Abstract
Individuals with obsessive-compulsive disorder often identify psychosocial stress as a factor that exacerbates their symptoms, and many trace the onset of symptoms to a stressful period of life or a discrete traumatic incident. However, the pathophysiological relationship between stress and obsessive-compulsive disorder remains poorly characterized: it is unclear whether trauma or stress is an independent cause of obsessive-compulsive disorder symptoms, a triggering factor that interacts with a preexisting diathesis, or simply a nonspecific factor that can exacerbate obsessive-compulsive disorder along with other aspects of psychiatric symptomatology. Nonetheless, preclinical research has demonstrated that stress has conspicuous effects on corticostriatal and limbic circuitry. Specifically, stress can lead to neuronal atrophy in frontal cortices (particularly the medial prefrontal cortex), the dorsomedial striatum (caudate), and the hippocampus. Stress can also result in neuronal hypertrophy in the dorsolateral striatum (putamen) and amygdala. These neurobiological effects mirror reported neural abnormalities in obsessive-compulsive disorder and may contribute to an imbalance between goal-directed and habitual behavior, an imbalance that is implicated in the pathogenesis and expression of obsessive-compulsive disorder symptomatology. The modulation of corticostriatal and limbic circuits by stress and the resultant imbalance between habit and goal-directed learning and behavior offers a framework for investigating how stress may exacerbate or trigger obsessive-compulsive disorder symptomatology.
Keywords: OCD, stress, habit, goal-directed, corticostriatal-limbic circuitry
Introduction
Obsessive-compulsive disorder (OCD) is characterized by chronic and interfering obsessions, compulsions, and avoidance.1 Obsessions are intrusive, distressing thoughts that are typically perceived as irrational. Intrusive thoughts are nearly ubiquitous in the general population,2,3 but individuals with OCD interpret their intrusions as more meaningful and distressing and, as such, habitually avoid situations or stimuli that may trigger the intrusions, or perform compulsive rituals in attempts to control obsessions and concomitant negative affect. OCD patients typically feel an urge to perform repetitive compulsions, often under strict, idiosyncratic guidelines; these behaviors are often irrational or performed to extreme excess.1 Despite being relatively uncommon in the general population—12-month and lifetime prevalence of OCD is estimated to be 1.2% and 2.3%, respectively4—OCD is a top cause of disability among all psychiatric conditions.5
Heritability studies have demonstrated that OCD has a significant genetic component but have also made clear that environmental factors contribute importantly to its etiology.6–8 There is considerable evidence that exposure to stressful and traumatic events constitutes an important environmental risk factor. We review the evidence for a relationship between stress and OCD and consider the different forms that this relationship may take; current evidence is not sufficient to arbitrate among several possibilities, which are not mutually exclusive. We then provide a selective review of current neurobiological and cognitive theories of the pathophysiology of OCD and consider how the effects of stress may interact with these mechanisms. Stress has striking effects on the corticostriatal and limbic circuitry, which are dysfunctional in OCD; this permits formulation of a general hypothesis as to how stress may trigger or exacerbate OCD symptomatology.
Stress, Trauma, and OCD
Numerous environmental events have been identified as potential contributors to OCD, and many of these can be considered stressful in a broad sense. These include, but are not limited to, perinatal and childbirth complications, age-related changes in reproductive systems, parental rearing styles, socioeconomic problems, and bodily insults or injuries.9 We focus here more specifically on the effects of psychosocial stress and stressful life events—including exposure to traumatic events—on OCD symptoms.10–14
Psychosocial stress interacts with underlying vulnerability factors in many neuropsychiatric conditions, including mood and anxiety disorders,15 as well as posttraumatic stress disorder (PTSD) and other trauma-related conditions.16,17 This has been captured in the concept of a “stress-diathesis” interaction in the development of psychopathology.18,19 A similar stressor can produce disparate effects on two individuals, producing pathology in one but minimal effects, or even enhanced resilience, in another. The term diathesis refers to this preexisting vulnerability to pathogenic effects of a stressor; such a vulnerability may derive from genetic factors, developmental events, or earlier experience and may be conceptualized as biological, psychological, or a combination of the two. It is presumed that all individuals have some level of diathetic vulnerability and that everyone experiences stress; the development of symptoms in a particular individual, therefore, derives from a complex interaction between the two.
In a particular condition, such as OCD, stress may interact with symptomatology in several different ways; these may co-exist within the population or even in an individual. First, in some individuals, stress may lead directly and causally to the development of symptomatology—that is, the vulnerability may be generic, and the stress may produce the specific illness. Second, an individual may have a specific vulnerability to a particular condition, but it may be latent until triggered by a stressful experience. Third, an illness may exist independently, but it may be exacerbated (either permanently or episodically) by elevated psychosocial stress. The type and severity of stressors may be of importance for all three of these examples; that is, specific types of stressors may differentially affect vulnerabilities and manifestations of symptoms. In considering the limited literature documenting an association between stressful life events and OCD symptomatology, it is useful to keep these different modes of interaction in mind.
Acutely, stress can induce obsessionality in both healthy and psychiatric populations.20–23 No study has experimentally examined the effects of acute stressors on obsessionality among OCD patients, but self-report studies indicate that 25–67% of OCD patients report significant life events (a majority of which are stressful) in relation to the onset of their OCD.24–27 Patients with OCD also report significantly more stressful life events 6 months14,28 and 12 months29,30 before disease onset, as well as over their lifetime,28,30 compared with nonclinical controls. Moreover, the severity of both obsessions and compulsions is significantly correlated with the number of stressful life events experienced in the year prior to OCD onset and over the patient's lifetime.28,30,31 Finally, OCD patients who report stressful life events prior to disease onset also report significantly more abrupt symptom onset and reduced likelihood of family history of OCD compared to OCD patients who report no such stressful life events.25,27 This may suggest a larger environmental contribution to pathophysiology in these cases.
