Abstract
The aim of this study was to provide an evidence-based answer to the question: “Is 3.6-mL volume of an anesthetic agent more effective than 1.8-mL volume in providing anesthesia for mandibular molars?” Following formulation of research question and keyword selection, a comprehensive search of the following databases was conducted: Cochrane library, PubMed, Scopus, Google Scholar, ProQuest, and Clinicaltrials.gov. Three-phase eligibility appraisal and quality assessment of the studies were carried out by 2 independent reviewers. To reduce clinical heterogeneity, the included studies were divided into 2 groups: studies on healthy teeth and studies on teeth with pulpitis. The data of included studies were statistically combined through meta-analysis using a fixed-effects model. A total of 20,778 records were initially retrieved from the search. Following screening and eligibility assessment, 8 studies met the eligibility criteria and were included for qualitative synthesis. Of those, 5 studies were qualified for meta-analysis. In the irreversible pulpitis group, increasing the volume of anesthetic agent from 1.8 to 3.6 mL significantly increased the success rate of inferior alveolar nerve block (risk ratio = 2.45, 95% CI: 1.67–3.59, p < .001). However, there was insufficient evidence to draw a conclusion regarding healthy teeth.
Key Words: Anesthetic success, Anesthetic volume, Inferior alveolar nerve block, Mandibular molar, Meta-analysis, Systematic review
Providing profound pulpal anesthesia is still a challenge in endodontics. This problem is more pronounced in inflamed mandibular posterior teeth. The inferior alveolar nerve block (IANB) is the most commonly used technique for anesthetizing mandibular posterior teeth. However, it has the highest percentage of clinical failure among mandibular anesthetic techniques, even when it is administered properly.1,2 Failure rates of 10–61% in uninflamed teeth to over 70% in teeth with inflamed pulps have been reported.3–8
Various methods have been proposed to increase the success rate of IANB, including supplemental buccal and/or lingual infiltrations,9 the use of various anesthetic agents and injection techniques,7,10–12 supplemental intraligamental or intraosseous injections,12 and increasing the volume of anesthetic agents.5,13–16 The latter approach is based on the hypothesis that a sufficient length of axon should be exposed to anesthetic agent for effective blockade of nerve impulses.11,13 In IANB, the anesthetic agent is deposited in pterygomandibular space to target the inferior alveolar nerve before entering the mandibular foramen. According to this hypothesis, increasing the volume of anesthetic agent exposes greater length of the inferior alveolar nerve to higher concentration of the agent, thereby providing more profound anesthesia.
The efficacy of this approach in clinical condition has been the focus of several studies. Yared and Dagher17 evaluated the success rate of IANB in teeth with uninflamed pulps using 3.6 mL of 2% lidocaine with 3 different concentrations of epinephrine. They found no difference between success rates of 3 formulations. The authors retrospectively compared the results of their study with the data of another study18 with similar methodology that used 1.8 mL of 2% lidocaine. They found a statistically higher success rate of IANB with 3.6-mL in comparison to 1.8-mL volume. However, 2 subsequent randomized controlled clinical trials (RCTs)14,15 failed to show any significant difference between 3.6 and 1.8 mL of anesthetic solution in healthy uninflamed mandibular teeth.
Similar studies on teeth with irreversible pulpitis also showed conflicting results. In a retrospective study on data of 7 previous researches, Fowler and Reader19 concluded that success rates of 3.6 and 1.8 mL of 2% lidocaine were not significantly different in teeth with irreversible pulpitis. This was in agreement with the results of an RCT by Parirokh et al.16 However, 2 subsequent RCTs carried out on patients presenting with irreversible pulpitis showed a higher success rate of 3.6- compared to 1.8-mL volume.5,13
The conflicting results of the studies regarding the effect of different volumes of anesthetic agents on the success rate of IANB and the lack of any systematic review on this issue justify conducting the present study. Therefore, this study was designed as a systematic review and meta-analysis of the available evidence to answer the question whether 3.6-mL volume of an anesthetic agent is more effective than 1.8-mL volume in providing anesthesia for mandibular molar teeth.
METHODS
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols.20,21
Formulating Research Question and Selection of Keywords
The Population, Intervention, Comparison, and Outcome question defined in this systematic review was: “Is 3.6-mL volume of an anesthetic agent (I) more effective than 1.8-mL volume (C) in providing anesthesia (O) for mandibular molar teeth (P) in IANB?”
