Abstract
Objectives
The previously observed inverse association between hog farming and risk of lung cancer in the Agricultural Health Study (AHS) has been attributed to endotoxin exposure, levels of which are particularly high in industrial hog confinement facilities. We conducted an investigation to explore the potential biological mechanisms underlying this association as well as other immunologic changes associated with hog farming.
Methods
Serum immune marker levels were measured using a multiplexed bead-based assay in 61 active hog farmers and 61 controls matched on age, phlebotomy date, and raising cattle. Both groups were comprised of non-smoking male AHS participants from Iowa. We compared natural log-transformed marker levels between hog farmers and controls using multivariate linear regression models.
Results
Circulating levels of macrophage-derived chemokine (MDC/CCL22), a chemokine previously implicated in lung carcinogenesis, were reduced among hog farmers (17% decrease; 95% confidence interval [CI]: −28%, −4%), in particular for those with the largest operations (>6,000 hogs: 26% decrease; 95% CI −39%, −10%; Ptrend=0.002). We also found that hog farmers had elevated levels of other immune markers including macrophage inflammatory protein-3 alpha (MIP-3A/CCL20; 111% increase, 95% CI 19%, 273%), basic fibroblast growth factor (FGF-2; 93% increase, 95% CI 10%, 240%), and soluble interleukin-4 receptor (sIL-4R; 12% increase, 95% CI 1%, 25%), with particularly strong associations for MIP-3A/CCL20 and FGF-2 in winter.
Conclusions
These results provide insights into potential immunomodulatory mechanisms through which endotoxin or other exposures associated with hog farming may influence lung cancer risk, and warrant further investigation with more detailed bioaerosol exposure assessment.
Keywords: hog farming, endotoxin, chemokines, immune markers
Introduction
Farmers raising hogs in confined facilities are often highly exposed to endotoxin, a component of the cell wall of Gram negative bacteria that provokes an acute inflammatory response (1–3). A reduced risk of lung cancer has been observed among individuals employed in various occupations with high levels of exposure to endotoxin (4–6), including agriculture (5, 6). This deficit has been attributed, in part, to potential anti-carcinogenic immune-related effects of endotoxin exposure (4). However, one study found an increased risk of lung cancer in relation to estimated exposure to endotoxin, although this association disappeared after adjusting for exposure to organic dust (7). In a prior investigation in the Agricultural Health Study (AHS), a prospective cohort that includes farmers in Iowa and North Carolina (8), the risk of lung cancer decreased with increasing numbers of livestock (9). This association was most apparent among those with at least 1,000 animals, and nearly all of the farmers in this category (93%) reported raising hogs. Although the specific biological mechanisms underlying this inverse association with lung cancer risk are not yet fully understood, it is suspected that immune alterations related to endotoxin exposure may play a role.
There is considerable experimental and epidemiologic evidence suggesting that immune and inflammatory responses influence lung carcinogenesis (10, 11). Prospective studies in the general U.S. population have shown an increased risk of lung cancer in relation to high pre-diagnosis circulating levels of several chemokines (B-cell attracting chemokine-1, BCA-1/CXCL13; macrophage-derived chemokine, MDC/CCL22; monokine induced by gamma interferon, MIG/CXCL9; thymus and activation regulated chemokine, TARC/CCL17), acute phase proteins (C-reactive protein, CRP; serum amyloid A, SAA), and other cytokines and growth factors (soluble tumor necrosis factor receptor-2, sTNFR2; transforming growth factor alpha, TGF-A) (12, 13).
To explore potential biological mechanisms underlying the inverse association between hog farming and lung cancer as well as other immunologic changes associated with hog farming, we investigated these and other biomarkers of immune function and inflammation among hog farmers in the study of Biomarkers of Exposure and Effect in Agriculture (BEEA), a molecular epidemiologic substudy in the AHS.
