Abstract
Aims
We aimed to determine the frequency of aortic valve surgery (AVR) with or without coronary artery bypass grafting (CABG), among patients with moderate/severe aortic stenosis (AS) and left ventricular systolic dysfunction (LVSD), and its relationship with survival.
Methods and results
The Duke Echocardiographic Database (N = 132 804) was queried for patients with mean gradient ≥25 mmHg and/or peak velocity ≥3 m/s and LVSD (left ventricular ejection fraction ≤50%) from 1 January 1995–28 February 2014. For analyses purposes, AS was defined both by mean gradient and calculated aortic valve area (AVA) criteria. Time-dependent indicators of AVR in multivariable Cox models were used to assess the relationship of AVR and all-cause mortality. A total of 1634 patients had moderate (N = 1090, 67%) or severe (N = 544, 33%) AS by mean gradient criteria. Overall, 287 (26%) patients with moderate AS and 263 (48%) patients with severe AS underwent AVR within 5 years of the qualifying echo. There were 863 (53%) deaths observed up to 5 years following index echo. After multivariable adjustment in an inverse probability weighted regression model, AVR was associated with higher 5-year survival amongst patients with moderate AS and severe AS whether classified by AVA or mean gradient criteria. Over all, AVR ± CABG compared with medical therapy was associated with significantly lower mortality [hazard ratio, HR = 0.49 (0.38, 0.62), P < 0.0001]. Compared with CABG alone, CABG + AVR was associated with better survival [HR = 0.18 (0.12, 0.27), P < 0.0001].
Conclusions
In patients with moderate/severe AS and LVSD, mortality is substantial and amongst those selected for surgery, AVR with or without CABG is associated with higher survival. Research is required to understand factors contributing to current practice patterns and the possible utility of transcatheter approaches in this high-risk cohort.
Keywords: Moderate aortic stenosis, severe aortic stenosis, aortic valve surgery, left ventricular systolic dysfunction, survival
Introduction
Aortic stenosis (AS) is the most common valve disorder leading to surgical intervention in developed countries.1,2 Aortic valve replacement (AVR) is the recommended treatment approach for patients with severe AS who have symptoms and/or evidence of left ventricular systolic dysfunction (LVSD).3 Aortic stenosis whether it is a bystander or cause of left ventricular dysfunction poses a significant hemodynamic afterload burden.4,5 Theoretically, among patients with a failing left ventricle, afterload reduction in the form of relief from significant AS may result in the greatest benefit in long-term survival. However, this must be weighed against the greater surgical risk in the oft-affected elderly patient with AS and LVSD.6–8
Prior small observational studies evaluating a subgroup of patients with AS and LVSD have suggested increased perioperative mortality risk but improved long-term survival with AVR.9–11 However, the current practice patterns in use of AVR and its relationship to mortality in patients with moderate and severe AS with concomitant LVSD are ill defined. With the emergence of transcatheter interventions to treat AS in high-risk populations,12–14 these data would be valuable to guide care decisions as well as to research alternative treatment approaches.
We sought to evaluate the frequency of AVR, with or without coronary artery bypass grafting (CABG), among patients with moderate/severe AS and LVSD, and study its relationship with survival. We hypothesized that AVR was associated with higher survival in patients with moderate or severe AS and LVSD.
Methods
Patient population
The Duke Echocardiographic Laboratory Database was queried for the period between 1 January 1995 and 28 February 2014 for adult patients with moderate or severe AS and concomitant LVSD. The setup of the Duke Echocardiography Laboratory Database (DELD) has been previously described.15 Briefly, DELD includes a prospectively maintained digital archive of all clinical echocardiograms performed at Duke University Hospital (DUH) and its satellite clinics linked to a corresponding searchable reporting database since 1995. The database also includes clinical information which is drawn from various sources: billing sources with demographic information, International Classification of Diseases, 9th revision (ICD-9) codes, Current Procedure Terminology codes; the Duke Databank of Cardiovascular Diseases16–18 with in-hospital data on all patients undergoing cardiac catheterization and/or cardiac surgery at DUH since 1969 as well as long-term follow-up information available through mailed questionnaires, telephone follow-up and searches of the national death index.19
Because inconsistencies in classification of AS exist and may affect patient management decisions, we classified AS by both mean gradients and calculated aortic valve area (AVA).20,21 Thus, a calculated AVA cut off of >1.0 cm2 was used to define moderate AS calculated AVA of ≤1.0 cm2 defined severe AS. Similarly, moderate AS was defined by a mean gradient of ≥25–39 mmHg and/or peak velocity of ≥3– < 4m/s. Severe AS was defined by a mean gradient of ≥40 mmHg and/or peak velocity of ≥4 m/s. Left ventricular systolic dysfunction was defined by a left ventricular ejection fraction (LVEF) of ≤50%. Mild–moderate LVSD was defined as LVEF 36–50%; severe LVSD as LVEF ≤35%. Left ventricular ejection fraction was derived from the clinical echocardiographic report and was visually estimated. Patients were excluded based on their qualifying echocardiogram if they had a history of any prior valvular intervention, congenital heart disease, rheumatic valve disease, prior solid organ transplantation, hypertrophic obstructive cardiomyopathy, missing aortic mean gradient, or a history of metastatic cancer. The index, or reference, echocardiogram for a patient is the first echo with moderate or severe AS and LVEF ≤50% meeting the necessary criteria above. To estimate surgical risk, the logistic EuroSCORE was calculated.22 This risk score was chosen to describe surgical risk based on the availability of clinical information required to compute the score.
The study was carried out under the approval of the Duke Institutional Review Board.
Statistical analysis
Baseline characteristics were stratified by AS severity and LVSD severity using percentages for categorical variables and medians and interquartile ranges for continuous variables. For each level of AS severity, we compared variables by LVSD status. Continuous variables were compared by t-tests or Wilcoxon rank-sum tests when appropriate; categorical variables were compared using χ2 or Fisher's exact test as appropriate.
The cumulative incidence of aortic valve surgery (AVS) within 5 years of the index echocardiogram was described by AS severity and LVSD severity. Cumulative incidence curves were plotted, accounting for the competing risk of mortality.
In order to examine the relationship of AVR with mortality up to 5 years after the index echocardiogram, a number of analyses were conducted examining the subsets of moderate and severe AS by calculated AVA and mean gradients as well as the entire population with resting mean gradients ≥25 mmHg. A Cox proportional hazards model was constructed where AVR was treated as a time-dependent covariate. The model was adjusted for LV dysfunction (≤35%/36–50%), age, sex, renal failure, history of ischaemic heart disease, COPD, hypertension, hyperlipidaemia, diabetes, moderate/severe MR, moderate/severe AR, and bicuspid valve disease. In the model using the entire population, adjustments were also made for AS severity. Adjustment covariates were assessed for linearity and proportional hazards assumptions and transformations were applied as needed.
