Abstract
Introduction
We evaluated TBS, a non-invasive method to evaluate trabecular bone quality at the lumbar spine, in adults with T1D compared to age-, sex- and BMI-matched adults without diabetes.
Methods
We calculated TBS from adults with T1D (n=47) and controls (n=47) who had a lumbar spine dual x-ray absorptiometry (DXA) at their third visit (2006–2009) of the ongoing “Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study.” The linear relationships of TBS and bone mineral density (BMD) with hemoglobin A1c, blood pressure, lipids and insulin resistance were evaluated using Pearson’s correlation coefficient. Multiple linear regression was used to test the association of TBS with sex and diabetes while adjusting for other potential confounders.
Results
TBS was significantly lower in adults with T1D compared to controls (1.42±0.12 vs 1.44±0.08, p=0.02) after adjusting for age, sex, current smoking status, and lumbar spine BMD, despite no difference in lumbar spine BMD between the groups. Components of the metabolic syndrome, including diastolic blood pressure, BMI, triglycerides and insulin resistance were negatively correlated with TBS among patients with T1D.
Conclusion
Trabecular bone score, an indirect measurement of trabecular bone quality, was lower in adults with T1D compared to controls. Components of metabolic syndrome and insulin resistance were associated with lower TBS in adults with T1D.
Keywords: Type 1 diabetes, Osteoporosis, Fracture, Bone mineral density, trabecular bone score, Insulin resistance and abdominal obesity
Introduction
Improvements in diabetes care have resulted in a reduction in life-threatening complications and increased longevity in people with type 1 diabetes (T1D) [1–2]. Generally, osteoporosis is recognized as a disease of elderly postmenopausal women. However, in T1D, osteoporotic fractures are common in both men and women and increased risk is apparent at a relatively young age (~ 50 years). [3–8]. In a meta-analysis of 14 observational studies with 27,300 subjects with T1D and 4,364,125 subjects without diabetes, we reported a three-fold higher fracture risk in people with T1D compared to people without diabetes [4]. In addition, fracture risk at the spine and hip was higher in both men and women with T1D compared to people without diabetes.
The observed fracture risk is higher than expected based on bone mineral density (BMD) in adults with T1D, suggesting a detrimental effect of diabetes on bone quality [7,8]. Bone histomorphometry and quantitative computed tomography (QCT) are standard research methods to evaluate bone microarchitecture. However, bone biopsy is invasive and QCT is associated with radiation exposure and high cost. Therefore, these tools are not widely used in clinical practice.
Trabecular bone score (TBS) is a non-invasive tool to measure trabecular microarchitecture from the lumbar spine dual x-ray absorptiometry (DXA) image [9]. Trabecular bone is metabolically more active than cortical bone. A population-based study showed that substantial bone loss starts earlier in the trabecular region compared to the cortical region at the lumbar spine, distal radius, and hip [10]. Trabecular bone quality measured by TBS has been shown to predict fracture risk independent of BMD [11,12]. Patients with type 2 diabetes (T2D) have lower TBS despite higher BMD at the lumbar spine [13,14]. In a study of patients with T1D, TBS was lower in those with prevalent fractures [15]. However, the study was limited by recruitment of younger subjects with T1D and shorter duration of diabetes. In addition, the factors affecting trabecular bone quality have not been studied in adults with T1D. Studies in patients with T2D suggest that higher body weight positively influences BMD [16,17]. However, higher insulin resistance is associated with lower BMD and lower bone strength at the femoral neck, suggesting a detrimental role of high fat mass and insulin resistance on bone density and quality [18]. Studies have reported higher insulin resistance in patients with T1D as compared with controls without diabetes [19,20]. However, the effects of body weight and insulin resistance on trabecular bone quality is unknown. The primary objective of the study was to compare TBS between adults with T1D and controls. The secondary objective was to examine the relationships of body mass index, waist circumference, lipids, and insulin resistance with lumbar spine BMD and TBS among adults with T1D.
