Abstract
The effects of short-term resistance training on performance and health variables associated with prolonged sedentary lifestyle and metabolic syndrome were investigated. Resistance training may alter a number of health-related, physiological and performance variables. As a result, resistance training can be used as a valuable tool in ameliorating the effects of a sedentary lifestyle including those associated with metabolic syndrome. Nineteen previously sedentary subjects (10 metabolic syndrome, 9 non-metabolic syndrome) underwent 8 weeks of supervised resistance training. Maximum strength was measured using an isometric mid-thigh pull and resulting force-time curve. Vertical jump height and power were measured using a force plate. Muscle cross-sectional area (CSA) and type were examined using muscle biopsy and standard analysis techniques. Aerobic power was measured on a cycle ergometer using a ParvoMedics 2400 Metabolic system. Endurance was measured as time to exhaustion on a cycle ergometer. After training, maximum isometric strength, jump height, jump power and V̇O2 peak increased by approximately 10% (or more) in both the metabolic and non-metabolic syndrome groups (both male and female subjects). Over 8 weeks of training, body mass did not change statistically, but percent body fat decreased in subjects with the metabolic syndrome and in females, and lean body mass increased in all groups (p<0.05). Few alterations were noted in fiber type. Males had larger CSA’s compared to females and there was a fiber-specific trend toward hypertrophy over time. In summary eight weeks of semi-block free-weight resistance training improved several performance variables and some cardiovascular factors associated with metabolic syndrome.
Keywords: strength training, strength, power, endurance, fitness
INTRODUCTION
Long-term lack of physical activity is a risk factor for developing a number of diseases including metabolic syndrome, diabetes, and coronary heart disease (58). Additionally, a prolonged sedentary lifestyle reduces the ability to perform daily tasks and can reduce quality of life (51) . Recently resistance training has been studied as to its impact on factors associated with prolonged physical inactivity (2). Resistance training may be important for reducing disease risk factors given the inverse relationship between maximum strength and mortality (35, 46) and between maximum strength and the development of metabolic syndrome (6). Indeed, among adults resistance training has been associated with alterations in physiological and performance variables including body composition, increased max V̇O2, stabilized or lower resting and exercise recovery blood pressures, improved insulin sensitivity and glucose tolerance, improved blood lipid profiles (10, 32), improved strength capabilities, and improved endurance (10, 24, 51). These factors make resistance training an attractive mode of physical training to improve health for many adults.
Normal human skeletal muscle contains a mixture of type I, IIa, and IIx muscle fibers. Each fiber type is suited for different types of physical activity. Type I fibers generally contain more mitochondria and are well suited for oxidative energy production and sustained activity. While type I fibers can provide energy for long periods of time, their relatively slow contraction speed and low force production make them best suited for long-term, low intensity activity (53). Conversely, type IIx fibers typically have larger cross sectional areas (CSA) and are better suited for energy production via phosphogens and fast glycolysis. Although aerobic capabilities are relatively low, type IIx fibers have the greatest velocity of contraction and the higher force and power production capabilities, making them well suited for short term, high intensity activity such as sprints or weight training. Type IIa fibers are an intermediate fiber type with some properties of both type I and type IIx fibers. Training, particularly with higher volume, can result in a shift from type IIx muscle fibers toward type IIa fibers, reflecting the altered energy need of the trained muscles (53). Decreases in training volume or a sufficient taper can produce a shift back toward type IIx with a reduction in type IIa (3, 4, 53). Long term training has not always resulted in alteration of the baseline proportion of type I and type II fibers in humans (15). As a result of divergent physiological and performance characteristics of skeletal muscle fiber types, it is not surprising that many of the adaptations to training are fiber type dependent (57). These fiber type adaptations may be related to separate and somewhat independent cell signaling pathways that predominate in each of the two principal fiber types (7). It is likely that fiber type and cell signaling pathways can be influenced in different manners or to different degrees depending on several factors including age, trained sate, the type of training program, exercise selection, volume, and intensity considerations (3,4,7,11). Indeed, substantial motor unit and muscle mass loss accompanies aging. However recent evidence indicates that long-term training among master athletes' appears to protect motor neurons from age related deterioration (20). Importantly, strength-power training appeared to have a substantial positive effect on muscle mass and could therefore be an effective method of training to prevent sarcopenia (20). Power training is easily incorporated into resistance training protocols, particularly those using free-weights (50).
Resistance training variables such as volume, intensity, and exercise selection can be manipulated to emphasize performance characteristics (e.g. strength-endurance, strength, power) or to alter physiology and metabolism through the use of large muscle mass exercises (39) and increased training volume (50). Most studies using previously sedentary and metabolic syndrome adults have used machine based training as the primary mode (22). However, while a few machine exercises may be advantageous for muscle isolation or use with severely disabled individuals, Stone and colleagues (50) argue that training exercises for sedentary populations should be primarily carried out with free weights for the same reasons that athletic populations should use them. Free weights may offer advantages particularly concerning athletic or daily activity task specificity. There is no reason to believe that the superior “transfer of training effect” that can often be realized from free weights using similar exercises to that of strength-power athletes would not be effective in improving strength, rate of force development, and power among sedentary populations provided appropriate training methodologies are incorporated (50).
Additionally evidence indicates block periodization schemes, particularly among athletes (17, 18, 43), can provide superior adaptive efficacy and training efficiency. Briefly: Periodization entails time-lines and fitness phases. Periodization schemes typically move from targeting more general fitness characteristics to more specific characteristics. Programming (sets, reps, exercises etc.) is the method by which the fitness phases are given structure and targeted fitness characteristics attained. Block periodization typically entails using a periodization “stage” containing three fitness phases (17, 18), Accumulation, Transmutation, and Realization. Programming for these three phases is different for different sports but would typically entail: Accumulation, generally corresponding to an emphasis on higher volume, less specific training that results in alterations in aspects such as work capacity, body composition, basic strength etc.; Transmutation entails somewhat more specific exercises, lower volume and somewhat higher intensities of training and can entail increases in maximum strength for specific exercises; Realization typically deals with very specific exercises and involves a taper. For strength-power sports the emphasis would generally be: accumulation, emphasis on strength endurance, work capacity and body composition alterations, particularly total muscle CSA and the muscle fiber type II/I CSA; Transmutation would be constructed to emphasize exercise specific strength gains and further target the II/CSA area; Realization would entail an emphasis on increasing power output in performance specific activities as well as a taper in order to dissipate fatigue and possibly alter MHC type from IIa to IIx (17, 18).
The purpose of this study was to explore the potential performance and physiological effects of free-weight training using exercises, modified-block periodization (Accumulation and Transmutation) concepts, and a programmed training routine more typical of that used by strength-power athletic populations during the accumulation and transmutation training phases. Additionally, by using blocks targeting work capacity and body composition parameters it may be possible that additional health related variables can be positively affected. Variables chosen for study were those typically showing substantial decay with prolonged inactivity. These variables include performance aspects (endurance, strength, explosiveness, and power) and physical and physiological factors (body mass and composition, aerobic power, blood pressure, muscle fiber type and CSA, and blood lipids). Additionally, comparisons were made between subjects with and without “metabolic syndrome” and between men and women. Our long-term plans are to optimize resistance training programs for clinical populations. Our first step here is to show feasibility and efficacy among a cohort of metabolic syndrome adults and those with significant risk of developing metabolic syndrome. Our next steps will be to compare block periodization to more traditional linear training protocols, and to compare free-weight training to machine based training.
METHODS
Experimental Approach to the Problem
Due to the unconventional methods of training and testing used with this sample, the study was considered to be exploratory in nature. Nineteen previously sedentary subjects (9 male, 10 female) were divided into metabolic syndrome (MS) and non-metabolic syndrome groups (NMS). All had been relatively sedentary for at least six months prior to the study. Subjects were encouraged throughout the study to eat their normal diet and not to lose body mass as the intent of the study was to examine the effects of resistance exercise independent of body mass loss. All subjects were familiarized with the exercises during the week prior to study initiation. Testing was performed pre and post 8 weeks of training. Tests consisted of a variety of physical characteristics, performance, and physiological measurements including muscle fiber type and size, body composition, maximum strength and power, blood pressure, and blood lipids. Statistical comparisons were made between males and females and between metabolic syndrome (MS) and non-metabolic syndrome (NMS) subjects.
Subjects
Subjects consisted of 19 men and women between 18 and 55 years of age. Average age for each comparison group was: MS 45±8.6 yrs; NMS 36±12.2 yrs; M 40±10.8 yrs; F 42±11.8 yrs. Separation into the two experimental groups was based upon the criteria as defined by the International Diabetes Federation (1, 21). The initial subject characteristics are shown in Table 1. Metabolic syndrome subjects were older, not statistically heavier, and had higher resting glucose concentrations.
Table 1.
