Abstract
Diabetic kidney disease (DKD) is the leading cause of end-stage renal disease (ESRD) and dialysis in the Western world. Early DKD, including microalbuminuria and renal hyperfiltration, are common in adolescents with type 2 diabetes (T2D). Furthermore, youth-onset T2D carries a higher risk of progressive DKD than adult-onset T2D of similar diabetes duration. DKD is characterized by a long clinically-silent period without signs of disease. Therefore, a major challenge in preventing DKD is the difficulty in identifying high-risk T2D patients at an early stage.
The Type 2 Diabetes in Adolescents and Youth (TODAY) study demonstrated a high initial prevalence that increased over time, irrespective of treatment arm. This key observation underscores the importance of discovering new therapeutic targets to supplement conventional management, in order to reduce DKD risk.
In this review, we focus on early DKD in T2D and summarize potential novel biomarkers and therapeutic targets.
Keywords: Glomerular filtration rate (GFR), cystatin C, creatinine, iohexol, diabetic kidney disease, renal hyperfiltration, rapid GFR decline, albuminuria, impaired GFR
Introduction
Diabetic kidney disease (DKD) remains a leading cause of morbidity and mortality in people with type 2 diabetes (T2D) (1–3). The 2011 US Renal Data System reported that DKD accounted for 44.5% of all cases of end-stage renal disease (ESRD) (4). In 2009, overall Medicare expenditure for people with chronic kidney disease (CKD) and diabetes accounted for $18 billion (4). The prevalence of DKD has remained fairly stable over the last 20 years, despite increasing prevalence of T2D (5, 6), likely related to improved glycemic, blood pressure and weight control, since evidence-based therapies directly targeting DKD are scarce. Markers of early DKD, including microalbuminuria and renal hyperfiltration, are common in youth with T2D (7). For example, we previously reported a prevalence of 34% for microalbuminuria and 24% for renal hyperfiltration in adolescents with T2D with a mean age of 15 years (7). Moreover, the Type 2 Diabetes in Adolescents and Youth (TODAY) study demonstrated that microalbuminuria is common in youth with an average T2D duration of only 6 months and reported a 2.5 fold increase in the occurrence of microalbuminuria over an average follow-up of 3.9 years (8). Since signs of DKDare already present at diabetes diagnosis in youth with T2D, early interventions may be the most likely to prevent progression of DKD.
Youth-onset T2D carries a particularly high risk of progressive DKD, which is significantly greater than youth with type 1 diabetes (T1D) or adults with T2D of similar disease duration (8–15). In fact, adolescents with T2D have a two-fold increased risk of microalbuminuria compared to youth with T1D (8, 10, 16). Risk factors for DKD in T2D include female sex, obesity, triglycerides, hyperglycemia, hypertension, cardiovascular disease, insulin resistance, and elevated uric acid (17–21) [Figure 1].
Figure 1.
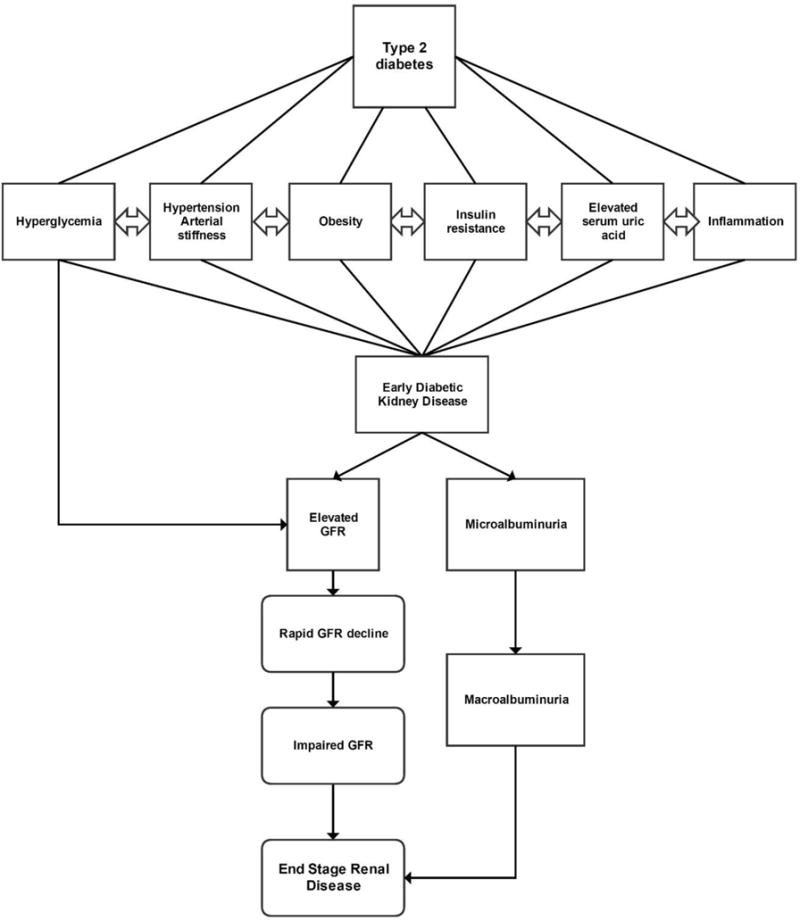
Risk factors for diabetic nephropathy in youth with type 2 diabetes
There are limited longitudinal data available on the natural history of DKD in youth with T2D (22). Microalbuminuria may precede the onset of T2D in insulin-resistant obese adolescents (23, 24). In addition, hyperfiltration is thought to be a major contributing factor for DKD in T2D, reflecting underlying increased intraglomerular pressure leading to structural changes over time, such as mesangial expansion and glomerular basement membrane thickening (25). Obesity and impaired glucose tolerance are associated with hyperfiltration (25–29), suggesting that renal injury occurs very early in the disease process (30), possibly prior to development of T2D. It is also noteworthy that a significant proportion of patients do not follow this classical trajectory of microalbuminuria and normal-to-elevated glomerular filtration rate (GFR), followed by proteinuria and GFR decline; instead, these patients exhibit an accelerated GFR decline in the absence of proteinuria (17–21). The loss of renal function in the absence of albuminuria highlights the need to identify alternate biomarkers that better capture early DKD risk.
By the time GFR is below 60mL/min/1.73m2, approximately half of renal function is lost, with well-established renal structural changes that are usually refractory to therapeutic strategies, including improved blood pressure and glycemic control (31, 32). While there are reports demonstrating associations between glycemic control, insulin sensitivity, and DKD in youth with T2D (7, 8), additional longitudinal data are required to further characterize these relationships and to identify novel and modifiable risk factors that contribute to the development and progression of early DKD. Understanding these risk factors may enable us to identify individuals at high risk of early DKD and to intervene prior to permanent kidney injury. In this review, we focus on early DKD in T2D and summarize risk factors, early biomarkers and therapeutic targets for this condition.
Risk factors for DKD in youth with T2D
i. Microalbuminuria
Microalbuminuria, defined as albumin-to-creatinine ratio ≥30mg/g or an albumin-excretion-rate ≥200ug/min, has been used as a marker of renal and systemic vascular dysfunction (33) and metabolic risk in adults and adolescents with prediabetes and T2D (34), (35). The implications of having microalbuminuria are, however, controversial, since microalbuminuria regresses to normoalbuminuria in a significant proportion of adults with T2D (36). Proposed determinants of albuminuria regression include blood pressure and glycemic control (36) but are not well defined in adolescents with T2D. In adults with T1D, estimated insulin sensitivity at baseline was predictive of microalbuminuria regression over 6-years of follow up [odds ratio: 2.5, 95% confidence interval 1.3–4.9, p= 0.006] (37). Similarly, in our cross-sectional analysis of adolescents with T2D, one standard deviation increase in measured insulin sensitivity by the hyperinsulinemic-euglycemic clamp technique was associated with lower odds of having microalbuminuria [odds ratio: 0.41, 95% confidence interval 0.17-0.99, p=0.047] (7). For these reasons, insulin sensitivity may hold promise as a modifiable risk factor for microalbuminuria in adolescents with pre-diabetes and T2D.
ii. Renal hyperfiltration
Early DKD phenotypes, such as renal hyperfiltration and rapid GFR decline, are considered strong risk factors for progression to CKD and ESRD and may predict progressive DKD prior to loss of renal function (38–42). For that reason, GFR may be a more clinically relevant measure of early nephropathy than albuminuria in diabetes. As a result, the American Diabetes Association, National Kidney Foundation and International Society of Nephrology recommend annual measurement of estimated GFR to identify and monitor DKD (43–45).