Based on this question, the following keywords and MeSH terms were selected: local anesthesia, mandibular block, inferior alveolar nerve block, mandibular molar, mandibular posterior teeth, anesthetic success, anesthetic volume(s), and anesthesia volume(s). Both spellings of “anesthesia” and “anaesthesia” were separately searched.
Eligibility Criteria
The included studies were RCTs that compared the anesthetic efficacy of different volumes of anesthetic agents for IANB in adult patients. Only the studies that evaluated the anesthetic success in mandibular molars, either with healthy pulp or with irreversible pulpitis, were included. The retrospective studies that used the data of similar previous studies were also included. The studies that used anesthetic delivery routes other than IANB were excluded.
Search Strategy
A comprehensive search of the following electronic databases was conducted: Cochrane library, PubMed, Scopus, and Google Scholar. The selected keywords and MeSH terms were individually searched or combined using Boolean operators. To cover the gray literature, ProQuest and Scopus were searched to identify relevant theses and conference proceedings, respectively. Additionally, the www.clinicaltrials.gov Web site was searched to identify unpublished completed RCTs. The search was also supplemented by hand searching major textbooks: Handbook of Local Anesthesia,22 Cohen's Pathways of the Pulp,23 and Ingle's Endodontics.24 Reference lists of the selected articles were also scanned to identify relevant studies (backward search). Forward reference searching was also performed to identify the relevant studies that cited the selected articles. No language or time restrictions were applied.
All searches were conducted on March 1–3, 2016. To identify the studies published from that time until the completion of critical assessment stage on July 1, 2016, automated search with e-mail alert was activated in each search engine.
Study Selection Process
The screening and eligibility assessment of studies were conducted in a 3-phase process. In initial screening (phases 1 and 2), the titles and abstracts of the retrieved studies were reviewed by 2 independent endodontists (first and second authors). In phase 3 (detailed appraisal), the full texts of selected articles were reviewed by the same reviewers. In each stage, the irrelevant studies that did not meet the eligibility criteria were excluded. Any disagreement between the reviewers was resolved by the means of discussion.
Data Extraction Process
For the purpose of data extraction, the included studies were divided into RCT or non-RCT studies. In the RCT group, one reviewer (first author) extracted the data directly from the full text of the articles to a structured data extraction table. The second reviewer (second author) verified the extracted data and confirmed its accuracy. Any disagreement between reviewers was resolved by discussion. The following data were extracted from each included study: main article information (authors, year, title, journal) and study characteristics (study design, sample size, sex distribution and mean age of the participants, preoperative pulp diagnosis, tooth type, anesthetic agent used and its dosage, definition of anesthetic success and method used to assess it, method of randomization, allocation concealment, and blindness, and findings of the study).
The non-RCT group included retrospective studies that compared the success rates of the 2 volumes in different previous studies. In each study, either 1.8 or 3.6 mL of anesthetic agent was used as control solution for IANB. Full texts of all the included articles in each retrospective study were appraised by 2 reviewers (first and third authors), and relevant data were extracted into an extraction table similar to RCT studies. The accuracy of the reported results was checked by critical assessment and statistical analysis of the data. Any disagreement between reviewers was resolved by discussion.
Quality Assessment
Risk of bias assessment of included studies was carried out by 2 independent reviewers (first and second authors) using a standard table based on the Cochrane tool for assessing risk of bias.25 Any disagreement between 2 reviewers was resolved by discussion. In this table, selection bias involves bias in random sequence generation and/or allocation concealment. To evaluate performance bias, blindness of participants and personnel is evaluated. To appraise detection bias, blindness of outcome assessors is evaluated. Attrition bias and reporting bias are also detected through incomplete outcome data and selective reporting, respectively.
Synthesis of the Results
The definition of anesthetic success in studies on healthy teeth was different from that in studies on teeth with irreversible pulpitis. Therefore, to reduce clinical heterogeneity, the included studies were divided into 2 groups: studies on healthy teeth and studies on teeth with irreversible pulpitis.
The outcome variable was dichotomous (success, failure). Thus, risk ratio (RR) was used as the outcome measure of study effects. Statistical heterogeneity was assessed by using I2 statistics. A value greater than 50% was considered an indicator of substantial heterogeneity between studies.26 In each group, the data of studies were statistically combined through meta-analysis using a fixed-effects model. The difference between 2 anesthetic volumes was graphically expressed in a forest plot. Finally, we carried out retrospective statistical power analysis in each group.
