Abstract
Purpose
Many anticancer drugs induce apoptosis in malignant cells, and resistance to apoptosis could lead to suboptimal or no therapeutic benefit. Two cytoplasmic proteins, B-cell lymphoma protein 2 (Bcl-2)-associated X (Bax) and Bcl-2, act as a promoter and an inhibitor of apoptosis, respectively. Both Bax and Bcl-2 as well as their ratio have been regarded as prognostic markers in various cancers. However, conflicting results have been reported. A clear understanding of apoptosis has also become crucial due to reports about anti-Bcl-2 chemotherapy. We explored the relationship of Bax and Bcl-2 gene expression and their ratio with the therapeutic response in acute myeloid leukemia (AML) patients.
Patients and methods
Bone marrow and/or blood samples from 90 AML patients treated with cytarabine and daunorubicin were included. Expression of Bax and Bcl-2 was determined through real-time polymerase chain reaction by using ΔΔCt method of relative expression.
Results
Bax and Bcl-2 expression among marrow and blood samples correlated with each other (rs=0.5, p<0.01). Although bone marrow expression of Bax and Bcl-2 tended to remain higher among responders (median 1.01 and 0.29, respectively) as compared to non-responders (median 0.66 and 0.24, respectively), the difference failed to reach statistical significance (U=784.5 and 733; p=0.68 and 0.28, respectively). Conversely, Bax/Bcl-2 ratio was higher among poor responders (median 3.07 vs 1.78), though again failed to reach statistical significance (U=698.5, p=0.07).
Conclusion
Expression of Bax and Bcl-2 does not differ significantly among AML patients treated with cytarabine and daunorubicin in terms of remission, relapse, resistance, overall survival, and disease-free survival, thus questioning the utility of emerging anti-Bcl-2 therapy.
Keywords: anthracyclines, cytarabine, Bcl-2, Bax Bcl-2 ratio, anti Bcl-2 therapy, BH3 mimetic inhibitors
Introduction
Acute myeloid leukemia (AML) is frequently a fatal malignancy in adults.1,2 Chemotherapy is the primary treatment of AML, which commonly employs cytarabine and anthracyclines such as daunorubicin.3 It is typically administered in two phases: an induction phase followed by a consolidation phase. After induction chemotherapy is given, the patient’s bone marrow and blood are analyzed for complete remission (CR) between days 21 and 28. Unfortunately, a substantial number of patients do not respond to chemotherapy. In addition, AML patients who respond to chemotherapy often relapse later.3–6 Relapse is defined as >5% blast cells in bone marrow, or reappearance of blast cells in blood, or development of blasts from any sites other than bone marrow after CR is achieved. Relapse occurs usually within first 3 years from the end of the chemotherapy,3 especially in young patients.5 Different relapse rates have been reported, ranging between 21% and 39%, from various parts of the world.7,8 Thus, resistance to chemotherapy is a common observation and major obstacle in treating AML patients. Even though patients may respond to anticancer drugs, their overall survival (OS) remains low.5,9
Anticancer drugs eradicate cancer cells either by disrupting cellular pathways vital for cell survival or by activating programmed cell death (apoptosis). Apoptosis is the final executer of many anticancer drugs. This cell suicide can be achieved by extrinsic or intrinsic pathways. The extrinsic pathway is mainly involved in controlling the cell turnover as well as eliminating mutant cells, while the intrinsic pathway is involved in antineoplastic drug action.10,11 DNA strand breaks accumulate after chemotherapy which trigger the intrinsic pathway of apoptosis in cancer cells.11 In apoptosis, B-cell lymphoma protein 2 (Bcl-2)-associated X (Bax) protein activates the cascade of reactions by releasing cytochrome c from the mitochondria that helps in successive activation of caspases and ultimately leads to cell death. Bcl-2 is believed to prevent Bax from releasing cytochrome c, thus restricting downstream activation of apoptotic machinery. This will result in cell survival but at the same time Bcl-2 is also involved in retiring proliferating cells back to G0 phase of the cell cycle.12 Both Bax and Bcl-2 are cytoplasmic proteins.13 Release of cytochrome c from mitochondrial matrix is considered to work as an inevitable call for cell death.14 DNA fragmentation activates Bax and inhibits Bcl-2 through p53.10,11
Dysregulation of apoptosis in cancer cells is considered to be one of the mechanisms of multidrug resistance.10 Many studies have investigated Bax and Bcl-2 in AML as well as other cancers and have yielded conflicting results. It has been proposed that high Bax and/or low Bcl-2 as well as high Bax/Bcl-2 ratio favors apoptosis and hence may lead to a favorable outcome,15–19 whereas others have reported contradictory observations.18,20,21 Thus, despite ongoing research, our understanding of this process and its impact on therapeutic outcome is still inadequate. This is further complicated by the current focus to develop anti-Bcl-2 drugs and their anticipated role in cancer therapy.22,23 Hence, we designed this study to explore the relationship of chemotherapeutic response to Bax and Bcl-2 gene expression in newly diagnosed AML patients using their bone marrow as well as peripheral blood samples and investigate their role as biomarkers of chemotherapy outcome.
Patients and methods
Patients and samples
We recruited 135 AML patients, diagnosed according to WHO criteria and treated at National Institute of Blood Diseases and Bone Marrow Transplantation (NIBD&BMT), Karachi, during 2011–2017. All prospective AML patients, including acute promyelocytic leukemia (M3), were inducted into the study if they received an induction chemotherapy comprising only standard 3+7 regimen (daunorubicin 45 mg/m2 on days 1–3; cytarabine 200 mg/m2 on days 1–7). High-dose Ara-C was administered in the consolidation phase after the patient achieved CR. The study was approved by the Ethical Review Board at NIBD&BMT in accordance with the Declaration of Helsinki. All patients provided written informed consent to participate in this research. Any participant under the age of 18 years had parental or legal guardian written informed consent confirmed. Bone marrow and blood samples of the patients were collected separately. Samples from 45 patients were excluded for various reasons, such as hemolysis or no RNA yield. Thus, samples from 90 AML patients were available for analysis. Of those, 82 patients provided bone marrow samples and 77 provided peripheral blood samples, while 70 patients provided both bone marrow and peripheral blood samples. Gene expression was analyzed in bone marrow and peripheral samples separately without pooling.
Cell separation and storage
A total of 2 mL of bone marrow and/or blood samples from patients and only blood sample from control subjects were collected in EDTA tubes. White blood cell pellets were isolated by using Lymphocyte Separation Medium (LSM®; Corning-Cellgro, Manassas, VA, USA), which contains Ficoll and sodium diatrizoate (density=1.077 g/mL). The cell pellets were immediately stored in RNALater® (Thermo Fisher Scientific, Waltham, MA, USA) at 4°C overnight and then stored at −80°C until RNA extraction.
RNA extraction, quantification, and cDNA synthesis
For RNA extraction, samples were thawed and washed with phosphate-buffered saline (PBS). Total RNA was extracted by using RNeasy Plus Mini Kit® from Qiagen (NV, Venlo, the Netherlands) according to the manufacturer’s instructions. RNA was quantified by using Qubit® RNA HS Assay Kits and Qubit® 2.0 fluorometer (Thermo Fisher Scientific) according to the manufacturer’s protocol. A total of 1 mg of RNA was utilized to synthesize cDNA by using Revert Aid First Strand cDNA Synthesis kit #K1622 (Thermo Fisher Scientific) according to the manufacturer’s protocol. The cDNA was either analyzed through real-time PCR or stored at −40°C until analysis.
