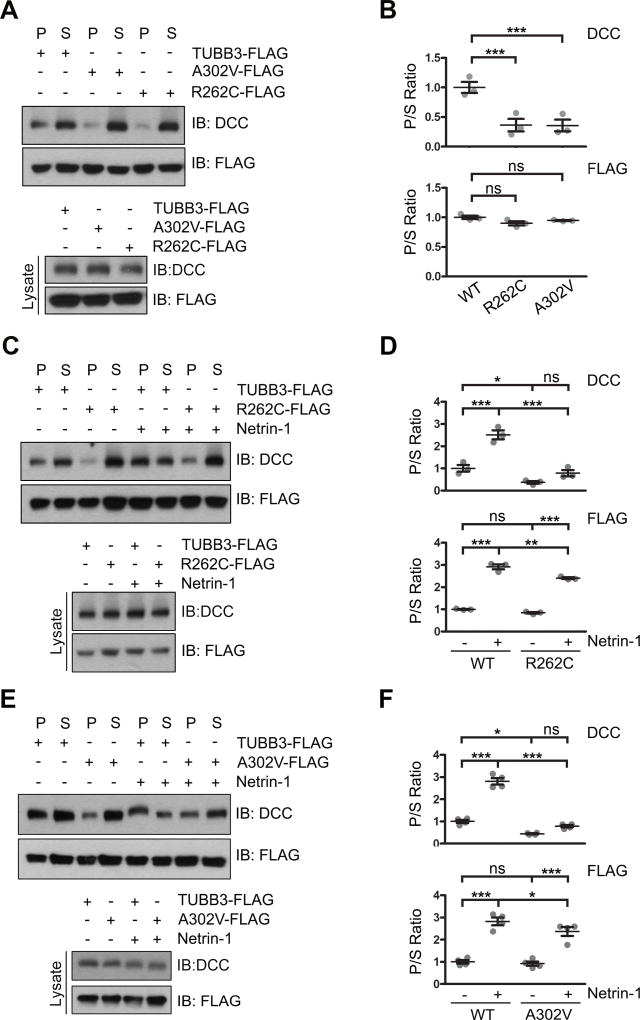
Figure 4.
TUBB3 mutations decrease the interaction of DCC with polymerized TUBB3 mutants. A. DCC was cotransfected with wild type human TUBB3, R262C or A302V into HeLa cells and a cosedimentation assay was performed. DCC and TUBB3 proteins in the pellet (P) and supernatant (S) fractions were analyzed by immunoblotting using anti-DCC and anti-FLAG antibodies, respectively. B. Quantification of P/S ratio of DCC and TUBB3 in A (three independent experiments). *** indicates p<0.0001, ns indicates no significant difference (one-way Anova and Tukey’s test for post-hoc comparisons). C-F. TUBB3 shRNAs were co-transfected with wild type TUBB3 (C–F), R262C (C and D) or A302V (E and F) into mouse E15 cortical neurons. Primary neurons were stimulated with purified netrin-1 or sham-purified control. The co-sedimentation assay of cell lysates was conducted with taxol to stabilize MTs in vitro. DCC and TUBB3-FLAG in the P and S fractions were examined by Western blotting. D and F are quantification of P/S ratio of DCC and TUBB3 in C and E, respectively. Data are mean ± s.e.m from three separate experiments. *p<0.05, **p<0.01, ***p<0.001, ns indicates no significant difference (one-way Anova and Tukey’s test for post-hoc comparisons). WT, wild type human TUBB3-FLAG.