Renal hyperfiltration is typically defined as a GFR of between 120 mL/min and 150 mL/min/1.73m2, or greater than 2 standard deviations above the mean GFR in normal, healthy individuals (46), and is thought to represent the earliest hemodynamic abnormality seen in diabetes (27, 38, 47). Individuals with T2D frequently exhibit a significant increase in GFR, with the prevalence of renal hyperfiltration reported to range between 5-40% (27, 38), predisposing this population to progressive renal disease (27). The pathogenesis of hyperfiltration in T2D is incompletely understood but has been attributed to glomerular hemodynamic and tubular factors (48). Hyperfiltration has also been documented to occur in individuals with glucose intolerance before the diagnosis of T2D (49, 50). Additionally, obesity and impaired glucose tolerance are associated with renal injury that is pathophysiologically and histologically similar to classical diabetic nephropathy (27–29, 51), suggesting that the renal insult may begin prior to the development of frank hyperglycemia (30). For example, a recent report demonstrated increased estimated GFR in adolescents with pre-diabetes and overweight adolescents compared to lean controls (35).
iii. Rapid GFR decline
Rapid GFR decline, commonly defined as annual loss greater than 3mL/min/1.73m2 or >3.3%/year, is considered a stronger predictor of progressive DKD than albuminuria in T1D (52–57), but data in T2D are less consistent (58). In Pima Indians with T2D, rapid GFR decline is frequently present prior to the onset of macroalbuminuria and the GFR slope over time is reported to be almost as predictive of ESRD as albuminuria (58). In contrast to data from adults with T1D, progression to ESRD was strongly dependent on progression to macroalbuminuria (58). Given the importance of rapid GFR decline and current lack of data, longitudinal assessments of GFR trajectories in adolescents with T2D are needed.
iv. Estimation and measurement of GFR in T2D
Although there are several equations available to estimate GFR in children and adolescents using endogenous filtration markers (serum creatinine and/or cystatin C), to our knowledge no single equation has been specifically developed or validated in adolescents with T2D. The Schwartz creatinine-based equation from 2009 is the most widely used in clinical practice, but with its most accurate range being between 25-75 mL/min/1.73m2 (59), this equation is less useful in adolescents with T2D who usually have GFR values above this range (27, 38, 40, 41). Stronger agreement with measured GFR was demonstrated with cystatin C (e.g. Zappitelli and Berg) (60–62) and combined creatinine and cystatin C equations (e.g. CKiD, Zappitelli, Schwartz, Bouvet combined creatinine and cystatin C equations) (59–61, 63, 64) compared to creatinine equations (59–64). While both serum creatinine and cystatin C are affected by factors other than GFR, cystatin C is considered to be less biased by age and weight compared to creatinine-based measurements and correlates more closely with direct measures of GFR over a wide spectrum of plasma glucose levels (65, 66). Despite the possible superiority of cystatin C compared to creatinine, currently available estimates of GFR remain imperfect (67–69) and there are no currently published equations validated against measured GFR in youth with T2D.
A recent DCCT-EDIC paper reported that changes in eGFR over a 3 year period may not reflect changes in measured GFR (70, 71). This is of particular concern in adolescents and young adults with diabetes, in whom renal hyperfiltration is present in approximately 50% of individuals (38, 46). The dissociation between changes in eGFR and measured GFR is of further concern since rapid changes in GFR may be missed due to a lack of acceptable screening methods for subtle changes in renal function (56). Perrin et al. reported that most GFR estimations fail to detect a significant proportion of hyperfiltration in patients with T1D based on measured GFR and concluded that estimated GFR cannot replace measured GFR in T1D patients with hyperfiltration (72). Recently, MacIsaac et al. demonstrated that, in adults with T1D and T2D, estimated GFR by creatinine significantly underestimated early decline in measured GFR (73). There is, thus, a clear need to improve calculation of GFR in the ambulatory setting. We recently demonstrated that iohexol clearance using dried capillary blood spots on filter paper measured GFR accurately in adults with T1D compared to the gold standard method of plasma iohexol measurement (74). This method was also piloted for feasibility in adolescents and adults with T1D (74, 75) and has the potential to be translated to screening for early kidney disease in adolescents with T2D in both clinical and research settings (74).
Novel biomarkers for the prediction of DKD
DKD is characterized by a long, clinically silent period without signs or symptoms of disease. However, while albuminuria and estimated glomerular filtration rate are currently the best means of screening for DKD in adolescents with T2D, there is a need for improved methods to detect early mediators of renal injury. Early detection would improve risk stratification and ultimately prevent initiation and progression to ESRD. Serum and urinary biomarkers that show promise in predicting DKD in adults with T2D are listed in Table 1. Circulating TNF Receptors 1 and 2 are particularly promising and strongly predicted ESRD in adults with T2D with and without proteinuria (76). Rather than examining single biomarkers, improved prediction may also be obtainable with panels of several urinary or serum biomarkers. Looker et al recently examined a broad set of 207 serum biomarkers in 154 Scottish T2D adults with incident cases of progressive GFR decline, and 153 non-progressing controls from the Genetics of Diabetes Audit and Research Tayside Study (GO-DARTS). A panel of 14 of these biomarkers (including FGF-21, SDMA, ADMA, β2-microglobulin, C16-acylcarnitine, and KIM-1) significantly improved upon the predictive performance of rapid progression by clinical data alone, with an increase in the area under the ROC curve from 0.706 to 0.868 (77). Similar analyses are needed in adolescents with T2D.
Table 1.
Serum and urinary biomarkers of DKD
| Biomarker | Reference | |
|---|---|---|
| Promising serum biomarkers | ||
| TNF receptor 1 and 2 | (139) | |
| Kidney injury molecule-1 | (77, 140) | |
| Fibroblast growth factor 21 and 23 | (77, 141) | |
| Symmetric dimethylarginine (SDMA) | (77) | |
| Asymmetric dimethylarginine (ADMA) | (77) | |
| Promising urinary biomarkers | ||
| Neutrophil gelatinase associated lipocalin (NGAL) | (142, 143) | |
| Metalloproteinases | (144) | |
| N-acetyl-beta-glucosaminidase | (145, 146) | |
| Nephrin | (147, 148) | |
| Alpha 1-microglobulin | (149, 150) |
Urinary proteomics is also a promising method of evaluating DKD risk early in the course of illness (78–81). CKD273, a panel of 273 urinary biomarkers, has shown to improve prediction of macroalbuminuria in individuals prior to an increase in albumin excretion (78–81). Furthermore, the addition of multi-peptide biomarkers to eGFR and albuminuria significantly improved prediction of CKD (80).
Another novel group of biomarkers are gasotransmitters, which include nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S). These gasotransmitters play important roles in the glomeruli for scavenging of reactive oxygen species, blood pressure regulation, and inflammation (82, 83). In diabetes, the bioavailability of gasotransmitters is generally lowered. For instance, deficiency of endothelial nitric oxide synthase (NOS) results in accelerated nephropathy in diabetic mice (84–86) and supplementation of tetrahydrobiopterin, a co-factor of NOS, reduces proteinuria and renal injury in T2D rats (87). Measurements of NO, CO, and H2S are not routinely available and remain technically challenging due to a relatively short half-life (82). Studies in humans are also needed to determine whether gasotransmitters are important risk factors for progression of DKD.
Novel therapeutic targets
Insulin sensitivity
Insulin resistance leads to important hemodynamic changes in the kidney, including increased sympathetic nervous system tone, hypertension, and accelerated atherosclerosis of the renal microvasculature. We previously demonstrated relationships between measured insulin sensitivity, albuminuria, and eGFR and also found lower odds of albuminuria with greater insulin sensitivity in adolescents with T2D (7). The association between insulin sensitivity and DKD is also increasingly recognized in adults with T2D, with reports demonstrating a cross-sectional relationship between measured insulin sensitivity and albuminuria (88), greater odds of albuminuria in adult T2D males with the highest quartile of HOMA-IR (89), and longitudinal associations between HOMA-IR and incident microalbuminuria over 5-years (90).
