Abstract
Purpose
The striatum is a well-known region affected in Huntington disease (HD). However, other regions including the visual cortex are implicated. We previously identified an abnormal energy response in the visual cortex of patients at an early stage of HD using 31P magnetic resonance spectroscopy (MRS). We therefore sought to further characterize these metabolic alterations with 1H MRS using a well-validated semi-localized by adiabatic selective refocusing (semi-LASER) sequence that allows measuring an expanded number of neurometabolites.
Materials and methods
Ten early affected patients (Unified Huntington Disease Rating Scale (UHDRS), total motor score = 13.6 ± 10.8) and ten healthy volunteers of similar age and BMI were recruited for the study. We performed 1H MRS in the striatum – the region that is primary affected in HD – and the visual cortex.
Results
The protocol allowed a reliable quantification of 10 metabolites in the visual cortex and 8 in the striatum, compared to 3–5 metabolites in prior 1H MRS studies performed in HD. We identified higher total creatine (p < 0.05) in the visual cortex and lower glutamate (p < 0.001) and total creatine (p < 0.05) in the striatum of HD patients compared to controls. Less abundant neurometabolites (glutamine, GABA, glutathione, aspartate) showed similar concentrations in both groups.
Conclusion
The protocol allowed measurement of several additional metabolites compared to standard vendor protocol. Our study points to early changes in metabolites involved in energy metabolism in the visual cortex and striatum of HD patients. Decreased striatal glutamate could reflect early neuronal dysfunction or impaired glutamatergic neurotransmission.
Keywords: Huntington disease, movement disorders, 1H MRS, semi-LASER, neurometabolite, neurochemical profile
INTRODUCTION
Huntington disease (HD) is a polyglutamine disorder caused by expansion in the glutamine-encoding cytosine-adenine-guanine (CAG) repeats1 and striatal atrophy is a prominent characteristic of the disease.2 Other brain regions including the thalamus, cerebral cortex, cerebellum3,4 and visual cortex5 are also involved in the pathological process as the disease progresses. Positron emission tomography studies have shown metabolic alterations in the striatum6 whilst 31P MRS studies have revealed abnormal energy response in the visual cortex of HD patients at an early stage of the disease.7,8 With the availability of genetic testing for HD, there exists a therapeutic window that can be taken advantage of before symptoms onset. However, biomarkers are critically needed to evaluate presymptomatic individuals and make use of this therapeutic window5 since the Unified Huntington Disease Rating Scale (UHDRS),10 the most commonly used measure to assess disease severity, is unable to evaluate pathological processes prior to motor symptoms onset. In the search for biomarkers, 1H MRS has been used to study neurometabolism in HD at different field strengths.11–15 Metabolic alterations start indeed in the very early stages of the disease,16 and represent important targets for neuroprotective therapies.8
Previous studies with 1H MRS in HD were carried out at field strengths between 0.5 T and 7 T (Table 1), with several studies performed on 3 T systems that are becoming common in hospitals.12,13,15,26 In general, these studies have focused on the most prominent metabolites in the 1H spectrum – total N-acetylaspartate (tNAA), total creatine (tCr), total choline (tCho), and to a lesser extent myo-inositol (myo-Ins) and glutamate (Glu) – but not on less obvious metabolites such as gamma amino-butyric acid (GABA), glutamine (Gln), or glutathione (GSH). Some studies reported metabolite ratios only, which further complicate data interpretation.12,13 Moreover, most of these studies focused mainly on the striatum, caudate or putamen, and only a few on the occipital cortex (Table 1) even though abnormal metabolic alterations are also present in this region.6–8 Furthermore, these studies used stimulated echo acquisition mode (STEAM) and point-resolved spectroscopy (PRESS) with long or short TE without prior test of robustness or reproducibility. The present study thus sought to measure an expanded neurochemical profile in both the visual cortex and the striatum of early affected HD patients at 3 T, using a previously optimised and validated semi-localized by adiabatic selective refocusing (semi-LASER) sequence with water as an internal concentration reference and correction of concentrations for cerebrospinal fluid (CSF) content..
Table 1.
Summary of previous 1H MRS studies in the striatum and occipital cortex in HD.
|
|
||||
|---|---|---|---|---|
| Striatum | Caudate | Putamen | Occipital cortex | |
|
|
||||
| Presymptomatic vs controls | ||||
|
| ||||
| NAA/tNAA |
 NAA −8% but not tNAA Sturrock 2010 (n=25)14 NAA −8% but not tNAA Sturrock 2010 (n=25)14No change in NAA but  tNAA Sturrock 2015 (n=25)17 tNAA Sturrock 2015 (n=25)17
|
|||
|
| ||||
| No change in any metabolite | Gomez-Anson 2007 (n=19)18 | Van den Bogaard 2011 (n=11 reported)15 | Van den Bogaard 2011 (n=9 reported)15 Van Oostrom 2007 (n=19)19 |
|
|
| ||||
| Patients vs controls | ||||
|
| ||||
| NAA/tNAA |
 Jenkins 1993 (tNAA/Cr, n=15)20 Jenkins 1993 (tNAA/Cr, n=15)20 Jenkins 1998 (tNAA/Cr, n=31)11 Jenkins 1998 (tNAA/Cr, n=31)11 Clarke 1998 (NAA, n=6)12 Clarke 1998 (NAA, n=6)12
|