A caveat to all of these studies is that they may be subject to recall bias: OCD symptom onset may occur irrespective of stressful events but be associated with symptoms post hoc as patients attempt to make sense of their experience. This confound can be partially addressed using a longitudinal design. One longitudinal study found that high school and middle school students who reported stressful life events were 21% more likely to go on to meet criteria for OCD 12 months later when compared to students who reported no such events.32 These findings suggest a causal association between stressful life events (or at least the subjective experience thereof) and the onset of OCD symptoms; although stressful life events may also serve as a marker for symptom development. It must be noted that a smaller longitudinal study failed to find significant relationships between stressful life events and the subsequent development of OCD symptoms; more work is needed here.33
Trauma represents an extreme form of psychosocial stress. There is a sizable body of literature linking exposure to traumatic events and development of OCD symptoms.9,34 Exposure to traumatic events appears to be associated with increased OCD symptom severity,35,36 particularly compulsions,37 and most research suggests that those exposed to such events are at an elevated risk for developing OCD.38 For example, individuals who retrospectively reported exposure to traumatic events in childhood are five to nine times more likely to meet criteria for OCD in adulthood than those without a trauma history.39,40 Similarly, two studies have shown that retrospective report of multiple childhood traumatic events (compared to no trauma) elevates adult OCD risk nearly fivefold, and that severity of childhood trauma correlates strongly with adult OCD symptom severity.39,41 Again, caveats apply to studies based on retrospective report; it is possible that a bias toward recalling or reporting past trauma exaggerates these numbers in individuals with OCD, or with anxious psychopathology in general.
Examination of specific trauma types has revealed trauma-related links with OCD. Sexual assault survivors are approximately four times more likely to meet criteria for OCD than matched controls or community members.42,43 OCD patients are also significantly more likely to report childhood sexual abuse than nonclinical controls;44,45 one study found that those who report such abuse are up to seven times more likely to meet criteria for OCD than those who report no such abuse.46 Furthermore, OCD patients retrospectively report more physical abuse and neglect in childhood than anxious and nonclinical samples,41,45,47 and significant correlations between severity of abuse/neglect and obsessive (but not compulsive) symptoms in adulthood have been reported.41
Some trauma-exposed individuals manifest overlapping OCD and PTSD symptoms.38 Individuals with OCD are significantly more likely than community controls to meet criteria for PTSD based on some studies,40,48 although at least one study has found no such elevation.49 Patients with PTSD who experienced criminal, combat, terror-related, and accidental man-made traumatic events (e.g., fires) have all been reported to have an elevated risk of developing OCD.42,50,51 Finally, patients with comorbid PTSD and OCD exhibit greater PTSD and OCD symptom levels than those with only PTSD or OCD.42,52
The reviewed literature does not establish whether psychosocial stress or specific types of stress and trauma are uniquely associated with OCD symptomatology or with psychopathology more generally. A nonspecific vulnerability factor such as neuroticism may be associated with stress reactivity across a wide range of mood- and anxiety-related disorders, with OCD representing only one particular example.53 In this case, the role of stress in the development of OCD symptomatology would be real, but not specific to OCD. Alternatively, there may be more specific vulnerability factors that determine whether similar stressors lead some individuals to develop OCD and others to develop different psychopathology. Addressing this question requires comparing the incidence and effects of stress in OCD to those in other clinical conditions, not just healthy controls. These comparisons have been made in a few studies,27,28,31,32 revealing some evidence for a disease-specific contribution of stress or trauma to OCD symptoms. However, more work is needed to clarify this question.
Neurocircuitry of OCD and the Role of Habit Learning
The most widely accepted neuroanatomical model of OCD implicates abnormal activity in corticostriatal-thalamocortical (CSTC) circuitry. In particular, hyperactivity has been reported in the medial frontal cortex (mFC) (especially the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC)) and the striatum.8,54–56 Meta-analyses have noted alterations in gray matter volumes within CSTC circuitry among OCD patients, including increased gray matter volume in the dorsal striatum (chiefly the putamen) and thalamus and decreased gray matter volume in the mFC (particularly the ACC), although results have been inconsistent across studies.57,58 Aberrant functional connectivity within the CSTC circuit has also been reported.57,59–62 For example, relative to psychiatrically healthy controls, OCD patients have increased global brain connectivity in the dorsal striatum (particularly the right putamen) and thalamus, increased functional connectivity between the ventral striatum and mPFC, but decreased global brain connectivity in the ventral striatum/nucleus accumbens.62 Altered activation of the OFC63–66 and other components of the CSTC circuitry57 has been reported during tests of cognitive control in OCD; the directionality of these effects appear quite sensitive to task and study design, with many reports suggesting hyperactivity at rest but reduced recruitment during certain cognitive tests.
Growing preclinical literature demonstrates that altered activity in these CSTC circuits can promote repetitive behaviors, which may model aspects of OCD. For example, elevated activity in both ventral67 and dorsal striatum68 can increase repetitive grooming, which has been proposed, with some important caveats, to recapitulate the repetitive nature of obsessions and compulsions.69 Stress modulates the activity and network properties of these same neurons, producing dendritic atrophy and a range of molecular alterations,70 and may thereby contribute to repetitive behavioral pathology.
