Abstract
Background
Diabetes mellitus (DM) has been identified to be both a risk factor and a prognostic factor in a variety of malignancies, but its association with the risk and outcome of nasopharyngeal carcinoma (NPC) is still unclear. To elucidate this issue, we systematically reviewed the evidence concerning the association between DM status and NPC.
Materials and methods
We identified studies by a literature search of PubMed, Embase, and ISI Web of Knowledge through May 31, 2017, and by searching the reference lists of pertinent articles. Odds ratios (ORs) and hazard ratios (HRs) with 95% CIs were used to estimate the effect size. Heterogeneity across studies was evaluated by the Cochran’s Q and I2 statistics.
Results
A total of nine studies were included. Four studies with a total sample size of 221,611 reported the effect of DM on NPC risk, and the other five studies with a sample size of 9,442 reported the impact of DM on survival in NPC patients. All included studies were retrospective, and mostly conducted in Asian populations. Meanwhile, condition of metformin usage was not considered in all studies. A pooled OR of 0.65 (95% CI: 0.43–0.98, P=0.04) revealed an inverse association between DM and NPC. Additionally, pooled analyses of studies investigating the prognosis value of DM revealed that preexisting DM had no effect on overall survival (HR =1.17, 95% CI: 0.94–1.46, P=0.16), local recurrence-free survival (HR =1.16, 95% CI: 0.80–1.67, P=0.44), and distant metastasis-free survival (HR =1.14, 95% CI: 0.92–1.40, P=0.22).
Conclusion
Our results suggested that DM patients might have decreased NPC risk, and have little impact on prognosis of NPC patients. This conclusion should be limited to Asian population. Our results also suggest that more attention should be paid to metformin medication in further studies in order to clarify whether the effects of DM on NPC risk and prognosis are influenced by the anticancer effect of metformin.
Keywords: diabetes mellitus, nasopharyngeal carcinoma, risk factor, prognosis factor, a meta-analysis, evidence based medicine
Introduction
The relationship between malignancies and diabetes mellitus (DM) has become a critically important area of study because of the concomitant increase in the incidence of DM.1 Epidemiological studies suggest that individuals with DM have an elevated risk of various types of cancers, such as hepatocellular carcinoma, cholangiocarcinoma, pancreatic duct adenocarcinoma, gastric cancer, colorectal cancer, renal carcinoma, bladder cancer, and breast cancer.2–7 Additionally, DM has been identified to be an adverse prognostic factor for various kinds of cancers, and many studies identified that cancer patients with preexisting diabetes are at increased risk for poor prognosis compared with those without diabetes.8,9 However, in contrast to the findings in most cancer types, DM appeared to have no impact on the prognosis of head and neck cancer,10 and a study even found that patients with DM had a weakly decreased risk of head and neck squamous cell cancer.11
Nasopharyngeal carcinoma (NPC) is a unique type of head and neck cancer, with a low incidence rate below 2 per 100,000 person-years in western countries.12 Conversely, NPC shows a particularly high incidence in Southeast Asia and its surrounding regions.13 NPC demonstrates distinct epidemiology, etiology, pathophysiology, clinical characteristics, and therapeutic model in comparison with other cancers, including other squamous cell carcinomas of the head and neck.14 Studies also reported controversial results about the relationship between DM and NPC.15–23 Therefore, the present study aimed to summarize results from relevant studies, and to provide insight into the relationship between DM and NPC.
Materials and methods
Search and filtration strategy
A systematic literature search of PubMed, Embase, and ISI Web of Knowledge was conducted to retrieve clinical studies up to May 2017. We used MeSH terms and text words related to both the exposure (diabetes, diabetes mellitus, blood glucose, hyperglycemia, and impaired glucose tolerance) and populations (nasopharynx cancer, nasopharynx carcinoma, nasopharynx neoplasm, nasopharyngeal cancer, nasopharyngeal carcinoma, nasopharyngeal neoplasm, pharyngeal cancer, pharyngeal carcinoma, and pharyngeal neoplasm) to search for related articles. The language of all publications was limited to English only. The initial selection was performed to exclude obviously irrelevant articles and retain potentially relevant articles about the effect of DM on NPC risk and/or outcome by an analysis of the title and abstract by two independent investigators (G Guo and M Fu). Thereafter, the full text was reviewed according to the including criteria: 1) prospective and retrospective studies that researched the relationship between DM and NPC risk; 2) one of the following risk indexes, including odds ratio (OR), relative risk (RR), rate difference, or attributable risk, along with the 95% CIs or P-values should be available. The following publications were excluded: duplicated literatures, duplicated reported data, letters, reviews, expert opinions, or case reports.