As a sensitivity analysis, initial AVR treatment was defined as AVR within 90 days of index echo. Cox proportional hazards regression assessed the benefit of initial AVR treatment on mortality where time zero was time of AVR for recipients and day 15 following echo for non-recipients in order to exclude early mortality or loss of follow-up which might preclude planned surgery. The model used inverse probability of treatment weighting to account for bias due to non-random treatment assignment.23 Probability of initial AVR treatment was estimated using logistic regression adjusting for age, sex, LV dysfunction, level of AS, prior PCI, prior MI, prior CABG, history of ischaemia, peripheral vascular disease, hypertension, hyperlipidemia, diabetes, smoking, cardiovascular disease, COPD, renal failure, congestive heart failure, and atrial fibrillation/flutter. Balance of all adjustment covariates was assessed for the propensity of treatment model; no violations of assumptions were detected. A similar sensitivity analysis was conducted defining initial AVR treatment within 30 days of index echo and again excluded patients with early mortality within 15 days of index echo. Finally, whether the relationship between AVR and survival differed based on index echo AS severity, was assessed, using interactions.
Results
Baseline characteristics
We identified a total of 1634/132 804 patient who fit inclusion criteria. Of these 1090 (67%) had moderate and 544 (33%) had severe AS and concomitant LVSD. Severe LVSD (LVEF ≤ 35%) was present in 35% of this cohort. Where Doppler data allowed calculation of AVA (n = 1338), 403 patients had moderate AS and 935 had severe AS. The median age of the cohort was 75 years (interquartile range, IQR 67–83), and the study population had a high prevalence of comorbidities including ischaemic heart disease (61.3%), hypertension (64.1%), diabetes mellitus (32.9%), peripheral vascular disease (14.5%), history of cerebrovascular disease (19.9%), and renal disease (17.6%). The median logistic EuroSCORE was 9.8 (IQR 5.5, 16.8).
Patients with moderate AS by mean gradient criteria were more likely to have a history of ischaemic heart disease (63.9 vs. 56.3%, P 0.0029), prior CABG (22.5 vs. 14.7%, P = 0.0002), prior PCI (13.2 vs. 6.6%, P < 0.0001), diabetes (36.0 vs. 26.7%, P = 0.0002), peripheral vascular disease (16.4 vs. 10.7%, P = 0.002), prior cerebrovascular disease (22.3 vs. 15.3%, P = 0.008), and renal disease (19.7 vs. 13.4%, P = 0.002) than those with severe AS. The mean AVA was 1.08 cm2 amongst patients with moderate AS and 0.72 cm2 amongst those with severe AS. Dobutamine stress testing was undertaken in a minority of the cohort (1.4%). The baseline characteristics for moderate and severe AS stratified by LVSD severity are described in Tables 1 and 2. The baseline characteristics of AS by AVA criteria are provided in Appendix, Table A1.
Table 1.
Baseline characteristics of patients with moderate aortic stenosis and stratified by left ventricular systolic dysfunction
| Characteristic | Moderate AS |
|||
|---|---|---|---|---|
| EF 36–50% (n = 687) | EF ≤ 35% (n = 403) | Overall (n = 1090) | P a | |
| Age, median (IQR) | 75 (67–82) | 75 (67–81) | 75 (67–82) | 0.411 |
| Female gender | 261 (38.0%) | 131 (32.5%) | 392 (36.0%) | 0.066 |
| History of ischaemic heart disease | 439 (63.9%) | 257 (63.8%) | 696 (63.9%) | 0.966 |
| History of hypertension | 463 (67.4%) | 254 (63.0%) | 717 (65.8%) | 0.142 |
| Diabetes | 232 (33.8%) | 160 (39.7%) | 392 (36.0%) | 0.049 |
| Peripheral vascular disease | 117 (17.0%) | 62 (15.4%) | 179 (16.4%) | 0.479 |
| Prior cerebrovascular disease | 161 (23.4%) | 82 (20.3%) | 243 (22.3%) | 0.237 |
| Renal disease | 143 (20.8%) | 72 (17.9%) | 215 (19.7%) | 0.238 |
| History of smoking | 227 (33.0%) | 131 (32.5%) | 358 (32.8%) | 0.856 |
| History of hyperlipidaemia | 330 (48.0%) | 196 (48.6%) | 526 (48.3%) | 0.848 |
| Congestive heart failure | 383 (55.7%) | 269 (66.7%) | 652 (59.8%) | <0.001 |
| Atrial fibrillation | 222 (32.3%) | 120 (29.8%) | 342 (31.4%) | 0.383 |
| Chronic obstructive pulmonary disease | 51 (7.4%) | 33 (8.2%) | 84 (7.7%) | 0.648 |
| Prior acute myocardial infarction | 253 (36.8%) | 175 (43.4%) | 428 (39.3%) | 0.031 |
| Prior percutaneous intervention | 96 (14.0%) | 48 (11.9%) | 144 (13.2%) | 0.332 |
| Prior coronary artery bypass graft surgery | 154 (22.4%) | 91 (22.6%) | 245 (22.5%) | 0.950 |
| Logistic EuroSCORE, median (IQR) | 10.0 (5.6–17.2) | 9.4 (5.0–16.6) | 9.8 (5.5–16.8) | 0.161 |
| Moderate-to-severe LVH | 257 (37.4%) | 96 (23.8%) | 353 (32.4%) | <0.001 |
| Bicuspid aortic valve | 22 (3.2%) | 6 (1.5%) | 28 (2.6%) | 0.084 |
| Aortic regurgitation (moderate–severe) | 127 (18.5%) | 49 (12.2%) | 176 (16.1%) | 0.006 |
| Mitral regurgitation (moderate–severe) | 157 (22.9%) | 137 (34.0%) | 294 (27.0%) | <0.001 |
| LVID diastole, median (IQR) | 4.9 (4.4–5.3) | 5.5 (5.0–6.1) | 5.1 (4.5–5.7) | <0.001 |
| LVID systole, median (IQR) | 3.6 (3.1–4.1) | 4.6 (3.9–5.3) | 3.9 (3.3–4.6) | <0.001 |
LVID, left ventricular internal dimension; LVH, left ventricular hypertrophy; AS is defined by mean gradient criteria.
aFor comparison of EF 36–50 vs. EF ≤ 35%.
Table 2.