Components of CSTC circuitry are implicated in the development of inflexible behavior patterns and habits.69–71 Habits are stimulus–response associations that become automatic over the course of extensive practice, such that they are executed irrespective of current motivational or behavioral state and, once initiated, proceed in a largely stereotyped sequence. These are contrasted with goal-directed actions, which are more flexible behaviors that take into account motivational state, anticipated outcomes and their desirability, and contextual variables. An overreliance on habitual behaviors has been proposed to be a core mechanism in the development of OCD symptomatology;71 individuals with OCD exhibit a bias toward inflexible habits, relative to more flexible, goal-directed behavioral control.62,64,66,72–74 This bias may derive from abnormalities in the mechanisms of habit and goal-directed control themselves,65,73,75 or from dysfunction of the executive systems that arbitrates between habitual and goal-directed behavioral strategies.76
Abnormalities in CSTC circuitry in OCD have been interpreted in the context of habit and goal-directed control. The dorsolateral or sensorimotor striatum (putamen) is implicated in habit formation and expression.69–71 The putamen is relatively larger in individuals with OCD, in proportion to the duration of symptoms.77 More precisely, the normal age-related loss of gray matter in the putamen is attenuated in individuals with OCD.77 Larger putamen volume has been interpreted as evidence for an overreliance on habits, which leads to hypertrophy of the associated circuitry over time. The dorsomedial or associative striatum (caudate), on the other hand, supports associative learning and flexible, goal-directed behavior.78–81 The OFC functions in concert with the dorsomedial striatum when switching from habitual to goal-directed actions and monitors changes in stimulus–response relationships.82,83 Similarly, the lateral PFC and parts of the mPFC, such as the frontal pole, ACC, and supplementary motor area are implicated in switching or arbitration between habitual and goal-directed actions.84 Abnormal activity in these brain regions in OCD may underlie dysregulation of the balance between habits and goal-directed actions.84
Some have extended the CSTC model of OCD to include structures more explicitly associated with the processing of fear and anxiety, including the amygdala and hippocampus.85,86 Amygdala responsivity is elevated in OCD in response to symptom provocation,87–89 negative stimuli unrelated to OCD,88 and an emotional face processing paradigm.90 Hippocampal metabolism at rest may be correlated with symptom severity,91 and several studies have reported aberrant hippocampal activation during implicit learning tasks.92,93 This fear circuitry is also implicated in the formation and modulation of habitual behavior.94–100 Fear induction and amygdala activation can acutely bias organisms toward the expression of habitual behavior, partly by interfering with regulatory or executive functions of the dorsolateral PFC.95,96 The central nucleus of the amygdala interacts with the dorsolateral striatum in regulating the acquisition of habits.101 Amygdala afferents to the dorsal striatum (particularly to neurochemically distinct compartments known as striosomes) may mediate this interaction.102,103 In contrast, the basolateral amygdala is vital for the more flexible updating of behavior following changes in environmental contingencies.104 Finally, hippocampal/parahippocampal regions, which project to the striatum,105,106 play a major role in complex spatial and explicit (conscious/declarative) learning and memory,106,107 and impairments in hippocampal function can bias toward habit-like behaviors.108,109
Modulation of Habit by Stress: A Possible Mechanism for Stress Effects on OCD Symptoms
Both acute and chronic stress can bias an organism toward rigid, habit-like patterns of behavior108,110–112 and impair flexible, goal-directed learning and behavioral control.108,110,113–115 In conjunction with the proposal that an overreliance on habit may contribute to OCD,71 this observation identifies a potential mechanistic locus for the interaction between stress and the development, exacerbation, and maintenance of OCD symptomatology. Both preclinical and human studies of the effects of stress on the brain support this idea.116
Preclinical work suggests that stress-induced structural and functional changes in corticostriatal and limbic circuits shift the balance between habitual and goal-directed behaviors.117,118 Excessive stress can cause neuronal atrophy and synaptic loss in the PFC119 (particularly the medial prefrontal cortex [mPFC] but also the OFC117,120–122), hippocampus,123 dorsomedial striatum (caudate),117,124,125, and ventral striatum (nucleus accumbens),70,126,127 and can impair neurogenesis in the hippocampus.108 Prolonged stress, particularly early-life stress, can significantly disrupt striatal and amygdala development and function,128 but can also cause neuronal hypertrophy and synaptic potentiation in the amygdala129 and dorsolateral striatum (putamen).114,117,129
Some human research suggests early-life stressors (including exposure to traumatic events) are associated with gray matter loss throughout corticostriatal-limbic circuitry.130 Other work has found that recent stressful life events, excessive adverse life events, and lifetime trauma lead more specifically to gray matter loss in the mPFC (including the ACC and OFC).131
Stress-induced changes in brain structure and function map remarkably well onto the anatomical substrates of goal-directed and habitual behavioral control. Dysfunction of mPFC, OFC, hippocampus, and dorsomedial striatum may impair goal-directed behavioral control. Hypertrophy and hyperfunction of the amygdala and dorsolateral striatum are consistent with excessive reliance on the habitual behavior patterns supported by this circuitry. This suggests a circuit-level mechanism whereby stress may induce or enhance a bias toward habit and thereby induce or exacerbate OCD symptomatology.132
The neurobiological literature briefly summarized above suggests five distinct mechanisms whereby this may occur.
Excessive stress can result in atrophy of the caudate, which may impair goal-directed control of behavior.133
Stress can have hypertrophic effects on the putamen, which may enhance sensorimotor habit133; a role for such an effect in OCD is supported by the relative preservation of putamen volume in patients over the course of disease.77
Stress may impair complex spatial or declarative learning and memory and thereby produce a bias toward habit via atrophy or disrupted neurogenesis of the hippocampus.115
Stress may impair goal-directed behavioral control and the flexible switching between habitual and goal-directed systems76 due to atrophy of the frontal cortices, particularly the mPFC and OFC.134,135
Finally, stress may have both acute and protracted effects on the balance between habit and goal-directed action due to amygdala hyperactivity and, over time, hypertrophy.
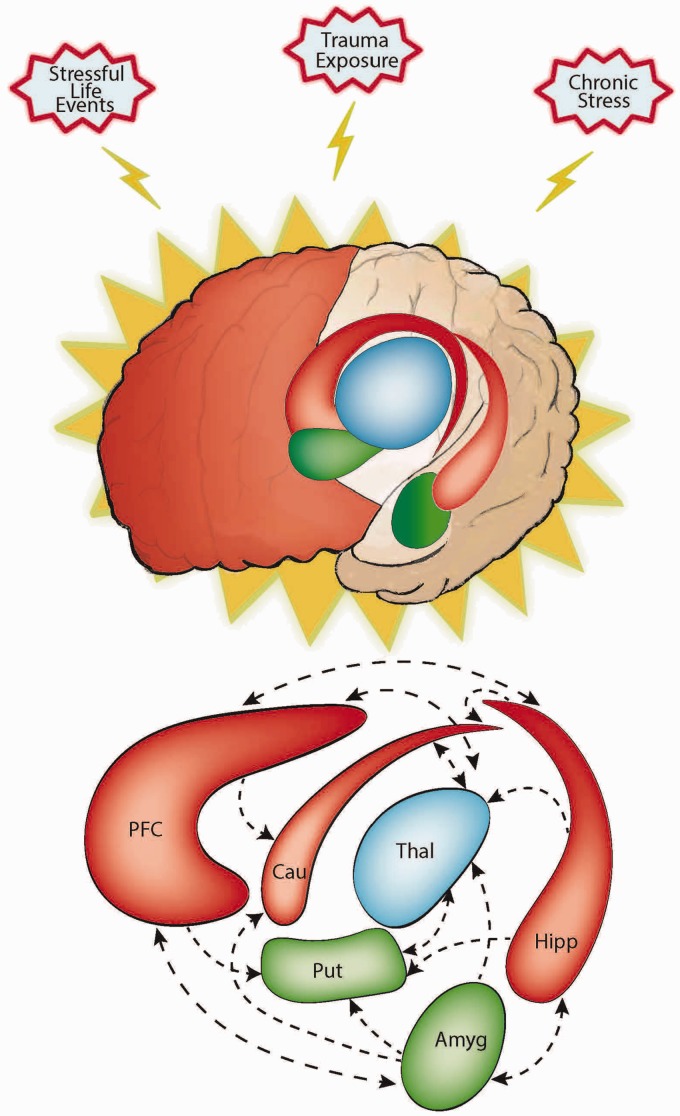
The specific anatomical and functional effects of stress in an individual are likely to depend on a complex interplay between underlying vulnerability factors (i.e., a genetic or developmental diatheses) and the nature, intensity, and duration of the stressful experience. Of course, excessive stress is likely to lead to interactive effects across the various anatomical regions listed above, which constitute an interacting circuitry. The overall effect of biasing the brain toward dependence on inflexible, habitual patterns of behavior may lead to the development and expression of habitual avoidance, compulsive behaviors, and repetitive thoughts (Figure 1).