Methods
Study population
The design and enrollment methods of the BEEA study have been described (14). Briefly, we recruited male AHS participants who were ≥50 years of age, had never been diagnosed with cancer (except for non-melanoma skin cancer), and had completed the questionnaires administered at AHS enrollment (1993–1997) and during two follow-up interviews (1998–2003 and 2005–2010). For the current investigation, we selected the study sample from the 955 participants who were enrolled in BEEA between June 2010 and September 2013. To minimize potential confounding and reduce heterogeneity in other factors that might have influenced immune marker levels, we restricted our selection to Iowa residents who did not smoke cigarettes and further excluded those who were raising poultry, which has also been associated with lung cancer risk in the AHS (9). Our hog farming group (N=61) was comprised of all participants who had raised at least 1,000 hogs in the past year, had spent time in a swine confinement facility in the past month, and had also reported raising hogs on each of the previously administered AHS questionnaires; all of those selected had been raising hogs for a minimum of 13 years at the time of phlebotomy. For the selection of controls, in addition to the exclusion criteria noted above (i.e., limiting eligibility to Iowa residents who were non-smokers and not raising poultry), we further restricted to those who had never raised hogs and matched to hog farmers with a 1:1 ratio on age (±2 years), date of phlebotomy (±60 days, N=52; relaxed to ±365 days, N=9), and whether they raised cattle (yes/no).
All BEEA participants provided written informed consent, and study procedures have been approved by Institutional Review Boards at the NCI and other relevant organizations.
Immune marker measurements
We performed assays measuring serum levels of 67 markers (including the 8 markers previously linked to lung cancer) across 6 Millipore multiplex bead-based panels at the Frederick National Laboratory for Cancer Research using methods that have been described and evaluated for sensitivity and reproducibility (15). All of the selected serum samples were collected, aliquoted, and stored at −80°C following the same protocol (14). Blinded replicate quality control (QC) samples amounting to 10% of the test samples were interspersed within and across batches. For three markers that were measured on multiple panels, we used the results from the high sensitivity panel, which had higher detectability and lower coefficients of variation (CVs). We further excluded five markers (TSLP, IL-3, IL-4, IL-29, IL-33) for which ≥50% of the test samples had levels below the lowest limit of quantification (LLOQ), and one marker (TNF-B) for which a CV could not be estimated because too few measurements in the QC samples were above the LLOQ. After these exclusions, there were 58 markers available for analysis. Most of these (83%) were detectable in >90% of the test samples; those with values below the LLOQ were assigned a level that was half of the LLOQ. The median overall CV for the evaluated markers was 3.7% (range 0.95–10.4%) (Supplementary Table 1).
Statistical analysis
Differences in the distributions of absolute marker levels between groups were assessed using Wilcoxon rank-sum tests. We evaluated departure from the normal distributions of untransformed and natural log-transformed marker levels using Quantile-Quantile plots, and found that natural log-transformed levels were generally more normally distributed. For our main analyses, we assessed differences in natural log-transformed marker levels between hog farmers and controls using multivariate linear regression models. All models were adjusted for matching factors, including age, season of phlebotomy (winter months: October-March; summer months: April-September), and exposure to cattle. In addition, body mass index (BMI) and recent use of aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) were selected as covariates a priori because they have been shown to influence immune marker levels in previous studies (16, 17), and recent history of respiratory infection was selected because it was considered likely to influence immune marker levels and differed between hog farmers and controls in these data. Analyses for selected markers with measurements below the LLOQ were also conducted using Tobit regression to account for interval censoring.
For markers that were associated with hog farming in the main analyses, we compared levels across categories of hog farmers, based on approximate tertiles of the number of hogs being raised (none, N=61; <3,000 hogs, N=18; 3,000–6,000 hogs, N=21; and >6,000 hogs, N=22), using linear regression models adjusted for the same covariates as described above. Tests for trend in marker levels were performed by assigning the within-category median for number of hogs and analyzing as a continuous variable. We also performed analyses of these immune markers after stratifying by other characteristics such as age and season of phlebotomy as these factors may influence immune markers or likelihood or exposure to endotoxin or other bioaerosols. Additional analyses were conducted to evaluate potential confounding by historical or current exposure to pesticides that are commonly used in hog confinement facilities (e.g., malathion, cyfluthrin, imidacloprid) (18) or have been previously associated with lung cancer risk in the AHS (19). We adjusted for selected pesticides that were reported by >10% of the study participants at AHS enrollment (chlorimuron-ethyl, pendimethalin, diazinon, and malathion) or in the 12 months prior to phlebotomy in BEEA (malathion and cyfluthrin). Finally, we conducted sensitivity analyses excluding hog farmers and controls who were raising cattle and those who reported having respiratory infections in the 7 days prior to phlebotomy.
All analyses were performed using Stata 14.0 (College Station, TX). Because this investigation focused on a few specific immune markers from across the selected panels that have been implicated previously in lung carcinogenesis, and analyses of other markers on those panels were exploratory, we considered findings to be statistically significant if P < 0.05.
