Abstract
The cytochrome P450 (CYP) enzymes are a diverse group of heme monooxygenases that, through the course of their reaction cycle, contribute to cellular reactive oxygen species (ROS). CYP enzymes play a crucial role in human physiology and are involved in drug and xenobiotic metabolism as well as biosynthesis of endogenous molecules and are expressed throughout the human body. However, during the course of the CYP catalytic cycle, ROS can be generated through uncoupling of the enzymatic cycle. ROS is known to modify endogenous molecules, included lipids, proteins, and nucleic acids, which can lead to cell damage and death and contribute to disease development. ROS has been implicated in a wide range of diseases and conditions, including cancer and ageing, but ROS also play a role in the normal physiological functions in the cell. Here, we discuss specific examples whereby ROS generated by CYPs contribute to or protect against various phenomena, such as hyperoxic lung injury, oxidative hepatic toxicity, formation of DNA adducts from lipid peroxidation products. We have also discussed the mechanistic roles of CYP enzymes belonging to various families, and their effect on cellular ROS production, in relation to normal cellular function as well as disease pathophysiology.
Keywords: Cytochrome P450, reactive oxygen species, reaction uncoupling
1. Introduction
Cytochrome P450 (CYP) enzymes are a diverse group of heme monooxygenases that have been actively studied in conjunction with their crucial roles in metabolism or biotransformation of drugs and xenobiotics, but also in the biosynthesis of sterols, fatty acids, eicosanoids, vitamins, etc. [1,2]. CYP enzymes play a prominent role in phase I metabolism of approximately three quarters of drug metabolism reactions in humans, with only five isoforms accounting for the majority of these reactions [3]. While these enzymes can conduct a wide range of reactions, the most common is the oxidation of a substrate (R) and will be described in greater depth later, but the general reaction mechanism is [4]:
CYP enzymes were originally identified and characterized in mammals with the initial observation of a pigment which, upon binding carbon monoxide, would exhibit a characteristic absorption band at 450 nm [5,6]. Omura and Sato found that the pigment was a novel cytochrome, as it contained a heme group and that was unique from cytochrome b5 in liver microsomal preparations [7,8]. These findings were then followed by subsequent work detailing a bacterial CYP enzyme [9]. We now know that, thanks to the increasing use of genome sequencing, CYP enzymes are evolutionarily conserved and exist in all domains of life, Bacteria, Archaea, and Eukarya, and potentially in viruses [10,11].
As the CYP field grew, there was a need to standardize the nomenclature of CYP enzymes to ensure that no duplications of gene names and that newly discovered CYP were assigned to the correct family and subfamily [12]. The P450 Nomenclature Committee determined that CYP enzymes should be grouped into families, with members of that family have greater than 40% amino acid similarity and are denoted by a number, i.e. CYP4 family. CYP enzymes that share >55% homology in mammals would then be grouped into the same subfamily denoted by a letter and number in the subfamily, e.g., CYP1A1 and CYP1A2. The completion of the human and mouse genomes identified 102 CYP sequences in mouse, with 88 pseudogenes, and 57 CYP sequences in human, with 58 pseudogenes, highlighting the need for a set nomenclature [13]. For further information on CYP nomenclature and sequence information on dozens of animal and plant species, Dr. David Nelson maintains a cytochrome P450 database which may be of interest http://drnelson.uthsc.edu/cytochromeP450.html (last accessed 10/02/17) [14].
In this review, we will describe the mechanism behind CYP-mediated generation of ROS and its contribution to disease (Figure 1). In subsequent sections, we will briefly describe the CYP reaction cycle and how ROS can directly be generated from unproductive reactions and how ROS generated by CYP enzymes, as well as their modified substrates, impact human disease.
Figure 1. Mechanism by which CYPs contribute to ROS-mediated human diseases.
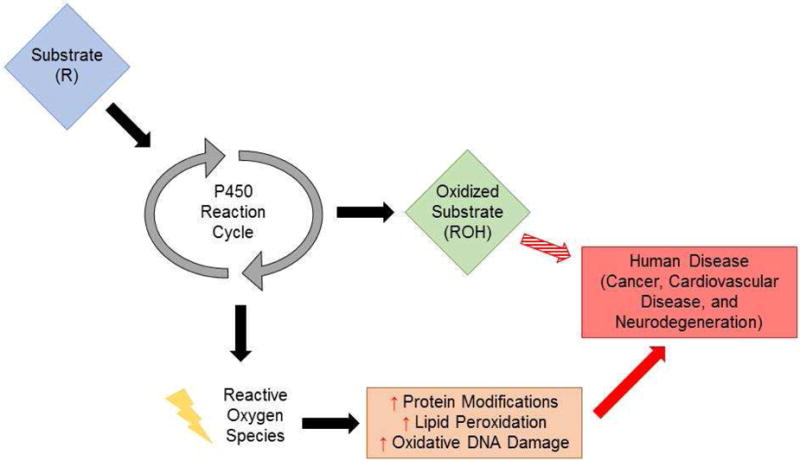
As CYP enzymes metabolize their substrates, they can produce ROS which can increase cellular damage via increases in protein, nucleic acid, and lipid modifications. These products, including lipid peroxidation produces and DNA damage, are known to contribute to numerous human pathologies, including cancer.