Figure 1.
Excessive stress adversely affects goal-directed control and enhances habit learning and behavior. This may be partly explained by the demonstrated effects of stress on limbic and cortico-striatal circuitry. More specifically, stress can result in the atrophy of brain regions associated with goal-directed control, shown here in red—the frontal cortex (particularly the medial and orbital frontal regions), hippocampus, and caudate—while also causing hypertrophy in regions associated with habit, shown here in green—the amygdala and putamen. Moreover, stress can disrupt functional connectivity between these various regions. Dysregulation of limbic and cortico-striatal circuitry has repeatedly been demonstrated in OCD. These abnormalities may be related to the neurocognitive impairments implicated in the pathogenesis and maintenance of OCD, including the acquisition, arbitration, and expression of goal-directed and habitual behaviors. Given the likely association between excessive stress and OCD, the adverse effects of stress on corticostriatal-limbic circuitry and the associated disruption in the balance between goal-directed control and habit may play a causal role in the pathogenesis and maintenance of OCD, at least for some patients.
Discussion
While the literature examining the relations between stress and OCD symptomatology is substantial, as reviewed above, most studies are cross-sectional and rely on retrospective report. There is a paucity of mechanistic research directly examining the relationship between stress and OCD symptomatology. The hypotheses developed above can be tested using a quasi-experimental design, comparing, for example, habit learning between OCD patients with and without a history of trauma.132 Such an approach could be extended using neuroimaging by probing whether stress or trauma potentiates abnormalities in corticostriatal-limbic circuits at rest, during symptom provocation, or during tests of habit and goal-directed control in OCD. Given the high prevalence of trauma in OCD and the fact that trauma exposure is typically assessed during standard clinical screenings, initial steps in this direction may be possible through secondary analyses of existing datasets.
There is a conspicuous lack of preclinical work employing stress-based models to elucidate the pathophysiology of OCD.136–138 Preclinical models of fear conditioning and chronic stress have been used to shed light on the neurobiology of other disorders with trauma-based etiology,139 which provides some insight into the ways in which stress might affect habitual fear-motivated behaviors.118 Advances in our understanding of the modulation of habit learning and behavioral control by acute and chronic stress are likely to shed light on how these processes can contribute to the pathophysiology of OCD.
It has been argued that the symptoms of OCD are analogous to displacement behaviors in animals, such as grooming, which has been shown to be modulated by stress.136 Much of this work has been done within the context of preclinical models of Tourette's syndrome (TS), which is highly comorbid with OCD; TS shares a number of neurobiological correlates, and is similarly exacerbated by stress.140–142 Stress has been shown to exacerbate grooming phenotypes in several pathophysiologically grounded models of TS.143–146 While these studies have focused on tic disorders, they highlight the possibility that animal models can be used to study the effects of stress on the etiology and pathophysiology of OCD.
Conclusion
A variety of survey studies suggest excessive stress may play a significant role in the etiology and maintenance of symptoms in many patients with OCD. However, our understanding of the mechanisms of this effect remains limited. It has been proposed that OCD symptomatology arises from an imbalance between goal-directed and habitual control of behavior in OCD; these processes have been associated with specific components of limbic and corticostriatal circuitry. As reviewed, stress has clear functional and anatomical impacts on this circuitry that are consistent with a net effect of biasing an organism toward increased reliance on habit; preclinical studies have revealed just such an effect. Perturbations in limbic and corticostriatal circuitry and associated imbalances between goal-directed and habitual control may represent specific mechanisms whereby stress can predispose toward or exacerbate OCD and related disorders.
The effect of stress on OCD, at both clinical and mechanistic levels, has received relatively little attention until recently.38,132 This is an important area for ongoing research; better insight into these relationships will have important consequences both for our understanding of pathophysiology and for the development of new strategies for treatment and prevention.
