Abstract
Fludarabine and melphalan (Flu/Mel) has emerged as a more tolerable chemotherapy-based conditioning regimen compared with busulfan and cyclophosphamide (Bu/Cy) for allogeneic stem cell transplant (allo-hematopoietic stem cell transplantation (HSCT)) patients with acute myeloge-nous leukemia (AML). We conducted a retrospective review of a single-institution database including patients with AML who received allo-HSCT following conditioning with Mel/Flu or Bu/Cy-based regimens. We performed descriptive statistical analysis to examine patient demographics and clinical outcomes. We identified 156 patients meeting criteria between 2005 and 2014. Overall, patients conditioned with Bu/Cy were significantly younger, but more likely to be treated in an earlier era than those receiving Flu/Mel. Regimen choice was not associated with relapse rates (RR), relapse-free survival (RFS), or overall survival (OS) on both univariate and multivariable analyses. Bu/Cy was associated with increased non-relapse mortality (NRM) on multi-variable analysis. These findings demonstrate that Flu/Mel provides non-inferior disease control and could be an appropriate regimen for selected patients.
Keywords: Transplant toxicity, chemotherapeutic approaches, myeloid leukemias and dysplasias
Introduction
Allogeneic hematopoietic stem cell transplant (allo-HCST) is a cornerstone of treatment for acute myelogenous leukemia (AML) and pre-transplant conditioning is integral to this procedure. Historically, myeloablative conditioning (MAC) with either total body irradiation (TBI)-based conditioning (TBIBC) or a busulfan-based regimen have been used [1,2]. Due to the poor tolerance of MAC in the elderly or infirm, reduced intensity conditioning (RIC) regimens have emerged to allow transplantation of such patients [3,4]. Such regimens may be based on low-dose TBI or on non-myeloablative chemotherapy regimens, such as fludarabine (Flu) and low dose Bu, or Flu and melphalan (Mel.) [5].
As expected, RIC has been shown in meta-analyses to be associated with less early toxicity and non-relapse mortality (NRM) in patients with leukemia [5], despite the older age of patients treated with RIC. Two meta-analyses suggest that RIC may be associated with higher relapse rate (RR) in acute leukemia [5,6]. Furthermore, in the LBA-8 study, which was a prospective randomized trial of MAC (chemotherapy-based or TBI-based) versus RIC regimens for allo-hematopoietic stem cell transplantation (HSCT) for myelodysplastic syndrome (MDS) or AML, RIC resulted in a significant reduction in NRM, whereas MAC resulted in significantly lower RR, higher relapse-free survival (RFS), and a trend toward improved overall survival (OS) [7]. These data support MAC as the preferred approach for those able to tolerate it. However, for the elderly or infirm patient, the risk of higher NRM with MAC may outweigh the benefit of improved RR. In two studies of AML, in patients over 50 years old, the reduced NRM associated with RIC compared with MAC overcame any advantage in RR with MAC, such that there was no significant difference in RFS or OS [5,8]. Thus, additional studies of RIC remain necessary.
In many studies of RIC, patients with AML and MDS are included together, limiting conclusions specific to AML [5]. In addition, previous studies have generally included a variety of RIC regimens [5], limiting conclusions about any specific regimen. For example, some data suggest that Flu/Mel may be associated with lower RR compared to Flu/Bu, despite both being grouped as RIC regimens [9]. In the LBA-8 study, separate outcomes were not reported for Flu/Mel and Flu/Bu [7]. In studies of MAC versus RIC, results of TBIBC and chemotherapy-based conditioning are often combined; however, patients with AML are increasingly being prepared for allo-HSCT with chemotherapy-based conditioning rather than TBIBC, and review of a large database showed a RFS and OS advantage associated with chemotherapy [10]. Thus, studies focused on chemotherapy-based conditioning are needed. We did not identify any studies comparing myeloablative Bu/Cy directly with reduced intensity Flu/Mel or any studies restricted to chemotherapy-based conditioning, comparing RIC versus MAC specifically for AML.