Methods
Study design
This was a retrospective cross-sectional study of adults with T1D and non-diabetic controls who had a lumbar spine DXA (n=109) at their third visit (2006–2009) of the ongoing “Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study” [21]. The inclusion criteria and patient selection were described in detail previously [21]. T1D was defined as: on insulin therapy within a year of diabetes diagnosis and currently on insulin therapy; diagnosed before age 30 or a clinical course consistent with T1D; and a diabetes duration of 4 years or greater. Adults without diabetes were frequency matched on age, sex, and body mass index (BMI) category as controls. All subjects provided informed consent and the study was approved by the Colorado Multiple Institutional Review Board.
Measures
Current height, weight, and waist circumference (WC, measured at the smallest point between the 10th rib and the iliac crest over the bare skin) were recorded, and BMI (weight/height2; kg/m2) was calculated. Resting systolic blood pressure (SBP) and fifth-phase diastolic blood pressure (DBP) were measured three times while the subjects were seated, and the second and the third measurements were averaged. Hypertension was defined as current SBP ≥140 mmHg or DBP ≥90 mmHg or current antihypertensive therapy. Participants completed a standardized questionnaire including medical history and medication inventory and current and past smoking status as described previously [21–23].
After an overnight fast, blood was collected and centrifuged, and separated plasma was stored at 4°C until assayed. Total cholesterol and triglyceride levels were measured using standard enzymatic methods. High density lipoprotein cholesterol (HDL-C) was separated using dextran sulfate, and low density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald formula. High-performance liquid chromatography was used to measure glycosylated hemoglobin (HbA1c) (HPLC; BioRad variant).
Dual X-ray absorptiometry (DXA, Hologic Discovery W) scans were performed for body composition and fat-free mass (FFM) and lumbar spine BMD just before the clamp study. All subjects underwent screening questions such as recent radiocontrast administration, implants or devices in measurement area before BMD testing. A single well-trained technician performed BMD at the lumbar spine per the guidelines of the International Society for Clinical Densitometry (ISCD) [24]. The coefficient of variation for total hip BMD, lumbar spine BMD, whole body fat mass and lean mass was 1.7%, 4.0%, 1.5% and 0.4%, respectively.
Trabecular bone score was measured at the lumbar spine using TBS iNsight software version 2.2.0.0 (TBS iNsight; Medimaps, Switzerland) per manufacturer instructions. TBS was calculated as the mean value of the individual measurements for vertebrae L1–L4, based on gray-level analysis of DXA images. All the DXA scans were reviewed by three authors (VNS, RS, JKS) for the accuracy of L1–L4 selection, scoliosis, spinal deformity, and any fractured and/or fused vertebrae before computation of TBS. Of the 109 subjects who had lumbar spine DXA done in the CACTI study, 15 subjects were excluded from the study due to inability to obtain TBS or to spinal pathologies.
Subjects (n=94) also underwent a hyperinsulemic-euglycemic clamp for measurement of glucose infusion rate (a measure of insulin sensitivity) as described previously [21]. In brief, subjects were admitted to the inpatient clinical research unit before dinner the evening before their study. Subjects with T1D were instructed to take their last long-acting insulin injections at least 12 hours before admission. Dinner was provided on the unit and subjects then fasted overnight and through the clamp. Subjects with T1D bolused for dinner per their usual regimen and were transitioned 3 hours later to a continuous insulin infusion overnight to optimize glycemic control with short-acting insulin. After a baseline blood sample was collected for insulin, glucose, and C-peptide measurement, a primed continuous infusion of insulin was administered at 4, 8, and then 40 mU/m2/min for 1.5 hours each. A variable infusion of 20% dextrose was infused to maintain blood glucose of 90 mg/dl. Arterialized blood was sampled every 5 min for bedside determination of glucose concentration (Analox, Lunenberg, MA) and the dextrose infusion adjusted as necessary. A hyperinsulemic-euglycemic steady state was achieved during the last 30 minutes of the high insulin infusion stage and mean glucose infusion rate ([GIR], mg/kg fat free mass/min) during this time was used as the measure of whole body insulin sensitivity. For example; lower the GIR, higher is the insulin resistance.
