Abstract
Background
Prevention of hepatitis C virus (HCV) transmission among people who inject drugs (PWID) is critical to eliminating HCV in Europe. We estimate impact of current and scaled-up HCV treatment with and without scaling-up opioid substitution therapy (OST) and needle and syringe programmes (NSP) across Europe over the next 10 years.
Methods
We collected data on PWID HCV treatment rates, PWID prevalence, HCV prevalence, OST and NSP coverage from 11 European settings. We parameterized a HCV transmission model to setting-specific data that projects chronic HCV prevalence and incidence among PWID.
Results
At baseline, chronic HCV prevalence varied from <25% (Slovenia/Czech Republic) to >55% (Finland/Sweden), and <2% (Amsterdam/Hamburg/Norway/Denmark/Sweden) to 5% (Slovenia/Czech Republic) of chronically infected PWID were treated annually. Current treatment rates using new direct acting antivirals (DAAs) may achieve observable reductions in chronic prevalence (38-63%) in 10 years in Czech Republic, Slovenia and Amsterdam. Doubling HCV-treatment rates will reduce prevalence in other sites (12-24%, Belgium/Denmark/Hamburg/Norway/Scotland) but is unlikely to reduce prevalence in Sweden and Finland. Scaling-up OST and NSP to 80% coverage with current treatment rates using DAAs could achieve observable reductions in HCV prevalence (18-79%) in all sites.
Using DAAs, Slovenia and Amsterdam are projected to reduce incidence to 2 per 100pyrs or less in 10 years. Moderate to substantial increases in current treatment rates are required to achieve the same impact elsewhere, from 1.4-3 times (Czech Republic/France), 5-17 times (France/Scotland/Hamburg/Norway/Denmark/Belgium/Sweden), to 200 times (Finland). Scaling-up OST and NSP coverage to 80% in all sites reduces treatment scale-up needed by 20-80%.
Conclusions
Scale-up of HCV treatment and other interventions is needed in most settings to minimise HCV transmission among PWID in Europe.
Introduction
Chronic hepatitis C virus (HCV) infection is a leading cause of liver disease and morbidity causing more deaths than HIV in the US and other high income countries [1–4]. Preventing HCV transmission among people who inject drugs (PWID) is critical for averting future liver disease in Europe and elsewhere [5] and new HCV infections in this group[6]. Primary prevention through opioid substitution treatment (OST) and high coverage needle and syringe programmes (NSP) can reduce HCV transmission among PWID [7, 8] and averts new HCV infections,[9] but substantial reductions in HCV prevalence are unlikely to be achieved without scaling up HCV treatment [10–15].
The arrival of highly effective and short duration direct acting antivirals (DAAs) with cure rates (sustained viral response or SVR) above 90% for all genotypes has made HCV “treatment as prevention” more than a theoretical possibility [16–18]. However, the current high cost of DAA regimes (often >€30,000 per treatment regime in higher income countries) is a barrier to scaling up treatment in most countries. European guidelines, that previously recommended prioritising DAAs for patients with advanced liver disease, now suggest HCV treatment should also be provided to people with a risk of transmitting HCV, such as PWID [19, 20]. Although a recent economic model suggested that in general it is more cost-effective to delay treatment of mild disease until more moderate stages of fibrosis[21], when these individuals have on-going transmission risk they should be prioritised for early treatment over other patient groups [22].
In this paper, we estimate the current HCV treatment rates and coverage of OST and NSP in PWID across 11 sites in Europe. We assess the impact of these and scaled-up HCV treatment rates and other primary prevention on HCV prevalence and incidence over the next ten years.
Methods
The model
We used a dynamic deterministic mathematical model of HCV transmission among PWID, stratifying PWID according to intervention status (no OST or NSP, on OST, NSP, or both) alongside HCV infection and treatment status (susceptible never infected, previously infected, chronically infected, on treatment, treatment failure [9, 23]). In three sites (Czech Republic, Finland and Sweden) PWID are also stratified by drug type (opioid or methamphetamine/amphetamine). PWID enter the model through a constant rate that individuals initiate injecting; all PWID are assumed initially susceptible to HCV infection (Figure 1a). Susceptible PWID can become infected at a per-capita rate proportional to the background prevalence of disease which changes as HCV treatment increases. Transmission is reduced by a fixed multiplicative cofactor dependent on OST and NSP status (Figure 1b). Once infected, PWID either transition to the chronically infected group (Ab+, RNA+) or spontaneously clear infection and transition to the previously infected group (Ab+, RNA−). This previously infected group are assumed to be re-infected and clear infection at the same rate as susceptible PWID[24–26]. Chronically infected PWID (both primary and re-infection) can be treated; if treatment is successful and SVR attained, PWID transition to the previously infected group. However, if SVR is not attained PWID transition to the treatment failure group. In the baseline model, treatment failures cannot be retreated (Figure 1a); once treatment is switched to DAAs we assume treatment failures can be retreated. PWID leave the model through permanent cessation of injecting or drug-related or non-drug related mortality. All PWID enter the model with no coverage of OST or NSP, and transition between the different intervention states (OST and/or NSP) at site-specific fixed per-capita rates (Figure 1b, Tables S1a-S1k). Further details of the model including model equations are in the Supplementary materials.
Figure 1. Schematics of HCV transmission (1a) and OST and NSP interventions (1b) in the model.