Thus, the aim of our analysis is to compare outcomes of MAC Bu/Cy versus. RIC Flu/Mel as regimens for allo-HSCT in patients with AML.
Methods
Patient data
After Institutional Review Board approval, we analyzed information from a prospectively-gathered transplant database at a single institution with a regional referral base for transplants. We assessed age, sex, and Karnofsky performance status (KPS) data [low (< 80) versus high (>80)], disease status [complete response (CR) 1 versus CR2 versus other] presence of extramedullary disease, cytogenetic classification [‘poor,’ ‘intermediate,’ or ‘favorable,’ defined per Southwest Oncology Group/Eastern Cooperative Oncology Group definitions (11) except ‘intermediate’ includes t(9:11), 11q23 and MLL rearrangements, and ‘poor’ and ‘intermediate’ exclude 20q], transplant type [matched related donor (MRD) versus matched unrelated donor (MUD)] human leukocyte antigen disparity (0° versus 1° or 2° of human leukocyte antigen (HLA) locus disparity), graft source (bone marrow versus peripheral blood stem cell), year of allo-HSCT [2005-09 versus 2010-14], and CD34 dose).
Study population
We reviewed patients with AML, 18 years and older, treated with allo-HSCT after conditioning with either Flu/Mel or Bu/Cy-based conditioning between 2005 and 2014 at our institution. Patients with haplo-identical transplants were excluded.
Transplant source and conditioning regimen
For the Bu/Cy-based regimen, patients typically received either intravenous busulfan 14.4mg/kg (0.9mg/kg per dose) or oral busulfan 16mg/kg (1 mg/kg per dose) in divided doses every 6 h over 4 consecutive days starting 7 d before the transplant. Busulfan drug levels were measured after the first dose, and pharmacokinetic-directed dosing was performed to achieve a predefined target area under the curve (AUC) of 20,000 μM-min for the aggregate AUC of all delivered doses. Starting 3 d before the transplant, patients received 120 mg/kg CY (60 mg/kg, on each of 2 consecutive days). A small cohort of Bu/Cy patients (see below) also received pre-transplant Fludarabine as a part of the regimen, per an institutional trial.
For the Flu/Mel regimen, patients typically received fludarabine 100 mg/m2 (25 mg/m2 per day for 4 consecutive days starting 6 d before transplant), and melphalan 140 mg/m2 (70 mg/m2 per day for 2 consecutive days starting 3 d before treatment), followed by a day of rest before infusion of the stem cell product. Patients transplanted from HLA mismatched donors additionally received pre-transplant anti-thymocyte globulin (thymoglobulin 6 mg/kg total dose).
The stem cell product consisted of either BM or PBSC from matched related or matched unrelated donors with HLA disparity < or =2 of 10 typed HLA loci. Graft-versus-host disease (GvHD) prophylaxis was per institutional protocol, and typically consisted of a calcineurin inhibitor plus short course methotrexate.
Outcome data
The outcomes of the study included RR, RFS, OS, and NRM. We assessed post-transplant length of hospital stay (LoHS) and admission to intensive care unit (ICU) within 30 d after transplant as indirect measures of conditioning toxicity. Acute GvHD onset and overall highest grade and chronic GvHD incidence were recorded. Days to engraftment represent the days from induction to absolute neutrophil count ≥500 cells/microliter. Chimerism data represent either 30 d, 100 d, 1 year, or 2 year bone marrow biopsy analysis for karyotype or variable nucleotide tandem repeat analysis. Samples with ≥95% donor chimerism were considered fully donor.
Statistics
OS is the time from transplant to either death or last follow-up, and those who are still alive are censored at last follow-up. RFS is the time from transplant to relapse, death, or last follow-up, where those alive without relapse or death are censored at last follow-up. NRM is the time from transplant to death or last follow-up without relapse. Deaths without prior relapse are considered events, and relapse is considered a competing event. Relapse is the time from transplant to relapse or last follow-up, where death without relapse is considered a competing event. Patients alive without death or relapse are censored at the time of last follow-up.