Statistical analysis
Descriptive statistics presented are mean ± standard deviation (SD), counts and frequencies. Variables were tested for normality using the Shapiro-Wilk test. Non-normally distributed (TBS, triglycerides, HDL-C, waist circumference, lean mass, fat mass, GIR and TBS) were log transformed before analysis. Continuous variables were compared using unpaired t-tests. Pearson’s correlation coefficient was used to evaluate the linear relationships of TBS and BMD with other clinical variables. Multiple linear regression was used to test the association of TBS with sex and diabetes while adjusting for other potential confounders. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC). All tests performed were two-sided and a p-value <0.05 was considered statistically significant.
Results
A total of 47 adults with T1D and 47 non-diabetic controls were included in this study. Characteristics of the study population are shown in Table 1 by diabetes status. Since there are well-recognized gender differences in lipids, waist circumference, fat and lean mass, insulin resistance and bone density, the differences in these variables by diabetes status and sex is provided in Supplementary Table 1.
Table 1.
Baseline characteristics of the participants with T1D and controls without diabetes
| Variables | Type 1 diabetes (n=47) | Controls without diabetes (n=47) |
|---|---|---|
| Age (years) | 43.4±8.7 | 44.7±6.9 |
| Duration of diabetes (years) | 28.7±7.5 | NA |
| HbA1c (%) | 7.7±1.0 | 5.4±0.3 |
| Insulin dose (units/kg/day) | 0.6±0.2 | NA |
| BMI ( kg/m2) | 26.1±4.0 | 25.7±4.1 |
| Waist circumference (cm) | 87.1±12.6 | 85.8±12.8 |
| Total fat mass (kg) | 21.8±8.2 | 22.6±7.9 |
| Lean body mass (kg) | 54.8±12.1 | 51.3±13.1 |
| GIR (mg/kg FFM/min) | 5.7±3.7 | 13.2±5.9 |
| SBP (mmHg) | 113.5±10.4 | 113.0±11.8 |
| DBP (mmHg) | 76.1±7.5 | 76.1±7.9 |
| Total Cholesterol (mg/dl) | 158.1±31.5 | 189.1±30.2 |
| HDL-C (mg/dl) | ||
| LDL-C (mg/dl) | 84.4±28.5 | 110.0±28.1 |
| Triglyceride (mg/dl) | ||
| Total hip BMD (g/cm2) | 0.99±0.14 | 0.97±0.15 |
| Lumbar spine BMD L1–L4 (g/cm2) |
1.04±0.15 | 1.02±0.14 |
| TBS | 1.42±0.12 | 1.44±0.08 |
[Statistics are mean ± SD unless specified. T1D; type 1 diabetes, HbA1c; glycated hemoglobin A1c, BMI; body mass index, GIR; glucose infusion rate, FFM; fat free mass, DBP, diastolic blood pressure, SBP; systolic blood pressure, HDL; high density lipoprotein cholesterol, LDL; low density lipoprotein cholesterol, BMD; bone mineral density]
There were no differences in BMI, WC, systolic or diastolic blood pressure between adults with and without diabetes within each sex [Table 1]. Only eight adults with T1D had some form of microvascular complications (nephropathy, proliferative retinopathy and/or diabetic neuropathy). The frequency of current smoking did not differ by diabetes status among either men or women. Statin use was more common among participants with T1D for both men and women. Total cholesterol, LDL-C, and triglyceride levels were lower in adults with T1D compared to controls, and GIR was significantly lower among T1D participants in both men and women. In a sensitivity analysis excluding participants on statin therapy, adults with T1D still had significantly lower total cholesterol (p=0.003) and LDL-C (p=0.01), triglycerides (p=0.008) and GIR (p=0.003).
Correlations between clinical measures and both TBS and lumbar spine BMD are shown in Table 2, by diabetes status. HbA1c was not correlated with either TBS or lumbar spine BMD in either group, but among participants with T1D, a higher insulin dose was significantly correlated with lower TBS and lower lumbar spine BMD. Components of the metabolic syndrome such as diastolic blood pressure, BMI,, and triglycerides were all negatively correlated with TBS but not with lumbar spine BMD among adults with T1D. WC and triglycerides were negatively correlated with TBS in non-diabetics. GIR, a measure of skeletal muscle insulin sensitivity, was positively correlated with TBS in participants with and without T1D.; insulin resistance (low GIR) was associated with lower TBS in participants irrespective of diabetes status. BMI was the only factor associated with lumbar spine BMD, and was positively correlated among non-diabetic participants.