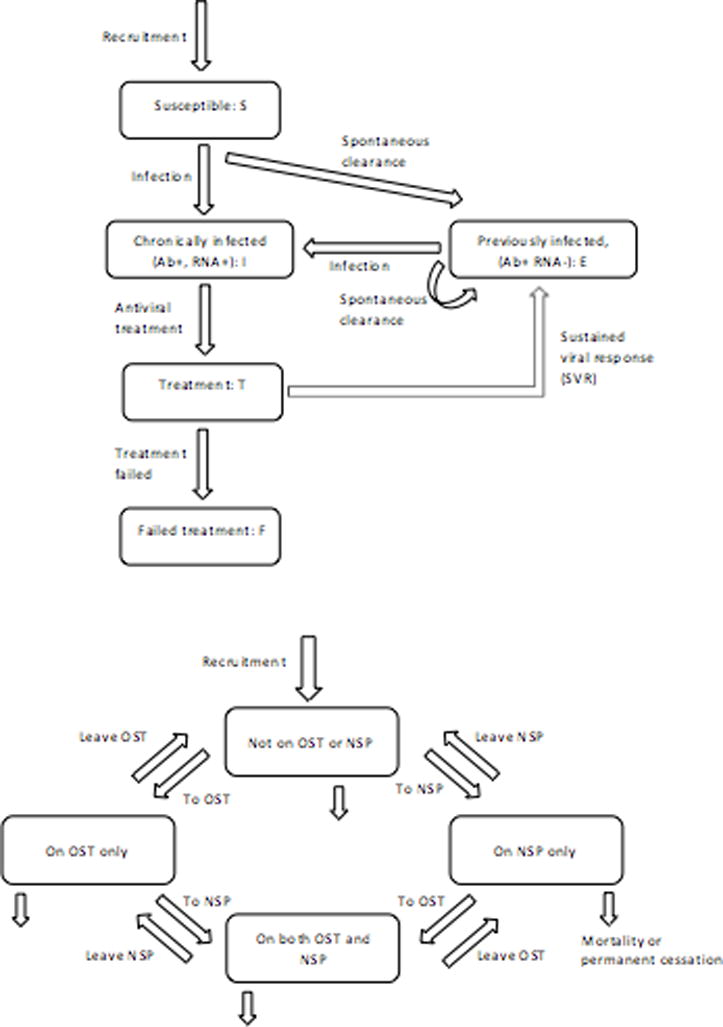
a: Infection component of the model
b: OST and NSP intervention component of the model
Model parameterisation and calibration
The model was parameterised to each of the 11 sites (Table 1 and Supplementary Tables S1a-S1l for site specific information).
Table 1.
Parameter table
| Parameter | Value | Notes | |
|---|---|---|---|
| PWID population size: | All sampled from a normal distribution | ||
| Amsterdam | 2621 (1946–3374) in 2009 1874 (1341–2455) in 2014 |
||
| Belgium | 9080 (6356 – 11804) | ||
| Czech Republic | 41816 – 46563 | Range – no point estimate available | |
| Denmark | 16500 (13000 – 19000) | ||
| Finland | 15611 (13770 – 22655) | ||
| France | 80000 (65000 – 95000) | ||
| Hamburg | 8492 (7582 – 9436) | ||
| Norway | 15500 (10500 – 20150) | ||
| Scotland | 16000 (11500 – 19400) | ||
| Slovenia | 6000 (4200 – 7800) | ||
| Sweden | 8021 – 26550 | Maximum and minimum PWID population size estimates. | |
| PWID mortality rate: | All sampled from a Poisson distribution | ||
| Amsterdam | 2.4% per year | ||
| Belgium | 2.5% per year | ||
| Czech Republic | 0.8% per year | ||
| Denmark | 2.0% per year | ||
| Finland | 2.0% per year | ||
| France | 1.3% per year | ||
| Hamburg | 0.7% per year | ||
| Norway | 1.9% per year | ||
| Scotland | 1.0% per year | ||
| Slovenia | 0.7% per year | ||
| Sweden | 2.0% per year | ||
| HCV antibody prevalence among PWID and year prevalence fit to | All sampled from a normal distribution. In all cases HCV antibody prevalence is adjusted to chronic prevalence by assuming a 26% (22–29%) spontaneous clearance rate [54] |
||
| Amsterdam | 59.4% (54.8 – 64.0%) | 2007 | |
| Belgium | 43.3% (34.3 – 52.4%) | 2012 | |
| Czech Republic | 35.0% (31.6 – 38.5%) | 2005 | |
| Finland | 76.0% (72.4–79.4%) | 2014 | |
| France | 66.4% (60.3–71.9%) | 2011 | |
| Hamburg | 67.7% (62.3–72.8%) | 2014 | |
| Scotland | 58.0% (55.8 – 60.2%) | 2013/14 | |
| Slovenia | 27.3% (19.1 – 35.5%) | mid-2010 | |
| Sweden | 81.7% (79.6 – 83.6%) | 2014 | |
| HCV chronic prevalence among PWID | |||
| Denmark | 35.0 – 45.0% | 2014 | |
| Norway | 45% (42.6 – 47.5%) | 2007 | |
| Number PWID treated per year: | 1. Only those on OST are initially eligible for treatment. 2. All PWID can be treated. |
||
| Total treated in each site per year | Number treated per 1000 PWID per year | ||
| Amsterdam | 2005–2016: 15 | 2005–2016: 6.1 – 11.2 | 1. |
| Belgium | 2004–2016: 30 | 2004–2016: 5.7 – 10.6 | 1. |
| Czech Republic | 2002–2011: 370 2011–2016: 540 |
2002–2011: 7.9 – 8.8 2011–2016: 11.6 – 12.9 |
2. |
| Denmark | 2002–2014: 53 2014–2015: 50 2014–2016: 100 |
2002–2014: 2.8 – 4.1 2014–2015:2.6–3.8 2014–2016: 5.3–7.7 |
1. |
| Finland | 2006–2016: 5 | 2006–2016: 0.06 | 1. |
| France | 2001–2016: 1705 (923 – 3148) |
2001–2016:10.5 – 43.3 | 1. Note: these are the calculated number treated based on the treatment rate for people who have injected at least one in the last year. |
| Hamburg | 2005–2011: 60 2011–2016: 72 |
2005–2011: 6.2 – 7.9 2011–2016: 7.6 – 9.5 |
1. |
| Norway | 2009–2016: 100 | 2009–2016: 5.0 – 9.5 | 70% treatment are amongst those on OST and 30% treatment amongst those not on OST. |
| Scotland | 2005–2008: 60 2008–2009: 90 2009–2016: 150 |
2005–2008: 3.1 – 5.2 2008–2009: 4.6–7.8 2009–2016: 7.7–13.0 |
1. |
| Slovenia | 1997–1999: 2 1999–2008: 5 2008–2016: 62 |
1997–1999: 0.3 – 0.5 1999–2008: 0.6 – 1.2 2008–2016: 7.9 – 14.8 |
2. |
| Sweden | 1997–2016: 90 | 1997–2016: 3.4 – 11.2 | 2. |
Key parameters used in the modelling for each of the sites. PWID population size and prevalence estimates shows mean (95% CI) unless otherwise stated. Mortality rates are given per year. The range for the number of PWID treated per 1000 PWID is estimated using the number of treatments in each site and the PWID population size. References are given in Tables S1a-S1k in Supplementary Material.