Covariates were compared between Bu/Cy chemo-based treatment and Flu/Mel chemo-based treatment as well as between ICU admission status (yes/no) using Fisher's exact tests or chi-squared tests, where appropriate, for categorical variables, and ANOVA for continuous variables. Mean length of hospital stay was compared across categorical variables using ANOVA, whereas the Pearson correlation coefficient was used to assess the relationship between continuous variables and length of hospital stay. Survival was estimated using the Kaplan–Meier method, and survival distributions were compared using log-rank tests. Cumulative incidence curves of relapse and NRM were generated and were compared between variables using Gray's tests. Cumulative incidence curves for time from transplant to GVHD were also generated.
The covariates were fit in univariate and multivariate Cox proportional hazards regression models for the OS and RFS endpoints. Backward elimination criteria for removal from the multivariate models were applied for transplant type, HLA, graft source, year of transplant, CD34 dose, and age at transplant with an alpha value of 0.05, while conditioning regimen, sex, status, cytogenetic classification, extramedullary disease, and KPS were fixed into the multivariate models as these variables have previously been identified as significant predictors of outcomes. Additionally, competing risks univariate and multivariate analyses were performed for NRM, with relapse considered a competing event, using a semiparametric proportional hazards model for the sub-distribution of a competing risk [11]. Removal criteria for the multivariate models were the same as OS and RFS. Likewise, competing risks univariate and multivariate analyses were performed for relapse, with death considered a competing event, where the removal criteria for the multivariate models were the same as OS, RFS, and NRM. Multivariate analyses were also performed for ICU admission and length of hospital stay using logistic regression and linear regression, respectively. Only conditioning regimen was fixed into each model, and other covariates were subject to backwards elimination as with the other defined endpoints. Survival analysis and regression assumptions were checked and verified. Significance was assessed at the 0.05 level, and statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). Kaplan-Meier plots and cumulative incidence curves were generated using R 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) with packages ‘survival’ and ‘cmprsk’ [12,13].
Results
Patient and transplant characteristics
A total of 156 patients met eligibility criteria, 73 conditioned with Bu/Cy and 83 with Flu/Mel. Of the 73 patients treated with Bu/Cy, nine also received Flu at 100 mg/m2, five received ATG, and one received Flu and ATG. Of the 83 Flu/Mel patients, 17 also received ATG. As shown in Table 1, patient characteristics were similar for both groups, except patients treated with Flu/Mel were older than those treated with Bu/Cy, and more likely to be treated in later years. The median follow-up time for Flu/Mel and Bu/Cy patients was 3.7 years and 5.5 years, respectively.
Table 1.
Patient characteristics.
| Conditioning regimens | p valuea | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Covariate | Statistics | Level | BU/CY N = 73 | FLU/MEL N = 83 | |
| Sex | N (Col %) | Female | 42 (57.53) | 38 (45.78) | .156 |
| N (Col %) | Male | 31 (42.47) | 45 (54.22) | ||
| Status | N (Col %) | CR1 | 36 (49.32) | 36 (43.37) | .424 |
| N (Col %) | CR2 | 12 (16.44) | 12 (14.46) | ||
| N (Col %) | All others | 25 (34.24) | 35 (42.17) | ||
| Cytogenetic classification | N (Col %) | Favorable | 7 (10) | 4 (5) | .246 |
| N (Col %) | Intermediate | 44 (62.86) | 43 (53.75) | ||
| N (Col %) | Poor | 19 (27.14) | 33 (41.25) | ||
| Extramedullary disease | N (Col %) | No | 68 (93.15) | 73 (87.95) | .119 |
| N (Col %) | Yes | 5 (6.85) | 10 (12.05) | ||
| KPSa | N (Col %) | Low (<80) | 23 (35.38) | 31 (38.75) | .537 |
| N (Col %) | High (80+) | 42 (64.62) | 49 (61.25) | ||
| Transplant type | N (Col %) | MRD | 27 (36.98) | 28 (33.73) | .376 |
| N (Col %) | MUD | 46 (63.02) | 55 (66.27) | ||
| HLA disparity | N (Col %) | 0 | 62 (84.93) | 64 (77.11) | .271 |
| N (Col %) | 1–2 | 11 (15.07) | 19 (22.89) | ||
| Graft source | N (Col %) | BM | 11 (15.07) | 13 (15.66) | .621 |
| N (Col %) | PBSC | 62 (84.93) | 70 (84.34) | ||
| Year of transplant | N (Col %) | 2005–2009 | 51 (69.86) | 27 (32.53) | <.001 |
| N (Col %) | 2010–2014 | 22 (30.14) | 56 (67.47) | ||
| Age at transplant | N | 73 | 83 | <.001 | |
| Mean | 39.32 | 56.99 | |||
| Median | 39.1 | 59 | |||
| CD34 dosea | N | 72 | 83 | .435 | |
| Mean | 11.55 | 10.35 | |||
| Median | 10.31 | 9.78 | |||
The p value is calculated by ANOVA for numerical covariates; and chi-square test or Fisher's exact for categorical covariates, where appropriate. BU: busul-fan; CY: cyclophosphasmide; FLU: fludarabine; MEL: melphalan; KPS: Karnofsky performance status; HLA: human leukocyte antigen.