RESULTS
Study Selection
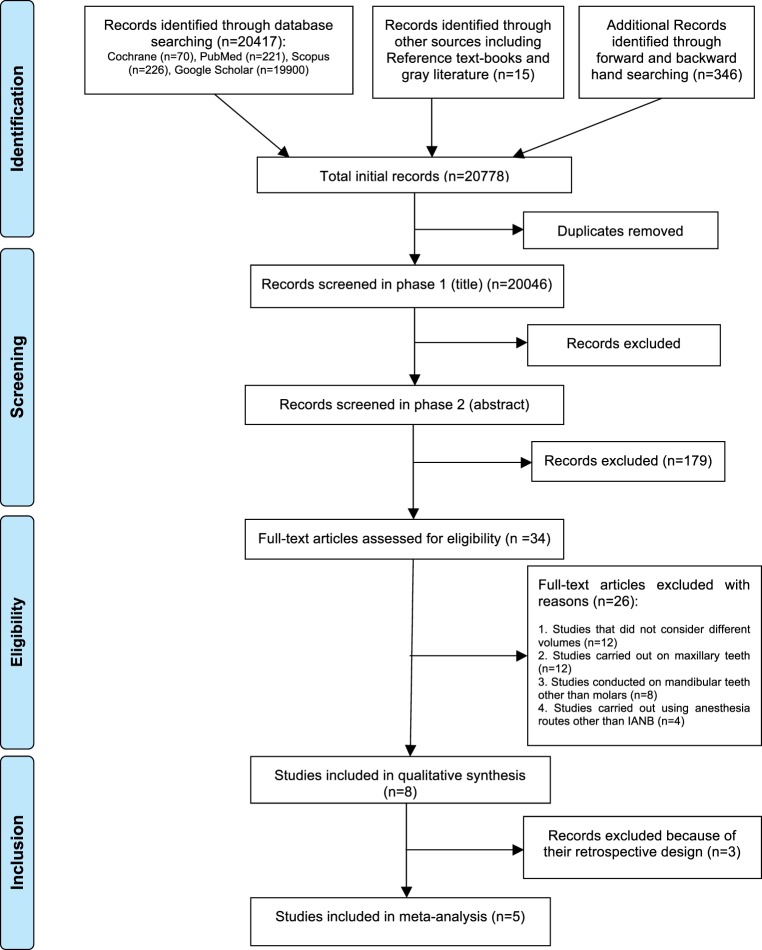
The Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols flow diagram depicting the process of article inclusion is shown in Figure 1. A total of 20,778 records were retrieved through initial search of electronic databases, main textbooks, gray literature, and forward and backward hand searching. Following exclusion of duplicates, 20,046 records were entered in phase 1 screening. A total of 20,012 records were excluded in the phase 1 and 2 screening process (interreviewer agreement, κ = 0.93). Full texts of the remaining 34 papers were assessed in phase 3.
Figure 1. .
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols flow diagram of study inclusion process.
Additionally, 87 articles were retrieved through automated search alerts during the eligibility assessment and critical appraisal process. All these recently published articles were excluded during phase 1 and 2 screening. Finally, 8 studies fulfilled the eligibility criteria and were included for qualitative synthesis. Of those, 5 studies were qualified for meta-analysis.
Characteristics of Studies
The main characteristics of included studies are summarized in Table 1. The studies consisted of 4 articles on healthy teeth and 4 articles on teeth with irreversible pulpitis. There was no limitation in definition of outcome—anesthetic success—in eligibility criteria. However, in all the studies on healthy teeth, anesthetic success had been defined as 2 consecutive negative responses to maximum output of electric pulp tester within 15 minutes that had been continuously sustained for 55–60 minutes.3,14,15,17 On the other hand, in all the papers on teeth with irreversible pulpitis, anesthetic success had been defined as no pain or mild/weak pain during endodontic access preparation and instrumentation according to patient-reported pain scores (Heft-Parker visual analogue scale ≤55 mm).5,13,16,19
Table 1. .
