Abstract
Introduction
The Ebola virus (EBOV) disease epidemic during 2014-16 in West Africa has accelerated the clinical development of several vaccine candidates that have demonstrated efficacy in the gold standard nonhuman primate (NHP) model, namely cynomolgus macaques.
Areas covered
This review discusses the pre-clinical research and if available, clinical evaluation of the currently available EBOV vaccine candidates, while emphasizing the translatability of pre-clinical data generated in the NHP model to clinical data in humans.
Expert opinion
Despite the existence of many successful EBOV vaccine candidates in the pre-clinical stages, only two platforms became the focus of Phase 2/3 efficacy trials in Liberia, Sierra Leone, and Guinea near the peak of the epidemic: the Vesicular stomatitis virus (VSV)-vectored vaccine and the chimpanzee adenovirus type 3 (ChAd3)-vectored vaccine. The results of three distinct clinical trials involving these candidates may soon pave the way for a licensed, safe and efficacious EBOV vaccine to help combat future epidemics.
Keywords: Clinical trials, Ebola virus, Nonhuman primates, Vaccines
1. Introduction
The Zaire ebolavirus species contain one member virus: Ebola virus (EBOV), which causes a severe, acute and often-fatal hemorrhagic fever in humans and non-human primates (NHPs). The virus has an incubation period of 2 to 21 days [1], and patients initially present non-specific, flu-like symptoms such as: sudden onset of high fever (39 – 40°C), headaches, muscle and joint pain in addition to general fatigue and malaise, lasting typically between 2 to 4 days [2]. The disease then progresses to more severe symptoms and manifestations including gastrointestinal, respiratory, vascular and neurological distress [1]. A maculopapular rash accompanied by erythema, petechiae or ecchymoses appears between 5 to 7 days, and uncontrolled bleeding may be observed from puncture sites and the mucosa [1]. Up to 90% of cases progress to terminal disease, which is characterized by severe metabolic imbalances, anuria, convulsions, and hypovolemic shock. Death occurs approximately 2 to 3 days afterwards due to multi-organ failure from massive tissue injury, as well as capillary extravasation from vascular permeability and diffuse coagulopathy [2]. Survivors undergo a prolonged convalescent phase including intense fatigue, loss of appetite, weight and memory loss, and migratory joint pains [2]. Sequelae from the disease are both physical and psychological in nature, and may include orchitis, myelitis, recurrent hepatitis, uveitis, as well as psychosis [1]. Shedding of live EBOV in bodily fluids, such as semen, is detectable for up to three months, and perhaps longer, after the onset of disease [3] [4].
Health authorities have been aware of EBOV since 1976, after the first outbreak killed 280 of 318 cases in what is presently the Democratic Republic of the Congo (DRC) [5]. There are few studies on EBOV epidemiology because the reservoir species is unknown; however a past report has implicated fruit bats as a probable host for EBOV [6]. Sporadic outbreaks have since occurred in the remote and humid rainforests of Gabon, the Republic of Congo and the DRC. Up until the end of 2013, 1,093 people had perished from 1,393 total cases, for an aggregate case fatality rate (CFR) of 78%. EBOV was therefore regarded as a minor public health concern localized to central Africa until March 25, 2014, when the World Health Organization (WHO) reported an ongoing EBOV epidemic in the western African nation of Guinea, with additional suspect cases in neighbouring Liberia and Sierra Leone [7].
The index case of the recent 2014-2016 EBOV epidemic is hypothesized to be a 2-year old boy who had died in Meliandou, South-eastern Guinea during December 2013 [8]. The virus quickly spread throughout Guinea, Liberia and Sierra Leone, reaching densely-populated capital cities of all three countries. By the end of 2014, six other countries (Mali, Nigeria, Senegal, Spain, the UK, and the USA) have been impacted by this epidemic, either through imported cases by air or road travel [9] [10] [11] [12], or local nurses who were exposed to EBOV while providing primary care to repatriated patients [13] [14]. The number of fatalities was eventually reported to be 11,310 out of 28,616 total cases (a CFR of 40%) [15], but the numbers are almost certainly underestimated, as difficulties in reporting the epidemiological data accurately has led some to believe that the actual toll from the epidemic is approximately 2.5 times the number of cases currently being reported by the WHO [16]. The response was hampered by the sizable number of healthcare workers who were infected by EBOV in the line of duty, with a toll standing at 512 fatalities out of 881 cases (a CFR of 58%) across Guinea, Liberia and Sierra Leone [17]. Furthermore, while the main mode of EBOV transmission occurs via direct contact with an infectious host, there are several studies that suggest that EBOV transmission may occasionally occur through direct contact with infectious material, such as bodily fluids, from an infected individual (reviewed in [18]) and the knowledge behind the mechanics of EBOV transmission is currently incomplete. The lack of experimental data on the epidemiology and transmission of EBOV indicates that it will be difficult to predict when and where the next EBOV outbreak or epidemic may take place. Thus, the licensure of an efficacious vaccine against EBOV is timely since immunization constitutes one of the best lines of defense against such an unpredictable pathogen.
To date, multiple vaccine platforms have demonstrated efficacy in NHPs, including: 1) DNA vaccines, 2) human adenovirus serotype 5 (Ad5) vectors, 3) human adenovirus serotypes 26 (Ad26) or 35 (Ad35) vectors, 4) chimpanzee adenovirus type 3 (ChAd3) vectors, 5) vesicular stomatitis virus (VSV) vectors, 6) EBOV virus like particles (VLPs), 7) human parainfluenza virus type 3 (HPIV3) vectors, 8) Venezuelan equine encephalitis virus (VEEV) vectors, 9) rabies virus (RABV) vectors, 10) replication-deficient EBOV lacking the VP30 gene (EBOVΔVP30), and 11) cytomegalovirus vectors (CMV, all vaccines summarized in Figure 1 and Table 1). The goal of this review is to discuss the pre-clinical and/or clinical evaluation of the EBOV vaccine candidates mentioned above, while emphasizing the translatability of pre-clinical data generated in the gold standard NHP model to clinical data in humans.
Figure 1. Structure and design of vaccine vectors against EBOV.
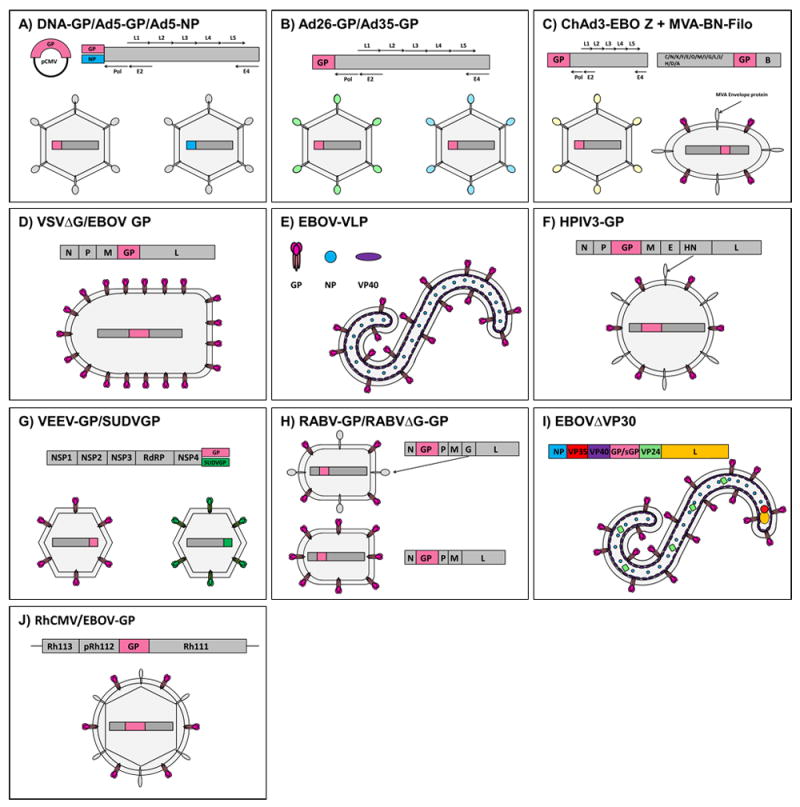
A) DNA- or Ad5-vectored vaccines, B) Ad26/Ad35-vectored vaccines, C) ChAd3-vectored vaccines with MVA-vectored boost, D) VSV-vectored vaccines, E) EBOV-VLPs, F) HPIV3-vectored vaccines, G) VEEV-vectored vaccines, H) RABV-vectored vaccines, I) EBOVΔVP30 vaccine and J) CMV-vectored vaccine.
Table 1.