2. Role of reactive oxygen species in biology and medicine
There are many enzymatic sources of reactive oxygen species (ROS), including CYP enzymes, that contribute to cellular oxidation-reduction (redox) balance, which play critical roles in normal cellular processes, including immune function and cell signaling [15,16]. Disruption of the normal redox balance results in oxidative stress and is involved in a number of disease processes, including carcinogenesis and ageing [17]. There are many types of ROS that are important to biology and medicine, including superoxide anion (O2−*), hydroxyl radial (OH*), hydrogen peroxide (H2O2), and even reactive carbonyls (RCO), as well as reactive nitrogen species (RNS), all of which can disrupt the redox balance and contribute to oxidative stress (expertly reviewed here [17]).
ROS enacts biological function, and elicits cellular damage, through modifications of lipids, nucleic acids, and proteins. Lipid peroxidation products such as F2-isoprostanes, which result from non-enzymatic oxidation of arachidonic acid by oxygen radicals [18], are often used as surrogate markers of oxidative stress and ROS levels, but also have their own biological effects [19]. ROS also can modify DNA, which can create mutations and errors in replication [20], and are known to play major roles in cancer, particularly pro-tumorigenic signaling, pro-survival signaling, and drug resistance [21]. CYP enzymes, notably CYP2E1, have been shown to generate ROS and lipid peroxidation and these lipid peroxidation products may interact with DNA, creating oxidative DNA adducts [22]. Excessive ROS levels and increases in oxidative stress can also modify proteins, in particular amino acid cysteine, which can in turn damage proteins and/or lead to downstream signaling in toxic pathways. In this review, we will focus on understanding how ROS generated by CYP enzymes may contribute to disease pathologies, but it is important to highlight the growing appreciation for ROS as a signaling molecule and the role ROS plays in normal physiology, which is reviewed elsewhere [4,15,23–25].
3. Cytochrome P450 enzymatic cycle
As mentioned previously, CYP enzymes are versatile catalysts for a wide range of biochemical reactions, but generally known for their role in substrate oxidation [26]. Due to their oxidation capacity, CYP enzymes play a major role in phase I metabolism of drugs and xenobiotics, increasing the substrate’s polarity and aiding in excretion. In this review, we will briefly describe the general reaction mechanism, however other more thorough biochemical and biophysical reviews on the reaction mechanism can be found here [27–29]. In addition, Mak and Denisov have recently published a detailed review of the various spectroscopic methods used to study CYP enzymes and the CYP reaction cycle [30]. Recent advances in CYP enzyme structure has greatly contributed to our understanding of the CYP reaction cycle [31]. Knowledge of the reaction cycle plays a crucial role in the understanding of how CYP enzymes can generate ROS and increase oxidative stress (Figure 1). The initial step (1) in the reaction is the binding of the substrate (R-H) to the ferric iron (Fe3+) of the home-thiolate group (Figure 2). The iron of the heme-thiolate group is then reduced (2), from Fe3+ to Fe2+, through the one electron reduction by NAPDH cytochrome P450 reductase (CPR). This facilitates the binding of oxygen (O2) to iron (3). CPR then reduces the Fe2+-O2 complex with the addition of a second election (4), activating the oxygen in the complex (Fe2+-O2−). Addition of two protons (H+) cleaves the O-O bond and releases H2O (5 & 6). The FeO3+ complex then removes a proton from the substrate (R), leaving a reactive intermediate, RFe3+.OH− (7). The hydroxyl group is then transferred to the substrate radical (8) and then the oxidized substrate is then released (9). While this is considered to be a generalized CYP reaction mechanism, other reaction mechanisms have been described and reviewed here [1]. However, one major research effort underway in CYP biology has been unravelling the involvement of the various reactive intermediates that are formed during the CYP reaction cycle and their effect on different types of CYP-mediated oxidation reactions [32].
Figure 2. Generalized CYP Reaction Mechanism.
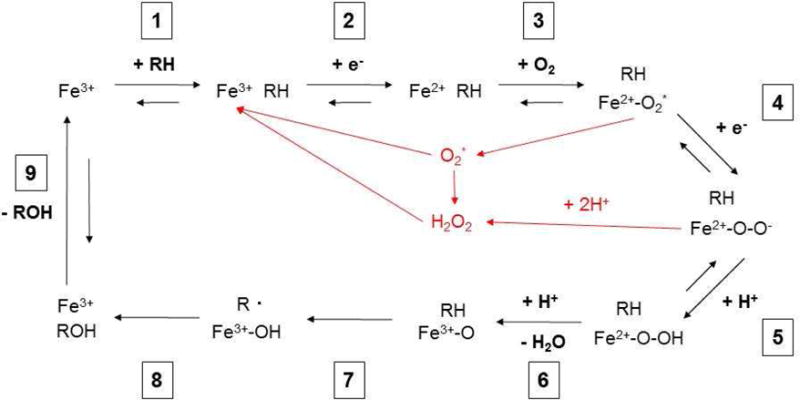
During the course of the CYP reaction mechanism, there are various steps that can generate ROS, shown in red. These ROS can then modify cellular components and contribute to disease. Reaction cycle adapted from refs [29] and [4].