Data extraction and quality assessment
Two investigators (G Guo and M Fu) independently extracted data from the full manuscript independently using a standardized form. The following items were collected from each study: first author, year of publication, study design, geographic areas of the study population, sample size, age and sex of patients, follow-up time, statistic model, and outcome measurements. For assessing the impact of DM on NPC risk, OR was preferred as the primary outcome. The hazard ratio (HR) was preferred for evaluating the survival outcome because it is time-to-event data. For studies that reported only survival curves, the HR values were obtained by contacting the authors or were estimated by the methods described by Tierney et al.24 Multivariate outcomes were used for meta-analyses, but univariate outcomes were used instead if no multivariate results were presented. The quality assessment of included studies was independently applied using the “Newcastle–Ottawa Scale (NOS)”, which includes three domains with eight items. Each item could be awarded 1 to a maximum of 2 score, and the total possible score was 9. Study with a score ≥6 was deemed as being of good quality.
Statistical analysis
All meta-analyses were performed using Review Manager software (Version 5.3; The Cochrane Collaboration, Copenhagen, Denmark). The heterogeneity of the included studies was evaluated using the Cochran’s Q-test and Higgins I2 statistic. Pooled analysis with a P≥0.10 or I2≤50% was considered to have low heterogeneity, and a fixed-effects model was subsequently applied. Otherwise, the random-effects model was applied for meta-analysis. Additionally, the funnel plot was used to evaluate the publication bias. A two-tailed P<0.05 was considered statistically significant. All the results are presented in the forest plots.
Results
Figure 1 shows the flow chart for study selection. Initially, 296 articles were retrieved using the search words, of which 80 were duplicates. After title and abstract screening, 65 studies were involved in the full-text review. Studies that did not report NPC risk or survival information, and those that were basic researches, reviews, case reports, commentaries, or meta-analyses were excluded from our analysis. The reference lists of retrieved reviews and meta-analyses were also examined for potential relevant studies, but no more articles were identified. After further full-test review, 56 articles were excluded and nine studies were ultimately included in the present meta-analysis,15–23 of which four studies reported association between DM and NPC risk (Table 1),15–18 and another five studies reported association between DM and NPC outcome (Table 2).19–23
Figure 1.
Literature screening flowchart.
Abbreviations: DM, diabetes mellitus; NPC, nasopharyngeal carcinoma.
Table 1.
Characteristics of included studies investigating the relationship between DM and NPC risk
| Author/year | Design | Sample size | Age | Male/female | Diabetes assessment/types | Matching factors | Adjusted factors | NOS |
|---|---|---|---|---|---|---|---|---|
| Hsieh et al,15 2014 | Case–control | 24,884*,a 10,096b 7,249c |
70.0±12.0d 61.4±13.1d 60.4±13.1d |
14,118/10,766 5,865/4,231 3,683/3,566 |
Medical record/type 2 | None | None | 7 |
| Tseng et al,16 2014 | Retrospective cohort | 178,178 | 55.4±15.1d | 94,224/83,954 | Medical record/type 1 and type 2 | Comorbidities (obesity, coronary artery disease, hyperlipidemia, and hypertension), sex, and age | Age, sex, hypertension, hyperlipidemia, coronary artery disease, chronic kidney disease, obesity, geographic region, and monthly income | 7 |
| Zucchetto et al,18 2016 | Case–control | 789 | 26.8% <44 17.5% ≥65 |
626/163 | Self-reported/type 1 and type 2 | Study center, area of residence, sex, age, and period of interview | Study center, area of residence, sex, age, period of interview, years of education, smoking and drinking habits | 8 |
| Chen et al,22 2016 | Case–control | 415 | 46 (39, 54)e | 294/121 | Medical record/type 1 and type 2 | None | Gender, age group, and ethnicity | 6 |
Notes:
Hsieh et al reported results from three subgroups instead of the whole cohort;
subgroup included normal-weight patients;
subgroup included overweight patients;
subgroup included obese patients.
Mean ± SD.
Median (IQR).
Abbreviations: DM, diabetes mellitus; IQR, interquartile range; NOS, Newcastle–Ottawa Scale; NPC, nasopharyngeal carcinoma.
Table 2.