Characteristics of Studies That Compared the Anesthetic Success Rate of 2 Volumes of Anesthetic Agents for Mandibular Molars
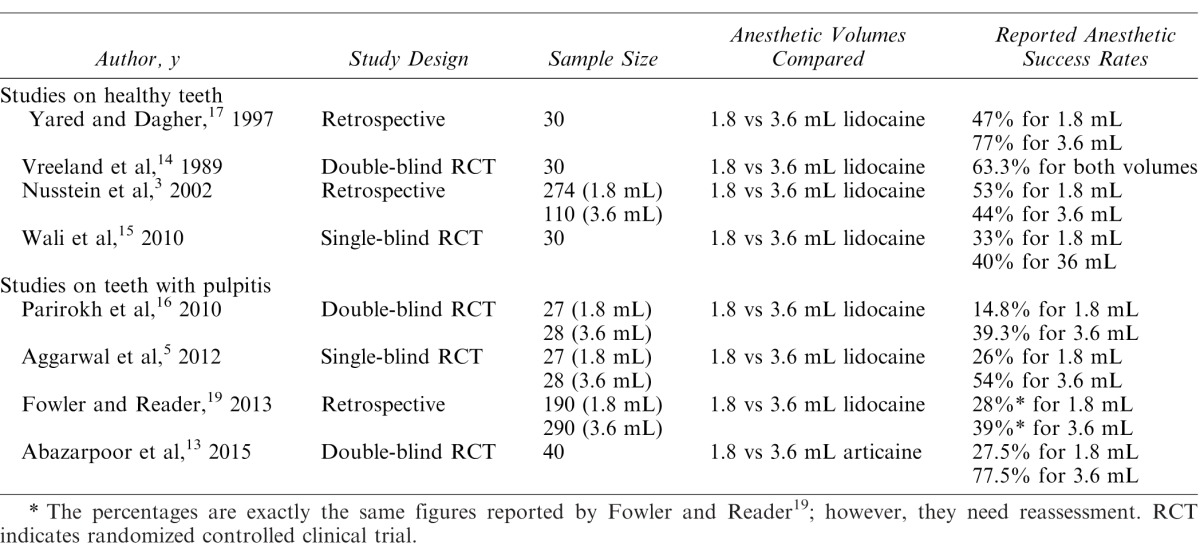
Of the included studies, 3 articles—2 studies on healthy teeth and 1 on teeth with pulpitis—were not RCT studies and had retrospective design. Therefore, they were not entered in the final meta-analysis.
Quality Assessment Results
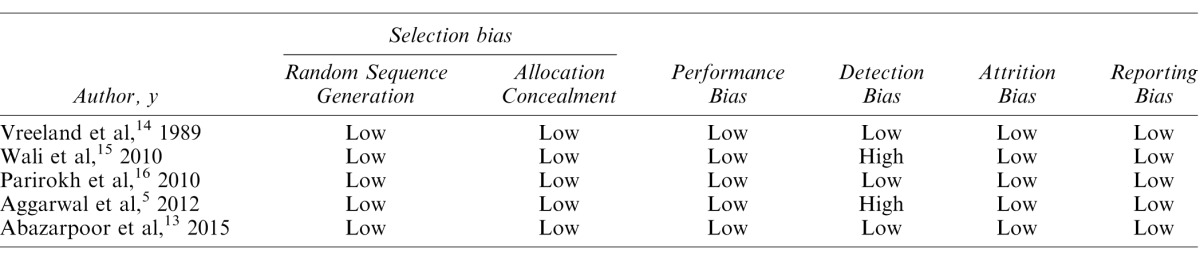
Risk of bias assessment of the included RCTs is shown in Table 2. In 3 studies, the risks were low across all categories.13,14,16 Two studies, one on healthy teeth and the other on irreversible pulpitis, had a single-blind design. Thus, risk of detection bias was evaluated as high in these studies. Any conflict of interest was denied in 4 studies3,5,13,19 and not mentioned in the other 4 studies.14–17
Table 2. .
Risk of Bias Assessment of Studies According to the Cochrane Collaboration's Tool
Synthesis of the Results
Heterogeneity test showed homogeneity of the results in healthy and pulpitis groups (I2 = 0.00). Therefore, in each group, the data were statistically combined using a fixed-effects model (Figure 2).
Figure 2. .
