Little is known about the interaction between Alzheimer’s disease and cerebrovascular disease with respect to network degeneration. Chong et al. demonstrate differential functional and structural network changes in patients with Alzheimer’s disease with and without cerebrovascular disease, suggesting that the two groups may have different underlying pathologies.
Keywords: Alzheimer’s disease, cerebrovascular disease, prodromal stage, intrinsic functional connectivity, structural covariance
Abstract
Network-sensitive neuroimaging methods have been used to characterize large-scale brain network degeneration in Alzheimer’s disease and its prodrome. However, few studies have investigated the combined effect of Alzheimer’s disease and cerebrovascular disease on brain network degeneration. Our study sought to examine the intrinsic functional connectivity and structural covariance network changes in 235 prodromal and clinical Alzheimer’s disease patients with and without cerebrovascular disease. We focused particularly on two higher-order cognitive networks—the default mode network and the executive control network. We found divergent functional connectivity and structural covariance patterns in Alzheimer’s disease patients with and without cerebrovascular disease. Alzheimer’s disease patients without cerebrovascular disease, but not Alzheimer’s disease patients with cerebrovascular disease, showed reductions in posterior default mode network functional connectivity. By comparison, while both groups exhibited parietal reductions in executive control network functional connectivity, only Alzheimer’s disease patients with cerebrovascular disease showed increases in frontal executive control network connectivity. Importantly, these distinct executive control network changes were recapitulated in prodromal Alzheimer’s disease patients with and without cerebrovascular disease. Across Alzheimer’s disease patients with and without cerebrovascular disease, higher default mode network functional connectivity z-scores correlated with greater hippocampal volumes while higher executive control network functional connectivity z-scores correlated with greater white matter changes. In parallel, only Alzheimer’s disease patients without cerebrovascular disease showed increased default mode network structural covariance, while only Alzheimer’s disease patients with cerebrovascular disease showed increased executive control network structural covariance compared to controls. Our findings demonstrate the differential neural network structural and functional changes in Alzheimer’s disease with and without cerebrovascular disease, suggesting that the underlying pathology of Alzheimer’s disease patients with cerebrovascular disease might differ from those without cerebrovascular disease and reflect a combination of more severe cerebrovascular disease and less severe Alzheimer’s disease network degeneration phenotype.
Introduction
Alzheimer’s disease is a neurodegenerative disorder associated with large-scale brain functional and structural network dysfunctions (Seeley et al., 2009). Using intrinsic functional connectivity approaches that measure correlated spontaneous activity between brain regions under task-free conditions, several intrinsic connectivity networks have been consistently identified in healthy individuals (Biswal et al., 1995; Fox and Raichle, 2007) and demonstrated to show aberrant changes in Alzheimer’s disease and its prodrome. Of these, decreased functional connectivity in the default mode network (DMN) is the most prominent in patients with Alzheimer’s disease (Greicius et al., 2004; Seeley et al., 2009; Zhou et al., 2010). More recently, aberrant loss of functional connectivity in other intrinsic connectivity networks has also been documented in Alzheimer’s disease patients, including the frontoparietal executive control network (ECN) (Brier et al., 2012; Wang et al., 2015). In patients with mild cognitive impairment, reduced connectivity in the DMN has also been reported (Rombouts et al., 2005; Bai et al., 2008; Zhou et al., 2008), with some evidence of increased frontoparietal connectivity that may be suggestive of a compensatory mechanism (Qi et al., 2010).
Structural brain networks have been characterized by analysing the co-varying grey matter volume or cortical thickness between different brain regions across individuals (He et al., 2007; Zielinski et al., 2010). Structural covariance networks derived using this technique have been shown to corroborate with intrinsic connectivity networks in both health and disease. Seeley and colleagues (2009), for example, demonstrated convergent intrinsic connectivity network and structural covariance network patterns in healthy individuals, which mirrored grey matter atrophy patterns in distinct neurodegenerative disorders. However, while some studies have examined structural covariance networks in Alzheimer’s disease (Spreng and Turner, 2013; Montembeault et al., 2016), little is known about its correspondence with intrinsic connectivity networks.
In addition, few studies have examined structural and functional network changes in Alzheimer’s disease patients that have comorbid cerebrovascular disease. Alzheimer’s disease frequently co-occurs with cerebrovascular disease (Toledo et al., 2013), which has emerged as the leading cause of age-related cognitive impairment (Schneider et al., 2009; Chen et al., 2016). On its own, cerebrovascular disease is associated with declines in executive function (Prins et al., 2005), reduced frontal lobe metabolism (Kuczynski et al., 2009), and disrupted functional connectivity in frontoparietal regions (Schaefer et al., 2014). Together, Alzheimer’s disease and cerebrovascular disease are proposed to have additive effects on cognitive decline (Zekry et al., 2002; Iadecola, 2010; Attems and Jellinger, 2014; Kalheim et al., 2017). For the same clinical severity of dementia, patients with pure Alzheimer’s disease exhibited more severe Alzheimer’s disease pathology (amyloid-β and tau) than those comorbid with cerebrovascular disease (Goulding et al., 1999; Zekry et al., 2002; Toledo et al., 2013). However, it is unknown whether the additive effects of Alzheimer’s disease and cerebrovascular disease would be observed in functional connectivity and structural covariance network changes at different stages of the disease as well.
In view of this gap, our study sought to use both intrinsic functional connectivity and structural covariance approaches to compare large-scale brain network changes between prodromal and clinical Alzheimer’s disease patients with and without cerebrovascular disease. We focused particularly on two higher-order cognitive networks, DMN and ECN. Given the proposed additive effects of Alzheimer’s disease and cerebrovascular disease on cognitive decline (Goulding et al., 1999; Zekry et al., 2002), we predict that Alzheimer’s disease patients with and without cerebrovascular disease would feature divergent connectivity changes across the networks. Specifically, given the same clinical severity, Alzheimer’s disease patients with cerebrovascular disease would feature greater cerebrovascular disease-related changes in the ECN and less Alzheimer’s disease-related changes in the DMN than those without cerebrovascular disease. Further, we predict that connectivity changes in the prodromal stages of Alzheimer’s disease would mirror that of Alzheimer’s disease. Finally, given the correspondence between structural covariance networks and intrinsic connectivity networks (Seeley et al., 2009), we expect that structural covariance networks would exhibit similar changes to those observed for intrinsic connectivity networks.