Table 2.
Correlations of trabecular bone score with clinical markers by diabetes status
| Participants with T1D | Non-diabetic controls | |||
|---|---|---|---|---|
| LogTBS | Lumbar BMD | Log TBS | Lumbar BMD | |
| HbA1c | 0.001 | 0.1 | 0.05 | −0.05 |
| Insulin dose | −0.4* | −0.3* | - | - |
| DBP | −0.3* | −0.06 | −0.2 | 0.01 |
| SBP | −0.2 | 0.1 | −0.3 | −0.03 |
| BMI | −0.3* | 0.1 | −0.2 | 0.3* |
| WC | −0.6 | −0.0 | −0.4* | 0.2 |
| Total Cholesterol | −0.05 | −0.02 | −0.2 | −0.08 |
| Log LDL-C | −0.1 | 0.1 | −0.2 | 0.01 |
| Log HDL-C | 0.2 | −0.1 | 0.3* | −0.03 |
| Log Triglyceride | −0.4* | −0.1 | −0.5* | −0.1 |
| Log GIR | 0.3* | 0.1 | 0.4* | 0.2 |
p<0.05
[T1D; type 1 diabetes, HbA1c; glycated hemoglobin A1c, TBS; trabecular bone score, WC; waist circumference, BMI; body mass index, GIR; glucose infusion rate, DBP, diastolic blood pressure, SBP; systolic blood pressure, HDL; high density lipoprotein cholesterol, LDL; low density lipoprotein cholesterol, BMD; bone mineral density]
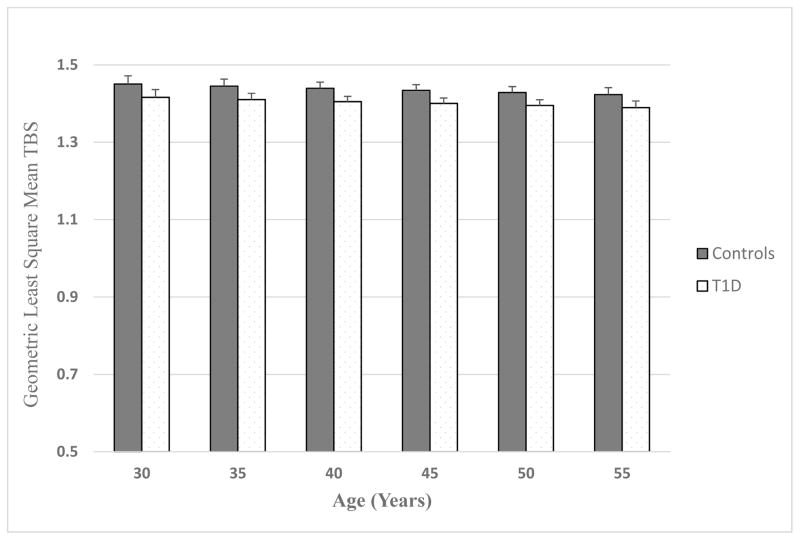
As shown in Table 1, there were no differences in BMD at the lumbar spine in adults with T1D and controls. In multiple linear regression, TBS at the lumbar spine was significantly lower in adults with T1D compared to controls (1.4±0.12 vs 1.44±0.08, p=0.02) after adjusting for age, sex, current smoking status, and lumbar spine BMD. TBS, though within normal range, was lower in adults with T1D compared to controls at any age, even in T1D patients as young as 30 years of age [Figure 1]. In this analysis, men had a lower TBS than women (p<0.0001) when adjusted for age, diabetes status, smoking and lumbar spine BMD. Smoking status (p=0.44) and age (p=0.11) were not significantly associated with TBS, but higher BMD at the lumbar spine was significantly associated with higher TBS (p<0.0001).
Figure 1.
Geometric Least Square Mean Trabecular Bone Score at Lumbar Spine in Adults with T1D and Controls
Shown in Table 3 are least-squares (LS) means for TBS by diabetes status in the multiple linear regression model adjusted for age, sex, smoking status and lumbar spine BMD, and then in subsequent models adjusted for each of the clinical factors correlated with TBS individually. TBS remained significantly lower in adults with T1D even when further adjusted for BMI, WC, HDL-C, triglycerides, systolic and diastolic blood pressure, but was attenuated and no longer significantly different by diabetes status when adjusted for GIR.