Results
Demographic and other characteristics of the selected study participants are shown in Table 1. As expected based on our matching criteria, hog farmers and controls were similar with respect to age, season of phlebotomy, and whether they reported raising cattle. Controls were more likely than hog farmers to have had a respiratory infection in the past 7 days (31% and 13%, respectively), but did not differ in terms of other characteristics such as race, level of education, BMI, or recent NSAID use.
Table 1.
Selected characteristics of hog farmers and controlsa
| Characteristic | Hog farmers N (%) |
Controls N (%) |
|---|---|---|
| No. of participants | 61 | 61 |
| Age at phlebotomy, median (range) | 56 (50–72) | 56 (50–70) |
| Race | ||
| Non-Hispanic white | 60 (98) | 59 (97) |
| Other/missing | 1 (2) | 2 (3) |
| Education | ||
| High school or less | 21 (34) | 30 (49) |
| Some college or vocational school | 20 (33) | 18 (30) |
| College graduate | 19 (31) | 12 (20) |
| Other/missing | 1 (2) | 1 (2) |
| Body mass index | ||
| <25 kg/m2 | 8 (13) | 6 (10) |
| 25–29.9 kg/m2 | 33 (54) | 31 (51) |
| ≥30–34.9 kg/m2 | 20 (33) | 24 (39) |
| Season of phlebotomy | ||
| January – March | 21 (34) | 26 (43) |
| April – June | 15 (25) | 11 (18) |
| July – September | 19 (31) | 15 (25) |
| October – December | 6 (10) | 9 (15) |
| Recent (past 7 days) respiratory infectionb | ||
| No | 53 (87) | 42 (69) |
| Yes | 8 (13) | 19 (31) |
| Recent (past 7 days) NSAID use | ||
| No | 24 (39) | 23 (38) |
| Yes | 37 (61) | 38 (62) |
| Raised cattle (past 12 months) | ||
| No | 40 (66) | 40 (66) |
| Yes | 21 (34) | 21 (34) |
Notes: NSAID, non-steroidal anti-inflammatory drugs
Reported as frequencies (%) unless otherwise noted.
P < 0.05, chi-square test
Findings for immune markers that were statistically significantly associated with hog farming are shown in Table 2. Circulating levels of macrophage-derived chemokine (MDC/CCL22) were lower among hog farmers compared with controls, whereas levels of macrophage inflammatory protein-3 alpha (MIP-3A/CCL20), basic fibroblast growth factor (FGF-2), and soluble interleukin-4 receptor (sIL-4R) were elevated (Table 2). The association with MDC/CCL22 remained statistically significant after adjustment for covariates; levels of this chemokine were estimated to be 17% lower (95% confidence interval [CI]: −28%, −4%) among hog farmers than among controls. Notably, as shown in Figure 1, we observed an exposure-response trend of decreasing MDC/CCL22 levels with increasing number of hogs (>6,000 hogs vs. controls: −26%; 95% CI −39%, −10%; Ptrend = 0.002). A similar pattern of association was observed among these participants for number of hogs reported in earlier AHS interviews (e.g., at first follow-up, >4,000 hogs vs. controls: −24%; 95% CI −38%, −7%; Ptrend = 0.006). The overall association between hog farming and MDC/CCL22 levels did not differ by season or age at phlebotomy (not shown), and remained statistically significant after excluding those who were raising cattle (−28%; 95% CI −41%, −13%).
Table 2.
Comparisons of selected immune marker levels among hog farmers and controls
| Marker | Bivariate analyses | Multivariate analyses | ||
|---|---|---|---|---|
|
|
|
|||
| Hog farmers, median (IQR)a | Controls, median (IQR)a | P-value | Percent difference (95% CI) between hog farmers and controlsb | |
| Decreased in hog farmers | ||||
| MDC/CCL22 | 969 (714–1270) | 1052 (864–1336) | 0.046 | −17% (−28%, −4%) |
| Increased in hog farmers | ||||
| MIP-3A/CCL20 | 9.08 (6.62–13.61) | 7.35 (4.28–11.10) | 0.032 | 111% (19%, 273%) |
| FGF-2 | 140.1 (56.7–260.8) | 63.0 (8.0–159.3) | 0.005 | 93% (10%, 240%) |
| sIL-4R | 825 (680–986) | 744 (622–877) | 0.058 | 12% (1%, 25%) |
Notes: MDC/CCL22, macrophage-derived chemokine; MIP-3A/CCL20, macrophage inflammatory protein 3-alpha; FGF-2, basic fibroblast growth factor; sIL-4R, soluble interleukin-4 receptor.