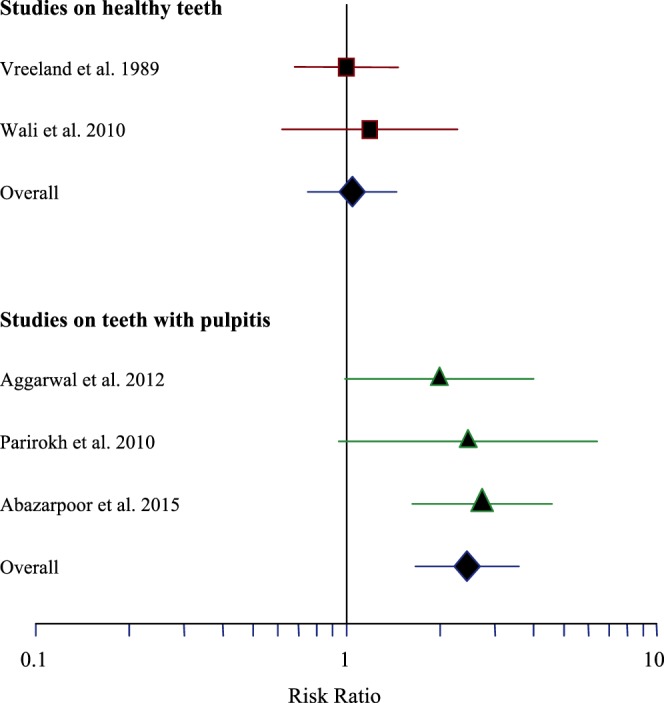
Forest plot of risk ratios (95% CI).
In the healthy teeth group, there was no statistically significant difference between success rates of 3.6- and 1.8-mL volumes (RR = 1.05, 95% CI: 0.75–1.45, p = .704).
In the irreversible pulpitis group, increasing the volume of anesthetic agent from 1.8 to 3.6 mL significantly increased the success rate of IANB (RR = 2.45, 95% CI: 1.67–3.59, p < .001).
To evaluate the effect of the study with high risk of bias5 on overall results, sensitivity analysis was performed in this group by conducting a meta-analysis that excluded that study. The sensitivity analysis showed that exclusion of the study in question did not substantially alter the overall study results (RR = 2.68, 95% CI: 1.70–4.22, p < .001, I2 =.0%).
The retrospective power analysis showed that the overall power was 85%; however, the statistical powers in healthy and pulpitis groups were 5 and 97%, respectively. Therefore, in the healthy group, given the number of studies and the average within-study sample size in those studies, power to detect the smallest important effect was low (approximately .05).
DISCUSSION
The aim of the present systematic review was to clarify whether doubling the volume of anesthetic agents for IANB provides more successful anesthesia for mandibular molars. To find an evidence-based answer, a comprehensive search of various databases was conducted using multiple search strategies. An important information source that is frequently overlooked in systematic reviews is gray literature. It consists of theses, reports, conference proceedings, and unpublished studies. Missing this source of information is a cause of publication bias in systematic reviews.27 To address the issue in the present study, a thorough search of Scopus and ProQuest databases and the www.clinicaltrials.gov Web site was carried out. Another potential source of publication bias in systematic reviews is duplicate studies.27 In the present study, the duplicates were detected and excluded using Endnote-X5 software (Thompson Reuters, NY) and carefully appraising the full texts of included studies.
In this systematic review, the included studies were categorized in 2 groups: healthy and pulpitis. Achieving profound pulpal anesthesia in inflamed teeth is more difficult than in teeth with healthy pulp.28 Meanwhile, as mentioned earlier, the definition of anesthetic success was different in these 2 groups. Therefore, this grouping of included studies resulted in substantial reduction of clinical heterogeneity within each group. Also, I2 statistics revealed statistical homogeneity in each group.
In the present study, 3 articles that met the inclusion criteria were not included in the final meta-analysis. Of these, 2 articles were on healthy teeth,3,17 and 1 was on teeth with irreversible pulpitis.19 In the first article, Nusstein et al3 used the data from the 13 previously reported studies on healthy teeth. In each study, either 1.8 or 3.6 mL of 2% lidocaine had been used as the control solution for the IANB. Nusstein et al3 compared the anesthetic efficacies of the 2 volumes in these studies. The authors concluded that the success rates of 1.8 and 3.6 mL for IANB were not significantly different (53 and 44%, respectively). That study was not a typical RCT because groups from different studies with different patient populations had been compared with each other. Therefore, it was not included in the final meta-analysis. Another study that was not included in the meta-analysis also used the data of 2 separate studies.17 It showed enhanced anesthetic efficacy with increasing anesthetic agent volume (47 and 77% success rate for 1.8 vs 3.6 mL, respectively). The third study that was not included in meta-analysis because of its retrospective design was Fowler and Reader's19 study. They used the data of 7 separate studies. Careful assessment of the included studies revealed that for some studies, the anesthetic volumes reported were not accurate.7,29 Meanwhile, some of the included studies also used supplemental long buccal injection.6,8,30,31
The first finding of the present meta-analysis was that increasing the anesthetic volume does not improve the anesthetic efficacy in healthy mandibular molars. However, as mentioned, given the small number of studies and the average within-study sample size in those studies, power to detect the smallest important effect was low. Therefore, the most reasonable conclusion to be drawn from this result is that we currently do not have enough information to judge adequately whether increasing the volume of anesthesia has a meaningful effect on success rate of IANB in healthy teeth.