Characteristics of included studies investigating the impact of DM on NPC prognosis
| Author/year | Design | Group | Sample size | Male/female | Diabetes assessment/types | Matching factors | Adjusted factors | Follow-up | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Liu et al,23 2006 | Retrospective | Diabetes Nondiabetes |
37 897 |
30/7 658/239 |
Medical record/type 2 | None | None | 34.6 months* | 7 |
| Chen et al,22 2016 | Retrospective | Diabetes Nondiabetes |
71 530 |
0/71 0/530 |
Medical record/type 2 | None | Age and sex | NA | 5 |
| OuYang et al,21 2014 | Retrospective | Normoglycemic Diabetes Prediabetes |
3,949 345 1,566 |
2,916/1,033 270/75 1,187/379 |
Medical record/type 2 | None | Age, sex, smoking, drinking, hypertension, heart diseases, BMI, levels of CHO, TG, HDL-C and LDL-C, titer of VCA-IgA and EA-IgA, histological type, T stage, N stage, chemotherapy, and radiotherapy with forward selection method | 55.6 months* (range: 3.1–119.2 months) | 7 |
| Peng et al,20 2016 | Retrospective | Diabetes Nondiabetes |
186 372 |
160/2 52/320 |
Medical record/type 2 | Sex, age (within 5 years), T stage, N stage, chemotherapy (with or not), and radiotherapy (two-dimensional radiotherapy or intensity-modulated radiation therapy) | Gender, age, T stage, N stage, overall stage, chemotherapy, and radiotherapy | 66 months* (range: 3–157 months) | 9 |
| Peng et al,19 2016 | Retrospective | Nondiabetes Diabetes Prediabetes |
1,240 81 168 |
914/326 64/17 132/36 |
Medical record/type 2 | None | Age, sex, pathology, T stage, N stage, pre-DNA, smoking, drinking, CHO, TG, LDL-C, HDL-C, hypertension, cardiovascular complications, and chemotherapy | 49.8 months* (range: 1.3–70.7 months) | 7 |
Note:
Median follow-up time.
Abbreviations: CHO, total cholesterol; DM, diabetes mellitus; EA-IgA, early antigen immunoglobulin A; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NA, not available; NOS, Newcastle–Ottawa Scale; NPC, nasopharyngeal carcinoma; TG, triglycerides; VCA-IgA, viral capsid antigen immunoglobulin A.
Association between DM and NPC risk
The baseline characteristics of the included studies reporting association between DM and NPC risk are summarized in Table 1. A total of 221,611 patients were included. All studies were retrospectively designed. Two studies were published in 2014, and the other two studies were published in 2016. Three studies were conducted in Southeast Asia, and one in Europe. Matched design was introduced in two studies,16,18 and three studies used multivariate analysis to calculate ORs.16–18 Hsieh et al reported ORs from three subgroups instead of the whole cohort.15 Based on the NOS, the quality scores of each study were more than 5, ranging from 6 to 8. The lack of comparability between groups was found in two studies.15,17 The study of Tseng et al did not exclude potential cancer patients in the control group at the initial time of identifying patients.16
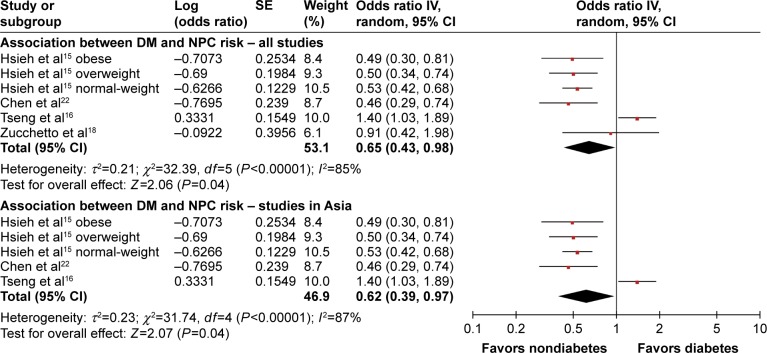
To evaluate the relationship between DM and NPC risk, we pooled the results of four relevant studies (Figure 2). We noted that Hsieh et al reported three RR values from three subgroups;15 because each of the three subgroups was from a different and independent population, we put them into the meta-analysis as three independent studies. The heterogeneity test showed that major heterogeneity exists (I2=85%) among these studies, and thus a random-effects model was used for the analysis. A pooled OR of 0.65 (95% CI: 0.43–0.98, P=0.04) revealed an inverse association between DM and NPC. Meta-analysis of three studies conducted in Asia also showed an inverse association between DM and NPC (OR =0.62, 95% CI: 0.39–0.97, P=0.04) (Figure 2).15–17 The above results suggest that DM appears to be correlated with a trend toward decreased NPC risk, which is quite different from the finding that DM is positively associated with an increased risk of most other cancers.