3.1 Reaction uncoupling in the CYP reaction cycle
Early work in CYP biology revealed that CYP enzymes were directly capable of generating superoxide and hydrogen peroxide [27]. It is now thought that two “shunts” exist within the CYP catalytic cycle which can generate ROS without completion of the substrate oxidation and is known as ‘reaction uncoupling’ [28]. The first possible release of ROS is superoxide radical (Figure 1, step 3), due to the loss of reduced oxygen (O2−), which then quickly dismutates to H2O2 formation [4,33]. The second possible release of ROS is after the addition of a proton to the reduced oxygen complex leading directly to H2O2 formation and not the release of H2O (Step 4) [4,33]. There are many factors which determine the coupling efficiency of a given CYP reaction with the substrate and reaction environment (pH, O2 concentration) playing significant roles [33]. There is also evidence that the different CYP isoforms have different rates of reaction uncoupling, and they can also be dependent on the substrate [34]. Harskamp and colleagues found that CYP1B1 and CYP1D1 in Danio rerio (zebrafish) had high rates of reaction uncoupling compared to other isoforms tested (CYP1A1/1C1/1C2), indicating that CYP1B1/1D1 are more prone to generating ROS due to reaction uncoupling. The differences in coupling efficiencies may be due, in part, to structural differences in the substrate binding pocket of the various CYP isoforms [35]. The ROS generated by CYPs through the course of their reactive cycle can then go on to modify cellular components which in turn leads to disease.
4. Role of CYP enzymes in ROS metabolism and diseases
As seen in Figures 1 and 2, CYP enzymes can increase ROS and alter the redox balance, creating oxidative stress, through their catalytic cycle and contribute to disease development. However, some substrates, modified by CYP enzymes to create reactive intermediates or products, can also contribute to disease development. We will now describe several physiological or disease states in which CYP generated ROS or reactive products are known to play a role.
4.1 CYP1 family in hyperoxic lung injury
Supplemental oxygen is often provided to premature infants suffering from pulmonary insufficiency and in adults with acute respiratory distress syndrome [36,37]. Despite the clinical utility of oxygen supplementation, excess oxygen can create a hyperoxic environment which can induce or exacerbate existing pulmonary injury in animal models and is thought to be due to increased ROS and oxidative stress levels [38–40]. Some groups initially hypothesized that increases in H2O2 could be due to increased CYP activity and possible reaction uncoupling. One such study found that cimetidine, a CYP inhibitor, treated lambs had reduced pulmonary injury, which they attributed to decreased CYP activity [41]. However, several subsequent studies, including ours, found that induction of CYP enzymes by 3-methylcholanthrene (3-MC) and β-napthoflavone (BNF) do, in fact, protect against hyperoxic lung injury and inhibition of CYP1 activity potentiates injury [42,43].
Our group has extensively investigated the role of the CYP1 family of enzymes in hyperoxic lung injury (42,45–52). The CYP1 enzymes were initially implicated in hyperoxic lung injury after Okamoto and colleagues found that CYP1A1/1A2 were selectively induced in the lungs and liver, respectively, of rats during hyperoxia exposure [44]. We have since shown that induction of CYP1A by β-napthoflavone (BNF) protect against hyperoxic lung injury in newborn and adult mouse and in adult rat models [45–47]. We have also found that 3-MC induces CYP1A via the aryl hydrocarbon receptor (AHR), a key transcription factor which regulates CYP1 gene expression [48]. Further, we found that adult Cyp1a1−/−, Cyp1a2−/−, and Ahr−/− mice all have increased pulmonary injury when exposed to hyperoxia (>95% O2), suggesting that CYP1A enzymes protect against hyperoxia induced lung injury [49–51]. This may be due to increased ROS, as we found increased levels of lipid peroxidation and oxidative DNA damage in the lungs of Cyp1a1−/− and Cyp1a2−/− mice [50–52]. While the mechanism underlying CYP1A mediated protection against hyperoxic lung injury is unclear, in vitro experimental evidence suggests that CYP1A2 metabolizes the F2-isoprostane PGF2-α to a dinor metabolite, which may reduce pulmonary injury [51]. We have also utilized –omics driven approaches and found that several biological pathways are altered in Cyp1a1−/− and Cyp1a2−/− mice during hyperoxia, including DNA repair and protein secretion, and the effect of CYP1A on these pathways is the subject of ongoing investigations [52]. The role of CYP1A in ROS generation and metabolism has been reviewed in depth here [53].
We are also investigating the role of CYP1B1 in hyperoxic lung injury. CYP1B1 is of particular interest because it has been shown by other groups to be involved in modulating oxidative stress, with some groups reporting that CYP1B1 may be involved in decreasing ROS in other experimental conditions [54,55]. Our initial study found that CYP1B1 may in fact contribute to hyperoxic toxicity. Cells overexpressing CYP1B1 showed increased hyperoxic toxicity in an MTT assay and cells in which CYP1B1 was knocked down showed decreased caspase 3/7 activity, a marker of apoptosis [56]. We have also found that Cyp1b1−/− mice are less susceptible to hyperoxic lung injury, suggesting that CYP1B1, unlike CYP1A, contributes to hyperoxic toxicity (Veith et al, unpublished data). These findings suggest that, despite homology between CYP1A/1B, there can be different effects on ROS production. Further, our data suggest that CYP1B1 increases ROS levels, while other groups show CYP1B1 decreases ROS levels, highlighting the importance of cellular environment, in addition to biochemical environment, on the effect of any given CYP in ROS production.
