Abstract
Behavioral inhibition (BI) is an early temperamental profile characterized by negative reactivity to novelty, withdrawal from social situations, and increased risk for social anxiety. Previous research associated BI assessed in early childhood to striatal hypersensitivity in mid-to-late adolescence. The present study examined this association among 10 year-olds, characterized with BI at ages 24 and 36 months on measures of temperamental reactivity. Participants (n = 40) were studied at age 10 using a reward processing task during functional magnetic resonance imaging (fMRI). Child- and maternal-report of social anxiety symptoms was collected at ages 10 and 13. Findings indicate greater caudate activation and stronger striatal connectivity in high, compared to low, behaviorally inhibited children. Caudate activation related to social anxiety symptoms at both ages. These findings suggest that enhanced striatal responsivity reliably manifests among high behaviorally inhibited children as early as age 10. This may reflect hyper-sensitivity to reward or excessive motivation to avoid errors.
Keywords: behavioral inhibition, reward, caudate, social anxiety, fMRI
Introduction
Behavioral inhibition (BI) is a temperament characterized in infancy and early childhood by a predisposition for heightened vigilance, negative affect and fearful responses to novelty (Kagan and Snidman, 1991; Fox et al., 2005). In infancy this temperament presents as negative reactivity to a wide range of novel stimuli. As they develop, many of these children exhibit excessive social reticence, are reluctant to initiate social interactions (Coplan et al., 1994), and are frequently disliked and rejected by their peers (Stewart and Rubin, 1995). This early temperament also predicts later behavior, personality and brain function, and increases risk for anxiety disorders, particularly social anxiety (Chronis-Tuscano et al., 2009). However, the term ‘behavioral inhibition’ is typically only used when referring to patterns manifested in early childhood.
While much attention focuses on relations between BI and the amygdala (Kagan et al., 1987; Fox et al., 2005), emerging work also examines the striatum and its sensitivity to reward, a positive incentive that reinforces behavior (Guyer et al., 2006; Bar-Haim et al., 2009; Helfinstein et al., 2011). Although cross-sectional studies show mixed results regarding age-related changes in striatal activation (Ernst et al., 2005; Bjork et al., 2010), recent longitudinal work suggests that activation of the striatum on some reward tasks increases with age (Lamm et al., 2014a). However, prior work on striatal circuitry in BI, only examined adolescents and young adults. Additional research is needed on the relations between striatal function and BI at earlier ages, and the present study examines these relations in 10 year olds.
Adolescents and adults characterized by BI in childhood exhibit reward hypersensitivity. For example, studies using functional magnetic resonance imaging (fMRI) and the monetary incentive delay task (MID) (Knutson et al., 2000) show that striatal activation to gain or loss cues is higher in adolescents with, relative to those without, early childhood BI (Guyer et al., 2006). Such enhanced activation occurs specifically to anticipatory cues signaling potential behavioral-contingent positive or negative outcomes (Bar-Haim et al., 2009). In terms of feedback, findings are less consistent. One study linked BI to striatal activation only to negative, but not positive, feedback (Helfinstein et al., 2011), whereas others found no associations with feedback (Guyer et al., 2006; Pérez-Edgar et al., 2014). Thus, BI-related hypersensitivity manifests most consistently in anticipation of reward, rather than in response to feedback.
Striatal function may play a key role in adolescent anxiety (Lago et al., 2017). A similar pattern of striatal hyper-sensitivity as observed in adolescents with a history of BI has been reported in a sample of adolescents seeking treatment for social anxiety (Guyer et al., 2012). These findings suggest comparable alterations in striatal response to incentives in BI and social anxiety during adolescence. Furthermore, high striatal activation has been found in adolescents with a combination of early childhood BI and high concurrent anxiety (Pérez-Edgar et al., 2014). Thus striatal hypersensitivity may moderate risk for anxiety among individuals with a history of high BI. Of note, adolescent anxiety is predicted by anxiety symptoms during school age (Pine, 2007). As a result, examining relations among BI in toddlerhood, striatal function at school age, and anxiety at school age and early adolescence ties together prior research on BI, anxiety, striatal function and development.
To study children, our group recently developed the piñata task, a child-friendly adaptation of the MID task. In an initial validation of the task (Helfinstein et al., 2013), healthy children between the ages of 8 and 14 years made speeded responses to an animal-shaped piñata target to gain a reward of varying levels, as compared to a no-reward condition. Children showed neural activation patterns consistent with those seen in adolescents and adults using the MID task, such that striatal activation was modulated by cue incentive value (Helfinstein et al., 2013).