For sites with opioid injecting only, 2,500 model parameter sets were randomly sampled from the parameter uncertainty distributions (see Tables S1a-S1l). For each parameter set, the model was fit to the PWID population size by varying the rate that individuals initiate injecting, to OST and NSP coverage levels by varying the recruitment rates onto OST and NSP, and to either the chronic or antibody HCV prevalence at a site-specific time-point by varying the transmission rate. For Czech Republic, Finland and Sweden the model was fit to more parameters - see Supplementary Materials for further details. HCV incidence was estimated from model inputs assuming a stable epidemic except for Amsterdam where additional data were available suggesting a decreasing PWID population size and declining incidence[15].
In sites with opioid and meth/amphetamine injecting, we assume that baseline risk of HCV transmission is the same for all PWID [27–29], there is equal NSP coverage across both types of injectors, but only opioid users can be recruited on to OST.
In all but four sites (Czech Republic, Finland, Sweden and Norway), HCV treatment of PWID was modelled only amongst those on OST for initial analyses as in these sites only PWID on OST are currently treated.
Model projections and analyses
Data on PWID treatment numbers for each site were scaled to give a rate per 1000 PWID as well as percentage of chronic HCV infections treated in 2015/16. By scaling to give a rate based on total PWID population size we can easily compare current and projected future treatment numbers between all sites. All known increases in treatment prior to 2015 were included in the model.
We used the model to project the change in prevalence and incidence between 2016 and 2026 if treatment is switched from IFN-based therapies to new DAAs (SVR rate 90% (85-95%) and current treatment rates per 1000 PWID are either maintained, doubled, or increased to 50 per 1000 PWID treated annually. Impact projections either assumed current coverages of OST and NSP are maintained or OST and NSP are scaled-up to 80% coverage (if not already achieved). We determined the annual treatment number (expressed as a rate of treatment per 1000 PWID) needed to reduce incidence to 2 per 100 person years (2%) by 2026. This is the number of treatments annually per 1000 PWID and is therefore constant when projecting to 2026.
We estimated the z-score associated with the mean difference in chronic prevalence given the uncertainty in chronic HCV generated by the model. We categorised a z-score less than 0.5 as a modest change (unlikely to be observed), between 0.5-1.5 as a moderate change (may be observable), and scores greater than 1.5 or 3.0 as changes that are increasingly and highly likely to be observed.
Uncertainty analysis
To consider the effect of uncertainty within the underlying parameters, we performed a linear regression analysis of covariance on the relative decrease in HCV prevalence and incidence between 2016 and 2026 when current treatment rates are doubled. For each site, the proportion of each model outcome’s sum-of-squares contributed by each parameter was calculated to estimate the importance of each parameter to the uncertainty[30].
Results
Baseline HCV Treatment Rates
HCV treatment of PWID started at different times across the sites, ranging from 1997 (Slovenia) to 2009 (Norway) with very few PWID having been treated in Finland. Figure 2 shows the percentage of chronic HCV prevalent cases among PWID that were treated in 2015/16 based on data from each site – varying from <0.1% in Finland, to 0.5-2% in Sweden, Denmark, Belgium, Norway and Amsterdam, and >5% in Czech Republic and Slovenia.
Figure 2. Percentage of estimated PWID with chronic HCV infections treated annually at baseline (2015/16) for each site.
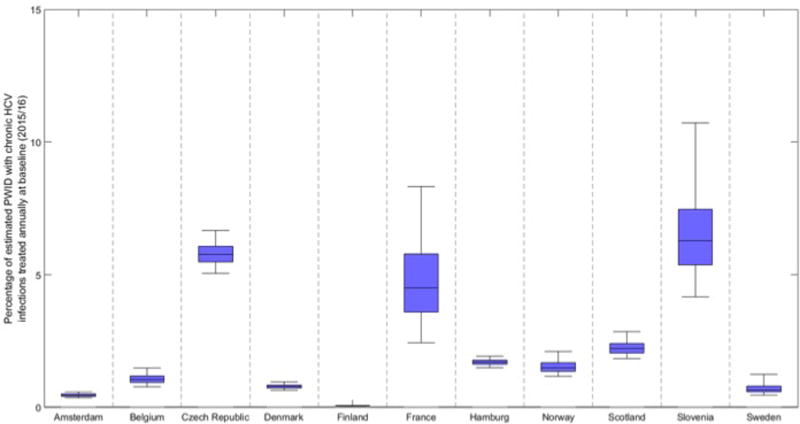
Bars indicate the median and interquartile range and whiskers show the 95% credibility intervals.
Model projections
(a) Chronic HCV prevalence among PWID
At baseline in 2016, projected chronic prevalence varied from <25% in Czech Republic (21% (95% CrI 18-24%)) and Slovenia (16% (11-22%)), to >55% in Finland (56% (53-59%)) and Sweden (60% (57-63%)). Figure 3 shows projected baseline and 10 year chronic HCV prevalence among PWID in each setting for different levels of scale-up of HCV treatment with new DAAs. Figure 4 shows the same projections but with scale-up in OST and NSP coverage to 80%.
Figure 3. Baseline and projected 10 year chronic HCV prevalence among PWID in multiple sites in Europe for various treatment intervention scenarios.
