Abstract
Introduction
The first drug selected for treatment of gastro-oesophageal reflux disease (GERD) and prevention of the recurrence is a proton pump inhibitor (PPI), but recently, a potassium-competitive acid blocker (P-CAB) was put on the market in Japan. Its onset of effect is faster than PPI, and it takes more than 2 days to recover acid secretion after the withdrawal period. Therefore, unlike PPI, the usefulness of every other day administration or discontinuous administration is expected.
Methods and analysis
This study is a prospective, multicentre, open-label, two-period randomised cross-over study to compare the efficacy and safety of PPI every other day administration and P-CAB every other day administration in 120 patients who receive erosive GERD maintenance therapy with PPI. Patients will be randomly allocated to receive 4 weeks P-CAB or PPI followed by 4 weeks cross over, where those on P-CAB will receive PPI and vice versa. The primary endpoint is proportion of asymptomatic patients. Secondary endpoints are suppressive effect of GERD symptoms, proportion of asymptomatic patients at each time point, safety and cost-saving effect of P-CAB every other day administration, compliance with every other day administration, and proportion of asymptomatic patients at the first month of study drug administration.
Ethics and dissemination
This study was approved by the National Hospital Organization Central Review Board for Clinical Trials (5 December 2017).
Discussion
If P-CAB every other day administration is established as one of GERD maintenance therapies, there is merit in both medical cost reduction and the safety to alleviate elevation in serum gastrin.
Trial registration number
UMIN000034701.
Keywords: acid, gastric acid secretion, gastro-esophageal reflux disease
Introduction
Gastro-oesophageal reflux disease (GERD) is a condition that caused symptoms such as heartburn and acid regurgitation due to backflow of stomach contents, and the prevalence rate of GERD has dramatically increased in Japan. The first drug for GERD treatment and prevention of the recurrence is a proton pump inhibitor (PPI).1 Recently, potassium-competitive acid blocker (P-CAB) which exerts stronger and more stable acid suppressive effect than PPI was put on the market in Japan.2 Owing to its pharmacological action, unlike PPI, P-CAB shows the maximum acid suppressive effect immediately after administration, and it has the characteristic that the acid suppressive effect lasts for a few days after discontinuation of the administration.3–5 Therefore, a single administration of P-CAB showed significant acid suppressive effect compared with double-dose administration of PPI, and its efficacy against PPI-resistant GERD has been confirmed.6–8 Since the incidence of relapse becomes high after the initial treatment of GERD, maintenance therapy with PPI is required to prevent recurrence. As maintenance therapy of GERD, there are continuous administration, intermittent administration, and discontinuous (on demand) administration of PPI.9 Also in GERD clinical practice guideline 2015, maintenance therapy for mild reflux esophagitis which accounts for 90% of the total of GERD recommends step-down therapy to reduce the dose to the minimum if symptom control is possible.1 If symptoms are suppressed by daily administration, it is common to take half-dose administration or every other day administration or intermittent therapy as the next step. From the pharmacological characteristics of P-CAB, it is presumed that there is no significant difference in acid suppressive effect between daily administration and every other day administration of P-CAB. On the other hand, since PPI has a slow onset of effect and maximum acid suppression is achieved on the 4–5 days after daily administration, it is considered that acid suppressive effect cannot be sufficiently obtained by PPI every other day administration.
For easily relapsing GERD in which recurrence of symptoms easily occurs due to discontinuation of PPI administration, it is difficult to transfer from continuous administration of PPI as GERD maintenance therapy to dosage reduction, intermittent administration, or discontinuous administration as step-down therapy. P-CAB every other day administration can be expected for such cases also.
There have been no studies showing the usefulness of PPI and P-CAB every other day in the maintenance therapy of GERD, and in the studies comparing P-CAB and PPI in maintenance therapy, there are only results of clinical trials for new drug applications.7
Methods and analysis
Study objectives
This study aims to clarify the usefulness of P-CAB every other day administration as GERD maintenance therapy by cross over with PPI every other day administration. If P-CAB every other day administration is established as one of GERD maintenance therapies, there is merit in both medical cost reduction and the safety to alleviate elevation in serum gastrin.
Study design
This study is a prospective, multicentre, open-label, two-period randomised cross-over study to compare the efficacy and safety of PPI every other day administration and P-CAB every other day administration in 120 patients who receive erosive GERD maintenance therapy with PPI.