Figure 2.
Forest plots for the association between diabetes mellitus and nasopharyngeal carcinoma.
Notes: The pooled result of all studies and the result of studies conducted in Asia are shown separately. Squares represent the study-specific odds ratio. The diamond denotes the pooled odds ratio. Horizontal lines represent the 95% CIs.
Abbreviations: DM, diabetes mellitus; NPC, nasopharyngeal carcinoma.
Association between DM and NPC outcome
Five studies reported the influence of DM on outcomes among patients with NPC.19–23 The main characteristics of these five included studies are listed in Table 2. The included studies were published between 2006 and 2017. All studies were conducted in Asian population, and 9,442 NPC cases were included. All studies retrospectively analyzed data. Three studies reported the median follow-up period,20,21,23 with a range between 34.6 and 66.0 months. Four studies adopted multivariate analysis method,19–22 but only one study used a matched design to select control patients.20 DM was identified in the cohorts using blood glucose test results validated by medical records in all five studies.
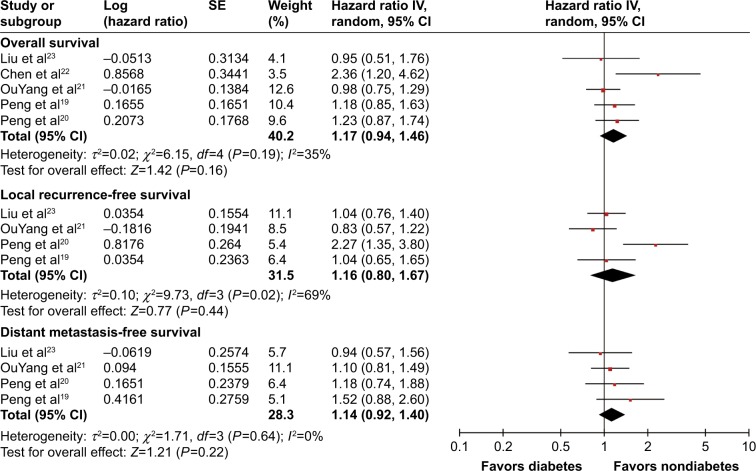
To investigate the impact of DM on prognosis of NPC patients, three outcome measurements, including overall survival (OS), local recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS), were quantitatively pooled. The meta-analysis results are displayed in Figure 3. Heterogeneity is illustrated in each forest plot. OS outcomes were available from all five studies, and the pooled result did not show significantly decreased OS in NPC patients with DM (HR =1.17, 95% CI: 0.94–1.46, P=0.16, I2=35%). Four studies provided sufficient data on LRFS outcome. Pooled results did not show a significantly higher risk of tumor local recurrence in patients with DM (HR =1.16, 95% CI: 0.80–1.67, P=0.44, I2=69%). Similarly, four studies provided DMFS information, and the DM history and DMFS were not found to be significantly associated in the pooled analysis (HR =1.14, 95% CI: 0.92–1.40, P=0.22, I2=0%). In summary, all of the meta-analysis results found no significant differences of OS, LRFS, and DMFS when comparing patients with diabetes to those with normoglycemia.
Figure 3.
Forest plot for the impact of diabetes mellitus on the prognosis of nasopharyngeal carcinoma patients.
Notes: The impact of diabetes on overall survival, local recurrence-free survival, and distant metastasis-free survival is demonstrated. Squares represent the study-specific hazard ratio. The diamond denotes the pooled hazard ratio. Horizontal lines represent the 95% CIs.
Publication bias
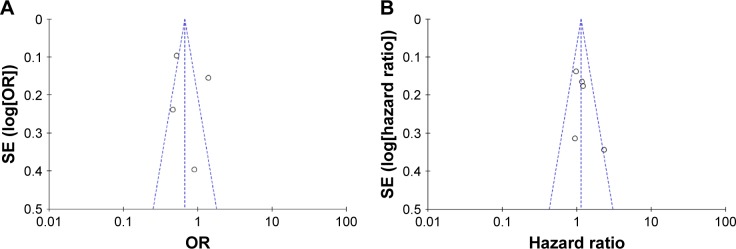
Funnel plots were introduced for estimating the publication bias. The shape of the funnel plots (Figure 4A and B) seemed unsymmetrical, suggesting that there was a potential publication bias. But due to the less number of studies selected in our meta-analysis, the funnel plot is of less significance.
Figure 4.
Funnel plot analysis of the included articles’ publication bias about diabetes mellitus and nasopharyngeal carcinoma.