Table 3.
Least square mean TBS and 95% CI by diabetes status in multivariable linear regression models
| T1D | Controls | p-value | |
|---|---|---|---|
| Model 1: age, sex, smoking status, lumbar BMD | 1.40 (1.38–1.43) | 1.44 (1.41–1.47) | 0.0394 |
| Model 1 + BMI | 1.40 (1.38–1.43) | 1.44 (1.41–1.46) | 0.0295 |
| Model 1 + WC | 1.40 (1.38–1.43) | 1.44 (1.41–1.46) | 0.0106 |
| Model 1 + HDL | 1.40 (1.38–1.43) | 1.44 (1.41–1.47) | 0.0198 |
| Model 1 + Triglycerides | 1.40 (1.37–1.43) | 1.45 (1.42–1.48) | 0.0048 |
| Model 1 + SBP | 1.41 (1.38–1.43) | 1.44 (1.41–1.47) | 0.0354 |
| Model 1 + DBP | 1.41 (1.38–1.44) | 1.44 (1.41–1.48) | 0.0297 |
| Model 1 + GIR | 1.41 (1.37–1.44) | 1.43 (1.40–1.47) | 0.2090 |
[T1D; type 1 diabetes, HbA1c; glycated hemoglobin A1c, TBS; trabecular bone score, BMI; body mass index, WC; waist circumference, GIR; glucose infusion rate, DBP, diastolic blood pressure, SBP; systolic blood pressure, HDL; high density lipoprotein cholesterol, LDL; low density lipoprotein cholesterol, BMD; bone mineral density]
Discussion
Our study showed that TBS, an indirect measure of trabecular bone quality, at lumbar spine was lower in adults with T1D compared to age-, BMI- and sex-matched subjects without diabetes, despite similar lumbar spine BMD. BMI, triglyceride levels and diastolic blood pressure were associated with lower TBS among adults with T1D. Insulin resistance was independently associated with lower TBS in adults with and without T1D, and adjustment for insulin resistance as measured using a hyperinsulinemic euglycemic clamp study attenuated the difference in TBS by diabetes status.
In this study, we did not find differences in lumbar spine or total hip BMD between adults with T1D and controls. This is in agreement with a recent meta-analysis reporting no differences in lumbar spine BMD between T1D and controls, after adjusting for age, sex and DXA instrument [8]. Similarly, higher BMD does not protect patients with type 2 diabetes from osteoporotic fractures [7]. Mechanisms associated with skeletal fragility in diabetes are therefore recognized as not directly associated with bone loss but rather with impaired bone quality. Studies in patients with T2D consistently showed lower TBS compared to controls and TBS adjusted FRAX improved fracture prediction in this population [13,14]. In a study by Neumann et al, there were no differences in TBS between adults with T1D and controls; however, TBS was lower in patients with T1D with a prior history of fractures [15]. The fact that participants with T1D in our study had longer duration of diabetes compared to the study by Neumann et al [15] may explain the differences in our results
Little is known about factors affecting bone quality in patients with diabetes. It is generally accepted that obesity has a protective effect on bone tissue [25]. However, many studies have shown higher fractures among obese patients [26]. The relationship between obesity and osteoporosis varies depending on how obesity is defined. Obesity defined on the basis of BMI or body weight appears to be a protective factor against bone mineral loss or vertebral fractures. However, obesity based on the percentage body fat may be a risk factor for osteoporosis [27]. Our study did show a positive relation between BMI as a measure of obesity and lumbar spine BMD in adults without diabetes; however, BMI was negatively related with TBS. This suggests that excess overall and central adiposity affects bone quality at the lumbar spine adversely despite normal BMD, whereas mechanical loading by higher weight may explain the positive association between BMI and BMD.
In our study, adults with T1D were more insulin resistant than controls as measured using the gold standard clamp technique, and greater insulin resistance was associated with lower TBS. Similar to previous studies [19,20], our study highlights that T1D is a highly insulin resistant state. Insulin resistance in patients with T1D is one of the potential explanations for compromised bone quality in patients with T1D.