 Van den Bogaard 2011 (n=5 reported)15 Van den Bogaard 2011 (n=5 reported)15
|
 −17%/−15% Sturrock 2010 (n=29)14 −17%/−15% Sturrock 2010 (n=29)14 Van den Bogaard 2011 (n=5 reported)15 Van den Bogaard 2011 (n=5 reported)15 NAA Hoang 1998 (n=15)21 NAA Hoang 1998 (n=15)21 Sturrock 2015 (n=23/24)17 Sturrock 2015 (n=23/24)17
|
 NAA/Cr Hoang 199821 NAA/Cr Hoang 199821
|
|
| ||||
| myo-Ins |
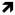 Hoang 1998 (n=15)21 Hoang 1998 (n=15)21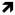 ~50% Sturrock 2010 (n=29)14 ~50% Sturrock 2010 (n=29)14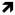 Sturrock 2015 (n=23/24)17 Sturrock 2015 (n=23/24)17
|
|||
|
| ||||
| tCr |
 Clarke 1998 (Cr) (n=6)12 Clarke 1998 (Cr) (n=6)12
|
 Van den Bogaard 2011 (n=5 reported)15 Van den Bogaard 2011 (n=5 reported)15
|
 Van den Bogaard 2011 (n=5 reported)15 Van den Bogaard 2011 (n=5 reported)15 Hoang 1998 (n=15)21 Hoang 1998 (n=15)21 −18% Sturrock 2010 (n=29)14 −18% Sturrock 2010 (n=29)14
|
|
|
| ||||
| Glu |
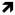 Glx Taylor-Robinson 1996 (Glx/tCr, n=5)22 Glx Taylor-Robinson 1996 (Glx/tCr, n=5)22
|
 Van den Bogaard 2011 (n=5 reported)15 Van den Bogaard 2011 (n=5 reported)15 ~10% Sturrock 2010 (n=29)14 ~10% Sturrock 2010 (n=29)14
|
||
|
| ||||
| Cho/tCho |
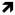 Jenkins 1993 (tCho/tCr, n=15)20 Jenkins 1993 (tCho/tCr, n=15)20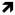 Jenkins 1998 (tCho/tCr, n=31)11 Jenkins 1998 (tCho/tCr, n=31)11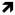 Clarke 1998 (Cho/Cr, n=6)12 Clarke 1998 (Cho/Cr, n=6)12
|
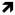 ~10% Sturrock 2010 (n=29)14 ~10% Sturrock 2010 (n=29)14 Hoang 1998 (n=15)21 Hoang 1998 (n=15)21
|
 Jenkins 1998 (n=31)11 Jenkins 1998 (n=31)11
|
|
|
| ||||
| Lac |
 Jenkins 1993 (n=15)20 Jenkins 1993 (n=15)20 Jenkins 1998 (n=31)11 Jenkins 1998 (n=31)11
|
 Jenkins 1993 (n=15)20 Jenkins 1993 (n=15)20 Jenkins 1998 (n=31)11 Jenkins 1998 (n=31)11
|
||
|
| ||||
| No change in any metabolite | Taylor-Robinson 199622 Hoang 1998 (abs conc)21 |
|||
|
| ||||
| Mixed presymptomatic/patients vs controls | ||||
|
| ||||
| NAA/tNAA |
 Sanchez-Pernaute 1999 (n=4 PMC, n=6 MC)23 Sanchez-Pernaute 1999 (n=4 PMC, n=6 MC)23
|
|||
|
| ||||
| myo-Ins | Trend
 Padowski 2014 (n=6 PMC, n=4 MC)24 Padowski 2014 (n=6 PMC, n=4 MC)24
|
|||
|
| ||||
| tCr |
 Sanchez-Pernaute 1999 (n=4 PMC, n=6 MC)23 Sanchez-Pernaute 1999 (n=4 PMC, n=6 MC)23
|
|||
|
| ||||
| Glu |
 Padowski 2014 (only when considering ratios) (n=6 PMC, n=4 MC)24 Padowski 2014 (only when considering ratios) (n=6 PMC, n=4 MC)24
|
|||
|
| ||||
| Mixed presymptomatic/patients longitudinal changes | ||||
|
| ||||
| NAA/tNAA |
 van den Bogaard 2014 (n=6 PMC, n=1 MC)25 van den Bogaard 2014 (n=6 PMC, n=1 MC)25
|
|||
|
| ||||
| myo-Ins |
 van den Bogaard 2014 (n=7 PMC, n=2 MC)25 van den Bogaard 2014 (n=7 PMC, n=2 MC)25
|
Trend myo-Ins/NAA
 Sturrock 2015 (n = 23/24 MC)17 Sturrock 2015 (n = 23/24 MC)17
|
||
|
| ||||
| Cr |
 van den Bogaard 2014 (n=7 PMC, n=2 MC)25 van den Bogaard 2014 (n=7 PMC, n=2 MC)25
|
|||
|
| ||||
| Cho |
 van den Bogaard 2014 (n=6 PMC, n=1 MC)25 van den Bogaard 2014 (n=6 PMC, n=1 MC)25
|
|||
|
| ||||
| No change in any metabolite | Sturrock 2015 (n=25/22 PMC, n= 23/24 MC)17 | |||
NAA: N-acetylaspartate, tNAA: total N-acetylaspartate. Cr: creatine, tCr: total creatine, Glu: glutamate, Glx: glutamine and glutamate, tCho: total choline, Lac: lactate, myo-Ins: myo-inositol, PMC: premanifest carrier; MC: manifest carrier, abs conc: absolute concentration;
 : increased concentration;
: increased concentration;
 : decreased concentration
: decreased concentration
MATERIAL AND METHODS
The study was designed to test whether we can reliably report on an expanded range of neurochemicals in HD using a validated sequence with minimal J-modulation, small chemical-shift displacement error, higher signal-to-noise ratio (SNR) and excellent outer volume suppression performance compared to common vendor-provided sequences.
Subjects
The local ethics committee approved this study and all subjects signed a written informed consent after the nature of the procedure had been fully explained before participating in the study. Motor dysfunction was evaluated with the total motor score (TMS) of UHDRS with a maximal worth score of 124. We recruited ten patients at the early stage of HD without medication as well as ten healthy volunteers with similar general characteristics – sex, age and BMI (Table 2).
Table 2.
Demographic and spectroscopic parameters of participants.
| Variable | Controls | HD patients |
|---|---|---|
| No of participants | 10.0 | 10.0 |
| Gender (M/F) | 3/7 | 3/7 |
| Age (years) | 38.9 ± 13.8 | 45.6 ± 12.7 |
| BMI (kg/m2) | 21.1 ± 1.7 | 21.6 ± 3.3 |
| TMS (UHDRS) | 0.9 ± 1.0 | 13.6 ± 10.8# |
| CAG length | 44.1 ± 4.2 | |
| Lw striatum (Hz) | 6.7 ± 1.0 | 8.4 ± 1.2 * |
| Lw visual cortex (Hz) | 4.6 ± 0.5 | 4.6 ± 0.5 |
| SNR striatum | 46.7 ± 11.3 | 39.0 ± 16.7 |
| SNR visual cortex | 65.6 ± 19.4 | 57.1 ± 14.5 |
| %CSF striatum | 4.5 ± 1.4 | 12.0 ± 8.4 * |
| %CSF visual cortex | 7.0 ± 4.8 | 17.0 ± 11.5 * |
Data are presented as mean ± standard deviation and compared by t-test. BMI: body mass index; TMS: total motor score; SNR: signal-to-noise ratio estimated by LCModel; Lw: spectral linewidth estimated by LCModel; CSF: cerebrospinal fluid.
p<0.05 and
p≤0.01 represent significant differences between HD patients and controls.