Real-time PCR, primers, and probes
We used VeriQuest Probe® qPCR Master Mix (Affymerix, Santa Clara, CA, USA), and real-time PCR was run by using Eco Illumina® System version 5.0.16.0 (Illumina, San Diego, CA, USA). The expression of the housekeeping glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as the internal control. Primers and probes were purchased from Integrated DNA Technologies (IDT, Coralville, IA, USA). The reporter dye in the probe was 6-carboxyfluorescein (FAM), and the quencher was 6-carboxytetramethylrhodamine (TAMRA) with an intermediate ZEN-BQI. The primers and probes used for Bcl-2 were 5′-TCCGATCAGGAAGGCTAGAGTT-3′ (forward), 5′-TCGGTCTCCTAAAAGCAGGC-3′ (reverse), 5′-(FAM)/56-FAM/CC CAG AGC A/ZEN/T CAG GCCGCCAC/3IABkFQ/(TAMRA)-3′ (Probe), for Bax were 5′-CCGCCGTGGACACAGAC-3′ (forward), 5′-CAGAAAACATGTCAGCTGCCA-3′ (reverse), 5′-(FAM)/56-FAM/CC CCC CGA G/ZEN/A GGT CTT TTTCCG AC/3IABkFQ/(TAMRA)-3′ (Probe), and for GAPDH were 5′-GAAGGTGAAGGTCGGAGTCA-3′ (forward), 5′-GAAGATGGTGATGGGATTTC-3′ (reverse), 5′-(FAM)/56-JOEN/CC GAC TCT T/ZEN/G CCC TTCGAA C/3IABkFQ/(TAMRA)-3′ (Probe).24 The concentration was 6 pmol/mL for primers and 4 pmol/mL for probes. The reaction volume was 20 mL, which consisted of 10 mL Master Mix, 2.4 mL cDNA, 4 mL PCR water, 1.2 mL each of both forward and reverse primers, and 1.2 mL of probe. Thermal profile for real-time PCR was 50°C for 2 minutes, 95°C for 10 minutes followed by 45 cycles of 95°C for 30 seconds and 60°C for 60 seconds. Remaining cDNA was stored at −40°C. Real-time PCR determined the threshold (Cq values) for Bcl-2, Bax, and GAPDH. Relative quantification of the genes was done by 2−ΔΔCt method.25
Statistical analysis
The statistical analyses were done on SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). The data were given as frequencies and percentages, or median and interquartile range, where applicable. Mann–Whitney U-tests were used to analyze the differences between the groups where applicable. Spearman’s correlations (rs) were also computed between variables. Kaplan–Meier survival analysis (log-rank test) was carried out to explore the relationship between gene expression and survival. The significance level was set at 5%, and a p-value <0.05 was considered significant.
Results
The baseline characteristics (Table 1) show that male to female ratio was 2.7 to 1, and the most common age group was 15–40 years (68.8%). The most common AML subtype was “AML with maturation” (M2) (48.9%), followed by acute promyelocytic leukemia (APL) (M3) (18.9%). Special investigations for diagnosis or prognosis were carried out in patients as part of their diagnostic workup. Such investigations could only be performed for limited number of patients because of financial constraints. The myeloperoxidase (MPO) test was carried out in 84.4% of patients to determine the myeloid linage, and the majority of patients were positive. FLT3 mutation and MLL translocation are considered adverse prognostic markers, while NPM1 mutation and PML-RAR translocation are considered favorable prognostic markers. The FLT3 test was performed in 42 patients out of which only seven were found positive, while MLL was performed in 15 patients out of which five were positive. NPM1 was investigated in 13 patients who were all negative. PML-RAR was performed in nine patients where five were positive. Karyotyping was performed in 49 patients, and the findings are given in Table 1. Karyotyping was categorized according to ELN classification. A total of 37.8% of patients showed resistance to chemotherapy, while 62.2% of patients achieved remission, and 34% of patients who achieved remission (21% overall) later presented with relapse. As described above, patients who achieved remission and did not relapse during the study period were labeled as “good responders” (GR) to therapy. All those patients who were either resistant to chemotherapy or relapsed later were grouped together as “poor responders” (PR). Survival of the study subjects was also noted. At the end of the study, 42 patients died while 44 were alive and four patients could not be traced for survival status.
Table 1.
Baseline characteristics of study population (N=90)
| Parameters | N | % |
|---|---|---|
| Age groups | ||
| <15 years | 3 | 3.3 |
| 15–40 years | 62 | 68.9 |
| 41–60 years | 24 | 26.7 |
| >60 years | 1 | 1.1 |
| Gender | ||
| Male | 66 | 73.3 |
| Female | 24 | 26.7 |
| AML classification (WHO) | ||
| APL (M3) with t 15:17 | 17 | 18.9 |
| Translocation 6:9 | 2 | 2.2 |
| AML with minimal differentiation (M0) | 2 | 2.2 |
| AML without maturation (M1) | 15 | 16.7 |
| AML with maturation (M2) | 44 | 48.9 |
| Acute myelomonocytic leukemia (M4) | 2 | 2.2 |
| Acute panmyelosis with fibrosis | 1 | 1.1 |
| Myeloid proliferations related to Down syndrome | 1 | 1.1 |
| Unknown | 6 | 6.7 |
| AML classification (prognostic) | ||
| APL (M3) | 17 | 18.9 |
| Other (poor) | 67 | 74.4 |
| Unknown | 6 | 6.7 |
| Ethnicity | ||
| Urdu | 36 | 40.0 |
| Sindhi | 28 | 31.1 |
| Pashto | 9 | 10.0 |
| Punjabi | 3 | 3.3 |
| Baloch | 6 | 6.7 |
| Gujrati/Katchhi | 4 | 4.4 |
| Unknown | 4 | 4.4 |
| MPO status | ||
| Negative | 14 | 15.6 |
| Positive | 62 | 68.9 |
| Unknown | 14 | 15.6 |
| FLT3 mutation | ||
| Negative | 35 | 38.9 |
| Positive | 7 | 7.8 |
| Unknown | 48 | 53.3 |
| NPM1 mutation | ||
| Negative | 13 | 14.4 |
| Unknown | 77 | 85.6 |
| PML-RAR mutation | ||
| Negative | 4 | 4.4 |
| Positive | 5 | 5.6 |
| Unknown | 81 | 90.0 |
| MLL mutation | ||
| Negative | 10 | 11.1 |
| Positive | 5 | 5.6 |
| Unknown | 75 | 83.3 |
| Karyotyping | ||
| Poor | 18 | 20.0 |
| Good | 7 | 7.8 |
| Normal | 24 | 26.7 |
| Unknown | 41 | 45.6 |
| Total | 90 | 100.0 |
| Karyotyping (prognostic) | ||
| Unfavorable karyotype | 18 | 20.0 |
| Favorable karyotype | 31 | 34.4 |
| Unknown | 41 | 45.6 |
| Sample type | ||
| Pre-chemotherapy sample | 34 | 37.8 |
| Post-chemotherapy sample | 56 | 62.2 |
| Therapeutic response | ||
| Resistant | 34 | 37.8 |
| Relapse | 19 | 21.1 |
| Persistent remission | 37 | 41.1 |
| Survival status | ||
| Dead | 42 | 46.7 |
| Alive | 44 | 48.9 |
| Unknown | 4 | 4.4 |
| Final outcome | ||
| Poor | 53 | 58.9 |
| Good | 37 | 41.1 |
Abbreviations: AML, acute myeloid leukemia; MPO, myeloperoxidase; APL, acute promyelocytic leukemia; PML-RAR, promyelocytic leukemia/retinoic acid receptor alpha; MLL, mixed lineage leukemia.
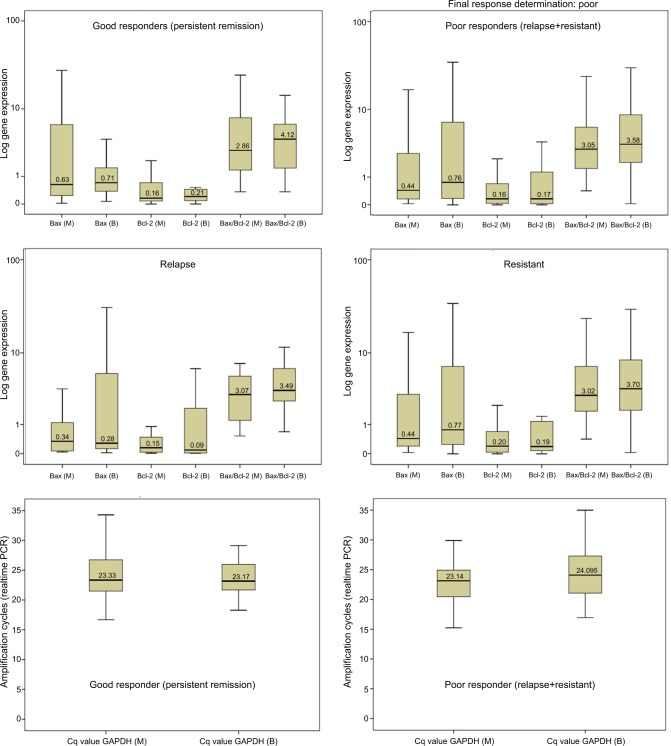
To determine whether gene expression in bone marrow and peripheral blood correlated, we computed Spearman correlation coefficients because of wide variation in expression levels (Table 2). It is evident that Bax and Bcl-2 in bone marrow correlated significantly with their corresponding blood levels, but not consistently with ratios. Difference in gene expression was explored by using analysis of variance with post hoc Dunnett’s test, which did not show any significant differences (Tables S1 and S2). Since, the gene expression data are not normally distributed, the data are presented using the median and interquartile range (Table 3) and expressed as boxplots on a logarithmic scale (Figure 1). Furthermore, we categorized the patients into either high or low expressers (HE or LE) of Bax and Bcl-2 based on the normalized expression, as described above25 being greater than or up to 1, respectively, for meaningful analysis. HE and LE were analyzed through the Mann–Whitney U-test (Table 4). Bax/Bcl-2 ratio in marrow was found significantly higher in all other AML subtypes combined as compared to APL (p<0.001) and in MPO positive as compared to MPO negative patients (p=0.03). Mean ranks for Bax and Bcl-2 were higher in the GR group as compared to the PR group suggesting an overall better apoptosis response, but failed to reach statistical significance. However, Bax/Bcl-2 ratio was higher in the PR group (mean rank 44.1 vs 37.9; p=0.07) apparently due to relatively lower Bcl-2 values. Overall, Bax and Bcl-2 were expressed higher among GR as compared to PR (Figure 1) but did not reach statistical significance. The median and interquartile ranges of Cq values of GAPDH, the housekeeping gene used as control in our experiments, are also considered for comparison. It is noteworthy that although GAPDH Cq values are plotted on a linear scale, Bax and Bcl-2 gene expression data could be plotted on logarithmic scale, suggesting a skewed distribution.