Highlights
Stress and trauma are associated with the development and expression of OCD symptoms
Stress effects corticostriatal and limbic circuitry
Corticostriatal and limbic circuits are involved in habit formation and expression
Habit learning and corticostriatal and limbic circuits are dysfunctional in OCD
This provides a framework for investigating the effects of stress and trauma on OCD
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The primary author was supported by NIMH awards 1K23MH111977 and 1L30MH111037.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), Lake St. Louis, MO: American Psychiatric Association, 2013. [Google Scholar]
- 2.Rachman S, de Silva P. Abnormal and normal obsessions. Behav Res Ther 1978; 16: 233–248. [DOI] [PubMed] [Google Scholar]
- 3.Muris P, Merckelbach H, Clavan M. Abnormal and normal compulsions. Behav Res Ther 1997; 35: 249–252. [DOI] [PubMed] [Google Scholar]
- 4.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010; 15: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayuso-Mateos JL. Global burden of obsessive-compulsive disorder in the year 2000. World Health Organization Global Program on Evidence of Health Policy, Global Burden of Disease, Geneva, Switzerland: World Health Organization, 2000. . [Google Scholar]
- 6.Taylor S. Etiology of obsessions and compulsions: a meta-analysis and narrative review of twin studies. Clin Psychol Rev 2011; 31: 1361–1372. [DOI] [PubMed] [Google Scholar]
- 7.Pinto R, Monzani B, Leckman JF, et al. Understanding the covariation of tics, attention-deficit/hyperactivity, and obsessive-compulsive symptoms: a population-based adult twin study. Am J Med Genet B Neuropsychiatr Genet 2016; 171: 938–947. . [DOI] [PubMed] [Google Scholar]
- 8.Pauls DL, Abramovitch A, Rauch SL, Geller DA. Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci 2014; 15: 410–424. [DOI] [PubMed] [Google Scholar]
- 9.Brander G, Perez-Vigil A, Larsson H, Mataix-Cols D. Systematic review of environmental risk factors for obsessive-compulsive disorder: a proposed roadmap from association to causation. Neurosci Biobehav Rev 2016; 65: 36–62. [DOI] [PubMed] [Google Scholar]
- 10.Vidal-Ribas P, Stringaris A, Rück C, Serlachius E, Lichtenstein P, Mataix-Cols D. Are stressful life events causally related to the severity of obsessive-compulsive symptoms? A monozygotic twin difference study. Eur Psychiatry 2015; 30: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cath DC, Van Grootheest DS, Willemsen G, Van Oppen P, Boomsma DI. Environmental factors in obsessive-compulsive behavior: evidence from discordant and concordant monozygotic twins. Behav Genet 2008; 38: 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maina G, Albert U, Bogetto F, Vaschetto P, Ravizza L. Recent life events and obsessive-compulsive disorder (OCD): the role of pregnancy/delivery. Psychiatry Res 1999; 89: 49–58. [DOI] [PubMed] [Google Scholar]
- 13.Ravizza L, Bogetto F, Maina G, Manfredi A, Vaschetto P. Stressful life-events and onset of obsessive-compulsive disorder. Clinical study. Minerva Psichiatrica 1996; 37: 109–116. . [Google Scholar]
- 14.Khanna S, Rajendra PN, Channabasavanna SM. Life events and onset of obsessive compulsive disorder. Int J Soc Psychiatry 1988; 34: 305–309. [DOI] [PubMed] [Google Scholar]
- 15.Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry 2005; 62: 182–189. [DOI] [PubMed] [Google Scholar]
- 16.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol 2000; 68: 748–766. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Powers A, Bradley B, Ressler KJ. Gene × Environment Determinants of stress- and anxiety-related disorders. Annu Rev Psychol 2016; 67: 239–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuckerman M. Vulnerability to Psychopathology: A Biosocial Model, Washington, DC: American Psychological Association, 1999. [Google Scholar]
- 19.Ingram RE, Luxton DD. Vulnerability-stress models. In: Development of Psychopathology: A Vulnerability-Stress Perspective. Thousand Oaks, CA: Sage; 2005, pp.32–46.
- 20.Horowitz MJ. Intrusive and repetitive thoughts after experimental stress. A summary. Arch Gen Psychiatry 1975; 32: 1457–1463. [DOI] [PubMed] [Google Scholar]
- 21.Horowitz MJ, Becker SS. Cognitive response to stress and experimental demand. J Abnorm Psychol 1971; 78: 86–92. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz MJ, Becker SS. Cognitive response to erotic and stressful films. Arch Gen Psychiatry 1973; 29: 81–84. [DOI] [PubMed] [Google Scholar]
- 23.Horowitz MJ, Becker SS, Moskowitz M, Rashid K. Intrusive thinking in psychiatric patients after stress. Psychol Rep 1972; 31: 235–238. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen SA, Tsuang MT. Clinical characteristics and family history in DSM-III obsessive-compulsive disorder. Am J Psychiatry 1986; 143: 317–322. [DOI] [PubMed] [Google Scholar]
- 25.Real E, Labad J, Alonso P, et al. Stressful life events at onset of obsessive-compulsive disorder are associated with a distinct clinical pattern. Depress Anxiety 2011; 28: 367–376. [DOI] [PubMed] [Google Scholar]
- 26.Lensi P, Cassano GB, Correddu G, Ravagli S, Kunovac JL, Akiskal HS. Obsessive-compulsive disorder familial-developmental history, symptomatology, comorbidity and course with special reference to gender-related differences. Br J Psychiatry 1996; 169: 101–107. [DOI] [PubMed] [Google Scholar]
- 27.Rosso G, Albert U, Asinari GF, Bogetto F, Maina G. Stressful life events and obsessive-compulsive disorder: clinical features and symptom dimensions. Psychiatry Res 2012; 197: 259–264. [DOI] [PubMed] [Google Scholar]
- 28.Sarkhel S, Praharaj SK, Sinha VK. Role of life events in obsessive compulsive disorder. Isr J Psychiatry Relat Sci 2011; 48: 182–185. [PubMed] [Google Scholar]
- 29.McKeon J, Roa B, Mann A. Life events and personality traits in obsessive-compulsive neurosis. Br J Psychiatry 1984; 144: 185–189. [DOI] [PubMed] [Google Scholar]
- 30.Hazra SK. Occurrence of life events in obsessive compulsive disorder and its impact on disease severity. Indian J Psychiatry 2015; 57: S49. [Google Scholar]
- 31.Briggs ES, Price IR. The relationship between adverse childhood experience and obsessive-compulsive symptoms and beliefs: the role of anxiety, depression, and experiential avoidance. J Anxiety Disord 2009; 23: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 32.Valleni-Basile LA, Garrison CZ, Waller JL, et al. Incidence of obsessive-compulsive disorder in a community sample of young adolescents. J Am Acad Child Adolesc Psychiatry 1996; 35: 898–906. [DOI] [PubMed] [Google Scholar]