Physical and Physiological Characteristics Over time (Mean ± SD)
| All ( n = 19) | Pre | Post | d | p value |
| Body Mass (kg) | 83.9 ± 20.9 | 85.1 ± 21.3 | −0.06 | 0.031 |
| Lean Body Mass (kg) | 52.8 ± 10.4 | 54.1 ± 10.6 | 0.12 | 0.000 |
| Percent Fat | 35.6 ± 10.2 | 34.9 ± 9.9 | 0.07 | 0.037 |
| Skinfold Sum (mm) | 255.4 ± 82.7 | 246.4 ± 73.6 | 0.12 | 0.124 |
| Waist Circumference (cm) | 105.1 ± 14.4 | 102.1 ± 15.5 | 0.20 | 0.019 |
| Glucose (mg/dL) | 96 ± 14 | 100 ± 11 | −0.29 | 0.238 |
| Insulin (mg/dL) | 11 ± 8 | 10 ± 8 | 0.14 | 0.101 |
|
| ||||
| MS ( n = 9) | Pre | Post | d | p value |
| Body Mass (kg) | 98.1 ± 13.4 | 99.3 ± 14.3 | −0.09 | 0.194 |
| Lean Body Mass (kg) | 56.8 ± 9.5 | 58.0 ± 10.1 | 0.12 | 0.047 |
| Percent Fat | 42.2 ± 5.4 | 41.7 ± 5.3 | 0.09 | 0.011 |
| Skinfold Sum (mm) | 299.7 ± 64.0 | 291.2 ± 52.8 | 0.14 | 0.347 |
| Waist Circumference (cm) | 115.9 ± 5.5 | 113.3 ± 7.9 | 0.39 | 0.102 |
| Glucose (mg/dL) | 103 ± 12 | 102 ± 12 | 0.12 | 0.769 |
| Insulin (mg/dL) | 15 ± 10 | 14 ± 8 | 0.09 | 0.543 |
|
| ||||
| NMS (n = 10) | Pre | Post | d | p value |
| Body Mass (kg) | 68.1 ± 15.7 | 69.3 ± 16.1 | −0.08 | 0.079 |
| Lean Body Mass (kg) | 48.3 ± 10.0 | 49.7 ± 9.9 | 0.14 | 0.003 |
| Percent Fat | 28.3 ± 9.4 | 27.5 ± 8.4 | 0.09 | 0.221 |
| Skinfold Sum (mm) | 206.2 ± 74.8 | 196.5 ± 61.1 | 0.14 | 0.297 |
| Waist Circumference (cm) | 93.1 ± 11.0 | 89.7 ± 11.7 | 0.30 | 0.143 |
| Glucose (mg/dL) | 89 ± 13 | 98 ± 9 | −0.84 | 0.014 |
| Insulin (mg/dL) | 8 ± 4 | 6 ± 4 | 0.43 | 0.014 |
|
| ||||
| Male ( n = 9) | Pre | Post | d | p value |
| Body Mass (kg) | 94.1 ± 18.1 | 95.7 ± 18.5 | −0.09 | 0.087 |
| Lean Body Mass (kg) | 61.3 ± 6.6 | 62.7 ± 6.9 | 0.21 | 0.050 |
| Percent Fat | 33.3 ± 10.5 | 33.0 ± 10.0 | 0.03 | 0.403 |
| Skinfold Sum (mm) | 228.9 ± 72.7 | 221.7 ± 65.5 | 0.10 | 0.283 |
| Waist Circumference (cm) | 106.9 ± 12.1 | 103.8 ± 12.0 | 0.26 | 0.007 |
| Glucose (mg/dL) | 99 ± 16 | 99 ± 13 | 0.03 | 0.940 |
| Insulin (mg/dL) | 13 ± 11 | 13 ± 8 | 0.02 | 0.870 |
|
| ||||
| Female ( n = 10) | Pre | Post | d | p value |
| Body Mass (kg) | 74.7 ± 19.7 | 75.5 ± 19.8 | −0.04 | 0.232 |
| Lean Body Mass (kg) | 45.1 ± 6.3 | 46.3 ± 6.3 | 0.19 | 0.000 |
| Percent Fat | 37.6 ± 10.1 | 36.7 ± 10.0 | 0.09 | 0.055 |
| Skinfold Sum (mm) | 279.3 ± 87.4 | 268.6 ± 76.6 | 0.13 | 0.305 |
| Waist Circumference (cm) | 103.5 ± 16.6 | 100.7 ± 18.6 | 0.16 | 0.227 |
| Glucose (mg/dL) | 94 ± 12 | 101 ± 9 | −0.70 | 0.009 |
| Insulin (mg/dL) | 10 ± 5 | 8 ± 7 | 0.32 | 0.007 |
Subject Selection
Nineteen subjects (9 males and 10 females) age 18–55. Subjects were split between 2 groups, consisting of 10 subjects at-risk for Type II diabetes (5m & 5f) and 9 non-metabolic syndrome subjects (4m & 5f). At-risk subjects were required to meet at least three of the five metabolic syndrome risk factors as defined by the International Diabetes Federation (1, 21). Subjects were recruited through printed ads and news releases in local news outlets. The research and consent documents were approved by the East Tennessee State University Institutional Review Board. Each subject gave signed informed consent. The exercise program was supervised by faculty and graduate students from the ETSU Department of Exercise and Sport Science. All training was performed in the ETSU Exercise and Sport Sciences Laboratory.
Procedures
Training Protocol
The study protocol consisted of 1 week of pre-intervention low volume and very low intensity (≤ 20 kg) exercise that served as an orientation and familiarization with the exercises chosen for the study. Additionally pre-intervention testing was also performed at the end of this week. Eight weeks of closely monitored resistance training followed (Table 2). The training consisted of two phases: Phase 1: weeks 1–4 used relatively light loads, high repetitions and emphasized strength-endurance and basic fitness; and Phase 2: Weeks 5–8 used heavier loading and fewer repetitions and emphasized maximum strength and power training. Loads were increased in weeks 1 through 4 by approximately 5–10% each week. Weeks 5 through 8 consisted of increased loading and increased training intensity. Similarly, loads were increased each week during weeks 5 through 7 by approximately 5–10% and decreased during the 8th week to allow for dissipation of fatigue (17, 18, 54). Post-testing was performed during the 9th week, 2–3 days after the last workout session. Exercises emphasized large muscle group, multi-joint movements. During weeks 2–8, in order to reduce the overreaching – overtraining potential, Fridays’ loads were always reduced by 15 – 20 % (compared to Monday) and during weeks 5–8 Tuesdays’ loads were reduce by 15–20% compared to Thursday. Higher velocity whole body power training was emphasized during the mid-thigh pulls, squat press, and vertical jumps. Additionally, higher velocity higher power was emphasized during the light days (17, 18). Subjects exercised 6 days per week with 3 to 4 of those days being dedicated to resistance exercises and 1 to 2 days dedicated to mid-section (abdominal) work or stretching. Rest between sets was self-selected, during spot checks by the investigators, and were typically just over 3 min during the first block and just under 3 min during the 2nd block.
Table 2.
Training Protocol
| Phase I (Weeks 1–4) 10 repetitions x 3sets | ||||||
|---|---|---|---|---|---|---|
| Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | |
| 1 | Squats | Bent-legged Sit-ups | Shoulder Shrugs | Bent-legged Situps | Squats | Vertical Jumps |
| 2 | Bench Press | Supine Windshield Wipers | Mid-thigh Pulls | Supine Windshield Wipers | Bench Press | Stretch |
| 3 | Seated Dumbbell Press | Stretch | Stiff-legged Deadlifts | Stretch | Seated Dumbbell Press | |
| 4 | Front Raises | Bent-over rows | Front Raises | |||
| 5 | - | - | Bicep Curls | - | - | - |
| Phase II (Weeks 5–8) 5 repetitions x 3sets | ||||||
|---|---|---|---|---|---|---|
| Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | |
| 1 | Squats | Shoulder Shrugs | Baskethangs 3x10 | Shoulder Shrugs | Squats | Stretch |
| 2 | Squat Press | Mid-thigh Pulls | Supine Windshield Wipers 3x10 | Mid-thigh Pulls | Squat Press | |
| 3 | Dumbbell Incline Press | Stiff-legged Deadlifts | Stretch | Stiff-legged Deadlifts | Dumbbell Incline Press | |
| 4 | Lateral Raises | Upright Row | Upright Row | Lateral Raises | ||
| 5 | - | Assisted Pull-ups | - | Assisted Pull-ups | - | - |
Subjects were weighed prior to each supervised training session and were encouraged to maintain the same weight throughout the study. Each training session, except Saturday, was closely monitored by investigators and any injury was noted. Subjects were questioned as to compliance with Saturday’s training session. Days missed were recorded; days missed were made up when possible. Only 3 subjects missed a day of training (2 MS and 1 NMS); 3 days were missed in total. No subject missed more than 1 day. No days were missed due to injury. All sets and repetitions and loads were recorded in order to calculate an estimate of work, volume load (sets x repetitions x load).
Apparatus and Test Protocol
Body Composition: Body mass was measured on a calibrated and certified digital scale to the nearest 0.1 kg and height was measured to the nearest 0.5 cm using a stadiometer. Body composition was measured using air displacement plethysmography (BodPod, Concord, CA) Also, a seven-site skinfold measurement was performed on each subject by an experienced technician (Lange Skinfold Caliper, Beta Technology Inc, Cambridge, MD). Waist circumference was measured just above the iliac crest to the nearest 0.1cm. As a precaution, on testing days, urinary specific gravity (USG) was measured for all subjects as an estimate of hydration status. If the USG was ≥1.020 the subjects were asked to drink 3–4 glasses of water before testing; USG was re-measured after 30 min, if USG was ≤ 1.020 testing proceeded. If the USG was still ≥ 1.020 more water was ingested and the subject re-tested every 30 min until a USG of < 1.020 was obtained. Although this procedure may not ensure complete hydration, the re-hydration process was started. Testing then proceeded as follows:
Jumps
Prior to testing each subject performed a standardized warm-up procedure consisting of 25 jumping jacks followed by one set of five mid-thigh pulls with a 20kg bar (Werksan Inc, Turkey). The subjects then performed three sets of five mid-thigh pulls with either 40kg for women or 60kg for men (34).
Jump height was used to determine power development and explosiveness using previously described methods (34). Following the warm-up, two types of jumps were performed (static [SJ] and countermovement [CMJ]). Briefly, the SJ condition was performed on a 91.4 x 91.4 cm force plate (Rice Lake Weighing Systems, Rice Lake, WI, USA) sampling at 1000 Hz. Subjects held a bar on their back just below the seventh cervical vertebrae to control for arm swing and to facilitate uniform technique. Subjects were instructed to squat to a knee angle of 90° as previously measured with a goniometer; a 3-second countdown was given followed by the “Jump” command. Two unloaded practice jumps were performed followed by two full effort trials. Subjects performed the jumps using two different external loads (0kg PVC pipe and 20kg barbell) with each subject performing two trials for each load. One minute of rest was given after the first trial and after each subsequent trial. Jump height and power output were recorded and averaged for the two trials under each respective loading condition.