Table 2.
Spearman correlation between gene expression in bone marrow and peripheral blood
| Parameters | Bone Marrow
|
Blood
|
||||||
|---|---|---|---|---|---|---|---|---|
| Bax | Bcl-2 | Bax/Bcl-2 | Bax | Bcl-2 | Bax/Bcl-2 | |||
| Bone marrow | Bax | rs | 1 | 0.906** | −0.215 | 0.510** | 0.432** | 0.053 |
| N | 82 | 82 | 82 | 69 | 69 | 69 | ||
| Bcl-2 | rs | 1 | −0.563** | 0.500** | 0.503** | −0.108 | ||
| N | 82 | 82 | 69 | 69 | 69 | |||
| Bax/Bcl-2 | rs | 1 | −0.233 | −0.405** | 0.414** | |||
| N | 82 | 69 | 69 | 69 | ||||
| Blood | Bax | rs | 1 | 0.847** | 0.097 | |||
| N | 77 | 77 | 77 | |||||
| Bcl-2 | rs | 1 | −0.331** | |||||
| N | 77 | 77 | ||||||
| Bax/Bcl-2 | rs | 1 | ||||||
| N | 77 | |||||||
Notes: Correlation is significant at the 0.05 level (two-tailed).
Correlation is significant at the 0.01 level (two-tailed).
Abbreviation: rs, Spearman rho.
Table 3.
Median and interquartile ranges of gene expression
| Grouping variable | n | Bone marrow
|
Blood
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bax | Bcl-2 | Bax/Bcl-2 | Bax | Bcl-2 | Bax/Bcl-2 | ||||||||
| Age group | 82 | ||||||||||||
| <40 years | 60 | 0.66 | (0.17–6.31) | 0.26 | (0.05–4.01) | 2.51 | (1.13–5.24) | 0.77 | (0.2–6.87) | 0.21 | (0.06–1.4) | 3.20 | (1.78–6.25) |
| >40 years | 22 | 1.01 | (0.25–13.41) | 0.25 | (0.08–1.34) | 5.42 | (1.58–13.74) | 0.62 | (0.35–3.9) | 0.19 | (0.08–0.78) | 4.12 | (1.83–7.21) |
| Gender | 82 | ||||||||||||
| Male | 58 | 1.11 | (0.23–10.6) | 0.30 | (0.08–4.13) | 2.76 | (1.15–6.72) | 0.77 | (0.29–4.42) | 0.21 | (0.06–1.4) | 3.66 | (1.83–6.84) |
| Female | 24 | 0.44 | (0.17–3.44) | 0.14 | (0.05–1.18) | 2.73 | (1.41–6.19) | 0.71 | (0.13–5.51) | 0.16 | (0.09–0.73) | 2.65 | (1.53–7.95) |
| AML classification | 82 | ||||||||||||
| APL (M3) | 17 | 1.20 | (0.23–20.56) | 0.71 | (0.12–22.23) | 1.13 | (0.87–2.2) | 0.59 | (0.14–1.72) | 0.22 | (0.07–0.46) | 2.54 | (1.12–5.88) |
| AML with maturation (M2) | 40 | 0.92 | (0.28–3.77) | 0.24 | (0.06–0.69) | 2.97 | (1.68–6.79) | 0.76 | (0.26–5.25) | 0.19 | (0.08–0.88) | 4.29 | (1.93–7.27) |
| AML without maturation (M1) | 14 | 0.58 | (0.19–6.22) | 0.33 | (0.04–1.66) | 3.99 | (1.93–16.4) | 0.55 | (0.35–10.44) | 0.09 | (0.04–1.83) | 4.99 | (1.67–20.99) |
| Other AML subtypes | 11 | 0.32 | (0.07–12.34) | 0.10 | (0.01–23.93) | 3.07 | (0.75–22.32) | 0.88 | (0.14–695.6) | 0.44 | (0.07–1923.3) | 1.85 | (0.87–2.68) |
| Pre-/post-chemotherapy sample | 82 | ||||||||||||
| Pre-chemotherapy | 32 | 1.02 | (0.17–9.11) | 0.33 | (0.06–6.52) | 2.21 | (1.13–4.18) | 0.71 | (0.25–4.12) | 0.16 | (0.06–1.16) | 4.12 | (1.39–5.89) |
| Post-chemotherapy | 50 | 0.57 | (0.23–6.28) | 0.25 | (0.06–0.87) | 3.12 | (1.15–7.2) | 0.78 | (0.22–5.25) | 0.20 | (0.08–0.94) | 2.79 | (1.81–7.62) |
| Myeloperoxidase status | 71 | ||||||||||||
| Negative | 13 | 1.20 | (0.26–20.56) | 0.20 | (0.04–16.92) | 3.82 | (0.87–9.46) | 0.95 | (0.21–25.93) | 0.18 | (0.06–23.08) | 5.50 | (1.26–8.9) |
| Positive | 58 | 0.53 | (0.19–3.25) | 0.21 | (0.06–0.7) | 3.05 | (1.6–5.79) | 0.62 | (0.2–1.95) | 0.16 | (0.05–0.52) | 3.66 | (1.92–7.24) |
| FLT3 mutation | 39 | ||||||||||||
| Negative | 33 | 0.76 | (0.21–6.33) | 0.15 | (0.06–3.68) | 3.02 | (1.27–7.65) | 0.73 | (0.26–7.79) | 0.20 | (0.06–1.22) | 3.89 | (1.45–7.52) |
| Positive | 6 | 0.55 | (0.14–14.23) | 0.26 | (0.05–4.24) | 2.47 | (1.46–5856.1) | 0.64 | (0–3.9) | 0.15 | (0–0.78) | 2.89 | (2.27–4.15) |
| Karyotyping | 46 | ||||||||||||
| Poor | 16 | 0.65 | (0.12–6.06) | 0.26 | (0.05–0.71) | 2.83 | (1.18–5.53) | 1.95 | (0.09–24.02) | 0.46 | (0.06–4.93) | 3.91 | (1.07–4.99) |
| Good | 7 | 1.02 | (0.31–6.38) | 0.41 | (0.11–8.69) | 1.33 | (0.88–3.12) | 0.59 | (0.13–0.8) | 0.07 | (0.02–0.35) | 3.75 | (1.32–1797.1) |
| Normal | 23 | 0.76 | (0.24–10.02) | 0.16 | (0.02–7.28) | 4.12 | (1.38–8.63) | 0.84 | (0.28–10.19) | 0.17 | (0.05–1.16) | 3.49 | (1.47–8.81) |
| Remission status | 82 | ||||||||||||
| Resistant | 32 | 0.48 | (0.19–3.63) | 0.22 | (0.04–0.7) | 3.07 | (1.85–8.22) | 0.77 | (0.2–7) | 0.19 | (0.07–1.16) | 2.89 | (1.82–8.34) |
| Relapse | 15 | 1.02 | (0.08–4.95) | 0.30 | (0.06–1.33) | 3.07 | (1.15–5.19) | 0.33 | (0.1–6.87) | 0.10 | (0.02–3.85) | 3.49 | (1.92–8.81) |
| Remission | 35 | 1.01 | (0.31–15.04) | 0.29 | (0.1–7.28) | 1.78 | (0.89–6.90) | 0.72 | (0.38–3.16) | 0.25 | (0.09–0.52) | 3.17 | (1.39–6.46) |
| Final outcome | 82 | ||||||||||||
| Poor | 47 | 0.66 | (0.19–3.81) | 0.24 | (0.05–0.9) | 3.07 | (1.33–5.57) | 0.76 | (0.13–6.90) | 0.18 | (0.03–1.22) | 3.35 | (1.89–8.46) |
| Good | 35 | 1.01 | (0.31–15.04) | 0.29 | (0.1–7.28) | 1.78 | (0.89–6.90) | 0.72 | (0.38–3.16) | 0.25 | (0.09–0.52) | 3.17 | (1.39–6.46) |
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia.