Materials and methods
Participants
Participants from five groups [Alzheimer’s disease with cerebrovascular disease (AD + CVD), Alzheimer’s disease without cerebrovascular disease (AD), cognitive impairment no dementia with cerebrovascular disease (CIND + CVD), cognitive impairment no dementia without cerebrovascular disease (CIND) and healthy controls] were selected from a dataset previously described in a published case-control study (Hilal et al., 2015). Detailed recruitment, diagnostic and inclusion/exclusion criteria for Alzheimer’s disease, cerebrovascular disease, cognitive impairment no dementia and healthy control subjects are provided in the Supplementary material. The study was approved by the National Healthcare Group Domain-Specific Review Board and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants in their preferred language prior to the start of the study. All participants underwent neuroimaging as well as an extensive clinical and neuropsychological evaluation, including the Mini-Mental State Examination (MMSE) and a neuropsychological assessment (Yeo et al., 1997), which assessed two memory domains (verbal and visual memory) and five non-memory domains (executive function, attention, language, visuomotor speed and visuoconstruction).
For this study, 235 participants were selected based on the following criteria: (i) met specific cut-offs on the Clinical Dementia Rating (CDR) scores: 0 for healthy controls, 0.5 for CIND and CIND + CVD participants and 1–2 for AD and AD + CVD participants; and (ii) had structural images that were visually assessed to be free of motion and susceptibility artefacts. Each group had an equal number of participants (n = 47) to ensure fair comparisons across groups for structural covariance, and there were no differences in disease severity and cognition between CVD and non-CVD groups at each disease stage (Table 1). For the functional connectivity analyses, a subset of 199 participants (of 235 participants) whose functional images met the motion criteria (maximum absolute displacement <3 mm, maximum relative displacement <1 mm) was used (Supplementary Table 1).
Table 1.
Participant demographic, clinical and neuropsychological characteristics
| AD + CVD | AD | CIND + CVD | CIND | HC | P-value | |
|---|---|---|---|---|---|---|
| (n = 47) | (n = 47) | (n = 47) | (n = 47) | (n = 47) | ||
| Age, years | 79.09 (5.80)a,b,c,d | 75.21 (7.81)a,b,c | 70.43 (8.11) | 69.96 (8.81) | 68.57 (5.14) | <0.001* |
| Gender, m/f | 16/31 | 17/30 | 24/23 | 22/25 | 22/25 | 0.375 |
| Handedness, R/L | 47/0 | 47/0 | 47/0 | 46/1 | 47/0 | 0.404 |
| Ethnicity, C/M/I/O | 34/8/5/0 | 38/6/1/2 | 35/6/3/3 | 39/1/7/0 | 43/1/3/0 | 0.019* |
| Education, years | 4.68 (4.90) | 4.62 (5.14) | 6.40 (4.31) | 7.47 (5.32) | 9.96 (4.49) | <0.001* |
| MMSE | 16.17 (4.53)a,b,c | 16.62 (5.17)a,b,c | 23.6 (3.46)a | 24.34 (3.36)a | 27.47 (2.03) | <0.001* |
| Global CDR | 1–2 | 1–2 | 0.5 | 0.5 | 0 | - |
| CDR-SB | 7.30 (3.09)a,b,c | 6.21 (2.24)a,b,c | 1.31 (0.96)a | 1.03 (0.63)a | 0.00 (0.00) | <0.001* |
| (n = 35) | (n = 37) | (n = 42) | (n = 43) | (n = 41) | ||
| Executive function | −0.946 (0.929)a,b,c | −0.782 (1.006)a,b,c | 0.074 (0.672)a | 0.369 (0.551)a | 0.812 (0.375) | <0.001* |
| Attention | −0.696 (0.865)a,b,c | −0.765 (1.098)a,b,c | 0.054 (0.722)a | 0.282 (0.557)a | 0.777 (0.500) | <0.001* |
| Language | −1.000 (0.763)a,b,c | −0.844 (0.884)a,b,c | 0.053 (0.505)a | 0.237 (0.493)a | 0.932 (0.508) | <0.001* |
| Verbal memory | −0.964 (0.336)a,b,c | −0.943 (0.571)a,b,c | −0.109 (0.708)a | −0.023 (0.780)a | 1.066 (0.548) | <0.001* |
| Visual memory | −1.009 (0.523)a,b,c | −1.004 (0.580)a,b,c | −0.049 (0.570)a | −0.017 (0.636)a | 1.171 (0.465) | <0.001* |
| Visuoconstruction | −0.927 (0.827)a,b,c | −0.749 (0.972)a,b,c | −0.053 (0.641)a | 0.297 (0.594)a | 0.857 (0.419) | <0.001* |
| Visuomotor speed | −0.877 (0.703)a,b,c | −0.830 (0.795)a,b,c | 0.011 (0.722)a | 0.288 (0.679)a | 0.866 (0.464) | <0.001* |
*A significant difference between groups.
aA significant difference from the healthy control group.
bA significant difference from the CIND group.
cA significant difference from the CIND + CVD group.
dA significant difference from the AD group.
C = Chinese; CDR = Clinical Dementia Rating; CDR-SB = clinical dementia rating – sum of boxes; f = female; HC = healthy controls; I = Indian; L = left; M = Malay; m = male; MMSE = Mini-Mental State Examination; O = others; R = right.
Image acquisition
Participants were scanned at the Clinical Imaging Research Centre, National University of Singapore, on a 3 T Siemens Magnetom Tim Trio scanner using a 32-channel head coil. The scan protocol included a T1-weighted MPRAGE (magnetization prepared rapid gradient recalled echo) sequence (repetition time = 2300 ms, echo time = 1.9 ms, inversion time = 900 ms, flip angle = 9°, 192 sagittal slices, matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm3), a FLAIR (fluid attenuated inversion recovery) sequence (repetition time = 9000 ms, echo time = 82 ms, inversion time = 2500 ms, flip angle = 180°, 48 transversal slices, matrix size = 256 × 232, voxel size = 1.0 × 1.0 × 3.0 mm3), and a 5-min T2*-weighted task-free functional MRI scan (T2*-weighted echo planar sequence with repetition time = 2300 ms, echo time = 25 ms, flip angle = 90°, field of view = 192 × 192 mm2, voxel size = 3.0 mm isotropic, slice thickness = 3 mm, 48 axial slices, interleaved collection).