CMJ condition trials were performed with the same loading procedures and same test equipment as mentioned above. However, during the CMJ trials the subjects started from a standing position. Subjects were given a 3-second countdown followed by the “Jump” command. CMJ trials were performed without a pause to a self-selected depth of knee and hip flexion. Subjects were allowed two trials for each load (0kg and 20kg), with 1 minute of rest given between trials. If any SJ or CMJ was perceived to be less than a maximal effort, the jump was repeated. Two maximum effort SJs and CMJs were recorded. Henry (25) and Stone and Sands (53) note that using the mean of two trials instead of the best effort can help give a clearer picture of an athlete’s typical performance and obviate some intra-subject variance problems. Jump height (JH), peak force (PF), rate of force development (RFD), peak velocity (PV), peak power (PP), and impulse (IM) were calculated for each jump using customized Labview software (National Instruments Co., Austin, TX).
Jump height was derived from flight time using the formula: Jump height = (g x flight time x flight time)/8. Previously established test-retest reliability for JH in our lab using athletes was ICCα = 0.98 (n = 128, CMJ) and ICCα = 0.96 (n = 128, SJ). Intraclass correlations for PF, RFD, PV, PP, and IM were ICCα > 0.9 for both types of jumps (n = 128).
Maximum Isometric Strength
Maximum strength was measured with an isometric mid-thigh pull (IMTP) using previously established testing protocols (23). Briefly, all isometric tests were performed on a custom built isometric rack that allows the bar to be fixed at any height above the floor using a combination of pins and hydraulic jacks. The isometric rack is anchored to the floor and placed over a 91.4 x 91.4 cm force plate (Rice Lake Weighing Systems, Rice Lake, WI, USA) sampling at 1000 Hz. A nonflexible steel bar was fixed in a static position above the force plate at a height which allowed each subject to attain an initial mid-thigh pull position similar to the one used for mid-thigh pulls in training (knee angle between 120°–135° and hip angle between 170°–175°). Knee and hand placement were similar to that used in clean pulls during training. The subjects’ hands were taped to the bar to control for grip strength. Each subject was allowed two warm up pulls (one at 50% and one at 75% of their perceived maximal effort) followed by two maximal effort pulls. A third maximal effort pull was performed by subjects who either performed a countermovement prior to the isometric pull or if a difference ≥250 N existed between the first two pulls. The two best trials were average and used for further analysis. Isometric Peak force (IPF) and isometric rate of force development (IRFD) were derived from force-time curves. For additional comparisons of strength across groups, allometric scaling (IPFa) was used, (IPF x body mass −0.67). Previously established reliability for this system showed excellent results (n = 200). Intra class correlations (ICCα) were, IPF, r > 0.99, IRFD r > 0.90.
Measures of Aerobic Fitness
Peak V̇O2 was assessed using a Monark Ergomedic 874E cycle ergometer (Monark Exercise AB, Vansbro, Sweden). All endurance testing was performed 20–24 h after all measures of explosiveness and maximum strength. After a brief warm-up with no resistance, a graded exercise test was performed using a progressive loading protocol until exhaustion. Each stage was 2 minutes in duration. Resistance began with 0.5kg and progressed by 0.5kg every 2 minutes until a resistance load of 1.5kg was reached. Thereafter, resistance was increased in increments of 0.2kg every 2 minutes until exhaustion. Expired gases were collected and analyzed using a ParvoMedics 2400 Metabolic system (ParvoMedics, Sandy, Utah, USA). Heart rate, V̇O2 peak (ml/kg/min), respiratory exchange ratio (RER), and time to exhaustion were recorded.
V̇O2 peak was considered to be achieved by meeting two of three requirements: a respiratory exchange ratio ≥ 1.05, a plateau in oxygen consumption, or achievement of estimated maximum heart rate (45).
Blood Pressure
Blood pressure was determined by a trained technician using a sphygmomanometer (Trimline, Somerville, NJ) and manual auscultation. All blood pressures were measured from the left arm at heart level after 5 minutes of quiet sitting before any warm-up, exercise, or other testing were performed.
Blood Lipids, Fasting Insulin, and Glucose
Measures of lipids, glucose, and insulin were performed after a minimum of 8 hours fasting. Lipids measured were total cholesterol (TC), high-density lipoprotein cholesterol (HDL), low density lipoprotein cholesterol (LDL), and triglycerides. These variables were measured at the ETSU Clinical Reference Laboratory.
Muscle Biopsy and Analyses
Biopsies were performed pre and post training. Percutaneous needle biopsies of right side vastus lateralis were performed after an overnight fast and 2 hours of quiet recumbency using a Bergstrom-Stille 5 mm muscle biopsy needle with suction (55). The second biopsy (post training) was performed 24–48 hours after the last training session. The muscle sample was mounted on cork and quickly frozen in an isopentane slurry cooled in liquid nitrogen. The cork-mounted piece was stored at −80°C and later sectioned on a cryostat (Leica, Wetzlet, Germany) for evaluation of fiber type composition.
Fiber composition was determined using methods described by Behan et al. (8). Muscle sections were stained for light microscopy in a 2-step method using commercial monoclonal antibodies to fast and slow isoforms of myosin heavy chain. After acetone fixation and incubation with 20% normal rabbit serum, the slow myosin antibody (Sigma clone NOQ7.5.4D, Sigma-Aldrich, St. Louis, MO) was applied, followed by a peroxidase-conjugated rabbit anti-mouse IgG antibody. The fast myosin antibody (Sigma clone MY-32 alkaline phosphatase conjugate) was then applied (8). Slides were alcohol dehydrated, cleared with xylene, and preserved in synthetic medium. This technique allows discrimination of type I, type IIa, and type IIx muscle fibers. All sections were coded and then quantified independently by 3 observers who were unaware of which subject or treatment the image represented. The observers’ data was averaged for statistical analyses.
Statistical Analysis
As this study was considered exploratory in nature and was atypical in that a unique approach to training was used, a somewhat less stringent statistical approach was used (28). Statistical analyses were performed using SPSS statistical analysis software (V.17). A 2x2x2 (group x sex x trial) repeated measures ANOVA was performed; follow-ups were performed using paired t-test (p ≤ 0.05). Effect sizes were calculated using Cohen’s d. Data are displayed as mean ± standard deviation, except where indicated otherwise.
RESULTS
Group Effects
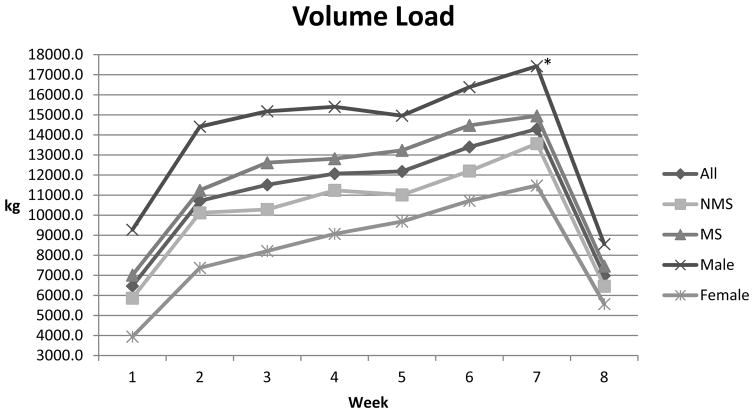
As a combined group (n = 19) statistically significant effects were noted over time (T1–T2) for several body composition variables (Table 1) such as body mass, lean body mass, percent fat, and waist circumference. These positive alterations in body composition occurred despite an increase in body mass. Performance variables such as isometric peak force (IPF), allometrically scaled isometric peak force (IPFa), rate of force development (RFD) (Table 3), and volume load (Figure 1); and all static and countermovement variables showed statistical improvement (Table 4). In addition, both V̇O2 peak and time to exhaustion showed statistically significant increases over time (Table 3). Blood pressure, total cholesterol, resting glucose, and insulin generally showed trends toward positive alterations over time but did not achieve statistical significance (Tables 1 and 5).
Table 3.
Strength and Endurance Performance Characteristics Over time (Mean ± SD)
| All ( n = 19) | Pre | Post | d | p value |
| Isometric Peak Force (N) | 2601 ± 963 | 2968 ± 1079 | 0.36 | 0.000 |
| IPFa (N/kg0.67) | 135 ± 37 | 152 ± 43 | 0.42 | 0.001 |
| Rate of Force Development (N/s) | 3665 ± 3132 | 4274 ± 3151 | 0.19 | 0.044 |
| VO2 peak (mL/kg/min) | 27.0 ± 5.4 | 29.5 ± 5.7 | 0.45 | 0.001 |
| Time to Exhaustion (min:sec) | 6:30 ± 2:04 | 8:48 ± 3:04 | 0.88 | 0.000 |
|
| ||||
| MS ( n = 9) | Pre | Post | d | p value |
| Isometric Peak Force (N) | 2910 ± 1037 | 3317 ± 1025 | 0.39 | 0.000 |
| IPFa (N/kg0.67) | 135 ± 40 | 153 ± 36 | 0.47 | 0.001 |
| Rate of Force Development (N/s) | 4098 ± 4198 | 4800 ± 4027 | 0.17 | 0.069 |
| VO2 peak (mL/kg/min) | 23.3 ± 3.1 | 26.0 ± 3.9 | 0.77 | 0.006 |
| Time to Exhaustion (min:sec) | 6:37 ± 1:11 | 9:02 ± 2:23 | 1.28 | 0.003 |
|
| ||||
| NMS (n = 10) | Pre | Post | d | p value |
| Isometric Peak Force (N) | 2257 ± 790 | 2581 ± 1058 | 0.35 | 0.033 |
| IPFa (N/kg0.67) | 134 ± 37 | 151 ± 51 | 0.38 | 0.074 |
| Rate of Force Development (N/s) | 3184 ± 1321 | 3689 ± 1832 | 0.32 | 0.322 |
| VO2 peak (mL/kg/min) | 31.0 ± 4.5 | 33.4 ± 4.9 | 0.51 | 0.059 |
| Time to Exhaustion (min:sec) | 6:21 ± 2:49 | 8:33 ± 3:49 | 0.66 | 0.021 |
|
| ||||
| Male ( n = 9) | Pre | Post | d | p value |
| Isometric Peak Force (N) | 3335 ± 776 | 3774 ± 817 | 0.55 | 0.001 |
| IPFa (N/kg0.67) | 161 ± 24 | 180 ± 23 | 0.81 | 0.001 |
| Rate of Force Development (N/s) | 5395 ± 3864 | 6234 ± 3536 | 0.23 | 0.104 |
| VO2 peak (mL/kg/min) | 29.1 ± 4.8 | 31.9 ± 4.0 | 0.63 | 0.013 |
| Time to Exhaustion (min:sec) | 7:22 ± 2:11 | 10:23 ± 3:25 | 1.05 | 0.000 |
|
| ||||
| Female ( n = 10) | Pre | Post | d | p value |
| Isometric Peak Force (N) | 1940 ± 544 | 2243 ± 709 | 0.48 | 0.013 |
| IPFa (N/kg0.67) | 111 ± 30 | 127 ± 40 | 0.45 | 0.003 |
| Rate of Force Development (N/s) | 2108 ± 809 | 2509 ± 1199 | 0.39 | 0.281 |
| VO2 peak (mL/kg/min) | 25.1 ± 5.5 | 27.3 ± 6.3 | 0.37 | 0.034 |
| Time to Exhaustion (min:sec) | 5:42 ± 1:41 | 7:23 ± 1:54 | 0.94 | 0.048 |
Figure 1.
