Fetal magnetocardiography (fMCG) is an emerging technology that has provided invaluable insight into the mechanisms of fetal arrhythmia. Its efficacy for diagnosis and management of serious fetal arrhythmia has been acknowledged in the recent American Heart Association Statement on Diagnosis and Treatment of Fetal Cardiac Disease (1).
FMCG is based on the principle that bioelectric currents generate surface magnetic fields, as well as surface potentials, which are proportional to the net current. The main technical requirement for fMCG sensors is magnetic field resolution ≤ 10 fT/(Hz)1/2 over a 0–100 Hz bandwidth. The number of sensors needed depends on the desired coverage of the maternal surface and the level of interference suppression required of the signal processing. Using a 2-shell magnetically-shielded room in a typical hospital environment, a minimum of 5–10 sensors is required.
A major barrier to clinical adoption of fMCG is the high cost and complexity of SQUID (Superconducting Quantum Interference Device) technology (2). Recently, however, a cheaper, more practical type of magnetometer, known as an optically-pumped magnetometer (OPM (3)), has become commercially available. In this study, we compare the quality of fMCG recordings made using SQUID-based and OPM-based systems.
The subjects were 15 healthy women, 8 with uncomplicated pregnancies and 7 with pregnancies complicated by fetal arrhythmia or a high risk of fetal arrhythmia.
The fMCG recordings were acquired within a 2-shell, magnetically-shielded room, using an FDA-cleared SQUID magnetometer system (Tristan 624 Biomagnetometer, Tristan Technologies, San Diego; Fig. 1A) and an array of OPM sensors (QuSpin Zero Field Magnetometer, QuSpin Inc., Louisville, Colorado; Fig. 1B). The OPM sensors are modular. A sensor array was formed by 3D-printing a holder to accommodate up to 8 sensors, arranged in a 3x3 square grid with 3.81 cm grid spacing with the center location occupied by a support post. The number of OPM sensors deployed increased from 3, initially, to 8, as additional sensors were acquired. At least 10 minutes of data were recorded with each device, moving the sensor at least once during the session.
Figure 1.
Photographs of (A) SQUID and (B) OPM fMCG systems. The OPM sensor array (circled) was supported by attaching it to a SQUID system.
