F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology 2012; 78: 1354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez A, Urquina H, Petroni A, Baez S, Lopez V, do Nascimento M, et al. Neural processing of emotional facial and semantic expressions in euthymic bipolar disorder (BD) and its association with theory of mind (ToM). PLoS One 2012; 7: e46877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges JR, Piguet O. Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain 2012; 135 (Pt 7): 2178–91. [DOI] [PubMed] [Google Scholar]
- Irish M, Hodges JR, Piguet O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain 2014a; 137 (Pt 4): 1241–53. [DOI] [PubMed] [Google Scholar]
- Irish M, Piguet O, Hodges JR, Hornberger M. Common and unique gray matter correlates of episodic memory dysfunction in frontotemporal dementia and Alzheimer's disease. Hum Brain Mapp 2014b; 35: 1422–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski KF, Takahashi H. Cognitive neuroscience of social emotions and implications for psychopathology: examining embarrassment, guilt, envy, and schadenfreude. Psychiatry Clin Neurosci 2014; 68: 319–36. [DOI] [PubMed] [Google Scholar]
- Kamminga J, Kumfor F, Burrell JR, Piguet O, Hodges JR, Irish M. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. J Neurol Neurosurg Psychiatry 2015; 86: 1082–8. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Nestor PJ, Acosta-Cabronero J, Arnold R, Hodges JR. Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain 2009; 132 (Pt 3): 592–603. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Roberts RO. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci 2011; 45: 330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E. Frontal pole function: what is specifically human? Trends Cogn Sci 2011; 15: 241. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Irish M, Hodges JR, Piguet O. The orbitofrontal cortex is involved in emotional enhancement of memory: evidence from the dementias. Brain 2013; 136 (Pt 10): 2992–3003. [DOI] [PubMed] [Google Scholar]
- Lai JM, Hawkins KA, Gross CP, Karlawish JH. Self-reported distress after cognitive testing in patients with Alzheimer's disease. J Gerontol A Biol Sci Med Sci 2008; 63: 855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, Sturm VE, Haase CM. Emotional and behavioral symptoms in neurodegenerative disease: a model for studying the neural bases of psychopathology. Annu Rev Clin Psychol 2014; 10: 581–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Ann Rev Psychol 2007; 58: 259–89. [DOI] [PubMed] [Google Scholar]
- Liljegren M, Naasan G, Temlett J, Perry DC, Rankin KP, Merrilees J, et al. Criminal behavior in frontotemporal dementia and Alzheimer disease. JAMA Neurol 2015; 72: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE. The challenges and promise of neuroimaging in psychiatry. Neuron 2012; 73: 8–22. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Siegel EH, Quigley KS, Barrett LF. The hundred-year emotion war: are emotions natural kinds or psychological constructions? Comment on Lench, Flores, and Bench (2011). Psychol Bull 2013; 139: 255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci 2012; 35: 121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni M, Billeke P, Baez S, Hesse E, de la Fuente L, Forno G, et al. Your perspective and my benefit: multiple lesion models of self-other integration strategies during social bargaining. Brain 2016; 139: 3022–40. http://dx.doi.org/10.1093/brain/aww231. [DOI] [PubMed] [Google Scholar]
- Melloni M, Lopez V, Ibanez A. Empathy and contextual social cognition. Cogn Affect Behav Neurosci 2014; 14: 407–25. [DOI] [PubMed] [Google Scholar]
- Mendez MF. The unique predisposition to criminal violations in frontotemporal dementia. J Am Acad Psychiatry Law 2010; 38: 318–23. [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Chen AK, Shapira JS, Miller BL. Acquired sociopathy and frontotemporal dementia. Dement Geriatr Cogn Disord 2005; 20: 99–104. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R. Moral judgments, emotions and the utilitarian brain. Trends Cogn Sci 2007; 11: 319–21. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ. Morals and the human brain: a working model. Neuroreport 2003; 14: 299–305. [DOI] [PubMed] [Google Scholar]
- Moll J, De Oliveira-Souza R, Zahn R. The neural basis of moral cognition: sentiments, concepts, and values. Ann N Y Acad Sci 2008; 1124: 161–80. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Bramati IE, Krueger F, Tura B, et al. Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. Neuroimage 2011a; 54: 1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: the neural basis of human moral cognition. Nat Rev Neurosci 2005; 6: 799–809. [DOI] [PubMed] [Google Scholar]
- Najle M. Delicious justice: schadenfreude toward atheists bound for hell. Theses and Dissertations–Psychology 61. 2015. http://uknowledge.uky.edu/psychology_etds/61. [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- Nathaniel-James D, Frith C. The role of the dorsolateral prefrontal cortex: evidence from the effects of contextual constraint in a sentence completion task. Neuroimage 2002; 16: 1094–102. [DOI] [PubMed] [Google Scholar]