4.2 CYP enzymes in ROS-mediated hepatotoxicity
One of the major functions of the CYP enzymes in human physiology is the metabolism and detoxification of xenobiotics and drugs. However, CYP mediated metabolism of drugs can also generate ROS, in addition to bioactivated intermediates, and increase oxidative stress, particularly in the liver, and contribute to liver pathology [57]. While 57 human CYP genes have been identified, five account for the majority of drug metabolism, CYP3A4, CYP2D6, CYP2C9, CYP1A2, and CYP2C19, which primarily occurs in the liver and small intestine where these enzymes are expressed [29]. While these CYP enzymes play a critical role in phase I metabolism in detoxifying drugs, hepatic CYP enzymes also produce ROS which contributes to a range of liver pathologies, including liver cancer and alcoholic liver disease.
4.2.1 CYP2 family in metabolism of ethanol and acetaminophen
Acute and chronic ethanol consumption is known to increase ROS levels which can contribute to liver cirrhosis and hepatocyte cell death [58]. Ethanol is primarily metabolized by alcohol dehydrogenase, but can also be metabolized by catalase and CYP enzymes, primarily CYP2E1 [58]. In addition to metabolizing ethanol, ethanol also induces CYP2E1 [59]. Metabolism of ethanol by CYP2E1 generates ROS, through reaction uncoupling, and increases hepatic oxidative stress, which in turn, can contribute to alcoholic liver disease [58]. CYP2E1 expression and ROS are associated with pro-carcinogenic etheno-DNA adducts in individuals which chronic ethanol consumption [22]. As CYP2E1 metabolizes ethanol, it generates a significant amount of ROS which results in lipid peroxidation. These lipid peroxides interact with DNA bases and create etheno-DNA adducts, potent mutagens that are linked to hepatocarcinogenesis [22]. In addition to CYP2E1’s role in cancer, ROS generated by CYP2E1 has also been implicated in non-alcoholic fatty liver disease (NAFLD) [60]. CYP2E1 has been shown to be a potent ROS source, as CYP2E1 can generate ROS in the absence of substrate due to reaction uncoupling, indicating that CYP2E1 may play a role in multiple liver pathologies [61].
CYP2E1 is also known to play a role in the metabolism of the common analgesic acetaminophen [61]. Acetaminophen metabolism begins with the conversion of acetaminophen to a reactive intermediate, N-acetyl-p-benzo-quinone-imine (NAPQI) via CYP oxidation, which is readily conjugated with glutathione by glutathione S-transferase, inactivating NAPQI [62]. Acetaminophen overdose and toxicity is caused by excessive amounts NAPQI being generated by CYP2E1 without the detoxification by GST, due to glutathione exhaustion, and can be rescued with administration of N-acetylcysteine [62]. CYP2E1 is thought to play a major role in the activation of acetaminophen to NAPQI, as Cyp2e1−/− mice are resistant to acetaminophen toxicity [63]. While CYP2E1 is thought to be the predominate CYP involved in NAPQI generation, other CYPs can also convert acetaminophen to NAPQI, including CYP3A4, CYP1A2, and CYP2D6 [64].
4.2.2 CYP3 family in drug metabolism
CYP3A4 plays a prominent role in drug metabolism and is estimated to metabolize approximately 50% of commercially available drugs [65]. CYP3A4’s broad substrate spectrum may also explain why CYP3A4 generates significant ROS during drug metabolism, as it has a flexible active site which may allow for increased rates of reaction uncoupling [66,67]. Generation of ROS by CYP3A4 has been implicated in altering protein secretion, particularly proteins involved in autocrine and paracrine signaling, and may play a role in liver carcinogenesis [68]. While ROS is known to contribute to carcinogenesis through increased proliferation and survival, excessive ROS can still lead to cellular damage and cytotoxicity, even in tumor cells [69]. Interestingly, one group harnessed CYP3A4’s propensity to generate ROS and utilized a drug which would be metabolized by CYP3A4, methyl 3-(4-nitrophenyl) propiolate (NPP), in tumor cells, increasing ROS levels to induce cytotoxicity [70].
4.3 CYP2 & 4 families in eicosanoid metabolism
CYP enzymes have also been shown to act as a third metabolic avenue for arachidonic acid, in addition to cyclooxygenase (COX) and lipoxygenase (LOX) pathways [71]. These metabolites are collectively known as eicosanoids and play roles in inflammation as well as respiratory and cardiovascular function and have been implicated in disease processes such as cancer [71]. There are several groups of metabolites generated from arachidonic acid (AA), prostaglandins (COX), leukotrienes (LOX), hydroxyeicosatetraenoic acids (HETEs) by LOX/CYP metabolic pathways, and epoxyeicosatrienoic acids (EETs) by the CYP metabolic pathway [71]. The CYP4A and CYP4F are known to play predominant roles in metabolizing AA into HETEs while the CYP2C and CYP2J families metabolize AA into EETs [71,72]. CYP metabolism of EETs and HETEs are known to play roles in several disease states, including cancer and cardiovascular disease. The role of EETs and HETEs in cardiovascular disease has been thoroughly reviewed [73]. Briefly, HETEs, specifically 20-HETE, generated by CYP4A and CYP4F enzymes is associated with inflammation and vasoconstriction which can lead to cardiac dysfunction while EETs generated by the CYP2C and CYP2J families are associated with angiogenesis and vasodilation and maintain cardiovascular health [73].