While insulin sensitivity can be modified by lifestyle changes (diet and exercise), drugs, such as metformin, have also been examined in renal studies. The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI-2D) study showed no benefit of an insulin sensitizing strategy on DKD in older adults with coronary artery disease and T2D (91) and the use of metformin in adults with T2D and stage 5 CKD has been associated with a significantly increased risk of all-cause mortality (92). While these studies showed no benefit of insulin sensitization on DKD in T2D, they were conducted in cohorts of older adults with multiple cardiovascular risk factors and longstanding nephropathy who may be less responsive to changes in insulin sensitivity than early DKD in adolescents with T2D. Therefore strategies to improve insulin sensitivity in T2D youth may still be of benefit to renal health and deserve further study.
Uric acid
Serum uric acid is a recognized risk factor for DKD in T2D (93, 94). Patients with T2D have elevated serum uric acid concentrations compared to their non-diabetic peers (95). Moreover, the metabolism of fructose, which is endogenously produced in diabetes from excess glucose via the polyol pathway, is associated with the generation of uric acid from a side chain reaction driven by ATP depletion and purine nucleotide turnover (96). Evidence from animal studies demonstrates that blocking uric acid production protects the kidney from tubulointerstitial injury, which may suggest a causal role for uric acid in the development of DKD (96). In an Italian cohort of T2D adults with normal kidney function and without overt proteinuria, the risk of CKD during a 5 year follow-up was significantly higher in participants with hyperuricemia compared with those without (93). In adults with T2D and DKD, serum uric acid was also found to predict progression of established DKD (94). From a renal therapeutic perspective, a post hoc analysis of the Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL) Trial found that lowering serum uric acid levels with losartan, which reduces serum uric acid levels by facilitating urinary uric acid excretion, accounted for 20% of the renoprotective benefit of this medication (97). More direct uric acid lowering with xanthine oxidase inhibitors, such as allopurinol, significantly reduces proteinuria in T2D patients and macroalbuminuria (98) and may also help maintain stable renal function and reduce cardiovascular risk in patients with T2D (99, 100). Studies examining the relationships between serum uric acid and DKD in adolescents with T2D are needed to determine if uric acid plays a role in the pathophysiology of pediatric T2D. To determine whether uric acid lowering translates into renal or cardiovascular protective effects, the Preventing Early Renal Function Loss in Diabetes (PERL) study is an on-going multi-center, double-blind, randomized clinical trial of allopurinol in individuals with T1D and either albuminuria or renal functional decline (101). If PERL produces promising results, similar studies should be considered in adolescents and young adults with T2D.
Vasopressin
Arginine vasopressin (AVP) plays an essential role in regulation of volume status and exerts important renal and cardiovascular effects in health and disease. It is recognized that AVP infusion induces hypertension, glomerular hyperfiltration, and albuminuria (102–104). Unfortunately, measuring AVP is technically difficult due to its relatively small size and short half-life. Copeptin is a more stable peptide derived from the same precursor molecule as AVP, is accepted as a surrogate marker for AVP, and is useful in the assessment of fluid and osmotic status in various diseases. AVP concentrations are higher in adults with T2D compared with healthy counterparts (105, 106). High concentrations of plasma AVP are known to preferentially stimulate vasopressin V1a receptors (107), which may contribute to the cardiovascular and renal complications associated with diabetes. For example, Fenske et al. recently reported that copeptin was strongly associated with cardiovascular events and mortality in adults with T2D (107). Similar findings were also demonstrated by Riphagen et al. who showed that copeptin correlated with cardiovascular and all-cause mortality in adults with T2D in the Zwolle Outpatient Diabetes project Integrating Available Care (ZODIAC-31) study (108). In adults with T2D, copeptin has also been associated with declining GFR in the type 2 DIABetes, Hypertension, CArdiovascular Events and Ramipril (DIABHYCAR) (109), and ZODIAC-33 studies (110). To our knowledge, the association between copeptin and renal health in youth with T2D has yet to be examined. The vasopressin system is not only a modifiable risk factor, but also a promising therapeutic target with the recent availability of vaptans (vasopressin receptor antagonists). Vaptans are generally well-tolerated, with most commonly reported adverse effects including dry mouth, thirst and increased daytime urination (111).
ACE2 and neprilysin
Another important system in DKD is the renin-angiotensin-aldosterone system (RAAS). However, RAAS inhibition does not halt or delay progression of DKD in T2D (112–114) as effectively as it does in T1D (113). While a primary prevention study failed to demonstrate benefit of RAAS inhibitors (2) and another showed harm with dual RAAS blockade (3), the identification of angiotensin-converting enzyme 2 (ACE2) has changed our understanding of RAAS and introduced potential new therapeutic targets (115). ACE2 is expressed in most tissues, but especially abundant in the kidney (116) and cleaves the C-terminal amino acid of Angiotensin II to generate the peptide Angiotensin 1-7, which is thought to provide renoprotection by counteracting the adverse effects of Angiotensin II (117). Angiotensin 1-7 is also thought to reduce oxidative stress, inflammation, and lipotoxicity (118). Diabetic animal models are associated with Angiotensin II over-activity (119, 120) and studies with downregulation of tubular ACE2 found significant albuminuria and tubular injury (121, 122). Furthermore, ACE2 activity at the podocytes can attenuate the development of DKD (123), suggesting a potential mechanism to counteract diabetes-associated Angiotensin II over-activity (119, 120). In fact, DKD is associated with reduced tubular ACE2 expression (124) and ACE2 activity is associated with glycemic control and glomerular filtration rate (GFR) in adults with DKD (125). For these reasons, studies have investigated ACE2 as a potential therapeutic target using recombinant ACE2 and Mas receptor modulators to diminish DKD progression, with promising preliminary results (126–130).
A system strongly related to RAAS is the natriuretic peptide (NP) system that counter-regulates the RAAS. Neprilysin is an enzyme responsible for degradation of NPs (131). Neprilysin inhibitors (NEPi) lead to natriuresis, vasodilatation, and reductions in both intraglomerular pressure and proteinuria (132, 133). The beneficial renal effects of NEPi may be enhanced when combined with RAAS blockade, which led to the development of combined NEPi/RAASi agents. While no large-scale human trials have been conducted with NEPi or NEPi/RAASi in a CKD cohort to date, animal models show promising results. For instance, in a 5/6 nephrectomy model (CKD animal model with unilateral nephrectomy and either partial infarction or amputation of the poles of the remaining kidney), AVE7688, a vasopeptidase blocking ACE and NEP, increased renal synthesis of nitric oxide, decreased synthesis of endothelin-1, and increased tubular ANP release, leading to with reduced renal vasoconstriction, proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis (134). LCZ696, a combined angiotensin-neprilysin inhibitor, was shown to be superior to enalapril in reducing the risks of death and hospitalization for heart failure in adults with and without diabetes in the PARADIGM-HF study (135, 136). Renal outcome studies using this emerging class are not yet available.
Sodium glucose co-transporter 2
Another important emerging therapeutic area relates to sodium glucose co-transporter 2 (SGLT2) inhibition. This class of agents has a strong mechanistic basis for renal protection in both T2D and T1D. In adults with T1D, SGLT2 inhibition with empagliflozin significantly attenuates renal hyperfiltration, likely by restoring the altered tubular-glomerular feedback mechanism leading to hyperfiltration (137). SGLT2 inhibition blocks proximal tubular glucose and sodium reabsorption, which leads to increased sodium delivery to the macula densa, thereby reducing GFR and renal blood flow (RBF) via afferent arteriolar vasoconstriction (137). Furthermore, in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG RENAL) trial, empagliflozin was well-tolerated, reduced HbA1c in adults with T2D and CKD, and exerted important blood pressure and anti-proteinuric effects in patients with and without DKD (138). Importantly, the EMPA-REG OUTCOME trial, with 7000 individuals from 42 countries observed for a median duration of 3.1 years, recently reported that empagliflozin is the first glycemic lowering therapy to reduce a composite cardiovascular endpoint (defined as time to first occurrence of either CV death, or non-fatal myocardial infarction or non-fatal stroke). To our knowledge, there are no studies demonstrating attenuation of hyperfiltration in T2D with SGLT2 inhibition, but it is likely that SGLT2 inhibitors will affect the tubular-glomerular feedback mechanisms similarly to what has been observed in T1D.