Levels of immune markers are expressed as pg/mL.
Differences in natural log-transformed marker levels between hog farmers and controls were estimated using multivariate linear regression models adjusted for age, season of phlebotomy, BMI, recent history of respiratory infection, recent NSAID use, and raising cattle. The percent difference was calculated as follows: exp(β) − 1
Multivariate analyses of other markers demonstrated that, relative to controls, hog farmers had approximately two-fold higher levels of MIP-3A/CCL20 and FGF-2 (increases of 111% [95% CI: 19%, 273%] and 93% [10%, 240%], respectively; Table 2). These two markers were only moderately correlated (spearman correlation coefficients of 0.2 among controls and 0.4 among hog farmers). Levels of sIL-4R were also elevated by 12% (1%, 25%) in hog farmers after covariate adjustment. Results for these markers were similar in analyses using Tobit regression that accounted for interval censoring when measurements were below the LLOQ (Supplementary Table 2). There were suggestive but non-statistically significant trends of increasing levels of MIP-3A/CCL20 and sIL-4R with greater numbers of hogs (Ptrend = 0.05 and 0.075, respectively; Figure 1), but not for FGF-2 (Ptrend = 0.28). After stratifying by season of phlebotomy, we observed particularly strong associations during the winter months for MIP-3A/CCL20 (330%; 95% CI 66%, 1013%) and FGF-2 (272%; 95% CI 64%, 740%) when endotoxin levels are typically highest (2, 3); neither marker was elevated among hog farmers during the summer months (Pinteraction = 0.02 and 0.07, respectively; Supplementary Figure 1).
Figure 1.
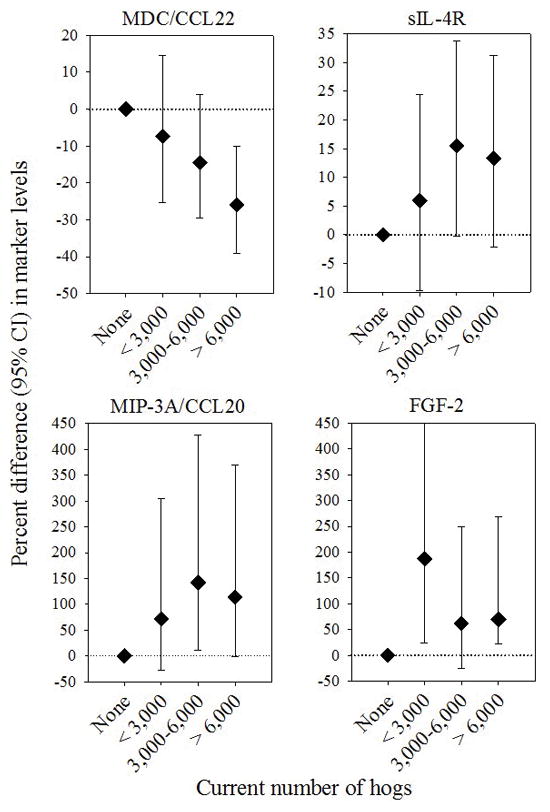
Differences in natural log-transformed marker levels between hog farmers and controls by number of hogs raised in the past year for MDC/CCL22 (Ptrend = 0.002), sIL-4R (Ptrend = 0.075), MIP-3A/CCL20 (Ptrend = 0.05), and FGF-2 (Ptrend = 0.28). Categories for number of hogs were defined as none (N=61), <3,000 (N=18), 3,000–6,000 (N=21), and >6,000 hogs (N=22). All analyses were adjusted for age, season of phlebotomy, BMI, recent history of respiratory infection, recent NSAID use, and exposure to cattle.
Our findings for MDC/CCL22, MIP-3A/CCL20, FGF-2, and sIL-4R were essentially unchanged in analyses adjusted for reported pesticide use at AHS enrollment or for current use (in past 12 months) at the time of enrollment in BEEA, although the association with sIL-4R was no longer statistically significant. In sensitivity analyses excluding those with recent respiratory infections (in the past 7 days), the associations with hog farming for these four markers were similar or only modestly attenuated.
Findings for other markers are shown in Supplementary Table 3. Hog farmers had somewhat lower levels of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) and B-cell attracting chemokine-1 (BCA-1/CXCL13), and somewhat higher levels of macrophage inflammatory protein-1 beta (MIP-1B/CCL4) and fractalkine (CX3CL1). However, these and other markers were not associated with hog farming after adjustment for covariates (Supplementary Table 3).