- Niemi L, Wasserman E, Young L. The behavioral and neural signatures of distinct conceptions of fairness. Soc Neurosci 2017; 1: 1–17. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C, Bertoux M, Irish M, Shine JM, Wong S, Spiliopoulos L, et al. Fair play: social norm compliance failures in behavioural variant frontotemporal dementia. Brain 2016; 139 (Pt 1): 204–16. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C, Hornberger M. Towards a neurocomputational account of social dysfunction in neurodegenerative disease. Brain 2017; 140: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortony A, Clore GL, Collins A. The cognitive structure of emotions. Cambridge University Press; 1990. http://www.cambridge.org/co/academic/subjects/psychology/cognition/cognitive-structure-emotions?format=PB&isbn=9780521386647#vrjKhhBUrU5qHDtw.97 [Google Scholar]
- Pan PL, Song W, Yang J, Huang R, Chen K, Gong QY, et al. Gray matter atrophy in behavioral variant frontotemporal dementia: a meta-analysis of voxel-based morphometry studies. Dement Geriatr Cogn Disord 2012; 33: 141–8. [DOI] [PubMed] [Google Scholar]
- Parkinson C, Sinnott-Armstrong W, Koralus PE, Mendelovici A, McGeer V, Wheatley T. Is morality unified? Evidence that distinct neural systems underlie moral judgments of harm, dishonesty, and disgust. J Cogn Neurosci 2011; 23: 3162–80. [DOI] [PubMed] [Google Scholar]
- Patil I, Silani G. Reduced empathic concern leads to utilitarian moral judgments in trait alexithymia. Front Psychol 2014; 5: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez A, Matias-Guiu JA, Caceres-Guillen I, Rognoni T, Valles-Salgado M, Fernandez-Matarrubia M, et al. The hayling test: development and normalization of the Spanish version. Arch Clin Neuropsychol 2016; 31: 411–19. [DOI] [PubMed] [Google Scholar]
- Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB. Functional network disruption in the degenerative dementias. Lancet Neurol 2011; 10: 829–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol 2011; 10: 162–72. [DOI] [PubMed] [Google Scholar]
- Plutchik R. A general psychoevolutionary theory of emotion. Theor Emotion 1980; 1: 4. [Google Scholar]
- Portmann J. When bad things happen to other people. New York, NY: Routledge; 2000. [Google Scholar]
- Portmann J. When bad things happen to other people. New York, London: Routledge; 2002. [Google Scholar]
- Radua J, Canales-Rodríguez EJ, Pomarol-Clotet E, Salvador R. Validity of modulation and optimal settings for advanced voxel-based morphometry. Neuroimage 2014; 86: 81–90. [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Soc Cogn Affect Neurosci 2006; 1: 203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134 (Pt 9): 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, et al. Executive function and fluid intelligence after frontal lobe lesions. Brain 2010; 133: 234–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci 2004; 5: 813–19. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002; 58: 198–208. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci 2013; 16: 1140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfeliu MC, Fernandez A. A set of 254 snodgrass-vanderwart pictures standardized for Spanish: norms for name agreement, image agreement, familiarity, and visual complexity. Behav Res Methods Instrum Comput 1996; 28: 537–55. [Google Scholar]
- Santamaria-Garcia H, Reyes P, Garcia A, Baez S, Martinez A, Santacruz JM, et al. First symptoms and neurocognitive correlates of behavioral variant frontotemporal dementia. J Alzheimers Dis 2016; 54: 957–70. [DOI] [PubMed] [Google Scholar]
- Scarpazza C, Tognin S, Frisciata S, Sartori G, Mechelli A. False positive rates in voxel-based morphometry studies of the human brain: should we be worried? Neurosci Biobehav Rev 2015; 52: 49–55. [DOI] [PubMed] [Google Scholar]
- Schlaffke L, Lissek S, Lenz M, Juckel G, Schultz T, Tegenthoff M, et al. Shared and nonshared neural networks of cognitive and affective theory-of-mind: a neuroimaging study using cartoon picture stories. Hum Brain Mapp 2015; 36: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeno L, Couto B, Garcia-Cordero I, Melloni M, Baez S, Morales Sepulveda JP, et al. Brain network organization and social executive performance in frontotemporal dementia. J Int Neuropsychol Soc 2016; 22: 250–62. [DOI] [PubMed] [Google Scholar]
- Sedeno L, Piguet O, Abrevaya S, Desmaras H, Garcia-Cordero I, Baez S, et al. Tackling variability: a multicenter study to provide a gold-standard network approach for frontotemporal dementia. Hum Brain Mapp 2017; 38: 3804–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009; 62: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Zhou J, Kim EJ. Frontotemporal dementia: what can the behavioral variant teach us about human brain organization? Neuroscientist 2012; 18: 373–85. [DOI] [PubMed] [Google Scholar]