Image preprocessing
Functional imaging
Task-free functional MRI images were preprocessed using the Analysis of Functional NeuroImages software (Cox, 1996) and the FMRIB (Oxford Centre for Functional MRI of the Brain) Software Library (FSL) (Jenkinson et al., 2012), following our previous protocol (Ng et al., 2016). The preprocessing steps comprised: (i) removal of the first five volumes to allow for magnetic field stabilization; (ii) motion correction; (iii) time series despiking; (iv) spatial smoothing; (v) grand mean scaling; (vi) bandpass temporal filtering; (vii) removal of linear and quadratic trends; (viii) co-registration of T1 image using boundary-based registration (Greve and Fischl, 2009) and subsequent registration of the functional image to Montreal Neurological Institute (MNI) 152 space using a non-linear registration tool (FNIRT); and finally (ix) regression of nine nuisance signals (white matter, CSF, global signals and six motion parameters) from the preprocessed functional images. To determine if global signal regression was preferred, we calculated the global negative index for each subject, taken as the percentage of voxels showing negative correlation with the global signal (Chen et al., 2012). A large proportion of our subjects (82.4%) had a global negative index of <3%, suggesting that the global signal was representative of non-neural noise and should be regressed out from the images.
Structural imaging
Voxel-based morphometry was applied to structural T1-weighted images using the VBM8 toolbox (Structural Brain Mapping Group; http://dbm.neuro.uni-jena.de/software/) for Statistical Parametric Mapping (SPM8; Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Individual grey matter volume probability maps were obtained from T1-weighted images using an approach that included: (i) segmentation of T1-weighted images into grey matter, white matter, and CSF using an adaptive Maximum A Posterior technique (Rajapakse et al., 1997), which eliminates the use of tissue priors; (ii) creation of a study-specific template using non-linear DARTEL (Diffeomophic Anatomical Registration Through Exponentiated Lie Algebra) registration of the affine-registered grey matter and white matter segments (Ashburner, 2007); (iii) registration of each grey matter/white matter probability map to the customized template in MNI standard space; (iv) modulation by multiplying voxels with the non-linear component of the Jacobian determinant to account for individual brain volumes; and (v) smoothing on the normalized grey matter images by an isotropic 8 mm Gaussian kernel.
Image analyses
Functional connectivity analyses
Individual-level intrinsic connectivity networks were obtained using a seed-based approach following our previous work (Zhou et al., 2012) (Supplementary Fig. 1). First, mean time series of two 4-mm spherical regions of interest were extracted from each participant’s preprocessed functional images. These regions of interest corresponded to core DMN and ECN regions and were selected from the peak foci of intrinsic connectivity networks reported in previous literature: left posterior cingulate cortex (DMN; MNI coordinates: −7, −43, 33) (Sridharan et al., 2008) and right dorsolateral prefrontal cortex (ECN; MNI coordinates: 44, 36, 20) (Seeley et al., 2007) (Supplementary Fig. 2). Pearson’s correlation was then computed between each voxel’s spontaneous BOLD (blood oxygen level-dependent signal) time series and the average time series from each region of interest, and converted to z-scores using Fisher’s r-to-z transformation. Separate second-level, random effects analyses for each region of interest were subsequently performed using the Biological Parametric Mapping toolbox (Casanova et al., 2007) on individual z-score maps to identify voxels showing group differences in intrinsic connectivity networks. To restrict the analyses to within-network group differences, group-averaged network maps were derived by performing a one-sample t-test on the z-score maps of healthy controls for each region of interest. Results from the analyses were then masked with the network maps and thresholded at a height threshold of P < 0.01 and a cluster-extent threshold of P < 0.05. Age, gender, years of education, handedness, ethnicity and voxelwise grey matter maps (for atrophy correction) were included as covariates, and groups were modelled as separate covariates in the analyses.
To ensure that the results are unaffected by differences in methodology, we repeated our analyses using (i) non-parametric statistical methods; (ii) functional images without global signal regression; and (iii) network masks derived from an independent healthy elderly dataset (n = 78) as described previously (Ng et al., 2016). Detailed information on these additional analyses is given in the Supplementary material.
We additionally examined if these functional connectivity changes were associated with hippocampal volume and white matter lesion measures in clinical and prodromal Alzheimer’s disease patients. Mean bilateral hippocampal grey matter volumes (Alzheimer’s disease marker) were extracted from the normalized preprocessed grey matter images using the mask defined in the automated anatomical labelling template (Tzourio-Mazoyer et al., 2002), and white matter lesions (cerebrovascular disease marker) were quantified using the age-related white matter changes scale (Kapeller et al., 2003). Mean functional connectivity z-scores of clusters that showed DMN and ECN differences between clinical Alzheimer’s disease patients and controls (DMN: AD < HC; ECN: AD + CVD > HC) were also extracted. After regressing out nuisance covariates (age, gender, education, handedness, ethnicity), residuals of functional connectivity measures were then correlated with hippocampal/white matter lesion markers in clinical (AD and AD + CVD) and prodromal Alzheimer’s disease patients (CIND and CIND + CVD) separately. As age-related white matter changes scores have a skewed distribution, correlation analyses with age-related white matter changes scores were performed using two-tailed Spearman’s correlation tests, while correlation analyses with hippocampal grey matter volumes were performed using two-tailed Pearson’s correlation tests.
Structural covariance analyses
Similar to the functional connectivity analyses, structural covariance analyses were performed using a seed-based approach with the same DMN and ECN regions of interest used in the functional connectivity analyses (Seeley et al., 2009; Zielinski et al., 2010). Mean grey matter volumes of the two 4-mm spherical regions of interest were first extracted from each participant’s preprocessed grey matter image. Separate regression analyses for each region of interest were then performed by entering mean region of interest grey matter volumes as the covariate of interest, group as the grouping variable, and age, gender, education, ethnicity and handedness as confounding variables. Following previous methods (Montembeault et al., 2016), we conducted specific contrasts to identify clusters with significant group differences in regression slopes (‘structural association’). Resultant maps were thresholded at a height threshold of P < 0.001 and a cluster-extent threshold of P < 0.05. To examine if these results were related to grey matter atrophy, group comparisons of grey matter volumes were additionally performed (Supplementary material). Resultant atrophy maps were subsequently overlaid on the group structural association difference maps to identify regions of overlap between structural covariance changes and grey matter atrophy. To ensure that the results remain unaffected by changes in methodology, the analyses were also repeated using non-parametric statistical methods (Supplementary material).