- 33.Lin H, Katsovich L, Ghebremichael M, et al. Psychosocial stress predicts future symptom severities in children and adolescents with Tourette syndrome and/or obsessive-compulsive disorder. J Child Psychol Psychiatry 2007; 48: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollitt J. Natural history of obsessional states; a study of 150 cases. Br Med J 1957; 1: 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cromer KR, Schmidt NB, Murphy DL. An investigation of traumatic life events and obsessive-compulsive disorder. Behav Res Ther 2007; 45: 1683–1691. [DOI] [PubMed] [Google Scholar]
- 36.Ivarsson T, Saavedra F, Granqvist P, Broberg AG. Traumatic and adverse attachment childhood experiences are not characteristic of OCD but of depression in adolescents. Child Psychiatry Hum Dev 2016; 47: 270–280. . [DOI] [PubMed] [Google Scholar]
- 37.Miller ML, Brock RL. The effect of trauma on the severity of obsessive-compulsive spectrum symptoms: a meta-analysis. J Anxiety Disord 2017; 47: 29–44. [DOI] [PubMed] [Google Scholar]
- 38.McKay D, Ojerskis R, Elhai JD. Psychological trauma exposure and obsessive-compulsive symptoms. In: Pittenger C. (ed). Obsessive-Compulsive Disorder: Phenomenology, Pathophysiology, and Treatment, New York, NY: Oxford University Press, 2017, pp. 613–622. . [Google Scholar]
- 39.Park S, Hong JP, Bae JN, et al. Impact of childhood exposure to psychological trauma on the risk of psychiatric disorders and somatic discomfort: single vs. multiple types of psychological trauma. Psychiatry Res. 2014; 219: 443–449. [DOI] [PubMed] [Google Scholar]
- 40.Lafleur DL, Petty C, Mancuso E, et al. Traumatic events and obsessive compulsive disorder in children and adolescents: is there a link?. J Anxiety Disord 2011; 25: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpenter L, Chung MC. Childhood trauma in obsessive compulsive disorder: the roles of alexithymia and attachment. Psychology and Psychotherapy: Theory, Research and Practice 2011; 84: 367–388. [DOI] [PubMed] [Google Scholar]
- 42.Boudreaux E, Kilpatrick DG, Resnick HS, Best CL, Saunders BE. Criminal victimization, posttraumatic stress disorder, and comorbid psychopathology among a community sample of women. J Trauma Stress 1998; 11: 665–678. [DOI] [PubMed] [Google Scholar]
- 43.Burnam MA, Stein JA, Golding JM, et al. Sexual assault and mental disorders in a community population. J Consult Clin Psychol 1988; 56: 843–850. [DOI] [PubMed] [Google Scholar]
- 44.Caspi A, Vishne T, Sasson Y, Gross R, Livne A, Zohar J. Relationship between childhood sexual abuse and obsessive-compulsive disorder: case control study. Isr J Psychiatry Relat Sci 2008; 45: 177–182. [PubMed] [Google Scholar]
- 45.Grisham JR, Fullana MA, Mataix-Cols D, Moffitt TE, Caspi A, Poulton R. Risk factors prospectively associated with adult obsessive-compulsive symptom dimensions and obsessive-compulsive disorder. Psychol Med 2011; 41: 2495–2506. [DOI] [PubMed] [Google Scholar]
- 46.Saunders BE. Child sexual assault as a risk factor for mental disorders among women a community survey. J Interpers Violence 1992; 7: 189–204. [Google Scholar]
- 47.Lochner C, Du Toit PL, Zungu-Dirwayi N, et al. Childhood trauma in obsessive-compulsive disorder, trichotillomania, and controls. Depress Anxiety 2002; 15: 66–68. [DOI] [PubMed] [Google Scholar]
- 48.Frydman I, do Brasil PE, Torres AR, et al. Late-onset obsessive-compulsive disorder: risk factors and correlates. J Psychiatr Res 2014; 49: 68–74. [DOI] [PubMed] [Google Scholar]
- 49.Grabe HJ, Ruhrmann S, Spitzer C, et al. Obsessive-compulsive disorder and posttraumatic stress disorder. Psychopathology 2008; 41: 129–134. [DOI] [PubMed] [Google Scholar]
- 50.Nacasch N, Fostick L, Zohar J. High prevalence of obsessive-compulsive disorder among posttraumatic stress disorder patients. Eur Neuropsychopharmacol 2011; 21: 876–879. [DOI] [PubMed] [Google Scholar]
- 51.Maes M, Mylle J, Delmeire L, Altamura C. Psychiatric morbidity and comorbidity following accidental man-made traumatic events: incidence and risk factors. Eur Archives Psychiatry Clin Neurosci 2000; 250: 156–162. [DOI] [PubMed] [Google Scholar]
- 52.Huppert JD, Moser JS, Gershuny BS, et al. The relationship between obsessive-compulsive and posttraumatic stress symptoms in clinical and non-clinical samples. J Anxiety Disord 2005; 19: 127–136. [DOI] [PubMed] [Google Scholar]
- 53.Barlow DH, Sauer-Zavala S, Carl JR, Bullis JR, Ellard KK. The nature, diagnosis, and treatment of neuroticism: back to the future. Clin Psychol Sci 2014; 2: 344–365. [Google Scholar]
- 54.Graybiel AM, Rauch SL. Toward a neurobiology review of obsessive-compulsive disorder. Neuron 2000; 28: 343. [DOI] [PubMed] [Google Scholar]
- 55.Saxena S, O'Neill J, Rauch SL. The role of cingulate cortex dysfunction in obsessive-compulsive disorder. In: Vogt B. (ed). Cingulate Neurobiology and Disease, Oxford, UK: Oxford University Press, 2009, pp. 588–606. [Google Scholar]
- 56.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev 2008; 32: 525–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eng GK, Sim K, Chen SH. Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: an integrative review. Neurosci Biobehav Rev 2015; 52: 233–257. [DOI] [PubMed] [Google Scholar]
- 58.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry 2009; 195: 393–402. [DOI] [PubMed] [Google Scholar]
- 59.Beucke JC, Kaufmann C, Linnman C, Gruetzmann R, Endrass T, Deckersbach T. Altered cingulostriatal coupling in obsessive-compulsive disorder. Brain Connect 2012; 2: 191–202. [DOI] [PubMed] [Google Scholar]
- 60.Beucke JC, Sepulcre J, Talukdar T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry 2013; 70: 619–629. [DOI] [PubMed] [Google Scholar]
- 61.Harrison BJ, Soriano-Mas C, Pujol J, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry 2009; 66: 1189–1200. [DOI] [PubMed] [Google Scholar]
- 62.Anticevic A, Hu S, Zhang S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry 2014; 75: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev 2008; 32: 525–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yücel M, Harrison BJ, Wood SJ, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry 2007; 64: 946–955. [DOI] [PubMed] [Google Scholar]
- 65.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron 2000; 28: 343–347. [DOI] [PubMed] [Google Scholar]
- 66.Chamberlain SR, Menzies L, Hampshire A, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science 2008; 321: 421–422. [DOI] [PubMed] [Google Scholar]