Signal processing was used to remove maternal and environmental interference. The quality of the SQUID and OPM recordings were compared by computing the signal-to-noise ratio (SNR) of the rhythm strips, defined as the peak-to-peak amplitude of the fetal QRS complex divided by the root-mean-square noise.
The quality of the SQUID recordings was good to excellent in all of the subjects. The SNR ranged from 12.8 to 48.2. Compared to the SQUID data, the OPM data showed significantly lower SNR for the first 11 fetuses studied, with SNR ratios ranging from 0.13 to 0.75. Most of these subjects were studied using 6 or fewer OPM sensors, and noise from the data acquisition system degraded the SNR by about a factor of two. For the last 4 subjects, however, the SNR ratios were much closer to unity, ranging from 0.74–1.04. These subjects were studied using 8 OPM sensors and a higher-performance data acquisition system.
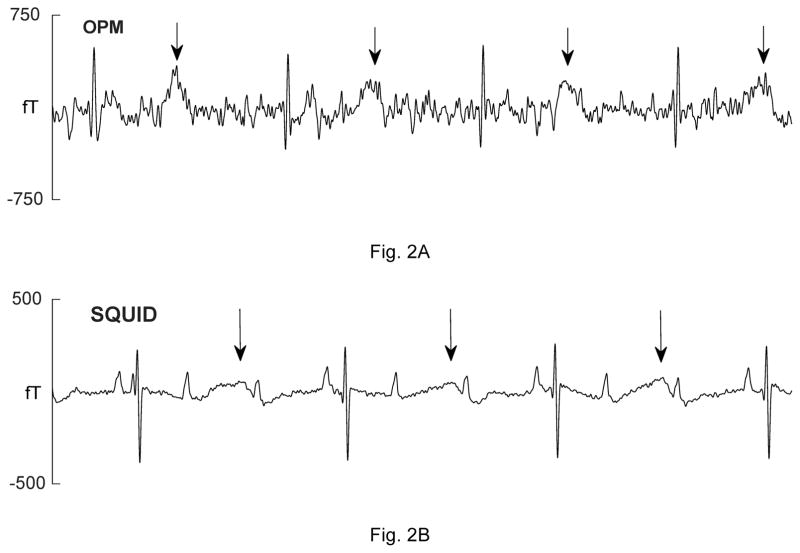
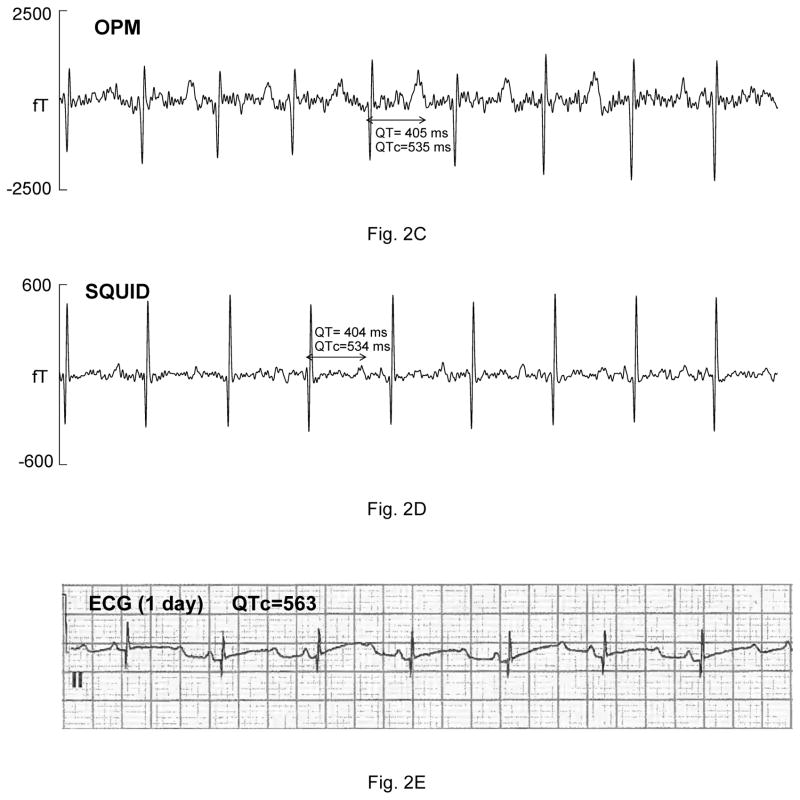
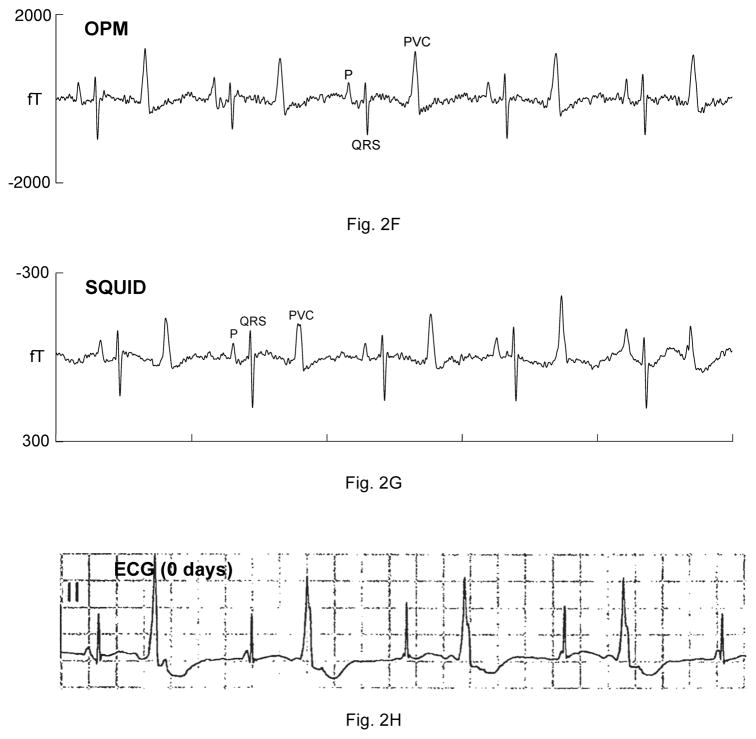
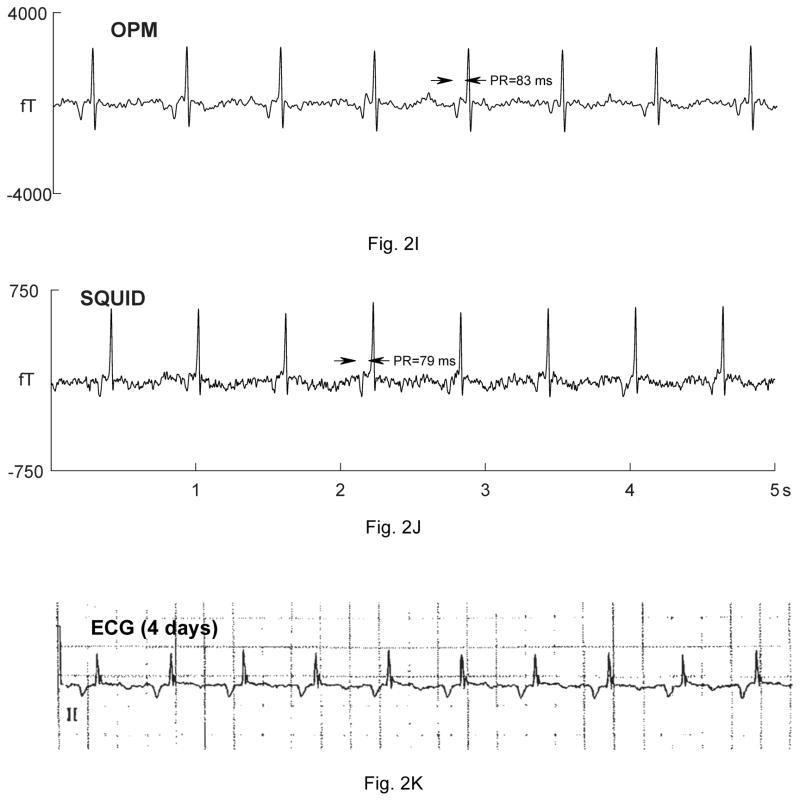
Five fetuses showed serious, sustained arrhythmias. The first had sinus bradycardia with intermittent atrial flutter (not shown). The second was referred with a diagnosis of AV block. The fMCG revealed the critical finding that the AV block was secondary to severe QTc prolongation (QTc>700 ms; Fig. 2A–B). The fetus was treated with oral magnesium, but died suddenly 10 days after the fMCG study. The third fetus had a mother with long QT syndrome (KCNQ1 mutation). The fMCG at 29 weeks’ gestation showed severe QT prolongation suggestive of LQTS (Fig. 2C–D), which was confirmed postnatally (Fig. 2E). The fourth showed a predominant rhythm of ventricular bigeminy (Fig. 2F–G) with occasional short runs of ventricular tachycardia and a virtual absence of normal sinus rhythm. The fifth had a low atrial rhythm, which resulted in a low heart rate and a short PR interval (Fig. 2I–J). The diagnosis of a low atrial rhythm prompted the referring physician to reexamine the fetus for heterotaxy, which was subsequently confirmed. Two others showed frequent ectopy due to premature ventricular and premature atrial contractions (not shown).
Figure 2.