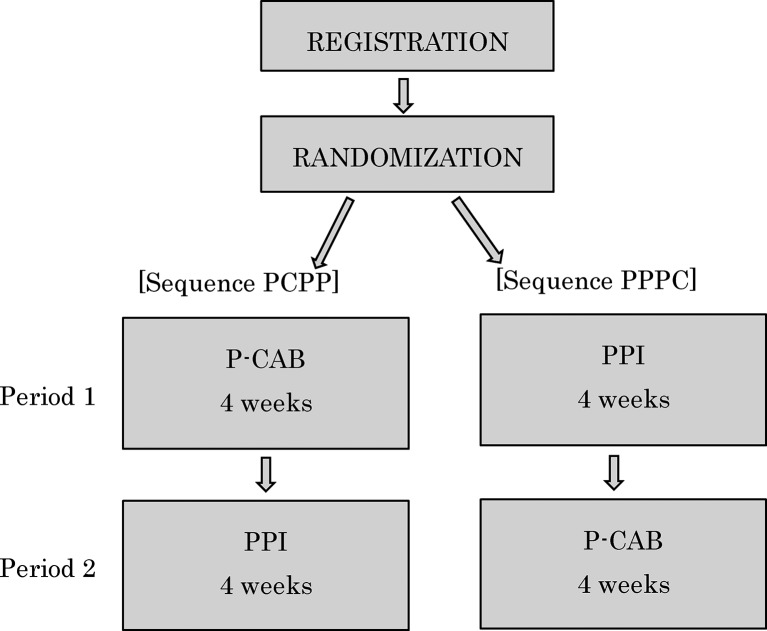
Patients will randomly and equally allocated to the two treatment sequences, sequence PCPP (P-CAB during period 1 and PPI during period 2) and sequence PPPC (PPI during period 1 and P-CAB during period 2). During the first 4 weeks period defined as period 1, patients in sequence PCPP and sequence PPPC will first receive P-CAB and PPI, respectively. After period 1, patients in sequences PCPP and PPPC will be crossed over to receive P-CAB and PPI, respectively, for 4 weeks as period 2 (figure 1). There is no washout period.
Figure 1.
Study design: treatment sequences and periods for sequence PCPP and sequence PPPC. P-CAB, potassium-competitive acid blocker; PCPP, P-CAB during period 1 and PPI during period 2; PPPC, PPI during period 1 and P-CAB during period 2; PPI, proton pump inhibitor.
Participating centres
National Hospital Organization Kanazawa Medical Center
National Hospital Organization Tokyo Medical Center
National Hospital Organization Osaka National Hospital
National Hospital Organization Kure Medical Center
National Hospital Organization Okayama Medical Center
National Hospital Organization Kyushu Medical Center
National Hospital Organization Osaka Minami Medical Center
National Hospital Organization Higashihiroshima Medical Center
National Hospital Organization Kochi National Hospital
National Hospital Organization Mie Chuo Medical Center
National Hospital Organization Kyoto Medical Center
National Hospital Organization Hokkaido Medical Center
National Hospital Organization Sendai Medical Center
National Hospital Organization Fukuoka-higashi Medical Center
National Hospital Organization Disaster Medical Center
National Hospital Organization Fukuyama Medical Center
National Hospital Organization Matsumoto Medical Center
National Hospital Organization Hakodate National Hospital
Eligibility criteria
Inclusion criteria
Patients who fulfil all of the following criteria are included:
patients with erosive GERD diagnosed with grade A to D by Los Angeles Classification by an endoscopic examination
receiving PPI maintenance therapy with reflux esophagitis
aged over 20 years at the time of consent
those provided written consent by their free will.
Exclusion criteria
Patients who fulfil any of the following criteria are excluded:
receiving P-CAB administration
for those PPI maintenance therapy is ineffective
having undergone gastro-oesophageal surgery
in very bad compliance
allergic to PPI or vonoprazan
with serious complications such as heart failure, renal failure or hepatic failure
pregnant, breastfeeding, or possibly pregnant
the patients who are administered drugs having influence to CYP2C19, for example, antidepressant, antiplatelet, anticoagulant and antifungal
deemed inappropriate for participation in this study by the principal investigator (PI)/subinvestigators.
Study outline
Study drug
Vonoprazan: a Takecab tablet 10 mg (Takeda Pharmaceutical) is administered orally once.
Lansoprazole: a Takepron once a day tablet 15 mg (Takeda Pharmaceutical) or a generic product of the same drug is administered orally once.