MR Protocol
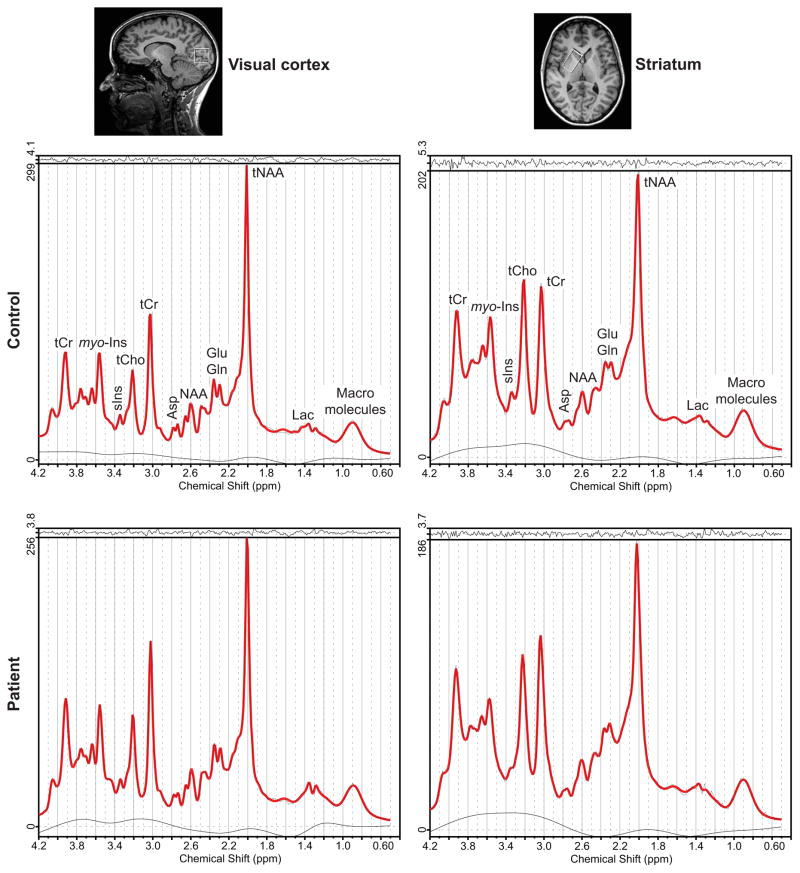
MRS data were acquired on a 3 T whole-body Siemens Magnetom Trio scanner (Siemens Medical Solutions, Erlangen, Germany). We used a modified semi-LASER 1H MRS protocol27 that has been previously tested in healthy controls for robustness and reproducibility in a bi-centric study and demonstrated highly reproducible data.28 Using a 32-channel receive head coil, 3D T1-weighted volumetric images (TR = 2530 ms, TE = 3.65 ms, 1 mm isotropic, FOV = 256 × 240 mm2, matrix size = 256 × 240) were acquired for spatial normalization and localization of brain volumes and for volumetric analysis of brain regions of interest. Shimming and spectra acquisition were performed on a 25 × 25 × 25 mm3 volume-of-interest (VOI) in the visual cortex and a 34 × 19 × 23 mm3 VOI in the striatum (Figure 1). Automatic B0 shimming in the voxels was performed with FAST(EST)MAP29 resulting in narrow linewidth. The calibration and acquisition steps were performed as described previously.27,28,30 The radiofrequency (RF) power for the asymmetric slice-selective 90° pulse (duration = 2 ms) of the semi-LASER sequence (TR = 5000 ms, TE = 28 ms, averages = 64, vector size = 2048, acquisition time = 6 min) was calibrated by determining the power that produced the maximum signal. The power for the 180° hyperbolic secant adiabatic full passage (AFP) pulses (duration = 4 ms) was set automatically based on the 90° pulse. For better suppression of unwanted coherences with shorter spoiler gradients, AFP pulses were interleaved. Water suppression pulses with the variable pulse power and optimized relaxation delays (VAPOR) were adjusted by following signal intensities to determine the power with maximum water suppression. The VAPOR pulses were interleaved with outer volume suppression (OVS) pulses to reduce contamination from other brain regions outside the VOI in the visual cortex (thickness: anterior = 200 mm, posterior = 40 mm) and the striatum (thickness: anterior = 80 mm, posterior = 80 mm). A 7 mm margin was left between the VOI and the OVS slices in order to avoid signal loss in the VOI due to the transition band of the OVS pulse profile. Two preparation scans were performed at the beginning of each spectral acquisition to achieve steady-state magnetization. Additionally, unsuppressed water spectra were acquired in each subject for eddy current correction (VAPOR on with RF off, OVS on) and another one (VAPOR and OVS off to avoid loss of water signal due to magnetization transfer effects) to use as an internal water concentration reference for metabolite quantification.28,30 The total scan time for each VOI including shimming, calibration and spectra acquisition was approximately 10 minutes. To evaluate the CSF contribution to VOI, we segmented the brain and estimated the %CSF content in the VOI based on T1 images as explained below. Metabolite concentrations were then corrected for regional CSF content assuming zero metabolite content in the CSF.
Figure 1.
Voxel positioning, spectra quality and model fitting by LCModel in the visual cortex and striatum. Spectra were acquired in an acquisition voxel of 25 × 25 × 25 mm3 in the visual cortex and 34 × 19 × 23 mm3 in the striatum using the modified semi-LASER sequence (TR = 5000 ms, TE = 28 ms, averages = 64). The black lines are the raw spectra, whilst the red lines are the LCModel fits. Asp: aspartate; Gln: glutamine; Glu: glutamate; Lac: lactate; myo-Ins: myo-inositol; NAA: N-acetylaspartate; sIns: scyllo-inositol; tCho: total choline; tCr: total creatine; tNAA: total N-acetylaspartate.