5. Conclusions and future directions
In summary, while CYPs have necessary and important biological roles in the human body, CYPs are also involved in cellular ROS generation and metabolism and can contribute to disease development. The biochemical properties of the heme group in CYP enzymes help to facilitate ROS generation through reaction uncoupling. CYPs can also generate reactive intermediates, NAPQI in CYP2E1 metabolism of acetaminophen for example, which can contribute to toxicity and/or disease development. These reactive intermediates, similar to ROS, can modify endogenous substrates, including lipids, proteins, and nucleic acids, leading to oxidative stress. However, as our understanding of redox biology grows, so too will our understanding of the normal biological and physiological roles of ROS, including CYP generated ROS, in the cell. This is becoming more evident as we appreciate the endogenous roles of CYPs which were previously thought to be only involved in metabolism of exogenous substances, such as xenobiotics [2]. Due to the high conservation of CYP enzymes throughout life, it is likely that they play important endogenous functions. Another exciting avenue for CYP research is further our understanding of the other types of reactions CYPs are involved in [32]. Unraveling the detailed catalytic mechanisms of action of various CYP enzymes, in relation to ROS formation/degradation, should lead to a better understanding of the role CYP enzymes in ROS metabolism, in relation to a myriad of human diseases in which ROS and oxidative stress play a contributory role.
Abbreviations
- CYP
Cytochrome P450
- ROS
reactive oxygen species
- NAPQI
N-acetyl-p-benzo-quinone-imine
- HETE
hydroxyeicosatetraenoic acid
- EET
epoxyeicosatrienoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isin EM, Guengerich FP. Complex reactions catalyzed by cytochrome p450 enzymes. Biochim Biophys Acta. 2007;1770(3):314–329. doi: 10.1016/j.bbagen.2006.07.003. doi: https://doi.org/10.1016/j.bbagen.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Guengerich FP. Intersection of the roles of cytochrome p450 enzymes with xenobiotic and endogenous substrates: Relevance to toxicity and drug interactions. Chem Res Toxicol. 2017;30(1):2–12. doi: 10.1021/acs.chemrestox.6b00226. https://doi.org/10.1021/acs.chemrestox.6b00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21(1):70–83. doi: 10.1021/tx700079z. https://doi.org/10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- 4.Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells. 2011;32(6):491–509. doi: 10.1007/s10059-011-0276-3. https://doi.org/10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omura T, Sato R. A new cytochrome in liver microsomes. J Biol Chem. 1962;237:1375–1376. [PubMed] [Google Scholar]
- 6.Klingenberg M. Pigments of rat liver microsomes. Arch Biochem Biophys. 1958;75(2):376–386. doi: 10.1016/0003-9861(58)90436-3. https://doi.org/10.1016/0003-9861(58)90436-3. [DOI] [PubMed] [Google Scholar]
- 7.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 8.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. Ii Solubilization, purification, and properties. J Biol Chem. 1964;239:2379–2385. [PubMed] [Google Scholar]
- 9.Katagiri M, Ganguli BN, Gunsalus IC. A soluble cytochrome p-450 functional in methylene hydroxylation. J Biol Chem. 1968;243(12):3543–3546. [PubMed] [Google Scholar]
- 10.Danielson PB. The cytochrome p450 superfamily: Biochemistry, evolution and drug metabolism in humans. Curr Drug Metab. 2002;3(6):561–597. doi: 10.2174/1389200023337054. [DOI] [PubMed] [Google Scholar]
- 11.Lamb DC, Lei L, Warrilow AG, Lepesheva GI, Mullins JG, Waterman MR, Kelly SL. The first virally encoded cytochrome p450. J Virol. 2009;83(16):8266–8269. doi: 10.1128/JVI.00289-09. https://doi.org/10.1128/JVI.00289-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson DR, Kamataki T, Waxman DJ, Guengerich FP, Estabrook RW, Feyereisen R, Gonzalez FJ, Coon MJ, Gunsalus IC, Gotoh O, et al. The p450 superfamily: Update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993;12(1):1–51. doi: 10.1089/dna.1993.12.1. https://doi.org/10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- 13.Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome p450 (cyp) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14(1):1–18. doi: 10.1097/00008571-200401000-00001. https://doi.org/10.1097/01.fpc.0000054151.92680.31. [DOI] [PubMed] [Google Scholar]