Discussion
This study is, to our knowledge, the first comprehensive investigation of alterations in lung cancer-related immune markers among hog farmers. We found that hog farming was associated with lower circulating levels of MDC/CCL22 and observed an exposure-response trend with increasing numbers of hogs. MDC/CCL22 has been implicated in lung cancer development in a recent pooled investigation involving 1,052 lung cancer cases from the Prostate, Lung, Colorectal and Ovarian (PLCO) cohort (13), and an earlier investigation in PLCO found that MDC/CCL22 was independently associated with lung cancer risk (12). Taken together with these prospective findings from PLCO, our results suggest that altered expression of MDC/CCL22 may contribute to the reduced risk of lung cancer observed previously among hog farmers in the AHS (9). The specific role of MDC/CCL22 in lung carcinogenesis has yet to be fully elucidated. This CC-motif chemokine is involved in the selective recruitment of lymphocytes through signaling in the C-C chemokine receptor type 4 pathway, and its expression is associated with a T helper 2 (Th2)-type immune response (20). Systemic levels of MDC/CCL22 have been associated with pulmonary inflammation in animal models (21), and elevated MDC/CCL22 levels have been observed in bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis (22, 23). Notably, MDC/CCL22 and its receptors are expressed in lung tumors (24, 25).
In contrast to our findings for MDC/CCL22, we also observed elevated levels of several markers including MIP-3A/CCL20, FGF-2, and sIL-4R. The particularly strong associations with MIP-3A/CCL20 and FGF-2 during the winter months are consistent with well-characterized patterns of higher levels of exposure to endotoxin and other organic dusts in winter when ventilation rates in hog confinement facilities are reduced (2, 3). Differences by season have been observed for other markers in prior studies of hog farm workers (26, 27). Although levels of these markers were elevated in overall analyses, the patterns were not monotonic with increasing number of hogs. This could indicate that intermediate levels of exposure might elicit a different response for these markers, or could be indicative of exposure misclassification as number of hogs is a crude surrogate of exposure to endotoxin and other bioaerosols. Future studies of these markers with quantitative estimation of exposure should consider evaluating differences by season and potential non-linear exposure-response patterns. Evidence from experimental studies supports an association with endotoxin exposure for these markers. Gene expression of MIP-3A/CCL20 was induced by bacterial endotoxin (lipopolysaccharide, LPS) (28), and treatment with recombinant FGF-2 protected against interferon (IFN)-gamma induced emphysema following LPS challenge in FGF-2 deficient mice (29). Expression of both MIP-3A/CCL20 and FGF-2 may be related to IFN-gamma production, which is an important mediator of endotoxin-induced immune responses (30). The underlying biological basis of the association with sIL-4R observed in multivariate analyses is less clear, although the binding of IL-4 to receptors on macrophages may downregulate IFN-gamma during immune response to glycans (31). We note that these findings may have implications for other acute and chronic health endpoints related to occupational endotoxin exposure, such as atopic asthma and chronic respiratory disease (1–3, 32).
Prior studies in hog farm workers (26, 27) and hog breeders (33) noted associations with circulating levels and/or mRNA expression for TNF-A and IL-6, -8, and -10. However, these findings were inconsistent across studies, which were limited to small numbers of hog-exposed workers (N≤28), evaluated short-term effects, and measured relatively few markers with no clear relevance to the etiology of lung cancer. None of these specific markers were associated with hog farming in the present study.
Our investigation had several notable strengths. This is the largest investigation to date of immune alterations among hog farmers, and the first to evaluate markers specifically linked to lung cancer development. We were able to maximize the exposure contrast between groups by selecting hog farmers who were most likely to have high current exposure (as indicated by raising ≥1,000 hogs and having spent time in a hog confinement building in the past month) as well as high cumulative lifetime exposure based on their reported history of hog farming on previous AHS questionnaires. We also minimized confounding by other factors by adjusting for exposure to cattle and restricting to BEEA participants who were not occupationally exposed to poultry, were non-smokers, and were living in Iowa. Further adjustment for exposure to pesticides commonly used in hog confinement facilities or linked to lung cancer did not change our findings. With a median age at phlebotomy of 56 years (interquartile range: 53–59 years), the measurements of immune markers reflect levels during an etiologically relevant time period for future risk of lung cancer, which may take years to develop into clinically manifest disease and is typically diagnosed at an older age (13).