Conclusion
The increasing prevalence of T2D worldwide has led to a concomitant rise in DKD (4, 56). Left untreated, patients with DKD have a high risk of progressing to ESRD and dialysis – a significant public health burden (4). Particularly worrisome is the decreasing age of onset of T2D and the presence of DKD even at time of diagnosis. This review examines the current literature and data addressing novel biomarkers and potential therapeutic targets in early DKD in adolescents with T2D. Longitudinal human research is required to develop improved methods of measuring renal function in adolescents with T2D, and to investigate the effect of novel pharmacotherapy on long-term clinical outcomes.
Acknowledgments
David Z. Cherney was also supported by a Canadian Diabetes Association-KRESCENT Program Joint New Investigator Award.
Footnotes
Author Contributions:
PB, DZC, DMM, KJN wrote, contributed to discussion, and reviewed/edited the manuscript.
Compliance with Ethics Guidelines
Conflict of Interest
Petter Bjornstad and Kristen J. Nadeau declare that they have no conflict of interest. David Z. Cherney has received speaker honoraria from Janssen, AstraZeneca, Boehringer-Ingelheim, Lill,y and Merck and has received research grant support from AstraZeneca, Merck, Astellas, and Boehringer-Ingelheim. David M. Maahs is on the advisory board for Insulet and his institution has received research support from Dexcom and Medtronic.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus–progress made, more to be done. J Clin Endocrinol Metab. 2006;91(10):3757–9. doi: 10.1210/jc.2006-1730. Epub 2006/10/10. [DOI] [PubMed] [Google Scholar]
- 2.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–9. doi: 10.1007/s00125-010-1860-3. Epub 2010/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. ‘United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl 1):A7,e1–420. doi: 10.1053/j.ajkd.2011.11.015. Epub 2011/12/30. [DOI] [PubMed] [Google Scholar]
- 4.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57(1 Suppl 1):A8, e1–526. doi: 10.1053/j.ajkd.2010.10.007. Epub 2010/12/28. [DOI] [PubMed] [Google Scholar]
- 5.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. Jama. 2011;305(24):2532–9. doi: 10.1001/jama.2011.861. Epub 2011/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. Jama. 2010;304(6):649–56. doi: 10.1001/jama.2010.1111. Epub 2010/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Bjornstad P, Maahs DM, Cherney DZ, Cree-Green M, West A, Pyle L, et al. Insulin Sensitivity Is an Important Determinant of Renal Health in Adolescents With Type 2 Diabetes. Diabetes care. 2014 doi: 10.2337/dc14-1331. Epub 2014/07/30. First report of a relationship between insulin resistance and renal health in adolescents with type 2 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes care. 2013;36(6):1735–41. doi: 10.2337/dc12-2420. Epub 2013/05/25. Report of incident microalbuminuria in adolescents with type 2 diabetes in the TODAY clinical trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alleyn CR, Volkening LK, Wolfson J, Rodriguez-Ventura A, Wood JR, Laffel LM. Occurrence of microalbuminuria in young people with Type 1 diabetes: importance of age and diabetes duration. Diabetic medicine: a journal of the British Diabetic Association. 2010;27(5):532–7. doi: 10.1111/j.1464-5491.2010.02983.x. Epub 2010/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eppens MC, Craig ME, Cusumano J, Hing S, Chan AK, Howard NJ, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes care. 2006;29(6):1300–6. doi: 10.2337/dc05-2470. Epub 2006/05/30. [DOI] [PubMed] [Google Scholar]
- 11.Kiess W, Bottner A, Bluher S, Raile K, Galler A, Kapellen TM. Type 2 diabetes mellitus in children and adolescents–the beginning of a renal catastrophe? Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19(11):2693–6. doi: 10.1093/ndt/gfh455. Epub 2004/09/24. [DOI] [PubMed] [Google Scholar]
- 12.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22(1):99–111. doi: 10.2337/diacare.22.1.99. Epub 1999/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoyama H, Okudaira M, Otani T, Takaike H, Miura J, Saeki A, et al. Existence of early-onset NIDDM Japanese demonstrating severe diabetic complications. Diabetes Care. 1997;20(5):844–7. doi: 10.2337/diacare.20.5.844. Epub 1997/05/01. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama H, Okudaira M, Otani T, Watanabe C, Takaike H, Miuira J, et al. High incidence of diabetic nephropathy in early-onset Japanese NIDDM patients. Risk analysis. Diabetes Care. 1998;21(7):1080–5. doi: 10.2337/diacare.21.7.1080. Epub 1998/07/08. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez BL, Dabelea D, Liese AD, Fujimoto W, Waitzfelder B, Liu L, et al. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. The Journal of pediatrics. 2010;157(2):245–51 e1. doi: 10.1016/j.jpeds.2010.02.021. Epub 2010/04/17. [DOI] [PubMed] [Google Scholar]
- 16••.Maahs DM, Snively BM, Bell RA, Dolan L, Hirsch I, Imperatore G, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes care. 2007;30(10):2593–8. doi: 10.2337/dc07-0450. Epub 2007/07/17. Report demonstrating greater prevalence of elevated albuminuria in youth with type 2 diabetes compared to youth with type 1 diabetes. [DOI] [PubMed] [Google Scholar]
- 17.Mottl AK, Kwon KS, Mauer M, Mayer-Davis EJ, Hogan SL, Kshirsagar AV. Normoalbuminuric diabetic kidney disease in the U.S. population. Journal of diabetes and its complications. 2013;27(2):123–7. doi: 10.1016/j.jdiacomp.2012.09.010. Epub 2012/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis JB. Renal Dysfunction in the Presence of Normoalbuminuria in Type 2 Diabetes: Results from the DEMAND Study. Cardiorenal medicine. 2012;2(1):1–10. doi: 10.1159/000333249. Epub 2012/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27(1):195–200. doi: 10.2337/diacare.27.1.195. Epub 2003/12/25. Report demonstrating nonalbuminuric renal insufficiency in adults with type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- 20.So WY, Kong AP, Ma RC, Ozaki R, Szeto CC, Chan NN, et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care. 2006;29(9):2046–52. doi: 10.2337/dc06-0248. Epub 2006/08/29. [DOI] [PubMed] [Google Scholar]
- 21.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA: the journal of the American Medical Association. 2003;289(24):3273–7. doi: 10.1001/jama.289.24.3273. Epub 2003/06/26. [DOI] [PubMed] [Google Scholar]
- 22.Svensson M, Sundkvist G, Arnqvist HJ, Bjork E, Blohme G, Bolinder J, et al. Signs of nephropathy may occur early in young adults with diabetes despite modern diabetes management: results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS) Diabetes Care. 2003;26(10):2903–9. doi: 10.2337/diacare.26.10.2903. Epub 2003/09/30. [DOI] [PubMed] [Google Scholar]
- 23.Adelman RD, Restaino IG, Alon US, Blowey DL. Proteinuria and focal segmental glomerulosclerosis in severely obese adolescents. J Pediatr. 2001;138(4):481–5. doi: 10.1067/mpd.2001.113006. Epub 2001/04/11. [DOI] [PubMed] [Google Scholar]
- 24••.Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. The New England journal of medicine. 2012;366(24):2247–56. doi: 10.1056/NEJMoa1109333. Epub 2012/05/01. Key article describing the TODAY study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116(2):288–96. doi: 10.1172/JCI27699. Epub 2006/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tantravahi J, Srinivas TR, Johnson RJ. Hyperfiltration: a sign of poor things to come in individuals with metabolic syndrome. Nature clinical practice Nephrology. 2007;3(9):474–5. doi: 10.1038/ncpneph0565. Epub 2007/07/20. [DOI] [PubMed] [Google Scholar]