Figure 1.
Median and interquartile ranges of gene expression.
Abbreviations: B, blood; M, bone marrow.
Table 4.
Gene expression–Mann–Whitney U-test
| Parameters | N | Groups | Groups, N | Mean rank | Sum of ranks | p-value |
|---|---|---|---|---|---|---|
| AML class: APL vs others (poor) | ||||||
| Bone marrow | ||||||
| Bax | 78 | APL | 17 | 43.94 | 747.00 | 0.29 |
| Others | 61 | 38.26 | 2334.00 | |||
| Bcl-2 | 78 | APL | 17 | 45.56 | 774.50 | 0.10 |
| Others | 61 | 37.81 | 2306.50 | |||
| Bax/Bcl-2 | 78 | APL | 17 | 28.94 | 492.00 | <0.001 |
| Others | 61 | 42.44 | 2589.00 | |||
| Peripheral blood | ||||||
| Bax | 72 | APL | 14 | 30.71 | 430.00 | 0.17 |
| Others | 58 | 37.90 | 2198.00 | |||
| Bcl-2 | 72 | APL | 14 | 34.14 | 478.00 | 0.50 |
| Others | 58 | 37.07 | 2150.00 | |||
| Bax/Bcl-2 | 72 | APL | 14 | 36.36 | 509.00 | 0.96 |
| Others | 58 | 36.53 | 2119.00 | |||
| MPO status | ||||||
| Bone marrow | ||||||
| Bax | 71 | Negative | 13 | 39.62 | 515.00 | 0.42 |
| Positive | 58 | 35.19 | 2041.00 | |||
| Bcl-2 | 71 | Negative | 13 | 41.65 | 541.50 | 0.13 |
| Positive | 58 | 34.73 | 2014.50 | |||
| Bax/Bcl-2 | 71 | Negative | 13 | 29.58 | 384.50 | 0.03 |
| Positive | 58 | 37.44 | 2171.50 | |||
| Peripheral blood | ||||||
| Bax | 67 | Negative | 12 | 38.75 | 465.00 | 0.26 |
| Positive | 55 | 32.96 | 1813.00 | |||
| Bcl-2 | 67 | Negative | 12 | 35.88 | 430.50 | 0.59 |
| Positive | 55 | 33.59 | 1847.50 | |||
| Bax/Bcl-2 | 67 | Negative | 12 | 32.92 | 395.00 | 0.72 |
| Positive | 55 | 34.24 | 1883.00 | |||
| Final response: poor vs good | ||||||
| Bone marrow | ||||||
| Bax | 82 | Poor | 47 | 40.69 | 1912.50 | 0.68 |
| Good | 35 | 42.59 | 1490.50 | |||
| Bcl-2 | 82 | Poor | 47 | 39.60 | 1861.00 | 0.28 |
| Good | 35 | 44.06 | 1542.00 | |||
| Bax/Bcl-2 | 82 | Poor | 47 | 44.14 | 2074.50 | 0.07 |
| Good | 35 | 37.96 | 1328.50 | |||
| Peripheral blood | ||||||
| Bax | 77 | Poor | 46 | 40.24 | 1851.00 | 0.49 |
| Good | 31 | 37.16 | 1152.00 | |||
| Bcl-2 | 77 | Poor | 46 | 39.54 | 1819.00 | 0.73 |
| Good | 31 | 38.19 | 1184.00 | |||
| Bax/Bcl-2 | 77 | Poor | 46 | 39.64 | 1823.50 | 0.64 |
| Good | 31 | 38.05 | 1179.50 | |||
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; MPO, myeloperoxidase.
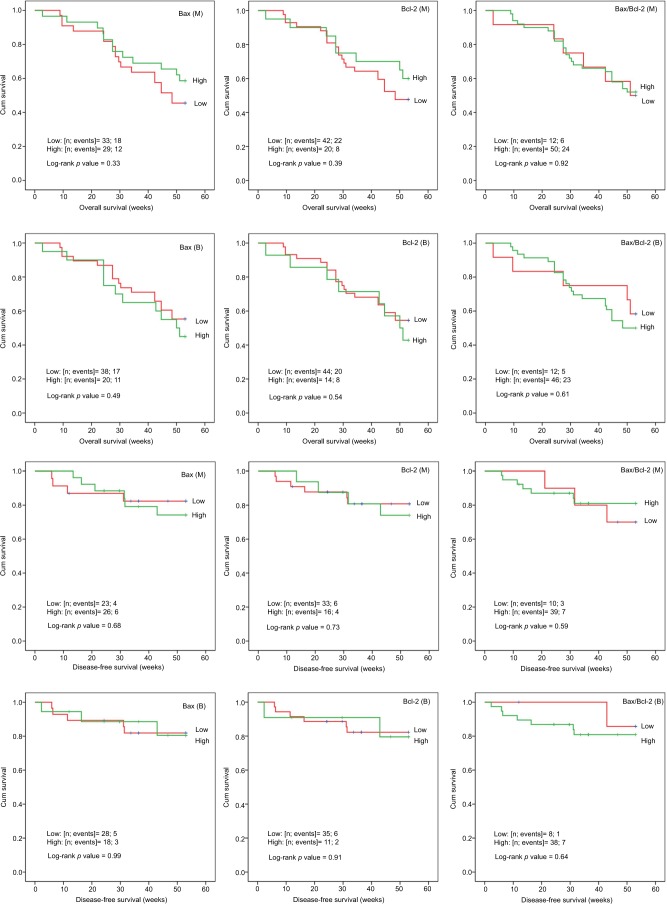
A Kaplan–Meier analysis (Figure 2) was carried out on categorically transformed gene expression to explore its relationship with overall as well as disease-free survival (DFS) up to 1 year (52 weeks). We found no significant difference in DFS or OS according to gene expression.
Figure 2.
Kaplan–Meier survival curves according to gene expression.
Abbreviations: Cum, cumulative; B, blood; M, bone marrow.
Discussion
In this study, we observed no significant association of Bax or Bcl-2 expression with remission, DFS, or OS among AML patients. However, a higher Bax/Bcl-2 ratio was associated with poor chemotherapy outcome, despite higher absolute values of Bax and Bcl-2 in “good response” group. However, there are certain trends that need to be discussed, despite not being statistically significant. It is interesting to note that while some studies, like ours, have shown that these markers have no association with clinical outcome, others have shown a positive or negative association. Table 5 compares various contemporary studies in this regard. A number of factors could underlie this contrast, such as sample size, technique used, and pathology of the patients.
Table 5.