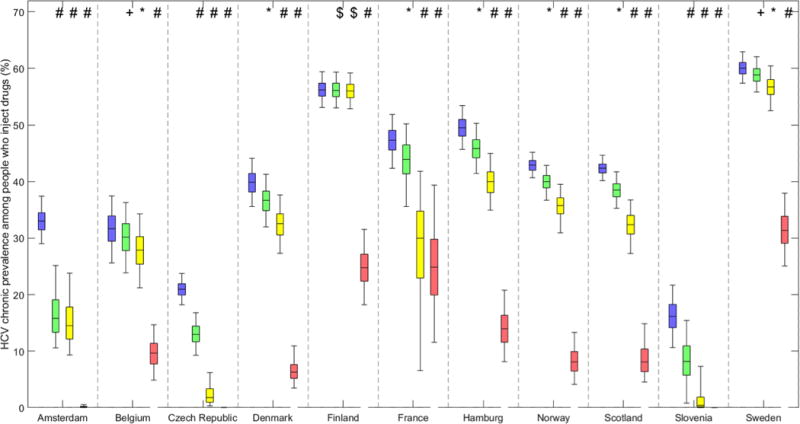
Baseline chronic prevalence (blue boxes) and projected 10 year chronic prevalence if either current treatment rates continue with new DAAs (green boxes), treatment rates are doubled with new DAAs (yellow boxes), or increased to 50 per 1000 PWID annually with new DAAs (pink boxes). Bars indicate the median and interquartile range and whiskers show the 95% credibility intervals.
Figure 4. Baseline and projected 10 year chronic HCV prevalence among PWID in multiple sites in Europe for various treatment intervention scenarios with OST and NSP scaled-up to 80% coverage.
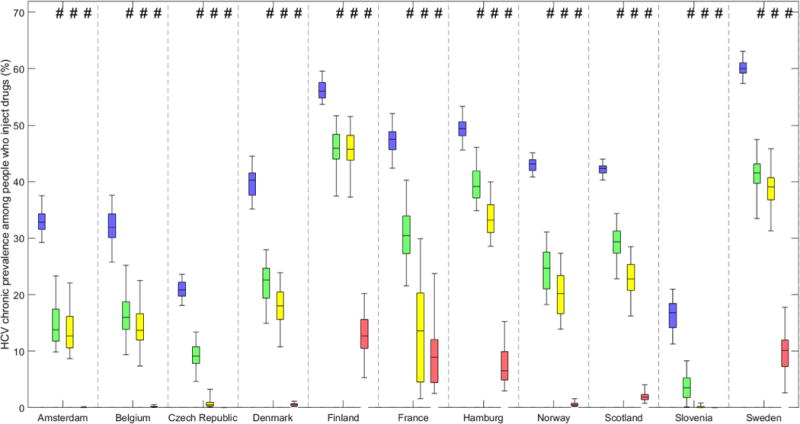
Baseline chronic prevalence (blue boxes) and projected 10-year chronic prevalence if either current treatment rates continue with new DAAs (green boxes), treatment rates are doubled with new DAAs (yellow boxes), or increased to 50 per 1000 PWID annually with new DAAs (pink boxes) with OST and NSP scaled-up to 80% coverage. Bars indicate the median and interquartile range and whiskers show the 95% credibility intervals.
(i) Switching to DAAs, treatment rate maintained
In the majority (8/11) of the sites the difference in projected chronic HCV prevalence after 10 years if current treatment rates with DAAs remain constant is <5%. In these sites the median absolute difference ranges from <1.5% in Finland, Sweden and Belgium up to 3-4% in Norway, Denmark, France, Hamburg, and Scotland. This difference is substantially smaller than the uncertainty in the baseline chronic HCV prevalence in the sites. This equates to a relative decrease of <10% at each site (see Supplementary Materials).
In the remaining three sites (Amsterdam, Czech Republic and Slovenia), there is a much greater relative decrease in chronic HCV prevalence between 2016 and 2026 from switching to DAAs; 37.5% (26.6-51.8%) in Czech Republic and 49.3% (25.0-98.0%) in Slovenia. In Amsterdam, the decreasing population size and concurrent decrease in transmission contribute around 90% of the relative decrease of 51.8% (28.7-65.7%) in chronic prevalence between 2016 and 2026. These sites have a z-score >3.0 indicating that an observable change in chronic prevalence will likely occur by switching to DAAs with current treatment rates.
If all sites switched to DAAs with current treatment rates and concurrently increased OST and NSP coverage to 80%, the model projects a reduction in prevalence in all sites, from less than 20% in Finland (17.6 (10.0-27.9%)) and Hamburg (19.5% (11.7-27.6%)), 30-50% in Scotland, Sweden, France, Norway, Denmark and Belgium, to >50% in Czech Republic and Amsterdam, and >75% in Slovenia. The differential benefit of scaling-up OST and NSP alongside treatment on reducing chronic HCV prevalence ranges from greater than 10 to less than 1.5 times because of baseline coverage. For example, in Finland, Sweden and Belgium scaling-up OST and NSP with current HCV treatment rates reduces chronic HCV prevalence in 10 years by 17.1%, 31.1% and 48.4% respectively, compared to 0.1%, 1.8% and 4.4% reduction without OST and NSP scale-up. In contrast, there is only a small projected improvement in Amsterdam and Czech Republic which already have high coverage of OST and NSP (Supplementary Materials Tables S1a-S1k); other sites are projected to improve reductions in chronic HCV prevalence from 2-3 times (Slovenia, Hamburg and Scotland) and 5-6 times in France, Denmark and Norway (Supplementary Materials Table S2).
(ii) Switching to DAAs, treatment rate doubled
For sites with high baseline chronic prevalence (>55% at baseline) and low treatment rates (<1% of chronic infections treated at baseline), for example Sweden and Finland, doubling DAA treatment rates has little effect on the projected prevalence in 2026 (0.4% (0.3-0.6%) and 5.2% (3.3-10.4%) relative decrease respectively) if OST and NSP are maintained at current coverage. For sites with moderate chronic prevalence (30-50% at baseline) and <2.5% of chronic infections being treated annually in 2015/16 (Belgium, Denmark, Hamburg, Norway, Scotland), doubling DAA treatment rates could reduce chronic HCV prevalence from 11.6% relative decrease (Belgium) up to 23.5% (Scotland).
France has a moderate chronic prevalence (47.3%) at baseline and high initial treatment rate (4.5% (2.4-8.3%) of all chronic infections treated annually). When their treatment rate is doubled with DAAs this yields a greater relative decrease in chronic prevalence than other sites with moderate prevalence (36.4% (16.7-85.5%)). The credibility intervals are wide because of uncertainty in the estimates of HCV treatment rates.
In Czech Republic and Slovenia, doubling DAA treatment rates is projected to reduce chronic prevalence by >90% (Figure 3), and in Amsterdam by 55.8% (32.8-69.6%).