The present study examined neural responses to reward in children characterized by BI in toddlerhood. To our knowledge, this is the first study to assess the neural processing of reward among school-age children characterized by early childhood BI. Children were followed prospectively from 4 months of age until age 10, when participants completed the fMRI piñata task. Child- and maternal-reported social anxiety symptoms were assessed at age 10 and 13. Prior work on BI and anxiety found striatal hypersensitivity in anticipation of incentives, rather than following reward receipt (Guyer et al., 2006; Bar-Haim et al., 2009). Accordingly, the study tested the hypothesis that high, compared to low, behaviorally inhibited children show increased striatal activation in anticipation of reward. The study also examined striatal connectivity. Since no prior study examined this issue, hypotheses are exploratory, merely predicting different patterns of striatal connectivity in children with, as opposed to without, a history of BI. Finally, given associations among BI, reward sensitivity, and social anxiety (Guyer et al., 2012; Pérez-Edgar et al., 2014), the study also considered if striatal activation and connectivity related to social anxiety symptoms at age 10 and 13.
Materials and methods
Participants and procedure
Children were drawn from a cohort participating in a longitudinal study of temperament and affect regulation. A total of 779 healthy, full-term infants were seen at 4 months of age for temperament screening, during which positive and negative affect and motor reactivity to novel stimuli were assessed (for a complete description of the recruitment and selection process see Hane et al., 2008). Of this sample, 291 infants (135 males, 156 females) representing the full range of reactivity were selected and invited to continue participation in the longitudinal study, with 64% white, 14% African American and 22% from other backgrounds. When children were 24 and 36 months, behavioral observations and maternal-report of behavioral inhibition were collected on 268 participants.
All 268 participants were contacted for a large testing wave at age 10, which included multiple laboratory and neuroimaging assessments. Interested participants were screened for eligibility. To participate in the study, participants had to be free from medical illness on the basis of medical history and physical examination, and free of contraindications for MRI. Of the 268 participants, 15 were not eligible to participate in neuroimaging due to medication or metallic objects (typically braces), 48 refused participation in neuroimaging and 92 either aged out or did not respond. For the neuroimaging portion of the study, we recruited only participants who also completed two laboratory visits not reported here (N = 84). Of these, 13 either completed different fMRI tasks or completed the piñata task on a different scanner, and thus are not included in the analysis. Additionally, nine refused to complete the task while in the scanner, and two participants experienced technical problems. Thus, 60 participants completed the piñata task, and 58 of these participants were trained in a mock scanner. Twenty participants were excluded, five due to poor behavioral performance (either a high frequency of random anticipatory responses or low frequency of responses in the no-reward condition), and 15 due to excessive movement. As a result, data from 40 participants (23 female; age M = 10.48, SD = 0.42) were analyzed. These 40 participants did not significantly differ from excluded participants on BI or gender, all Ps > 0.30.
Additionally at age 10, participants were assessed using maternal- and child-report on the Screen for Child Anxiety Related Emotional Disorders (SCARED) (Birmaher et al., 1999). Participants were re-assessed again using maternal- and child-report on the SCARED at 13 years of age.
All participants provided informed assent, and participants’ guardians provided informed consent. The study was approved by the Institutional Review Boards of the National Institute of Mental Health and the University of Maryland, College Park.
Behavioral inhibition
BI was observed at 24 and 36 months of age during 3 episodes (stranger, robot, tunnel) involving unfamiliar people and objects (Calkins et al., 1996; Fox et al., 2001). Observations were coded for each episode, and interrater reliability (intraclass correlations) ranged from 0.72 to 0.98 (mean = 0.87; 19% overlap; 2 coders) for 2-year coding and from 0.93 to 1.00 (mean = 0.98; 10% overlap; 2 coders) for 3-year coding. Maternal-report of social fear was obtained using the Toddler Behavior Assessment Questionnaire (TBAQ) (Goldsmith, 1996). As in previous work, measures from both 24 and 36 months were standardized and averaged to create a BI composite (Lahat et al., 2014; Lamm et al., 2014b; Walker et al., 2014). In order to create high and low BI groups, the BI composite variable was median split. By definition, the high BI group scored significantly higher than the low BI group on the BI composite, t(38) = −6.88, P < 0.0001 (Table 1). No significant differences were found between the high and low BI groups in age, gender, IQ, ethnicity or mother’s education, all Ps > 0.44 (Table 1).
Table 1.