Summary of various candidate vaccines against EBOV.
| Vaccine candidate | Dosage | Dose Regimens | Efficacy in NHPs | Rapid immunity in NHPs | Long-term immunity in NHPs | Pre-existing immunity concerns | Safety and immunogeni city in human trials | Testing in human efficacy trials |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| DNA-GP prime, Ad5-EBOV boost | 100 μg DNA IM, 1010 PFU Ad5, IM | 3 doses of DNA at 32, 28 and 24 weeks and 1 dose of Ad5 at 12 weeks before challenge | 4/4 (100%) | No | Not investigated | Yes a | N/A | N/A |
|
| ||||||||
| Ad5-NP and Ad5-EBOV | 1 × 1012 viral particles Ad5-NP and 1 × 1012 viral particles Ad5-EBOV combined, IM | 1 dose at 4 weeks before challenge | 4/4 (100%) | No | Not investigated | Yes | N/A | N/A |
|
| ||||||||
| Ad5-EBOV | 1 × 1010 viral particles, IM | 1 dose at 4 weeks before challenge | 3/3 (100%) | Yes d | Yes e | Yes c | Phase 1: Safe and immunogenic | N/A |
|
| ||||||||
| Ad5-MakGP | 4 × 1010 viral particles, IM | 1 dose at 4 weeks before challenge | 3/3 (100%) | No | Not investigated | Yes | Phase 1: Safe and immunogenic | N/A |
|
| ||||||||
| Ad26.ZEBO V | 1 × 1011 viral particles, IM | 1 dose at 4 weeks before challenge | 2/4 (50%) | No | No | No | Phase 1: Safe and Immunogenic when provided with MVA-BN-Filo boost | N/A |
|
| ||||||||
| Ad26.ZEBO V/Ad35-GP | 1 × 1011 viral particles Ad26, 1 × 1011 viral particles Ad35, IM | 1 dose Ad26 at 8 weeks and 1 dose Ad35 at 4 weeks before challenge | 4/4 (100%) | No | Not investigated | No | N/A | N/A |
|
| ||||||||
| ChAd3-EBO Z | 1 × 1011 viral particles, IM | 1 dose at 5 weeks before challenge | 2/4 (50%) | No | No | No | Phase 1: Safe and immunogenic | PREVAIL Phase 2/3: Phase 2 results pending, Phase 3 study halted |
|
| ||||||||
| ChAd3-EBO Z + MVA- BN-Filo | 1 × 1011 viral particles ChAd3, 1 × 108 PFU MVA, IM | 1 dose ChAd3 at 10 months and 1 dose of MVA at 8 months before challenge | 4/4 (100%) | No | Yes | No | Phase 1: Safe and immunogenic | N/A |
|
| ||||||||
| VSVΔG/EBO VGP | 1 × 107 PFU, IM | 1 dose at 4 weeks before challenge | 4/4 (100%) | Yes f | Yes g | No | Phase 1: Safe and immunogenic [6,7] | PREVAIL Phase 2/3: Phase 2 results pending, Phase 3 study halted |
| 5 × 107 PFU, IM | 1 dose at 28, 21, 14, 7 and 3 days before challenge | 3/3 (100%) in the 28, 21, 14, and 7 days group. 2/3 (67%) in the 3 days group. | STRIVE Phase 2/3: No infections or deaths reported amongst vaccinated individuals | |||||
| Ebola ca suffit! Phase 2/3: 100% efficacy | ||||||||
|
| ||||||||
| EBOV VLPs | 250 μg IM | 3 doses at 16, 10 and 4 weeks before challenge | 5/5 (100%) | No | Not investigated | No | N/A | N/A |
|
| ||||||||
| HPIV3-EbovZ GP | 2 × 107 TCID50, IN/IT | 2 doses at 14 and 10 weeks before challenge | 3/3 (100%) | No | Not investigated | Yes h | Phase 1: Results pending | N/A |
|
| ||||||||
| VEEV-SUDVGP and VEEV-GP | 1 × 1010 FFU of VEEV-SUDVGP and 1010 FFU VEEV-EBOVGP, IM | 1 dose per vaccine per leg at 4 weeks before challenge | 3/3 (100%) | No | Not investigated | No | N/A | N/A |
|
| ||||||||
| RABV-GP (live-attenuated) | 5 × 107 FFU, IM | 1 dose at 70 days before challenge | 4/4 (100%) | No | Not investigated | No | N/A | N/A |
|
| ||||||||
| RABV-GP (inactivated) | 250 μg, IM | 2 dose at 70 and 42 days before challenge | 2/4 (50%) | No | No | No | N/A | N/A |
|
| ||||||||
| RABVΔG-GP | 1 × 107 FFU, IM | 1 dose at 70 days before challenge | 2/4 (50%) | No | No | No | N/A | N/A |
|
| ||||||||
| EBOVΔVP30 | 1 × 107 FFU, IM | 2 doses at 56 and 28 days before challenge | 4/4 (100%) | No | Not investigated | No | N/A | N/A |
|
| ||||||||
| EBOVΔVP30 (H2O2-inactivated) | 1 × 107 FFU, IM | 2 doses at 56 and 28 days before challenge | 2/2 (100%) | No | Not investigated | No | N/A | N/A |
|
| ||||||||
| rhCMV/EBO V-GP | 1 × 107 PFU, SC | 1 dose at 16 weeks and 1 dose at 4 weeks before challenge | 3/4 (75%) | No | Not investigated | Yes | N/A | N/A |
Pre-existing immunity documented for Ad5 vectors only.
Phase 1 clinical trials completed for Ad5-EBOV only.
PEI can be bypassed with IN/IT administration of Ad5-EBOV combined with Ad5-IFN-α as an adjuvant.
Ad5-EBOV mixed with Ad5-IFN-α adjuvant was protective in 6/9 (67%) of NHPs when administered 30 minutes after EBOV challenge.
Ad5-EBOV administered IN/IT was protective in 3/3 (100%) of NHPs when given 21 weeks prior to EBOV challenge.
4/8 (50%) survival when VSVΔG/EBOVGP is given at 30 min after challenge.
Durable protection was observed at up to 18 months in guinea pigs.
PEI can be bypassed with IN/IT administration of HPIV3-EbovZ GP in guinea pigs.
2. Pre-clinical Testing Of EBOV Vaccines In Non-human Primates
The development and licensure of an efficacious EBOV vaccine requires the consideration of many factors, but first and foremost, an experimental vaccine must undergo rigorous pre-clinical testing and demonstrate protection in an appropriate animal model of the disease. This animal model should be susceptible to infection when challenged with the pathogen, or closely related strains, and should ideally recapitulate hallmarks of the human disease [19]. For EBOV, a mouse adapted (MA)-EBOV challenge virus in BALB/c mice has been studied extensively and is a suitable animal model for screening the protective efficacy of vaccines against EBOV infection [20]. The results are then confirmed using a guinea pig adapted (GA)-EBOV challenge model [21] before studies in NHPs. Ideally, the vaccine should demonstrate immunogenicity and protective efficacy in the current gold-standard animal model for EBOV infection, cynomolgus macaques, which replicate the major hallmarks of human EBOV infections [22], before progressing to clinical trials. Rhesus macaques, while also used occasionally for vaccine studies, have a delayed disease course compared to cynomolgus macaques and are primarily used for testing EBOV-specific therapies [23].
2.1. DNA- and Ad5-vectored vaccines
DNA vaccines consist of plasmids that have been genetically engineered to express a desired antigen in order to produce an immunological response, and are not infectious agents. Adenoviruses are primarily respiratory pathogens which cause mild, cold-like symptoms in the upper respiratory tract; however, they have also been known to cause gastroenteritis and conjunctivitis depending on the virus serotype. Replication-defective Ad5 vectors lack the E1 and E3 genes in their viral genome, and the Ad5-vectored EBOV vaccine (Ad5-EBOV) contains GP in place of E1. The earliest success of an experimental EBOV vaccine in NHPs was from a DNA vector expressing EBOV GP (DNA-GP) followed by a boost from Ad5-EBOV (Figure 1A). Three 100 μg doses of DNA-GP were administered via the intramuscular (IM) route every 4 weeks before an Ad5-EBOV boost of 1010 plaque forming units (PFU) IM at 20 weeks after the first DNA dose. Challenge with EBOV at 32 weeks after the initiation of vaccination yielded protection in all vaccinated cynomolgus macaques [24]. A second study showed that cynomolgus macaques immunized with a single IM dose of Ad5-EBOV and Ad5 expressing NP mixed at a 1:1 ratio (totalling 2 × 1012 viral particles) resulted in complete protection when challenged 4 weeks after vaccination [25]. DNA vaccines have not yet been evaluated as a stand-alone candidate, however a third study showed that a single IM administration of 1010 viral particles of Ad5-EBOV given 4 weeks before challenge protected 100% of cynomolgus macaques from EBOV [26]. Additionally, an Ad5-vectored vaccine expressing the GP from a clinical isolate of the West African epidemic (Ad5-MakGP) was found to fully protect cynomolgus macaques at four weeks after immunization, when given as a single IM injection at a dose of 4 × 1010 viral particles [27].
An advantage to Ad5-EBOV vaccination includes the ability to induce protective immunity in NHPs with a single injection. Ad5-EBOV is also a well-characterized clinical product which is not usually associated with disease in humans; however one person had died from complications after receiving an Ad5-based treatment during a previous gene therapy trial [28]. The main concern with Ad5-based vaccines is pre-existing immunity (PEI) to the vaccine vector, as serological surveys performed in North America showed that 30-50% of the population is seropositive for anti-Ad5 antibodies [29], and the number rises to 90% in South America, Asia and sub-Saharan Africa [30] [31]. Pre-existing antibodies against Ad5 has resulted in reduced vaccine potency during a previous clinical trial with an Ad5-based vaccine against human immunodeficiency virus (HIV) [32]. In response to this, a research group demonstrated that PEI did not impact the efficacy of adjuvanted Ad5-EBOV, consisting of Ad5-EBOV mixed with Ad5 expressing IFN-α (Ad5- IFN-α), in cynomolgus macaques if administration was via the intranasal/intratracheal (IN/IT) route [33]. The protection was found to be sustained, as all cynomolgus macaques vaccinated with a single IN/IT dose of Ad5-EBOV at 109 infectious viral particles per kg survived a lethal EBOV challenge 21 weeks after vaccination [34]. Additionally, a single IM dose of adjuvanted Ad5-EBOV [2 × 1010 infectious forming units (IFU) of Ad5-ZGP and 3 × 109 PFU Ad5- IFN-α] given 30 minutes after EBOV challenge resulted in 67% survival of cynomolgus macaques, suggesting that protective immunity can be rapidly established with this vaccine [35]. As a result, Ad5-vectored vaccines are promising as a mucosal vaccine, but the IN/IT administration of Ad5-EBOV should be investigated in clinical trials in volunteers with or without PEI before use.