Metabolite quantification
Data pre-processing consisted of eddy current correction and shot-to-shot phase and frequency correction in MATLAB as previously described.28,30 Summed spectra were quantified with LCModel.31 The basis set was simulated using density matrix calculation in MATLAB and included: alanine, ascorbate, aspartate, creatine, γ-aminobutyric acid (GABA), glycerophosphorylcholine (GPC), phosphocholine (PCho), phosphocreatine (PCr), glucose (Glc), glutamine (Gln), glutamate (Glu), glutathione (GSH), myo-inositol (myo-Ins), scyllo-inositol (sIns), lactate, N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphorylethanolamine (PE) and taurine. The basis set also included macromolecule spectra that were acquired in healthy volunteers in a previous study.28 By using water from the same VOI as an internal concentration reference, we limited errors that otherwise could arise from the use of an external reference such as those related to B1 homogeneities and coil loading. Metabolite concentrations were corrected for CSF content and gray matter (GM)/white matter (WM) partial volume fraction assuming 100% water content in CSF, 78% water content in GM and 65% water content in WM as recommended in the LCModel manual. Even though atrophy is observed in HD, tissue water content seems to remain unchanged in HD.32 We thus assumed identical tissue water content in GM and WM in controls and patients. In principle, increased CSF fraction should not bias the quantification, since metabolite concentrations are corrected for CSF content. However, T2 relaxation of water during the semi-LASER protocol could introduce some bias. While we used a short TE of 28 ms, there is non-negligible relaxation of water especially in tissue, since T2 of water in tissue (GM and WM) is much shorter than T2 of water in CSF. If CSF content differs significantly among subjects, a small bias is introduced to the quantification (the more CSF, the less the water reference signal relaxes during TE). We thus corrected the water reference signal intensity for T2 relaxation during TE using T2 of water in CSF of 740 ms, and T2 of water in tissue (GM and WM) to be 100 ms33 using the formula:
Where fCSF, fGM and fWM are fractions of CSF, GM and WM in the voxel and T2,CSF, T2,GM and T2,WM are T2 relaxation times of CSF, GM, and WM.
While the reported T2 of water in brain tissue are approximately 80 ms, we increased these values by 25% to account for longer T2 during the Carr-Purcell-Meiboom-Gill train in LASER. The metabolite signal was corrected for relaxation using T2(metab,tissue) = 200 ms for all metabolites. While some metabolites have a longer T2 (e.g. tNAA), the small bias introduced by using the same T2 would be the same in the control and patient group and would not affect comparisons between the two groups.
Metabolites were considered reliably quantified when Cramér-Rao lower bounds (CRLB) ≤ 20% were observed in at least half the subject population as this threshold allows the selection of the most reliably quantified metabolites as recommended in the LCModel manual. However, in order to avoid quantification bias, we reported the average of all concentration values for each reliably quantified metabolite, including those with CRLB < 999%. For metabolites exhibiting high cross-correlation (correlation coefficient < −0.7) – e.g. Cr and PCr; and NAA and NAAG – only the sum was reported (e.g. tCr for Cr and PCr; tNAA for NAA and NAAG). All spectra had metabolite linewidth < 10 Hz, as estimated by LCModel (average ~5 Hz in visual cortex and ~7–8 Hz in striatum). Pre-processing and quantification steps took approximately 3 minutes per spectrum to complete.
Brain tissue volume estimation
To obtain reliable volume estimates, 3D T1 images were automatically segmented using Freesurfer v5.3 (https://surfer.nmr.mgh.harvard.edu/). The GM/WM interface as well as the GM volume segmentations were examined and reprocessed if corrections were needed. For each subject, VOIs were registered to their respective 3D T1 image and the volume fractions of the GM, WM and CSF within each VOI were extracted and quantified using tools available from Freesurfer.
Statistical analysis
We applied a student t-test to compare neurometabolite concentrations between patients and controls. Pearson correlations were performed between neurometabolite concentrations and TMS, CAG repeat length and brain volume fractions in the VOI with Holm-Bonferroni multiple comparison correction. Probability values of p < 0.05 were considered significant.
RESULTS
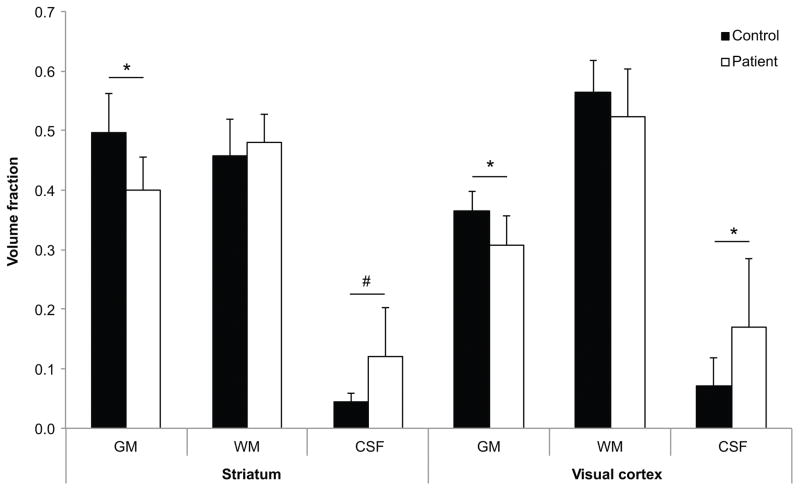
Reduced GM volume fractions and increased CSF fractions were observed in both striatum and visual cortex in HD patients compared to controls (Figure 2). Each subject’s volume fractions were used to correct for partial volume effects in determining metabolite concentrations. Correction for CSF content and for T2 relaxation greatly reduced the SD of metabolite concentrations and minimized sources of bias.
Figure 2.
Brain tissue volume fraction in VOI in the striatum and visual cortex. Gray matter (GM) was markedly reduced in the striatum and visual cortex whilst cerebrospinal fluid (CSF) was significantly increased. *p<0.05 and #p≤0.01 represent significant differences between patients with HD and controls.