- 67.Ahmari SE, Spellman T, Douglass NL, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science 2013; 340: 1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rapanelli M, Frick L, Bito H, Pittenger C. Histamine modulation of the basal ganglia circuitry in the development of pathological grooming. Proc Natl Acad Sci USA 2017; 114: 6599–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pittenger C, Dulawa S, Thompson SL. Animal models of OCD: a conceptual framework. Obsessive-compulsive Disorder: Phenomenology, Pathophysiology, and Treatment. 2017, pp. 323. [Google Scholar]
- 70.Francis TC, Chandra R, Gaynor A, et al. Molecular basis of dendritic atrophy and activity in stress susceptibility. Mol Psychiatry 2017; 22: 1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gillan CM, Robbins TW, Sahakian BJ, van den Heuvel OA, van Wingen G. The role of habit in compulsivity. Eur Neuropsychopharmacol 2016; 26: 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gillan CM, Papmeyer M, Morein-Zamir S, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry 2011; 168: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gillan CM, Papmeyer M, Morein-Zamir S, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry 2011; 168: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burguière E, Monteiro P, Mallet L, Feng G, Graybiel AM. Striatal circuits, habits, and implications for obsessive–compulsive disorder. SI: Neuropsychiatry 2015; 30: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gillan CM, Apergis-Schoute AM, Morein-Zamir S, et al. Functional neuroimaging of avoidance habits in obsessive-compulsive disorder. Am J Psychiatry 2015; 172: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gruner P, Anticevic A, Lee D, Pittenger C. Arbitration between action strategies in obsessive-compulsive disorder. Neuroscientist 2016; 22: 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Wit SJ, Alonso P, Schweren L, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry 2014; 171: 340–349. [DOI] [PubMed] [Google Scholar]
- 78.Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 2005; 22: 513–523. [DOI] [PubMed] [Google Scholar]
- 79.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci 2005; 25: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Wit S, Corlett PR, Aitken MR, Dickinson A, Fletcher PC. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. Journal of Neuroscience 2009; 29: 11330–11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci 2012; 32: 12066–12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valentin VV, Dickinson A, O'Doherty JP. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci 2007; 27: 4019–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun 2013; 4: 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gruner P, Vo A, Argyelan M, et al. Independent component analysis of resting state activity in pediatric obsessive-compulsive disorder. Hum Brain Mapp 2014; 35: 5306–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci 2012; 16: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Posner J, Marsh R, Maia TV, Peterson BS, Gruber A, Simpson HB. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp 2014; 35: 2852–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mataix-Cols D, van den Heuvel OA. Common and distinct neural correlates of obsessive-compulsive and related disorders. Psychiatr Clin North Am 2006; 29: 391–410. viii. [DOI] [PubMed] [Google Scholar]
- 88.Simon D, Kaufmann C, Musch K, Kischkel E, Kathmann N. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology 2010; 47: 728–738. [DOI] [PubMed] [Google Scholar]
- 89.van den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Amygdala activity in obsessive-compulsive disorder with contamination fear: a study with oxygen-15 water positron emission tomography. Psychiatry Res 2004; 132: 225–237. [DOI] [PubMed] [Google Scholar]
- 90.Cardoner N, Harrison BJ, Pujol J, et al. Enhanced brain responsiveness during active emotional face processing in obsessive compulsive disorder. World J Biol Psychiatry 2011; 12: 349–363. [DOI] [PubMed] [Google Scholar]
- 91.Kwon JS, Kim J-J, Lee DW, et al. Neural correlates of clinical symptoms and cognitive dysfunctions in obsessive-compulsive disorder. Psychiatry Res: Neuroimaging 2003; 122: 37–47. [DOI] [PubMed] [Google Scholar]
- 92.Rauch SL, Savage CR, Alpert NM, et al. Probing striatal function in obsessive-compulsive disorder: a PET study of implicit sequence learning. J Neuropsychiatry Clin Neurosci 1997; 9: 568–573. [DOI] [PubMed] [Google Scholar]
- 93.Marsh R, Tau GZ, Wang Z, et al. Reward-based spatial learning in unmedicated adults with obsessive-compulsive disorder. Am J Psychiatry 2015; 172: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arnsten A, Wang M, Paspalas C. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 2012; 76: 223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 2009; 10: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elliott AE, Packard MG. Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol Learn Mem 2008; 90: 616–623. [DOI] [PubMed] [Google Scholar]
- 97.Gasbarri A, Pompili A, Packard MG, Tomaz C. Habit learning and memory in mammals: behavioral and neural characteristics. Neurobiol Learn Mem 2014; 114: 198–208. [DOI] [PubMed] [Google Scholar]
- 98.Wingard JC, Packard MG. The amygdala and emotional modulation of competition between cognitive and habit memory. Behav Brain Res 2008; 193: 126–131. [DOI] [PubMed] [Google Scholar]
- 99.Packard MG, Teather LA. Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. Neurobiol Learn Mem 1998; 69: 163–203. [DOI] [PubMed] [Google Scholar]
- 100.Packard MG, Wingard JC. Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol Learn Mem 2004; 82: 243–252. [DOI] [PubMed] [Google Scholar]
- 101.Lingawi NW, Balleine BW. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J Neurosci 2012; 32: 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat 2011; 5: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ragsdale CW, Jr, Graybiel AM. Fibers from the basolateral nucleus of the amygdala selectively innervate striosomes in the caudate nucleus of the cat. J Comp Neurol 1988; 269: 506–522. [DOI] [PubMed] [Google Scholar]
- 104.Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci 2003; 23: 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci 2015; 16: 317–331. [DOI] [PubMed] [Google Scholar]
- 106.Pennartz CM, Ito R, Verschure PF, Battaglia FP, Robbins TW. The hippocampal-striatal axis in learning, prediction and goal-directed behavior. Trends Neurosci 2011; 34: 548–559. [DOI] [PubMed] [Google Scholar]