2.2. Adenovirus-vectored vaccines derived from other human serotypes
Another strategy to avoid PEI against Ad5 in vaccine recipients includes the characterization and testing of other replication-defective, human-origin adenovirus serotype platforms (Figure 1B). A previous study showed that a prime-boost scheme consisting of a vaccine prime with 1011 focus forming units (FFU) of Ad26 expressing EBOV GP (Ad26.ZEBOV), followed by a boost of 1011 FFU of Ad35 expressing EBOV GP (Ad35-GP) as a boost 4 weeks later, is 100% efficacious in cynomolgus macaques when challenged 4 weeks after the boost [36]. Durable immunity has not yet been tested with this vaccine candidate. While adenovirus-vectored vaccines share many of the same benefits and drawbacks with each other, an advantage with Ad26/Ad35-based vaccines is that PEI against these serotypes is not as prevalent in the human population. However, a disadvantage is the difficulty of implementing a prime-boost schedule during outbreak or epidemic situations. Furthermore, both Ad26.ZEBOV and Ad35-GP have lower efficacy rates compared to Ad5-EBOV as a stand-alone vaccine: an IM injection of 1011 viral particles of Ad26.ZEBOV or Ad35-GP protected only 50% and 0% of NHPs, respectively, when challenged with EBOV 4 weeks later. Therefore, more characterization is required before advancing this vaccine to clinical trials.
2.3. Adenovirus-vectored vaccines derived from nonhuman origins
A third strategy to avoid PEI against Ad5 is to characterize and test replication-defective adenovirus platforms derived from nonhuman origins, such as nonhuman primates (Figure 1C). A first report dating back to 2006 established that an adenovirus of simian origin (AdC7) can be amenable to induce protective immunity against EBOV in vivo [37]. A recent study demonstrated that a single IM administration of 1 × 1011 viral particles of chimpanzee ChAd3 expressing EBOV GP (ChAd3-EBO Z), followed 2 months later with an modified vaccinia Ankara expressing EBOV GP (MVA-BN-Filo) boost, results in complete protection in cynomolgus macaques when challenged with EBOV 10 months after the initial vaccination [38]. In this study, specific antibody responses appear to be a reliable correlate of protection [38]. An advantage of this strategy is the low to undetectable levels of PEI associated with simian adenoviruses in the human population. However, a single IM dose of 1 × 1011 viral particles of ChAd3-EBO Z only resulted in 50% protection in NHPs when challenged 4 weeks after immunization [38], suggesting that ChAd3-EBO Z will play a bigger role as a preventive vaccine for residents of EBOV-endemic countries, rather than as a rapid vaccine used in outbreak or epidemic scenarios.
2.4. Vesicular stomatitis virus-based vaccines
VSV causes disease in cattle, horses and swine characterized by the presence of vesicles and ulcers on the foot and mouth [39]. Human infections are rare and usually limited to those exposed to infected livestock [40], but the disease in humans is either asymptomatic or mild and flu-like [39] [41]. The VSV-vectored vaccine against EBOV (VSVΔG/EBOVGP) is a recombinant replication-competent virus in which the wild-type glycoprotein was deleted from the viral genome and replaced with EBOV GP (Figure 1D). A single IM injection of 107 PFU VSVΔG/EBOVGP was shown to fully protect cynomolgus macaques if administered 4 weeks before challenge [42]. This protection was rapid, as evidenced by the protection observed in 50% of rhesus macaques when given the vaccine as a postexposure prophylactic at 30 minutes after the EBOV challenge [43]. Additionally, complete and partial (67%) protection was achieved with a single IM dose of 5 × 107 PFU VSVΔG/EBOVGP given to cynomolgus macaques as late as 7 and 3 days before challenge, respectively [44]. While durable immunity has not been demonstrated directly with the VSVΔG/EBOVGP vaccine in NHPs, guinea pigs inoculated with VSVΔG/EBOVGP are still fully protected at 18 months after vaccination [45]. The VSV vaccine platform has also been shown to demonstrate sustained efficacy against Marburg virus (MARV), a virus closely related to EBOV, for up to 14 months post-vaccination in cynomolgus macaques [46]. IgG antibodies against EBOV, and in particular GP, are critical for vaccine mediated protection in cynomolgus macaques [47] and statistically correlates with protection [48]. A potential drawback to VSV-vectored vaccines is safety, as intrathalamic inoculation of cynomolgus macaques with wild-type VSV resulted in severe neurological disease [49]. However, subsequent studies have shown that intrathalamic inoculation of cynomolgus macaques with VSVΔG/EBOVGP does not result in neurovirulence [50]. Furthermore, VSVΔG/EBOVGP was shown to be safe and partially efficacious in immunocompromised (SHIV-infected) rhesus macaques, protecting 4 of 6 (67%) of animals when challenged with EBOV one month after vaccination [51].
2.5. EBOV virus-like particles
EBOV VLPs are empty virion shells consisting of EBOV GP, NP and VP40 as structural proteins (Figure 1E). Since VLPs do not contain a viral genome, there is no possibility for vaccine replication in living organisms. In a past study, NHPs were immunized three times at 6-week intervals with IM injections of VLPs, with each dose containing a total of 250 μg protein. Challenge of cynomolgus macaques with EBOV at 4 weeks after the last injection resulted in full protection [52]. Elevated levels of EBOV-specific antibodies that function by neutralization, complement-mediated cell lysis, and antibody-dependent cell-mediated cytotoxicity were detected in the sera of NHPs after each vaccination with VLPs, but it was unknown whether humoral immunity correlated with protection [52]. Advantages with protein-based vaccines, such as VLPs, include vaccine stability compared to recombinant viral vaccines, safety due to its non-replicative property, and lack of pre-existing immunity amongst the vaccine recipients. Disadvantages include production costs, because protein-based vaccines are more expensive to manufacture on a large scale, as well as the vaccination regimens: three injections spaced over 18 weeks will be difficult to implement, and would not be useful as a rapid vaccine. The capacity of VLPs to confer durable immunity remains unknown. VLPs have been proposed and tested as a vaccine for captive chimpanzees, in order to prevent outbreaks in the wild NHP population, subsequently decreasing potential exposure to humans in endemic areas [53].
2.6. Human parainfluenza influenza virus type 3
HPIV3 is a common human pathogen which causes infections in the lower respiratory tracts of children [54]. The HPIV3 vaccine expressing EBOV GP (HPIV3-EbovZ GP) vaccine is a replication-competent, recombinant virus in which the EBOV GP was inserted into the genome between the phosphoprotein and the matrix genes (Figure 1F). The demand for needle-free vaccinations resulted in the development of HPIV3-EbovZ GP for use as a potential mucosal vaccine against EBOV. A study was published in which two doses of 2 × 107 median tissue culture infective dose (TCID50) HPIV3-EbovZ GP administered IN/IT at 14 and 10 weeks before challenge resulted in complete protection against EBOV in rhesus macaques [55]. While PEI to HPIV3 may negatively impact the immunogenicity of the vaccine in humans, a study in rhesus macaques demonstrated that the IN/IT administration of both HPIV3-EbovZ GP doses is able to bypass host PEI and induce similar levels of EBOV-specific antibodies in both naive and HPIV3-immune animals [56]. The same study also showed that the magnitude of EBOV-specific IgG serum titres correlated with survival [56]. HPIV3-EbovZ GP utilizes a non-invasive route of administration, however the requirement for two doses to achieve potency means it is more suited as a long-term vaccine. Before advancing this experimental vaccine to clinical trials, the duration of immunity provided by HPIV3-EbovZ GP still needs to be further characterized.
2.7. Venezuelan equine encephalitis virus
VEEV is a mosquito-borne, positive-sense RNA pathogen which mainly causes severe disease in horses, donkeys and zebras, but only a mild, flu-like disease in humans [57]. VEEV-EBOV GP are enveloped, single cycle replicons in which the structural protein genes have been replaced with the EBOV GP gene (Figure 1G). VEEV-SUDVGP is a recombinant VEEV which encodes the glycoprotein of Sudan virus (SUDV), a pathogen closely related to EBOV. VEEV-based vaccines were initially investigated against EBOV due to a past study describing the ability of recombinant alphavirus replicons expressing GP and/or NP to protect cynomolgus macaques against MARV infection [58]. In a past study, 1010 FFU of VEEV-EBOVGP and 1010 FFU of VEEV-SUDVGP was given IM to NHPs as two separate injections. The vaccinated cynomolgus macaques survived a lethal challenge with EBOV or SUDV at 4 weeks after immunization [59]. While GP-specific antibody titers provided a useful measure of seroconversion to the vaccine vector in NHPs, the study lacks a comprehensive evaluation of both humoral and cellular immunity and therefore it is unclear which compartment of the adaptive immune response is responsible for protection [59]. It is unknown whether VEEV is able to provide rapid or long-term immunity, and therefore these factors will need to be further evaluated before progression to clinical trials.
2.8. Rabies virus
RABV causes an acute inflammation of the brain in humans and other warm-blooded animals, and killed approximately 26,000 people in 2010 [60]. The RABV vaccine vector is derived from the Street Alabama Dufferin (SAD) B19 RABV strain (Figure 1H), which is already approved for veterinary use and has been used as an oral vaccine to virtually eradicate RABV from Western Europe as well as parts of eastern Europe [61] [62]. The live-attenuated RABV-GP vaccine contains EBOV GP inserted between the nucleoprotein and the matrix genes in the RABV genome. A past study showed that rhesus macaques administered IM with a single dose of 5 × 107 FFU RABV-GP were completely protected from EBOV challenge 70 days after vaccination [63]. Live-attenuated, recombinant RABV-GP will cause significant safety concerns if used in humans, therefore it has been suggested that RABV-GP be used as a bivalent vaccine for use in wildlife, such as fruit bats in order to prevent potential animal-to-human RABV or EBOV transmission [64]. A live-attenuated version of RABV, in which the RABV glycoprotein was removed from the genome (RABVΔG-GP), as well as a chemically inactivated version of RABV-GP was created for potential use in humans. NHPs were administered either 1 × 107 FFU of RABVΔG-GP, or a prime-boost regimens involving 250 μg of the inactivated RABV-GP as prime followed by an identical boost 28 days later. EBOV challenge at 70 days after the initial vaccination yielded 50% survival in each group [63]. High levels of GP-specific antibodies were shown to be beneficial for the control of EBOV infection [63]. It is unknown whether RABV-vectored vaccines are able to provide durable immunity against EBOV, although the immunogenicity of the vaccine will need to be enhanced, either with an adjuvant or an increased dosage, before advancement to clinical trials.