Bold values represent variables reaching statistical significance with p value < .05.
Data were not available for CD34 dose in 1/73 BuCy patient; KPS for 8/73 Bu/Cy and 3/83 Flu/Mel patients; and cytogenetics in 1/73 Bu/Cy and 3/83 Flu/Mel patients.
OS
As shown in Figure 1, the median OS for patients treated with Bu/Cy was 2.2 years versus 5 years for those treated with Flu/Mel (p = .485). No association of conditioning regimen with OS emerged on univariate or multivariate analysis, shown in Table 2. Disease status was most strongly associated with improved OS on both UV and MV analyses (HR 0.45, 95% CI: 0.28–0.74, p = .002; and HR 0.50, 95% CI 0.28–0.88, p = .016, respectively, comparing CR1 to all others), with CR1 patients performing best. Additionally, MRD was significantly associated with better OS compared with MUD on UV (HR 1.64, 95% CI: 1.01–2.67, p = .046) and MV (HR 1.93, 95% CI: 1.07–3.46, p = .029) analyses. Later year of transplant was associated with improved OS on UV analysis (HR 0.53, 95% CI 0.33–0.84, p = .008).
Figure 1.
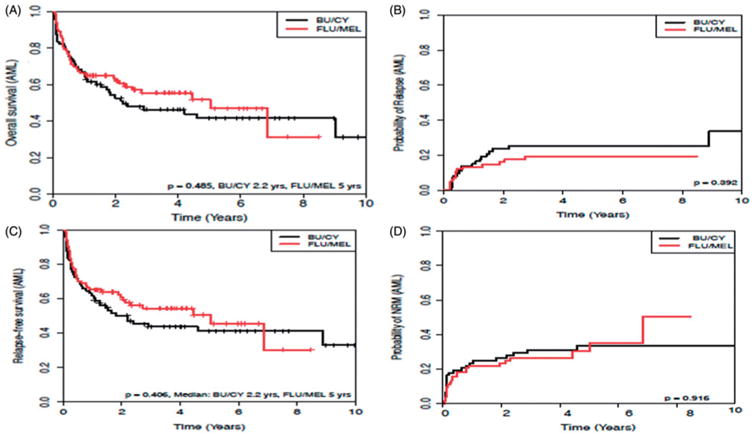
Cumulative incidence of (A) overall survival, (B) relapse rate, (C) relapse-free survival, and (D) non-relapse mortality, of patients with (acute myelogenous leukemia) AML undergoing hematopoietic stem cell transplantation (HSCT). Black line represents Bu/Cy conditioning, red line represents Flu/Mel conditioning. p values as indicated.
Table 2.