Baseline characteristics of patients with severe aortic stenosis and stratified by left ventricular systolic dysfunction
| Characteristic | Severe AS |
|||
|---|---|---|---|---|
| EF 36–50% (n = 374) | EF ≤ 35% (n = 170) | Overall (n = 544) | P a | |
| Age, median (IQR) | 77 (68–83) | 74 (64–82) | 76 (67–83) | 0.032 |
| Female gender | 152 (40.6%) | 70 (41.2%) | 222 (40.8%) | 0.906 |
| History of ischaemic heart disease | 218 (58.3%) | 88 (51.8%) | 306 (56.3%) | 0.155 |
| History of hypertension | 236 (63.1%) | 95 (55.9%) | 331 (60.8%) | 0.110 |
| Diabetes | 99 (26.5%) | 46 (27.1%) | 145 (26.7%) | 0.886 |
| Peripheral vascular disease | 47 (12.6%) | 11 (6.5%) | 58 (10.7%) | 0.033 |
| Prior cerebrovascular disease | 60 (16.0%) | 23 (13.5%) | 83 (15.3%) | 0.450 |
| Renal disease | 45 (12.0%) | 28 (16.5%) | 73 (13.4%) | 0.159 |
| History of smoking | 108 (28.9%) | 43 (25.3%) | 151 (27.8%) | 0.387 |
| History of hyperlipidaemia | 151 (40.4%) | 62 (36.5%) | 213 (39.2%) | 0.387 |
| Congestive heart failure | 213 (57.0%) | 114 (67.1%) | 327 (60.1%) | 0.026 |
| Atrial fibrillation | 109 (29.1%) | 55 (32.4%) | 164 (30.1%) | 0.450 |
| Chronic obstructive pulmonary disease | 23 (6.1%) | 7 (4.1%) | 30 (5.5%) | 0.336 |
| Prior acute myocardial infarction | 119 (31.8%) | 42 (24.7%) | 161 (29.6%) | 0.092 |
| Prior percutaneous intervention | 28 (7.5%) | 8 (4.7%) | 36 (6.6%) | 0.227 |
| Prior coronary artery bypass graft surgery | 63 (16.8%) | 17 (10.0%) | 80 (14.7%) | 0.037 |
| Logistic EuroSCORE, median (IQR) | 9.9 (5.8–16.9) | 9.3 (4.8–18.1) | 9.7 (5.5–17.3) | 0.294 |
| Moderate-to-severe LVH | 200 (53.5%) | 61 (35.9%) | 261 (48.0%) | <0.001 |
| Bicuspid aortic valve | 10 (2.7%) | 7 (4.1%) | 17 (3.1%) | 0.370 |
| Aortic regurgitation (moderate–severe) | 80 (21.4%) | 44 (25.9%) | 124 (22.8%) | 0.247 |
| Mitral regurgitation (moderate–severe) | 65 (17.4%) | 67 (39.4%) | 132 (24.3%) | <0.001 |
| LVID diastole, median (IQR)a | 4.8 (4.2–5.3) | 5.3 (4.8–5.9) | 4.9 (4.4–5.5) | <0.001 |
| LVID systole, median (IQR)a | 3.6 (3.0–4.0) | 4.4 (3.8–5.0) | 3.8 (3.2–4.4) | <0.001 |
LVID, left ventricular internal dimension; LVH, left ventricular hypertrophy; AS is defined by mean gradient criteria.
aFor comparison of EF 36–50 vs. EF ≤ 35%.
Among patients with moderate AS, most baseline characteristics were evenly distributed between the groups of LVSD, although a history of congestive heart failure, and concomitant moderate–severe mitral regurgitation occurred more commonly among those with severe LVSD. Compared with mild–moderate LVSD, patients with severe LVSD had larger left ventricular dimensions (Table 1).
Similarly, among patients with severe AS, most baseline characteristics were evenly distributed between the groups of LVSD, although moderate-to-severe LVH was more commonly present among those with mild–moderate LVSD and significant MR was more frequently seen among those with severe LVSD (Table 2).
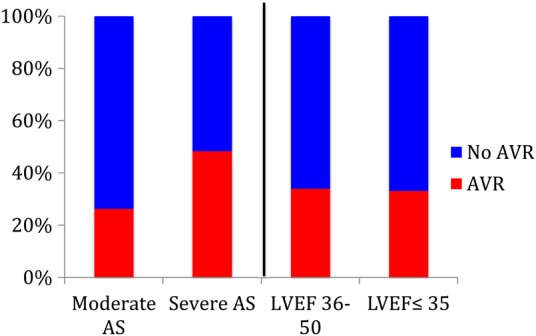
Use of aortic valve surgery
A total of 550 (34% of 1634 total) patients underwent AVR within 5 years of the index echo. This included 287/1090 (26%) patients with moderate AS by mean gradient criteria of which 108 (37%) had severe LVSD (ejection fraction, EF ≤ 35%) who underwent AVR. Of the 403 patients with calculated AVA > 1.0 cm2, 31% underwent AVR within 5 years of the qualifying echo. The median time to surgery from qualifying echo among patients with moderate AS was 28 days (IQR 5, 255). Of the 1090 patients with moderate AS by mean gradient criteria, 135 underwent isolated AVR and 152 had AVR + CABG. Of the 135 patients with moderate AS by mean gradient criteria who underwent AVR alone, 6/135 had concomitant severe MS, 17/135 had concomitant moderate or severe AR, 12/135 with concomitant moderate or severe MR. A total of 6/135 patients had dobutamine challenges in the cathlab documenting contractile reserve and truly severe AS and 30/135 patients had a subsequent echo prior to AVR, documenting increase in mean gradients >40 mmHg. The rest, either had AVA < 1.0 cm2 on echo or confirmed in the cathlab, had severely thickened leaflets on the echo or had peak velocities >3 m/s with calculated AVA < 1.0 cm2.
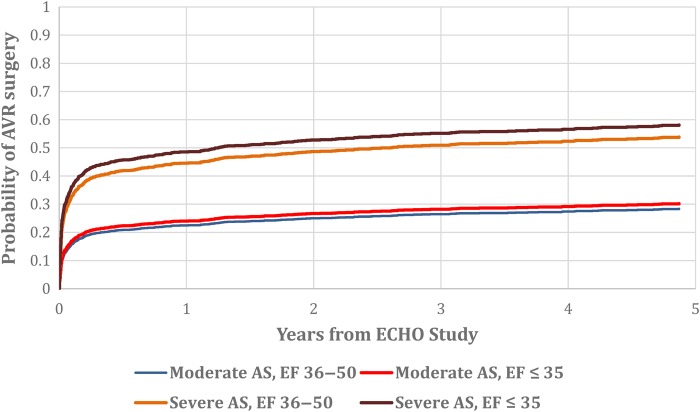
Among patients with severe AS by mean gradient criteria, 263/544 (48%) underwent AVR within 5 years of the qualifying echo with a median time to surgery of 8 days (IQR 4, 41). Of the patients with severe AS by mean gradient criteria who underwent AVR, 82 (31%) had severe LVSD (EF ≤ 35%) (Figure 1). Of the 544 patients with severe AS by mean gradient criteria, 145 had isolated valve surgery and 118 had AVR + CABG. Of the 935 patients with calculated AVA ≤ 1.0 cm2, 39% underwent AVR within 5 years.
Figure 1.
Use of aortic valve surgery among entire cohort by aortic stenosis severity is described on the left of the vertical bar and by severity of left ventricular systolic dysfunction on the right. Aortic stenosis severity is defined by mean gradients.
Among patients with moderate or severe AS, 190/573 (33.2%) with severe LVSD underwent AVR while 360/1061 (33.9%) patients with mild–moderate LVSD underwent AVR. The cumulative incidence of AVR amongst patients with moderate or severe AS and LVSD is shown in Figure 2. Baseline characteristics of patients stratified by AVR use within 5 years of the index echo are provided in Table 3.
Figure 2.
Cumulative incidence of aortic valve surgery by aortic stenosis severity and left ventricular systolic dysfunction.
Table 3.