2.9. EBOVΔVP30
EBOVΔVP30 is a replication-defective EBOV, Mayinga variant, lacking the VP30 gene (Figure 1I). EBOVΔVP30 is genetically stable with no chance for reversion to wild-type and can only grow in cells engineered to express VP30 [65]. Cynomolgus macaques were administered a prime of 1 × 107 FFU of EBOVΔVP30, followed by an identical boost 28 days later. EBOV challenge at 56 days after the initial vaccination yielded 100% survival [66]. All surviving animals showed a robust IgG response specific to the GP antigen. To add an extra layer of protection, H2O2-inactivated EBOVΔVP30 was also tested using the same vaccination regimens in NHPs. Although the resulting IgG concentrations were slightly lower than that of non-chemically treated EBOVΔVP30, the immunized NHPs still survived the challenge with no clinical symptoms of EBOV disease [66]. While it is unlikely that EBOVΔVP30 will provide rapid immunity, the long-term immunity of this vaccine should be evaluated before progression to clinical trials.
2.10. Cytomegalovirus
CMV is a common β-herpesvirus which that does not cause disease in an immunocompetent host, but may cause neurological damage in those with weakened immune systems (such as the immunocompromised or newborn). The CMV vaccine vector is derived from the rhesus macaque RhCMV strain 68-1, in which the full-length EBOV GP was inserted in place of the Rh112 gene (Figure 1J) to create the recombinant vaccine RhCMV/EBOV-GP. CMV-seropositive rhesus macaques received a single SC injection of 1 × 107 PFU of RhCMV/EBOV-GP, with an identical boost at 12 weeks later. At 16 weeks after the initial vaccination, the animals were challenged with EBOV, resulting in 75% protection. These results indicate that RhCMV/EBOV-GP is effective in NHPs despite pre-existing immunity to the vaccine vector, and survival of these animals appeared to correlate with total levels of EBOV GP-specific IgG, since the non-surviving animal showed the lowest antibody levels [67]. To further advance this candidate to clinical trials, the immunization regimens should be modified and tested to achieve optimum levels of protection first.
3. Safety and Immunogenicity Of EBOV Vaccine Candidates In Phase 1 and 2 Clinical Trials
Once shown to be immunogenic and protective in pre-clinical studies, a vaccine candidate may then proceed to evaluation in human clinical trials. Phase 1 trials for the evaluation of vaccines primarily aim to test the safety of the vaccine in escalating doses in healthy human subjects. This is followed by Phase 2 clinical trials, which further evaluate the safety of a vaccine candidate as well as its immunogenicity in a larger study population. Despite the availability of potential vaccine candidates with promising results in pre-clinical studies, evaluation of these candidates in Phase 1 trials was delayed partially due to insufficient public sector investment supporting the assessment of vaccine candidates against relatively rare diseases. However, an international ethical panel assembled by the World Health Organization (WHO) declared the 2014-2016 EBOV epidemic a “Public Health Emergency of International Concern” and expedited the clinical evaluation of multiple EBOV vaccine candidates as well as therapeutics [68] [69] [70]. As a result, several candidates underwent Phase 1 and 2 clinical trials in the search for a safe and immunogenic vaccine.
3.1. VRC-EBODNA023-00-VP
The VRC-EBODNA023-00-VP, a DNA-vectored vaccine, was evaluated at 4 mg doses given at 0, 4, and 8 weeks post-enrollment in a Phase 1b trial in Uganda. Overall, the vaccine was well-tolerated in most participants. 56 of 60 (93%) of participants who received the vaccine experienced mild to moderate local reactions. 83% of participants who received the vaccine experienced mild-moderate systemic reactions. EBOV GP-specific antibodies were induced in 17 of 30 (57%) participants in the vaccine group 4 weeks after completion of the vaccine regimen. In addition, 63% of vaccinees elicited T-cell responses to EBOV GP [71].
3.2. Ad5-EBOV
The safety and immunogenicity of Ad5-EBOV was evaluated in a Phase 1 clinical trial in China at a low dose of 4 × 1010 viral particles (vp) and high dose of 1.6 × 1011 vp. 68% of participants who received either dose reported at least one adverse reaction, such as mild pain or redness at the injection site, fever, headache, joint pain, diarrhea, muscle pain, throat pain and cough, within 7 days of vaccination. However, the incidences were not statistically significant from the placebo control group. In terms of immunogenicity, EBOV GP-specific IgG antibodies were induced by 14 days post-vaccination (dpv) in 93 and 100% of those vaccinated with the lose dose and high dose of Ad5-EBOV, respectively [72].
3.3. Heterologous prime-boost regimen: Ad26.ZEBOV and MVA-BN-Filo
A heterologous prime-boost vaccine regimen comprising Ad26.ZEBOV at a dose of 5 × 1010 vp and MVA-BN-Filo at a dose of 1 × 108 TCID50 was evaluated in a Phase 1 trial performed in Oxford, United Kingdom. In terms of safety, there were no vaccine-related serious adverse events. However, adverse events were observed in 45 of 60 individuals (75.0%) after receiving Ad26.ZEBOV as a prime or boost. 40 of 59 (67.8%) people experienced adverse events after receiving MVA-BN-Filo as a prime or boost. However, these were mainly mild or moderate pain, swelling, warmth, or pruritus at the injection site. The systemic effects were mainly mild or moderate fatigue, headache, myalgia, chills, nausea, arthralgia, rash, or pruritus. Four instances of grade three adverse effects were noted. One of the grade 3 adverse effects in vaccinated individuals was experienced by an individual after Ad26.ZEBOV prime and included myalgia, headache, and pain at the injection site. The other grade 3 adverse effects in vaccinated individuals occurred after receiving Ad26.ZEBOV as a boost and included injection site erythema, swelling, and/or nausea. In terms of immunogenicity, EBOV GP-specific IgG was induced after 28 days in 28 of 29 (97%) and 7 of 30 (23%) participants who received Ad26.ZEBOV and MVA-BN-Filo as a prime immunization. All vaccine recipients had specific IgG detectable 21 days post-boost and at 8-months follow-up [73].
3.4. ChAd3-EBO Z
Phase 1 and 2 trials have been initiated in the USA, the UK, Switzerland and Mali to test the safety and immunogenicity of the vaccine [74] in response to the EBOV epidemic in West Africa. Preliminary results from the UK clinical trial is available and doses ranging from 1 × 1010 to 5 × 1010 viral particles were well tolerated without significant serious adverse effects in volunteers [75]. In around 45-50% of subjects, mild reactogenicity was observed in the form of local tenderness, malaise, and headache [76]. In conjunction with the ChAd3-EBO Z prime, the boost component of the vaccine regimen utilizes a highly attenuated vaccinia strain (MVA-BN-Filo) [77] in order to extend the duration of vaccine-induced protection [78]. Despite its lack of efficacy as a stand-alone EBOV vaccine platform, its use as a booster was rationalized for its ability to induce long-lasting CD4+ T cell responses, while overcoming pre-existing vector immunity in humans [38]. MVA-based vaccines are currently undergoing clinical trials for H5N1 influenza [79], tuberculosis [80] and smallpox [81], with results showing that recombinant MVA is well-tolerated, even in immunocompromised humans [81].
A Phase 1/2a study in Switzerland evaluated ChAd3-EBO Z at 2.5 × 1010 and 5 × 1010 viral particles. In this study, local adverse events were reported in 33 of 42 (79%) low dose vaccinees, 30 of 40 (79%) high-dose vaccinees. These events also occurred in 5 of 20 (25%) of participants receiving the placebo control. The most common systemic adverse events were fatigue or malaise, which occurred in 62% and 60% of the high- and low-dose participants, respectively. Headache was reported in 57% and 60% in these groups, respectively. In addition, fever occurred within 24 hours of vaccination in 30% of high-dose participants, and 26% of low dose participants. In terms of immunogenicity, EBOV GP-specific antibodies were induced in 96% of both high-dose and low-dose participants. EBOV GP-specific CD4+ T cells were induced in 57 and 61% of high-dose and low-dose participants, while EBOV GP-specific CD8+ T cells were induced in 67% and 69% of high-dose and low-dose participants, respectively [82].
3.5. VSVΔG/EBOVGP
The first uses of VSVΔG/EBOVGP in humans occurred under compassionate circumstances to a German researcher who may have been infected in a laboratory accident during 2009 [83] and a physician upon a needle stick at an EBOV treatment unit during 2014, both of whom showed good tolerance toward the vaccine [84]. To further characterize VSVΔG/EBOVGP in a clinical setting, Phase 1 trials were conducted in Gabon, Kenya, Germany, and Switzerland. In these trials, the safety and immunogenicity of VSVΔG/EBOVGP was evaluated at doses ranging from 3 × 105 to 5 × 107 PFU. While no serious vaccine-related adverse events were observed, mild-moderate reactogenicity to the vaccine was frequent but resolving, and fever occurred in up to 30% of vaccinated individuals. EBOV GP-specific antibody responses were elicited in all vaccinated participants, with similar GP-binding antibody titres lasting through 180 dpv in all vaccinees. Significantly higher neutralizing antibody titres were elicited at higher immunization doses [85]. The clinical trial in Switzerland was halted due to safety concerns from joint pain and pathology (e.g. arthralgia, arthritis) in several volunteers, but then allowed to continue with a lower dose of vaccine. It will be necessary to determine if additional attenuation of the vaccine could lower the severity of the side effects while conserving protective efficacy. Other VSV-based variants with increased attenuation phenotypes have already been generated and tested for neurovirulence in NHPs [86], and are also currently being developed as potential vaccine candidates against EBOV [87].
Another Phase 1 trial conducted in the USA evaluated the immunogenicity and safety of VSVΔG/EBOVGP at a dose of 3 × 106 PFU or 2 × 107 PFU. Even at the high dose, the vaccine was well-tolerated, with the most common adverse events being pain at the injection site, myalgia, and fatigue. By 28 dpv, all vaccinated individuals were positive for EBOV GP-specific IgG. However, IgG titers were highest in those receiving the high vaccine dose. By ELISA, mean antibody titres induced by the high and low doses were, 4079 and 1300, respectively, while mean neutralizing antibody titers were 441 and 223, respectively [88].