Mandibular anesthesia via the IANB has a relatively low success rate.3–8,11 This was also shown in the present systematic review. Several reasons have been proposed for this low success rate, including patient anxiety and fear,11 inaccurate injections,11,28 needle deflection,11,28 cross-innervations,4 and accessory innervations from long buccal, lingual, mylohyoid, or transverse cervical nerve.11,32–35 However, the exact reason is still unclear.
The second finding of this meta-analysis is that doubling the anesthetic volume for IANB improves the anesthetic efficacy in irreversibly inflamed mandibular molars. The success rate of IANB in teeth with irreversible pulpitis is substantially lower than that in healthy teeth.34,36,37 One hypothesis to explain this lower success rate is that the nerves arising from the inflamed pulps have altered resting potential and reduced excitability threshold.28,38,39 Thus, the anesthetic agents have trouble in preventing the transmission of impulses.38,40 This hypothesis may also explain the second finding of the present study. There is another theory that can help explain the second finding. As mentioned, sufficient length of nerve trunk should be exposed to anesthetic agents for complete blockade of impulse transmission.41 Therefore, it has been suggested to fill the pterygomandibular space with the anesthetic solutions so that the maximum length of the inferior alveolar nerve is exposed to the agent.5,42 Based on this theory, 3.6 mL of anesthetic solution theoretically provides higher amount of anesthetic agent around inferior alveolar nerve trunk.5,42
Certainly, this systematic review has its own limitations. First, only the studies on mandibular molars were included in this review. Thus, it is recommended not to generalize the findings of this study to all mandibular teeth. Second, the available studies on different anesthetic volumes only evaluated lidocaine and articaine, and there are no data on other anesthetic agents. It is unclear whether these results differ with different anesthetic agents or not. Therefore, the findings of this systematic review should be generalized to other anesthetic agents with caution.
In conclusion, increasing the anesthetic volume improves the anesthesia in mandibular molars with irreversible pulpitis. However, more studies are needed to draw a conclusion on healthy teeth. The overall anesthetic success rate for irreversibly inflamed mandibular molars even with greater volumes is low, and supplemental anesthetic techniques are frequently required to ensure pain-free endodontic treatment of these teeth.
REFERENCES
- 1. . Kanaa MD, Whitworth JM, Corbett IP, Meechan JG. . Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block. Int Endod J. 2009; 42: 238– 246. [DOI] [PubMed] [Google Scholar]
- 2. . Kaufman E, Weinstein P, Milgrom P. . Difficulties in achieving local anesthesia. J Am Dent Assoc. 1984; 108: 205– 208. [DOI] [PubMed] [Google Scholar]
- 3. . Nusstein J, Reader A, Beck FM. . Anesthetic efficacy of different volumes of lidocaine with epinephrine for inferior alveolar nerve blocks. Gen Dent. 2002; 50: 372– 375; quiz 376–377. [PubMed] [Google Scholar]
- 4. . Yonchak T, Reader A, Beck M, Meyers WJ. . Anesthetic efficacy of unilateral and bilateral inferior alveolar nerve blocks to determine cross innervation in anterior teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 92: 132– 135. [DOI] [PubMed] [Google Scholar]
- 5. . Aggarwal V, Singla M, Miglani S, Kohli S, Singh S. . Comparative evaluation of 1.8 mL and 3.6 mL of 2% lidocaine with 1:200,000 epinephrine for inferior alveolar nerve block in patients with irreversible pulpitis: a prospective, randomized single-blind study. J Endod. 2012; 38: 753– 756. [DOI] [PubMed] [Google Scholar]