Multivariable analysis of overall survival, relapse rate and non-relapse mortality for patients with acute myelogenous leukemia.
| Outcomes | Covariate | Level | Hazard ratio | HR p value | Type3 p value |
|---|---|---|---|---|---|
| Overall survival | Conditioning regimen | BU/CY | 1.30 (0.77–2.20) | .321 | .321 |
| FLU/MEL | – | – | – | ||
| Sex | Male | 1.14 (0.69–1.89) | .615 | .615 | |
| Female | – | – | – | ||
| Status | CR1 | 0.50 (0.28–0.88) | .016 | .050 | |
| CR2 | 0.89 (0.41–1.90) | .754 | |||
| All others | – | – | |||
| Cytogenetic classification | Poor | 2.74 (0.92–8.17) | .070 | .015 | |
| Intermediate | 1.31 (0.46–3.77) | .612 | |||
| Favorable | – | – | |||
| Extramedullary disease | Yes | 0.82 (0.29–2.34) | .707 | .707 | |
| No | – | – | |||
| KPS | High (80+) | 0.61 (0.37–1.00) | .052 | .707 | |
| Low (<80) | – | – | |||
| Transplant type | MUD | 1.93 (1.07–3.46) | .029 | .029 | |
| MRD | – | – | |||
| Relapse rate (competing risks) | Conditioning regimen | BU/CY | 1.78 (0.86–3.69) | .120 | .120 |
| FLU/MEL | – | – | – | ||
| Sex | Male | 0.94 (0.45–1.98) | .870 | .870 | |
| Female | – | – | – | ||
| Status | CR1 | 0.44 (0.20–0.97) | .041 | .119 | |
| CR2 | 0.65 (0.23–1.81) | .412 | |||
| All others | – | – | |||
| Cytogenetic classification | Poor | 1.83 (0.89–3.74) | .099 | .099 | |
| Other | – | – | |||
| Extramedullary disease | Yes | 1.53 (0.43–5.46) | .509 | .509 | |
| No | – | – | |||
| KPS | High (80+) | 0.63 (0.30–1.31) | .215 | .215 | |
| Low (<80) | – | – | |||
| Non-relapse mortality (competing risks) | Conditioning regimen | BU/CY | 2.65 (1.08–6.50) | .033 | .033 |
| FLU/MEL | – | – | – | ||
| Sex | Male | 1.00 (0.48–2.09) | .993 | .993 | |
| Female | – | – | – | ||
| Status | CR1 | 0.83 (0.39–1.75) | .621 | .727 | |
| CR2 | 1.26 (0.43–3.71) | .677 | |||
| All others | – | – | |||
| Cytogenetic classification | Poor | 1.72 (0.85–3.48) | .133 | .133 | |
| Other | – | – | |||
| Extramedullary disease | Yes | 0.53 (0.10–2.77) | .449 | .449 | |
| No | – | – | |||
| KPS | High (80+) | 0.57 (0.29–1.12) | .105 | .105 | |
| Low (<80) | – | – | |||
| Transplant type | MUD | 5.55 (2.37–12.99) | <.001 | <.001 | |
| MRD | – | – | |||
| Age at transplant | 1.07 (1.02–1.11) | .002 | .002 |
FLU: fludarabine; MEL: melphalan; KPS: Karnofsky performance status; MUD: matched unrelated donor; MRD: matched related donor. Bold values represent variables reaching statistical significance with p value < .05.
RR
No significant difference was seen in RR between conditioning regimens (Figure 1). Disease status was significantly associated with RR on UV (HR 0.46, 95% CI: 0.22–0.97, p = .041) and MV analysis (HR 0.44, 95% CI: 0.20–0.97, p = .041, comparing CR1 to all others, Table 2). Additionally, later year of transplant was associated with RR on UV analysis (HR 0.35, 95% CI: 0.16–0.75, p = .007). Of 34 patients who relapsed, 32 (94%) died with a median survival of 10.6 months.
RFS
As shown in Figure 1, there was no difference in RFS across regimens, and no difference in RFS was seen on either UV (HR 1.20, CI: 0.78–1.87, p = .407) or MV analysis (HR 1.30, CI: 0.78–2.15, p = .319). Disease status was associated with RFS on both UV (HR 0.49, CI: 0.30–0.79, p = .003) and MV analyses (HR 0.48 CI: 0.28–0.82, p = .007) with CR1 patients performing the best. On MV but not UV analysis, cytogenetic classification was significantly associated with lower RFS (HR 3.02, 95% CI: 1.03–8.89, p = .045, comparing poor to favorable).