Several limitations of the present study should also be acknowledged. Although it is likely that the observed immunologic changes may be attributable to exposure to endotoxin or other bioaerosols, we did not have quantitative estimates of these exposures. It is also possible that expression of some markers could be influenced by other exposures related to hog farming including zoonotic pathogens, particulate matter, and hydrogen sulfide (18, 32, 34–36). Future studies of these immune markers and other intermediate endpoints would benefit from detailed characterization of endotoxin and other exposures among farmers who are raising hogs or other animals (e.g., dairy and beef cattle, poultry), using different kinds of animal confinement facilities (e.g., indoor vs. outdoor), or engaged in other farming activities that are likely to influence levels of exposure. While this study is the largest of its kind and was sufficiently powered to detect relatively modest differences in immune marker levels between hog farmers and controls, it is possible that some of the associations may have arisen due to chance given the number of immune markers that were evaluated; in particular, the findings of exploratory analyses among those markers not previously associated with risk of lung cancer should be interpreted cautiously. As such, replication of our findings of altered immune marker levels in hog farmers and extension of this work to other kinds of animal production and farming activities involving exposure to endotoxin and other bioaerosols is warranted. Finally, previous studies have noted a lower prevalence of allergic diseases in some farming populations that may be attributable to early life farm exposures including endotoxin (reviewed in ref. 37). Future investigations of these and other markers related to Th1/Th2 balance and expression of IFN-gamma in studies with detailed characterization of early life farm exposures could provide mechanistic insights into persistent immunologic changes that may influence the risk of allergic diseases and other health endpoints.
In summary, we found that hog farmers had decreased circulating levels of MDC/CCL22 and increased levels of MIP-3A/CCL20, FGF-2, and sIL-4R; these immunologic alterations may be attributable to high exposure to endotoxin or other bioaerosols associated with hog farming. Our finding of reduced circulating levels of MDC/CCL22 among hog farmers supports the biological plausibility of the previously observed inverse association between hog farming and lung cancer risk, and may provide insights into the underlying biological mechanisms through which endotoxin or other bioaerosol exposures influence lung cancer development.
Supplementary Material
What this paper adds.
1. What is already known about this subject?
Occupational exposure to endotoxin exposure has been consistently associated with a reduced risk of lung cancer, although the underlying biological mechanisms remain unclear. Levels of endotoxin exposure are particularly high in industrial hog confinement facilities, and hog farming has been inversely associated with lung cancer risk in the Agricultural Health Study cohort.
2. What are the new findings?
We found that circulating levels of MDC/CCL22, a chemokine that has been prospectively linked to lung cancer development, were significantly reduced among hog farmers in the Agricultural Health Study. Several other endotoxin-related immunologic markers (e.g., MIP-3A/CCL20, FGF-2) were elevated in hog farmers.
3. How might it impact on policy or clinical practice in the foreseeable future?
This study is, to the best of our knowledge, the first comprehensive investigation of lung cancer-related immunologic markers among endotoxin-exposed farmers. Our findings provide insights into the potential mechanisms of action through which endotoxin prevents lung carcinogenesis, thereby informing our understanding of lung cancer etiology and prevention.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute (Z01CP010119) and National Institute of Environmental Health Sciences (Z01ES049030). We gratefully acknowledge Amy Miller, Kate Torres, Emily Tristani, Linda Gowen, Himanshi Singh, Marsha Dunn and other staff at Westat, Inc. (Rockville, Maryland) for study coordination, data management, and field research efforts. We thank Debra Lande, Debra Podaril, and Jennifer Hamilton from the field research team in Iowa for their efforts on this study. We also thank Peter Hui, Michael Curry, and other staff at Information Management Services, Inc. for data management support. The ongoing participation of the Agricultural Health Study participants is indispensable and sincerely appreciated.
Footnotes
Contributors: All contributors meet the criteria for authorship.
Competing interests: None declared.