- 6. . Bigby J, Reader A, Nusstein J, Beck M. . Anesthetic efficacy of lidocaine/meperidine for inferior alveolar nerve blocks in patients with irreversible pulpitis. J Endod. 2007; 33: 7– 10. [DOI] [PubMed] [Google Scholar]
- 7. . Claffey E, Reader A, Nusstein J, Beck M, Weaver J. . Anesthetic efficacy of articaine for inferior alveolar nerve blocks in patients with irreversible pulpitis. J Endod. 2004; 30: 568– 571. [DOI] [PubMed] [Google Scholar]
- 8. . Matthews R, Drum M, Reader A, Nusstein J, Beck M. . Articaine for supplemental buccal mandibular infiltration anesthesia in patients with irreversible pulpitis when the inferior alveolar nerve block fails. J Endod. 2009; 35: 343– 346. [DOI] [PubMed] [Google Scholar]
- 9. . Aggarwal V, Jain A, Kabi D. . Anesthetic efficacy of supplemental buccal and lingual infiltrations of articaine and lidocaine after an inferior alveolar nerve block in patients with irreversible pulpitis. J Endod. 2009; 35: 925– 929. [DOI] [PubMed] [Google Scholar]
- 10. . Aggarwal V, Singla M, Kabi D. . Comparative evaluation of anesthetic efficacy of Gow-Gates mandibular conduction anesthesia, Vazirani-Akinosi technique, buccal-plus-lingual infiltrations, and conventional inferior alveolar nerve anesthesia in patients with irreversible pulpitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109: 303– 308. [DOI] [PubMed] [Google Scholar]
- 11. . Potocnik I, Bajrovic F. . Failure of inferior alveolar nerve block in endodontics. Endod Dent Traumatol. 1999; 15: 247– 251. [DOI] [PubMed] [Google Scholar]
- 12. . Hargreaves KM, Keiser K. . Local anesthetic failure in endodontics. Endod Topics. 2002; 1: 26– 39. [Google Scholar]
- 13. . Abazarpoor R, Parirokh M, Nakhaee N, Abbott PV. . A comparison of different volumes of articaine for inferior alveolar nerve block for molar teeth with symptomatic irreversible pulpitis. J Endod. 2015; 41: 1408– 1411. [DOI] [PubMed] [Google Scholar]
- 14. . Vreeland DL, Reader A, Beck M, Meyers W, Weaver J. . An evaluation of volumes and concentrations of lidocaine in human inferior alveolar nerve block. J Endod. 1989; 15: 6– 12. [DOI] [PubMed] [Google Scholar]
- 15. . Wali M, Drum M, Reader A, Nusstein J. . Prospective, randomized single-blind study of the anesthetic efficacy of 1.8 and 3.6 milliliters of 2% lidocaine with 1:50,000 epinephrine for inferior alveolar nerve block. J Endod. 2010; 36: 1459– 1462. [DOI] [PubMed] [Google Scholar]
- 16. . Parirokh M, Satvati SA, Sharifi R, et al. . Efficacy of combining a buccal infiltration with an inferior alveolar nerve block for mandibular molars with irreversible pulpitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109: 468– 473. [DOI] [PubMed] [Google Scholar]
- 17. . Yared GM, Dagher FB. . Evaluation of lidocaine in human inferior alveolar nerve block. J Endod. 1997; 23: 575– 578. [DOI] [PubMed] [Google Scholar]
- 18. . Dagher FB, Yared GM, Machtou P. . An evaluation of 2% lidocaine with different concentrations of epinephrine for inferior alveolar nerve block. J Endod. 1997; 23: 178– 180. [DOI] [PubMed] [Google Scholar]
- 19. . Fowler S, Reader A. . Is a volume of 3.6 mL better than 1.8 mL for inferior alveolar nerve blocks in patients with symptomatic irreversible pulpitis? J Endod. 2013; 39: 970– 972. [DOI] [PubMed] [Google Scholar]
- 20. . Moher D, Liberati A, Tetzlaff J, Altman DG. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009; 62: 1006– 1012. [DOI] [PubMed] [Google Scholar]
- 21. . Shamseer L, Moher D, Clarke M, et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015; 349:g7647. [DOI] [PubMed]
- 22. . Malamed SF. . Handbook of Local Anesthesia. St. Louis, Missouri: Elsevier Health Sciences; 2014. [Google Scholar]
- 23. . Hargreaves KM, Berman LH. . Cohen's Pathways of the Pulp Expert Consult. St. Louis, Missouri: Elsevier Health Sciences; 2015. [Google Scholar]