Comparison of contemporary literature on Bax, Bcl-2, and Bax/Bcl-2 ratio with our study
| Study | Disease | No. of subjects | Technique | Findings |
|---|---|---|---|---|
| Bax expression | ||||
| Bekhet et al26 | AML | 34 | RT-PCR | ↑ Bax associated with ↑ CR |
| Garcia et al37 | Hepatocellular carcinoma | 70 | IHC | ↑ Bax associated with ↑ OS |
| Prokop et al15 | ALL | 14 | Immunoblotting | ↓ Bax associated with relapse |
| Hogarth and Hall27 | ALL | 47 | WB | ↑ Bax associated with ↓ DFS |
| Köhler et al28 | AML | 232 | RT-PCR | ↑ Bax associated with ↓ OS |
| Kaparou et al29 | AML | 26 | RT-PCR | No correlation with outcome |
| Peiró et al45 | Endometrial cancer | 89 | IHC | No correlation with outcome |
| Bcl-2 | ||||
| Kornblau et al31 | AML | 198 | WB | Expression associated with ↓ median survival if favorable/intermediate cytogenetics & ↑ median survival if poor cytogenetics |
| Aref et al30 | AML and ALL | 16 (ALL); 24 (AML) | FCM | ↓ Expression → ↑ CR only in ALL, expression → ↑relapse and blast % in both AML and ALL No significant correlation with OS in both AML and ALL |
| Sahu and Jena32 | AML | 110 | IHC; blood only | Bcl-2 positive → better OS |
| Huang et al33 (meta-analysis) | Colorectal carcinoma | 7658 | IHC; 40 articles | ↑ Bcl-2 associated with longer OS/DFS/RFS, if received adjuvant therapy before surgery |
| Hwang et al34 | Breast cancer | 7230 | Meta-analysis | Positive Bcl-2 → favorable clinicopathologic features, OS & DFS |
| Kaparou et al29 | AML & ALL | 26 | RT-PCR | No significant correlation between Bcl-2 expression and clinical outcome |
| Khor et al17 | Prostate cancer | 502 | IHC | |
| Prokop et al15 | ALL | 14 | Immunoblotting | |
| Peiro et al45 | Endometrial cancer | 89 | IHC | |
| Casado et al35 | Head and neck cancers | 43 | IHC | |
| Sagarra et al36 | Epithelial and serous ovarian cancer | 90 | IHC | |
| Palmer et al21 | 132 | IHC | ||
| Garcia et al37 | Hepatocellular carcinoma | 70 | IHC | |
| Katkoori et al18 | Colorectal adenocarcinoma | 112 | IHC | |
| Pierce et al38 | Mouse liver | Bcl-2 inhibits carcinogenesis and delays the growth of proliferative foci | ||
| Bax/Bcl-2 | ||||
| Scopa et al46 | Rectal carcinoma | 49 | IHC | ↓ Bax/Bcl-2 → radioresistance |
| Del Poeta et al40 | AML | 255 | FCM | ↑ Bax/Bcl-2 ratio is associated with ↑ CR and ↓ Bax/Bcl-2 ratio is associated with AML-M0 & M1 and CD34 >20% |
| Köhler et al28 | AML | 232 | RT-PCR | ↓ Bax/Bcl-2 associated with ↓ OS |
| Kasimir-Bauer et al39 | AML | 124 | FCM | A combination of at least two proteins out of Bcl-2, P-gp, MDR, p53, and hsp27 correlates with OS |
| Kaparou et al29 | ALL | 26 | RT-PCR | ↑ Bax/Bcl-2 at diagnosis than at remission in high-risk group patients, no such association observed in the medium-risk group At diagnosis, Bax/Bcl-2 ratio in children >10 years and with WBC count, DNA index <1.16 (at diagnosis & remission) & del(9p) |
| Prokop et al15 | ALL | 14 | Immunoblotting | ↓ Bax/Bcl-2 ratio → relapse |
| Hogarth and Hall27 | ALL | 47 | WB | ↑ Bcl-2/Bax and Mcl-1/Bax in B lineage as compared to T lineage. Bax → relapse |
| Katkoori et al18 | Colorectal adenocarcinoma | 112 | Immunophenotyping | ↓ Bax/Bcl2 ratio → better OS |
| Khodapasand et al41 | Colon rectal cancers | 22 | RT-PCR | The Bax/Bcl2 ratio decreased with increasing age and in colon cancers (vs sigmoid colon and rectosigmoid) |
| Khor et al17 | Prostate cancer | 502 | IHC | “Negative Bcl-2/Normal Bax” → better response rate to short-term androgen therapy (not long-term androgen therapy) |
| Our study | AML | 90 | RT-PCR | Bax/Bcl-2 ratio did not differ among GR and PR ↓ Bax/Bcl-2 ratio in APL (vs others) in marrow ↓ Bax/Bcl-2 ratio in MPO negative in marrow samples |
Abbreviations: AML, acute myeloid leukemia; RT-PCR, real-time polymerase chain reaction; IHC, immunohistochemistry; ALL, acute lymphoblastic leukemia; WB, Western blotting; GR, good responders; PR, poor responders; MPO, myeloperoxidase; OS, overall survival; DFS, disease-free survival; CR, complete remission.
Studies have shown that increased Bax expression is a good predictor being a pro-apoptotic marker, as it is associated with a higher CR rate (n=34) in AML patients,26 while in acute lymphoblastic leukemia (ALL), a low Bax expression was associated (n=14, immunoblotting) with relapse.15 In contrast, increased Bax expression has been found in association with decreased DFS (n=47, Western blotting)27 and OS (n=232, real-time PCR).28 However, a recent study conducted on ALL patients (n=26; real-time PCR)29 and another study on endometrial cancer (n=89; immunohistochemistry) have shown no significant correlation of Bax expression with chemotherapy outcome, in agreement with our observations.20
Similarly, Bcl-2 has been investigated in many cancers, yielding conflicting results. A recent study reported that decreased expression of Bcl-2 was found to be associated with a better CR rate in ALL but not in AML patients, which supports our observations.30 However, they found that increased Bcl-2 expression was associated with relapse and higher bone marrow blast counts in both AML and ALL patients. It is notable that they had a small sample size (ALL=16 and AML=24) investigated using flow cytometry. Similarly, Kornblau et al31 has reported in a larger sample of AML patients (n=198; Western blotting) that high Bcl-2 expression was associated with a shorter median survival in the favorable and intermediate cytogenetic group, while a longer median survival as well as remission duration in the poor cytogenetic group. We found that there was a tendency of higher Bcl-2 levels in APL patients as compared to other patient subtypes, as well as in those with persistent remission as compared to poor responders, although it failed to reach statistical significance (Table 4). However, others have reported significant differences. For example, it has been observed that Bcl-2-positive patients had better OS as compared to Bcl-2-negative patients in AML,32 colorectal carcinoma,33 and breast cancer34 patients. In contrast, another study with a smaller number of ALL and AML patients (n=26) showed no significant correlation with CR rate, DFS, or OS.29 Similarly a larger study (n=502) conducted on prostate patients using immunohistochemistry did not find any significant correlation between Bcl-2 expression and clinical outcome.17 Likewise, other studies on malignancies such as ALL,15 endometrial cancer,20 head and neck cancers,35 ovarian cancer,21,36 hepatocellular carcinoma,37 and colorectal carcinoma18 did not find significant association of Bcl-2 expression and clinical outcome. It is noteworthy that although being anti-apoptotic, it is easy to understand that a lower expression of Bcl-2 could translate into a better clinical outcome, but the paradoxical association with poor clinical outcome is difficult to explain. A partial explanation could be given by an observation that in mouse liver, Bcl-2 inhibits carcinogenesis and delays the growth of proliferative foci, thereby leading to a favorable outcome.38 This is contrary to the widely accepted notion that Bcl-2 may be involved in the development of cancer and chemoresistance. Interestingly, Kasimir-Bauer et al,39 who investigated the expression of Bcl-2, P-glycoprotein, multidrug resistance-related protein, p53, and heat shock protein 27 in AML patients (n=124), reported that although the individual markers did not correlate with OS, a combination of at least two proteins does so.