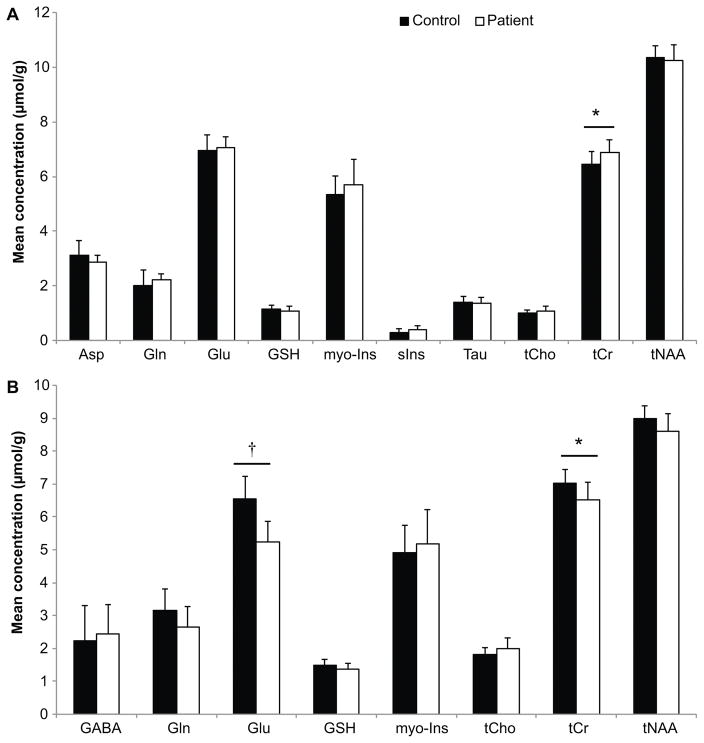
None of the datasets were excluded on the basis of spectral linewidth. We could not acquire one dataset from the striatum of a patient due to scanning time constraints. Hence nine datasets are reported from the striatum in patients. None of the metabolites reported in Figure 3 had CRLB = 999 for any subject. In the visual cortex, HD patients displayed a 7% increase in tCr relative to controls (p < 0.05) (Figure 3A). All other neurometabolites (Asp, Gln, Glu, GSH, myo-Ins, sIns, Tau, tCho and tNAA) had similar concentrations in both groups.
Figure 3.
Mean metabolite concentrations obtained in the A) visual cortex and B) striatum. Fewer metabolites are reported for the striatum since they did not meet the quality control threshold unlike in the visual cortex. Asp: aspartate, Gln: glutamine, Glu: glutamate, GSH: glutathione, myo-Ins: myo-inositol, sIns: scyllo-inositol, Tau: taurine, tCho: total choline, tCr: total creatine, tNAA: total N-acetylaspartate. Error bars represent standard deviations. *p< 0.05, †p≤ 0.001.
In the striatum, HD patients showed a large 20% decrease in Glu concentration (p < 0.001) and a 12% decrease in tCr (p < 0.05). All other metabolites were comparable to controls (Figure 3B). Total NAA showed a 4% trend to decrease in striatum (p = 0.08).
In order to examine less concentrated neurometabolites (aspartate, ascorbic acid, scyllo-inositol, lactate and taurine), the CRLB threshold was increased to 50%. These additional metabolites with the exception of aspartate in the striatum did not show any significant differences between patients and controls. (Supplementary Figure).
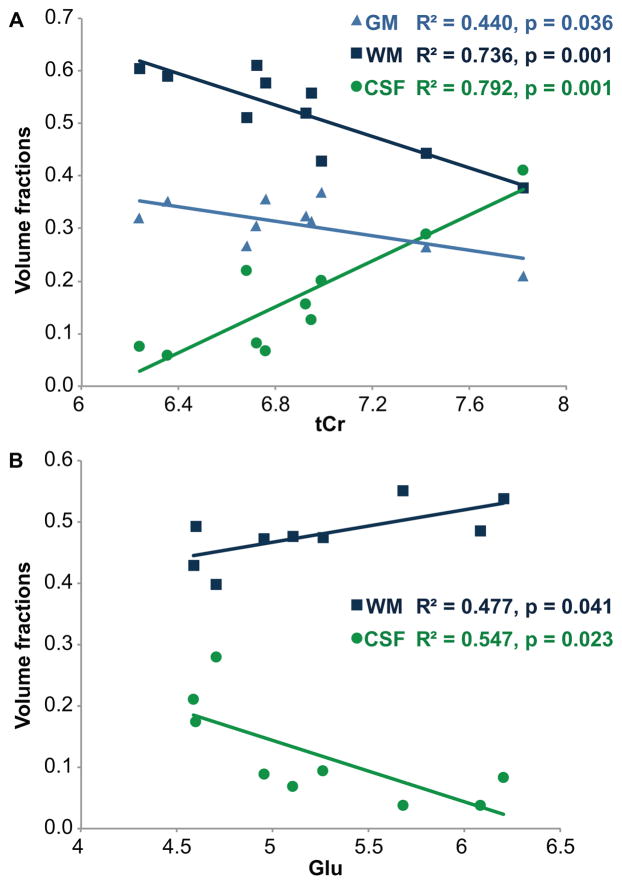
In the visual cortex of patients, tCr correlated positively with CSF fraction (p < 0.001), and negatively with GM (p < 0.05) and WM (p < 0.001) volume fractions (Figure 4A) but not with TMS, CAG repeat length or age. In the striatum of patients, Glu correlated negatively with CSF fraction (p < 0.05) and positively with WM volume fraction (p < 0.05) (Figure 4B) but not with TMS, CAG repeat length or age.
Figure 4.
Neurometabolite correlations with brain volume fractions of patients. A) tCr correlated positively with CSF fraction (p = 0.001), and negatively with GM (p < 0.05) and WM (p < 0.001) volume fractions in the visual cortex. B) Glu correlated negatively with CSF fraction (p < 0.05) and positively with WM volume fraction (p < 0.05) in the striatum.