- 107.Turnock M, Becker S. A neural network model of hippocampal-striatal-prefrontal interactions in contextual conditioning. Brain Res 2008; 1202: 87–98. [DOI] [PubMed] [Google Scholar]
- 108.Ferragud A, Haro A, Sylvain A, Velazquez-Sanchez C, Hernandez-Rabaza V, Canales JJ. Enhanced habit-based learning and decreased neurogenesis in the adult hippocampus in a murine model of chronic social stress. Behav Brain Res 2010; 210: 134–139. [DOI] [PubMed] [Google Scholar]
- 109.Lee AS, Duman RS, Pittenger C. A double dissociation revealing bidirectional competition between striatum and hippocampus during learning. Proc Natl Acad Sci USA 2008; 105: 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwabe L, Wolf OT, Oitzl MS. Memory formation under stress: quantity and quality. Neurosci Biobehav Rev 2010; 34: 584–591. [DOI] [PubMed] [Google Scholar]
- 111.Arnsten A, Lee D, Pittenger C. Risky business: the circuits that impact stress-induced decision making. Cell 2017. In press. [DOI] [PubMed] [Google Scholar]
- 112.Park H, Lee D, Chey J. Stress enhances model-free reinforcement learning only after negative outcome. PLoS ONE 2017; 12(7): ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwabe L, Wolf OT. Stress prompts habit behavior in humans. J Neurosci 2009; 29: 7191–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soares JM, Sampaio A, Ferreira LM, et al. Stress-induced changes in human decision-making are reversible. Transl Psychiatry 2012; 2: e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patterson TK, Craske MG, Knowlton BJ. The effect of early-life stress on memory systems supporting instrumental behavior. Hippocampus 2013; 23: 1025–1034. [DOI] [PubMed] [Google Scholar]
- 116.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 2008; 33: 88. [DOI] [PubMed] [Google Scholar]
- 117.Dias-Ferreira E, Sousa JC, Melo I, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science 2009; 325: 621–625. [DOI] [PubMed] [Google Scholar]
- 118.Goodman J, Leong KC, Packard MG. Emotional modulation of multiple memory systems: implications for the neurobiology of post-traumatic stress disorder. Rev Neurosci 2012; 23: 627–643. [DOI] [PubMed] [Google Scholar]
- 119.De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry 2002; 52: 1066–1078. [DOI] [PubMed] [Google Scholar]
- 120.Seib LM, Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. Neurosci Lett 2003; 337: 29–32. [DOI] [PubMed] [Google Scholar]
- 121.Liston C, Miller MM, Goldwater DS, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 2006; 26: 7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Radley JJ, Rocher AB, Miller M, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex 2006; 16: 313–320. [DOI] [PubMed] [Google Scholar]
- 123.Han F, Xiao B, Wen L. Loss of Glial Cells of the Hippocampus in a rat model of post-traumatic stress disorder. Neurochem Res 2015; 40: 942–951. [DOI] [PubMed] [Google Scholar]
- 124.Kang HJ, Voleti B, Hajszan T, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 2012; 18: 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 2012; 73: 962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Christoffel DJ, Golden SA, Dumitriu D, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci 2011; 31: 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Golden SA, Christoffel DJ, Heshmati M, et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med 2013; 19: 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fareri DS, Tottenham N. Effects of early life stress on amygdala and striatal development. Dev Cogn Neurosci 2016; 19: 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 2004; 128: 667–673. [DOI] [PubMed] [Google Scholar]
- 130.Edmiston EE, Wang F, Mazure CM, et al. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med 2011; 165: 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry 2012; 72: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fontenelle LF, Cocchi L, Harrison BJ, et al. Towards a post-traumatic subtype of obsessive-compulsive disorder. J Anxiety Disord 2012; 26: 377–383. [DOI] [PubMed] [Google Scholar]
- 133.Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci 2015; 16: 719–732. [DOI] [PubMed] [Google Scholar]
- 134.Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex 2003; 13: 400–408. [DOI] [PubMed] [Google Scholar]
- 135.Arnsten AFT, Raskind MA, Taylor FB, Connor DF. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress 2015; 1: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Albelda N, Joel D. Current animal models of obsessive compulsive disorder: an update. Neuroscience 2012; 211: 83–106. [DOI] [PubMed] [Google Scholar]
- 137.Pittenger, C. Animal models of Tourette syndrome and obsessive-compulsive disorder. In: Animal Models of Movement Disorders. Burlington, MA: Elsevier Academic Press; 2014, pp.748–766.
- 138.Pittenger C. The neurobiology of tic disorders and obsessive-compulsive disoder: human and animal models. In: Charney DS, Sklar P, Buxbaum JD, Nestler EJ (eds.) Neurobiology of Mental Illness. New York, NY: Oxford University Press; 2017, pp.879–889.
- 139.Goswami S, Rodriguez-Sierra O, Cascardi M, Pare D. Animal models of post-traumatic stress disorder: face validity. Front Neurosci 2013; 7: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette's disorder. J Child Adolesc Psychopharmacol 2010; 20: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Buse J, Kirschbaum C, Leckman JF, Munchau A, Roessner V. The modulating role of stress in the onset and course of Tourette's syndrome: a review. Behav Modif 2014; 38: 184–216. [DOI] [PubMed] [Google Scholar]
- 142.Godar SC, Bortolato M. What makes you tic? Translational approaches to study the role of stress and contextual triggers in Tourette syndrome. Neurosci Biobehav Rev 2017; 76: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pittenger C. Histidine Decarboxylase Knockout Mice as a Model of the Pathophysiology of Tourette Syndrome and Related Conditions. In: Hattori Y, Seifert R (eds.) Histamine and Histamine Receptors in Health and Disease. Handbook of Experimental Pharmacology. Cham, Switzerland: Springer; 2017, pp.189–215. [DOI] [PMC free article] [PubMed]
- 144.Xu M, Li L, Ohtsu H, Pittenger C. Histidine decarboxylase knockout mice, a genetic model of Tourette syndrome, show repetitive grooming after induced fear. Neurosci Lett 2015; 595: 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu M, Li L, Pittenger C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience 2016; 324: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu M, Kobets A, Du JC, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci USA 2015; 112: 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]