Likewise, Bax and Bcl-2 as a ratio (Bax/Bcl-2 or otherwise) has been widely studied as a prognostic marker of chemotherapy success, as discussed below. One of the reasons to study Bax and Bcl-2 as a ratio was lack of clear evidence of them being a reliable marker when used alone, as mentioned earlier. A study on AML patients has shown that a higher Bax/Bcl-2 ratio is associated with a higher CR rate and a lower Bax/Bcl-2 ratio is associated with a poor outcome.40 The authors also reported that a low Bax/Bcl-2 ratio was characterized by relatively immature cell types (AML-M0 and M1 subtypes; CD34 >20%) and poor risk cytogenetics (complex patterns, hyperploid or polyploidy, numerical or structural deletions of chromosome 5 or 7, trisomy 8, t(9;22), 11q23 rearrangements, etc). In another large study on AML patients, lower Bax/Bcl-2 was associated with lower OS.28
Other hematological malignancies have been studied in relation to the Bax/Bcl-2 ratio. In a study on a limited number of ALL patients (n=26), the high-risk group (n=9) had a higher Bax/Bcl-2 ratio at diagnosis than at remission, whereas no such association was observed in the median risk group.29 Others reported that decreased Bax/Bcl-2 ratio was associated with relapse in ALL patients (n=14).15 Hogarth and Hall observed an increased Bcl-2/Bax ratio (reversed ratio, translating into low Bax/Bcl-2) and myeloid cell leukemia factor 1 (Mcl-1)/Bax ratio in B lineage as compared to T lineage cells.27 They also reported that high Bax expression was associated with a higher probability of relapse in ALL patients (n=47). Similarly, the Bax/Bcl-2 ratio has been studied in solid organ tumors, again with conflicting results. In rectal carcinoma (n=49), a lower Bax/Bcl-2 ratio was found associated with radio resistance,16 while others reported that a lower Bax/Bcl-2 ratio correlated with better OS in colorectal adenocarcinoma (n=112).18 However, the ratio shows variability with age and site of involvement, as reported by a small study (n=22).41 Considering the skewed distribution of data in many studies and its possible role in conflicting results, some authors have attempted to rather categorize the expression data. In a large study among prostate cancer patients (n=502), a multivariate analysis found that Bcl-2 overexpression was not related independently to mortality and metastasis. However, a combination of “negative bcl-2 and normal Bax” was associated with slightly better response rate to short-term androgen therapy but not with long-term androgen therapy.17
In our study, Bax/Bcl-2 ratio was not significantly different among good and poor responders; however, APL, a subtype of AML with better prognosis than all other subtypes combined, showed significantly lower Bax/Bcl-2 ratio as compared to other subtypes (mostly M0, M1, and M2) (p<0.001) in bone marrow samples. When considering Bax and Bcl-2 expression separately rather than as a ratio, it was observed that their expression was higher in APL patients as compared to other patient subtypes, although it failed to reach statistical significance. It is noteworthy that APL patients had a favorable outcome as denoted by significantly lower rates of relapse (χ2(1) =6.3, p=0.012), poor final therapeutic response (χ2(1) =15.5, p<0.001), and mortality (χ2(1) =4.3, p=0.05) as compared to other AML subtypes combined (data not shown). Likewise, bone marrow Bax/Bcl-2 ratio was lower in MPO-negative as compared to MPO-positive patients (p=0.03), where MPO was significantly positive among AML subtypes with poor prognosis (subtypes other than APL) (χ2(1) =13.7, p<0.001), and those who had relapse (χ2(1) =4.3, p=0.033), or had poor final therapeutic response (χ2(1) =8, p=0.007) (data not shown). Thus, APL subtype and negative MPO were found as good prognostic factors but the Bax/Bcl-2 ratio was lower in those subgroups.
Thus, we can conclude from the above discussion that the expression pattern of Bax, Bcl-2, and their ratio not only differs between various cancers but even within the same cancer. Such conflicting reports might suggest a more complicated role of Bax, Bcl-2, or their ratio and needs further investigation. It is evident that some of the inconsistencies could be explained by the type of cancer, the source and size of the sample, the variance of data, and treatment options or techniques used.
Recently, multiple studies have focused on the development of anti-Bcl-2 drugs.22,23 Although in vitro results show some positive findings, the ultimate efficacy needs to be evaluated cautiously due to the conflicting results discussed above. Hence, we recommend that the process of apoptosis should first be explored in depth to understand the role of different members of apoptotic pathway before conducting clinical trials on patients with new potential therapeutic agents.
Our study has some limitations as discussed below. We included patients available from a single hematology center. The survival analysis was available up to only 1 year. A long-term follow-up is required for more conclusive results. Another limitation is that we focused on only Bax and Bcl-2 among various apoptosis-related proteins. As discussed above, both are frequently studied members of apoptosis-related proteins but still have conflicting evidence in terms of utility in cancer chemotherapy, thus making them a logical choice to study in resource-constrained situations like ours. Recently, Mcl-1, a Bcl-2 family member has gained importance as a regulator of apoptosis.42 Mcl-1 has multiple variants due to alternative splicing. One of them is “long isoform” (Isoform 1) that inhibits apoptosis while two other “short isoforms” (Isoforms 2 and 3) promote apoptosis. This is interesting that the same gene codes for both anti- and pro-apoptotic protein isoforms which regulate each other as well.43 However, it has been shown in vitro that Bcl-2 rather than Mcl-1 is a predominant marker in leukemia.44 Further studies should be designed to investigate the role of Mcl-1 and other members of apoptosis regulating proteins in relation to chemotherapy response in AML patients.
This study has important advantages. The sample size was larger than many contemporary studies. We used a dual approach to study both the actual expression and their ranking, as discussed above. Furthermore, we analyzed the expression in bone marrow and blood separately, thus avoiding the bias of sample pooling. Such an approach is based on the fact that AML is a disease in bone marrow rather than peripheral blood; therefore, bone marrow but not peripheral blood should logically be the appropriate substrate in such studies. It is also evident that a difference of results between the two types of samples supports our approach. Another advantage was inclusion of concurrent bone marrow and blood samples from the majority of patients. Therefore, we were able to compare the two types of samples confidently. Finally, the patients in our study received similar treatment, thus allowing direct statistical comparisons to be made. We used real-time PCR which is more sensitive and accurate than immunohistochemistry, Western blotting, and flow cytometry that are used in many studies.
Conclusion
The findings of our study suggest that there is no significant association between the expression of Bax and Bcl-2 and their ratio with clinical response, or with 1 year DFS and OS. Although both Bax and Bcl-2 had higher expression in those with persistent remission (good response group), a higher Bax/Bcl-2 ratio was more common among those who were resistant or had relapse (poor response group) (Table 4). The overall picture reflects a complex balance between Bax and Bcl-2, and hence their ratio could be misleading if interpreted incautiously. Based on our findings, we recommend that development of anti Bcl-2 therapy should be pursued with extreme caution in patients until the role of Bcl-2 is more clearly understood in cancer-specific apoptosis. Our findings suggest that for these types of analyses, pooling of bone marrow and blood samples is likely to generate a bias. Thus, the body of literature that reported findings based on pooled bone marrow and blood samples should be revisited to confirm their conclusions.
Supplementary materials
Table S1.
One-way ANOVA test to analyze Bax and Bcl-2 expression difference between various outcomes
| Gene expression | Remission status | N | Mean | SE | 95% CI
|
p-value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Bax expression marrow | Resistant | 31 | 7.23 | 13.25 | −0.24 | 14.71 | 0.49 |
| Relapse | 15 | 3.75 | 1.69 | 0.12 | 7.39 | ||
| Remission | 31 | 13.35 | 304.25 | −0.57 | 27.28 | ||
| Total | 77 | 9.02 | 130.31 | 2.78 | 15.26 | ||
| Bcl-2 expression marrow | Resistant | 31 | 4.89 | 5.90 | −2.06 | 11.85 | 0.53 |
| Relapse | 15 | 3.48 | 1.94 | −0.69 | 7.65 | ||
| Remission | 31 | 12.65 | 362.11 | −4.07 | 29.37 | ||
| Total | 77 | 7.74 | 155.35 | 0.60 | 14.88 | ||
| Ratio Bax/Bcl-2 marrow | Resistant | 31 | 3609.67 | 2792.84 | 2276.34 | 9495.67 | 0.32 |
| Relapse | 15 | 3.18 | 0.55 | 1.99 | 4.37 | ||
| Remission | 31 | 5.56 | 1.08 | 3.15 | 7.97 | ||
| Total | 77 | 1456.10 | 1095.80 | −867.58 | 3779.78 | ||
| Bax expression blood | Resistant | 30 | 10.59 | 11.89 | −2.08 | 23.26 | 0.56 |
| Relapse | 14 | 4.14 | 1222.59 | −0.70 | 8.99 | ||
| Remission | 28 | 4.66 | 1330.16 | −0.45 | 9.78 | ||
| Total | 72 | 7.03 | 583.28 | 1.48 | 12.59 | ||
| Bcl-2 expression blood | Resistant | 30 | 3.84 | 11.44 | −1.28 | 8.95 | 1.00 |
| Relapse | 14 | 4.14 | 239.17 | −2.93 | 11.21 | ||
| Remission | 28 | 3.92 | 2608.46 | −2.92 | 10.76 | ||
| Total | 72 | 3.93 | 1051.18 | 0.42 | 7.43 | ||
| Ratio Bax/Bcl-2 blood | Resistant | 30 | 659.46 | 10.43 | −673.06 | 1991.98 | 0.68 |
| Relapse | 14 | 9.93 | 54.06 | −2.62 | 22.48 | ||
| Remission | 28 | 259.33 | 6846.27 | −263.95 | 782.61 | ||
| Total | 72 | 377.56 | 2759.30 | −196.11 | 951.23 | ||
Abbreviation: SE, standard error.
Table S2.