References
- 1.Schenker MB, Christiani D, Cormier Y, Dimich-Ward H, Goekes G, Dosman J, et al. Respiratory health hazards in agriculture. American journal of respiratory and critical care medicine. 1998;158(5 Pt 2):S1–S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- 2.Basinas I, Schlunssen V, Takai H, Heederik D, Omland O, Wouters IM, et al. Exposure to inhalable dust and endotoxin among danish pig farmers affected by work tasks and stable characteristics. Ann Occup Hyg. 2013;57(8):1005–19. doi: 10.1093/annhyg/met029. [DOI] [PubMed] [Google Scholar]
- 3.Basinas I, Sigsgaard T, Kromhout H, Heederik D, Wouters IM, Schlunssen V. A comprehensive review of levels and determinants of personal exposure to dust and endotoxin in livestock farming. J Expo Sci Environ Epidemiol. 2015;25(2):123–37. doi: 10.1038/jes.2013.83. [DOI] [PubMed] [Google Scholar]
- 4.Lundin JI, Checkoway H. Endotoxin and cancer. Environ Health Perspect. 2009;117(9):1344–50. doi: 10.1289/ehp.0800439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenters V, Basinas I, Beane-Freeman L, Boffetta P, Checkoway H, Coggon D, et al. Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Causes Control. 2010;21(4):523–55. doi: 10.1007/s10552-009-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben Khedher S, Neri M, Matrat M, Cenée S, Sanchez M, Menvielle G, et al. Occupational exposure to endotoxins and lung cancer risk: Results of the ICARE Study. Occup Environ Med. 2017;74(9):667–79. doi: 10.1136/oemed-2016-104117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters S, Kromhout H, Olsson AC, Wichmann HE, Bruske I, Consonni D, et al. Occupational exposure to organic dust increases lung cancer risk in the general population. Thorax. 2012;67(2):111–6. doi: 10.1136/thoraxjnl-2011-200716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. The Agricultural Health Study. Environ Health Perspect. 1996;104(4):362–9. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beane Freeman LE, Deroos AJ, Koutros S, Blair A, Ward MH, Alavanja M, et al. Poultry and livestock exposure and cancer risk among farmers in the agricultural health study. Cancer Causes Control. 2012;23(5):663–70. doi: 10.1007/s10552-012-9921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clinical lung cancer. 2003;5(1):46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- 11.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert review of anticancer therapy. 2008;8(4):605–15. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 12.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. 2013;105(24):1871–80. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiels MS, Katki HA, Hildesheim A, Pfeiffer RM, Engels EA, Williams M, et al. Circulating Inflammation Markers, Risk of Lung Cancer, and Utility for Risk Stratification. J Natl Cancer Inst. 2015;107(10) doi: 10.1093/jnci/djv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann JN, Beane Freeman LE, Lynch CF, Andreotti G, Thomas KW, Sandler DP, et al. The Biomarkers of Exposure and Effect in Agriculture (BEEA) Study: Rationale, Design, Methods, and Participant Characteristics. Journal of toxicology and environmental health Part A. 2015;78(21–22):1338–47. doi: 10.1080/15287394.2015.1091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi AK, Kemp TJ, Pfeiffer RM, Biancotto A, Williams M, Munuo S, et al. Evaluation of multiplexed cytokine and inflammation marker measurements: a methodologic study. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1902–11. doi: 10.1158/1055-9965.EPI-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitahara CM, Trabert B, Katki HA, Chaturvedi AK, Kemp TJ, Pinto LA, et al. Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2840–9. doi: 10.1158/1055-9965.EPI-14-0699-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang Kuhs KA, Hildesheim A, Trabert B, Kemp TJ, Purdue MP, Wentzensen N, et al. Association between Regular Aspirin Use and Circulating Markers of Inflammation: A Study within the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2015;24(5):825–32. doi: 10.1158/1055-9965.EPI-14-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agricultural Chemical Usage: Swine and Swine Facilities. National Agricultural Statistics Service, United States Department of Agriculture; Washington, DC: Dec, 2006. [Google Scholar]
- 19.Bonner MR, Beane Freeman LE, Hoppin JA, Koutros S, Sandler DP, Lynch CF, et al. Occupational Exposure to Pesticides and the Incidence of Lung Cancer in the Agricultural Health Study. Environ Health Perspect. 2016 Jul 6; doi: 10.1289/EHP456. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantovani A, Gray PA, Van Damme J, Sozzani S. Macrophage-derived chemokine (MDC) Journal of leukocyte biology. 2000;68(3):400–4. [PubMed] [Google Scholar]