DISCUSSION
We showed increased tCr in the visual cortex and decreased Glu and tCr in the striatum of patients at an early stage of HD, but no significant changes in less prominent metabolites such as GABA, Gln or GSH. Although the striatum is at the center of structural and metabolic changes in HD, we confirmed that the visual cortex is also involved in the early stage of the disease at both structural and metabolic levels. The extended analysis of 10 neurometabolites in the visual cortex and 8 in the striatum was possible due to a previously validated semi-LASER sequence providing high SNR, small chemical shift displacement and minimal J-modulation. The lower number of metabolites reported for the striatum compared to the visual cortex is due in part to broader linewidth (metabolite linewidth ~7–8 Hz in striatum compared to ~5 Hz in visual cortex). In contrast, GABA was quantified with CRLB < 20% in the striatum but not in the visual cortex due to its two-fold higher concentration in the striatum. Metabolites of low concentration such as Asp, GSH, sIns are considered difficult to quantify in short TE spectra at 3 T due to their small resonance peaks. In addition, small changes to the baseline could influence their quantification even when excellent spectral resolution and SNR are achieved. Nonetheless, we are providing concentrations for all metabolites with the less stringent threshold CRLB < 50% as Supplementary Figure, to be considered with appropriate caution. The finding of a significantly reduced aspartate in the striatum in patients will need to be confirmed in a larger cohort.
Most clinical protocols use PRESS and STEAM sequences, which are vendor provided sequences. In the previous studies compiled in Table 1, long TE protocols19,22 allowed only a limited number of metabolites to be reported since J-coupled and shorter T2 metabolites could not be measured. Other studies used short TE STEAM12,15,25 and PRESS14,17,24 protocols to report “absolute” (i.e. water-referenced) concentrations or ratios of metabolites. However, STEAM produces spectra with ~two-fold lower SNR and PRESS suffers from high chemical shift displacement error.34 In semi-LASER, the two pairs of 180° AFP pulses minimize J-modulation and allow measurement of J-coupled as well as singlet metabolites with optimal sensitivity.28
Cr and PCr whose summed resonance peak is referred to as total creatine (tCr), serve as an energetic marker and maintains brain energy homeostasis.35 The importance of tCr may stem from the limited glucose storage capability of the brain.36 Increased tCr has been reported in the brain of HD mouse models37 and this increase preceded the depletion of ATP.38 Patients with spinocerebellar ataxia type 1, 2 and 3, another group of polyglutamine disorders that share common pathophysiological pathways with HD,39 also displayed increased tCr in the vermis and the pons.30 In addition, the relative concentrations of components of energy metabolism – ATP, PCr and inorganic phosphate (Pi) – were previously measured using 31P MRS in the visual cortex of patients with HD before, during and after visual stimulation.7,8 Healthy controls exhibited a normal profile, with increased Pi/PCr ratio during visual stimulation, followed by a return to baseline levels during recovery. In contrast, HD patients displayed no significant change in Pi/PCr ratio in two independent studies.7,8 The higher tCr in the visual cortex reported here is in agreement with these studies. Altogether, these studies indicate that creatine metabolism is modified in the visual cortex of HD patients, a region that is particularly enriched in mitochondria,7,8 at an early stage of the disease and may signify a compensatory mechanism. In the striatum however, we observed a decrease in tCr concentration which is in line with some previous studies.12,14,15,21 This decrease might point to a failure to initiate energetic compensatory mechanisms as observed in the visual cortex. It should be noted that the p values reported for tCr and Glu were not corrected for multiple comparisons within groups. It is therefore possible that our observations on tCr, with p values close to statistical significance, might more likely be a trend to increase. Our interpretation of tCr changes must therefore be taken with caution considering the small sample size.
The significant decrease in the neuronal marker Glu in the striatum is in agreement with previous reports from the putamen.14,15 Decreased Glu could be due in part to the lower GM content in the striatal VOI. However, assuming that Glu is two-fold higher in GM than in WM,40 we calculated that the measured decrease in GM content would have resulted in a less than 5% decrease in Glu concentration in the VOI, and therefore did not explain the 20% decrease observed in our study. Since it has been shown that the striatum is highly affected in HD, even at the presymptomatic stage,2 decreased Glu concentrations could reflect neuronal dysfunction as a result of atrophy. Furthermore, Glu levels have been linked to metabolic activity.35 Twenty percent of brain glucose metabolism is directed to Glu synthesis through the Gln-Glu cycle.41 As patients with HD display glucose hypometabolism in the striatum and cortex,42 decreased Glu levels could reflect decreased metabolic activity in HD patients.
Consistent with several other studies reported in Table 1, which reported changes in tNAA, we observed a decreasing trend in tNAA in the striatum (from 9.0 ± 0.4 to 8.6 ± 0.5 μmol/g), although it did not reach statistical significance (p = 0.08). This could be due to the fact that our cohort was at a very early stage of the disease or that we had a relatively small sample size. Some previous studies reported no change in tNAA but a decrease in NAA.12,14,15,21 In fact, in our study, NAA was significantly decreased in the striatum (from 8.6 ± 0.3 to 7.8 ± 0.5 μmol/g, p < 0.001), but this decrease was partly compensated by an increase in NAAG (from 0.4 ± 0.3 to 0.8 ± 0.4 μmol/g, p < 0.02). However, individual NAA and NAAG concentrations are generally considered unreliable at 3 Tesla due to their high cross-correlation, and we consider that only the sum tNAA can be quantified reliably.
GABA is another important metabolite in HD since the medium-sized spiny neurons that contain GABA undergo selective degeneration in the striatum.43 Although spectral editing is often performed to quantify GABA by MRS, GABA can also be quantified from high-quality short TE 1H spectra such as obtained with LASER or semi-LASER, especially in brain regions that have higher GABA concentration.44 Here GABA was quantified with good precision in striatum due to its higher concentration (~2.2 – 2.4 μmol/g), but not in visual cortex (~1 μmol/g). One previous study reported GABA in striatum in HD at 7 Tesla and also reported no change.45
Reports of changes in myo-Ins and tCho in the striatum in early HD are mixed. Some studies showed increased myo-Ins and/or tCho, but some did not (see Table 1). Our study showed no evidence of changes in tCho or myo-Ins in the striatum, in agreement with the only reported measurements in striatum in HD at 7 Tesla,15 a higher magnetic field expected to provide improved accuracy. Our study is also consistent with the main findings of that study,15 namely lower Glu, lower tCr and lower tNAA (or in our case trend to lower tNAA) in the striatum. The main evidence for increased myo-Ins in the striatum in early HD is from Sturrock et al.14 However, in that study, spectra from early HD patients appear much broader than spectra from controls and presymptomatic individuals, and the degraded spectral quality could conceivably affect myo-Ins and tCho concentrations determined by LCModel. Some earlier studies reported increased tCho based on increased tCho/tCr ratio; however, more recent studies using water as a concentration reference suggest that the increased ratio actually results from decreased tCr rather than increased tCho.