Results
Group differences in functional intrinsic connectivity networks
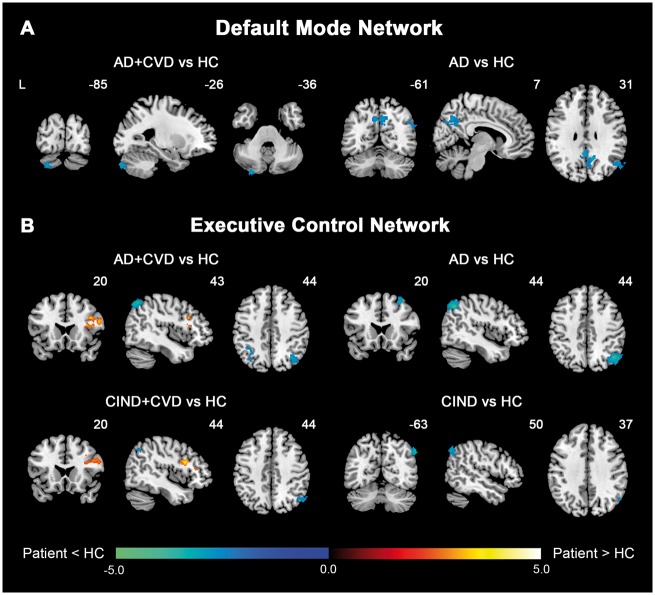
Both intrinsic connectivity networks featured divergent connectivity changes between cerebrovascular disease and non-cerebrovascular disease groups. In the DMN, AD patients exhibited widespread functional connectivity decreases in core posterior regions of the network compared to controls, including the bilateral precuneus and posterior cingulate cortex, and right angular gyrus. By comparison, AD + CVD patients showed only connectivity decreases in the left cerebellum crus, while CIND and CIND + CVD patients demonstrated no DMN connectivity changes relative to controls (Fig. 1A and Supplementary Table 3). In agreement with these findings, functional connectivity decreases in the posterior DMN were also found in AD patients compared to AD + CVD patients (Fig. 2A).
Figure 1.
Patients with and without cerebrovascular disease feature divergent changes in DMN and ECN functional connectivity compared to controls. Group functional connectivity difference maps, overlaid on the MNI template brain, highlight regions showing increased (hot colour) or decreased functional connectivity (cold colour) in patient groups compared to healthy controls for each intrinsic connectivity network. (A) In the DMN, AD patients showed reduced connectivity in key posterior regions of the default mode network, whereas AD + CVD patients showed only connectivity decreases in the left cerebellum crus. No significant changes, however, were found in CIND and CIND + CVD patients compared to controls. (B) In the executive control network, AD patients exhibited reduced connectivity in both frontal and parietal regions of the network, while AD + CVD patients showed reduced connectivity in the parietal regions but increased connectivity in the frontal regions. These divergent changes in the executive control network were also largely recapitulated in CIND and CIND + CVD patients. Results are displayed at a height threshold of P < 0.01 and a cluster-extent threshold of P < 0.05. Colour bars indicate t-scores. HC = healthy controls.
Figure 2.
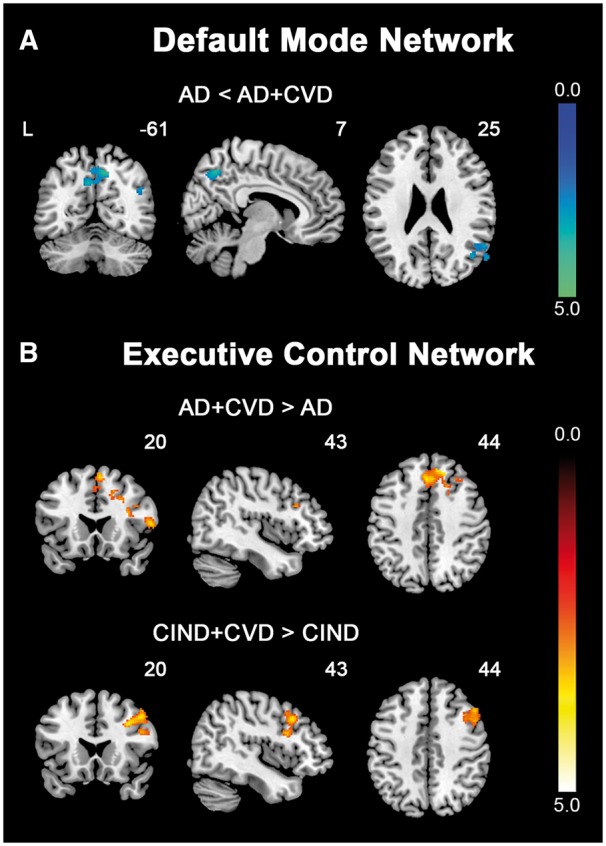
Direct comparisons between patients with and without cerebrovascular disease reveal distinct intrinsic functional connectivity patterns. Group functional connectivity difference maps, overlaid on the MNI template brain, indicate regions showing functional connectivity differences between patients with and without cerebrovascular disease for each intrinsic connectivity network. (A) In the DMN, AD patients showed reduced (cold colour) functional connectivity in key posterior DMN regions, including the precuneus, posterior cingulate cortex and angular gyrus, compared to AD + CVD patients. (B) In the ECN, AD + CVD patients exhibited increased (hot colour) connectivity in the frontal regions compared to AD patients. This increase (hot colour) in frontal connectivity was also observed in CIND + CVD patients compared to CIND patients. Results are displayed at a height threshold of P < 0.01 and a cluster-extent threshold of P < 0.05. Colour bars indicate t-scores.