Baseline characteristics of all patients stratified by aortic valve surgery
| Characteristic | AVR surgery within 5 years of index echo |
P | ||
|---|---|---|---|---|
| No AVR surgery (n = 1084) | AVR surgery (n = 550) | Total (n = 1634) | ||
| Age, median (IQR) | 77 (69–84) | 71 (64–78) | 75 (67–82) | <0.001 |
| Female gender | 440 (40.6%) | 174 (31.6%) | 614 (37.6%) | <0.001 |
| History of hypertension | 701 (64.7%) | 347 (63.1%) | 1048 (64.1%) | 0.530 |
| Diabetes | 371 (34.2%) | 166 (30.2%) | 537 (32.9%) | 0.100 |
| Peripheral vascular disease | 183 (16.9%) | 54 (9.8%) | 237 (14.5%) | <0.001 |
| Prior cerebrovascular disease | 238 (22.0%) | 88 (16.0%) | 326 (20.0%) | 0.004 |
| Renal disease | 218 (20.1%) | 70 (12.7%) | 288 (17.6%) | <0.001 |
| History of smoking | 321 (29.6%) | 188 (34.2%) | 509 (31.2%) | 0.059 |
| History of hyperlipidaemia | 496 (45.8%) | 243 (44.2%) | 739 (45.2%) | 0.546 |
| Congestive heart failure | 632 (58.3%) | 347 (63.1%) | 979 (59.9%) | 0.062 |
| Atrial fibrillation | 322 (29.7%) | 184 (33.5%) | 506 (31.0%) | 0.121 |
| Chronic obstructive pulmonary disease | 88 (8.1%) | 26 (4.7%) | 114 (7.0%) | 0.011 |
| Prior acute MI | 418 (38.6%) | 171 (31.1%) | 589 (36.0%) | 0.003 |
| Prior percutaneous intervention | 136 (12.5%) | 44 (8.0%) | 180 (11.0%) | 0.006 |
| Prior coronary artery bypass graft surgery | 246 (22.7%) | 79 (14.4%) | 325 (19.9%) | <0.001 |
| Logistic EuroSCORE, median (IQR) | 11.3 (6.3–19.0) | 7.3 (4.4–12.8) | 9.7 (5.5–17.0) | <0.001 |
| Moderate-to-severe LVH | 398 (36.7%) | 216 (39.3%) | 614 (37.6%) | 0.313 |
| Bicuspid aortic valve | 16 (1.5%) | 29 (5.3%) | 45 (2.8%) | <0.001 |
| Aortic regurgitation (moderate–severe) | 179 (16.5%) | 121 (22.0%) | 300 (18.4%) | 0.007 |
| Mitral regurgitation (moderate–severe) | 293 (27.0%) | 133 (24.2%) | 426 (26.1%) | 0.215 |
| LVID diastole, median (IQR) | 4.9 (4.4–5.6) | 5.1 (4.6–5.7) | 5.0 (4.5–5.6) | <0.001 |
| LVID systole, median (IQR) | 3.8 (3.2–4.5) | 4.0 (3.4–4.6) | 3.8 (3.3–4.5) | <0.001 |
| History of ischaemic heart disease | 650 (60.0%) | 352 (64.0%) | 1002 (61.3%) | 0.113 |
| Aortic valve area, median (IQR) | 0.8 (0.6–1.1) | 0.7 (0.6–1.0) | 0.8 (0.6–1.1) | 0.078 |
| Mean gradient, median (IQR) | 28.0 (23.0–37.5) | 36.0 (28.0–46.0) | 31.0 (24.0–41.0) | <0.001 |
LVID, left ventricular internal dimension.
Relationship of aortic valve surgery with survival in the entire cohort
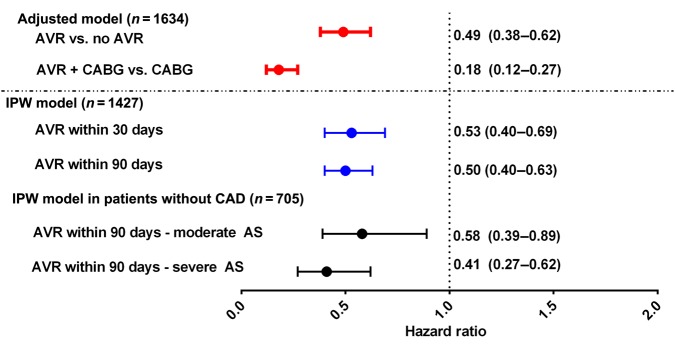
Mortality was evaluated up to 5 years from the index echo. Over that 5-year period, the median follow-up time was 1.2 years (IQR 0.2–3.9). There were 863 (53%) deaths observed up to 5 years following index echo. Aortic valve surgery with or without CABG, compared with medical therapy was associated with lower mortality [hazard ratio, HR = 0.49 (0.38, 0.62), P < 0.0001] in the entire cohort, after adjusting for AS (moderate or severe), LV dysfunction (EF ≤ 35% vs. 36–50%), age, sex, renal failure, history of ischaemic heart disease, COPD, hypertension, hyperlipidaemia, diabetes, moderate or severe MR, moderate or severe AR, bicuspid valves, and left ventricular dimensions. Compared with CABG alone, the combination of AVR + CABG (HR = 0.18 [0.12, 0.27], P < 0.0001) was associated with significantly higher survival. In the multivariable model, age >75 years (HR = 1.23 (1.15, 1.32) P < 0.0001), concomitant moderate or severe MR (HR = 1.47 (1.26, 1.71) P < 0.0001), diabetes (HR = 1.35 (1.15, 1.57) P = 0.0002), renal failure (HR = 1.6 (1.34, 1.91) P < 0.0001), and LVSD (HR = 1.79 (1.47, 2.19) P < 0.0001) were independently associated with increased risk of mortality.
Inverse probability treatment weighting propensity model in the entire cohort
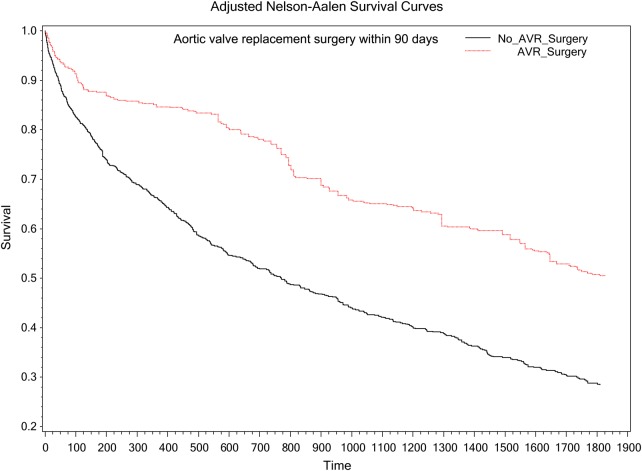
Of the original 1634 patients, 1427 had sufficiently complete data on all covariates allowing for inclusion in the sensitivity analysis using an inverse probability treatment weighting propensity model. Aortic valve surgery (within 90 days) was associated with significantly lower mortality (HR = 0.50, 95% CI = 0.40–0.63, P < 0.001) (Figure 3). Aortic valve surgery (within 30 days) was associated with a significantly lower mortality (HR = 0.53, 95% CI = 0.40–0.69, P < 0.0001) (Figure 4). These associations remained significant even when calculated AVAs were substituted for mean gradients in the model.
Figure 3.
Survival curves stratified by aortic valve surgery.
Figure 4.