3.6. HPIV3-EbovZ GP
A Phase 1 clinical trial has been launched to investigate the safety and immunogenicity of a homologous prime-boost regimen of HPIV-EbovZ GP, with 106 or 107 PFU administered as a prime and 106 or 107 PFU administered as a boost 4-6 weeks dpv. This trial is currently ongoing, but not recruiting [89] and results are not yet available.
4. Efficacy Of EBOV Vaccine Candidates In Phase 2/3 Clinical Trials
Typically, a vaccine candidate must demonstrate success in preliminary Phase 1 and 2 trials before it can be evaluated in Phase 3 clinical trials, which aim to determine the efficacy of the candidate against disease. However, Phase 3 clinical trials have not yet been performed prior to the 2014-2016 EBOV epidemic, primarily due to the lack of prior Phase 1/2 efficacy testing of these candidates. Additionally, vaccine protection cannot be properly evaluated in the absence of EBOV cases. During the 2014-2016 EBOV epidemic, the lack of an approved effective vaccine combined with the dire circumstances in West Africa led to an expedition of vaccine trials from Phase 1 safety studies directly to Phase 3 efficacy studies, alongside Phase 2 studies [68] [70]. Due to the practical limitations of front-line medical workers to adhere to classic clinical trial standards and the increased ethical need to provide protection to the high-risk population, these Phase 3 trials utilized alternative efficacy trial designs [90] [91] [92]. In West Africa, traditional randomized control trials, as well as adaptive trials such as ring vaccination, stepped-wedge, and cluster-randomized trials were employed for the evaluation of the leading vaccine candidates, ChAd3-EBO Z, VSVΔG/EBOVGP, and a heterologous prime-boost regimen comprising Ad26.ZEBOV and MVA-BN-Filo.
4.1. EBOVAC-Salone for the heterologous Ad26.ZEBOV prime, MVA-BN-Filo boost regimen
EBOVAC-Salone is another Phase 3 randomized control trial has also been launched in Kambia and Rokupr, Sierra Leone to evaluate the efficacy of an Ad26.ZEBOV prime, MVA-BN-Filo boost regimen. Participants are to be randomized to receive either the EBOV vaccine group or the control MENVEO vaccine against Meningococcal Groups A, C, Y and W-135. The EBOV vaccine group will prime participants with 5 × 1010 vp Ad26.ZEBOV, followed by a boost of 1 × 108 IFU MVA-BN-Filo at 57 dpv. The MENVEO group will prime participants with MENVEO followed by a placebo at 57 dpv, or a boost of MENVEO for children under the age of 2. This trial is ongoing and recruiting participants [93].
4.2. PREVAIL trial for ChAd3-EBO Z and VSVΔG/EBOVGP
The Partnership for Research on Ebola Vaccines in Liberia I (PREVAIL I, NCT02344407) is an ongoing randomized clinical trial to compare the efficacy of ChAd3-EBO Z and VSVΔG/EBOVGP, while further investigating safety and immunogenicity in Monrovia, Liberia [94] [95] [96]. Due to the limited amount of Phase 1 safety data prior to the trial, the first set of volunteers was enrolled in the Phase 2 sub-study to characterize immune responses, as well as analyze adverse effects and immunogenicity at 1 week and 1 month post-vaccination. Upon success in the initial phase, the remaining volunteers were to be evaluated in the Phase 3 study, which would report the incidence of adverse effects and EBOV disease (EVD) monthly for a span of 8-12 months [96]. However, as recruitment for the Phase 2 trial continued, the outbreak in Liberia diminished and Liberia was briefly declared EBOV-free in May 2015. Since the decline in EVD incidence in Liberia would impede the calculation of vaccine efficacy, the Phase 3 component of the trial was discontinued following recommendations of the US Food and Drug Administration (FDA) and Data Safety Monitoring Board (DSMB), as well as two institutional review boards. Instead, the Phase 2 trial was expanded to 1,500 participants, which was achieved by the end of April 2015. In addition, follow-up of participants was extended to obtain more safety data, with additional immunogenicity testing at 6 and 12 months post-vaccination. The results of this trial are pending [97].
4.3. STRIVE trial for VSVΔG/EBOVGP
Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE; NCT02378753) is currently being conducted by the Centers for Disease Control (CDC, USA) and MOH (Sierra Leone) to evaluate the efficacy of VSVΔG/EBOVGP. As of December 2015, over 8,650 of the original goal of 6,000 frontline EBOV workers in Freetown, Sierra Leone are participating in an open label, randomized, stepped-wedged trial design, with phased introduction into the target demographic [68] [98]. The target population of this study includes 18 healthcare facilities or frontline teams at high risk of exposure, such as personnel working in EVD treatment facilities and non-EVD healthcare facilities, surveillance teams, ambulance teams, and laboratory workers who swab the deceased [94]. Upon enrollment, participants are individually randomized to “immediate” or “deferred” vaccination, receiving 2 × 107 PFU of the vaccine by IM injection within 7 days or 18-24 weeks post-enrollment, respectively. In order to evaluate vaccine efficacy, incidence rate of the vaccinated population (IRV) will be calculated using the incidences of EVD that occur within 21 days after immunization of the immediate arm, while the incidence rate of the unvaccinated population (IRU) will be calculated using incidences of EVD that occur within 21 days of enrollment of the deferred arm. In addition, all participants of the study will be followed for 6 months after vaccination to monitor for occurrence of serious adverse effects and EVD [99]. As of April 2016, no cases of EBOV infection nor vaccine-related serious adverse events (as defined by death, life-threatening illness, hospitalization or prolongation of hospitalization, or permanent disability), were reported from the participants in the study [100].
4.4. Ebola ça suffit trial for VSVΔG/EBOVGP
Another Phase 3 trial evaluating the efficacy of VSVΔG/EBOVGP, named Ebola ca suffit (“Ebola, that’s enough”) (PACTR201503001057193), is being conducted by the WHO and MOH (Guinea), Médecins sans Frontières (MSF), EPICENTRE, and the Norwegian Institute of Public Health [101]. The highly collaborative endeavor involves additional support from the Public Health Agency of Canada, the Canadian Institutes of Health Research, and the Wellcome Trust (UK). In Conakry, Guinea, and surrounding areas known as Basse Guinée, 10,000 individuals are participating in an unusual trial developed by an international panel of experts. The observational stage, occurring in the first weeks of the trial, focuses on vaccinating frontline workers in the study area, to strengthen safety and immunogenicity data [68] [91] [101]. Successively, the ring vaccination stage, which was inspired by the strategy used to eradicate smallpox, involves the immunization of approximately 190 “rings” of 50-100 close contacts and contacts of contacts of laboratory-confirmed EBOV patients, including family, friends, neighbors, and co-workers who provide informed consent. The concept of ring vaccination is based on the idea that those with close contact to an infected person are at increased risk for infection [102]. After an index case is identified, rings are cluster randomized such that all members of a ring receive “immediate vaccination” or “delayed vaccination” with a 2 × 107 PFU dose of VSVΔG/EBOVGP in a 1:1 ratio, occurring immediately or 21 days after enrolment, respectively. The primary focus to assess the protective efficacy of VSVΔG/EBOVGP is accomplished by comparing laboratory-confirmed EBOV infection occurring after enrollment of the deferred group and laboratory-confirmed EBOV infection occurring 10 or more days post-vaccination in the immediate vaccination group. This design allows for vaccine efficacy calculation by comparing IRV observed in the immediate arm and IRU observed in the delayed arm prior to vaccination instead of using a placebo arm. In addition, the trial aims to determine whether or not contact vaccination is able to create a buffer that prevents further spread of infection [101] [103]. Interim results from July 2015 showed no incidence of EVD in the immediate group (n=4,123) and 16 laboratory-confirmed cases of EHF in the deferred group prior to receiving vaccination (n=3,528), demonstrating a vaccine efficacy of 100% (95% confidence interval 74.7–100.0; p=0.0036) at 6 dpv. These preliminary results suggest that protection conferred by VSVΔG/EBOVGP may be induced quickly (6–21 days) and potently by a single injection of vaccine. Secondly, it demonstrates the potential effectiveness of VSVΔG/EBOVGP at the population level when administered using the ring vaccination strategy. However, 43 serious adverse events were reported in trial participants. Of these, 1 participant experienced febrile reaction cerebrovascular accident (CVA) with hemiplegia at 3 dpv, which was considered to be causally related to vaccination. The other serious adverse events were considered unrelated to vaccination [104]. The findings from the Ebola ça suffit trial were recently published and confirmed the preliminary results, with no cases of EBOV disease occurring among vaccinated individuals from day 10 after vaccination [105].
5. Expert opinion
5.1. Determining vaccine effectiveness
In order to bring a vaccine to market, it is crucial that an immune marker correlating with protection can be reliably used to predict vaccine efficacy. This is especially important in the context of EBOV. In addition to basic immunological interest, studies into the correlates of protection will allow researchers to predict protection on an individual and population level without having to challenge the patients with a live pathogen, to provide an indicator of declining immunity resulting the need for booster immunizations, and may be used to discourage clinical trials that are deemed to have a low chance of success [106]. Such a marker must be consistently and reliably related to protection as evidenced by statistical analysis, and is not necessarily the same as the mechanism that is responsible for eliminating EBOV from the host [107]. While the strength of the immune correlate required to support licensing a vaccine candidate is not specified in the animal rule [19], factors such as survival versus non-survival, the risk of infections, and the urgency of public health needs will play a role in determining the threshold levels required for clinical acceptance [108]. Currently, the majority of studies summarized in this review suggest that specific IgG antibody levels play the major role for protection in immunized NHPs. Recent breakthroughs in the use of GP-specific IgG antibodies as a therapeutic for EBOV infection in NHPs [109] [110] [111] also highlight the contribution of antibodies towards survival from EBOV.