AD and AD + CVD patients also showed dissociations in ECN connectivity, particularly in the frontal regions (Figs 1B, 2B and Supplementary Table 4). Compared to controls, AD patients exhibited reduced functional connectivity in both lateral parietal and lateral prefrontal regions of the ECN. In contrast, while AD + CVD patients similarly showed reduced parietal connectivity relative to controls, they instead showed increased frontal connectivity compared to controls and AD patients. More interestingly, these connectivity changes were recapitulated in CIND and CIND + CVD patients. CIND patients displayed reduced parietal connectivity, while CIND + CVD patients displayed reduced parietal connectivity and increased frontal connectivity in the ECN relative to controls. Similarly, CIND + CVD patients showed increased frontal functional connectivity compared to CIND patients (Fig. 2B).
Similar results were obtained using (i) non-parametric statistical methods (Supplementary Fig. 3 and Supplementary Tables 5, 6, 9 and 10); (ii) functional images without global regression (Supplementary Fig. 4 and Supplementary Tables 7–10); and (iii) network maps generated from an independent healthy elderly dataset as masks (Supplementary Figs 5 and 6, and Supplementary Tables 11 and 12), thereby suggesting that our results are largely unaffected by methodological differences.
Association with hippocampal grey matter volumes and age-related white matter changes scores
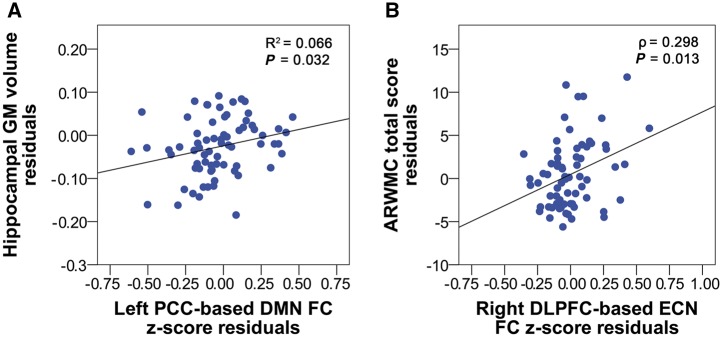
Across AD + CVD and AD patients, greater mean hippocampal grey matter volumes correlated with higher DMN (r = 0.257, P = 0.032) (Fig. 3A) and higher frontal ECN functional connectivity z-scores (r = 0.266, P = 0.026), while higher age-related white matter changes scores correlated with higher frontal ECN (ρ = 0.298, P = 0.013) (Fig. 3B) but not DMN functional connectivity z-scores (ρ = 0.185, P = 0.127). In comparison, mean hippocampal grey matter volumes or age-related white matter changes scores in CIND + CVD and CIND patients did not correlate with DMN or ECN functional connectivity z-scores (P > 0.05).
Figure 3.
Higher DMN functional connectivity z-scores are associated with greater hippocampal grey matter volumes and higher ECN functional connectivity z-scores are associated with age-related white matter changes scores in Alzheimer’s disease patients with and without cerebrovascular disease. (A) Across AD and AD + CVD patients, higher left posterior cingulate cortex-based DMN functional connectivity z-score residuals correlated with greater hippocampal grey matter volume residuals. (B) In contrast, higher right dorsolateral prefrontal cortex-based ECN functional connectivity z-score residuals correlated with higher age-related white matter changes total score residuals. Age, gender, education, handedness and ethnicity were controlled for in all analyses. DLPFC = dorsolateral prefrontal cortex; GM = grey matter; FC = functional connectivity; PCC = posterior cingulate cortex.
Structural covariance network comparisons between Alzheimer’s disease patients with and without cerebrovascular disease
We found divergent changes in structural association between AD and AD + CVD patients that partly resembled the functional connectivity changes (Fig. 4 and Supplementary Tables 13 and 14). In the DMN, AD patients featured greater structural association in posterior brain regions (left superior and middle occipital gyrus, cuneus and precuneus; right lingual gyrus, hippocampus, calcarine gyrus and parahippocampal gyrus) compared to controls (Fig. 4A). Superimposition of this contrast over the same contrast performed for the functional connectivity analysis revealed that some of these brain regions were in close proximity to regions showing DMN functional connectivity differences between AD patients and controls (Fig. 4A). In contrast, AD + CVD patients showed no change in the structural association of the DMN compared to controls.
Figure 4.
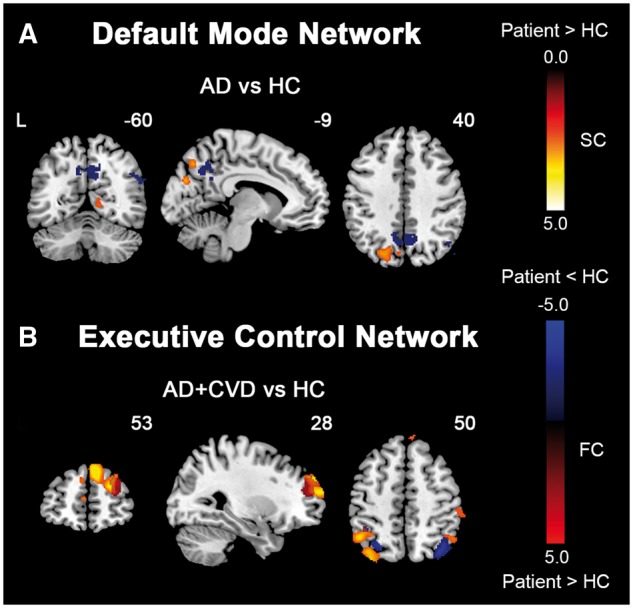
Alzheimer’s disease patients with and without cerebrovascular disease feature divergent changes in structural covariance networks compared to controls. Group structural association difference maps, indicating regions showing increased (hot colour) structural association in patient groups compared to healthy controls for each network, are superimposed on the group functional connectivity difference maps (red colour: patients > controls; blue colour: patients < controls). (A) In the DMN, AD patients showed increased structural association in posterior regions, which are located close to key posterior default mode network regions showing reduced functional connectivity in AD patients compared to controls (HC). AD + CVD patients, in contrast, did not show any significant changes compared to controls. (B) In the ECN, AD + CVD patients showed increased structural association in regions including the frontal and parietal cortices, which overlapped to some extent with regions showing functional connectivity changes in AD + CVD patients compared to controls. By comparison, AD patients did not show any significant changes in structural association. Results are displayed at a height threshold of P < 0.01 and a cluster-extent threshold of P < 0.05. Colour bars indicate t-scores. FC = functional connectivity; SC = structural covariance.