- 14.Nelson DR. The cytochrome p450 homepage. Hum Genomics. 2009;4(1):59–65. doi: 10.1186/1479-7364-4-1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones DP, Sies H. The redox code. Antioxid Redox Signal. 2015;23(9):734–746. doi: 10.1089/ars.2015.6247. https://doi.org/10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15(6):411–421. doi: 10.1038/nrm3801. https://doi.org/10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 17.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;8:715–748. doi: 10.1146/annurev-biochem-061516-045037. https://doi.org/10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 18.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin f2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87(23):9383–9387. doi: 10.1073/pnas.87.23.9383. https://doi.org/10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milne GL, Dai Q, Roberts LJ., 2nd The isoprostanes–25 years later. Biochim Biophys Acta. 2015;1851(4):433–445. doi: 10.1016/j.bbalip.2014.10.007. https://doi.org/10.1016/j.bbalip.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362(6422):709–715. doi: 10.1038/362709a0. https://doi.org/10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 21.Moloney JN, Cotter TG. Ros signalling in the biology of cancer. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.05.023. https://doi.org/10.1016/j.semcdb.2017.05.023. [DOI] [PubMed]
- 22.Linhart K, Bartsch H, Seitz HK. The role of reactive oxygen species (ros) and cytochrome p-450 2e1 in the generation of carcinogenic etheno-DNA adducts. Redox Biol. 2014;3:56–62. doi: 10.1016/j.redox.2014.08.009. https://doi.org/10.1016/j.redox.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Autreaux B, Toledano MB. Ros as signalling molecules: Mechanisms that generate specificity in ros homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–824. doi: 10.1038/nrm2256. https://doi.org/10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 24.Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7(8):504–511. doi: 10.1038/nchembio.607. https://doi.org/10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schieber M, Chandel NS. Ros function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–462. doi: 10.1016/j.cub.2014.03.034. https://doi.org/10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guengerich FP. Common and uncommon cytochrome p450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol. 2001;14(6):611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 27.White RE, Coon MJ. Oxygen activation by cytochrome p-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. https://doi.org/10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- 28.Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and chemistry of cytochrome p450. Chem Rev. 2005;105(6):2253–2277. doi: 10.1021/cr0307143. https://doi.org/10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 29.Furge LL, Guengerich FP. Cytochrome p450 enzymes in drug metabolism and chemical toxicology: An introduction. Biochem Mol Biol Educ. 2006;34(2):66–74. doi: 10.1002/bmb.2006.49403402066. https://doi.org/10.1002/bmb.2006.49403402066. [DOI] [PubMed] [Google Scholar]
- 30.Mak PJ, Denisov IG. Spectroscopic studies of the cytochrome p450 reaction mechanisms. Biochim Biophys Acta. 2017 doi: 10.1016/j.bbapap.2017.06.021. https://doi.org/10.1016/j.bbapap.2017.06.021. [DOI] [PMC free article] [PubMed]
- 31.Guengerich FP, Waterman MR, Egli M. Recent structural insights into cytochrome p450 function. Trends Pharmacol Sci. 2016;37(8):625–640. doi: 10.1016/j.tips.2016.05.006. https://doi.org/10.1016/j.tips.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modi AR, Dawson JH. Oxidizing intermediates in p450 catalysis: A case for multiple oxidants. Adv Exp Med Biol. 2015;851:63–81. doi: 10.1007/978-3-319-16009-2_2. https://doi.org/10.1007/978-3-319-16009-2_2. [DOI] [PubMed] [Google Scholar]
- 33.Zangar RC, Davydov DR, Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome p450. Toxicol Appl Pharmacol. 2004;199(3):316–331. doi: 10.1016/j.taap.2004.01.018. https://doi.org/10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Harskamp J, Britz-McKibbin P, Wilson JY. Functional screening of cytochrome p450 activity and uncoupling by capillary electrophoresis. Anal Chem. 2012;84(2):862–866. doi: 10.1021/ac202787n. https://doi.org/10.1021/ac202787n. [DOI] [PubMed] [Google Scholar]
- 35.Loida PJ, Sligar SG. Molecular recognition in cytochrome p-450: Mechanism for the control of uncoupling reactions. Biochemistry. 1993;32(43):11530–11538. doi: 10.1021/bi00094a009. [DOI] [PubMed] [Google Scholar]
- 36.Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388(10058):2416–2430. doi: 10.1016/S0140-6736(16)00578-X. https://doi.org/10.1016/S0140-6736(16)00578-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buczynski BW, Maduekwe ET, O’Reilly MA. The role of hyperoxia in the pathogenesis of experimental bpd. Semin Perinatol. 2013;37(2):69–78. doi: 10.1053/j.semperi.2013.01.002. https://doi.org/10.1053/j.semperi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank L, Bucher JR, Roberts RJ. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol Respir Environ Exerc Physiol. 1978;45(5):699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- 39.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256(21):10986–10992. [PubMed] [Google Scholar]