Group characteristics and demographics
| Characteristic | Low BI | High BI |
|---|---|---|
| N (Gender) | 18 (8 male, 10 female) | 22 (9 male, 13 female) |
| Mean age (SD) | 10.43 (0.31) | 10.53 (0.49) |
| BI continuous score (SD) | −0.52 (0.51)* | 0.62 (0.53)* |
| Age 10 social anxiety-m (SD) | 2.94 (3.30) | 4.89 (4.25) |
| Age 10 social anxiety-c (SD) | 5.33 (3.73) | 4.37 (2.65) |
| Age 10 social anxiety-c & m (SD) | −0.05 (0.82) | 0.04 (0.78) |
| Age 13 social anxiety-maternal report m (SD) | 3.09 (3.05) | 4.00 (3.59) |
| Age 13 social anxiety-child report c (SD) | 4.38 (4.01) | 3.89 (2.77) |
| Age 13 social anxiety-c & m (SD) | −0.09 (0.92) | 0.01 (0.82) |
| IQ (SD) | 114.94 (14.70) | 118.14 (11.03) |
| Child ethnicity N | ||
| Caucasian | 14 | 15 |
| African American | 3 | 3 |
| Other | 1 | 4 |
| Mother’s education | ||
| High school graduate | 2 | 4 |
| College graduate | 11 | 10 |
| Graduate school graduate | 4 | 7 |
| Other | 1 | 1 |
Note: BI—behavioral inhibition; c-child report; m-maternal report; c & m—child and maternal report averaged;
P < 0.0001.
Piñata task
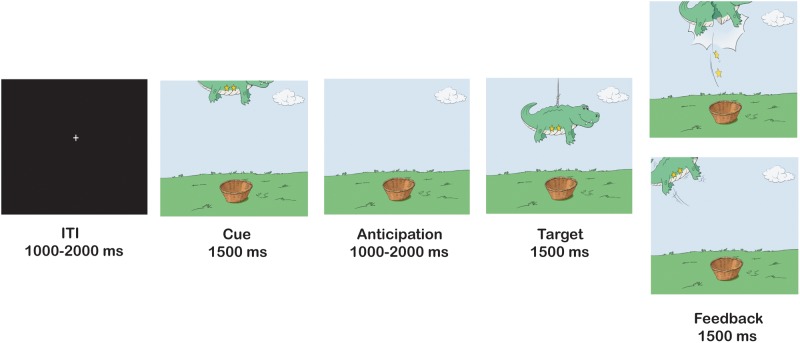
At age 10, participants completed the fMRI piñata task (Helfinstein et al., 2013), a child-friendly version of the MID task (Figure 1; Knutson et al., 2000). Thus, reward incentives in the piñata task parallel those of the MID task, ranging from no reward to a large reward. Participants saw animal-shaped piñatas, and were told to ‘whack’ the piñatas by pressing a button as fast as possible to earn stars inside the piñatas. They were told that the more stars they earned the larger would be the monetary reward. Each trial contained three stages: in the anticipation stage, participants saw the piñata partially revealed at the top of the screen (cue) where the number of stars inside the piñata was visible; in the response stage, the piñata dropped to the center of the screen (target) and the participant made a speeded button press; in the feedback stage, participants either saw the piñata cracked open with falling stars that were won (positive feedback), or they saw the intact piñata swinging away, indicating a miss. The cue appeared for 1500 ms, followed by a cue-free anticipatory period that varied between 1000 and 2000 ms. The target appeared for a variable period of time, followed by a delay period with a duration generating events such that the combined target and delay periods were 1500 ms. Finally, the feedback appeared for 1500 ms. Inter-trial interval (ITI) in the form of a white fixation cross on a black background varied between 1000 and 2000 ms. The task consisted of one practice run of 22 trials, followed by six task runs of 22 trials each, for a total of 132 task trials. Trials were divided evenly between the four incentive levels (0 stars, 1 star, 2 stars and 4 stars) for a total of 33 trials at each incentive level.
Fig. 1.
Trial structure of piñata task.
Following Helfinstein et al. (2013), we used a dynamic algorithm that adjusted target duration trial by trial to maintain a performance accuracy level of approximately 60%. Response window duration increased by 50 ms when the subject’s cumulative accuracy for all trials in the run was < 60%, and decrease by 50 ms when cumulative accuracy was > 60%. Participants were told that they would receive money based on the amount of stars they earned, up to $15. Participants received a minimum of $3, plus an additional $3 for every 47 stars they captured, up to a maximum of $15, but they were never informed of this exact payment structure.
Social anxiety symptoms
At ages 10 and 13, both children (10 year N = 178; 13 year N = 154) and mothers (10 year N = 181; 13 year N = 147) completed the SCARED, a standardized, reliable measure of anxiety symptoms (Birmaher et al., 1999). Based on prior work (Chronis-Tuscano et al., 2009; Clauss and Blackford, 2012), our main focus was on the social anxiety symptoms scale, which ranges in score from 0 to 14, with a clinical cut-off ≥ 8 (Birmaher et al., 1999; Table 1). In addition to examining child- and maternal-report separately, we also calculated cross-informant indices by standardizing and averaging scores across informants (cross-informant correlations: age 10 r = 0.27, P < 0.001; age 13 r = 0.43, P < 0.0001). This convention also is consistent with prior work (Chronis-Tuscano et al., 2009).