Increasing OST and NSP to 80% coverage and doubling DAA treatment rates is projected to reduce chronic prevalence between 17.9% (10.3-28.2%) in Finland to 99.5% (91.8%–99.9%) in Slovenia. In sites with high baseline treatment rates (Czech Republic and Slovenia), the decrease in prevalence is primarily due to doubling treatment rates (97.3% and 91.6% decrease in Czech Republic with and without scaled-up OST and NSP, respectively, and 99.5% and 97.4% in Slovenia). For sites with low baseline treatment rates and low coverage of OST and NSP, it is the increase in OST and NSP that drives the decrease in chronic prevalence rather than the doubling in treatment rates – in Finland the decrease changes from 0.4% to 17.9% when additionally scaling-up OST and NSP, from 5.2% to 35.5% in Sweden and from 11.6% to 55.6% in Belgium.
(iii) DAA treatment rate 50 per 1000 PWID
Increasing annual DAA treatment rates to 50 per 1000 PWID with current OST and NSP coverage leads to substantial reductions in chronic HCV in all sites (Figure 3). In the high prevalence sites of Finland and Sweden chronic prevalence reduces by about half by 2026. Conversely, in most moderate prevalence sites (Belgium, Hamburg, Scotland, Norway and Denmark) chronic HCV prevalence decreases by 70% or more, although in France the decrease is smaller and more uncertain (47.6% (21.7-73.8%)). In low prevalence settings (Czech Republic and Slovenia), chronic prevalence is projected to decrease by around 99% by 2026.
In projections with OST/NSP scale-up to 80%, prevalence decreases by more than three-quarters in all sites, with 7/11 sites (Scotland, Denmark, Norway, Belgium, Amsterdam, Czech Republic and Slovenia) projecting a decrease of >95%.
(b) HCV Incidence among PWID
Baseline projections of incidence before 2015 agree with observed incidence estimates where data were available (Supplementary Materials). Projected changes in incidence from 2016 to 2026 are shown in Supplementary Figures 2 and 3, without and with scale-up of OST and NSP to 80% coverage using DAAs. HCV incidence is projected to remain largely unchanged with current OST and NSP coverage in all but 3 sites if current HCV treatment rates are maintained using DAAs, however if OST and NSP are scaled-up to 80% coverage, projections estimate a relative decrease in incidence of over 35% at all sites.
Figure 5 shows the treatment number per 1000 PWID required in 2016/17 to reduce incidence to 2 per 100 pyrs (2%) among PWID by 2026 with and without scale-up of OST and NSP to 80% coverage. In Amsterdam, an incidence of 2% (1-3%) was already estimated in 2016, and so just switching to DAAs ensured an incidence <2% by 2026 in 99% of model runs. In Slovenia, just switching to the new DAAs and maintaining current treatment rates is likely to decrease incidence to <2% by 2026 (projected by 78% of model fits), with an increase in treatments rates by 20% being needed to ensure this impact in the other 22% of model fits. In Czech Republic switching to DAAs would achieve 2% incidence in <10% of model fits, and increasing current treatment rates by 43% over all model runs would ensure the decrease. In all other sites a substantial increase in HCV treatment rates (in the absence of any increase in OST and NSP coverage) is needed to reduce HCV incidence to 2%, ranging from 3 to 5 times the current treatment rates in France and Scotland, to between 6-9 times in Hamburg, Norway, Denmark, Belgium, 17 times in Sweden, and 200 times in Finland. If OST and NSP are scaled-up to 80% coverage, maintaining current treatment rates is sufficient to achieve an incidence of 2% in 2026 in Amsterdam and Slovenia (100% of model fits), may achieve this impact in Belgium, Czech Republic (84% and 50% of model fits), but is unlikely to (<10% of model fits) in other settings. Alongside increased OST and NSP, France, Denmark, Norway, Scotland, Hamburg, Sweden and Finland would need to scale-up their baseline treatment rates by 2.2, 2.8, 2.8, 3.6, 4.7, 10.3 and 159-fold, respectively. This is 20-60% less than if OST and NSP had not been scaled-up.
Figure 5. Current and projected number of treatments per 1000 PWID to reduce incidence to 2 per 100 pyrs by 2026.
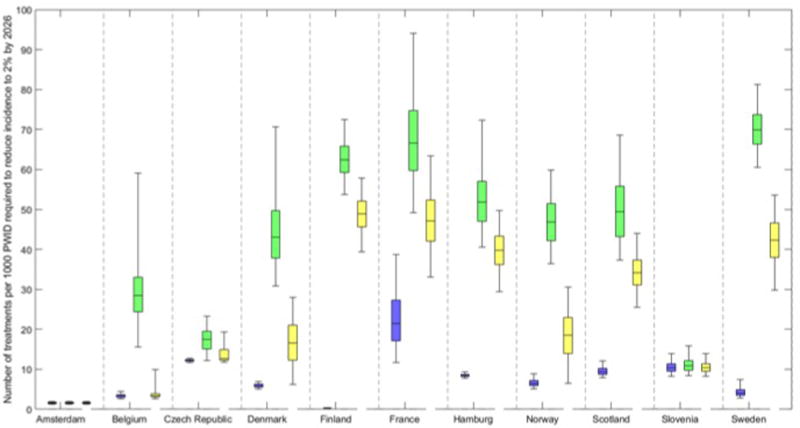
Current number of treatments per 1000 PWID at baseline (2015/16, blue) and required scale-up in number of treatments per 1000 PWID initially needed per year (2016/17) if current OST and NSP coverage is maintained (green, median and 95% credibility interval shown in figure) or if OST and NSP are scaled to 80% coverage (yellow) to reduce incidence to 2 per 100 pyrs (2%) by 2026. Based on data from the sites we have: 1Treatment initially given only to those on OST. 2Treatment initially given to all PWID. 370% treatment to PWIDs on OST, 30% treatment to PWIDs not on OST.