Other important factors to consider when proceeding towards licensure include vaccine safety, ease of administration, time to protective immunity, potential for long-term immunity, pre-existing immunity towards the delivery vector in the vaccine recipient population, vaccine stability, as well as the costs to produce a vaccine that complies with good manufacturing practices - especially when up to 500 million people residing in the endemic countries of Western and Central Africa may require this vaccine. In other words, the ideal vaccine should be able to establish rapid, durable immunity without adverse effects after a single non-invasive administration, can be used and re-used without concerns of pre-existing immunity, maintain stability in locations without cold-chain storage, and be economical to produce on a large scale. It is important to note that depending on the situation at hand, vaccines that only satisfy a portion of these factors may still be able to play a major role in limiting the number of cases in an EBOV outbreak or epidemic.
5.2. Translation of pre-clinical studies to human clinical trials
The strategy for testing medical countermeasures against EBOV is to use small animals, such as mice and guinea pigs, to screen the effectiveness in against an EBOV challenge before testing the most promising compounds in NHPs. Any compounds that were efficacious in NHPs would theoretically have the highest chance of also being protective in humans since EBOV-infected NHPs display signs of disease similar to human EBOV infections. However, adverse effects from the experimental compound may not be accurately predicted by NHP models and toxicological studies should be performed in other animals, such as rabbits, before proceeding to Phase 1 clinical trials. Encouragingly, the 100% efficacy of VSVΔG/EBOVGP observed in NHPs adequately predicted the 100% efficacy of the vaccine observed in the Ebola ça suffit trial in human subjects. Other experimental vaccines such as Ad5-EBOV, Ad26.ZEBOV, ChAd3-EBO Z±MVA-BN Filo, were also shown to be safe in Phase 1 clinical trials, demonstrated immunogenicity similar to that observed in pre-clinical NHP studies and thus should be promising candidates in Phase 3 trials.
5.3. Regulatory challenges
The 2014-2016 EBOV epidemic in West Africa is proof that any pathogen, no matter how seemingly minor, will have the capacity to cause significant public health concerns if left effectively ignored for an extended period of time. Due to the status of EBOV as a neglected tropical disease, it was previously difficult for many research groups to attract and secure sufficient finances from funding organizations, in order to perform the necessary pre-clinical studies for advancing a particular vaccine candidate towards clinical trials. Commercial vaccine manufacturers may need to be incentivized by governing bodies to take on the production and testing of a vaccine in which there is likely to be no business case in the absence of a major outbreak or epidemic. These are just some of the bottlenecks that have delayed the development of a licensed EBOV vaccine in the past.
VSVΔG/EBOVGP, which was developed in 2005, encountered more obstacles due to concerns about its safety, and clinical trials only started in the second half of 2014 in response to the magnitude of the 2014-16 EBOV epidemic. The sudden push of ChAd3- and VSV-based vaccines into clinical testing indicated that the regulatory body believed that any potential adverse effects that patients may experience from immunization is now an acceptable risk in light of the potential negative impacts from the EBOV epidemic. It is clear that the process from the end of pre-clinical testing to the start of Phase 1 clinical trials can be accelerated, as demonstrated in the case of VSVΔG/EBOVGP. Therefore, a discussion on the threshold required to advance promising vaccines against a high impact pathogen of low public health interest towards clinical trials is an important consideration to better prepare and prevent the impact of potentially high-consequence pathogens on public health in future infectious disease outbreaks or epidemics.
HIGHLIGHTS.
An abundance of vaccine candidates have performed well with pre-clinical testing in NHPs.
Some candidates have hurdles to overcome before advancement to clinical trials due to safety reasons (RABV and EBOVΔVP30, and at one point live, replicating recombinant viruses such as VSV).
Phase 1 trials have demonstrated the safety and immunogenicity of Ad5-EBOV, ChAd3-EBO Z, and VSVΔG/EBOVGP vaccines.
Clinical trials to evaluate the efficacy of ChAd3-EBO Z, VSVΔG/EBOVGP, and the Ad26.ZEBOV and MVA-BN-Filo heterologous prime-boost regimen were initiated in affected areas of West Africa, most were unable to elucidate efficacy due to the decline of the epidemic by late 2015.
Only VSVΔG/EBOVGP has demonstrated preliminary efficacy in a clinical trial setting, demonstrating 100% efficacy by the end of 2015.
Acknowledgments
Funding:
This work is supported by the National Key Research and Development Program of China (2016YFE0205800), the National Key Program for Infectious Disease of China (2016ZX10004222), the Sanming Project of Medicine in Shenzhen (ZDSYS201504301534057), the Shenzhen Science and Technology Research and Development Project (JCYJ20160427151920801), and the Public Health Agency of Canada, partially supported by grants from the National Institutes of Health (U19AI109762-1 to GP Kopinger and X Qiu), Canadian Institutes of Health Research (IER-143487 to X Qiu), and National Natural Science Foundation of China International Cooperation and Exchange Program (816110193 to G Wong).
Footnotes
Declaration of Interest:
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Feldmann H, Sanchez A, Geisbert TW. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley P, editors. Fields Virology. Philadelphia, PA, USA: Lippincott Williams and Wilkins; 2013. [Google Scholar]
- 2.Nkoghe D, Leroy EM, Toung-Mve M, et al. Cutaneous manifestations of filovirus infections [Review] Int J Dermatol. 2012 Sep;51(9):1037–43. doi: 10.1111/j.1365-4632.2011.05379.x. [DOI] [PubMed] [Google Scholar]
- 3.Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. The Journal of infectious diseases. 1999 Feb;179(Suppl 1):S28–35. doi: 10.1086/514318. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. The Journal of infectious diseases. 1999 Feb;179(Suppl 1):S170–6. doi: 10.1086/514291. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. Ebola haemorrhagic fever in Zaire, 1976. Report of an International Commission. Bull World Health Organ. 1978;56(2):271–93. [PMC free article] [PubMed] [Google Scholar]
- 6.Leroy EM, Kumulungui B, Pourrut X, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005 Dec 1;438(7068):575–6. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 7.Previous Updates: 2014 West Africa Outbreak (February 25, 2015) [8 November 2017];Centers for Disease Control and Prevention. 2015 Available at: http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/previous-updates.html.
- 8.Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus disease in Guinea [Research Support, Non-U.S. Gov’t] N Engl J Med. 2014 Oct 09;371(15):1418–25. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 9.Nigeria is now free of Ebola virus transmission. [8 November 2017];World Health Organization. 2014 Available from: http://www.who.int/mediacentre/news/ebola/20-october-2014/en/index2.html.
- 10.The outbreak of Ebola virus disease in Senegal is over. [8 November 2017];World Health Organization. 2014 Available from: http://www.who.int/mediacentre/news/ebola/17-october-2014/en/
- 11.New York City Reports Positive Test for Ebola in Volunteer International Aid Worker. [8 November 2017];Centers for Disease Control and Prevention. 2014 Available from: http://www.cdc.gov/media/releases/2014/s1023-ebola-nyc.html.
- 12.EBOLA UPDATE (03): AFRICA, WORLD, USA, UK, SUSPECTED, DRUGS, VACCINES. [8 November 2017];ProMED mail. 2015 Available from: http://www.promedmail.org/direct.php?id=3069311.
- 13.EBOLA VIRUS DISEASE - ex AFRICA (27): USA (TEXAS) SECOND NURSE BETTER, TEST, QUARANTINE. [8 November 2017];ProMED mail. 2014 Available from: http://www.promedmail.org/direct.php?id=2910297.
- 14.EBOLA VIRUS DISEASE - ex AFRICA (32): SPANISH NURSE RECOVERED, USA SEEKS PATENT. [8 November 2017];ProMED mail. 2014 Available from: http://www.promedmail.org/direct.php?id=2939861.
- 15.Ebola Situation Report - 26 May 2016. [8 November 2017];World Health Organization. 2016 Available from: http://apps.who.int/iris/bitstream/10665/206924/1/ebolasitrep_26May2016_eng.pdf?ua=1.
- 16.Meltzer MI, Atkins CY, Santibanez S, et al. Estimating the future number of cases in the Ebola epidemic--Liberia and Sierra Leone, 2014-2015. MMWR Surveill Summ. 2014 Sep 26;63(Suppl 3):1–14. [PubMed] [Google Scholar]
- 17.Bol GF. Risk communication in times of crisis: Pitfalls and challenges in ensuring preparedness instead of hysterics. EMBO Rep. 2016 Jan;17(1):1–9. doi: 10.15252/embr.201541678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osterholm MT, Moore KA, Kelley NS, et al. Transmission of ebola viruses: what we know and what we do not know. MBio. 2015;6(2) doi: 10.1128/mBio.00137-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidance for industry product development under the animal rule. [8 November 2017];US Food and Drug Administration. 2014 Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf.