- 27.Tomaszewski M, Charchar FJ, Maric C, McClure J, Crawford L, Grzeszczak W, et al. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney international. 2007;71(8):816–21. doi: 10.1038/sj.ki.5002160. Epub 2007/03/03. [DOI] [PubMed] [Google Scholar]
- 28.Ritz E. Metabolic syndrome: an emerging threat to renal function. Clinical journal of the American Society of Nephrology: CJASN. 2007;2(5):869–71. doi: 10.2215/CJN.02350607. Epub 2007/08/19. [DOI] [PubMed] [Google Scholar]
- 29.Ritz E. Metabolic syndrome and kidney disease. Blood purification. 2008;26(1):59–62. doi: 10.1159/000110566. Epub 2008/01/10. [DOI] [PubMed] [Google Scholar]
- 30•.Melsom T, Mathisen UD, Ingebretsen OC, Jenssen TG, Njolstad I, Solbu MD, et al. Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes care. 2011;34(7):1546–51. doi: 10.2337/dc11-0235. Epub 2011/05/20. Report demonstrating a relationship between impaired fasting glucose and renal hyperfiltration in general population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauer M, Drummond K. The early natural history of nephropathy in type 1 diabetes: I. Study design and baseline characteristics of the study participants. Diabetes. 2002;51(5):1572–9. doi: 10.2337/diabetes.51.5.1572. Epub 2002/04/30. [DOI] [PubMed] [Google Scholar]
- 32.Osterby R, Gall MA, Schmitz A, Nielsen FS, Nyberg G, Parving HH. Glomerular structure and function in proteinuric type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36(10):1064–70. doi: 10.1007/BF02374500. Epub 1993/10/01. [DOI] [PubMed] [Google Scholar]
- 33.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH. Diabetic nephropathy. Diabetes care. 2003;26(Suppl 1):S94–8. doi: 10.2337/diacare.26.2007.s94. Epub 2002/12/28. [DOI] [PubMed] [Google Scholar]
- 34.Mogensen CE, Schmitz O. The diabetic kidney: from hyperfiltration and microalbuminuria to end-stage renal failure. Med Clin North Am. 1988;72(6):1465–92. doi: 10.1016/s0025-7125(16)30717-9. Epub 1988/11/01. [DOI] [PubMed] [Google Scholar]
- 35.Bartz SK, Caldas MC, Tomsa A, Krishnamurthy R, Bacha F. Urine Albumin to Creatinine Ratio: A Marker of Early Endothelial Dysfunction in Youth. The Journal of clinical endocrinology and metabolism. 2015:JC20152230. doi: 10.1210/JC.2015-2230. Epub 2015/07/16. [DOI] [PubMed] [Google Scholar]
- 36.Yamada T, Komatsu M, Komiya I, Miyahara Y, Shima Y, Matsuzaki M, et al. Development, progression, and regression of microalbuminuria in Japanese patients with type 2 diabetes under tight glycemic and blood pressure control: the Kashiwa study. Diabetes care. 2005;28(11):2733–8. doi: 10.2337/diacare.28.11.2733. Epub 2005/10/27. [DOI] [PubMed] [Google Scholar]
- 37.Bjornstad P, Maahs DM, Johnson RJ, Rewers M, Snell-Bergeon JK. Estimated insulin sensitivity predicts regression of albuminuria in Type 1 diabetes. Diabet Med. 2014 doi: 10.1111/dme.12572. Epub 2005/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac RJ. The clinical significance of hyperfiltration in diabetes. Diabetologia. 2010;53(10):2093–104. doi: 10.1007/s00125-010-1794-9. Epub 2010/05/25. [DOI] [PubMed] [Google Scholar]
- 39.Levine DZ, Iacovitti M, Robertson SJ, Mokhtar GA. Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R975–81. doi: 10.1152/ajpregu.00693.2005. Epub 2005/12/13. [DOI] [PubMed] [Google Scholar]
- 40.Ekinci EI, Hughes JT, Chatfield MD, Lawton PD, Jones GR, Ellis AG, et al. Hyperfiltration in Indigenous Australians with and without diabetes. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2015 doi: 10.1093/ndt/gfv230. Epub 2015/07/05. [DOI] [PubMed] [Google Scholar]
- 41•.Premaratne E, Verma S, Ekinci EI, Theverkalam G, Jerums G, MacIsaac RJ. The impact of hyperfiltration on the diabetic kidney. Diabetes & metabolism. 2015;41(1):5–17. doi: 10.1016/j.diabet.2014.10.003. Epub 2014/12/03. Comprehensive review of renal hyperfiltration in diabetic kidney disease. [DOI] [PubMed] [Google Scholar]
- 42.Bjornstad P, Cherney DZ, Snell-Bergeon JK, Pyle L, Rewers M, Johnson RJ, et al. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv121. Epub 2015/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Standards of medical care in diabetes–2013. Diabetes care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. Epub 2013/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005. Epub 2007/02/06. [DOI] [PubMed] [Google Scholar]
- 45.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30. doi: 10.7326/0003-4819-158-11-201306040-00007. Epub 2013/06/05. [DOI] [PubMed] [Google Scholar]
- 46.Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes. 2012;3(1):1–6. doi: 10.4239/wjd.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52(4):691–7. doi: 10.1007/s00125-009-1268-0. Epub 2009/02/10. [DOI] [PubMed] [Google Scholar]
- 48.Cherney DZ, Scholey JW, Miller JA. Insights into the regulation of renal hemodynamic function in diabetic mellitus. Current diabetes reviews. 2008;4(4):280–90. doi: 10.2174/157339908786241151. Epub 2008/11/11. [DOI] [PubMed] [Google Scholar]
- 49.Pruijm M, Wuerzner G, Maillard M, Bovet P, Renaud C, Bochud M, et al. Glomerular hyperfiltration and increased proximal sodium reabsorption in subjects with type 2 diabetes or impaired fasting glucose in a population of the African region. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(7):2225–31. doi: 10.1093/ndt/gfq008. Epub 2010/02/04. [DOI] [PubMed] [Google Scholar]
- 50.Wuerzner G, Pruijm M, Maillard M, Bovet P, Renaud C, Burnier M, et al. Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2010;56(2):303–12. doi: 10.1053/j.ajkd.2010.03.017. Epub 2010/06/12. [DOI] [PubMed] [Google Scholar]
- 51.Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. The New England journal of medicine. 1999;341(15):1127–33. doi: 10.1056/NEJM199910073411506. Epub 1999/10/08. [DOI] [PubMed] [Google Scholar]
- 52.Maahs DM, Ogden LG, Kretowski A, Snell-Bergeon JK, Kinney GL, Berl T, et al. Serum cystatin C predicts progression of subclinical coronary atherosclerosis in individuals with type 1 diabetes. Diabetes. 2007;56(11):2774–9. doi: 10.2337/db07-0539. Epub 2007/07/31. [DOI] [PubMed] [Google Scholar]
- 53.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. The New England journal of medicine. 2013;369(10):932–43. doi: 10.1056/NEJMoa1214234. Epub 2013/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bjornstad P, Maahs DM, Rivard CJ, Pyle L, Rewers M, Johnson RJ, et al. Serum uric acid predicts vascular complications in adults with type 1 diabetes: the coronary artery calcification in type 1 diabetes study. Acta Diabetol. 2014 doi: 10.1007/s00592-014-0611-1. Epub 2014/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krolewski AS, Niewczas MA, Skupien J, Gohda T, Smiles A, Eckfeldt JH, et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes care. 2014;37(1):226–34. doi: 10.2337/dc13-0985. Epub 2013/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):279–86. doi: 10.1097/MED.0000000000000074. Epub 2014/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bjornstad P, Cherney DZI, Snell-Bergeon J, Pyle L, Rewers M, Johnson RJ, et al. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with type 1 diabetes. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavkov ME, Knowler WC, Lemley KV, Mason CC, Myers BD, Nelson RG. Early renal function decline in type 2 diabetes. Clin J Am Soc Nephrol. 2012;7(1):78–84. doi: 10.2215/CJN.07610711. Epub 2011/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–53. doi: 10.1038/ki.2012.169. Epub 2012/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL. Pediatric GFR estimating equations applied to adolescents in the general population. Clinical journal of the American Society of Nephrology: CJASN. 