In conclusion, this study used an optimized semi-LASER protocol to help understand the early neurometabolic alterations that occur in HD. We reported absolute metabolite concentrations and not ratios,11,20–22,24 in order to be able to analyse the impact of each individual metabolite. Unlike all the aforementioned studies summarized in Table 1, our semi-LASER protocol allowed us to obtain an expanded neurochemical profile of up to 10 metabolites, which is promising to find robust biomarkers in HD.
Supplementary Material
Acknowledgments
Grant support: This study was funded by NIH grants (P41EB015894 and P30NS076408) and the Ecole des Neurosciences de Paris. The study also received funding from Ipsen (NCT01696708) and the program “Investissements d’avenir” ANR-10-IAIHU-06 and ANR-11-INBS-0006.
The authors wish to thank warmly the patients and volunteers who participated in this study. The authors are also grateful to collaborators from the Center for Neuroimaging Research, France, and the Center for Magnetic Resonance Research, USA.
List of abbreviations
- AFP
Adiabatic full passage
- HD
Huntington disease
- CAG
Cytosine-adenine-guanine trinucleotide
- UHDRS
Unified Huntington Disease Rating Scale
- GM
Gray matter
- WM
White matter
- CSF
Cerebrospinal fluid
- OVS
Outer volume suppression
- VAPOR
Variable power and optimized relaxation delays
- VOI
Volume of interest
- RF
Radiofrequency
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
No disclosure: Adanyeguh, Monin, Rinaldi.
Freeman: Research support by grants from the National Multiple Sclerosis Society.
Durr: Research support by grants from the French Agency for Research, Fondation pour la recherche médicale (FRM), and Pfizer Inc.
Lehericy: Received grants from Agence Nationale de la Recherche (ANRMNP 2009, Nucleipark), DHOS-Inserm (2010, Nucleipark), France Parkinson (2008), Ecole Neuroscience de Paris, ‘Investissements d’avenir’ [grant number ANR-10-IAIHU-06 and ANR-11-INBS-0006] during the conduct of the study. Outside of this study, he received commercial research support from Servier and Pfizer, funding for travel from Siemens and General Electric and honoraria from Pileje, Lundbeck and Roche.
Henry: Research support by grants from NIH (P41 EB015894, P30 NS076408), Friedreich’s Ataxia Research Alliance and CureFA foundation.
Mochel: Research support by grants from INSERM, Carnot Institutes, ASL Foundation and Ultragenyx Pharmaceutical.
References
- 1.Kremer B, Goldberg P, Andrew SE, et al. A worldwide study of the Huntington’s disease mutation. The sensitivity and specificity of measuring CAG repeats. N Engl J Med. 1994;330(20):1401–1406. doi: 10.1056/NEJM199405193302001. [DOI] [PubMed] [Google Scholar]
- 2.Tabrizi SJ, Scahill RI, Owen G, et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12:637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- 3.Rosas HD, Koroshetz WJ, Chen YI, et al. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60:1615–1620. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- 4.Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel JP, Faull RL. The Neuropathology of Huntington’s Disease. Curr Top Behav Neurosci. 2015;22:33–80. doi: 10.1007/7854_2014_354. [DOI] [PubMed] [Google Scholar]
- 5.Rub U, Seidel K, Vonsattel JP, et al. Huntington’s Disease (HD): Neurodegeneration of Brodmann’s Primary Visual Area 17 (BA17) Brain Pathol. 2015;25(6):701–711. doi: 10.1111/bpa.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigin A, Leenders KL, Moeller JR, et al. Metabolic network abnormalities in early Huntington’s disease: an [(18)F]FDG PET study. J Nucl Med. 2001;42(11):1591–1595. [PubMed] [Google Scholar]
- 7.Mochel F, N’Guyen TM, Deelchand D, et al. Abnormal response to cortical activation in early stages of Huntington disease. Mov Disord. 2012;27:907–910. doi: 10.1002/mds.25009. [DOI] [PubMed] [Google Scholar]
- 8.Adanyeguh IM, Rinaldi D, Henry PG, et al. Triheptanoin improves brain energy metabolism in patients with Huntington disease. Neurology. 2015;84:490–495. doi: 10.1212/WNL.0000000000001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corvol JC. Neuroprevention: a new challenge? Rev Neurol (Paris) 2012;168:796–801. doi: 10.1016/j.neurol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Huntington Study Group. Unified Huntington’s disease rating scale: Reliability and consistency. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins BG, Rosas HD, Chen YC, et al. 1H NMR spectroscopy studies of Huntington’s disease: correlations with CAG repeat numbers. Neurology. 1998;50:1357–1365. doi: 10.1212/wnl.50.5.1357. [DOI] [PubMed] [Google Scholar]
- 12.Clarke CE, Lowry M, Quarrell OWJ. No change in striatal glutamate in Huntington’s disease measured by proton magnetic resonance spectroscopy. Parkinsonism Relat Disord. 1998;4:123–127. doi: 10.1016/s1353-8020(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 13.Ruocco HH, Lopes-Cendes I, Li LM, Cendes F. Evidence of thalamic dysfunction in Huntington disease by proton magnetic resonance spectroscopy. Mov Disord. 2007;22:2052–2056. doi: 10.1002/mds.21601. [DOI] [PubMed] [Google Scholar]
- 14.Sturrock A, Laule C, Decolongon J, et al. Magnetic resonance spectroscopy biomarkers in premanifest and early Huntington disease. Neurology. 2010;75:1702–1710. doi: 10.1212/WNL.0b013e3181fc27e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Bogaard SJ, Dumas EM, Teeuwisse WM, et al. Exploratory 7-Tesla magnetic resonance spectroscopy in Huntington’s disease provides in vivo evidence for impaired energy metabolism. J Neurol. 2011;258:2230–2239. doi: 10.1007/s00415-011-6099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liot G, Valette J, Pépin J, Flament J, Brouillet E. Energy defects in Huntington’s disease: Why “in vivo” evidence matters. Biochem Biophys Res Commun. 2017;483:1084–1095. doi: 10.1016/j.bbrc.2016.09.065. [DOI] [PubMed] [Google Scholar]