Multivariate and propensity adjusted models examining the impact of aortic valve surgery on survival. All P < 0.001; analysis includes patients with mean aortic valve gradients ≥25 mmHg.
Interaction of aortic valve surgery with severity of aortic stenosis
Using the inverse probability of treatment weighted models, there was a significant interaction between AVR surgery (within 90 days) and AS in relation to mortality (P = 0.0174). Overall the analysis found that AVR surgery (within 90 days) was associated with a significant decreased risk of mortality; however, this decrease in mortality risk associated with AVR surgery was much stronger in patients with severe AS (HR = 0.35, 95% CI = 0.26–0.48) compared with patients with moderate AS (HR = 0.59, 95% CI = 0.44–0.78) as defined by mean gradients.
In inverse probability weighted models, AVR was associated with a lower hazard for death in both patients with moderate and severe AS regardless of the grading scheme employed to classify patients (see Table 4).
Table 4.
Relationship of aortic valve surgery with survival in patients with moderate and severe aortic stenosis
| Model | Aortic stenosis by mean gradient |
Aortic stenosis by aortic valve area |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderate AS (N = 1090) | Severe AS (N = 544) | Moderate AS (N = 403) | Severe AS (N = 935) | |||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| AVR vs. no AVR (within 5 years) | 0.68 | 0.52–0.90 | 0.0072 | 0.26 | 0.18–0.38 | <0.0001 | 0.65 | 0.42–1.00 | 0.0521 | 0.42 | 0.32–0.57 | <0.0001 |
| AVR + CABG vs. CABG (within 5 years) | 0.29 | 0.20–0.44 | <0.0001 | 0.04 | 0.01–0.11 | <0.0001 | 0.37 | 0.20–0.67 | 0.0011 | 0.14 | 0.08–0.25 | <0.0001 |
| IPW model | ||||||||||||
| AVR (within 90 days) | 0.59 | 0.44–0.78 | 0.0002 | 0.35 | 0.26–0.48 | <0.0001 | 0.56 | 0.36–0.87 | 0.0097 | 0.47 | 0.36–0.62 | <0.0001 |
| IPW model with interaction | ||||||||||||
| AVR (within 90 days)* aortic stenosis | 0.59 | 0.44–0.78 | 0.35 | 0.26–0.48 | 0.0174* | 0.56 | 0.36–0.87 | 0.47 | 0.36–0.62 | 0.4093* | ||
*P-value for interaction of AVR surgery and aortic stenosis.
Aortic valve surgery and severity of aortic stenosis among patients without coronary artery disease
To evaluate whether the effect of AVR extended across moderate and severe AS among LVSD patients without coronary artery disease (CAD), an additional sensitivity analysis was conducted. A landmark of AVR within 90 days of index echo and patients known alive without AVR at Day 15 was chosen for the sensitivity analysis. Patients with known CAD defined as having a history of prior myocardial infarction, percutaneous intervention, CABG, or significant CAD on cardiac catheterization were excluded from the analysis. Of the original 1427 patients in the inverse probability weighting (IPW) population, 705 were included in this sensitivity analysis. A Cox regression using weights found in the previous IPW analysis was conducted to determine the relationship between AVR within 90 days on all-cause mortality, in addition we included the interaction of AVR surgery (within 90 days) and level of AS (moderate or severe); in patients without known CAD. This sensitivity analysis found no significant interaction between AVR surgery and AS in relation to mortality (P = 0.2391). Overall, the analysis found that AVR surgery (within 90 days) was associated with a significant decreased risk of mortality; this decrease in mortality risk associated with AVR surgery is similar in both patients with severe AS (HR = 0.41, 95% CI = 0.27–0.62) (Figure 4, Appendix 3) and patients with moderate AS (HR = 0.58, 95% CI = 0.39–0.89) (Figure 4, Appendix 2).
Discussion
This study is novel in its exploration of the relationship of AVR with survival in patients with moderate and severe AS and concomitant left ventricular dysfunction. The main findings of this study can be summarized as follows: (i) AVR is used infrequently; (ii) AVR with or without CABG is associated with a significant mortality benefit compared with medical management; and (iii) the mortality benefit associated with AVR extends to patients without CAD and among patients with calculated AVA > 1.0 cm2
In the last few decades, a deeper understanding of the pathophysiology and haemodynamic effects of AS, coupled with technological advancements in surgery have allowed surgical AVR to be a safe corrective option for severe AS.24–26 Even among patients with left ventricular dysfunction, AVR offers a survival benefit.9 In this study from a single major academic centre, AVR was used infrequently; even among patients with severe AS and mean aortic gradients >40 mmHg with LVSD, who would traditionally otherwise meet ACC/AHA guidelines class I indications for AVR, surgery was undertaken at our institution in <50% of cases within 5 years of the qualifying examination. National rates of AVR use for this indication are unknown and may be difficult to gather due to lack of detailed information in larger population datasets. Potential reasons for such low operative rates may be advanced age, high prevalence of comorbidities and high median logistic euroSCORE (a score of >6 defining high risk).22,27 Certainly, similar driving factors were noted in the Euro heart survey, which found that amongst patients with symptomatic single valve disease, an intervention was not undertaken in ∼30% of cases.2 Iung and colleagues reported that the most frequent reasons stated for a lack of intervention included old age (27.6%) chronic obstructive pulmonary disease (13.6%), renal failure (6.1%), and short life expectancy (19.3%).2 In a subanalysis of the Euro heart survey including patients with AS over 75 years of age, surgery was not undertaken in 33%.28 Older age and LVSD were hallmark characteristics of those who were denied surgery.28
A high mortality rate was observed in our cohort with 53% deaths within 5 years of the qualifying echo, a fact that highlights the poor prognosis associated with AS in the setting of LVSD. While patients with severe AS and left ventricular dysfunction were historically thought to be too high surgical risk, studies have suggested survival benefit with surgery compared with medical therapy alone.29 The studies by Conolly et al. and Pereira et al. allowed a paradigm shift where truly severe AS in the setting of LV dysfunction is routinely sought and treatments offered.8,9 More recently, investigations have pointed to LVEF recovery and improved survival comparable with surgical treatment, with the use of transcatheter aortic valve replacement among patients with severe AS and left ventricular dysfunction.14,30,31 Data in the present investigation confirm and extend the findings of previous investigators in noting a survival benefit among patients with severe AS and LVSD across both low- and high-gradient groups.
At present, dobutamine stress testing is recommended among patients with AS but low gradients to distinguish ‘pseudosevere’ vs. severe AS so that those with truly severe AS can be offered AVR.32–37 What has remained an enigma thus far is whether ‘pseudosevere’ AS could benefit from AVR.38 While an attempt to distinguish severe vs. ‘pseudosevere’ AS with a dobutamine challenge during echocardiography was not made in a majority (98.6%) of our patients, it is likely that ‘pseudosevere’ AS existed among those with calculated AVA < 1.0 cm2.39 While there was an interaction between AVR (within 90 days) and AS severity where the survival benefit associated with AVR was greater amongst those with higher gradients vs. lower gradients, the finding of benefit of AVR across gradients starting from ≥25 mmHg raises important considerations around the possible benefits of correcting even moderate or pseudo severe AS in the setting of left ventricular dysfunction. As further corroborated by the sensitivity analyses, this treatment association was noted even in patients without a history of CAD and among those with calculated valve areas of >1.0 cm2. Our finding of higher survival associated with use of AVR across aortic valve mean gradients ≥25 mm Hg in the setting of left ventricular dysfunction also lends credence to the hypothesis that in the setting of LV dysfunction, AS, whether it is a consequence or a coincident comorbid condition, poses a haemodynamic burden and mechanical relief from it is thus associated with a survival benefit.