Notes: (A) Funnel plots based on pooled analyses of OR for evaluating the association between diabetes and nasopharyngeal carcinoma risk; (B) funnel plots based on pooled analyses of hazard ratio for evaluating the impact of diabetes on the prognosis of nasopharyngeal carcinoma patients.
Abbreviation: OR, odds ratio.
Discussion
Numerous studies have identified close associations between DM and a variety of cancers in various populations, suggesting that DM appears to be a risk factor for various kinds of cancers, and patients diagnosed with cancer who have preexisting diabetes are at increased risk for long-term, all-cause mortality compared with those without diabetes.25–29 The potential link between DM and cancer has been hypothesized to be related to insulin, insulin-like growth factor, inflammatory status, metabolic characteristics, and even certain treatments of the DM.30,31 High levels of insulin (including the use of exogenous insulin) and IGF-1 can activate receptors and the downstream pathways associated with cell proliferation and subsequently increase cancer risk and promote cancer development.32–34 Other factors and potential mechanism involved chronic subclinical inflammation, abnormal carbohydrate and lipid metabolism, abnormalities in sex hormone metabolism, and excessive activation of Akt/mammalian target of rapamycin and Wnt/Beta-catenin pathways.35–37 However, in contrast to the relationship between DM and most cancers, our present study showed an inverse association between DM and NPC, and found that the diabetic NPC patients had prognosis similar to non-DM NPC patients.
Of all the included studies concerning the association between DM and NPC risk, only Tseng et al found that DM is associated with an increased risk of developing NPC;16 however, the study was limited by high risk of selection bias. They did not exclude cancer patients while selecting study population. In Figure 1 of their manuscript, the cumulative incidence curve of cancer in patients with DM showed a sharply rising curve at the time the study began, which suggested that there were more preexisting cancer patients in the DM cohort than in the non-DM cohort. Besides, the regular medical visits in patients with DM might have increased the chances of an early diagnosis of cancer. The negative association between diabetes and risk of head and neck cancer from the prior reports,11 along with the inverse relationship between diabetes and development of larynx cancer,3,38 also indirectly suggested that DM could not increase NPC risk.
NPC is a unique type of head and neck cancer, which exhibits distinct endemic distribution, close association with Epstein–Barr virus, and relative sensitivity to radiation and chemotherapy in contrast to other head and neck malignancies.13 For example, in high-incidence areas, undifferentiated NPC is the most frequent histological subtype, and differentiated cases are extremely rare, while in western countries, differentiated NPC cases were common and can make up to 25% of all NPCs.39–41 The peculiar geographic distribution of NPC reflects the differences in NPC subtypes and epidemiological patterns of known risk factors. In the study conducted in Italy, Zucchetto et al reported that the prevalence of DM in differentiated NPC patients was 8.7% compared to a rate of 4.4% for the undifferentiated cases;18 they also concluded that metabolic disorders could increase the risk of differentiated NPC, but not undifferentiated NPC. Therefore, as most included studies were conducted in Southeast Asia where differentiated NPC was rare, whether the negative pooled result was due to histology distribution needs further study.
Many studies have reported a variety of potential mechanisms linking diabetes to carcinogenic processes in various kinds of malignancies.30 So far, five studies have examined cancer-specific mortality among patients with NPC with or without diabetes.19–23 Our current meta-analysis of the present five studies did not find a clear correlation between poor prognosis of patients with NPC and DM. Overall, with respect to mortality among NPC patients with or without DM, only Chen et al reported that the diabetes group had a significantly increased mortality rate than the non-DM group.22 All of the other four studies reported that the diabetic NPC patients had similar OS rate to normoglycemic patients.19–21,23 We noted that, in the study of Chen et al,22 the follow-up time was not reported, and the mortality rate of diabetic NPC patients in their cohort was 18%, which was the highest among all cancer types in their cohort, even higher than the mortality rate (14%) of pancreatic cancer patients reported in their cohort. These questioned their results. Besides, regarding the LRFS and DMFS, Peng et al reported that the DM group had a worse LRFS than the non-DM, but they found that the DM NPC patients had similar OS and DMFS to non-DM NPC patients. Other studies also did not find that DM had prognostic impact on LRFS or DMFS of NPC patients.