2011;6(6):1427–35. doi: 10.2215/CJN.06460710. Epub 2011/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bacchetta J, Cochat P, Rognant N, Ranchin B, Hadj-Aissa A, Dubourg L. Which creatinine and cystatin C equations can be reliably used in children? Clinical journal of the American Society of Nephrology: CJASN. 2011;6(3):552–60. doi: 10.2215/CJN.04180510. Epub 2010/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berg UB, Nyman U, Back R, Hansson M, Monemi KA, Herthelius M, et al. New standardized cystatin C and creatinine GFR equations in children validated with inulin clearance. Pediatr Nephrol. 2015 doi: 10.1007/s00467-015-3060-3. Epub 2015/04/24. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. Journal of the American Society of Nephrology: JASN. 2009;20(3):629–37. doi: 10.1681/ASN.2008030287. Epub 2009/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fadrowski JJ, Furth SL. GFR estimation in children: questions and answers (and questions) Clinical journal of the American Society of Nephrology: CJASN. 2011;6(8):1810–2. doi: 10.2215/CJN.05900611. Epub 2011/07/26. [DOI] [PubMed] [Google Scholar]
- 65.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Newman AB, Siscovick DS, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol. 2009;30(3):171–8. doi: 10.1159/000212381. Epub 2009/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cherney DZ, Sochett EB, Dekker MG, Perkins BA. Ability of cystatin C to detect acute changes in glomerular filtration rate provoked by hyperglycaemia in uncomplicated Type 1 diabetes. Diabet Med. 2010;27(12):1358–65. doi: 10.1111/j.1464-5491.2010.03121.x. Epub 2010/11/10. [DOI] [PubMed] [Google Scholar]
- 67.Maahs DM, Jalal D, McFann K, Rewers M, Snell-Bergeon JK. Systematic shifts in cystatin C between 2006 and 2010. Clin J Am Soc Nephrol. 2011;6(8):1952–5. doi: 10.2215/CJN.11271210. Epub 2011/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maahs DM, Jalal D, Chonchol M, Johnson RJ, Rewers M, Snell-Bergeon JK. Impaired Renal Function Further Increases Odds of 6-Year Coronary Artery Calcification Progression in Adults With Type 1 Diabetes: The CACTI study. Diabetes care. 2013;36(9):2607–14. doi: 10.2337/dc12-2538. Epub 2013/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maahs DM, Prentice N, McFann K, Snell-Bergeon JK, Jalal D, Bishop FK, et al. Age and sex influence cystatin C in adolescents with and without type 1 diabetes. Diabetes care. 2011;34(11):2360–2. doi: 10.2337/dc11-0829. Epub 2011/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Zinman B, et al. Longitudinal changes in estimated and measured GFR in type 1 diabetes. Journal of the American Society of Nephrology: JASN. 2014;25(4):810–8. doi: 10.1681/ASN.2013050557. Epub 2013/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Boer IH. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes care. 2014;37(1):24–30. doi: 10.2337/dc13-2113. Epub 2013/12/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perrin NE, Berg UB. Estimated glomerular filtration rates cannot replace measured GFR in type 1 diabetes patients with hyperfiltration. Acta Paediatr. 2015 doi: 10.1111/apa.12993. Epub 2015/03/06. [DOI] [PubMed] [Google Scholar]
- 73.Macisaac R, Ekinci E, Premaratne E, Lu ZX, Seah J, Li Y, et al. The Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation does not improve the underestimation of Glomerular Filtration Rate (GFR) in people with diabetes and preserved renal function. BMC nephrology. 2015 doi: 10.1186/s12882-015-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maahs DM, Bushman L, Kerr B, Ellis SL, Pyle L, McFann K, et al. A practical method to measure GFR in people with type 1 diabetes. J Diabetes Complications. 2014;28(5):667–73. doi: 10.1016/j.jdiacomp.2014.06.001. Epub 2014/07/17. [DOI] [PubMed] [Google Scholar]
- 75•.Bjornstad P, Anderson PL, Maahs DM. Measuring glomerular filtration rate by iohexol clearance on filter paper is feasible in adolescents with type 1 diabetes in the ambulatory setting. Acta Diabetol. 2015 doi: 10.1007/s00592-015-0764-6. Epub 2015/05/12. Novel methodology measuring GFR in the ambulatory setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. Journal of the American Society of Nephrology: JASN. 2012;23(3):507–15. doi: 10.1681/ASN.2011060627. Epub 2012/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Looker HC, Colombo M, Hess S, Brosnan MJ, Farran B, Dalton RN, et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney international. 2015 doi: 10.1038/ki.2015.199. Epub 2015/07/23. [DOI] [PubMed] [Google Scholar]
- 78.Zurbig P, Jerums G, Hovind P, Macisaac R, Mischak H, Nielsen SE, et al. Urinary Proteomics for Early Diagnosis in Diabetic Nephropathy. Diabetes. 2012 doi: 10.2337/db12-0348. Epub 2012/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maahs DM, Siwy J, Argiles A, Cerna M, Delles C, Dominiczak AF, et al. Urinary collagen fragments are significantly altered in diabetes: a link to pathophysiology. PloS one. 2010;5(9) doi: 10.1371/journal.pone.0013051. Epub 2010/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schanstra JP, Zurbig P, Alkhalaf A, Argiles A, Bakker SJ, Beige J, et al. Diagnosis and Prediction of CKD Progression by Assessment of Urinary Peptides. Journal of the American Society of Nephrology: JASN. 2015;26(8):1999–2010. doi: 10.1681/ASN.2014050423. Epub 2015/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siwy J, Schanstra JP, Argiles A, Bakker SJ, Beige J, Boucek P, et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(8):1563–70. doi: 10.1093/ndt/gfu039. Epub 2014/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wallace JL, Ianaro A, Flannigan KL, Cirino G. Gaseous mediators in resolution of inflammation. Seminars in immunology. 2015;27(3):227–33. doi: 10.1016/j.smim.2015.05.004. Epub 2015/06/23. [DOI] [PubMed] [Google Scholar]
- 83.Snijder PM, van den Berg E, Whiteman M, Bakker SJ, Leuvenink HG, van Goor H. Emerging role of gasotransmitters in renal transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(12):3067–75. doi: 10.1111/ajt.12483. Epub 2013/11/26. [DOI] [PubMed] [Google Scholar]
- 84.Kamijo H, Higuchi M, Hora K. Chronic inhibition of nitric oxide production aggravates diabetic nephropathy in Otsuka Long-Evans Tokushima Fatty rats. Nephron Physiol. 2006;104(1):12–22. doi: 10.1159/000093276. Epub 2006/05/13. [DOI] [PubMed] [Google Scholar]
- 85.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. Journal of the American Society of Nephrology: JASN. 2006;17(10):2664–9. doi: 10.1681/ASN.2006070798. Epub 2006/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. Journal of the American Society of Nephrology: JASN. 2007;18(2):539–50. doi: 10.1681/ASN.2006050459. Epub 2007/01/05. [DOI] [PubMed] [Google Scholar]
- 87.Okumura M, Masada M, Yoshida Y, Shintaku H, Hosoi M, Okada N, et al. Decrease in tetrahydrobiopterin as a possible cause of nephropathy in type II diabetic rats. Kidney Int. 2006;70(3):471–6. doi: 10.1038/sj.ki.5000431. Epub 2006/06/16. [DOI] [PubMed] [Google Scholar]
- 88.Parvanova AI, Trevisan R, Iliev IP, Dimitrov BD, Vedovato M, Tiengo A, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55(5):1456–62. doi: 10.2337/db05-1484. Epub 2006/04/29. [DOI] [PubMed] [Google Scholar]
- 89.De Cosmo S, Minenna A, Ludovico O, Mastroianno S, Di Giorgio A, Pirro L, et al. Increased urinary albumin excretion, insulin resistance, and related cardiovascular risk factors in patients with type 2 diabetes: evidence of a sex-specific association. Diabetes care. 2005;28(4):910–5. doi: 10.2337/diacare.28.4.910. Epub 2005/03/29. [DOI] [PubMed] [Google Scholar]