Behavioral data analysis
Behavioral performance in the scanner was examined to determine whether participants responded differently to low- and high-incentive cues, and whether these behavioral responses differed as a function of BI grouping. We conducted 2 (BI Group) × 4 (Incentive Value) repeated measures ANOVAs to test three performance scores: hit rate (the proportion of trials on which the participant responded quickly enough to earn the reward), reaction time (RT; the average response time across all trials) and premature response rate (the proportion of trials on which the participant responded during the anticipation stage, before the target appeared).
Image acquisition and pre-processing
Image Acquisition
Participants were scanned in a General Electric (Waukesha, WI, USA) Signa 3 Tesla magnet. Task stimuli were displayed via back-projection from a head-coil mounted mirror to a screen at the foot of the scanner bed. Foam padding was used to constrain head movement. Behavioral data were recorded using a hand-held two-button response box. Forty-seven sagittal slices (3.0-mm thickness) per volume were obtained using a T2*-weighted echo-planar sequence (echo time, 25 ms; flip angle, 50°; 96 × 96 matrix; field of view, 240 mm; in-plane resolution, 2.5 mm × 2.5 mm; repetition time was 2300 ms). A total of 77 volumes were collected in each run. To improve the localization of activations, a high-resolution structural image was also collected from each participant during the same scanning session using a T1-weighted standardized magnetization prepared spoiled gradient recalled echo sequence with the following parameters: 124 1.2-mm axial slices; repetition time, 8100 ms; echo time, 32 ms; flip angle, 15°; 256 × 256 matrix; field of view, 240 mm; in-plane resolution, 0.86 mm × 0.86 mm; NEX, 1; bandwidth, 31.2 kHz.
Image Pre-processing
Analysis of fMRI data was performed using Analysis of Functional and Neural Images (AFNI) software version 2.56 b (Cox, 1996). Echo-planar images (EPI) were visually inspected to confirm image quality and minimal movement. Standard pre-processing of EPI data included slice-time correction, motion correction and spatial smoothing with a 6-mm full-width half-maximum Gaussian smoothing kernel. Each subject’s data were transformed to a percent signal change using each subject’s voxel-wise time series mean blood oxygen level-dependent (BOLD) activity as a baseline. Images were analyzed using an event-related design. Time series data for each individual were analyzed using multiple regression (Neter et al., 1996). The entire trial was modeled using a gamma-variate basis function, including five cue events (0 star cues, 1 star cues, 2 star cues, 4 star cues and cues from premature response trials), five feedback events (0 star feedback, 1 star feedback, 2 star feedback, 4 star feedback and feedback on trials where subjects made a premature response) and one target event. The model also included six nuisance variables modeling the effects of residual translational (motion in the x, y and z planes) and rotational motion (roll, pitch and yaw), and regressors for both baseline (any part of the time series not specifically modeled by the incentive cue or feedback events) and parametric linear trend. The 0 star, 1 star, 2 star and 4 star cues were our regressors of interest.
fMRI data analysis
Based on prior research (Guyer et al., 2006; Bar-Haim et al., 2009; Helfinstein et al., 2011, 2013), the study targeted striatal regions in anticipation of reward. fMRI data analysis examined this region in three ways: (1) mirroring the analytical approach with BI adolescents (Guyer et al., 2006), a region of interest (ROI) analysis was carried out, using anatomically defined striatal regions; (2) voxel-based analysis with a smaller volume correction for striatum, and a larger volume correction for the whole brain; (3) generalized psychophysiological interaction (gPPI) analysis in order to examine functional connectivity of anatomical striatal regions, using both the smaller and larger volume corrections. The statistical threshold for individual voxels was set to P < 0.005, uncorrected. Small volume correction for multiple comparisons was applied following Monte Carlo simulations to determine appropriate cluster thresholds of 18 voxels for striatum, and 75 voxels for the whole brain to achieve a corrected alpha level of P < 0.05.
Anatomical ROI analysis
To specifically isolate striatal activation, an ROI analysis was conducted examining significant activation in the left and right nucleus accumbens, left and right putamen, as well as left and right ventral and dorsal caudate. These regions were defined by creating a striatal map containing each region, as defined by Talairach anatomical boundaries provided by AFNI. For each region, a t-test was carried out in SPSS with BI Group as the independent variable and extracted parametrically weighted beta values as the dependent variables.
Voxel-based analysis
Voxel-based analyses were done with weighted parametric values. The weighted parametric contrast of BOLD activity was created using the four incentive cues, such that each cue type was weighted in proportion to its incentive value. These analyses were conducted using 3dMVM with BI group as a factor.
gPPI
A generalized psychophysiological interaction (gPPI) set of analyses was used to examine functional connectivity (http://afni.nimh.nih.gov/sscc/gangc/CD-CorrAna.html). This set of analyses modeled differences in connectivity between a ‘seed’ region and other brain regions across the different psychological events (incentive cues) in the task. Based on prior research with the piñata task (Helfinstein et al., 2013), we used four seeds, which were further anatomically defined by the Talairach atlas: left dorsal caudate, right dorsal caudate, left ventral caudate, and right ventral caudate. The gPPI term was created for the weighted parametric activity of the incentive cues. Individual-level GLMs were created using the same regressors as in the whole brain analysis, the eigenvariate time series for the ‘seed’, and psychophysiological interaction terms for each incentive cue.