Comparison of OPM and SQUID recordings from four fetuses with sustained arrhythmia. Postnatal ECGs are shown for the last three. All of the tracings are 5 seconds long. A) and B) are from a fetus at 33-4/7 weeks’ with extreme QTc prolongation (QTc> 700 ms), resulting in 3:1 AV block. The T-waves are indicated by arrows. C) and D) are from a fetus at 29 weeks’ with QTc prolongation. F) and G) are from a fetus at 33-6/7 weeks’ with a complex, irregular rhythm. As shown, the predominant rhythm was ventricular bigeminy, in which a sinus beat alternates with a premature ventricular contraction. I) and J) are from a fetus at 30-1/7 weeks’ with a low atrial rhythm, characterized by a low heart rate, inverted P-wave, and short PR interval.
The main finding of this study is that OPMs can detect fetal rhythm abnormalities with efficacy similar to that of an FDA-approved SQUID magnetometer. Despite the modest number of subjects, we also demonstrated the ability of the OPMs to detect abnormal repolarization, a critical and unique capability of fMCG. Efforts are underway to operate OPMs in small, person-sized magnetic shields, which are less expensive and portable. We are optimistic that the lower cost and practicality of OPMs will enable fMCG to be much more widely available in the near future.
Acknowledgments
FINANCIAL SUPPORT
National Institutes of Health, Bethesda, Maryland 20892, USA, grant numbers R44 HD080045 and R01 HL63174
The funding organizations had no role in any of the following: design and conduct of the study; collection, analysis, and interpretation of the data; or the preparation, review, and approval of the manuscript.
RELATIONSHIPS WITH INDUSTRY
The study was supported in part by a Small Business Innovative Research grant (R44 HD080045) awarded to QuSpin, Inc. Vishal Shah is the principle investigator of the grant; the other authors are co-investigators. Shah is founder and Chief Scientist of QuSpin, Inc. Orang Alem is Principal Physicist at QuSpin Inc.
Abbreviations
- FHR
fetal heart rate
- fMCG
fetal magnetocardiography
- ICA
independent component analysis
- LQTS
long QT syndrome
- OPM
optically-pumped magnetometer
- SQUID
superconducting quantum interference device
- SNR
signal-to-noise ratio
References
- 1.Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 2.Fagaly RL. Neuromagnetic instrumentation. Adv Neurol. 1990;54:11–32. [PubMed] [Google Scholar]
- 3.Budker D, Romalis M. Optical magnetometry. Nat Phys. 2007;3:227–234. [Google Scholar]