NRM
NRM was similar between conditioning regimens (Figure 1). While NRM was not associated with conditioning regimen on univariate analysis, Bu/Cy was associated with increased NRM (HR 2.65, 95% CI: 1.08–6.50, p = .033) on MV analysis.
There was a higher rate of NRM among patients receiving MUD grafts on both UV (HR 2.43, 95% CI: 1.24–4.76, p = .01) and MV analysis (HR 5.55, 95% CI: 2.37–12.99, p = .001.) Older age transplant was also significantly associated with increased NRM (HR 1.04, 95% CI: 1.02–1.11, p = .002) on MV, but not UV analysis.
Subgroup analysis, chimerism and graft kinetics, graft-versus-host disease, ICU admissions, VOD, and length of stay
When assessing cohorts of patients who survived ≥1, 3, or 5 years, there was no significant difference in OS or PFS (p = .49 and p = .41, respectively) between regimens. There was no significant difference in achievement of full donor chimerism between regimens (82/85 Bu/Cy versus 71/72 Flu/Mel, p = .39); however, there was a significant difference between median days to engraftment (14 d Bu/Cy versus 15 d Flu/Mel, p = .03). There were no differences between regimens on the number of ICU admissions, and conditioning regimen did not predict for length of stay on UV or MV analyses. On both UV and MV analyses, factors associated with increased LOS were: disease status, transplant type, graft source, and year of transplant. Factors associated with increased LOS were disease status, transplant type, graft source, and year of transplant. There was no difference in acute (Bu/Cy 48% versus Flu/Mel 59%; p = .138) or chronic GvHD (Bu/Cy 47.8% versus. Flu/Mel 49.4%, p = .60) incidence. There was a significant difference in acute GvHD severity with a propensity of Flu/Mel patients developing level 3 or 4 reactions (Bu/Cy 11.0% versus Flu/Mel 20.5%, p = .05); however, there was no difference in severity of chronic GvHD (p = .73). Veno-occlusive disease occurred in 13% of patients receiving busulfan-based regimens.
Discussion
For patients with AML who are young and healthy, MAC regimens such as Bu/Cy have been favored over RIC regimens such as Flu/Mel because they may be associated with a lower risk of post-transplant relapse. On one hand, a recent randomized clinical trial, BMT CTN 0901, was closed early due to increased relapse and a trend towards worse overall survival observed among subjects randomized to reduced intensity conditioning [7]. Surprisingly, in our study, relapse rates were not worse with Flu/Mel compared to Bu/Cy despite a clear selection bias whereby patients who received Flu/Mel were significantly older. On the other hand, as may be expected with RIC regimens, our findings demonstrate that Flu/Mel was associated with lower NRM compared to Bu/Cy on multivariate analysis. Our finding of similar OS across conditioning regimens suggest that Flu/Mel remains an appropriate conditioning regimen for older and infirm patients, and may provide similar leukemia control compared to Bu/Cy for AML.
Weaknesses of our study include a relatively small number of patients, and problems inherent in a retrospective analysis such as selection bias. Although Flu/Mel patients were older than the Bu/Cy patients, other unappreciated covariates may have led to selection biases in favor of Flu/Mel. Patients with Flu/Mel were more likely to be treated in later years, and it is possible that advances, such as better supportive care, may have also contributed to improved results for Flu/Mel. Regarding engraftment kinetics our findings demonstrate that the majority of patients achieve full donor chimerism, regardless of regimen. Patients who received busulfan had a rate of VOD similar to previous reports [14].
The shift in preference toward reduced intensity conditioning for middle-aged patients, as well as an upward extension of the eligible age at transplant likely account for the significant differences in age, and year of transplant, between regimens.