- 90.Hsu CC, Chang HY, Huang MC, Hwang SJ, Yang YC, Tai TY, et al. Association between insulin resistance and development of microalbuminuria in type 2 diabetes: a prospective cohort study. Diabetes care. 2011;34(4):982–7. doi: 10.2337/dc10-1718. Epub 2011/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.August P, Hardison RM, Hage FG, Marroquin OC, McGill JB, Rosenberg Y, et al. Change in albuminuria and eGFR following insulin sensitization therapy versus insulin provision therapy in the BARI 2D study. Clin J Am Soc Nephrol. 2014;9(1):64–71. doi: 10.2215/CJN.12281211. Epub 2013/11/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hung SC, Chang YK, Liu JS, Kuo KL, Chen YH, Hsu CC, et al. Metformin use and mortality in patients with advanced chronic kidney disease: national, retrospective, observational, cohort study. The lancet Diabetes & endocrinology. 2015;3(8):605–14. doi: 10.1016/S2213-8587(15)00123-0. Epub 2015/06/22. [DOI] [PubMed] [Google Scholar]
- 93.Zoppini G, Targher G, Chonchol M, Ortalda V, Abaterusso C, Pichiri I, et al. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes care. 2012;35(1):99–104. doi: 10.2337/dc11-1346. Epub 2011/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Altemtam N, Russell J, El Nahas M. A study of the natural history of diabetic kidney disease (DKD) Nephrol Dial Transplant. 2012;27(5):1847–54. doi: 10.1093/ndt/gfr561. Epub 2011/11/08. [DOI] [PubMed] [Google Scholar]
- 95.Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes care. 2008;31(2):361–2. doi: 10.2337/dc07-1276. Epub 2007/11/06. [DOI] [PubMed] [Google Scholar]
- 96.Bjornstad P, Lanaspa MA, Ishimoto T, Kosugi T, Kume S, Jalal D, et al. Fructose and uric acid in diabetic nephropathy. Diabetologia. 2015 doi: 10.1007/s00125-015-3650-4. Epub 2015/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miao Y, Ottenbros SA, Laverman GD, Brenner BM, Cooper ME, Parving HH, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58(1):2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. Epub 2011/06/03. [DOI] [PubMed] [Google Scholar]
- 98.Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4(2):128–32. Epub 2010/04/21. [PubMed] [Google Scholar]
- 99.Liu P, Chen Y, Wang B, Zhang F, Wang D, Wang Y. Allopurinol treatment improves renal function in patients with type 2 diabetes and asymptomatic hyperuricemia: 3-year randomized parallel-controlled study. Clin Endocrinol (Oxf) 2014 doi: 10.1111/cen.12673. Epub 2014/11/18. [DOI] [PubMed] [Google Scholar]
- 100.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clinical journal of the American Society of Nephrology: CJASN. 2010;5(8):1388–93. doi: 10.2215/CJN.01580210. Epub 2010/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maahs DM, Caramori L, Cherney DZ, Galecki AT, Gao C, Jalal D, et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13(4):550–9. doi: 10.1007/s11892-013-0381-0. Epub 2013/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bankir L, Kriz W. Adaptation of the kidney to protein intake and to urine concentrating activity: similar consequences in health and CRF. Kidney Int. 1995;47(1):7–24. doi: 10.1038/ki.1995.2. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- 103.Gellai M, Silverstein JH, Hwang JC, LaRochelle FT, Jr, Valtin H. Influence of vasopressin on renal hemodynamics in conscious Brattleboro rats. Am J Physiol. 1984;246(6 Pt 2):F819–27. doi: 10.1152/ajprenal.1984.246.6.F819. Epub 1984/06/11. [DOI] [PubMed] [Google Scholar]
- 104.Bardoux P, Martin H, Ahloulay M, Schmitt F, Bouby N, Trinh-Trang-Tan MM, et al. Vasopressin contributes to hyperfiltration, albuminuria, and renal hypertrophy in diabetes mellitus: study in vasopressin-deficient Brattleboro rats. Proc Natl Acad Sci U S A. 1999;96(18):10397–402. doi: 10.1073/pnas.96.18.10397. Epub 1999/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zerbe RL, Vinicor F, Robertson GL. Plasma vasopressin in uncontrolled diabetes mellitus. Diabetes. 1979;28(5):503–8. doi: 10.2337/diab.28.5.503. Epub 1979/05/01. [DOI] [PubMed] [Google Scholar]
- 106.Tallroth G, Ryding E, Ekman R, Agardh CD. The response of regulatory peptides to moderate hypoglycaemia of short duration in type 1 (insulin-dependent) diabetes mellitus and in normal man. Diabetes Res. 1992;20(3):73–85. Epub 1992/01/01. [PubMed] [Google Scholar]
- 107.Fenske W, Wanner C, Allolio B, Drechsler C, Blouin K, Lilienthal J, et al. Copeptin levels associate with cardiovascular events in patients with ESRD and type 2 diabetes mellitus. Journal of the American Society of Nephrology: JASN. 2011;22(4):782–90. doi: 10.1681/ASN.2010070691. Epub 2011/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Riphagen IJ, Boertien WE, Alkhalaf A, Kleefstra N, Gansevoort RT, Groenier KH, et al. Copeptin, a Surrogate Marker for Arginine Vasopressin, Is Associated With Cardiovascular and All-Cause Mortality in Patients With Type 2 Diabetes (ZODIAC-31) Diabetes care. 2013 doi: 10.2337/dc12-2165. Epub 2013/06/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Velho G, Bouby N, Hadjadj S, Matallah N, Mohammedi K, Fumeron F, et al. Plasma copeptin and renal outcomes in patients with type 2 diabetes and albuminuria. Diabetes care. 2013;36(11):3639–45. doi: 10.2337/dc13-0683. Epub 2013/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110•.Boertien WE, Riphagen IJ, Drion I, Alkhalaf A, Bakker SJ, Groenier KH, et al. Copeptin, a surrogate marker for arginine vasopressin, is associated with declining glomerular filtration in patients with diabetes mellitus (ZODIAC-33) Diabetologia. 2013;56(8):1680–8. doi: 10.1007/s00125-013-2922-0. Epub 2013/04/30. Report demonstrating copeptin as a risk factor for declining glomerular filtration rate in adults with type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- 111.Berl T, Quittnat-Pelletier F, Verbalis JG, Schrier RW, Bichet DG, Ouyang J, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. Journal of the American Society of Nephrology: JASN. 2010;21(4):705–12. doi: 10.1681/ASN.2009080857. Epub 2010/02/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. The New England journal of medicine. 2001;345(12):861–9. doi: 10.1056/NEJMoa011161. Epub 2001/09/22. [DOI] [PubMed] [Google Scholar]
- 113.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. The New England journal of medicine. 1993;329(20):1456–62. doi: 10.1056/NEJM199311113292004. Epub 1993/11/11. [DOI] [PubMed] [Google Scholar]
- 114.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. The New England journal of medicine. 2001;345(12):851–60. doi: 10.1056/NEJMoa011303. Epub 2001/09/22. [DOI] [PubMed] [Google Scholar]
- 115.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circulation research. 2000;87(5):E1–9. doi: 10.1161/01.res.87.5.e1. Epub 2000/09/02. [DOI] [PubMed] [Google Scholar]
- 116.Bernardi S, Burns WC, Toffoli B, Pickering R, Sakoda M, Tsorotes D, et al. Angiotensin-converting enzyme 2 regulates renal atrial natriuretic peptide through angiotensin-(1-7) Clin Sci (Lond) 2012;123(1):29–37. doi: 10.1042/CS20110403. [DOI] [PubMed] [Google Scholar]
- 117.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8258–63. doi: 10.1073/pnas.1432869100. Epub 2003/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mori J, Patel VB, Ramprasath T, Alrob OA, DesAulniers J, Scholey JW, et al. Angiotensin 1-7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. American journal of physiology Renal physiology. 2014;306(8):F812–21. doi: 10.1152/ajprenal.00655.2013. Epub 2014/02/21. [DOI] [PubMed] [Google Scholar]
- 119.Marquez E, Riera M, Pascual J, Soler MJ. Renin-angiotensin system within the diabetic podocyte. Am J Physiol Renal Physiol. 2015;308(1):F1–10. doi: 10.1152/ajprenal.00531.2013. [DOI] [PubMed] [Google Scholar]