Volume Load by week. kg = average volume load (repetitions x load); * females increased loading at a faster rate than males (p ≤ 0.05)
Table 4.
Jump Performance Characteristics Over time (Mean ± SD)
| All ( n = 19) | Pre | Post | d | p value |
| SJ Height 0kg (cm) | 16.6 ± 6.1 | 19.8 ± 5.4 | 0.56 | 0.000 |
| SJ Height 20kg (cm) | 10.1 ± 5.4 | 13.9 ± 4.8 | 0.74 | 0.000 |
| SJ Height Percent Difference | −36.7 ± 13.4 | −30.6 ± 7.7 | 0.56 | 0.012 |
| SJ Peak Power 0kg (W) | 2585 ± 1083 | 3303 ± 1079 | 0.66 | 0.000 |
| SJ Peak Power 20kg (W) | 2143 ± 726 | 2975 ± 1072 | 0.91 | 0.000 |
| CMJ Height 0kg (cm) | 19.7 ± 6.1 | 21.6 ± 5.6 | 0.32 | 0.007 |
| CMJ Height 20kg (cm) | 12.6 ± 5.6 | 15.2 ± 5.7 | 0.46 | 0.000 |
| CMJ Height Percent Difference | −37.3 ± 15.3 | −30.7 ± 14.0 | 0.45 | 0.003 |
| CMJ Peak Power 0kg (W) | 3445 ± 1264 | 3986 ± 1302 | 0.42 | 0.012 |
| CMJ Peak Power 20kg (W) | 2865 ± 941 | 3714 ± 1335 | 0.74 | 0.000 |
|
| ||||
| MS ( n = 9) | Pre | Post | d | p value |
| SJ Height 0kg (cm) | 16.1 ± 6.4 | 19.2 ± 5.6 | 0.52 | 0.000 |
| SJ Height 20kg (cm) | 10.9 ± 5.6 | 14.3 ± 5.0 | 0.64 | 0.000 |
| SJ Height Percent Difference | −34.8 ± 12.6 | −26.5 ± 5.6 | 0.85 | 0.022 |
| SJ Peak Power 0kg (W) | 2640 ± 1255 | 3485 ± 1162 | 0.70 | 0.001 |
| SJ Peak Power 20kg (W) | 2055 ± 649 | 3192 ± 1154 | 1.21 | 0.001 |
| CMJ Height 0kg (cm) | 19.2 ± 6.1 | 21.0 ± 5.0 | 0.32 | 0.065 |
| CMJ Height 20kg (cm) | 12.9 ± 5.6 | 15.9 ± 5.1 | 0.56 | 0.002 |
| CMJ Height Percent Difference | −34.3 ± 13.7 | −25.4 ± 8.8 | 0.77 | 0.016 |
| CMJ Peak Power 0kg (W) | 3636 ± 975 | 4408 ± 1109 | 0.74 | 0.007 |
| CMJ Peak Power 20kg (W) | 3068 ± 903 | 4135 ± 1117 | 1.05 | 0.000 |
|
| ||||
| NMS (n = 10) | Pre | Post | d | p value |
| SJ Height 0kg (cm) | 17.2 ± 6.0 | 20.4 ± 5.5 | 0.56 | 0.003 |
| SJ Height 20kg (cm) | 10.9 ± 5.4 | 13.5 ± 4.8 | 0.51 | 0.004 |
| SJ Height Percent Difference | −38.8 ± 14.6 | −35.1 ± 7.3 | 0.32 | 0.276 |
| SJ Peak Power 0kg (W) | 2344 ± 863 | 2814 ± 768 | 0.58 | 0.007 |
| SJ Peak Power 20kg (W) | 2102 ± 841 | 2474 ± 743 | 0.47 | 0.013 |
| CMJ Height 0kg (cm) | 20.3 ± 6.4 | 22.3 ± 6.5 | 0.31 | 0.075 |
| CMJ Height 20kg (cm) | 12.3 ± 6.0 | 14.4 ± 6.5 | 0.34 | 0.006 |
| CMJ Height Percent Difference | −40.6 ± 17.0 | −36.7 ± 16.7 | 0.23 | 0.010 |
| CMJ Peak Power 0kg (W) | 3234 ± 1559 | 3518 ± 1401 | 0.19 | 0.393 |
| CMJ Peak Power 20kg (W) | 2639 ± 982 | 3248 ± 1463 | 0.49 | 0.026 |
|
| ||||
| Male ( n = 9) | Pre | Post | d | p value |
| SJ Height 0kg (cm) | 21.1 ± 4.4 | 23.8 ± 3.8 | 0.67 | 0.009 |
| SJ Height 20kg (cm) | 15.2 ± 3.9 | 17.7 ± 3.6 | 0.67 | 0.003 |
| SJ Height Percent Difference | −29.0 ± 5.4 | −26.1 ± 6.6 | 0.48 | 0.086 |
| SJ Peak Power 0kg (W) | 3409 ± 979 | 4169 ± 815 | 0.84 | 0.013 |
| SJ Peak Power 20kg (W) | 2728 ± 493 | 3817 ± 875 | 1.53 | 0.013 |
| CMJ Height 0kg (cm) | 23.9 ± 3.6 | 25.8 ± 4.8 | 0.45 | 0.111 |
| CMJ Height 20kg (cm) | 17.3 ± 3.3 | 19.7 ± 4.2 | 0.64 | 0.003 |
| CMJ Height Percent Difference | −28.1 ± 8.5 | −23.6 ± 6.3 | 0.60 | 0.057 |
| CMJ Peak Power 0kg (W) | 4375 ± 1104 | 4993 ± 889 | 0.62 | 0.142 |
| CMJ Peak Power 20kg (W) | 3592 ± 615 | 4765 ± 833 | 1.60 | 0.001 |
|
| ||||
| Female ( n = 10) | Pre | Post | d | p value |
| SJ Height 0kg (cm) | 12.6 ± 4.2 | 16.1 ± 3.8 | 0.87 | 0.000 |
| SJ Height 20kg (cm) | 7.1 ± 3.1 | 10.5 ± 2.5 | 1.21 | 0.000 |
| SJ Height Percent Difference | −43.6 ± 14.8 | −34.6 ± 6.4 | 0.79 | 0.042 |
| SJ Peak Power 0kg (W) | 1843 ± 453 | 2524 ± 563 | 1.33 | 0.000 |
| SJ Peak Power 20kg (W) | 1617 ± 432 | 2216 ± 519 | 1.25 | 0.000 |
| CMJ Height 0kg (cm) | 15.9 ± 5.3 | 17.9 ± 3.1 | 0.46 | 0.041 |
| CMJ Height 20kg (cm) | 8.5 ± 3.5 | 11.0 ± 3.1 | 0.76 | 0.003 |
| CMJ Height Percent Difference | −45.6 ± 15.5 | −37.2 ± 16.1 | 0.53 | 0.025 |
| CMJ Peak Power 0kg (W) | 2609 ± 688 | 3080 ± 873 | 0.60 | 0.015 |
| CMJ Peak Power 20kg (W) | 2210 ± 655 | 2769 ± 922 | 0.70 | 0.009 |
Table 5.