The treatment of choice among patients with AS and LVSD likely also depends on the need for other interventions like CABG. This is particularly important among those with moderate AS severity where the surgical risk of AVR is unlikely to be taken unless there is need for correction of concomitant obstructive coronary disease or other severe valve disease. More than half the cohort of patients with moderate AS who underwent AVR also had concomitant CABG (52%). Aortic valve surgery for moderate AS when CABG is anticipated is currently a Class IIA European/ACC/AHA guideline indication.3 This indication is based on the likelihood of progression to more severe and symptomatic disease requiring intervention within 5 years of finding moderate AS and supported by a decision analysis by Smith and colleagues.40–43 Our findings are also consistent with the work of Pereira et al. who evaluated patients with mild–moderate AS undergoing CABG at their institution. In a propensity analysis, patients who underwent AVR and CABG did better at Years 1 and 8 following surgery compared with those who underwent CABG alone. This survival benefit was limited to those who had moderate AS at the time of CABG. The mean LVEF in their cohort was ∼50%.44 Our observational data lends further support to this guideline indication especially in the case of the higher risk moderate AS patient with more severe left ventricular dysfunction.
Clinical implications
The implications of our findings are several fold. Factors associated with avoidance of AVR in this population need to be explored at the institutional as well as national level, as they will inform shared patient–physician therapeutic decision-making and contribute to setting of quality standards in valve disease management.
The relief of AS, using a procedure that has established safety and acceptable risk, should translate into improvement in symptoms, and eventually hard outcomes such as long-term survival. The advent of safer surgical approaches and transcatheter therapies12–14 that are quite capable of achieving these ends, raises the question of whether AVR using transcatheter and surgical approaches among patients with moderate AS in the setting of LVSD should be performed routinely and needs to be prospectively evaluated.
Limitations
While there are several strengths to this study including the size of the data set, sophistical statistical analyses and the importance of the question addressed, there are limitations that need to be acknowledged. These data represent practice patterns at a single major medical centre. While conclusions cannot be made regarding generalizability of practice patterns, this study raises important questions regarding the need for national level information.
Our models were adjusted for all known and measured confounders, yet as with most observational datasets, the possibility of unmeasured confounders exists. Although a true randomized comparison would be ideal, we used propensity methods to account for biases associated with non-random treatment assignment.
Details of drug therapy were not captured in this study and differences in therapy across the treatment groups may have implications for survival that were thus not studied.
Since cardiac catheterization was not pursued in all cases, STS PROM score could not be calculated. We, therefore, chose to describe surgical risk using the logistic EuroSCORE. While each scoring system has its unique advantages and demerits, neither has more than moderate discrimination with regards to TAVR outcome.45
Conclusions
Aortic valve surgery, although performed infrequently, is associated with a significant survival benefit across moderate AS (mean AoV gradient ≥25 <40 mmHg) and severe AS (≥40 mmHg) amongst patients with LVSD. Which factors contribute to current practice patterns and whether the availability of transcatheter approaches will modify this high-risk cohort, requires further study.
Funding
This study was funded by an investigator-initiated Boston Scientific-Duke Strategic Alliance for Research grant award to Z.S. Boston Scientific was not involved in the study design, the collection, analysis, and interpretation of data, the writing of the report, or in the decision to submit the article for publication.
Conflict of interest: Z.S.: salary support through a National Heart, Lung, and Blood Institute (NHLBI) research grant, American Society of Echocardiography research grant, Boston Scientific-Duke Strategic Alliance for Research; J.K.: speaker for Phillips, E.J.V.: salary support through an NHLBI research grant; research grants from Abbott Laboratories, Evalve, and Ikaria; receiving consulting fees from Boehringer Ingelheim, Gilead, and Novartis.
Appendix 1
Table A1.
Baseline characteristics by aortic stenosis (defined by calculated aortic valve area)
| Characteristic | Moderate AS (n = 403) | Severe AS (n = 935) | Total (n = 1338) | P* |
|---|---|---|---|---|
| Age, median (IQR) | 73 (64–80) | 76 (68–83) | 75 (67–82) | <0.001 |
| Female gender | 126 (31.3%) | 371 (39.7%) | 497 (37.1%) | 0.003 |
| History of hypertension | 272 (67.5%) | 622 (66.5%) | 894 (66.8%) | 0.730 |
| Diabetes | 156 (38.7%) | 295 (31.6%) | 451 (33.7%) | 0.011 |
| Peripheral vascular disease | 69 (17.1%) | 132 (14.1%) | 201 (15.0%) | 0.158 |
| Prior cerebrovascular disease | 91 (22.6%) | 194 (20.7%) | 285 (21.3%) | 0.453 |
| Renal failure | 95 (23.6%) | 163 (17.4%) | 258 (19.3%) | 0.009 |
| History of smoking | 143 (35.5%) | 290 (31.0%) | 433 (32.4%) | 0.109 |
| History of hyperlipidaemia | 223 (55.3%) | 428 (45.8%) | 651 (48.7%) | 0.001 |
| Congestive heart failure | 260 (64.5%) | 581 (62.1%) | 841 (62.9%) | 0.409 |
| Atrial fibrillation | 130 (32.3%) | 296 (31.7%) | 426 (31.8%) | 0.829 |
| Chronic obstructive pulmonary disease | 43 (10.7%) | 51 (5.5%) | 94 (7.0%) | <0.001 |
| Prior acute MI | 155 (38.5%) | 326 (34.9%) | 481 (35.9%) | 0.209 |
| Prior PCI | 54 (13.4%) | 95 (10.2%) | 149 (11.1%) | 0.084 |
| Prior CABG | 87 (21.6%) | 178 (19.0%) | 265 (19.8%) | 0.283 |
| Logistic EuroSCORE, median (IQR) | 9.1 (4.8–15.4) | 10.1 (5.9–17.4) | 9.8 (5.7–16.7) | 0.005 |
| Moderate-to-severe LVH | 154 (38.2%) | 374 (40.0%) | 528 (39.5%) | 0.540 |
| Bicuspid aortic valve | 18 (4.5%) | 21 (2.2%) | 39 (2.9%) | 0.027 |
| Aortic regurgitation (moderate–severe) | 74 (18.4%) | 171 (18.3%) | 245 (18.3%) | 0.975 |
| Mitral regurgitation (moderate–severe) | 85 (21.1%) | 264 (28.2%) | 349 (26.1%) | 0.006 |
| LVID diastole, median (IQR) | 5.1 (4.6–5.7) | 5.0 (4.4–5.6) | 5.0 (4.5–5.6) | 0.003 |
| LVID systole, median (IQR) | 4.0 (3.3–4.6) | 3.8 (3.3–4.6) | 3.9 (3.3–4.6) | 0.337 |
| History of ischaemic heart disease | 255 (63.3%) | 596 (63.7%) | 851 (63.6%) | 0.870 |
*P-value is for comparison between moderate AS and severe AS.