Our finding of non-inferior RR and RFS with RIC compared with MAC for AML contrasts with the findings of the Wahid meta-analysis, which showed worse RR and improved RFS with RIC. In a series by Ringden et al, (included in the meta-analysis), RIC was associated with worse RR on MV analysis for the overall group, but in a subset of patients, those ≥50 years of age; relapse rates were not significantly different. There was no difference in RFS across regimens [12]. Aoudjhane et al. demonstrated that RIC was associated with worse RR but not with inferior RFS [8]. The randomized BMT CTN 0901 study also showed worse RR and worse RFS with RIC. This study excluded patients with high comorbidity scores, making the findings less applicable to the infirm [7]. In the study by Shimoni et al., a series in the meta-analysis comparing only chemotherapy-based regimens of RIC and MAC for AML and MDS also showed worse relapse with RIC [6].
The difference in outcomes between our study and others may be due to differences in patient populations or in the specific conditioning regimens studied. For example, in the study by Shimon et al. comparing RIC versus MAC, the RIC regimen was composed of Flu/Bu, not Flu/Mel as in our study [6]. Compared with our study, the BMT CTN 0901 randomized study included a more heterogeneous patient population, including patients with MDS. Additionally MAC regimens included multiple chemotherapy-based conditioning regimens as well as TBIBC, whereas RIC regimens were either Flu/Mel or Bu/Flu [9]. Regarding the Wahib meta-analysis, most studies incorporated TBI in the conditioning regimens, and there were heterogeneous chemotherapy-based conditioning regimens. Consequently, generalizations about specific chemotherapy-based regimens such as Flu/Mel are limited. In contrast, the regimens in our study are relatively homogenous.
Conclusions
In conclusion, our study adds to the evidence that Flu/Mel is an appropriate conditioning regimen for allo-HSCT in the elderly or infirm patient with AML. Our findings suggest that Flu/Mel may be a particularly valuable regimen, as it was associated with reduced NRM, but was not associated with an increase in RR compared with Bu/Cy. These findings suggest that the Flu/Mel regimen should included in future studies of RIC for allo-HSCT for AML.
Acknowledgments
Funding: Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1361027.
References
- 1.McCune JS, Holmberg LA. Busulfan in hematopoietic stem cell transplant setting. Expert Opin Drug Metab Toxicol. 2009;5:957–969. doi: 10.1517/17425250903107764. [DOI] [PubMed] [Google Scholar]
- 2.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 3.Bacigalupo A. Hematopoietic stem cell transplants after reduced intensity conditioning regime (RI-HSCT): report of a workshop of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2000;25:803–805. doi: 10.1038/sj.bmt.1702385. [DOI] [PubMed] [Google Scholar]
- 4.Carella AM, Champlin R, Slavin S, et al. Mini-allografts: ongoing trials in humans. Bone Marrow Transplant. 2000;25:345–350. doi: 10.1038/sj.bmt.1702204. [DOI] [PubMed] [Google Scholar]
- 5.Wahid SFA, Ismail NA, Mohd-Idris MR, et al. Comparison of reduced-intensity and myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia and acute lymphoblastic leukemia: a meta-analysis. Stem Cells Dev. 2014;23:2535–2552. doi: 10.1089/scd.2014.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimoni A, Hardan I, Shem-Tov N, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 7.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–1161. doi: 10.1200/JCO.2016.70.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron F, Labopin M, Peniket A, et al. Reduced-intensity condition in myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromesg with fludarabine and busulfan versus fludarabine and melphalan for patients with acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2015;121:1048–1055. doi: 10.1002/cncr.29163. [DOI] [PubMed] [Google Scholar]
- 9.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122:3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimoni A, Hardan I, Shem-Tov N, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21:2109–2116. doi: 10.1038/sj.leu.2404886. [DOI] [PubMed] [Google Scholar]
- 11.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 12.Therneau T. A package for survival analysis in S version 2.38. 2015 [Google Scholar]
- 13.Gray RJ. cmprsk: Subdistribution analysis of competing risks. 2014 [Google Scholar]
- 14.Nagler A, Labopin M, Shimoni A, et al. Intravenous busulfan-based conditioning prior to allogeneic stem cell transplantation in adults patients with AML – an ALWP-EBMT survey. Blood. 2007;110:1995. [Google Scholar]