Resting Physiological Characteristics Over time (Mean ± SD)
| All ( n = 19) | Pre | Post | d | p value |
| Systolic Blood Pressure (mmHg) | 123 ± 18 | 121 ± 12 | 0.13 | 0.642 |
| Diastolic Blood Pressure (mmHg) | 80 ± 9 | 79 ± 8 | 0.12 | 0.669 |
| Total Cholesterol (mg/dL) | 178 ± 41 | 170 ± 35 | 0.15 | 0.244 |
| HDL (mg/dL) | 44 ± 11 | 42 ± 11 | 0.13 | 0.093 |
| LDL (mg/dL) | 101 ± 34 | 99 ± 33 | 0.04 | 0.733 |
| Triglycerides (mg/dL) | 159 ± 135 | 142 ± 86 | 0.14 | 0.387 |
| TC:HDL | 4.3 ± 1.5 | 4.3 ± 1.7 | 0.00 | 0.700 |
|
| ||||
| MS ( n = 9) | Pre | Post | d | p value |
| Systolic Blood Pressure (mmHg) | 131 ± 20 | 129 ± 6 | 0.14 | 0.618 |
| Diastolic Blood Pressure (mmHg) | 82 ± 9 | 84 ± 6 | −0.26 | 0.686 |
| Total Cholesterol (mg/dL) | 192 ± 49 | 183 ± 38 | 0.19 | 0.298 |
| HDL (mg/dL) | 40 ± 9 | 39 ± 9 | 0.13 | 0.434 |
| LDL (mg/dL) | 112 ± 41 | 109 ± 38 | 0.08 | 0.600 |
| Triglycerides (mg/dL) | 198 ± 173 | 175 ± 101 | 0.16 | 0.519 |
| TC:HDL | 5.0 ± 1.5 | 5.1 ± 1.8 | 0.06 | 0.846 |
|
| ||||
| NMS (n = 10) | Pre | Post | d | p value |
| Systolic Blood Pressure (mmHg) | 113 ± 10 | 113 ± 11 | 0.00 | 0.900 |
| Diastolic Blood Pressure (mmHg) | 77 ± 10 | 74 ± 7 | 0.35 | 0.161 |
| Total Cholesterol (mg/dL) | 158 ± 19 | 155 ± 27 | 0.12 | 0.643 |
| HDL (mg/dL) | 48 ± 11 | 46 ± 11 | 0.15 | 0.010 |
| LDL (mg/dL) | 87 ± 16 | 88 ± 23 | −0.03 | 0.921 |
| Triglycerides (mg/dL) | 114 ± 52 | 106 ± 51 | 0.16 | 0.383 |
| TC:HDL | 3.5 ± 1.1 | 3.6 ± 1.1 | 0.09 | 0.698 |
|
| ||||
| Male ( n = 9) | Pre | Post | d | p value |
| Systolic Blood Pressure (mmHg) | 123 ± 8 | 124 ± 9 | −0.12 | 0.583 |
| Diastolic Blood Pressure (mmHg) | 81 ± 6 | 80 ± 5 | 0.18 | 0.587 |
| Total Cholesterol (mg/dL) | 174 ± 31 | 176 ± 37 | −0.07 | 0.715 |
| HDL (mg/dL) | 38 ± 11 | 38 ± 12 | 0.01 | 0.976 |
| LDL (mg/dL) | 110 ± 31 | 110 ± 39 | 0.00 | 1.000 |
| Triglycerides (mg/dL) | 127 ± 50 | 139 ± 78 | −0.18 | 0.583 |
| TC:HDL | 4.8 ± 1.4 | 5.1 ± 1.9 | 0.18 | 0.366 |
|
| ||||
| Female ( n = 10) | Pre | Post | d | p value |
| Systolic Blood Pressure (mmHg) | 123 ± 25 | 119 ± 13 | 0.20 | 0.484 |
| Diastolic Blood Pressure (mmHg) | 79 ± 12 | 78 ± 10 | 0.09 | 0.901 |
| Total Cholesterol (mg/dL) | 177 ± 49 | 164 ± 34 | 0.31 | 0.085 |
| HDL (mg/dL) | 49 ± 8 | 46 ± 8 | 0.32 | 0.003 |
| LDL (mg/dL) | 92 ± 35 | 89 ± 24 | 0.08 | 0.645 |
| Triglycerides (mg/dL) | 187 ± 179 | 145 ± 97 | 0.29 | 0.178 |
| TC:HDL | 3.8 ± 1.5 | 3.7 ± 1.1 | −0.08 | 0.446 |
Group Skeletal Muscle Fiber Composition
Muscle biopsies of the vastus lateralis were performed at baseline and after training. Fiber CSA alterations resulting from training did not reach statistical significance for any grouping, (MS Vs NMS or male versus female). However there was a small-moderate effect size (d = 0.40) for Type IIx fibers over time for the whole group (n = 19) suggesting a small gain in IIx CSA. Training tended to cause a shift in fiber composition from type IIx to type IIa in some subjects; however, the effect sizes (n = 19) were trivial.
Metabolic Syndrome Versus Non-metabolic Syndrome
As shown in Figures 2a and 2b and 3a and 3b, compared to NMS, the MS subjects (n =10) tended to have a larger mean muscle fiber CSA (73.6 ±17.5 vs 66.5± 14.3 μM; p = 0.18; d = 0.42) and had a lower percentage of type I muscle fibers than NMS (n = 9) at baseline (36.3 ± 10.2% vs. 50.0 ± 17.7%, p = 0.03; d= 7.9). Although not statistically significant, data suggests that the MS group tended to possess a greater percentage of type II fibers (65.9 ± 10.2% vs 52.9 ± 12.5%; p = 0.22; d = 1.2), particularly type IIx fibers
Figure 2.
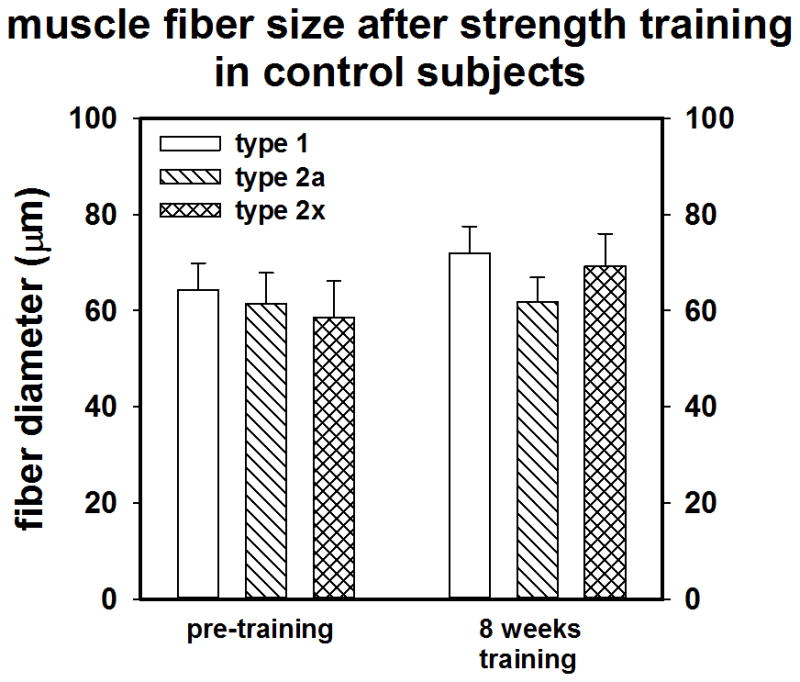
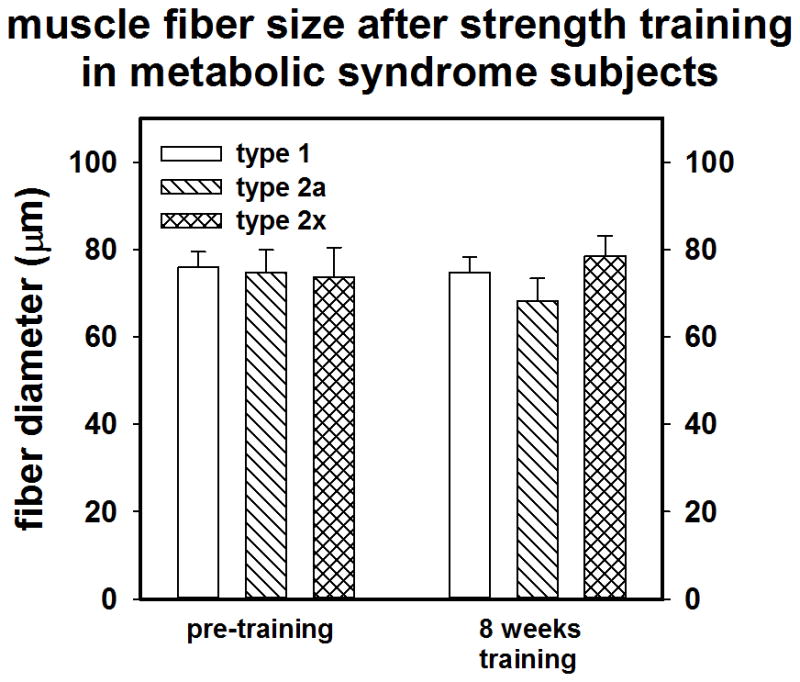
A) Pre-post training muscle fiber CSA of NMS (control). B) muscle fiber CSA of MS.
Figure 3.
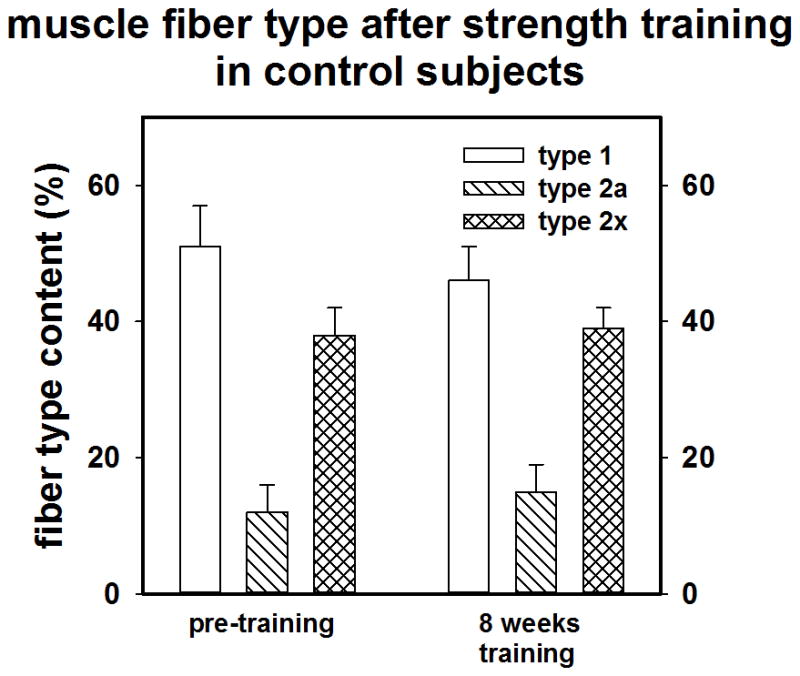
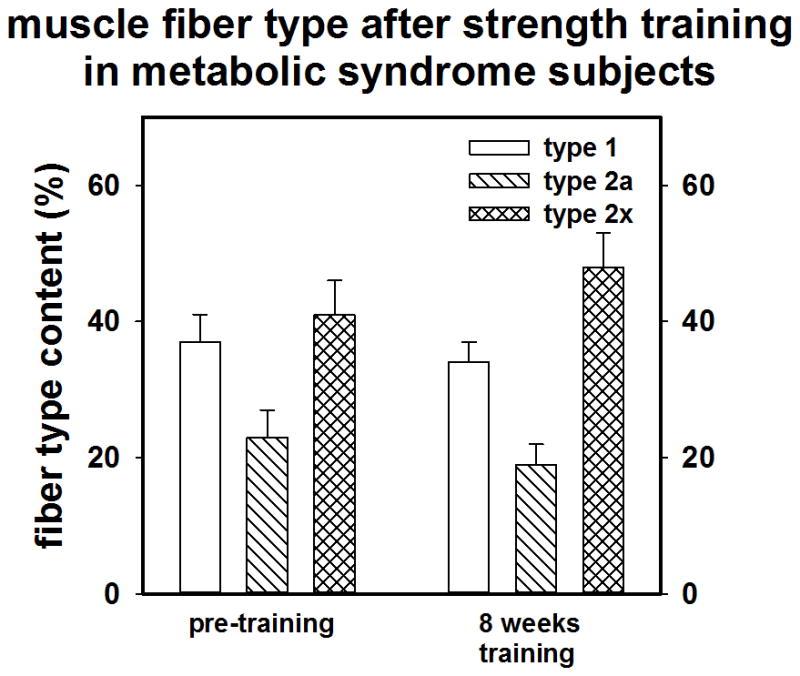
A) Pre-post training muscle fiber type of NMS (control). B) muscle fiber type of MS.