- 20.Bray M, Davis K, Geisbert T, et al. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever [Research Support, U.S. Gov’t, Non-P.H.S.] The Journal of infectious diseases. 1998 Sep;178(3):651–61. doi: 10.1086/515386. [DOI] [PubMed] [Google Scholar]
- 21.Connolly BM, Steele KE, Davis KJ, et al. Pathogenesis of experimental Ebola virus infection in guinea pigs. The Journal of infectious diseases. 1999 Feb;179(Suppl 1):S203–17. doi: 10.1086/514305. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama E, Saijo M. Animal models for Ebola and Marburg virus infections. Front Microbiol. 2013;4:267. doi: 10.3389/fmicb.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geisbert TW, Strong JE, Feldmann H. Considerations in the Use of Nonhuman Primate Models of Ebola Virus and Marburg Virus Infection. J Infect Dis. 2015 Oct 01;212(Suppl 2):S91–7. doi: 10.1093/infdis/jiv284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan NJ, Sanchez A, Rollin PE, et al. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000 Nov 30;408(6812):605–9. doi: 10.1038/35046108. **The first published vaccine against Ebola virus in NHPs. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan NJ, Geisbert TW, Geisbert JB, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003 Aug 07;424(6949):681–4. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan NJ, Geisbert TW, Geisbert JB, et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 2006 Jun;3(6):e177. doi: 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S, Kroeker A, Wong G, et al. An Adenovirus Vaccine Expressing Ebola Virus Variant Makona Glycoprotein Is Efficacious in Guinea Pigs and Nonhuman Primates. The Journal of infectious diseases. 2016 Aug 4; doi: 10.1093/infdis/jiw250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000 Nov;1(2):91–9. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 29.Chirmule N, Propert K, Magosin S, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999 Sep;6(9):1574–83. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 30.Mast TC, Kierstead L, Gupta SB, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010 Jan 22;28(4):950–7. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 31.Nwanegbo E, Vardas E, Gao W, et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States [Research Support, U.S. Gov’t, P.H.S.] Clin Diagn Lab Immunol. 2004 Mar;11(2):351–7. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng C, Wang L, Gall JG, et al. Decreased pre-existing Ad5 capsid and Ad35 neutralizing antibodies increase HIV-1 infection risk in the Step trial independent of vaccination. PloS one. 2012;7(4):e33969. doi: 10.1371/journal.pone.0033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson JS, Pillet S, Bello AJ, et al. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates [Comparative Study Research Support, Non-U.S. Gov’t] Journal of virology. 2013 Apr;87(7):3668–77. doi: 10.1128/JVI.02864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JH, Jonsson-Schmunk K, Qiu X, et al. A Single Dose Respiratory Recombinant Adenovirus-Based Vaccine Provides Long-Term Protection for Non-Human Primates from Lethal Ebola Infection. Mol Pharm. 2014 Nov 14; doi: 10.1021/mp500646d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong G, Richardson JS, Pillet S, et al. Adenovirus-vectored vaccine provides post-exposure protection to Ebola virus-infected nonhuman primates. Journal of infectious diseases J Infect Dis. 2015 Oct 1;212(Suppl 2):S379–83. doi: 10.1093/infdis/jiv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geisbert TW, Bailey M, Hensley L, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol. 2011 May;85(9):4222–33. doi: 10.1128/JVI.02407-10. *A study demonstrating Ad26 and Ad35-vectored vaccines are efficacious against EBOV infections in NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobinger GP, Feldmann H, Zhi Y, et al. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology. 2006 Mar 15;346(2):394–401. doi: 10.1016/j.virol.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nature medicine. 2014 Oct;20(10):1126–9. doi: 10.1038/nm.3702nm.3702. *A study demonstrating ChAd3-vectored vaccines are efficacious and stimulate long-term immunity against EBOV infections in NHPs. [DOI] [PubMed] [Google Scholar]
- 39.Brandly CA, Hanson RP. Epizootiology of vesicular stomatitis. Am J Public Health Nations Health. 1957 Feb;47(2):205–9. doi: 10.2105/ajph.47.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brody JA, Fischer GF, Peralta PH. Vesicular stomatitis virus in Panama. Human serologic patterns in a cattle raising area. Am J Epidemiol. 1967 Jul;86(1):158–61. doi: 10.1093/oxfordjournals.aje.a120721. [DOI] [PubMed] [Google Scholar]
- 41.Johnson KM, Vogel JE, Peralta PH. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus (VSV) Am J Trop Med Hyg. 1966 Mar;15(2):244–6. doi: 10.4269/ajtmh.1966.15.244. [DOI] [PubMed] [Google Scholar]
- 42.Jones SM, Feldmann H, Stroher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] Nature medicine. 2005 Jul;11(7):786–90. doi: 10.1038/nm12583. *A study demonstrating VSV-vectored vaccines are effective against EBOV infections in NHPs. [DOI] [PubMed] [Google Scholar]
- 43.Feldmann H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection [Clinical Trial Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] PLoS pathogens. 2007 Jan;3(1):e2. doi: 10.1371/journal.ppat.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marzi A, Robertson SJ, Haddock E, et al. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] Science. 2015 Aug 14;349(6249):739–42. doi: 10.1126/science.aab3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong G, Audet J, Fernando L, et al. Immunization with vesicular stomatitis virus vaccine expressing the Ebola glycoprotein provides sustained long-term protection in rodents [Research Support, Non-U.S. Gov’t] Vaccine. 2014 Sep 29;32(43):5722–9. doi: 10.1016/j.vaccine.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mire CE, Geisbert JB, Agans KN, et al. Durability of a vesicular stomatitis virus-based marburg virus vaccine in nonhuman primates [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] PloS one. 2014;9(4):e94355. doi: 10.1371/journal.pone.0094355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marzi A, Engelmann F, Feldmann F, et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural] Proc Natl Acad Sci U S A. 2013 Jan 29;110(5):1893–8. doi: 10.1073/pnas.1209591110. *A study demonstrating antibodies are important for protection against EBOV infections in VSV-vaccinated NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong G, Richardson JS, Pillet S, et al. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates [Research Support, Non-U.S. Gov’t] Sci Transl Med. 2012 Oct 31;4(158):158ra146. doi: 10.1126/scitranslmed.3004582. **A study demonstrating that IgG antibodies statistically correlate with survival against EBOV infections in NHPs, independent of vaccine vector choice, as well as timing or route of immunization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson JE, Nasar F, Coleman JW, et al. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology. 2007 Mar 30;360(1):36–49. doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mire CE, Miller AD, Carville A, et al. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS neglected tropical diseases. 2012;6(3):e1567. doi: 10.1371/journal.pntd.0001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, et al. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] PLoS pathogens. 2008 Nov;4(11):e1000225. doi: 10.1371/journal.ppat.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warfield KL, Swenson DL, Olinger GG, et al. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge [Research Support, Non-U.S. Gov’t] The Journal of infectious diseases. 2007 Nov 15;196(Suppl 2):S430–7. doi: 10.1086/520583. *A study demonstrating VLPs are effective against EBOV infections in NHPs. [DOI] [PubMed] [Google Scholar]
- 53.Warfield KL, Goetzmann JE, Biggins JE, et al. Vaccinating captive chimpanzees to save wild chimpanzees [Research Support, Non-U.S. Gov’t] Proc Natl Acad Sci U S A. 2014 Jun 17;111(24):8873–6. doi: 10.1073/pnas.1316902111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denny FW, Clyde WA., Jr Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986 May;108(5 Pt 1):635–46. doi: 10.1016/s0022-3476(86)81034-4. [DOI] [PubMed] [Google Scholar]
- 55.Bukreyev A, Rollin PE, Tate MK, et al. Successful topical respiratory tract immunization of primates against Ebola virus [Research Support, N.I.H., Intramural] Journal of virology. 2007 Jun;81(12):6379–88. doi: 10.1128/JVI.00105-07. *A study demonstrating HPIV3-vectored vaccines are effective against EBOV infections in NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bukreyev AA, Dinapoli JM, Yang L, et al. Mucosal parainfluenza virus-vectored vaccine against Ebola virus replicates in the respiratory tract of vector-immune monkeys and is immunogenic. Virology. 2010 Apr 10;399(2):290–8. doi: 10.1016/j.virol.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor KG, Paessler S. Pathogenesis of Venezuelan equine encephalitis. Vet Microbiol. 2013 Nov 29;167(1-2):145–50. doi: 10.1016/j.vetmic.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 58.Hevey M, Negley D, Pushko P, et al. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology. 1998 Nov 10;251(1):28–37. doi: 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 59.Herbert AS, Kuehne AI, Barth JF, et al. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. Journal of virology. 2013 May;87(9):4952–64. doi: 10.1128/JVI.03361-12. *A study demonstrating VEEV-vectored vaccines are effective against EBOV infections in NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cliquet F, Aubert M. Elimination of terrestrial rabies in Western European countries. Dev Biol (Basel) 2004;119:185–204. [PubMed] [Google Scholar]
- 62.Cliquet F, Robardet E, Must K, et al. Eliminating rabies in Estonia. PLoS neglected tropical diseases. 2012;6(2):e1535. doi: 10.1371/journal.pntd.0001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blaney JE, Marzi A, Willet M, et al. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural] PLoS pathogens. 2013;9(5):e1003389. doi: 10.1371/journal.ppat.1003389. *A study demonstrating RABV-vectored vaccines are effective against EBOV infections in NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong G, Kobinger G. A strategy to simultaneously eradicate the natural reservoirs of rabies and Ebola virus [Comment] Expert Rev Vaccines. 2012 Feb;11(2):163–6. doi: 10.1586/erv.11.179. [DOI] [PubMed] [Google Scholar]