- 17.Sturrock A, Laule C, Wyper K, et al. A longitudinal study of magnetic resonance spectroscopy Huntington’s disease biomarkers. Mov Disord. 2015;30:393–401. doi: 10.1002/mds.26118. [DOI] [PubMed] [Google Scholar]
- 18.Gómez-Ansón B, Alegret M, Muñoz E, et al. Decreased frontal choline and neuropsychological performance in preclinical Huntington disease. Neurology. 2007;68:906–910. doi: 10.1212/01.wnl.0000257090.01107.2f. [DOI] [PubMed] [Google Scholar]
- 19.van Oostrom JC, Sijens PE, Roos RA, Leenders KL. 1H magnetic resonance spectroscopy in preclinical Huntington disease. Brain Res. 2007;1168:67–71. doi: 10.1016/j.brainres.2007.05.082. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins BG, Koroshetz WJ, Beal MF, Rosen BR. Evidence for impairment of energy metabolism in vivo in Huntington’s disease using localized 1H NMR spectroscopy. Neurology. 1993;43:2689–2695. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- 21.Hoang TQ, Bluml S, Dubowitz DJ, et al. Quantitative proton-decoupled 31P MRS and 1H MRS in the evaluation of Huntington’s and Parkinson’s diseases. Neurology. 1998;50:1033–1040. doi: 10.1212/wnl.50.4.1033. [DOI] [PubMed] [Google Scholar]
- 22.Taylor-Robinson SD, Weeks RA, Bryant DJ, et al. Proton magnetic resonance spectroscopy in Huntington’s disease: evidence in favour of the glutamate excitotoxic theory. Mov Disord. 1996;11:167–173. doi: 10.1002/mds.870110209. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez-Pernaute R, García-Segura JM, del Barrio Alba A, et al. Clinical correlation of striatal 1H MRS changes in Huntington’s disease. Neurology. 1999;53:806–812. doi: 10.1212/wnl.53.4.806. [DOI] [PubMed] [Google Scholar]
- 24.Padowski JM, Weaver KE, Richards TL, et al. Neurochemical correlates of caudate atrophy in Huntington’s disease. Mov Disord. 2014;29:327–335. doi: 10.1002/mds.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Bogaard SJ, Dumas EM, Teeuwisse WM, et al. Longitudinal metabolite changes in Huntington’s disease during disease onset. J Huntingtons Dis. 2014;3:377–386. doi: 10.3233/JHD-140117. [DOI] [PubMed] [Google Scholar]
- 26.Tkác I, Öz G, Adriany G, Uğurbil K, Gruetter R, et al. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62:868–879. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Öz G, Tkáč I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: Validation in the cerebellum and brainstem. Magn Reson Med. 2011;65:901–910. doi: 10.1002/mrm.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deelchand DK, Adanyeguh IM, Emir UE, et al. Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single-voxel MRS at 3T. Magn Reson Med. 2014;73:1718–1725. doi: 10.1002/mrm.25295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;4(2):319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Adanyeguh IM, Henry PG, Nguyen TM, et al. In vivo neurometabolic profiling in patients with spinocerebellar ataxia types 1, 2, 3, and 7. Mov Disord. 2015;30:662–670. doi: 10.1002/mds.26181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 32.Zacharoff L, Tkac I, Song Q, et al. Cortical metabolites as biomarkers in the R6/2 model of Huntington’s disease. J Cereb Blood Flow Metab. 2012;32:503–514. doi: 10.1038/jcbfm.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, Golay X, van Zijl PC, et al. Inverse T(2) contrast at 1.5 Tesla between gray matter and white matter in the occipital lobe of normal adult human brain. Magn Reson Med. 2001;46:401–406. doi: 10.1002/mrm.1204. [DOI] [PubMed] [Google Scholar]
- 34.Boer VO, van Lier AL, Hoogduin JM, Wijnen JP, Luijten PR, Klomp DW. 7-T 1H MRS with adiabatic refocusing at short TE using radiofrequency focusing with a dual-channel volume transmit coil. NMR Biomed. 2011;24:1038–1046. doi: 10.1002/nbm.1641. [DOI] [PubMed] [Google Scholar]
- 35.Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014;39:1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- 36.Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem. 2000;74:1968–1978. doi: 10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- 37.Tkac I, Dubinsky JM, Keene CD, Gruetter R, Low WC, et al. Neurochemical changes in Huntington R6/2 mouse striatum detected by in vivo 1H NMR spectroscopy. J Neurochem. 2007;100:1397–1406. doi: 10.1111/j.1471-4159.2006.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mochel F, Durant B, Meng X, et al. Early alterations of brain cellular energy homeostasis in Huntington disease models. J Biol Chem. 2012;287:1361–1370. doi: 10.1074/jbc.M111.309849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet. 2005;6:743–755. doi: 10.1038/nrg1691. [DOI] [PubMed] [Google Scholar]
- 40.Hassel B, Dingledine R. Glutamate. In: Siegel GJ, Albers RW, Brady S, Price D, editors. Basic neurochemistry: molecular, cellular and medical aspects. 7. San Diego: Elsevier Academic Press; 2005. pp. 267–290. [Google Scholar]
- 41.Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Topics Behav Neurosci. 2012;11:199–251. doi: 10.1007/7854_2011_197. [DOI] [PubMed] [Google Scholar]
- 42.Pagano G, Niccolini F, Politis M. Current status of PET imaging in Huntington’s disease. Eur J Nucl Med Mol Imaging. 2016;43:1171–1182. doi: 10.1007/s00259-016-3324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57(5):369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Oz G, Terpstra M, Tkác I, et al. Proton MRS of the unilateral substantia nigra in the human brain at 4 tesla: detection of high GABA concentrations. Magn Reson Med. 2006;55:296–301. doi: 10.1002/mrm.20761. [DOI] [PubMed] [Google Scholar]
- 45.Unschuld PG, Edden RA, Carass A, et al. Brain metabolite alterations and cognitive dysfunction in early Huntington’s disease. Mov Disord. 2012;27:895–902. doi: 10.1002/mds.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.