- Shahid H, Sebastian R, Schnur TT, Hanayik T, Wright A, Tippett DC, et al. Important considerations in lesion-symptom mapping: illustrations from studies of word comprehension. Hum Brain Mapp 2017; 38: 2990–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Ahronberg-Kirschenbaum D, Bauminger-Zviely N. There is no joy like malicious joy: schadenfreude in young children. PLoS One 2014; 9: e100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tibi-Elhanany Y, Aharon-Peretz J. The green-eyed monster and malicious joy: the neuroanatomical bases of envy and gloating (schadenfreude). Brain 2007; 130 (Pt 6): 1663–78. [DOI] [PubMed] [Google Scholar]
- Sinnott-Armstrong W. The disunity of morality. In: Moral brains: the neuroscience of morality. Oxford University Press, 2016. p. 331. [Google Scholar]
- Smith RH, Kim SH. Comprehending envy. Psychol Bull 2007; 133: 46–64. [DOI] [PubMed] [Google Scholar]
- Smith RH, Powell CAJ, Combs DJY, Schurtz DR. Exploring the when and why of Schadenfreude. Soc Personal Psychol Compass 2009; 3: 530–546. doi: 10.1111/j.1751-9004.2009.00181.x. [Google Scholar]
- Snodgrass JG, Feenan K. Priming effects in picture fragment completion: support for the perceptual closure hypothesis. J Exp Psychol Gen 1990; 119: 276. [DOI] [PubMed] [Google Scholar]
- Sollberger M, Stanley CM, Wilson SM, Gyurak A, Beckman V, Growdon M, et al. Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia 2009; 47: 2812–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Yokoyama JS, Eckart JA, Zakrzewski J, Rosen HJ, Miller BL, et al. Damage to left frontal regulatory circuits produces greater positive emotional reactivity in frontotemporal dementia. Cortex 2015; 64: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Matsuura M, Mobbs D, Suhara T, Okubo Y. When your gain is my pain and your pain is my gain: neural correlates of envy and schadenfreude. Science 2009; 323: 937–9. [DOI] [PubMed] [Google Scholar]
- Tangney JP, Stuewig J, Mashek DJ. Moral emotions and moral behavior. Annu Rev Psychol 2007; 58: 345–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralva T, Roca M, Gleichgerrcht E, Lopez P, Manes F. INECO Frontal Screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia. J Int Neuropsychol Soc 2009; 15: 777–86. [DOI] [PubMed] [Google Scholar]
- Tosun D, Rosen H, Miller BL, Weiner MW, Schuff N. MRI patterns of atrophy and hypoperfusion associations across brain regions in frontotemporal dementia. Neuroimage 2012; 59: 2098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoucalas G, Bourelia S, Kalogirou V, Giatsiou S, Mavrogiannaki E, Gatos G, et al. End-stage dementia spark of life: reliability and validity of the “GATOS” questionnaire. Curr Alzheimer Res 2015; 12: 179–88. [DOI] [PubMed] [Google Scholar]
- Van den Stock J, De Winter FL, de Gelder B, Rangarajan JR, Cypers G, Maes F, et al. Impaired recognition of body expressions in the behavioral variant of frontotemporal dementia. Neuropsychologia 2015; 75: 496–504. [DOI] [PubMed] [Google Scholar]
- van Dijk WW, Ouwerkerk JW. Schadenfreude: understanding pleasure at the misfortune of others. Clays, St Ives plc, UK: Cambridge University Press; 2014. [Google Scholar]
- Van Dijk WW, Ouwerkerk JW, Goslinga S, Nieweg M. Deservingness and Schadenfreude. Cogn Emotion 2005; 19: 933–9. [Google Scholar]
- van Dijk WW, Ouwerkerk JW, Goslinga S, Nieweg M, Gallucci M. When people fall from grace: reconsidering the role of envy in Schadenfreude. Emotion 2006; 6: 156–60. [DOI] [PubMed] [Google Scholar]
- van Veen V, Krug MK, Schooler JW, Carter CS. Neural activity predicts attitude change in cognitive dissonance. Nat Neurosci 2009; 12: 1469–74. [DOI] [PubMed] [Google Scholar]
- Vendetti MS, Bunge SA. Evolutionary and developmental changes in the lateral frontoparietal network: a little goes a long way for higher-level cognition. Neuron 2014; 84: 906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario CM, Rafal RD, Martino D, Avenanti A. Core, social and moral disgust are bounded: a review on cognitive and neural bases of repugnance in clinical disorders. Neurosci Biobehav Rev 2017; 80: 185–200. [DOI] [PubMed] [Google Scholar]
- Volle E, Gonen-Yaacovi G, de Lacy Costello A, Gilbert SJ, Burgess PW. The role of rostral prefrontal cortex in prospective memory: a voxel-based lesion study. Neuropsychologia 2011; 49: 2185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain 2009; 132 (Pt 11): 2932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Camerer CF, Fujie S, Kato M, Matsuda T, Takano H, et al. Neural circuits in the brain that are activated when mitigating criminal sentences. Nat Commun 2012; 3: 759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Arch Gen Psychiatry 2009; 66: 986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 2011; 8: 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KJ, Decety J. The good, the bad, and the just: justice sensitivity predicts neural response during moral evaluation of actions performed by others. J Neurosci 2014; 34: 4161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN, Ochsner KN, Plutchik R, Klöppel S, Stonnington CM, et al. Envy, politics, and age. Emotion 2015; 11: 187–208. [Google Scholar]
- Zamboni G, Grafman J, Krueger F, Knutson K, Huey E. Anosognosia for behavioral disturbances in frontotemporal dementia and corticobasal syndrome: a voxel-based morphometry study. Dement Geriatr Cogn Disord 2010; 29: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.