(c) Uncertainty analysis
The sensitivity analysis indicates that for most sites, uncertainty in three main factors contribute to variation in the relative decrease in chronic prevalence and incidence between 2016 and 2026 when treatment rates are doubled, but with differing levels of influence between the sites (Supplementary Materials Figure S4). The PWID population size contributes 34-63% of the variation in Finland, Belgium, Scotland, Slovenia and Norway, and 80% in Sweden, whilst the prevalence estimates contribute 32-53% of the variation in five of the sites (Slovenia, Belgium, Czech Republic, Denmark and Hamburg). The duration of injecting is most important in Amsterdam, contributing 85% of the variation, but also contributes 25-48% in Scotland, Hamburg, Denmark, Norway, Belgium and Finland. In France, the estimated treatment rate contributes 80% of the variation.
Discussion
Main Findings
Treatment scale-up is needed to achieve observable reductions in chronic HCV prevalence among PWID in most sites in Europe, even with new DAAs. Doubling DAA treatment rates may lead to observable reductions (12-24% decrease) in chronic prevalence by 2026 in Belgium, Denmark, Hamburg, Norway and Scotland; but not in Finland or Sweden. Exceptions include Czech Republic, Slovenia, and Amsterdam, which at current HCV treatment rates are projected to reduce chronic HCV prevalence from a third to a half by 2026. This is due to the low or decreasing prevalence of infection in these settings. Alternatively, increasing OST and NSP coverage to 80% with current HCV treatment rates would reduce chronic HCV prevalence by 17-20% in Finland and Hamburg and 30-79% in all other sites. Reducing HCV incidence to less than 2% by 2026 requires little action in Amsterdam, Czech Republic and Slovenia, whereas in Belgium, Denmark, Hamburg, Norward and Scotland it will require at least a 5-fold increase in current HCV treatment rates, or 1.8-4.7-fold if OST and NSP are scaled up to 80% coverage.
Strengths and Limitations
Our model projections and their interpretation are influenced strongly by uncertainty in the parameters and evidence base. First, we collected information from a range of sources and obtained data not routinely collected across Europe (e.g. number of PWID treated for HCV) [6, 31]. Unfortunately, data collection was inconsistent across sites, particularly estimates of PWID population size which were used to estimate HCV treatment rates. Reliable PWID population size estimates are difficult to obtain and except for Amsterdam where evidence suggests a falling population [15, 32], we had to assume stable populations.
Second, uncertainty in the chronic HCV prevalence among PWID contributed substantially to the uncertainty in our projections, with estimates generated from a diverse range of sources and rarely (except for Scotland) from ongoing community based surveillance [6, 33]. Third, the duration of injecting drug use is difficult to estimate precisely and contributed to model uncertainty. We sampled the average injecting duration from a range extending from 6 to over 20 years, and in the absence of clear evidence assumed that opioid and methamphetamine injectors had similar durations. If the true duration is towards the higher end of our ranges, scaling-up HCV treatment will have greater impact on transmission, and if towards the lower end, scaling-up OST and NSP will have a greater contribution on reducing transmission [34].
Fourth, DAA SVRs for PWID in “real world” settings are yet to emerge, and so we assumed a range of 85-95%[35, 36]. Given the short treatment duration and early treatment of predominantly mild disease, it is likely that SVRs will be very high, although it is possible that as treatment is scaled-up among more vulnerable PWID this SVR may reduce. In general, the impact of HCV treatment in our projections is relatively robust to variations in SVR, although uncertainty in SVR becomes more influential in settings with lower chronic prevalence and higher HCV treatment rates. Furthermore, we assumed that PWID who had either cleared HCV spontaneously or after successful treatment had the same risk of re-infection as the susceptible population of PWID i.e. the per capita transmission probability of re-infection was the same as for primary infection. There is some evidence to suggest that previous spontaneous clearance could result in higher rates of clearance for subsequent re-infection[24], but similar data surrounding spontaneous clearance of re-infections after SVR does not exist, and infrequent testing intervals can contribute bias as some re-infections may go unnoticed[24, 37]. Observational studies have reported that re-infection after SVR can be of a similar, higher or lower rate than the background rate of infection[38–42], indicating uncertainty in the evidence. However, if re-infection risk was lower than primary infection for people achieving SVR, or spontaneous clearance higher for re-infections, then our model projections represent conservative estimates for the number of treatments needed to reduce prevalence and incidence across the different sites.
Fifth, we recorded substantial differences in coverage of OST and NSP between sites which are incorporated into the baseline model. In subsequent intervention scenarios, we either considered no scale-up of these interventions, or assumed their scale-up to 80% coverage. This optimistic scenario may over-estimate the likely impact of what could be achieved from scaling-up OST and NSP, although some of our sites demonstrate such coverage is possible. However, even if this scale-up is possible, it is unlikely that it would be achieved quickly, so these projections may over-estimate the real reduction in HCV that could be achieved from scaling-up OST and NSP. Sixth, the model does not incorporate information on HCV case-finding and any future difficulty in diagnosing and treating PWID with chronic HCV when HCV transmission and prevalence have fallen to low levels, but this limitation will only affect a small number of the most optimistic model projections.
Seventh, we have not modelled HIV co-infection which varies across Europe and may impact both on linkage to services and morbidity outcomes. Finally, we assume no change in injecting risk behaviour following HCV treatment – apart from through exposure to OST and NSP which is also included prior to HCV treatment. If injecting risk was reduced following treatment [43], our assumption provides conservative projections of impact.
Implications and Comparisons with Other Literature
Multiple studies in specific countries and across Europe have used statistical and mathematical model projections to suggest that new DAA treatments need to increase in order to reverse trends in End Stage Liver Disease [44–48]. However, to project impact on HCV transmission, a dynamic transmission model that can track both re-infection and prevention of future infections is required, alongside information on the number and proportion of individuals from key populations like PWID treated for HCV infection. Consequently, there are fewer analyses that project impact on HCV transmission.
An earlier study revealed a 2-3 fold difference in chronic HCV prevalence and 4-5 fold difference in baseline HCV treatment rates in seven cities in the UK [49]. We also found considerable heterogeneity between sites in Europe. For example, Czech Republic and Slovenia both have baseline chronic prevalence of less than 30% [28, 50, 51], whilst in Finland and Sweden it is over 55%. Treatment rates also varied 2-3 fold. At baseline, 8/11 sites had low treatment rates (<10/1000 PWID treated annually), whereas France had a much higher treatment rate (21/1000 PWID treated annually), a consequence of the high access to HCV treatment in France compared to other countries[52]. Our results also show a greater decrease in prevalence for Amsterdam than other studies have projected [15, 53], however this could be due to differences in modelling the decreasing epidemic to achieve the incidence estimate, and differences modelling the PWID population and transmission dynamics.