- 21.Richter JR, Sutton JM, Belizaire RM, Friend LA, Schuster RM, Johannigman TA, et al. Macrophage-derived chemokine (CCL22) is a novel mediator of lung inflammation following hemorrhage and resuscitation. Shock. 2014;42(6):525–31. doi: 10.1097/SHK.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue T, Fujishima S, Ikeda E, Yoshie O, Tsukamoto N, Aiso S, et al. CCL22 and CCL17 in rat radiation pneumonitis and in human idiopathic pulmonary fibrosis. The European respiratory journal. 2004;24(1):49–56. doi: 10.1183/09031936.04.00110203. [DOI] [PubMed] [Google Scholar]
- 23.Yogo Y, Fujishima S, Inoue T, Saito F, Shiomi T, Yamaguchi K, et al. Macrophage derived chemokine (CCL22), thymus and activation-regulated chemokine (CCL17), and CCR4 in idiopathic pulmonary fibrosis. Respir Res. 2009;10:80. doi: 10.1186/1465-9921-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71(20):6391–9. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi T, Imaizumi K, Hasegawa Y, Kawabe T, Hashimoto N, Okamoto M, et al. Expression of macrophage-derived chemokine (MDC)/CCL22 in human lung cancer. Cancer immunology, immunotherapy: CII. 2006;55(11):1320–9. doi: 10.1007/s00262-006-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonlokke JH, Meriaux A, Duchaine C, Godbout S, Cormier Y. Seasonal variations in work-related health effects in swine farm workers. Annals of agricultural and environmental medicine: AAEM. 2009;16(1):43–52. [PubMed] [Google Scholar]
- 27.Bonlokke JH, Cormier Y, Veillette M, Radu A, Meriaux A, Duchaine C. Immunologic mechanisms in the adaptation of swine farm workers to their work environment. Innate immunity. 2013;19(4):403–10. doi: 10.1177/1753425912466576. [DOI] [PubMed] [Google Scholar]
- 28.Schutyser E, Struyf S, Menten P, Lenaerts JP, Conings R, Put W, et al. Regulated production and molecular diversity of human liver and activation-regulated chemokine/macrophage inflammatory protein-3 alpha from normal and transformed cells. J Immunol. 2000;165(8):4470–7. doi: 10.4049/jimmunol.165.8.4470. [DOI] [PubMed] [Google Scholar]
- 29.Lee BJ, Moon HG, Shin TS, Jeon SG, Lee EY, Gho YS, et al. Protective effects of basic fibroblast growth factor in the development of emphysema induced by interferon-gamma. Exp Mol Med. 2011;43(4):169–78. doi: 10.3858/emm.2011.43.4.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varma TK, Lin CY, Toliver-Kinsky TE, Sherwood ER. Endotoxin-induced gamma interferon production: contributing cell types and key regulatory factors. Clin Diagn Lab Immunol. 2002;9(3):530–43. doi: 10.1128/CDLI.9.3.530-543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tundup S, Srivastava L, Harn DA., Jr Polarization of host immune responses by helminth-expressed glycans. Ann N Y Acad Sci. 2012;1253:E1–E13. doi: 10.1111/j.1749-6632.2012.06618.x. [DOI] [PubMed] [Google Scholar]
- 32.Schinasi L, Horton RA, Guidry VT, Wing S, Marshall SW, Morland KB. Air pollution, lung function, and physical symptoms in communities near concentrated Swine feeding operations. Epidemiology. 2011;22(2):208–15. doi: 10.1097/EDE.0b013e3182093c8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabibi R, Corsini E, Brambilla G, Bonizzi L, Melzi d’Eril G, Rabozzi G, et al. Immune changes in animal breeders: a pilot study conducted in northern Italy. Annals of agricultural and environmental medicine: AAEM. 2012;19(2):221–5. [PubMed] [Google Scholar]
- 34.Gray GC, McCarthy T, Capuano AW, Setterquist SF, Olsen CW, Alavanja MC. Swine workers and swine influenza virus infections. Emerg Infect Dis. 2007;13(12):1871–8. doi: 10.3201/eid1312.061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vegosen L, Breysse PN, Agnew J, Gray GC, Nachamkin I, Sheikh K, et al. Occupational Exposure to Swine, Poultry, and Cattle and Antibody Biomarkers of Campylobacter jejuni Exposure and Autoimmune Peripheral Neuropathy. PLoS One. 2015;10(12):e0143587. doi: 10.1371/journal.pone.0143587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wardyn SE, Forshey BM, Farina SA, Kates AE, Nair R, Quick MK, et al. Swine Farming Is a Risk Factor for Infection With and High Prevalence of Carriage of Multidrug-Resistant Staphylococcus aureus. Clinical infectious diseases. 2015;61(1):59–66. doi: 10.1093/cid/civ234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10(12):861–8. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