Brain function and social anxiety
Pearson correlations examined the relation between brain function and social anxiety. Variables considered included child-report, maternal-report and an average score of child- and maternal-report of social anxiety using the SCARED at ages 10 and 13, as well as beta values extracted from the striatal regions that exhibited significant BI group differences.
Results
Behavioral results
No significant main effects or interactions with BI group were found for hit rate (M = 58.05%, SD = 3.20%), RT (M = 454.53 ms, SD = 86.63 ms) or premature response rate (M = 7.25%, SD = 4.92%).
Anatomical ROI analysis
An examination of the ROIs, revealed that only the t-test for the right dorsal caudate showed a significant effect of BI group, t (38) = −3.56, P < 0.001. Greater activation was found for high (M = 0.10, SD = 0.06) than low behaviorally inhibited children (M = 0.04, SD = 0.05). No significant BI group differences were found for the other ROIs.
Voxel-based analysis
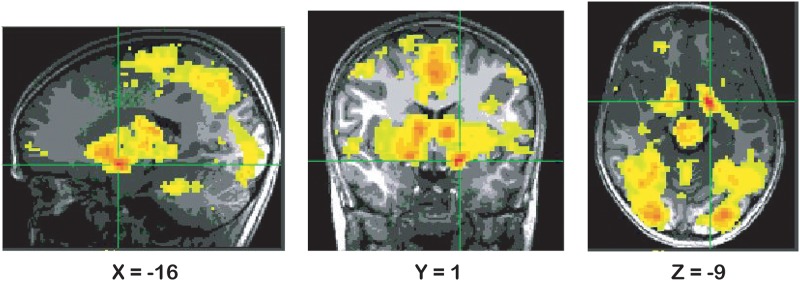
The weighted parametric analysis for the main effect of incentive revealed a large cluster in ventral striatum (Table 2 and Figure 2), and additional clusters in left middle frontal gyrus and left cuneus (Table 2). These findings reflect the fact that activation in these clusters tracks incentive value (greater activation with greater incentives). They document an expected pattern of activation in response to reward incentives across the sample as a whole. No BI group differences were observed for either the striatum or the whole brain.
Table 2.
Significant results from the voxel-based analysis
| Region | Cluster size (voxels) | Coordinates (x,y,z) | Peak activation | F | P |
|---|---|---|---|---|---|
| Incentiveparametric | |||||
| Ventral striatum | 24108 | −16,1,−9 | 9.45 | 2.984 | 0.005 |
| Left middle frontal gyrus | 148 | −39,26,41 | 4.26 | ||
| Left cuneus | 76 | 1,−91,31 | 4.75 | 2.984 | 0.005 |
| 2.984 | 0.005 |
Fig. 2.
Results from the voxel-based parametric analysis displaying effect of incentive (parametric) in ventral striatum, P < 0.005 (Note: right is left).
gPPI
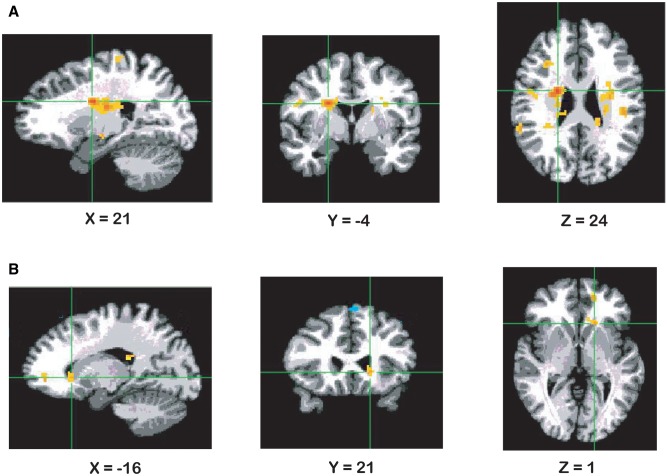
The gPPI analysis for the left dorsal caudate seed yielded a cluster in the right caudate for the group parametric analysis (Table 3 and Figure 3A). Data extracted from this cluster revealed stronger connectivity in high (M = 0.17, SD = 0.38) compared to low (M = −0.19, SD = 0.30) behaviorally inhibited children, t (38) = −3.22, P < 0.01. The gPPI analysis for this seed also revealed a cluster in right superior frontal gyrus (Table 3).
Table 3.