Abdominal obesity is associated with higher triglyceride levels and insulin resistance [28]. In our study, diastolic blood pressure and triglyceride levels were negatively associated with TBS among adults with T1D. The findings from our study provide further evidence that abdominal obesity and related metabolic consequences are associated with compromised bone quality despite normal BMD at the lumbar spine. Abdominal obesity is associated with higher inflammatory markers such as IL-6 and TNF-α [29] that might result in increased bone resorption from the trabecular structure in the spine resulting in lower TBS.
To our knowledge, this was the first study to evaluate the effects of central obesity and insulin resistance on trabecular bone quality at the lumbar spine in adults with T1D. The well-characterized cohort of adults with long-standing T1D and non-diabetic controls with similar levels of obesity from the ongoing CACTI study was a major strength of this study. Small sample size, relatively young age of subjects, variable duration of diabetes, and single time point measurement of insulin resistance were some of the limitations of the study. In addition, participants with T1D were well controlled and only a small number of participants with T1D (n=8) had microvascular complications, limiting the generalization of our findings.
In conclusion, our study showed that trabecular bone score, an indirect measure of trabecular bone quality at the lumbar spine was lower in adults with T1D compared to non-diabetic controls after adjusting for age, sex, smoking status, and lumbar spine BMD. Components of metabolic syndrome such as body weight, triglyceride levels, diastolic blood pressure and insulin resistance were associated with lower TBS in adults with T1D. Further studies are needed to clarify the relationship between the components of metabolic syndrome, insulin resistance and trabecular bone quality.
Supplementary Material
Baseline characteristics of participants by diabetes status and gender.
Summary.
We evaluated trabecular bone score (TBS) and factors affecting TBS in adults with type 1 diabetes (T1D) compared to age, sex and body mass index (BMI) matched adults without diabetes. Adults with T1D had lower TBS compared to controls. Abdominal obesity and insulin resistance are associated with lower TBS.
Acknowledgments
The study was supported from NHLBI grants HL61753, HL79611, and HL113029, DERC Clinical Investigation Core P30 DK57516, JDRF grant 17-2013-313, American Diabetes Association Career Development Award to Dr. Snell-Bergeon (7-13-CD-50) and a Center for Women’ Health Research Seed Grant to Dr. Shah. The study was performed at the Adult CTRC at UCD supported by NIH-M01-RR00051 and CTSA Grant UL1 TR001082 (IES), at the Barbara Davis Center for Childhood Diabetes and at Colorado Heart Imaging Center in Denver, CO.
Footnotes
Conflict of Interest: VNS, RS, PJ, LP, PJ, WMK, IES and JKS declares no conflict of interest related to this work. JKS is the guarantor of this work.
References
- 1.Pambianco G, Costacou T, Ellis D, et al. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 2.Harding JL, Shaw JE, Peeters A, et al. Mortality trends among people with type 1 and type 2 diabetes in Australia: 1997–2010. Diabetes Care. 2014;37:2579–86. doi: 10.2337/dc14-0096. [DOI] [PubMed] [Google Scholar]
- 3.Dhaon P, Shah VN. Type 1 diabetes and osteoporosis: A review of literature. Indian J Endocrinol Metab. 2014;18:159–65. doi: 10.4103/2230-8210.129105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah VN, Shah CS, Snell-Bergeon JK. Risk for fracture in type 1 diabetes: A meta-analysis and review of the literature. Diabet Med. 2015;32:1134–42. doi: 10.1111/dme.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hothersall EJ, Livingstone SJ, Looker HC, et al. Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res. 2014;29:1054–60. doi: 10.1002/jbmr.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN) Diabetes Care. 2015;38:1913–20. doi: 10.2337/dc15-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 8.Shah VN, Harrall KK, Shah CS, Gallo TL, Joshee P, Snell-Bergeon JK, Kohrt WM. Bone mineral density at femoral neck and lumbar spine in adults with type 1 diabetes: a meta-analysis and review of the literature. Osteoporos Int. 2017 Jun 3; doi: 10.1007/s00198-017-4097-x. [DOI] [PubMed] [Google Scholar]