- 40.Turrens JF, Freeman BA, Crapo JD. Hyperoxia increases h2o2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982;217(2):411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- 41.Hazinski TA, France M, Kennedy KA, Hansen TN. Cimetidine reduces hyperoxic lung injury in lambs. J Appl Physiol (1985) 1989;67(6):2586–2592. doi: 10.1152/jappl.1989.67.6.2586. [DOI] [PubMed] [Google Scholar]
- 42.Moorthy B, Parker KM, Smith CV, Bend JR, Welty SE. Potentiation of oxygen-induced lung injury in rats by the mechanism-based cytochrome p-450 inhibitor, 1-aminobenzotriazole. J Pharmacol Exp Ther. 2000;292(2):553–560. [PubMed] [Google Scholar]
- 43.Mansour H, Levacher M, Azoulay-Dupuis E, Moreau J, Marquetty C, Gougerot-Pocidalo MA. Genetic differences in response to pulmonary cytochrome p-450 inducers and oxygen toxicity. J Appl Physiol (1985) 1988;64(4):1376–1381. doi: 10.1152/jappl.1988.64.4.1376. [DOI] [PubMed] [Google Scholar]
- 44.Okamoto T, Mitsuhashi M, Fujita I, Sindhu RK, Kikkawa Y. Induction of cytochrome p450 1a1 and 1a2 by hyperoxia. Biochem Biophys Res Commun. 1993;197(2):878–885. doi: 10.1006/bbrc.1993.2561. https://doi.org/10.1006/bbrc.1993.2561. [DOI] [PubMed] [Google Scholar]
- 45.Sinha A, Muthiah K, Jiang W, Couroucli X, Barrios R, Moorthy B. Attenuation of hyperoxic lung injury by the cyp1a inducer beta-naphthoflavone. Toxicol Sci. 2005;87(1):204–212. doi: 10.1093/toxsci/kfi226. https://doi.org/10.1093/toxsci/kfi226. [DOI] [PubMed] [Google Scholar]
- 46.Couroucli XI, Liang YH, Jiang W, Wang L, Barrios R, Yang P, Moorthy B. Prenatal administration of the cytochrome p4501a inducer, beta-naphthoflavone (bnf), attenuates hyperoxic lung injury in newborn mice: Implications for bronchopulmonary dysplasia (bpd) in premature infants. Toxicol Appl Pharmacol. 2011;256(2):83–94. doi: 10.1016/j.taap.2011.06.018. https://doi.org/10.1016/j.taap.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maturu P, Wei-Liang Y, Jiang W, Wang L, Lingappan K, Barrios R, Liang Y, Moorthy B, Couroucli XI. Newborn mice lacking the gene for cyp1a1 are more susceptible to oxygen-mediated lung injury, and are rescued by postnatal beta-naphthoflavone administration: Implications for bronchopulmonary dysplasia in premature infants. Toxicol Sci. 2017;157(1):260–271. doi: 10.1093/toxsci/kfx036. https://doi.org/10.1093/toxsci/kfx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Couroucli XI, Welty SE, Geske RS, Moorthy B. Regulation of pulmonary and hepatic cytochrome p4501a expression in the rat by hyperoxia: Implications for hyperoxic lung injury. Mol Pharmacol. 2002;61(3):507–515. doi: 10.1124/mol.61.3.507. https://doi.org/10.1124/mol.61.3.507. [DOI] [PubMed] [Google Scholar]
- 49.Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B. Disruption of the ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes p4501a expression and exacerbates hyperoxic lung injury. J Pharmacol Exp Ther. 2004;310(2):512–519. doi: 10.1124/jpet.103.059766. https://doi.org/10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- 50.Lingappan K, Jiang W, Wang L, Wang G, Couroucli XI, Shivanna B, Welty SE, Barrios R, Khan MF, Nebert DW, Roberts LJ, et al. Mice deficient in the gene for cytochrome p450 (cyp)1a1 are more susceptible than wild-type to hyperoxic lung injury: Evidence for protective role of cyp1a1 against oxidative stress. Toxicol Sci. 2014;141(1):68–77. doi: 10.1093/toxsci/kfu106. https://doi.org/10.1093/toxsci/kfu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Lingappan K, Jiang W, Couroucli XI, Welty SE, Shivanna B, Barrios R, Wang G, Firoze Khan M, Gonzalez FJ, Jackson Roberts L, et al. Disruption of cytochrome p4501a2 in mice leads to increased susceptibility to hyperoxic lung injury. Free Radic Biol Med. 2015;82:147–159. doi: 10.1016/j.freeradbiomed.2015.01.019. https://doi.org/10.1016/j.freeradbiomed.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lingappan K, Maity S, Jiang W, Wang L, Couroucli X, Veith A, Zhou G, Coarfa C, Moorthy B. Role of cytochrome p450 (cyp)1a in hyperoxic lung injury: Analysis of the transcriptome and proteome. Sci Rep. 2017;7(1):642. doi: 10.1038/s41598-017-00516-x. https://doi.org/10.1038/s41598-017-00516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moorthy B. The cyp1a subfamily. In: Ioannides C, editor. Cytochrome p450: Role in the metabolism and toxicity of drugs and other xenobiotics. 2008. pp. 98–123. [Google Scholar]