Significant results from the gPPI analysis
| Region | Cluster Size (voxels) | Coordinates (x,y,z) | Peak activation | F | P |
|---|---|---|---|---|---|
| Left dorsal caudate seed | |||||
| Group parametric | |||||
| Right caudate | 208 | 21,−4,24 | 5.01 | 2.984 | 0.005 |
| Incentive parametric | |||||
| Right superior frontal gyrus | 129 | 1,6,49 | −3.99 | 2.984 | 0.005 |
| Left ventral caudate seed | |||||
| No significant findings | |||||
| Right dorsal caudate seed | |||||
| No significant findings | |||||
| Right ventral caudate seed | |||||
| Group parametric | |||||
| Left caudate | 21 | −16,21,1 | 3.93 | 2.984 | 0.005 |
Fig. 3.
Results from the gPPI analysis displaying: (A) effect of BI group (parametric) for connectivity between left dorsal caudate seed and right caudate, P < 0.005 (Note: right is left); (B) effect of BI group (parametric) for connectivity between right ventral caudate seed and left caudate, P < 0.005 (Note: right is left).
The gPPI analysis for the right ventral caudate seed yielded a cluster in left caudate (Table 3 and Figure 3B). Data extracted from this cluster revealed stronger connectivity between right ventral caudate and left caudate in high (M = 0.17, SD = 0.34) compared to low (M = −0.35, SD = 0.42) behaviorally inhibited children, t (38) = −4.33, P < 0.0001. Results for the left ventral caudate or right dorsal caudate seeds did not reveal any significant findings within striatal regions.
Brain function and social anxiety
Pearson correlations with social anxiety were conducted for striatal regions that exhibited significant BI group differences. These regions included the right dorsal caudate anatomical ROI, right caudate (associated with the left dorsal caudate seed) and left caudate (associated with the right ventral caudate seed). The results revealed positive correlations between activation in the right dorsal caudate anatomical ROI and maternal report of social anxiety at both ages 10 and 13. Activation in this region was also positively correlated with the averaged child-maternal report social anxiety score at age 13. Furthermore, BI also was positively correlated with maternal report of social anxiety at both ages 10 and 13 (Table 4).
Table 4.
Pearson correlations between brain activation/connectivity and social anxiety symptoms
| Variable | BI | BI Group | Age 10 (c) | Age 10 (m) | Age 10 (c & m) | Age 13 (c) | Age 13 (m) | Age 13 (c & m) | R dCaud Parametric (anatomical ROI) | R Caud Parametric (gPPI) | L Caud Parametric (gPPI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BI (continuous) | 1 | 0.74** | −0.05 | 0.38* | 0.19 | 0.09 | 0.45* | 0.30 | 0.51* | 0.19 | 0.51** |
| BI Group | 1 | −0.15 | 0.25 | 0.06 | −0.08 | 0.13 | 0.06 | 0.50** | 0.46* | 0.57** | |
| Age 10 social anxiety (c) | 1 | 0.25 | 0.80** | 0.42* | 0.33 | 0.32 | 0.07 | −0.07 | −0.04 | ||
| Age 10 social anxiety (m) | 1 | 0.80** | 0.52** | 0.71** | 0.67** | 0.38* | −0.12 | 0.15 | |||
| Age 10 social anxiety (c & m) | 1 | 0.58** | 0.62** | 0.62** | 0.27 | −0.12 | 0.07 | ||||
| Age 13 social anxiety (c) | 1 | 0.47* | 0.87** | 0.20 | −0.02 | −0.07 | |||||
| Age 13 social anxiety (m) | 1 | 0.86** | 0.39* | −0.03 | 0.12 | ||||||
| Age 13 social anxiety (c & m) | 1 | 0.33* | 0.001 | 0.10 | |||||||
| R dCaud Parametric (anatomical ROI) | 1 | 0.11 | 0.34* | ||||||||
| R Caud Parametric (gPPI) | 1 | 0.21 | |||||||||
| L Caud Parametric (gPPI) | 1 |
Note: c—child report; m—maternal report; c & m—child and maternal report averaged; R dCaud—right dorsal caudate; R Caud—right caudate; L Caud—Left Caudate;
P < 0.001; *P < 0.05.
Discussion
The neural responses to reward anticipatory cues were examined in a longitudinal sample of children characterized by BI in toddlerhood. This cohort was followed prospectively from 4 months to 13 years of age. Participants completed an fMRI reward-processing task when they were 10 years old. In addition, child- and maternal-report of social anxiety symptoms were obtained when children were 10 and 13 years old. Although no BI group differences were observed for behavioral performance, ROI-based and gPPI analyses revealed significant differences between high and low behaviorally inhibited children. Specifically, high behaviorally inhibited children showed greater activation than low behaviorally inhibited children in the right dorsal caudate. Furthermore, high behaviorally inhibited children displayed stronger connectivity than low behaviorally inhibited children between the left dorsal caudate seed and right caudate, as well as between the right ventral caudate seed and left caudate. This pattern occurred for parametrically-weighted incentive values, which weighs each incentive level based on its value and reflects a linear trend. Thus, regions and connectivity sensitive to increasingly high reward cues responded differently in high vs. low behaviorally inhibited children. Finally, positive correlations were observed between caudate activation and social anxiety symptoms at both ages 10 and 13. These findings extend previous research on adolescents characterized by childhood BI to behaviorally inhibited children as young as 10 years of age.