The metabolic syndrome (MS) group (n=10) and the non-metabolic syndrome (NMS) group (n = 9) showed an initial statistical difference for several body composition variables (Table 1). The metabolic syndrome group had higher values for body mass, lean body mass, percent fat, skinfold sum, and waist circumference. MS subjects also tended to have higher values for several performance variables such as IPF, RFD (Table 3), and static jump peak power at 0 and 20kg (Table 4) while having statistically significant lower values for V̇O2 peak (Table 3). Additionally, the MS subjects had higher values for several physiological variables including systolic and diastolic blood pressure and total cholesterol to HDL ratio (Table 5), and insulin (Table 1). These data generally indicate that: 1) the MS subjects had higher levels of body fat and less favorable body composition values compared to sedentary controls, 2) MS subjects had higher levels of lean body mass with concurrently higher levels of absolute strength and some measures of power when compared to NMS subjects, 3) MS subjects were less aerobically fit when compared to their NMS counterparts and 4) MS subjects had substantially higher values for several physiologically related metabolic syndrome risk factors.
Neither the MS nor the NMS group reached statistical significance for change in body mass, but showed improvements over time for body composition variables (Table 1) maximum strength (Table 3) jump variables (Table 4), V̇O2 peak and time to exhaustion (TTE) (Table 3). Further, variables relating to health parameters, blood lipids, glucose, and insulin, showed no statistically significant alterations. However, in the NMS group both HDL and insulin showed a statistically significant decrease (Tables 1 and 5). Interestingly, there was a trend toward increased blood glucose in the NMS (d = 0.84).
Alterations over time resulted in few statistical differences between MS and NMS subjects. These differences included fasting glucose (Table 1) and static jump peak power at 0kg and 20kg external load (Table 4). These data suggest that short-term resistance training can lead to improvements in body composition, strength and explosiveness levels, jump heights, power outputs, and measures of aerobic fitness regardless of metabolic risk factors in previously sedentary middle-aged adults.
Males Versus Females
Statistically significant differences were observed between males (n = 9) and females (n = 10) for several measures in this study. As expected, mean fiber CSA (and the CSA of the individual fibers types) were larger in the males (Mean CSA: 79.2 ± 14.3 vs 61.8 vs 13.8 μM, p = 0.002, d = 1.3). No other statistically significant differences in fiber type were noted.
Males had higher body mass and higher lean body mass when compared to females (Table 1). Males also displayed higher levels of strength, both absolutely and scaled, and higher levels of explosiveness (RFD) when compared to females (Table 3). Accordingly, males also displayed greater jumping ability, power output for both static and countermovement jumps at both 0 and 20 kg external load, smaller differences in jump height between loading conditions (Table 4), and used a greater volume load in training (Figure 1). Further, while not statistically significant, males tended to have higher values for measures of aerobic fitness and TTE (Table 3). HDL values were higher in female subjects and LDL values were lower when compared to males (Table 5). These data indicate that sedentary male subjects were larger, stronger (absolutely and scaled), more explosive, and more aerobically fit when compared to sedentary females.
Over time, statistically significant differences between males and females were evident for two factors measured in this study. Males increased peak power output over time for the CMJ 20kg loading condition. Also, statistically different increases in volume load were observed between males and females. Females increased volume load at a faster rate than did male subjects (Figure 1). Higher training volumes may have led to the larger effect sizes compared to males suggesting beneficial alterations in females for factors such as HDL, triglycerides, and insulin (Tables 1 and 5).
DISCUSSION
This exploratory study suggests that a unique approach to resistance training among a sedentary adult population can affect beneficial alterations in health-related parameters, and particularly, performance variables over a relatively short training period (8 weeks). Furthermore, this training program resulted in no injuries and a 99% adherence rate. Anecdotally, all subjects reported that their daily living activities were easier to perform and they generally had feelings of greater energy levels. Further, the subjects reported that the training sessions were enjoyable and that they looked forward to the sessions.
In the present study, alterations in body mass and body composition were similar to those noted in other short-term training programs using untrained adults (10, 32). Obesity (excess fat content) often accompanies the sedentary lifestyle. As is the case with other physical fitness characteristics, obesity typically begins in childhood and is related to a variety of environmental and genetic factors. Obese children often become obese adults. In the development of child to adult, if obesity is not obviated, there is a strong potential for the development of obesity-related inflammation, metabolic syndrome, Type II Diabetes, cardiovascular disease, certain types of cancer, and arthritis (12, 31). Interestingly, the gain in LBM encompassed all groups but loss of percent fat was most prominent among the females, which could be related to their faster gain in training volume load, which may reflect greater relative increase in energy expenditure.
Sarcopenia, particularly for type II fibers is a hallmark of inactivity and aging (14, 44, 58). Indeed the development of comorbidities such as kidney disease, atherosclerosis, and diabetes, which may contribute to accelerated sarcopenia, are not uncommon as the population ages (14, 44). Sarcopenia is related to a decrease in life quality and can lead to substantial loss of strength and power and eventually frailty (14). Thus developing superior methods of obviating the typical age related development of sarcopenia would be beneficial. While strength training can result in substantial muscle hypertrophy and increases in lean body mass, muscle fiber CSA alterations can be difficult to detect in only a few weeks (16). The 8 week training program did tend to cause a small increase in fiber CSA particularly in type IIx fibers. Although, the MS subjects had a higher initial proportion of type II fibers, which might have potentiated adaptations to strength training, this study did not find a distinct training advantage for this group.
The training induced alteration in body composition may be explained by several factors. First, activation of hypertrophic mechanisms such as the mTOR intracellular signaling system can stimulate protein synthesis and produce increases in LBM (11, 36). Second, resistance training may produce a substantial total (exercise + recovery) energy consumption and an increased lipid oxidation post-exercise (40) that could lead to reductions in body fat. Additionally, from a theoretical standpoint, an increase of 1 kg of muscle can result in an increased resting metabolic rate of about 21 kcal x kg−1 of new muscle (59). The average gain in LBM among the subjects (n = 19) was 1.3 kg; assuming this represents about 0.5 – 1 kg of muscle gain, the additional increase in resting metabolic rate that may have occurred could eventually lead to alterations in body composition including fat loss. Additionally, excess fat and obesity can reduce mechanical and metabolic efficiency (31). For obese people it is more difficult to move about and also, for any given amount of external work, there is typically a greater energy requirement. Indeed people with larger amounts of adipose tissue have to expend more energy due to their mechanically inefficient movement patterns and extra body mass (31, 51). Reduction in body fat and an increase in lean tissue may have contributed to the alterations noted in strength and power production.
Increased strength and power, as noted in the present study, have a strong potential to carry over into athletic as well as daily activities (51, 52). Substantial gains in peak force (maximum strength) and trends toward increased rate of force development (RFD) were noted in all groups. Increased strength is associated with greater explosiveness (RFD) and power and endurance capabilities (5, 48). Increases in maximum strength and related capabilities may explain the increased jump performance and high power outputs noted among the groups, particularly for the loaded jumps (34). Among athletes, Kraska et al. (34) noted that the decrease in jump height and power between unloaded and loaded jumps was smaller among stronger athletes. Kraska et al. (34) also noted that a smaller drop off was potentially related to superior performance in a variety of athletic activities including speed and agility. In the present study an increase in strength over time was also associated with smaller drop offs in unloaded versus loaded jump performance. It should be noted that the training program used in this study elicited gains in a wide variety of performance variables including, maximum strength (IPF and IPFa), explosive strength (RFD) and power output. These data suggest that long-term training of this nature might prevent the loss of LBM and Type II fiber CSA, loss of strength, RFD, and particularly power output that accompanies aging. It is also possible that alterations in these strength related variables represent an adaptation(s) that would relate to superior performance in daily activities that require exertion of force against increased resistance such as rapid stair climbing, recovery of balance, or recreational activities such as playing with children or pets.
Among sedentary adults the ability to use oxygen during work (as measured by V̇O2 peak testing) has been shown to decline by approximately 1% per year after age 30 (27). Furthermore, sedentary lifestyle and factors directly associated with metabolic syndrome such as obesity may further impair oxygen dependent substrate use (60). Therefore, methods of improving V̇O2 peak should be an integral part of any exercise program.
In the past, resistance training was generally considered to have relatively little or no impact on measures of aerobic efficiency (V̇O2 Peak) compared to aerobic training (30). However, based on both descriptive and longitudinal data, reviews of the literature indicate that resistance training can have substantial effects in previously sedentary populations (51). For example, competitive weightlifters and highly trained strength-power athletes have been shown to have average (37) or statistically greater V̇O2 peak compared to sedentary controls (47), suggesting an aerobic training effect.
Several resistance training studies have shown small (< 5%) V̇O2 peak increases over short-term (8–16 weeks) periods among relatively young or middle-aged subjects (26, 49). Among sedentary middle-aged and older subjects in resistance training studies lasting short periods (8–16 weeks) V̇O2 peak has shown increases as much as 6%–12% (24). Generally, these studies indicate that frequency (2 vs. 3 or 4 d/wk) and volume of training play a significant role in V̇O2 peak alterations over time. Circuit weight training using short rest periods and high repetitions is a method of raising metabolism. Typically, somewhat larger increases have been found (V̇O2 peak) when comparing circuit training to traditional methods of resistance training; conversely strength and power gains for circuit training were not as large as those of traditional resistance training (13). Among patients with cardiovascular disease (CVD), circuit weight-training as well as traditional methods of resistance training have shown even larger improvements in V̇O2 peak of as much as 10%–19% (56). Interestingly, resistance training has been shown to be safe and can produce fewer CV abnormalities during training than aerobic training (9). Additionally, resistance training has been shown to produce marked improvements in long-term endurance that are to an extent disassociated from alterations in V̇O2 peak (26, 38, 49). For example, Marcinik and colleagues (38) found increases in endurance on a cycle ergometer (time to failure) of 33% despite no statistically significant alterations in V̇O2 peak. These observations indicate that factors (such as maximum strength) other than the cardiovascular (CV) system are involved in endurance (38, 48).