Daily administration
A Takecab tablet 10 mg is administered orally once every other day, and it is administered 14 times in 28 days.
A Takepron once a day tablet 15 mg or a generic product of the same drug is administered orally once every other day for 14 times in 28 days.
Standard for change of study treatment
If there is a relapse of symptoms during the treatment period, the same drug as initial allocation is administered according to the symptoms on the days that are supposed to be rest days.
Allocation
Allocation of the study subjects to each treatment group is performed by the central registration system. The enrolled patients will be allocated randomly on electronic data capture (EDC) according to the random number table.
Assessments
Symptoms such as heartburn and acid regurgitation and the number of consumed tables will be assessed for all patients by diary records.
F scale, Gastrointestinal Symptom Rating Scale (GSRS) and serum gastrin level will be measured at baseline and at 4 weeks and at 8 weeks.
Data collection and monitoring
Data management
In this study, EDC system is used. The PI or a person nominated by the PI logs into EDC using a strictly controlled individual electronic signature (ID and password), promptly inputs the collected case information to EDC, and sends it to the data centre. The transmitted electronic data is regarded as a case report. The PI or the person nominated by the PI performs all inputs and corrections, makes inquiries within the EDC as necessary, and responds to inquiries prepared by the data manager. The PI confirms that all data inputs are accurate.
Monitoring
The data manager of this study will monitor the input data that caused doubt by EDC at any time by the central monitoring method. We will perform annual periodical monitoring report on the study progress.
Endpoints
Primary endpoint
Proportion of asymptomatic patients.
We defined an asymptomatic patient as a patient being free of symptoms according to a symptom diary record for 6 or more days per week.
Secondary endpoints
Suppressive effect of GERD symptoms (F scale and GSRS)
Proportion of asymptomatic patients at each time point
Safety and cost-saving effect of P-CAB every other day administration
Compliance with every other day administration
Statistical analysis
Sample size
From the previous study,10 proportions of asymptomatic patients for more than 6 days with reflux esophagitis at 4 weeks in the comparative study between PPI continuous therapy and PPI on-demand therapy were about 80% and 50%, respectively. It is presumed that there is no significant difference in acid suppressive effect between PPI daily administration and P-CAB every other day administration in terms of their pharmacological characteristics, and the dose of PPI every other day administration is about the same on average as that of on-demand therapy in the previous study.10 Accordingly, it is estimated that proportions of asymptomatic patients at 4 weeks in P-CAB administration cases (sequence PCPP) and in PPI administration cases (sequence PPPC) are 80% and 50%, and those at 8 weeks followed by cross over for 4 weeks are 50% and 80%, respectively. In that case, the proportion of asymptomatic patients is 30%–50% when P-CAB administration in asymptomatic cases is changed to PPI administration, and proportion of asymptomatic patients is 0%–20% when P-CAB administration in symptomatic cases is changed to PPI administration. In sample size calculation using McNemar’s test in which the former proportion is 50% and the latter proportion is 20%, the required number of patients with alpha level of 5% and 90% power or more was 84 patients in total. Taking into consideration the dropout patients and ineligible patients which are found after registration, 60 patients in each group (120 patients in total) are taken as the target number of patients.
Analysis sets
Full analysis set (FAS) consists of all patients who are randomly allocated in the study, excluding those who are in serious violation of the study protocol (eg, provided no consent and in serious procedural violation). The primary endpoint analysis and all efficacy analyses will be conducted in FAS.
Per-protocol set consists of all FAS patients who receive at least one dose of study medication and did not have any major protocol violations. Major protocol violation will include patients who take more than 21 tablets during the each 4-week administration period.
Safety analysis set (SAF) consists of all patients who received at least one dose of study medication. SAF will be used for all safety analyses.
Efficacy analysis
Primary endpoint is proportion of asymptomatic patients. Proportion of asymptomatic patients will be presented as a 2×2 table for paired data and analysed using McNemar’s test for paired data to estimate the difference in sequence PCPP compared with sequence PPPC.
Proportion of asymptomatic patients will be summarised using descriptive statistics at each time point per week and for each treatment group. Between-group analysis will be performed using Fisher’s exact test at 4 weeks and at 8 weeks. Fisher’s exact test will be conducted against a two-sided alternative hypothesis, employing a significance level of 0.05.
For suppressive effect in GERD, F scale and GSRS will be summarised using descriptive statistics at each time point and for each treatment group.