The above inconsistent results among the included studies of our present meta-analysis may be due to the difference in the sample sizes, selection bias, and the confounding factors caused by the retrospective nature. Additionally, the use of metformin, an insulin sensitizer from the family of the biguanides, was not considered in all studies. Metformin has been widely used in the treatment of DM for decades and is used as a first-line therapy in type 2 diabetes.42 Recently, its anticancer potential has also been discovered. Metformin shows inhibitory effect on some pathways that play an important role in cancer cell proliferation and angiogenesis, thereby inhibiting cancer cell growth and development.43–47 Emerging evidences showed that the use of metformin in cancer patients is related to a survival benefit.48–51 Therefore, whether the oncogenic effect of DM on NPC was diminished by metformin medication, and thus induced decreased risk of NPC in DM population, and a similar survival among NPC patients with and without DM need further study. Another limitation of the currently available studies on the topic of association between DM and NPC risk is that they rarely differentiated type 1 DM and type 2 DM, which may have an effect on the results as type 1 diabetes and type 2 diabetes have totally different pathogenesis. Due to this limitation, the present meta-analysis for DM and NPC risk also cannot perform a separate analysis for type 1 DM and type 2 DM. It is very necessary to differentiate type1 DM and type 2 DM in future studies.
DM is a chronic disease that can cause hyperglycemia-related comorbidities such as hypertension, hyperlipidemia, nerve damage, and cardiovascular complications.52 It has been established that amount and degree of comorbidities significantly affect the prognosis of NPC patients.53,54 Interestingly, Kiderlen et al found that patients with diabetes without other comorbidity had a similar OS as patients without any comorbidity.55 Peng et al found that patients with diabetes-related hyperlipidemia exhibited poor physical condition, resulting in poor prognosis, while diabetes did not have impact on survival.19 These findings suggest that the additional comorbidity in patients with diabetes, but not the diabetes itself, plays a major role in affecting the survival of patients. More attention should be paid to diabetes-related comorbidities, both in clinical management of NPC and in further studies involving the effect of diabetes on survival of NPC patients.
Conclusion
In our current study, diabetes was found to have neither significant impact on NPC risk nor clear association with survival of NPC patients. This conclusion should be limited to Asian population. Our results also suggest that more attentions should be paid to metformin medication in further studies in order to clarify whether the effects of DM on NPC risk and prognosis are influenced by the anticancer effect of metformin.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Viner R, White B, Christie D. Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. Lancet. 2017;389(10085):2252–2260. doi: 10.1016/S0140-6736(17)31371-5. [DOI] [PubMed] [Google Scholar]
- 2.La Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994;70(5):950–953. doi: 10.1038/bjc.1994.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosetti C, Rosato V, Polesel J, et al. Diabetes mellitus and cancer risk in a network of case-control studies. Nutr Cancer. 2012;64(5):643–651. doi: 10.1080/01635581.2012.676141. [DOI] [PubMed] [Google Scholar]
- 4.Rousseau MC, Parent ME, Pollak MN, Siemiatycki J. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, Canada. Int J Cancer. 2006;118(8):2105–2109. doi: 10.1002/ijc.21600. [DOI] [PubMed] [Google Scholar]
- 5.O’Mara BA, Byers T, Schoenfeld E. Diabetes mellitus and cancer risk: a multisite case-control study. J Chronic Dis. 1985;38(5):435–441. doi: 10.1016/0021-9681(85)90139-0. [DOI] [PubMed] [Google Scholar]
- 6.Sasazuki S, Charvat H, Hara A, et al. Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci. 2013;104(11):1499–1507. doi: 10.1111/cas.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 2013;104(1):9–14. doi: 10.1111/cas.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95(3):727–748. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300(23):2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchini C, Ciorba A, Aimoni C, et al. Head and neck cancer patients: impact of diabetes mellitus on surgical outcomes. J BUON. 2016;21(3):580–587. [PubMed] [Google Scholar]