The lack of ongoing surveillance data, including PWID prevalence and HCV treatment rates amongst PWID, in many European settings and comparable indicators between countries is important and a public health concern. Our model projections show that scaling-up OST and NSP combined with switching to DAAs with comparatively small increases in the number of PWID treated could generate substantial observable reductions in HCV prevalence in several sites. However, robust HCV surveillance data among PWID were not always available and chronic HCV prevalence was uncertain. To ensure that empirical evidence of the impact of HCV treatment as prevention can be generated, it is important that more attention is given to establishing robust surveillance systems to reduce the uncertainty surrounding chronic HCV prevalence among PWID. The potential and relative costs of introducing effective HCV surveillance are trivial compared to the costs of HCV treatment – and need to be encouraged across Europe.
Supplementary Material
Lay summary.
Measuring the amount of HCV in the population of people who inject drugs is uncertain. To reduce HCV infection to minimal levels in Europe will require scale-up of both HCV treatment and other interventions that reduce injecting risk (especially opioid substitution treatment and provision of sterile injecting equipment).
Acknowledgments
Funding: The study was funded by European Commission Drug Prevention and Information Programme (DIPP) [JUST/2013/DPIP/AG/4812]. In addition we acknowledge support from the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Evaluation of Interventions at the University of Bristol in partnership with Public Health England. NM, PV, and MH were supported by the National Institute for Drug Abuse [grant number R01 DA037773-01A1]. NM was additionally supported by the University of California San Diego Center for AIDS Research(CFAR)), a National Institute of Health (NIH) funded program [grant number P30 AI036214]
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
VM: institutional support no. PRVOUK-P03/LF1/9 and the project Nr. LO1611 with a financial support from the MEYS under the NPU I program.
Footnotes
Contribution of authors
All authors contributed to interpreting the results, writing and editing the paper.
HF undertook modelling supervised by PV, MH NM and drafted the paper.
MH, NM and PV conceived the study with support from all co-authors.
Disclosures: NM,MH,PV has received unrestricted research grants from Gilead unrelated to this work, and honoraria from Gilead, Merck, and AbbVie. HF has received an honorarium from MSD. HM Consultant/advisor for Abbvie and Gilead. Speaker fees from Abbvie, Gilead, MSD and Medivir. MP has served as a speaker and has worked on several research projects unrelated to this study for which her institute received unrestricted grants from Gilead, Roche, MSD and AbbVie.
References
- 1.Williams R, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384(9958):1953–97. doi: 10.1016/S0140-6736(14)61838-9. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PK, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ly KN, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of internal medicine. 2012;156(4):271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 4.Cowie B, Allard N, MacLachlan J. O86 EUROPEAN RESPONSES IN FOCUS: COMPARING VIRAL HEPATITIS AND HIV RELATED DEATHS IN EUROPE 1990–2010 IN THE GLOBAL BURDEN OF DISEASE STUDY 2010. Journal of Hepatology. 2014;1(60):S35–S36. [Google Scholar]
- 5.ECDC and EMCDDA. Prevention and control of infectious diseases among people who inject drugs. Stockholm: ECDC; 2011. pp. 4–5. [Google Scholar]
- 6.European Monitoring Centre for Drugs and Drug Addition. EMCDDA Insights. Vol. 23. Luxembourg: Publications Office of the European Union; 2016. Hepatitis C among drug users in Europe: epidemiology, treatment and prevention. [Google Scholar]
- 7.Turner KM, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106(11):1978–1988. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Den Berg C, et al. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102(9):1454–1462. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickerman P, et al. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction. 2012;107(11):1984–1995. doi: 10.1111/j.1360-0443.2012.03932.x. [DOI] [PubMed] [Google Scholar]
- 10.Martin NK, et al. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(Suppl 2):S39–S45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin NK, et al. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J Hepatol. 2011;54(6):1137–1144. doi: 10.1016/j.jhep.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Martin NK, et al. HCV treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–1609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wedemeyer H, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21(Suppl 1):60–89. doi: 10.1111/jvh.12249. [DOI] [PubMed] [Google Scholar]
- 14.De Vos A, Kretzschmar M. Benefits of hepatitis C virus treatment: A balance of preventing onward transmission and re-infection. Mathematical biosciences. 2014;258:11–18. doi: 10.1016/j.mbs.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 15.de Vos AS, et al. Decline in incidence of HIV and hepatitis C virus infection among injecting drug users in Amsterdam; evidence for harm reduction? Addiction. 2013;108(6):1070–81. doi: 10.1111/add.12125. [DOI] [PubMed] [Google Scholar]
- 16.Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of “perfectovir”. Clinical Infectious Diseases. 2015;60(12):1829–1836. doi: 10.1093/cid/civ197. [DOI] [PubMed] [Google Scholar]
- 17.Everson G, et al. Safety and efficacy of treatment with the interferon-free ribavirin-free combination of sofosbuvir+ GS-5816 for 12 weeks in treatment naive patients with genotype 1-6 HCV infection. 49th European Association for the Study of the Liver International Liver Congress (EASL 2014) 2014 [Google Scholar]
- 18.Martin NK, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association for the Study of the, L. EASL recommendations on treatment of hepatitis C 2014. J Hepatol. 2014;61(2):373–95. doi: 10.1016/j.jhep.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver. Electronic address, e.e.e. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63(1):199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Leidner AJ, et al. Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology. 2015;61(6):1860–9. doi: 10.1002/hep.27736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin NK, et al. How should HCV treatment be prioritized in the direct-acting antiviral era? An economic evaluation including population prevention benefits. Journal of hepatology. 2016 doi: 10.1016/j.jhep.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin NK, et al. Combination Interventions to Prevent HCV Transmission Among People Who Inject Drugs: Modeling the Impact of Antiviral Treatment, Needle and Syringe Programs, and Opiate Substitution Therapy. Clinical Infectious Diseases. 2013;57:S39–S45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vickerman P, et al. The more you look, the more you find: effects of hepatitis C virus testing interval on reinfection incidence and clearance and implications for future vaccine study design. The Journal of infectious diseases. 2012;205(9):1342–1350. doi: 10.1093/infdis/jis213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin NK, et al. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J Hepatol. 2011;54(6):1137–44. doi: 10.1016/j.jhep.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 26.Martin NK, Vickerman P, Hickman M. Mathematical modelling of hepatitis C treatment for injecting drug users. J Theor Biol. 2011;274(1):58–66. doi: 10.1016/j.jtbi.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 27.Zábranský T, Mravčík V, Chomynová P. Overal mortality of drug users in the Czech Republic. 1st. ResAd/EMCDDA; 2009. [Google Scholar]