- 9.Harvey NC, Glüer CC, Binkley N, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–24. doi: 10.1016/j.bone.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riggs BL, Melton LJ, Robb RA, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–14. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture Risk Prediction by Non-BMD DXA Measures: the 2015 ISCD Official Positions Part 2: Trabecular Bone Score. J Clin Densitom. 2015;18:309–30. doi: 10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Schacter GI, Leslie WD. DXA-Based Measurements in Diabetes: Can They Predict Fracture Risk? Calcif Tissue Int. 2017;100:150–164. doi: 10.1007/s00223-016-0191-x. [DOI] [PubMed] [Google Scholar]
- 13.Choi YJ, Ock SY, Chung YS. Trabecular Bone Score (TBS) and TBS-Adjusted Fracture Risk Assessment Tool are Potential Supplementary Tools for the Discrimination of Morphometric Vertebral Fractures in Postmenopausal Women With Type 2 Diabetes. J Clin Densitom. 2016;19:507–514. doi: 10.1016/j.jocd.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014;25:1969–73. doi: 10.1007/s00198-014-2704-7. [DOI] [PubMed] [Google Scholar]
- 15.Neumann T, Lodes S, Kästner B, Lehmann T, Hans D, Lamy O, Müller UA, Wolf G, Sämann A. Trabecular bone score in type 1 diabetes-a cross-sectional study. Osteoporos Int. 2016;27:127–33. doi: 10.1007/s00198-015-3222-y. [DOI] [PubMed] [Google Scholar]
- 16.Kim K-C, Shin D-H, Lee S-Y, Im J-A, Lee D-C. Relation between Obesity and Bone Mineral Density and Vertebral Fractures in Korean Postmenopausal Women. Yonsei Medical Journal. 2010;51:857–863. doi: 10.3349/ymj.2010.51.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007;18:1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- 18.Srikanthan P, Crandall CJ, Miller-Martinez D, Seeman TE, Greendale GA, Binkley N, Karlamangla AS. Insulin resistance and bone strength: findings from the study of midlife in the United States. J Bone Miner Res. 2014;29:796–803. doi: 10.1002/jbmr.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cree-Green M, Newcomer BR, Brown MS, et al. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. 2015;64:383–392. doi: 10.2337/db14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira RI, Snell-Bergeon JK, Erickson C, Schauer IE, Bergman BC, Rewers M, Maahs DM. Adiponectin dysregulation and insulin resistance in type 1 diabetes. J Clin Endocrinol Metab. 2012;97(4):E642–7. doi: 10.1210/jc.2011-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60:306–14. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, et al. Coronary Artery Calcification in Type 1 Diabetes Study. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–9. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 23.Snell-Bergeon JK, Hokanson JE, Jensen L, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care. 2003;26:2923–8. doi: 10.2337/diacare.26.10.2923. [DOI] [PubMed] [Google Scholar]
- 24.Lewiecki EM, Gordon CM, Baim S, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone. 2008;43:1115–21. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 25.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31:547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 26.Gonnelli S, Caffarelli C, Nuti R. Obesity and fracture risk. Clin Cases Miner Bone Metab. 2014;11:9–14. doi: 10.11138/ccmbm/2014.11.1.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y-h, Xu Y, Wen Y-b, Guan K, Ling W-h, He L-p, et al. Association of Weight-Adjusted Body Fat and Fat Distribution with Bone Mineral Density in Middle-Aged Chinese Adults: A Cross-Sectional Study. PLoS ONE. 2013;8(5):e63339. doi: 10.1371/journal.pone.0063339. https://doi.org/10.1371/journal.pone.0063339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Specht BJ, Wadwa RP, Snell-Bergeon JK, Nadeau KJ, Bishop FK, Maahs DM. Estimated insulin sensitivity and cardiovascular disease risk factors in adolescents with and without type 1 diabetes. J Pediatr. 2013;162:297–301. doi: 10.1016/j.jpeds.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt FM, Weschenfelder J, Sander C, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One. 2015;10:e0121971. doi: 10.1371/journal.pone.0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of participants by diabetes status and gender.