Post hoc analysis (Dunnett’s two-tailed): persistent remission was considered as control group
| Dependent variable | Remission status (a) | Remission status (b) | Mean difference (a–b) | SE | 95% CI
|
p-value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Bax expression marrow | Resistant | Remission | −6.12 | 7.01 | −21.99 | 9.75 | 0.60 |
| Relapse | Remission | −9.60 | 8.68 | −29.25 | 10.05 | 0.45 | |
| Bcl-2 expression marrow | Resistant | Remission | −7.76 | 8.03 | −25.94 | 10.42 | 0.54 |
| Relapse | Remission | −9.17 | 9.94 | −31.68 | 13.34 | 0.57 | |
| Ratio Bax/Bcl-2 marrow | Resistant | Remission | 3604.11 | 2595.18 | −2274.13 | 9482.35 | 0.29 |
| Relapse | Remission | −2.38 | 3213.55 | −7281.28 | 7276.52 | 1.00 | |
| Bax expression blood | Resistant | Remission | 5.93 | 6.25 | −8.24 | 20.09 | 0.55 |
| Relapse | Remission | −0.52 | 7.78 | −18.17 | 17.13 | 1.00 | |
| Bcl-2 expression Blood | Resistant | Remission | −0.08 | 3.97 | −9.09 | 8.93 | 1.00 |
| Relapse | Remission | 0.23 | 4.95 | −11.00 | 11.45 | 1.00 | |
| Ratio Bax/Bcl-2 Blood | Resistant | Remission | 400.13 | 647.12 | −1067.06 | 1867.32 | 0.77 |
| Relapse | Remission | −249.40 | 806.11 | −2077.05 | 1578.25 | 0.94 | |
Abbreviation: SE, standard error.
Acknowledgments
The authors acknowledge Alfaisal University, Riyadh, Saudi Arabia, for the financial support through grant IRG-302111603131 as a partial support of this project. We also thank Dr Peter MB Cahusac, Associate Professor of Pharmacology and Biostatistics, Alfaisal University, for his kind support in reviewing the article for English language.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Singh G, Parmar P, Kataria SP, Kataria SP, Singh S, Sen R. Spectrum of acute and chronic leukemia at a tertiary care hospital, Haryana, India. Int J Res Med Sci. 2016;4:1115–1118. [Google Scholar]
- 3.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 5.Lerch E, Espeli V, Zucca E, et al. Prognosis of acute myeloid leukemia in the general population: data from southern Switzerland. Tumori. 2009;95:303–310. doi: 10.1177/030089160909500306. [DOI] [PubMed] [Google Scholar]
- 6.Bahl A, Sharma A, Raina V, et al. Long-term outcomes for patients with acute myeloid leukemia: a single-center experience from AIIMS, India. Asia Pac J Clin Oncol. 2015;11:242–252. doi: 10.1111/ajco.12333. [DOI] [PubMed] [Google Scholar]
- 7.Weltermann A, Fonatsch C, Haas OA, et al. Impact of cytogenetics on the prognosis of adults with de novo AML in first relapse. Leukemia. 2004;18:293–302. doi: 10.1038/sj.leu.2403243. [DOI] [PubMed] [Google Scholar]
- 8.Ullah K, Ahmed P, Raza S, et al. Management of acute myeloid leukaemia-5 years experience at Armed Forces Bone Marrow Transplant Centre, Rawalpindi. J Pak Med Assoc. 2007;57:434–439. [PubMed] [Google Scholar]
- 9.Maynadié M, De Angelis R, Marcos-Gragera R, et al. Survival of European patients diagnosed with myeloid malignancies: a HAEMACARE study. Haematologica. 2013;98:230–238. doi: 10.3324/haematol.2012.064014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schimmer AD, Pedersen IM, Kitada S, et al. Functional blocks in caspase activation pathways are common in leukemia and predict patient response to induction chemotherapy. Cancer Res. 2003;63:1242–1248. [PubMed] [Google Scholar]
- 11.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 12.Janumyan Y, Cui Q, Yan L, Sansam CG, Valentin M, Yang E. G0 function of BCL2 and BCL-xL requires BAX, BAK, and p27 phosphorylation by Mirk, revealing a novel role of BAX and BAK in quiescence regulation. J Biol Chem. 2008;283:34108–34120. doi: 10.1074/jbc.M806294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 14.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prokop A, Wieder T, Sturn I, et al. Relapse in childhood acute lymphoblastic leukemia is associated with a decrease of the Bax/Bcl-2 ratio and loss of spontaneous caspase-3 processing in vivo. Leukemia. 2000;14:1606–1613. doi: 10.1038/sj.leu.2401866. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto H, Wada T, Fukunaga K, Yoshihiro S, Matsuyama H, Naito K. Bax to Bcl-2 ratio and Ki-67 index are useful predictors of neoadjuvant chemoradiation therapy in bladder cancer. Jpn J Clin Oncol. 2004;34:124–130. doi: 10.1093/jjco/hyh026. [DOI] [PubMed] [Google Scholar]
- 17.Khor LY, Moughan J, Al-Saleem T, et al. Bcl-2 and Bax expression predict prostate cancer outcome in men treated with androgen deprivation and radiotherapy on radiation therapy oncology group protocol 92-02. Clin Cancer Res. 2007;13:3585–3590. doi: 10.1158/1078-0432.CCR-06-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katkoori VR, Suarez-Cuervo C, Shanmugam C, et al. Bax expression is a candidate prognostic and predictive marker of colorectal cancer. J Gastrointest Oncol. 2010;1:76–89. doi: 10.3978/j.issn.2078-6891.2010.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh L, Pushker N, Saini N, et al. Expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins in human retinoblastoma. Clin Exp Ophthalmol. 2015;43:259–267. doi: 10.1111/ceo.12397. [DOI] [PubMed] [Google Scholar]
- 20.Appel ML, Edelweiss MI, Fleck J, et al. P53 and BCL-2 as prognostic markers in endometrial carcinoma. Pathol Oncol Res. 2008;14:23–30. doi: 10.1007/s12253-008-9000-9. [DOI] [PubMed] [Google Scholar]
- 21.Palmer JE, Sant Cassia LJ, Irwin CJ, Morris AG, Rollason TP. P53 and bcl-2 assessment in serous ovarian carcinoma. Int J Gynecol Cancer. 2008;18:241–248. doi: 10.1111/j.1525-1438.2007.01000.x. [DOI] [PubMed] [Google Scholar]
- 22.Paoluzzi L, Gonen M, Bhagat G, et al. The BH3-only mimetic ABT-737 synergizes the antineoplastic activity of proteasome inhibitors in lymphoid malignancies. Blood. 2008;112:2906–2916. doi: 10.1182/blood-2007-12-130781. [DOI] [PubMed] [Google Scholar]
- 23.Khaw SL, Mérino D, Anderson MA, et al. Both leukaemic and normal peripheral B lymphoid cells are highly sensitive to the selective Pharmacological inhibition of prosurvival Bcl-2 with ABT-199. Leukemia. 2014;28:1207–1215. doi: 10.1038/leu.2014.1. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, Kazui T, Yoshida M, et al. Drug-induced apoptosis and p53, BCL-2 and BAX expression in breast cancer tissues in vivo and in fibroblast cells in vitro. Jpn J Clin Oncol. 1999;29:323–331. doi: 10.1093/jjco/29.7.323. [DOI] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Bekhet MM, Fouad NTA, Fathey H, El-Sayed E, Hendawy LM, Khattab D. Prognostic value of Bcl2-associated X protein (Bax) expression in adult Egyptian patients with acute myeloid leukemia. Indian J Med Res Pharm Sci. 2016;3:1–7. [Google Scholar]
- 27.Hogarth LA, Hall AG. Increased BAX expression is associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood. 1999;93:2671–2678. [PubMed] [Google Scholar]
- 28.Köhler T, Schill C, Deininger MW, et al. High Bad and Bax mRNA expression correlate with negative outcome in acute myeloid leukemia (AML) Leukemia. 2002;16:22–29. doi: 10.1038/sj.leu.2402340. [DOI] [PubMed] [Google Scholar]