- 65.Halfmann P, Ebihara H, Marzi A, et al. Replication-deficient ebolavirus as a vaccine candidate [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Journal of virology. 2009 Apr;83(8):3810–5. doi: 10.1128/JVI.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marzi A, Halfmann P, Hill-Batorski L, et al. Vaccines. An Ebola whole-virus vaccine is protective in nonhuman primates [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] Science. 2015 Apr 24;348(6233):439–42. doi: 10.1126/science.aaa4919. *A study demonstrating EBOVΔVP40 vaccines are effective against EBOV infections in NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marzi A, Murphy AA, Feldmann F, et al. Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection [Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] Sci Rep. 2016 Feb 15;6:21674. doi: 10.1038/srep21674. *A study demonstrating CMV-vectored vaccines are effective against EBOV infections in NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Enserink M. The Ebola Epidemic. High hopes for Guinean vaccine trial [News] Science. 2015 Jan 16;347(6219):219–20. doi: 10.1126/science.347.6219.219. [DOI] [PubMed] [Google Scholar]
- 69.Mendoza EJ, Qiu X, Kobinger GP. Progression of Ebola Therapeutics During the 2014-2015 Outbreak [Research Support, Non-U.S. Gov’t Review] Trends Mol Med. 2016 Feb;22(2):164–73. doi: 10.1016/j.molmed.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Tully CM, Lambe T, Gilbert SC, et al. Emergency Ebola response: a new approach to the rapid design and development of vaccines against emerging diseases [Research Support, Non-U.S. Gov’t Review] The Lancet Infectious diseases. 2015 Mar;15(3):356–9. doi: 10.1016/S1473-3099(14)71071-0. [DOI] [PubMed] [Google Scholar]
- 71.Kibuuka H, Berkowitz NM, Millard M, et al. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial [Clinical Trial, Phase I Randomized Controlled Trial Research Support, N.I.H., Intramural Research Support, U.S. Gov’t, Non-P.H.S.] Lancet. 2015 Apr 18;385(9977):1545–54. doi: 10.1016/S0140-6736(14)62385-0. [DOI] [PubMed] [Google Scholar]
- 72.Zhu FC, Hou LH, Li JX, et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial [Randomized Controlled Trial Research Support, Non-U.S. Gov’t] Lancet. 2015 Jun 6;385(9984):2272–9. doi: 10.1016/S0140-6736(15)60553-0. [DOI] [PubMed] [Google Scholar]
- 73.Milligan ID, Gibani MM, Sewell R, et al. Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial [Clinical Trial, Phase I Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Jama. 2016 Apr 19;315(15):1610–23. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 74.Cooper CL, Bavari S. A race for an Ebola vaccine: promises and obstacles. Trends in microbiology. 2015 Feb;23(2):65–6. doi: 10.1016/j.tim.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Rampling T, Ewer K, Bowyer G, et al. A Monovalent Chimpanzee Adenovirus Ebola Vaccine - Preliminary Report. The New England journal of medicine. 2015 Jan 28; doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ledgerwood JE, DeZure AD, Stanley DA, et al. Chimpanzee Adenovirus Vector Ebola Vaccine - Preliminary Report. The New England journal of medicine. 2014 Nov 26; doi: 10.1056/NEJMoa1410863. **First published preliminary report on clinical trials with a candidate vaccine during the 2014-16 EBOV epidemic. [DOI] [PubMed] [Google Scholar]
- 77.Antoine G, Scheiflinger F, Dorner F, et al. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998 May 10;244(2):365–96. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Y, Sullivan NJ. Immunology and evolvement of the adenovirus prime, MVA boost Ebola virus vaccine [Research Support, N.I.H., Intramural Review] Curr Opin Immunol. 2015 Aug;35:131–6. doi: 10.1016/j.coi.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 79.Kreijtz JH, Goeijenbier M, Moesker FM, et al. Safety and immunogenicity of a modified-vaccinia-virus-Ankara-based influenza A H5N1 vaccine: a randomised, double-blind phase 1/2a clinical trial. Lancet Infect Dis. 2014 Dec;14(12):1196–207. doi: 10.1016/S1473-3099(14)70963-6. [DOI] [PubMed] [Google Scholar]
- 80.Satti I, Meyer J, Harris SA, et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial. Lancet Infect Dis. 2014 Oct;14(10):939–46. doi: 10.1016/S1473-3099(14)70845-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greenberg RN, Overton ET, Haas DW, et al. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. The Journal of infectious diseases. 2013 Mar 1;207(5):749–58. doi: 10.1093/infdis/jis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Santis O, Audran R, Pothin E, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study [Clinical Trial, Phase I Clinical Trial, Phase II Randomized Controlled Trial Research Support, Non-U.S. Gov’t] The Lancet Infectious diseases. 2016 Mar;16(3):311–20. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 83.Gunther S, Feldmann H, Geisbert TW, et al. Management of accidental exposure to Ebola virus in the biosafety level 4 laboratory, Hamburg, Germany. The Journal of infectious diseases. 2011 Nov;204(Suppl 3):S785–90. doi: 10.1093/infdis/jir298. [DOI] [PubMed] [Google Scholar]
- 84.Lai L, Davey R, Beck A, et al. Emergency postexposure vaccination with vesicular stomatitis virus-vectored Ebola vaccine after needlestick [Case Reports Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] Jama. 2015 Mar 24-31;313(12):1249–55. doi: 10.1001/jama.2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 Trials of rVSV Ebola Vaccine in Africa and Europe [Clinical Trial, Phase I Randomized Controlled Trial Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] The New England journal of medicine. 2016 Apr 28;374(17):1647–60. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clarke DK, Nasar F, Chong S, et al. Neurovirulence and immunogenicity of attenuated recombinant vesicular stomatitis viruses in nonhuman primates. Journal of virology. 2014 Jun;88(12):6690–701. doi: 10.1128/JVI.03441-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mire CE, Matassov D, Geisbert JB, et al. Single-dose attenuated Vesiculovax vaccines protect primates against Ebola Makona virus. Nature. 2015 Apr 30;520(7549):688–691. doi: 10.1038/nature14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Regules JA, Beigel JH, Paolino KM, et al. A Recombinant Vesicular Stomatitis Virus Ebola Vaccine - Preliminary Report. The New England journal of medicine. 2015 Apr 1; doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.National Institute of Allergy and Infectious Diseases (NIAID) ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [2016 March 29]. Evaluating the Safety of and Immune Response to a Human Parainfluenza Virus Type 3 Ebola Virus Vaccine (HPIV3-EbovZ GP) in Healthy Adults. Available from: https://clinicaltrials.gov/ct2/show/NCT02564575. NLM Identifier: NCT02564575. [Google Scholar]
- 90.Kuehn BM. As Ebola epidemic begins to slow, trials of drugs and vaccines speed up [News] Jama. 2015 Mar 10;313(10):1000–2. doi: 10.1001/jama.2015.0942. [DOI] [PubMed] [Google Scholar]
- 91.Butler D. Ebola experts seek to expand testing [News] Nature. 2014 Dec 11;516(7530):154–5. doi: 10.1038/516154a. [DOI] [PubMed] [Google Scholar]
- 92.Caplan AL, Plunkett C, Levin B. Selecting the right tool for the job. Am J Bioeth. 2015;15(4):4–10. doi: 10.1080/15265161.2015.1010993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Janssen Vaccines & Prevention B.V. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [2016 March 30]. Staged Phase 3 Study to Assess the Safety and Immunogenicity of Ebola Candidate Vaccines Ad26.ZEBOV and MVA-BN-Filo During Implementation of Stages 1 and 2. Available from: https://clinicaltrials.gov/ct2/show/record/NCT02509494. NLM Identifier: NCT02509494. [Google Scholar]
- 94.Mohammadi D. Ebola vaccine trials back on track [News] Lancet. 2015 Jan 17;385(9964):214–5. doi: 10.1016/S0140-6736(15)60035-6. [DOI] [PubMed] [Google Scholar]
- 95.Gulland A. Ebola vaccine to be tested on 30,000 volunteers [News] Bmj. 2015;350:h448. doi: 10.1136/bmj.h448. [DOI] [PubMed] [Google Scholar]
- 96.National Institute of Allergy and Infectious Diseases (NIAID) ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [2016 March 25]. Partnership for Research on Ebola Vaccines in Liberia (PREVAIL) Available from: https://clinicaltrials.gov/ct2/show/NCT02344407. NML Identifier: NCT02344407. [Google Scholar]
- 97.Kennedy SB, Neaton JD, Lane HC, et al. Implementation of an Ebola virus disease vaccine clinical trial during the Ebola epidemic in Liberia: Design, procedures, and challenges. Clin Trials. 2016 Feb;13(1):49–56. doi: 10.1177/1740774515621037. [DOI] [PubMed] [Google Scholar]
- 98.Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) [8 November 2017];Centers for Disease Control and Prevention. 2015 Available from: http://www.cdc.gov/vhf/ebola/strive/qa.html.
- 99.Centers for Disease Control and Prevention. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [2016 March 30]. STRIVE (Sierra Leone Trial to Introduce a Vaccine Against Ebola) Available from: https://clinicaltrials.gov/ct2/show/NCT02378753. NML Identifier: NCT02378753. [Google Scholar]
- 100.Widdowson MA, Schrag SJ, Carter RJ, et al. Implementing an Ebola Vaccine Study - Sierra Leone. MMWR Suppl. 2016 Jul 08;65(3):98–106. doi: 10.15585/mmwr.su6503a14. [DOI] [PubMed] [Google Scholar]
- 101.Questions and Answers – Ebola Phase III Vaccine Trial in Guinea. [8 November 2017];World Health Organization. 2015 Available from: http://www.who.int/medicines/ebola-treatment/q_a_vaccine_trial_guinea/en.
- 102.Muller J, Schonfisch B, Kirkilionis M. Ring vaccination [Comparative Study] J Math Biol. 2000 Aug;41(2):143–71. doi: 10.1007/s002850070003. [DOI] [PubMed] [Google Scholar]
- 103.Ebola ça Suffit Ring Vaccination Trial Consortium. The ring vaccination trial: a novel cluster randomised controlled trial design to evaluate vaccine efficacy and effectiveness during outbreaks, with special reference to Ebola. BMJ. 2015 Jul 27;351:h3740. doi: 10.1136/bmj.h3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial [Randomized Controlled Trial Research Support, Non-U.S. Gov’t] Lancet. 2015 Aug 29;386(9996):857–66. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 105.Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!) Lancet. 2017 Feb 04;389(10068):505–518. doi: 10.1016/S0140-6736(16)32621-6. **Clinical findings from the Ebola ça suffit trial clinical trial showing 100% efficacy with the VSV-vectored vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination [Research Support, N.I.H., Extramural] Clin Infect Dis. 2012 Jun;54(11):1615–7. doi: 10.1093/cid/cis238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plotkin SA. Vaccines: correlates of vaccine-induced immunity [Review] Clin Infect Dis. 2008 Aug 1;47(3):401–9. doi: 10.1086/589862. *An important review providing the definition of immune correlates induced by vaccination by Dr. Stanley Plotkin. [DOI] [PubMed] [Google Scholar]
- 108.Sullivan NJ, Martin JE, Graham BS, et al. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol. 2009 May;7(5):393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olinger GG, Jr, Pettitt J, Kim D, et al. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):18030–5. doi: 10.1073/pnas.1213709109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qiu X, Audet J, Wong G, et al. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies [Research Support, Non-U.S. Gov’t] Sci Transl Med. 2012 Jun 13;4(138):138ra81. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- 111.Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] Nature. 2014 Oct 2;514(7520):47–53. doi: 10.1038/nature13777. **Landmark study which shows a monoclonal antibody cocktail can reverse advanced EBOV disease in nonhuman primates. [DOI] [PMC free article] [PubMed] [Google Scholar]