- 28.Zabransky T, et al. Hepatitis C virus infection among injecting drug users in the Czech Republic–prevalence and associated factors. European addiction research. 2006;12(3):151–160. doi: 10.1159/000092117. [DOI] [PubMed] [Google Scholar]
- 29.ŠVŮGEROVÁ H. Spotřeba injekčního materiálu klienty pražských harm reduction služeb v závislosti na vzorcích užívání (Consumption of material for injection clients of Prague harm reduction services depending on use patterns) Mgr, Univerzita Karlova v Praze, 1 lékařská fakulta, obor adiktologie (MADI) 2015 [Google Scholar]
- 30.Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. In: Gray A, Briggs A, editors. Handbooks in Health Economic Evaluation Series. Oxford: Oxford University Press; 2006. [Google Scholar]
- 31.ECDC, editor. European Centre for Disease Prevention and Control. Hepatitis C surveillance in Europe - 2013. Stockholm: 2015. [Google Scholar]
- 32.Jones HE, et al. Recapture or precapture? Fallibility of standard capture-recapture methods in the presence of referrals between sources. American journal of epidemiology. 2014:kwu056. doi: 10.1093/aje/kwu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmateer NE, et al. Rapid decline in HCV incidence among people who inject drugs associated with national scale-up in coverage of a combination of harm reduction interventions. PLoS One. 2014;9(8):e104515. doi: 10.1371/journal.pone.0104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin NK, et al. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(Suppl 2):S39–45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grebely J, et al. Efficacy and safety of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic HCV genotype 1 infection receiving opioid substitution therapy: Analysis of Phase 3 ION trials. Clinical Infectious Diseases. 2016:ciw580. doi: 10.1093/cid/ciw580. [DOI] [PubMed] [Google Scholar]
- 36.Dore GJ, et al. Elbasvir–Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist TherapyA Randomized TrialElbasvir–Grazoprevir in Persons With HCV Receiving OAT. Annals of internal medicine. 2016;165(9):625–634. doi: 10.7326/M16-0816. [DOI] [PubMed] [Google Scholar]
- 37.Sacks-Davis R, et al. Many hepatitis C reinfections that spontaneously clear may be undetected: Markov-chain Monte Carlo analysis of observational study data. Journal of The Royal Society Interface. 2015;12(104):20141197. doi: 10.1098/rsif.2014.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta SH, et al. Protection against persistence of hepatitis C. The Lancet. 2002;359(9316):1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 39.Grebely J, et al. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. The Lancet Infectious Diseases. 2012;12(5):408–414. doi: 10.1016/S1473-3099(12)70010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Micallef J, et al. High incidence of hepatitis C virus reinfection within a cohort of injecting drug users. Journal of viral hepatitis. 2007;14(6):413–418. doi: 10.1111/j.1365-2893.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 41.Aitken CK, et al. High incidence of hepatitis C virus reinfection in a cohort of injecting drug users. Hepatology. 2008;48(6):1746–1752. doi: 10.1002/hep.22534. [DOI] [PubMed] [Google Scholar]
- 42.Simmons B, et al. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clinical Infectious Diseases. 2016:civ948. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alavi M, et al. Injecting risk behaviours following treatment for hepatitis C virus infection among people who inject drugs: The Australian Trial in Acute Hepatitis C. International Journal of Drug Policy. 2015;26(10):976–983. doi: 10.1016/j.drugpo.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duberg AS, et al. The future disease burden of hepatitis C virus infection in Sweden and the impact of different treatment strategies. Scandinavian Journal of Gastroenterology. 2015;50(2):233–244. doi: 10.3109/00365521.2014.990505. [DOI] [PubMed] [Google Scholar]
- 45.Razavi H, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(Suppl 1):34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 46.Harris R, et al. Increased uptake and new therapies are needed to avert rising hepatitis C-related end stage liver disease in England: modelling the predicted impact of treatment under different scenario. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.05.008. doi: http://dx.doi.org/10.1016/j.jhep.2014.05.008. [DOI] [PubMed]
- 47.Mullhaupt B, et al. Modeling the Health and Economic Burden of Hepatitis C Virus in Switzerland. Plos One. 2015;10(6):13. doi: 10.1371/journal.pone.0125214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Innes H, et al. Strategies for the treatment of Hepatitis C in an era of interferon-free therapies: what public health outcomes do we value most? Gut. 2015;64(11):1800–9. doi: 10.1136/gutjnl-2014-308166. [DOI] [PubMed] [Google Scholar]
- 49.Martin NK, et al. HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. J Viral Hepat. 2015;22(4):399. doi: 10.1111/jvh.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mravčík V, et al. In: Výroční zpráva o stavu ve věcech drog v České republice v roce 2013 [Annual Report on Drug Situation 2013 – Czech Republic] MRAVČÍK V, editor. Praha: Úřad vlády České republiky; 2014. [Google Scholar]
- 51.D A, editor. Reitox National Focal Point. Report on the drug situation 2013 of the republic of Slovenia. 2013. [Google Scholar]
- 52.Razavi H, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. Journal of viral hepatitis. 2014;21(s1):34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 53.van Santen DK, et al. Cost-Effectiveness of Hepatitis C Treatment for People Who Inject Drugs and the Impact of the Type of Epidemic; Extrapolating from Amsterdam, the Netherlands. PloS one. 2016;11(10):e0163488. doi: 10.1371/journal.pone.0163488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. Journal of Viral Hepatitis. 2006;13(1):34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