- 29.Kaparou M, Choumerianou D, Perdikogianni C, Martimianaki G, Kalmanti M, Stiakaki E. Enhanced levels of the apoptotic BAX/BCL-2 ratio in children with acute lymphoblastic leukemia and high-risk features. Genet Mol Biol. 2013;36:7–11. doi: 10.1590/S1415-47572013005000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aref S, Salama O, Al-Tonbary Y, Mansour A. Assessment of bcl-2 expression as modulator of fas mediated apoptosis in acute leukemia. Hematology. 2004;9:113–121. doi: 10.1080/1024533042000205496. [DOI] [PubMed] [Google Scholar]
- 31.Kornblau SM, Thall PF, Estrov Z, et al. The prognostic impact of BCL2 protein expression in acute myelogenous leukemia varies with cytogenetics. Clin Cancer Res. 1999;5:1758–1766. [PubMed] [Google Scholar]
- 32.Sahu G, Jena RK. Clinical significance of P53 and Bcl-2 in acute myeloid leukemia patients of Eastern India. Hematol Rep. 2011;3:e28. doi: 10.4081/hr.2011.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Q, Li S, Cheng P, et al. High expression of anti-apoptotic protein Bcl-2 is a good prognostic factor in colorectal cancer: result of a meta-analysis. World J Gastroenterol. 2017;23:5018–5033. doi: 10.3748/wjg.v23.i27.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang KT, Woo JW, Shin HC, et al. Prognostic influence of BCL2 expression in breast cancer. Int J Cancer. 2012;131:E1109–E1119. doi: 10.1002/ijc.27539. [DOI] [PubMed] [Google Scholar]
- 35.Casado S, Forteza J, Dominguez S, et al. Predictive value of P53, BCL-2, and BAX in advanced head and neck carcinoma. Am J Clin Oncol. 2002;25:588–590. doi: 10.1097/00000421-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Sagarra RA, Andrade LA, Martinez EZ, et al. P53 and Bcl-2 as prognostic predictors in epithelial ovarian cancer. Int J Gynecol Cancer. 2002;12:720–727. doi: 10.1046/j.1525-1438.2002.01135.x. [DOI] [PubMed] [Google Scholar]
- 37.Garcia EJ, Lawson D, Cotsonis G, et al. Hepatocellular carcinoma and markers of apoptosis (bcl-2, bax, bcl-x): prognostic significance. Appl Immunohistochem Mol Morphol. 2002;10:210–217. doi: 10.1097/00129039-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Pierce RH, Vail ME, Ralph L, Campbell JS, Fausto N. Bcl-2 expression inhibits liver carcinogenesis and delays the development of proliferating foci. Am J Pathol. 2002;160:1555–1560. doi: 10.1016/S0002-9440(10)61101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasimir-Bauer S, Beelen D, Flasshove M, Noppeney R, Seeber S, Scheulen ME. Impact of the expression of P glycoprotein, the multidrug resistance-related protein, bcl-2, mutant p53, and heat shock protein 27 on response to induction therapy and long-term survival in patients with de novo acute myeloid leukemia. Exp Hematol. 2002;30:1302–1308. doi: 10.1016/s0301-472x(02)00926-8. [DOI] [PubMed] [Google Scholar]
- 40.Del Poeta G, Venditti A, Del Principe MI, et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML) Blood. 2003;101:2125–2131. doi: 10.1182/blood-2002-06-1714. [DOI] [PubMed] [Google Scholar]
- 41.Khodapasand E, Jafarzadeh N, Farrokhi F, Kamalidehghan B, Houshmand M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J. 2015;19:69–75. doi: 10.6091/ibj.1366.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belmar J, Fesik SW. Small molecule Mcl-1 inhibitors for the treatment of cancer. Pharmacol Ther. 2015;145:76–84. doi: 10.1016/j.pharmthera.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JH, Sim SH, Ha HJ, Ko JJ, Lee K, Bae J. MCL-1ES, a novel variant of MCL-1, associates with MCL-1L and induces mitochondrial cell death. FEBS Lett. 2009;583:2758–2764. doi: 10.1016/j.febslet.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010;1:e40. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peiró G, Diebold J, Baretton GB, Kimmig R, Löhrs U. Cellular apoptosis susceptibility gene expression in endometrial carcinoma: correlation with Bcl-2, Bax, and caspase-3 expression and outcome. Int J Gynecol Pathol. 2001;20(4):359–367. doi: 10.1097/00004347-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Scopa CD, Vagianos C, Kardamakis D, Kourelis TG, Kalofonos HP, Tsamandas AC. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with rectal cancer. Appl Immunohistochem Mol Morphol. 2001;9:329–334. doi: 10.1097/00129039-200112000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
One-way ANOVA test to analyze Bax and Bcl-2 expression difference between various outcomes
| Gene expression | Remission status | N | Mean | SE | 95% CI
|
p-value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Bax expression marrow | Resistant | 31 | 7.23 | 13.25 | −0.24 | 14.71 | 0.49 |
| Relapse | 15 | 3.75 | 1.69 | 0.12 | 7.39 | ||
| Remission | 31 | 13.35 | 304.25 | −0.57 | 27.28 | ||
| Total | 77 | 9.02 | 130.31 | 2.78 | 15.26 | ||
| Bcl-2 expression marrow | Resistant | 31 | 4.89 | 5.90 | −2.06 | 11.85 | 0.53 |
| Relapse | 15 | 3.48 | 1.94 | −0.69 | 7.65 | ||
| Remission | 31 | 12.65 | 362.11 | −4.07 | 29.37 | ||
| Total | 77 | 7.74 | 155.35 | 0.60 | 14.88 | ||
| Ratio Bax/Bcl-2 marrow | Resistant | 31 | 3609.67 | 2792.84 | 2276.34 | 9495.67 | 0.32 |
| Relapse | 15 | 3.18 | 0.55 | 1.99 | 4.37 | ||
| Remission | 31 | 5.56 | 1.08 | 3.15 | 7.97 | ||
| Total | 77 | 1456.10 | 1095.80 | −867.58 | 3779.78 | ||
| Bax expression blood | Resistant | 30 | 10.59 | 11.89 | −2.08 | 23.26 | 0.56 |
| Relapse | 14 | 4.14 | 1222.59 | −0.70 | 8.99 | ||
| Remission | 28 | 4.66 | 1330.16 | −0.45 | 9.78 | ||
| Total | 72 | 7.03 | 583.28 | 1.48 | 12.59 | ||
| Bcl-2 expression blood | Resistant | 30 | 3.84 | 11.44 | −1.28 | 8.95 | 1.00 |
| Relapse | 14 | 4.14 | 239.17 | −2.93 | 11.21 | ||
| Remission | 28 | 3.92 | 2608.46 | −2.92 | 10.76 | ||
| Total | 72 | 3.93 | 1051.18 | 0.42 | 7.43 | ||
| Ratio Bax/Bcl-2 blood | Resistant | 30 | 659.46 | 10.43 | −673.06 | 1991.98 | 0.68 |
| Relapse | 14 | 9.93 | 54.06 | −2.62 | 22.48 | ||
| Remission | 28 | 259.33 | 6846.27 | −263.95 | 782.61 | ||
| Total | 72 | 377.56 | 2759.30 | −196.11 | 951.23 | ||
Abbreviation: SE, standard error.
Table S2.
Post hoc analysis (Dunnett’s two-tailed): persistent remission was considered as control group
| Dependent variable | Remission status (a) | Remission status (b) | Mean difference (a–b) | SE | 95% CI
|
p-value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Bax expression marrow | Resistant | Remission | −6.12 | 7.01 | −21.99 | 9.75 | 0.60 |
| Relapse | Remission | −9.60 | 8.68 | −29.25 | 10.05 | 0.45 | |
| Bcl-2 expression marrow | Resistant | Remission | −7.76 | 8.03 | −25.94 | 10.42 | 0.54 |
| Relapse | Remission | −9.17 | 9.94 | −31.68 | 13.34 | 0.57 | |
| Ratio Bax/Bcl-2 marrow | Resistant | Remission | 3604.11 | 2595.18 | −2274.13 | 9482.35 | 0.29 |
| Relapse | Remission | −2.38 | 3213.55 | −7281.28 | 7276.52 | 1.00 | |
| Bax expression blood | Resistant | Remission | 5.93 | 6.25 | −8.24 | 20.09 | 0.55 |
| Relapse | Remission | −0.52 | 7.78 | −18.17 | 17.13 | 1.00 | |
| Bcl-2 expression Blood | Resistant | Remission | −0.08 | 3.97 | −9.09 | 8.93 | 1.00 |
| Relapse | Remission | 0.23 | 4.95 | −11.00 | 11.45 | 1.00 | |
| Ratio Bax/Bcl-2 Blood | Resistant | Remission | 400.13 | 647.12 | −1067.06 | 1867.32 | 0.77 |
| Relapse | Remission | −249.40 | 806.11 | −2077.05 | 1578.25 | 0.94 | |
Abbreviation: SE, standard error.