- 11.Stott-Miller M, Chen C, Schwartz SM. Type II diabetes and metabolic syndrome in relation to head and neck squamous cell carcinoma risk: a SEER-Medicare database study. Cancer Epidemiol. 2013;37(4):428–433. doi: 10.1016/j.canep.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Petersson F. Nasopharyngeal carcinoma: a review. Semin Diagn Pathol. 2015;32(1):54–73. doi: 10.1053/j.semdp.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Chua ML, Wee JT, Hui EP, Chan AT. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 14.Caponigro F, Longo F, Ionna F, Perri F. Treatment approaches to nasopharyngeal carcinoma: a review. Anticancer Drugs. 2010;21(5):471–477. doi: 10.1097/CAD.0b013e328337160e. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh SH, Chiou WK, Wang MH, Lin JD. Association of body weight with the risk for malignancies in hospitalized patients with or without diabetes mellitus in Taiwan. J Investig Med. 2014;62(1):37–42. doi: 10.2310/JIM.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 16.Tseng KS, Lin C, Lin YS, Weng SF. Risk of head and neck cancer in patients with diabetes mellitus: a retrospective cohort study in Taiwan. JAMA Otolaryngol Head Neck Surg. 2014;140(8):746–753. doi: 10.1001/jamaoto.2014.1258. [DOI] [PubMed] [Google Scholar]
- 17.Roujun C, Yanhua Y, Bixun L. High prevalence of diabetes mellitus and impaired glucose tolerance in liver cancer patients: a hospital based study of 4610 patients with benign tumors or specific cancers. F1000Res. 2016;5:1397. doi: 10.12688/f1000research.8457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zucchetto A, Taborelli M, Bosetti C, et al. Metabolic disorders and the risk of nasopharyngeal carcinoma: a case-control study in Italy. Eur J Cancer Prev. 2016 Jul 29; doi: 10.1097/CEJ.0000000000000286. Epub. [DOI] [PubMed] [Google Scholar]
- 19.Peng H, Chen L, Zhang Y, et al. Prognostic value of diabetes in patients with nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Sci Rep. 2016;6:22200. doi: 10.1038/srep22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng XS, Xie GF, Qiu WZ, Tian YH, Zhang WJ, Cao KJ. Type 2 diabetic mellitus is a risk factor for nasopharyngeal carcinoma: a 1:2 matched case-control study. PLoS One. 2016;11(10):e0165131. doi: 10.1371/journal.pone.0165131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.OuYang PY, Su Z, Tang J, et al. Diabetes, prediabetes and the survival of nasopharyngeal carcinoma: a study of 5,860 patients. PLoS One. 2014;9(10):e111073. doi: 10.1371/journal.pone.0111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JY, Chiou WK, Chou WY, Lin JD. The impact of type 2 diabetes mellitus on mortality in hospitalized female cancer patients in Taiwan. Asia Pac J Clin Oncol. 2016;12(1):e75–e81. doi: 10.1111/ajco.12084. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Xia Y, Cui N. Impact of diabetes mellitus on treatment outcomes in patients with nasopharyngeal cancer. Med Oncol. 2006;23(3):341–346. doi: 10.1385/MO:23:3:341. [DOI] [PubMed] [Google Scholar]
- 24.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu B, Wu X, Wu B, Pei D, Zhang L, Wei L. The relationship between diabetes and colorectal cancer prognosis: a meta-analysis based on the cohort studies. PLoS One. 2017;12(4):e0176068. doi: 10.1371/journal.pone.0176068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu L, Cao H, Zhang T, et al. The effect of diabetes mellitus on lung cancer prognosis: a PRISMA-compliant meta-analysis of cohort studies. Med. 2016;95(17):e3528. doi: 10.1097/MD.0000000000003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Zhang X, Gu C, Xia J. Diabetes mellitus is associated with breast cancer: systematic review, meta-analysis, and in silico reproduction. Panminerva Med. 2015;57(3):101–108. [PubMed] [Google Scholar]
- 29.Wang YG, Wang P, Wang B, Fu ZJ, Zhao WJ, Yan SL. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2014;9(5):e95485. doi: 10.1371/journal.pone.0095485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher EJ, LeRoith D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care. 2013;36(Suppl 2):S233–S239. doi: 10.2337/dcS13-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shlomai G, Neel B, LeRoith D, Gallagher EJ. Type 2 diabetes mellitus and cancer: the role of pharmacotherapy. J Clin Oncol. 2016;34(35):4261–4269. doi: 10.1200/JCO.2016.67.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmadieh H, Azar ST. Type 2 diabetes mellitus, oral diabetic medications, insulin therapy, and overall breast cancer risk. ISRN Endocrinol. 2013;2013:181240. doi: 10.1155/2013/181240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller BS, Yee D. Type I insulin-like growth factor receptor as a therapeutic target in cancer. Cancer Res. 2005;65(22):10123–10127. doi: 10.1158/0008-5472.CAN-05-2752. [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim YH, Yee D. Insulin-like growth factor-I and breast cancer therapy. Clin Cancer Res. 2005;11(2 Pt 2):944s–950s. [PubMed] [Google Scholar]