- 24. . Ingle JI. . Ingle's Endodontics 6. Ontario, Canada: PMPH-USA; 2008. [Google Scholar]
- 25. . Higgins JP, Altman DG, Gotzsche PC, et al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. [DOI] [PMC free article] [PubMed]
- 26. . Leonardi Dutra K, Haas L, Porporatti AL, et al. . Diagnostic accuracy of cone-beam computed tomography and conventional radiography on apical periodontitis: a systematic review and meta-analysis. J Endod. 2016; 42: 356– 364. [DOI] [PubMed] [Google Scholar]
- 27. . Glasziou P, Irwig L, Bain C, Colditz G. . Systematic reviews in health care: a practical guide. Cambridge, England: Cambridge University Press; 2001. [Google Scholar]
- 28. . Nusstein JM, Reader A, Drum M. . Local anesthesia strategies for the patient with a “hot” tooth. Dent Clin North Am. 2010; 54: 237– 247. [DOI] [PubMed] [Google Scholar]
- 29. . Stanley W, Drum M, Nusstein J, Reader A, Beck M. . Effect of nitrous oxide on the efficacy of the inferior alveolar nerve block in patients with symptomatic irreversible pulpitis. J Endod. 2012; 38: 565– 569. [DOI] [PubMed] [Google Scholar]
- 30. . Oleson M, Drum M, Reader A, Nusstein J, Beck M. . Effect of preoperative ibuprofen on the success of the inferior alveolar nerve block in patients with irreversible pulpitis. J Endod. 2010; 36: 379– 382. [DOI] [PubMed] [Google Scholar]
- 31. . Simpson M, Drum M, Nusstein J, Reader A, Beck M. . Effect of combination of preoperative ibuprofen/acetaminophen on the success of the inferior alveolar nerve block in patients with symptomatic irreversible pulpitis. J Endod. 2011; 37: 593– 597. [DOI] [PubMed] [Google Scholar]
- 32. . Frommer J, Mele FA, Monroe CW. . The possible role of the mylohyoid nerve in mandibular posterior tooth sensation. J Am Dent Assoc. 1972; 85: 113– 117. [DOI] [PubMed] [Google Scholar]
- 33. . Wilson S, Johns P, Fuller PM. . The inferior alveolar and mylohyoid nerves: an anatomic study and relationship to local anesthesia of the anterior mandibular teeth. J Am Dent Assoc. 1984; 108: 350– 352. [DOI] [PubMed] [Google Scholar]
- 34. . Meechan JG. . How to overcome failed local anaesthesia. Br Dent J. 1999; 186: 15– 20. [DOI] [PubMed] [Google Scholar]
- 35. . Lin K, Uzbelger Feldman D, Barbe MF. . Transverse cervical nerve: implications for dental anesthesia. Clin Anat. 2013; 26: 688– 692. [DOI] [PubMed] [Google Scholar]
- 36. . Modaresi J, Mozayeni MA, Dianat O. . Comparing the quality of anaesthesia in normal and inflamed teeth by pulp testing. Aust Endod J. 2005; 31: 120– 122. [DOI] [PubMed] [Google Scholar]
- 37. . Boopathi T, Sebeena M, Sivakumar K, Harikaran J, Karthick K, Raj A. . Supplemental pulpal anesthesia for mandibular teeth. J Pharm Bioallied Sci. 2013; 5: S103– S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. . Wallace JA, Michanowicz AE, Mundell RD, Wilson EG. . A pilot study of the clinical problem of regionally anesthetizing the pulp of an acutely inflamed mandibular molar. Oral Surg Oral Med Oral Pathol. 1985; 59: 517– 521. [DOI] [PubMed] [Google Scholar]
- 39. . Byers MR, Taylor PE, Khayat BG, Kimberly CL. . Effects of injury and inflammation on pulpal and periapical nerves. J Endod. 1990; 16: 78– 84. [DOI] [PubMed] [Google Scholar]
- 40. . Modaresi J, Dianat O, Soluti A. . Effect of pulp inflammation on nerve impulse quality with or without anesthesia. J Endod. 2008; 34: 438– 441. [DOI] [PubMed] [Google Scholar]
- 41. . Franz DN, Perry RS. . Mechanisms for differential block among single myelinated and non-myelinated axons by procaine. J Physiol. 1974; 236: 193– 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. . Kohler BR, Castellon L, Laissle G. . Gow-Gates technique: a pilot study for extraction procedures with clinical evaluation and review. Anesth Prog. 2008; 55: 2– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]