Appendix 2
Figure A1.
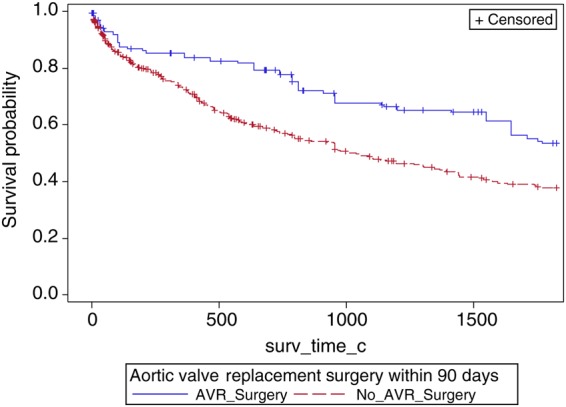
Adjusted product limit survival estimates of patients with moderate aortic stenosis and left ventricular systolic dysfunction and no known coronary artery disease stratified by aortic valve surgery. Aortic stenosis is defined by mean gradient criteria.
Appendix 3
Figure A2.
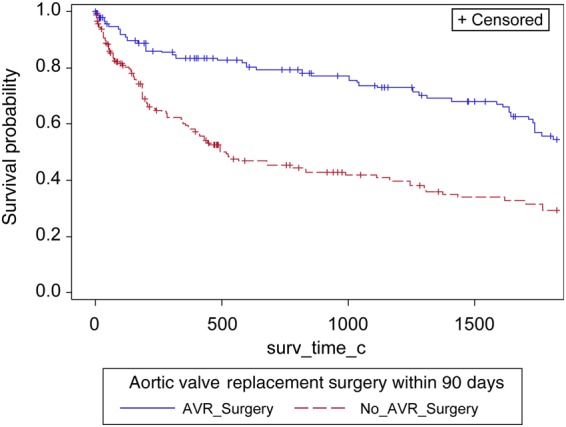
Adjusted product-limit survival estimates of patients with severe aortic stenosis and left ventricular systolic dysfunction and no known coronary artery disease stratified by aortic valve surgery. Aortic stenosis is defined by mean gradient criteria.
Appendix 4
Figure A3.
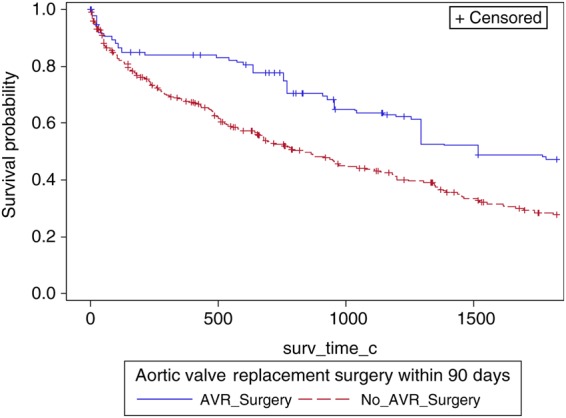
Adjusted product-limit survival estimates of patients with AVA > 1.0 cm2 and left ventricular systolic dysfunction stratified by aortic valve surgery.
References
- 1. Rankin JS, Hammill BG, Ferguson TB Jr, Glower DD, O'Brien SM, DeLong ER, Peterson ED, Edwards FH. Determinants of operative mortality in valvular heart surgery. J Thoracic Cardiovasc Surg 2006;131:547–557. [DOI] [PubMed] [Google Scholar]
- 2. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The euro heart survey on valvular heart disease. Eur Heart J 2003;24:1231–1243. [DOI] [PubMed] [Google Scholar]
- 3. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD, Members AATF. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 4. Ross J., Jr Afterload mismatch in aortic and mitral valve disease: implications for surgical therapy. J Am College Cardiol 1985;5:811–826. [DOI] [PubMed] [Google Scholar]
- 5. Wisenbaugh T, Booth D, DeMaria A, Nissen S, Waters J. Relationship of contractile state to ejection performance in patients with chronic aortic valve disease. Circulation. 1986;73:47–53. [DOI] [PubMed] [Google Scholar]
- 6. Hwang MH, Hammermeister KE, Oprian C, Henderson W, Bousvaros G, Wong M, Miller DC, Folland E, Sethi G. Preoperative identification of patients likely to have left ventricular dysfunction after aortic valve replacement. Participants in the veterans administration cooperative study on valvular heart disease. Circulation. 1989;80:I65–I76. [PubMed] [Google Scholar]
- 7. Logeais Y, Langanay T, Roussin R, Leguerrier A, Rioux C, Chaperon J, de Place C, Mabo P, Pony JC, Daubert JC. Surgery for aortic stenosis in elderly patients. A study of surgical risk and predictive factors. Circulation. 1994;90:2891–2898. [DOI] [PubMed] [Google Scholar]
- 8. Connolly HM, Oh JK, Schaff HV, Roger VL, Osborn SL, Hodge DO, Tajik AJ. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation. 2000;101:1940–1946. [DOI] [PubMed] [Google Scholar]
- 9. Pereira JJ, Lauer MS, Bashir M, Afridi I, Blackstone EH, Stewart WJ, McCarthy PM, Thomas JD, Asher CR. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am College Cardiol 2002;39:1356–1363. [DOI] [PubMed] [Google Scholar]
- 10. Connolly HM, Oh JK, Orszulak TA, Osborn SL, Roger VL, Hodge DO, Bailey KR, Seward JB, Tajik AJ. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation. 1997;95:2395–2400. [DOI] [PubMed] [Google Scholar]
- 11. Powell DE, Tunick PA, Rosenzweig BP, Freedberg RS, Katz ES, Applebaum RM, Perez JL, Kronzon I. Aortic valve replacement in patients with aortic stenosis and severe left ventricular dysfunction. Arch Inter Med. 2000;160:1337–1341. [DOI] [PubMed] [Google Scholar]
- 12. Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB, Investigators PT. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. New Engl J Med 2012;366:1696–1704. [DOI] [PubMed] [Google Scholar]
- 13. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, Investigators PT. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. New Engl J Med 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 14. Clavel MA, Webb JG, Rodes-Cabau J, Masson JB, Dumont E, De Larochelliere R, Doyle D, Bergeron S, Baumgartner H, Burwash IG, Dumesnil JG, Mundigler G, Moss R, Kempny A, Bagur R, Bergler-Klein J, Gurvitch R, Mathieu P, Pibarot P. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122:1928–1936. [DOI] [PubMed] [Google Scholar]
- 15. Ersboll M, Schulte PJ, Al Enezi F, Shaw L, Kober L, Kisslo J, Siddiqui I, Piccini J, Glower D, Harrison JK, Bashore T, Risum N, Jollis JG, Velazquez EJ, Samad Z. Predictors and progression of aortic stenosis in patients with preserved left ventricular ejection fraction. Am J Cardiol. 2015;115:86–92. [DOI] [PubMed] [Google Scholar]
- 16. Harris PJ, Lee KL, Harrell FE Jr, Behar VS, Rosati RA. Outcome in medically treated coronary artery disease. Ischemic events: Nonfatal infarction and death. Circulation. 1980;62:718–726. [DOI] [PubMed] [Google Scholar]
- 17. Califf RM, Harrell FE Jr, Lee KL, Rankin JS, Hlatky MA, Mark DB, Jones RH, Muhlbaier LH, Oldham HN Jr, Pryor DB. The evolution of medical and surgical therapy for coronary artery disease. A 15-year perspective. J Am Med Assoc. 1989;261:2077–2086. [PubMed] [Google Scholar]
- 18. Mark DB, Nelson CL, Califf RM, Harrell FE Jr, Lee KL, Jones RH, Fortin DF, Stack RS, Glower DD, Smith LR. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–2025. [DOI] [PubMed] [Google Scholar]
- 19. Boyle CA, Decoufle P. National sources of vital status information: Extent of coverage and possible selectivity in reporting. Am J Epidemiol 1990;131:160–168. [DOI] [PubMed] [Google Scholar]
- 20. Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J 2008;29:1043–1048. [DOI] [PubMed] [Google Scholar]