These results replicate patterns previously seen in a different, older cohort, where adolescents with a history of BI were studied using similar methods appropriate for older subjects than studied in the current report (Guyer et al., 2006; Bar-Haim et al., 2009). Detecting replicable associations has been a major challenge for fMRI, and observing comparable findings across two cohorts is a significant accomplishment. Moreover, the current findings suggest that previously observed patterns of increased striatal sensitivity in BI at adolescence and adulthood already manifest as early as 10 years of age. This is earlier than has been previously observed in prior work on striatal function in BI. This raises questions about the degree to which similar patterns of striatal function manifest in younger children with behavioral inhibition, such as toddlers. Finally, two prior studies link striatal hyper-sensitivity to adolescent anxiety, one of which examined adolescents with a history of BI (Pérez-Edgar et al., 2014) the other of which examined anxiety patients seeking treatment. Detection of a comparable pattern in this third study suggests that striatal function might be an underlying mechanism that plays a role in the development of anxiety. Given that behaviorally inhibited children are at increased risk for social anxiety, this may inform attempts to identify risk factors in these children.
The effect of BI group on striatal activation and within-striatal connectivity in response to reward is consistent with hyper-sensitivity of the striatal system. It is possible that instead of implying a hyper-sensitivity to reward, the striatal finding might reflect a motivation to avoid errors (Lago et al., 2017). Such interpretation is consistent with other findings in two prior reports in adolescents with behavioral inhibition. First, the report by Helfinstein et al. (2011) showed that adolescents with behavioral inhibition exhibited greater caudate activation to negative than positive feedback. Second, the report by Bar-Haim et al. (2009) showed greater striatal activation in the BI than the non-BI group in conditions when outcome was contingent on the participant’s action. Taken together, these findings are in line with the notion that BI is characterized by hyper-sensitivity to environmental stimuli, a characteristic that has typically been understood in terms of enhanced reactivity of the fear circuitry to novel stimuli, expressed as behavioral avoidance (Kagan et al., 1987; Fox et al., 2005). However, here we demonstrate enhanced neural sensitivity of brain regions that also facilitate approach behavior (reward-related behavior; Knutson et al., 2000). More work is needed to explore this motivation-related interpretation.
Finally, we examined whether activation and connectivity within striatal regions were associated with social anxiety. The findings indicated positive correlations between activation in the right dorsal caudate ROI and social anxiety symptoms at both ages 10 and 13. These correlations were obtained for social anxiety as reported by mothers at both ages, as well as for the mother-child social anxiety averaged score at age 13. This composite score takes into account the perspectives of both mother and child. However, the correlations seem to be driven mostly by maternal-reported social anxiety scores. Our results also revealed a positive correlation between BI measured in toddlerhood and social anxiety at age 13. These associations with social anxiety are consistent with previous reports on the role of striatal hyper-sensitivity in the link between BI and adolescent anxiety (Pérez-Edgar et al., 2014), as well as enhanced striatal activation in adolescents with social anxiety (Guyer et al., 2012). Taken together, these findings suggest that reward sensitivity may be an underlying mechanism that plays a role in the development of social anxiety.
Behaviorally, our results revealed no significant findings for incentive condition on RTs. The lack of significant behavioral findings are not uncommon in fMRI research and are consistent with the piñata task version used by Helfinstein et al. (2013) in the scanner, where target duration was dynamically adjusted, as done in our study. Furthermore, we did not observe significant BI group behavioral differences, which may be a result of the dynamic algorithm. This suggests that children high and low in BI maintained comparable levels of task performance despite differences in neural responses, and eliminates the possibility that behavioral performance confounded our results. Furthermore, the lack of behavioral group differences may suggest that neural responses are more sensitive than behavioral performance alone.
Some limitations of the present study should be noted. First, although almost all of our participants trained in a mock scanner, a good number of participants were excluded from the analyses due to excessive motion. Although this fact is quite typical for an fMRI study with children, it is possible that even more extensive motion training in a mock scanner would be beneficial to minimize data loss. Second, the piñata task used in the present study included only reward trials; there were no ‘loss’ trials where participants might have lost points they have earned. Both trial types are included in the MID task, and striatal activation has been observed in adolescents with a history of BI in response to both reward and punishment (Guyer et al., 2006). Thus, although our data with 10 year old children are comparable to adolescent BI data, the paradigms were different. However, our interest in the present study was specifically focused on striatal-based reward sensitivity because findings are generally stronger with reward than punishment trials (Knutson et al., 2001; Guyer et al., 2006). Furthermore, a task with reward-only trials is shorter and increases the likelihood that young children will remain engaged in the task.