As for the ECN, AD + CVD patients showed increased structural association in lateral parietal and prefrontal cortices compared to controls (Fig. 4B). Several regions were in close proximity or overlapped with regions showing ECN functional connectivity changes in AD + CVD patients (Fig. 4B). AD patients, in contrast, showed no change in the structural association of the ECN compared to controls.
We repeated the above analyses using non-parametric statistical methods and obtained similar results. Details of these results are provided in Supplementary Fig. 7 and Supplementary Tables 15 and 16.
Discussion
In this study, we examined the changes in functional connectivity and structural covariance of two higher-order cognitive networks in prodromal and clinical stages of Alzheimer’s disease with and without cerebrovascular disease. As hypothesized, we found differential functional connectivity and structural covariance changes between cerebrovascular disease and non-cerebrovascular disease groups. Specifically, DMN functional connectivity was reduced in AD but not in AD + CVD patients. Conversely, AD + CVD patients showed greater ECN connectivity in frontal regions compared to both controls and AD patients. Importantly, these ECN connectivity changes were recapitulated at the prodromal stage. Higher DMN and ECN functional connectivity z-scores also correlated with greater hippocampal volumes and white matter lesions in clinical Alzheimer’s disease patients, respectively. Supporting the functional connectivity findings, we found similar divergent structural covariance changes in the ECN and DMN between the two groups: AD patients showed only increased DMN structural association, while AD + CVD patients showed only increased ECN structural association compared to controls. Our findings highlight the differential structural and functional network disruptions in the two disorders, thereby implying that the pathology of cerebrovascular disease groups differs from non-cerebrovascular disease groups and is likely to reflect a network degeneration pattern of more cerebrovascular disease and less Alzheimer’s disease phenotype.
Divergent default mode network changes in Alzheimer’s disease patients with and without cerebrovascular disease
In agreement with previous studies (Greicius et al., 2004; Zhou et al., 2010), AD patients showed reduced left posterior cingulate cortex-based functional connectivity in the posterior DMN compared to both controls and AD + CVD patients. By comparison, reductions in posterior DMN connectivity were conspicuously absent in AD + CVD patients. In parallel, AD, but not AD + CVD patients, showed greater structural association of the DMN (seeded at left posterior cingulate cortex) in posterior brain regions compared to controls. Core DMN regions, including the posterior cingulate cortex, precuneus, prefrontal and temporal cortices, have been linked to autobiographical and episodic memory retrieval (Spreng et al., 2009) and are among the first to show elevated amyloid deposition (Mintun et al., 2006; Sheline and Raichle, 2013), both of which are hallmarks of Alzheimer’s disease. Additionally, higher DMN functional connectivity z-scores across clinical Alzheimer’s disease patients correlated with greater hippocampal grey matter volumes, another well-known marker of Alzheimer’s disease. The lack of functional connectivity and structural covariance disruptions in DMN in AD + CVD patients thus suggests that the Alzheimer’s disease pathology of these patients may be less severe than that of AD patients with similar clinical severity.
We did not find any DMN functional connectivity changes in CIND and CIND + CVD patients compared to controls. Additionally, no association was found between mean grey matter hippocampal volumes and DMN functional connectivity z-scores in these patients. While reduced DMN connectivity has been frequently found in patients at prodromal stages of Alzheimer’s disease (Rombouts et al., 2005; Bai et al., 2008; Zhou et al., 2008), there are also reports of increased frontal connectivity (Qi et al., 2010; Damoiseaux et al., 2012) and even increased posterior DMN connectivity in these patients (Esposito et al., 2013), suggesting the presence of a compensatory mechanism. The mixed functional connectivity changes in prodromal Alzheimer’s disease patients, coupled with the relative heterogeneity of the CIND and CIND + CVD participants in our sample (i.e. not only amnestic impairment), could have therefore resulted in a lack of DMN changes and a lack of association between DMN connectivity and hippocampal grey matter volumes in these patients.
Divergent ECN changes in prodromal and clinical Alzheimer’s disease patients with and without cerebrovascular disease
Divergent connectivity changes of the ECN between AD and AD + CVD patients, on the other hand, suggest the involvement of cerebrovascular disease pathology in patients with cerebrovascular disease. AD patients showed reduced frontal and parietal functional connectivity in the ECN compared to controls. In contrast, while AD + CVD patients similarly showed reduced functional connectivity between the right dorsolateral prefrontal cortex and parietal cortices compared to controls, they instead exhibited prefrontal functional connectivity increases relative to both controls and AD patients. Increased frontal ECN functional connectivity in AD + CVD and AD patients also correlated with increased age-related white matter changes scores (i.e. more severe cerebrovascular disease pathology) and increased grey matter hippocampal volumes (i.e. less severe Alzheimer’s disease pathology), further supporting the divergence between Alzheimer’s disease and cerebrovascular disease pathology. In parallel, the ECN showed greater structural association in the lateral parietal and prefrontal cortices in AD + CVD patients, but no changes in AD patients compared to controls. These greater ECN connectivity disruptions in AD + CVD patients support the idea that cerebrovascular disease is associated with frontoparietal executive dysfunctions (Prins et al., 2005; Kuczynski et al., 2009), and thus suggest the involvement of more extensive cerebrovascular disease-related changes in AD + CVD patients.
Our findings of decreased parietal ECN functional connectivity in both AD and AD + CVD patients are also consistent with past reports of decreased ECN functional connectivity in Alzheimer’s disease (Brier et al., 2012; Wang et al., 2015) and decreased parietal functional connectivity in small vessel disease (Schaefer et al., 2014). However, while other studies have reported decreased frontal functional connectivity in small vessel disease (Schaefer et al., 2014) and mixed dementia patients (Kim et al., 2015), we instead found increased frontal ECN functional connectivity in AD + CVD patients. This difference in findings could be due to differences in patient group (inclusion of patients with cortical infarcts in our study versus subcortical cerebrovascular disease in previous studies) and methodology (right dorsolateral prefrontal cortex-based connectivity in our study versus independent component analysis and graph theory approaches in previous studies). Moreover, another study has reported increased inter-regional frontal functional connectivity for the slow-four frequency band in vascular dementia patients compared to controls (Zhang et al., 2013). Further, we found an association between higher ECN functional connectivity z-scores and greater age-related white matter changes scores in clinical Alzheimer’s disease patients. Given that increased frontal functional connectivity has also been reported in patients with mild cognitive impairment and Alzheimer’s disease and suggested to act as a compensatory mechanism (Qi et al., 2010; Agosta et al., 2012), our observation of increased right dorsolateral prefrontal cortex—frontal connectivity may hence indicate a compensatory increase in local ECN frontal connectivity to cope with the increasing severity of cerebrovascular disease. Future research could be done to probe the origins of these connectivity changes and examine if these changes are indeed compensatory.