- 54.Tang Y, Scheef EA, Gurel Z, Sorenson CM, Jefcoate CR, Sheibani N. Cyp1b1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress. Am J Physiol Cell Physiol. 2010;298(3):C665–678. doi: 10.1152/ajpcell.00153.2009. https://doi.org/10.1152/ajpcell.00153.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jennings BL, George LW, Pingili AK, Khan NS, Estes AM, Fang XR, Gonzalez FJ, Malik KU. Estrogen metabolism by cytochrome p450 1b1 modulates the hypertensive effect of angiotensin ii in female mice. Hypertension. 2014;64(1):134–140. doi: 10.1161/HYPERTENSIONAHA.114.03275. https://doi.org/10.1161/HYPERTENSIONAHA.114.03275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dinu D, Chu C, Veith A, Lingappan K, Couroucli X, Jefcoate CR, Sheibani N, Moorthy B. Mechanistic role of cytochrome p450 (cyp)1b1 in oxygen-mediated toxicity in pulmonary cells: A novel target for prevention of hyperoxic lung injury. Biochem Biophys Res Commun. 2016;476(4):346–351. doi: 10.1016/j.bbrc.2016.05.125. https://doi.org/10.1016/j.bbrc.2016.05.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhattacharyya S, Sinha K, Sil PC. Cytochrome p450s: Mechanisms and biological implications in drug metabolism and its interaction with oxidative stress. Curr Drug Metab. 2014;15(7):719–742. doi: 10.2174/1389200215666141125121659. https://doi.org/10.2174/1389200215666141125121659. [DOI] [PubMed] [Google Scholar]
- 58.Louvet A, Mathurin P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12(4):231–242. doi: 10.1038/nrgastro.2015.35. https://doi.org/10.1038/nrgastro.2015.35. [DOI] [PubMed] [Google Scholar]
- 59.Roberts BJ, Song BJ, Soh Y, Park SS, Shoaf SE. Ethanol induces cyp2e1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of cyp2e1. J Biol Chem. 1995;270(50):29632–29635. doi: 10.1074/jbc.270.50.29632. [DOI] [PubMed] [Google Scholar]
- 60.Leung TM, Nieto N. Cyp2e1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58(2):395–398. doi: 10.1016/j.jhep.2012.08.018. https://doi.org/10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez FJ. Role of cytochromes p450 in chemical toxicity and oxidative stress: Studies with cyp2e1. Mutat Res. 2005;569(1–2):101–110. doi: 10.1016/j.mrfmmm.2004.04.021. https://doi.org/10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez FJ. The 2006 bernard b. Brodie award lecture Cyp2e1. Drug Metab Dispos. 2007;35(1):1–8. doi: 10.1124/dmd.106.012492. https://doi.org/10.1124/dmd.106.012492. [DOI] [PubMed] [Google Scholar]
- 63.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of cyp2e1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271(20):12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 64.Laine JE, Auriola S, Pasanen M, Juvonen RO. Acetaminophen bioactivation by human cytochrome p450 enzymes and animal microsomes. Xenobiotica. 2009;39(1):11–21. doi: 10.1080/00498250802512830. https://doi.org/10.1080/00498250802512830. [DOI] [PubMed] [Google Scholar]
- 65.Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32(3):210–258. doi: 10.2165/00003088-199732030-00004. https://doi.org/10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 66.Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome p450 enzymes. Free Radic Biol Med. 1998;24(7–8):1324–1330. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 67.Grinkova YV, Denisov IG, McLean MA, Sligar SG. Oxidase uncoupling in heme monooxygenases: Human cytochrome p450 cyp3a4 in nanodiscs. Biochem Biophys Res Commun. 2013;430(4):1223–1227. doi: 10.1016/j.bbrc.2012.12.072. https://doi.org/10.1016/j.bbrc.2012.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zangar RC, Bollinger N, Weber TJ, Tan RM, Markillie LM, Karin NJ. Reactive oxygen species alter autocrine and paracrine signaling. Free Radic Biol Med. 2011;51(11):2041–2047. doi: 10.1016/j.freeradbiomed.2011.09.001. https://doi.org/10.1016/j.freeradbiomed.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reczek CR, Chandel NS. The two faces of reactive oxygen species in cancer. Annual Review of Cancer Biology. 2017;1(1):79–98. https://doi.org/10.1146/annurev-cancerbio-041916-065808. [Google Scholar]
- 70.Sun X, Ai M, Wang Y, Shen S, Gu Y, Jin Y, Zhou Z, Long Y, Yu Q. Selective induction of tumor cell apoptosis by a novel p450-mediated reactive oxygen species (ros) inducer methyl 3-(4-nitrophenyl) propiolate. J Biol Chem. 2013;288(13):8826–8837. doi: 10.1074/jbc.M112.429316. https://doi.org/10.1074/jbc.M112.429316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panigrahy D, Kaipainen A, Greene ER, Huang S. Cytochrome p450-derived eicosanoids: The neglected pathway in cancer. Cancer Metastasis Rev. 2010;29(4):723–735. doi: 10.1007/s10555-010-9264-x. https://doi.org/10.1007/s10555-010-9264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson AL, Edson KZ, Totah RA, Rettie AE. Cytochrome p450 omega-hydroxylases in inflammation and cancer. Adv Pharmacol. 2015;74:223–262. doi: 10.1016/bs.apha.2015.05.002. https://doi.org/10.1016/bs.apha.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jamieson KL, Endo T, Darwesh AM, Samokhvalov V, Seubert JM. Cytochrome p450-derived eicosanoids and heart function. Pharmacol Ther. 2017 doi: 10.1016/j.pharmthera.2017.05.005. https://doi.org/10.1016/j.pharmthera.2017.05.005. [DOI] [PubMed]