- 35.Ali Kamkar MM, Ahmad R, Alsmadi O, Behbehani K. Insight into the impact of diabetes mellitus on the increased risk of hepatocellular carcinoma: mini-review. J Diabetes Metab Dis. 2014;13:57. doi: 10.1186/2251-6581-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Nishihara R, Zhang X, Ogino S, Qian ZR. Energy sensing pathways: bridging type 2 diabetes and colorectal cancer? J Diabetes Complications. 2017;31(7):1228–1236. doi: 10.1016/j.jdiacomp.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lecarpentier Y, Claes V, Vallee A, Hebert JL. Interactions between PPAR Gamma and the Canonical Wnt/Beta-Catenin Pathway in Type 2 Diabetes and Colon Cancer. PPAR Res. 2017;2017:5879090. doi: 10.1155/2017/5879090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko S, Yoon SJ, Kim D, Kim AR, Kim EJ, Seo HY. Metabolic risk profile and cancer in Korean Men and Women. J Prev Med Public Health. 2016;49(3):143–152. doi: 10.3961/jpmph.16.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faivre S, Janot F, Armand JP. Optimal management of nasopharyngeal carcinoma. Curr Opin Oncol. 2004;16(3):231–235. doi: 10.1097/00001622-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Sanguineti G, Corvo R. Treatment of nasopharyngeal carcinoma: state of the art and new perspectives (review) Oncol Rep. 1999;6(2):377–391. [PubMed] [Google Scholar]
- 41.Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–3364. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60(9):1586–1593. doi: 10.1007/s00125-017-4336-x. [DOI] [PubMed] [Google Scholar]
- 43.Storozhuk Y, Hopmans SN, Sanli T, et al. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108(10):2021–2032. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griss T, Vincent EE, Egnatchik R, et al. Metformin antagonizes cancer cell proliferation by suppressing mitochondrial-dependent biosynthesis. PLoS Biol. 2015;13(12):e1002309. doi: 10.1371/journal.pbio.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato K, Gong J, Iwama H, et al. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012;11(3):549–560. doi: 10.1158/1535-7163.MCT-11-0594. [DOI] [PubMed] [Google Scholar]
- 46.Bao B, Wang Z, Ali S, et al. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila) 2012;5(3):355–364. doi: 10.1158/1940-6207.CAPR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gui DY, Sullivan LB, Luengo A, et al. Environment dictates dependence on mitochondrial complex I for NAD+ and aspartate production and determines cancer cell sensitivity to metformin. Cell Metab. 2016;24(5):716–727. doi: 10.1016/j.cmet.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitburn J, Edwards CM, Sooriakumaran P. Metformin and prostate cancer: a new role for an old drug. Curr Urol Rep. 2017;18(6):46. doi: 10.1007/s11934-017-0693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia C, Yao A, Camacho F, Balkrishnan R, Cantrell LA. A SEER-Medicare analysis of the impact of metformin on overall survival in ovarian cancer. Gynecol Oncol. 2017;146(2):346–350. doi: 10.1016/j.ygyno.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Lopez FR, Pasupuleti V, Gianuzzi X, Palma-Ardiles G, Hernandez-Fernandez W, Hernandez AV. Systematic review and meta-analysis of the effect of metformin treatment on overall mortality rates in women with endometrial cancer and type 2 diabetes mellitus. Maturitas. 2017;101:6–11. doi: 10.1016/j.maturitas.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Martin M, Marais R. Metformin: a diabetes drug for cancer, or a cancer drug for diabetics? J Clin Oncol. 2012;30(21):2698–2700. doi: 10.1200/JCO.2012.42.1677. [DOI] [PubMed] [Google Scholar]
- 52.Jelinek HF, Osman WM, Khandoker AH, et al. Clinical profiles, comorbidities and complications of type 2 diabetes mellitus in patients from United Arab Emirates. BMJ Open Diabetes Res Care. 2017;5(1):e000427. doi: 10.1136/bmjdrc-2017-000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo R, Chen XZ, Chen L, et al. Comorbidity predicts poor prognosis in nasopharyngeal carcinoma: development and validation of a predictive score model. Radiother Oncol. 2015;114(2):249–256. doi: 10.1016/j.radonc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Guo R, Mao YP, Chen L, et al. Implication of comorbidity on the initiation of chemotherapy and survival outcomes in patients with locoregionally advanced nasopharyngeal carcinoma. Oncotarget. 2017;8(6):10594–10601. doi: 10.18632/oncotarget.8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiderlen M, de Glas NA, Bastiaannet E, et al. Diabetes in relation to breast cancer relapse and all-cause mortality in elderly breast cancer patients: a FOCUS study analysis. Ann Oncol. 2013;24(12):3011–3016. doi: 10.1093/annonc/mdt367. [DOI] [PubMed] [Google Scholar]