Importantly, ECN functional connectivity changes of patients at the prodromal stage largely mirrored those observed at the clinical stage. As with AD and AD + CVD patients, CIND patients exhibited reduced ECN functional connectivity in parietal regions, while CIND + CVD patients showed reduced parietal ECN functional connectivity but increased frontal ECN functional connectivity compared to controls and CIND patients. Our results indicate that differential changes in ECN connectivity between cerebrovascular disease and non-cerebrovascular disease groups, particularly within frontal regions, are present even at prodromal stages of Alzheimer’s disease. However, given that we did not observe an association between frontal ECN connectivity and age-related white matter changes scores in prodromal patients (CIND and CIND + CVD), it is likely that the compensatory mechanism described above becomes more prominent at a later disease stage where there is possibly greater interaction between cerebrovascular disease and Alzheimer’s disease pathology. Nevertheless, in view that both AD + CVD and CIND + CVD patients showed increased right dorsolateral prefrontal cortex functional connectivity to frontal regions, our findings further support frontal ECN hyperconnectivity as a distinctive feature of patients with cerebrovascular disease.
Taken together, our findings suggest that cerebrovascular disease and non-cerebrovascular disease groups exhibit divergent functional connectivity and structural covariance changes in the DMN and ECN, implying that different pathologies may underlie the two groups. More specifically, AD + CVD patients appear to be associated with more extensive cerebrovascular disease-related and less Alzheimer’s disease-related network changes than AD patients at the same level of dementia severity, which is in agreement with the idea that the effects of Alzheimer’s disease and cerebrovascular disease are additive (Zekry et al., 2002; Attems and Jellinger, 2014).
Correspondence between functional connectivity and structural covariance network changes
Interestingly, some of the structural covariance findings showed close proximity or even overlapped with the functional connectivity findings (Fig. 4), implying some correspondence between structural covariance and functional connectivity. This is consistent with previous studies showing that each neurodegenerative disorder targets a specific neural network with good functional and structural correspondence in health (Seeley et al., 2009), i.e. brain regions with structural covarying linkage might also be functionally connected with one another. It has been proposed that structural covariance is partly influenced by functional connectivity (Alexander-Bloch et al., 2013a), with evidence that functional connectivity accounts for a large proportion of the variance of structural covariance in humans (Alexander-Bloch et al., 2013a, b). However, despite the similarities, there remain differences between the functional connectivity and structural covariance findings. Some studies have similarly reported limited correspondence between intrinsic connectivity networks and structural covariance networks (Liao et al., 2013; Clos et al., 2014), possibly reflecting differences in noise, measurement error and biological processes underlying these networks (Clos et al., 2014).
In addition, grey matter atrophy is likely to influence the structural covariance changes observed in our study. Both AD and AD + CVD patients showed extensive grey matter atrophy throughout the brain (Supplementary Fig. 8), and there is significant overlap between structural covariance changes and grey matter atrophy for both AD and AD + CVD patients (Supplementary Fig. 9). The similarity between structural covariance and atrophy patterns might in turn explain why we found increased structural covariance in patients, rather than decreased structural covariance, as reported in previous studies where participants may be at a relatively early disease stage and have less severe grey matter atrophy (Spreng and Turner, 2013; Montembeault et al., 2016). Therefore, changes in the structural covariance networks of our AD and AD + CVD patients possibly reflect a combination of correlated grey matter atrophy as well as changes in functional connectivity.
Limitations and future directions
There remain several limitations in this study. First, subjects were not age or education-matched. However, we controlled for the effects of age and education in all the analyses. Second, our study is a cross-sectional study. Future longitudinal studies would be better equipped to track individual-level changes in functional connectivity and structural covariance networks as the disease progresses with time. Third, as we are using a seed-based approach, our results should be interpreted as changes in connectivity to the seed region as opposed to whole-network connectivity derived from independent component analysis. Finally, as no amyloid imaging was performed, we are unable to establish whether patients in this study have substantial amounts of amyloid-β.
In conclusion, we found divergent functional connectivity and structural covariance network changes between cerebrovascular disease and non-cerebrovascular disease groups. Specifically, AD patients showed reduced posterior DMN functional connectivity, while AD + CVD patients showed greater frontal ECN functional connectivity. Importantly, ECN functional connectivity changes in AD and AD + CVD patients were largely mirrored in CIND and CIND + CVD patients, respectively, suggesting that functional connectivity changes are present even at prodromal stages of Alzheimer’s disease. Additionally, higher DMN functional connectivity z-scores correlated with greater hippocampal volumes, while higher ECN functional connectivity z-scores correlated with greater white matter lesions in AD and AD + CVD patients. In parallel, AD patients showed increased DMN structural association in the posterior regions, while AD + CVD patients showed increased ECN structural association in the frontal and parietal regions compared to controls. Our findings thus support the idea that cerebrovascular disease groups have more cerebrovascular disease-related and less Alzheimer’s disease-related network disruptions than non-cerebrovascular disease groups at the same level of dementia severity. Further developed, the combination of structural and functional network imaging assays could shed more light on the differential network phenotype between cerebrovascular disease and non-cerebrovascular disease groups.