- 21. Samad Z, Armour A, Tinnermore A, Minter S, Strub K, Douglas PS, Velazquez EJ. Correlation between echocardiographic parameters reporting on aortic stenosis. J Am Soc Echocardiogr. 2015;28:B78. [Google Scholar]
- 22. Roques F, Michel P, Goldstone AR, Nashef SA. The logistic euroscore. Eur Heart J 2003;24:881–882. [DOI] [PubMed] [Google Scholar]
- 23. Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindman BR, Bonow RO, Otto CM. Current management of calcific aortic stenosis. Circ Res 2013;113:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carabello BA. Clinical practice. Aortic stenosis. New Engl J Med 2002;346:677–682. [DOI] [PubMed] [Google Scholar]
- 26. Brown JM, O'Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the society of thoracic surgeons national database. J Thoracic Cardiovasc Surg 2009;137:82–90. [DOI] [PubMed] [Google Scholar]
- 27. Michel P, Roques F, Nashef SA, Euro SPG. Logistic or additive euroscore for high-risk patients? Eur J Cardio-Thoracic Surg 2003;23:684–687. Discussion 687. [DOI] [PubMed] [Google Scholar]
- 28. Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P, Gohlke-Barwolf C, Boersma E, Ravaud P, Vahanian A. Decision-making in elderly patients with severe aortic stenosis: Why are so many denied surgery? Eur Heart J 2005;26:2714–2720. [DOI] [PubMed] [Google Scholar]
- 29. Carabello BA, Green LH, Grossman W, Cohn LH, Koster JK, Collins JJ Jr. Hemodynamic determinants of prognosis of aortic valve replacement in critical aortic stenosis and advanced congestive heart failure. Circulation. 1980;62:42–48. [DOI] [PubMed] [Google Scholar]
- 30. Elmariah S, Palacios IF, McAndrew T, Hueter I, Inglessis I, Baker JN, Kodali S, Leon MB, Svensson L, Pibarot P, Douglas PS, Fearon WF, Kirtane AJ, Maniar HS, Passeri JJ, Investigators P. Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: results from the placement of aortic transcatheter valves (partner) trial (cohort a). Circ Cardiovasc Intervent 2013;6:604–614. [DOI] [PubMed] [Google Scholar]
- 31. O'Sullivan CJ, Stortecky S, Heg D, Pilgrim T, Hosek N, Buellesfeld L, Khattab AA, Nietlispach F, Moschovitis A, Zanchin T, Meier B, Windecker S, Wenaweser P. Clinical outcomes of patients with low-flow, low-gradient, severe aortic stenosis and either preserved or reduced ejection fraction undergoing transcatheter aortic valve implantation. Eur Heart J 2013;34:3437–3450. [DOI] [PubMed] [Google Scholar]
- 32. Schwammenthal E, Vered Z, Moshkowitz Y, Rabinowitz B, Ziskind Z, Smolinski AK, Feinberg MS. Dobutamine echocardiography in patients with aortic stenosis and left ventricular dysfunction: Predicting outcome as a function of management strategy. Chest. 2001;119:1766–1777. [DOI] [PubMed] [Google Scholar]
- 33. Monin JL, Quere JP, Monchi M, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Tribouilloy C, Gueret P. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108:319–324. [DOI] [PubMed] [Google Scholar]
- 34. Brogan WC 3rd, Grayburn PA, Lange RA, Hillis LD. Prognosis after valve replacement in patients with severe aortic stenosis and a low transvalvular pressure gradient. J Am College Cardiol 1993;21:1657–1660. [DOI] [PubMed] [Google Scholar]
- 35. Smith RL, Larsen D, Crawford MH, Shively BK. Echocardiographic predictors of survival in low gradient aortic stenosis. Am J Cardiol 2000;86:804–807, A810. [DOI] [PubMed] [Google Scholar]
- 36. Quere JP, Monin JL, Levy F, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Gueret P, Tribouilloy C. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation. 2006;113:1738–1744. [DOI] [PubMed] [Google Scholar]
- 37. Levy F, Laurent M, Monin JL, Maillet JM, Pasquet A, Le Tourneau T, Petit-Eisenmann H, Gori M, Jobic Y, Bauer F, Chauvel C, Leguerrier A, Tribouilloy C. Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: a European multicenter study. J Am College Cardiol 2008;51:1466–1472. [DOI] [PubMed] [Google Scholar]
- 38. Carabello BA. Ventricular function in aortic stenosis: how low can you go? J Am College Cardiol. 2002;39:1364–1365. [DOI] [PubMed] [Google Scholar]
- 39. Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am College Cardiol 2012;60:1845–1853. [DOI] [PubMed] [Google Scholar]
- 40. Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake-Hull CY, Schwaegler RG. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997;95:2262–2270. [DOI] [PubMed] [Google Scholar]
- 41. Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J 2004;25:199–205. [DOI] [PubMed] [Google Scholar]
- 42. Smith WTt, Ferguson TB Jr, Ryan T, Landolfo CK, Peterson ED. Should coronary artery bypass graft surgery patients with mild or moderate aortic stenosis undergo concomitant aortic valve replacement? A decision analysis approach to the surgical dilemma. J Am College Cardiol 2004;44:1241–1247. [DOI] [PubMed] [Google Scholar]
- 43. Gillinov AM, Garcia MJ. When is concomitant aortic valve replacement indicated in patients with mild to moderate stenosis undergoing coronary revascularization? Curr Cardiol Reports 2005;7:101–104. [DOI] [PubMed] [Google Scholar]
- 44. Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005;118:735–742. [DOI] [PubMed] [Google Scholar]
- 45. Durand E, Borz B, Godin M, Tron C, Litzler PY, Bessou JP, Dacher JN, Bauer F, Cribier A, Eltchaninoff H. Performance analysis of Euroscore II compared to the original logistic euroscore and STS scores for predicting 30-day mortality after transcatheter aortic valve replacement. Am J Cardiol 2013;111:891–897. [DOI] [PubMed] [Google Scholar]