In the present study V̇O2 peak and TTE on the cycle ergometer showed substantial increases, particularly among the MS group. However, the exact mechanism(s) underlying the alterations in V̇O2 peak and TTE are not clear. The data from this study indicate that short-term resistance training can have a small to moderate effect on V̇O2 peak. The underlying mechanism(s) for improvement, as a result of resistance exercise, are not completely clear but is likely a combination of central and peripheral effects. It is possible that among very sedentary populations, resistance training activates the aerobic system enough to cause adaptation. Of particular interest are the potential effects of intra-cellular signals on “aerobic” adaptations resulting from resistance training. Stimulation of mTOR as a result of hormonal or autocrine and paracrine mechanisms affect increased protein synthesis, tissue remodeling, and hypertrophy (11, 36). AMPK is stimulated by a decreased energy supply (7) and results in mitochondrial biogenesis and a shift of myosin heavy chains from MHC IIx toward MHC I (7, 42). Some studies indicate that AMPK activation can partially deactivate mTOR redirecting energy toward the AMPK pathway (7, 11, 42). Conversely, mTOR may indirectly inhibit AMPK activation through S6K1(36). Although endurance exercise has been associated with AMPK activation and resistance training with activation of mTOR, it is more likely that these pathways are activated within a continuum of energy requirements (36). If the volume of training increases, AMPK is more activated; if the volume decreases and intensity of exercise increases, mTOR’s activation level tends to increase. These observations may partially explain why more dynamic, higher volume resistance training appears to have a greater effect on V̇O2 peak and other endurance-related parameters (51). It is also possible that strength level is a limiting factor among sedentary adults and increases in the ability to produce force allows a “truer” V̇O2 peak to be expressed (51).
Although previous research has demonstrated that resistance training can beneficially alter health-related variables (51), no statistically significant alterations were noted in the present study among blood pressure and blood lipids. However, HDL cholesterol showed a 3.5% decrease in the NMS and females dropped by 5.3 %. Indeed most of the drop in the NMS was due to alterations in the female group. This drop in HDL among the females may be partially explained by a 7.4 % decrease in total cholesterol among the females. Females also showed the largest drop in triglycerides (22%). Interestingly, evidence suggests that lipoprotein-lipid alterations resulting from resistance training are genotype dependent (29). In the present study subjects in all groups, particularly females, tended to show some positive alterations in blood lipids and it is possible that these group changes reflected alterations among individuals with the appropriate genotype. Further study linking genotype to specific lipid alterations is obviously necessary. However, resting glucose and insulin did show alterations over time. Resistance training can affect alterations in insulin sensitivity and GLUT protein concentrations potentially enhancing glucose disposal (36). However, in the present study resting glucose was largely unaltered (n=19) but increased in the non-metabolic syndrome (NMS) group, and among the females a substantial increase in resting glucose was observed. Paradoxically a decrease in insulin was observed in the same groups. While the decreased insulin may represent a beneficial adaptation to the training program (40), there is no clear explanation for this observation. However, it is known that catecholamines antagonize insulin release; it is possible that anxiety related elevations in catecholamines prior to blood collection may have altered glucose and insulin concentrations (40).
It should be noted that the training program lasted only 8 weeks. The amount of work, intensity, and training effort are factors that affect alterations in health-related parameters during resistance training (51). Although the subjects were familiarized with the exercises, the short training period and the initial sedentary nature of the subjects did not allow substantial increases in the volume of work (Figure 1) which may be necessary in creating significant alterations in health related variables. Volume loads of 20,000–40,000 kg/week are not uncommon among strength-power athletes using a similar training protocol (43). By dividing the volume load (VL) by maximum whole body strength values (IPF) an estimate of the relative training load can be made. Among athletes, a ratio of VL/IPF ranging from 3.5–6.0 per week is typical. In the present study the ratio ranged from an initial value of 2.7 to a final value (week 7) of 4.6 for the males and from 2.1 to 4.7 for females. Therefore, it is possible that the subjects in the present study reached loading values, intensities, and reasonable efforts that potentially could affect health-related physiological alterations only during the last few weeks of the study. A longer training period may be necessary to achieve full benefit.
In summary, the results of this study indicate that a short-term unique training protocol using free-weights and large muscle mass exercises and a form of block periodization, can improve several physical, physiological, and performance variables that indicate partial amelioration of a sedentary lifestyle and metabolic syndrome. These improvements include maximum strength, power, V̇O2 peak, and time to exhaustion. Although there were indications of trends toward improvement of physiological variables related to health (e.g. blood lipids) and muscle CSA, it is probable that longer training periods and greater levels of work are necessary (12, 51).
PRACTICAL APPLICATIONS
These data indicate that short-term resistance training, using free weights and semi-block periodization, can markedly alter several variables associated with increased risk for cardiovascular disease in both sedentary normal and especially among those with metabolic syndrome. Thus training in a manner more similar to athletes is a viable alternative to the more traditional methods of training middle-aged adults including those with metabolic syndrome. Although metabolic syndrome is not normally associated with athletes, recent research indicates that some athletes, particularly large strength-power athletes, for example, American Football linemen, may be at risk upon retirement (33, 41) or perhaps even during their athletic career (19). Re-training these athletes upon retirement, along with weight reduction can reduce the risk of development of metabolic syndrome among these athletes (33). Higher volume resistance training, along with exercises typically used by athletes, as used in this study, may be beneficial to athletes susceptible to metabolic syndrome upon retirement.
Acknowledgments
Supported in part by a grant from The National Institutes of Health DK 080488.
Footnotes
CONFLICT OF INTEREST No conflict of interest exists for any of the authors listed on this publication.
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Albu JB, Heilbronn LK, Kelley DE, Smith SR, Azuma K, Berk ES, Pi-Sunyer FX, Ravussin E. Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes. 2010;59:627–633. doi: 10.2337/db09-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson JL, Aagaard P. Myosin heavy chain IIx overshoot in human skeletal muscle. Muscle and Nerve. 2000;23:1095–1104. doi: 10.1002/1097-4598(200007)23:7<1095::aid-mus13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Andersen LL, Andersen JL, Magnusson SP, Suetta C, Madsen JL, Christensen LR, Aagaard P. Changes in the human muscle force-velocity relationship in response to resistance training and subsequent detraining. J Appl Physiol. 2005;99:87–94. doi: 10.1152/japplphysiol.00091.2005. [DOI] [PubMed] [Google Scholar]
- 5.Andersen LL, Aagaard P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol. 2006;96:46–52. doi: 10.1007/s00421-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 6.Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA Members of the Florey Adelaide Male Ageing Study. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009;58:1013–1022. doi: 10.1016/j.metabol.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Baar K. Training for endurance and strength: lessons from cell signaling. Med Sci Sports Exerc. 2006;38(11):1939–1944. doi: 10.1249/01.mss.0000233799.62153.19. [DOI] [PubMed] [Google Scholar]
- 8.Behan WM, Cossar DW, Madden HA, McKay IC. Validation of a simple, rapid, and economical technique for distinguishing type 1 and 2 fibres in fixed and frozen skeletal muscle. J Clin Pathol. 2002;55(5):375–380. doi: 10.1136/jcp.55.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjarnason-Wehrens B, Mayer-Berger W, Meister ER, Baum K, Hambrecht R, Gielen S. Recommendations for resistance exercise in cardiac rehabilitation. Recommendations of the German Federation for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2004;11(4):352–61. doi: 10.1097/01.hjr.0000137692.36013.27. [DOI] [PubMed] [Google Scholar]
- 10.Blessing D, Stone MH, Byrd R, Wilson D, Rozenek R, Pushparani D, Lipner H. Blood lipid and hormonal changes from Jogging and weight training of middle-aged men. J Appl Sports Sci Res. 1987;1(2):25–9. [Google Scholar]
- 11.Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38(11):1950–7. doi: 10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- 12.Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006;113(22):2642–50. doi: 10.1161/CIRCULATIONAHA.105.584060. [DOI] [PubMed] [Google Scholar]
- 13.Brentano MA, Cadore EL, Da Silva EM, Ambrosini AB, Coertjens M, Petkowicz R, Viero I, Kruel LF. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res. 2008;22(6):1816–25. doi: 10.1519/JSC.0b013e31817ae3f1. [DOI] [PubMed] [Google Scholar]