- 120.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, et al. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55(7):2132–9. doi: 10.2337/db06-0033. Epub 2006/06/29. [DOI] [PubMed] [Google Scholar]
- 121.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, et al. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. The American journal of pathology. 2007;171(2):438–51. doi: 10.2353/ajpath.2007.060977. Epub 2007/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72(5):614–23. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- 123.Nadarajah R, Milagres R, Dilauro M, Gutsol A, Xiao F, Zimpelmann J, et al. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int. 2012;82(3):292–303. doi: 10.1038/ki.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney international. 2008;74(12):1610–6. doi: 10.1038/ki.2008.497. Epub 2008/11/27. [DOI] [PubMed] [Google Scholar]
- 125.Anguiano L, Riera M, Pascual J, Valdivielso JM, Barrios C, Betriu A, et al. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30(7):1176–85. doi: 10.1093/ndt/gfv025. Epub 2015/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu CX, Hu Q, Wang Y, Zhang W, Ma ZY, Feng JB, et al. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol Med. 2011;17(1–2):59–69. doi: 10.2119/molmed.2010.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, et al. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59(2):529–38. doi: 10.2337/db09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu P, Costa-Goncalves AC, Todiras M, Rabelo LA, Sampaio WO, Moura MM, et al. Endothelial dysfunction and elevated blood pressure in MAS gene-deleted mice. Hypertension. 2008;51(2):574–80. doi: 10.1161/HYPERTENSIONAHA.107.102764. Epub 2008/01/09. [DOI] [PubMed] [Google Scholar]
- 129.Simoes e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. British journal of pharmacology. 2013;169(3):477–92. doi: 10.1111/bph.12159. Epub 2013/03/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Silveira KD, Barroso LC, Vieira AT, Cisalpino D, Lima CX, Bader M, et al. Beneficial effects of the activation of the angiotensin-(1-7) MAS receptor in a murine model of adriamycin-induced nephropathy. PloS one. 2013;8(6):e66082. doi: 10.1371/journal.pone.0066082. Epub 2013/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilkins MR, Redondo J, Brown LA. The natriuretic-peptide family. Lancet. 1997;349(9061):1307–10. doi: 10.1016/S0140-6736(96)07424-7. Epub 1997/05/03. [DOI] [PubMed] [Google Scholar]
- 132.Cao Z, Burrell LM, Tikkanen I, Bonnet F, Cooper ME, Gilbert RE. Vasopeptidase inhibition attenuates the progression of renal injury in subtotal nephrectomized rats. Kidney international. 2001;60(2):715–21. doi: 10.1046/j.1523-1755.2001.060002715.x. Epub 2001/07/28. [DOI] [PubMed] [Google Scholar]
- 133.Taal MW, Nenov VD, Wong W, Satyal SR, Sakharova O, Choi JH, et al. Vasopeptidase inhibition affords greater renoprotection than angiotensin-converting enzyme inhibition alone. Journal of the American Society of Nephrology: JASN. 2001;12(10):2051–9. doi: 10.1681/ASN.V12102051. Epub 2001/09/20. [DOI] [PubMed] [Google Scholar]
- 134.Benigni A, Zoja C, Zatelli C, Corna D, Longaretti L, Rottoli D, et al. Vasopeptidase inhibitor restores the balance of vasoactive hormones in progressive nephropathy. Kidney international. 2004;66(5):1959–65. doi: 10.1111/j.1523-1755.2004.00982.x. Epub 2004/10/22. [DOI] [PubMed] [Google Scholar]
- 135.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. The New England journal of medicine. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. Epub 2014/09/02. [DOI] [PubMed] [Google Scholar]
- 136.Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54–61. doi: 10.1161/CIRCULATIONAHA.114.013748. Epub 2014/11/19. [DOI] [PubMed] [Google Scholar]
- 137.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. The Renal Hemodynamic Effect of SGLT2 Inhibition in Patients with Type 1 Diabetes. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.005081. Epub 2013/12/18. [DOI] [PubMed] [Google Scholar]
- 138.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. The lancet Diabetes & endocrinology. 2014;2(5):369–84. doi: 10.1016/S2213-8587(13)70208-0. Epub 2014/05/06. [DOI] [PubMed] [Google Scholar]
- 139.Pavkov ME, Nelson RG, Knowler WC, Cheng Y, Krolewski AS, Niewczas MA. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney international. 2015;87(4):812–9. doi: 10.1038/ki.2014.330. Epub 2014/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. Journal of the American Society of Nephrology: JASN. 2014;25(10):2177–86. doi: 10.1681/ASN.2013070758. Epub 2014/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stein S, Bachmann A, Lossner U, Kratzsch J, Bluher M, Stumvoll M, et al. Serum levels of the adipokine FGF21 depend on renal function. Diabetes Care. 2009;32(1):126–8. doi: 10.2337/dc08-1054. Epub 2008/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Garg V, Kumar M, Mahapatra HS, Chitkara A, Gadpayle AK, Sekhar V. Novel urinary biomarkers in pre-diabetic nephropathy. Clinical and experimental nephrology. 2015 doi: 10.1007/s10157-015-1085-3. Epub 2015/01/31. [DOI] [PubMed] [Google Scholar]
- 143.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Fazio MR, Nicocia G, et al. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney & blood pressure research. 2009;32(2):91–8. doi: 10.1159/000209379. Epub 2009/03/27. [DOI] [PubMed] [Google Scholar]
- 144.McKittrick IB, Bogaert Y, Nadeau K, Snell-Bergeon J, Hull A, Jiang T, et al. Urinary matrix metalloproteinase activities: biomarkers for plaque angiogenesis and nephropathy in diabetes. Am J Physiol Renal Physiol. 2011;301(6):F1326–33. doi: 10.1152/ajprenal.00267.2011. Epub 2011/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Uslu S, Efe B, Alatas O, Kebapci N, Colak O, Demirustu C, et al. Serum cystatin C and urinary enzymes as screening markers of renal dysfunction in diabetic patients. Journal of nephrology. 2005;18(5):559–67. Epub 2005/11/22. [PubMed] [Google Scholar]
- 146.Sheira G, Noreldin N, Tamer A, Saad M. Urinary biomarker N-acetyl-beta-D-glucosaminidase can predict severity of renal damage in diabetic nephropathy. Journal of diabetes and metabolic disorders. 2015;14:4. doi: 10.1186/s40200-015-0133-6. Epub 2015/02/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Weil EJ, Lemley KV, Mason CC, Yee B, Jones LI, Blouch K, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney international. 2012;82(9):1010–7. doi: 10.1038/ki.2012.234. Epub 2012/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.do Nascimento JF, Canani LH, Gerchman F, Rodrigues PG, Joelsons G, dos Santos M, et al. Messenger RNA levels of podocyte-associated proteins in subjects with different degrees of glucose tolerance with or without nephropathy. BMC nephrology. 2013;14:214. doi: 10.1186/1471-2369-14-214. Epub 2013/10/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hong CY, Hughes K, Chia KS, Ng V, Ling SL. Urinary alpha1-microglobulin as a marker of nephropathy in type 2 diabetic Asian subjects in Singapore. Diabetes Care. 2003;26(2):338–42. doi: 10.2337/diacare.26.2.338. Epub 2003/01/28. [DOI] [PubMed] [Google Scholar]
- 150.Petrica L, Vlad A, Gluhovschi G, Gadalean F, Dumitrascu V, Gluhovschi C, et al. Proximal tubule dysfunction is associated with podocyte damage biomarkers nephrin and vascular endothelial growth factor in type 2 diabetes mellitus patients: a cross-sectional study. PloS one. 2014;9(11):e112538. doi: 10.1371/journal.pone.0112538. Epub 2014/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]