In sum, after having demonstrated in earlier work the association of BI and striatal hyper-sensitivity in adolescents (Guyer et al., 2006; Bar-Haim et al., 2009; Helfinstein et al., 2011), we now demonstrate that this neural pattern holds for children as young as 10 years of age. Furthermore, this reward sensitivity was associated with social anxiety symptoms in childhood. Future research should include even younger children to test at what age this neural perturbation emerges. Given the association with social anxiety, the present work may have important implications for early prevention and intervention efforts.
Acknowledgements
The authors would like to thank Sarah Helfinstein for her assistance with data analysis.
Funding
This project was supported by a grant from the National Institute of Mental Health (NIMH) U01MH093349 to NAF, by the NIMH Intramural Research Program, and by a Banting Post-Doctoral Fellowship from the Social Sciences and Humanities Research Council awarded to AL.
Conflict of interest. None declared.
References
- Bar-Haim Y., Fox N.A., Benson B., et al. (2009). Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science, 20(8), 1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B., Brent D.A., Chiappetta L., Bridge J., Monga S., Baugher M. (1999). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child & Adolescent Psychiatry, 38(10), 1230–6. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. (2010). Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One, 5(7), 1–14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins S.D., Fox N.A., Marshall T.R. (1996). Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development, 67(2), 523–40. [PubMed] [Google Scholar]
- Chronis-Tuscano A., Degnan K.A., Pine D.S., et al. (2009). Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 48(9), 928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J.A., Blackford J.U. (2012). Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child & Adolescent Psychiatry, 51(10), 1066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan R.J., Rubin K.H., Fox N.A., Calkins S.D., Stewart S.L. (1994). Being alone, playing alone, and acting alone: distinguishing among reticence and passive and active solitude in young children. Child Development, 65(1), 129–37. [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., et al. (2005). Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage, 25(4), 1279–91. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Henderson H.A., Marshall P.J., Nichols K.E., Ghera M.M. (2005). Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology, 56, 235–62. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Henderson H.A., Rubin K.H., Calkins S.D., Schmidt L.A. (2001). Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development, 72(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Goldsmith H. (1996). Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Development, 67(1), 218–35. [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Detloff A., et al. (2012). Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. American Journal of Psychiatry, 169(2), 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Nelson E.E., Perez-Edgar K., et al. (2006). Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience, 26(24), 6399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane A.A., Fox N.A., Henderson H.A., Marshall P.J. (2008). Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology, 44(5), 1491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein S.M., Benson B., Perez-Edgar K., et al. (2011). Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia, 49(3), 479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein S.M., Kirwan M.L., Benson B.E., et al. (2013). Validation of a child-friendly version of the monetary incentive delay task. Social Cognitive and Affective Neuroscience, 8(6), 720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J., Reznick J.S., Snidman N. (1987). The physiology and psychology of behavioral inhibition in children. Child Development, 58, 1459–73. [PubMed] [Google Scholar]
- Kagan J., Snidman N. (1991). Temperamental factors in human development. American Psychologist, 46(8), 856.. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage, 12(1), 20–7. [DOI] [PubMed] [Google Scholar]
- Lago T., Davis A., Grillon C., Ernst M. (2017). Striatum on the anxiety map: Small detours into adolescence. Brain Research, 1654(Pt B), 177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A., Lamm C., Chronis-Tuscano A., Pine D.S., Henderson H.A., Fox N.A. (2014). Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child & Adolescent Psychiatry, 53(4), 447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Benson B.E., Guyer A., et al. (2014a). Longitudinal study of striatal activation to reward and loss anticipation from mid-adolescence into late adolescence/early adulthood. Brain and Cognition, 89, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Walker O.L., Degnan K.A., et al. (2014b). Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Developmental Science, 17(5), 667–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neter J., Kutner M.H., Nachtsheim C.J., Wasserman W. (1996). Applied Linear Statistical Models. Chicago, IL: Richard D. Irwin, Inc. [Google Scholar]
- Pérez-Edgar K., Hardee J.E., Guyer A.E., et al. (2014). DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Social Cognitive and Affective Neuroscience, 9(4), 445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine D.S. (2007). Research review: a neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry, 48(7), 631–48. [DOI] [PubMed] [Google Scholar]
- Stewart S.L., Rubin K.H. (1995). The social problem-solving skills of anxious-withdrawn children. Development and Psychopathology, 7, 323–36. [Google Scholar]
- Walker O.L., Henderson H.A., Degnan K.A., Penela E.C., Fox N.A. (2014). Associations between behavioral inhibition and children’s social problem-solving behavior during social exclusion. Social Development, 23(3), 487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]