- 14.Buford TW, Anton sD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Maini TM. Ageing Research review. 2010;9:369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72:1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 16.DeFreitas JM, Beck TW, Stock MS, Dillon MA, Kasishke PR. An examination of the time course of training-induced skeletal muscle hypertrophy. Eur JAppl Physiol. 2010;111:2785–2790. doi: 10.1007/s00421-011-1905-4. [DOI] [PubMed] [Google Scholar]
- 17.DeWeese BH, Hornsby G, Stone M, Stone MH. The training process: Planning for strength–power training in track and field. Part 1: Theoretical aspects. Journal of Sport and Health Science. 2015;4:308–317. [Google Scholar]
- 18.DeWeese BH, Hornsby G, Stone M, Stone MH. The training process: Planning forstrength–power training in track and field. Part 2: Practical and applied aspects. J Sport and Health Sci. 2015;4:318–324. [Google Scholar]
- 19.Dobrosielski DA, Rosenbaum D, Wooster BM, Merrril lM, Swanson J, Moore JB, Brubaker PH. Assesment of cardiovascular risk in collegiate football players and nonathletes. J Am Coll Health. 2011;59:224–227. doi: 10.1080/07448481.2010.483719. [DOI] [PubMed] [Google Scholar]
- 20.Drey M, Sieber CC, Degens H, McPhee J, Korhonen MT, Müller K, Ganse B, Rittweger J. Relation between muscle mass, motor units and type of training in master athletes. Clin Physiol Funct Imaging. 2016;36:70–76. doi: 10.1111/cpf.12195. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Giles WH. A comparison of the prevalence of the metabolic syndrome using two proposed definitions. Diabetes Care. 2003;26:575–581. doi: 10.2337/diacare.26.3.575. [DOI] [PubMed] [Google Scholar]
- 22.Gordon BA, Benson AC, Bird SR, Fraser SF. Resistance training improves metabolic health in type 2 diabetes: a systematic review. Diabetes Res Clin Pract. 2009;83(2):157–175. doi: 10.1016/j.diabres.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Haff GG, Stone MH, O'Bryant HS, Harman E, Dinan, Johnson R, Han Ki. Hoon Force-Time dependent characteristics of dynamic and isometric muscle actions. J Strength Cond Res. 1997;11:269–272. [Google Scholar]
- 24.Hagerman FC, Walsh SJ, Staron RS, Hikida RS, Gilders RM, Murray TF, Toma K, Ragg KE. Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci. 2000;55:B336–346. doi: 10.1093/gerona/55.7.b336. [DOI] [PubMed] [Google Scholar]
- 25.Henry FM. 'Best' versus 'average' individual scores. The Research Quarterly. 1967;38:317–320. [Google Scholar]
- 26.Hickson RC. Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol. 1980;45:255–263. doi: 10.1007/BF00421333. [DOI] [PubMed] [Google Scholar]
- 27.Hodgson JL, Buskirk ER. Physical fitness and age, with emphasis on cardiovascular function in the elderly. J Am Geriatr Soc. 1977;25(9):385–392. doi: 10.1111/j.1532-5415.1977.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 28.Huberty CJ, Morris JD. Multivariate analysis versus multiple univariate analyses. Psychol Bull. 1989;105:302–308. [Google Scholar]
- 29.Hurley BF, Hanson ED, Sheaff AK. Strength training as a countermeasure to aging muscle and chronic disease. Sport Med. 2011;41:289–306. doi: 10.2165/11585920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Hurley BF, Seals DR, Ehsani AA, Cartier LJ, Dalsky GP, Hagberg JM, Holloszy JO. Effects of high-intensity strength training on cardiovascular function. Med Sci Sports Exerc. 1994;16:483–488. doi: 10.1249/00005768-198410000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13:46–51. doi: 10.1097/MCO.0b013e32833309cf. [DOI] [PubMed] [Google Scholar]
- 32.Johnson CC, Stone MH, Lopez SA, Hebert JA, Kilgore LT, Byrd RJ. Diet and exercise in middle-aged men. J Am Diet Assoc. 1982;81(6):695–701. [PubMed] [Google Scholar]
- 33.Judge LW, Stone MH, Craig B. Reconditioning the postcompetitive football lineman: recognizing the problem. Strength Cond J. 2010;32:28–32. [Google Scholar]
- 34.Kraska J, Ramsey M, Haff GG, Fethke N, Sands WA, Stone ME, Stone MH. Relationship between strength characteristics and unweighted and weighted vertical jump height. Int J Sports Physiol Perform. 2009;4:461–473. doi: 10.1123/ijspp.4.4.461. [DOI] [PubMed] [Google Scholar]
- 35.Kraschnewski JL, Sciamanna C, Poger JM, Rovniak LM, Lehman EB, Cooper AB, Ballentine NH, Ciccolo JT. Is strength training associated with mortality benefits? A 15 year cohort study of US older adults. Prevent Med. 2016;87:121–127. doi: 10.1016/j.ypmed.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 36.Layne AS, Nasrallah S, South MA, Howell ME, McCurry MP, Ramsey MW, Stone MH, Stuart CA. Impaired Muscle AMPK Activation in the Metabolic Syndrome May Attenuate Improved Insulin Action after Exercise Training. J Clin Endocrinol Metab. 2011;96:1815–1826. doi: 10.1210/jc.2010-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacFarlane N, Northridge DB, Wright AR, Grant S, Dargie HJ. A comparative study of left ventricular structure and function in elite athletes. Br J Sports Med. 1991;25:45–48. doi: 10.1136/bjsm.25.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcinik EJ, Potts J, Schlabach G, Will S, Dawson P, Hurley BF. Effects of strength training on lactate threshold and endurance performance. Med Sci Sports Exerc. 1991;23:739–743. [PubMed] [Google Scholar]
- 39.McCaulley GO, McBride JM, Cormie P, Hudson MB, Nuzzo JL, Quindry JC, Triplett NT. Acute hormonal and neuromuscular responses to hypertrophy, strength and power type resistance exercise. Eur J Appl Physiol. 2009;105:695–704. doi: 10.1007/s00421-008-0951-z. [DOI] [PubMed] [Google Scholar]
- 40.McMillan J, Stone MH, Sartain J, Marple D, Keith R, Lewis D, Brown C. The 20-hr Hormonal Response to a Single Session of Weight Training. J Strength and Cond Res. 1993;7:9–21. [Google Scholar]
- 41.Miller MA, Croft LB, Belanger AR, Romero-Corral A, Somers VK, Roberts AJ, Goldman ME. Prevalence of metabolic syndrome in retired national football league players. Am J Cardiol. 2008;101:1281–1284. doi: 10.1016/j.amjcard.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 42.Nader GA. Concurrent strength and endurance training: from molecules to man. Med Sci Sports Exerc. 2006;38:1965–1970. doi: 10.1249/01.mss.0000233795.39282.33. [DOI] [PubMed] [Google Scholar]
- 43.Painter KB, Haff G, Ramsey MW, McBride J, Triplett T, Sands WA, Lamont HS, Stone ME, Stone MH. Strength Gains: Block Vs DUP Weight-Training among Track and Field Athletes. Int J Sport Physiol Perf. 2012;7:161–169. doi: 10.1123/ijspp.7.2.161. [DOI] [PubMed] [Google Scholar]
- 44.Perkisas S, Vandewoude M. Where frailty meets diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):261–267. doi: 10.1002/dmrr.2743. [DOI] [PubMed] [Google Scholar]
- 45.Robergs RA, Dwyer D, Astorino T. Recommendations for improved data processing from expired gas analysis indirect calorimetry. Sports Med. 2010;40:95–111. doi: 10.2165/11319670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 46.Singh N, Liu K, Tian L, Criqui MH, Guralnik JM, Ferrucci L, Liao Y, McDermott MM. Leg strength predicts mortality in men but not in women with peripheral arterial disease. J Vasc Surg. 2010;52(3):624–531. doi: 10.1016/j.jvs.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stone MH, Smith D, Ward T, Carter D. In: Olympic weightlifting: I. Physiological characteristics of the athlete. Terauds J, editor. Del Mar, California: Academic Publishers; 1979. pp. 45–54. [Google Scholar]
- 48.Stone MH, Stone ME, Sands WA, Pierce KC, Newton RU, Haff GG, Carlock J. Maximum strength and strength training: a relationship to endurance? Strength Cond. 2006;28:44–53. [Google Scholar]
- 49.Stone MH, Wilson GD, Blessing D, Rozenek R. Cardiovascular responses to short-term Olympic style weight training in young men. Can J Appl Sports Sci. 1983;8(3):134–139. [PubMed] [Google Scholar]
- 50.Stone M, Plisk S, Collins D. Training principles: evaluation of modes and methods of resistance training--a coaching perspective. Sport Biomech. 2002;1:79–103. doi: 10.1080/14763140208522788. [DOI] [PubMed] [Google Scholar]
- 51.Stone MH, Fleck SJ, Triplett NT, Kraemer WJ. Health- and performance-related potential of resistance training. Sports Med. 1991;11(4):210–3. doi: 10.2165/00007256-199111040-00002. [DOI] [PubMed] [Google Scholar]
- 52.Stone MH, Sands WA, Pierce KC. Relationship of Maximum Strength to Weightlifting Performance. Med Sci Sports Exerc. 2005;37(6):1037–43. [PubMed] [Google Scholar]
- 53.Stone MH, Stone ME, Sands WA. Principles and Practice of Resistance Training. Champaign, IL: Human Kinetics; 2007. [Google Scholar]
- 54.Storey AG, Birch NP, Fan V, Smith HK. Stress responses to short-term intensified and reduced training in competitive weightlifters. Scand J Med Sci Sports. 2016;26:29–40. doi: 10.1111/sms.12400. [DOI] [PubMed] [Google Scholar]
- 55.Stuart CA, Yin D, Howell ME, Dykes RJ, Laffan JJ, Ferrando AA. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab. 2006;291:E1067–E1073. doi: 10.1152/ajpendo.00250.2006. [DOI] [PubMed] [Google Scholar]
- 56.Svendahl K, Haennel RG, Hudec R, Habib N, Gebhart V. The effects of circuit training on the physical fitness profile of post-myocradial infarction (MI) patients. Med Sci Sports Exerc. 1994;26:185. [Google Scholar]
- 57.Takekura H, Yoshioka T. Specific Mitochondrial Responses to Running Training are Induced in Each Type of Rat Single Muscle Fibers. Jpn J Physiol. 1989;39:497–509. doi: 10.2170/jjphysiol.39.497. [DOI] [PubMed] [Google Scholar]
- 58.Warner SO, Linden MA, Liu Y, Thyfault JP, Whaley-Connell AT, Chockalingam A, Hinton PS, Dellsperge KC, Thomas TR. The effects of resistance training on metabolic health with weight regain. J Clin Hypertens (Greenwich) 2010;12(1):64–72. doi: 10.1111/j.1751-7176.2009.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinsier RL, Schutz Y, Bracco D. Reexamination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am J Clin Nutr. 1992;55:790–794. doi: 10.1093/ajcn/55.4.790. [DOI] [PubMed] [Google Scholar]
- 60.Wells GD, Noseworthy MD, Hamilton J, Tarnopolski M, Tein I. Skeletal muscle metabolic dysfunction in obesity and metabolic syndrome. Can J Neurol Sci. 2008;35:31–40. doi: 10.1017/s0317167100007538. [DOI] [PubMed] [Google Scholar]