Funding
This research was supported by an National Medical Research Council Centre Grant (NMRC/CG/013/2013 and NMRC/CG/NUHS/2010 to C.C.), the Biomedical Research Council, Singapore (BMRC 04/1/36/372 to J.Z.), the National Medical Research Council, Singapore (NMRC/CIRG/1390/2014 to J.Z.), and Duke-NUS Medical School Signature Research Program funded by Ministry of Health, Singapore.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviations
- AD
Alzheimer's disease without CVD
- AD+CVD
Alzheimer’s disease with CVD
- CIND
cognitive impairment no dementia without CVD
- CIND+CVD
cognitive impairment no dementia with CVD
- CVD
cerebrovascular disease
- DMN
default mode network
- ECN
executive control network
References
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol Aging 2012; 33: 1564–78. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci 2013a; 14: 322–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J. The convergence of maturational change and structural covariance in human cortical networks. J Neurosci 2013b; 33: 2889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38: 95–113. [DOI] [PubMed] [Google Scholar]
- Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease—lessons from pathology. BMC Med 2014; 12; 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Yu H, Shi Y, Yuan Y, Zhu W, et al. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci Lett 2008; 438: 111–15. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995; 34: 537–41. [DOI] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci 2012; 32: 8890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, et al. Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. Neuroimage 2007; 34: 137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Homma A, Mok VC, Krishnamoorthy E, Alladi S, Meguro K, et al. Alzheimer's disease with cerebrovascular disease: current status in the Asia-Pacific region. J Intern Med 2016; 280: 359–74. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen G, Xie C, Ward BD, Li W, Antuono P, et al. A method to determine the necessity for global signal regression in resting-state fMRI studies. Magn Reson Med 2012; 68: 1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Rottschy C, Laird AR, Fox PT, Eickhoff SB. Comparison of structural covariance with functional connectivity approaches exemplified by an investigation of the left anterior insula. Neuroimage 2014; 99: 269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29: 162–73. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Prater KE, Miller BL, Greicius MD. Functional connectivity tracks clinical deterioration in Alzheimer's disease. Neurobiol Aging 2012; 33: 828 e19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R, Mosca A, Pieramico V, Cieri F, Cera N, Sensi SL. Characterization of resting state activity in MCI individuals. PeerJ 2013; 1: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007; 8: 700–11. [DOI] [PubMed] [Google Scholar]
- Goulding J, Signorini D, Chatterjee S, Nicoll J, Stewart J, Morris R, et al. Inverse relation between Braak stage and cerebrovascular pathology in Alzheimer predominant dementia. J Neurol Neurosurg Psychiatry 1999; 67: 654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 2004; 101: 4637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009; 48: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 2007; 17: 2407–19. [DOI] [PubMed] [Google Scholar]
- Hilal S, Chai YL, Ikram MK, Elangovan S, Yeow TB, Xin X, et al. Markers of cardiac dysfunction in cognitive impairment and dementia. Medicine 2015; 94: e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 2010; 120: 287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage 2012; 62: 782–90. [DOI] [PubMed] [Google Scholar]
- Kalheim LF, Bjornerud A, Fladby T, Vegge K, Selnes P. White matter hyperintensity microstructure in amyloid dysmetabolism. J Cereb Blood Flow Metab 2017; 37: 356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller P, Barber R, Vermeulen RJ, Ader H, Scheltens P, Freidl W, et al. Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke 2003; 34: 441–5. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Cha J, Lee JM, Shin JS, Jung NY, Kim YJ, et al. Distinctive resting state network disruptions among Alzheimer's disease, subcortical vascular dementia, and mixed dementia patients. J Alzheimers Dis 2015; 50: 709–18. [DOI] [PubMed] [Google Scholar]
- Kuczynski B, Jagust W, Chui HC, Reed B. An inverse association of cardiovascular risk and frontal lobe glucose metabolism. Neurology 2009; 72: 738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Mantini D, Xu Q, Wang Z, Chen G, et al. Relationship between large-scale functional and structural covariance networks in idiopathic generalized epilepsy. Brain Connect 2013; 3: 240–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006; 67: 446–52. [DOI] [PubMed] [Google Scholar]
- Montembeault M, Rouleau I, Provost JS, Brambati SM; Alzheimer's Disease Neuroimaging Initiative. Altered gray matter structural covariance networks in early stages of Alzheimer's disease. Cereb Cortex 2016; 26: 2650–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KK, Lo JC, Lim JK, Chee MW, Zhou J. Reduced functional segregation between the default mode network and the executive control network in healthy older adults: a longitudinal study. Neuroimage 2016; 133: 321–30. [DOI] [PubMed] [Google Scholar]
- Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005; 128 (Pt 9): 2034–41. [DOI] [PubMed] [Google Scholar]
- Qi Z, Wu X, Wang Z, Zhang N, Dong H, Yao L, et al. Impairment and compensation coexist in amnestic MCI default mode network. Neuroimage 2010; 50: 48–55. [DOI] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging 1997; 16: 176–86. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp 2005; 26: 231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Quinque EM, Kipping JA, Arelin K, Roggenhofer E, Frisch S, et al. Early small vessel disease affects frontoparietal and cerebellar hubs in close correlation with clinical symptoms—a resting-state fMRI study. J Cereb Blood Flow Metab 2014; 34: 1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009; 66: 200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009; 62: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer's disease. Biol Psychiatry 2013; 74: 340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci 2009; 21: 489–510. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Turner GR. Structural covariance of the default network in healthy and pathological aging. J Neurosci 2013; 33: 15226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 2008; 105: 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain 2013; 136 (Pt 9): 2697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–89. [DOI] [PubMed] [Google Scholar]
- Wang P, Zhou B, Yao H, Zhan Y, Zhang Z, Cui Y, et al. Aberrant intra- and inter-network connectivity architectures in Alzheimer's disease and mild cognitive impairment. Sci Rep 2015; 5: 14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo D, Gabriel C, Chen C, Lee S, Loenneker T, Wong M. Pilot validation of a customized neuropsychological battery in elderly Singaporeans. Neurol J South East Asia 1997; 2: 123. [Google Scholar]
- Zekry D, Duyckaerts C, Moulias R, Belmin J, Geoffre C, Herrmann F, et al. Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol 2002; 103: 481–7. [DOI] [PubMed] [Google Scholar]
- Zhang D, Liu B, Chen J, Peng X, Liu X, Fan Y, et al. Determination of vascular dementia brain in distinct frequency bands with whole brain functional connectivity patterns. PLoS One 2013; 8: e54512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012; 73: 1216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 2010; 133 (Pt 5): 1352–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Dougherty JH Jr, Hubner KF, Bai B, Cannon RL, Hutson RK. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer's disease and mild cognitive impairment. Alzheimers Dement 2008; 4: 265–70. [DOI] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci USA 2010; 107: 18191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.