Safety analysis
Serum gastrin level will be summarised using descriptive statistics at each time point by the treatment groups. Two comparisons will be performed by each treatment group: (1) baseline and at 4 weeks and (2) baseline and at 8 weeks. A two-sided t-test basis will be used to test the null hypothesis at the significance level of 0.05.
Adverse event
Serious adverse event (SAE) will be summarised using descriptive statistics by the treatment groups.
Other analyses
Patient demographics/other baseline characteristics
The demographic variables will be summarised by the treatment groups. Qualitative data will be summarised by means of contingency tables, and quantitative data will be summarised by appropriate descriptive statistics.
Compliance/cost reduction effect
The number of consumed tables per week recorded in the diary will be summarised by the treatment groups. The quantitative data will be summarised by appropriate descriptive statistics.
Sensitivity analysis
The sensitivity analysis will be performed for proportion of asymptomatic patients. Analysis of asymptomatic patients will be conducted assuming that the patients with the reason for discontinuation as ‘maintenance therapy by every other day administration is impossible: daily administration for more than 10 days is required’ will also be considered as symptomatic patients.
Additional analyses will be selected to test whether the differences in proportion of asymptomatic patients, F scale, GSRS, serum gastrin level and the number of consumed tables per week recorded in the diary between the two groups vary by each time point.
Interim analysis
No interim analysis will be performed.
Ethics and dissemination
Ethics
The present study complies with the World Medical Association’s Declaration of Helsinki, Ethical Guidelines for Medical and Health Research Involving Human Subjects, and Act on the Protection of Personal Information. It was approved by the National Hospital Organization Central Review Board for Clinical Trials (5 December 2017).
Prior to participation in the study, patients must have the study fully explained to them by a study investigator or research team member using an explanation and consent form and provide written consent of their own free will. The explanation and consent form meets the requirements of Ethical Guidelines for Medical and Health Research Involving Human Subjects, and its use has been approved by the Central Review Board for Clinical Trials.
Patient safety
All SAEs from the start to the completion (at 8 weeks) of the study treatment or to the withdrawal from the study will be collected.
After the initial SAE report or the report of an important non-SAE as defined in the package insert, the PI or subinvestigators must follow-up the event until the event is confirmed (death, recovery, or loss to follow-up).
The PI or subinvestigators will evaluate adverse events of the study subjects, and if signs including laboratory test values or symptoms are included in the diagnosis, the diagnosis name shall be used as much as possible rather than individual signs or symptoms in the case report.
The PI or subinvestigators must have records as the original materials for clinical tests and questions to the study subjects conducted according to the schedule for safety assessment.
Discussion
GERD is a disease that is susceptible to recurrence, and many patients need maintenance therapy for prevention of recurrence. As a maintenance therapy of GERD, ‘step-down’ of PPI is performed currently if symptoms are controlled, but many patients still cannot reach the dose reduction from PPI daily administration. If the efficacy of P-CAB every other day is indicated in this study, it will be established as an option for GERD maintenance therapy and described in the GERD clinical practice guidelines.1 One of the benefits from P-CAB every other day administration is that it reduces medical cost by halving the dose. Since the age of Helicobacter pylori negativity is coming and the rapid increase of GERD patients is predicted, the effect of medical cost reduction is expected. As another merit, hypergastrinemia is caused by the feedback of strong acid suppressive effect of P-CAB, but P-CAB every other day administration can be expected to reduce the gastrin elevation.2 Increased enterochromaffin-like cells in hypergastrinemia concern development of carcinoid tumours. There is no report that hypergastrinemia caused by PPI or P-CAB directly produces carcinoid tumours in humans, but since hypergastrinemia caused by P-CAB exceeds that caused by PPI, P-CAB every other day administration as a device to suppress the elevation in serum gastrin level, even a little, is considered widely accepted in general clinic. In addition, in transition from daily administration to every other day administration, sufficient acid suppressive effect may not be obtained by PPI every other day administration. On the other hand, in the P-CAB every other day administration, since it is possible to obtain the maximum acid suppressive effect by a single administration of P-CAB, it is considered easy to transit from daily administration to every other day administration.
Footnotes
Contributors: MK finalised the protocol and managed the entire study through the steering committee. NI was responsible for statistical analysis in this study. MD performed case registration, data management and central monitoring in this study. KK, KM, and NH contributed to the protocol.
Funding: This study is being conducted using an operating expense grant for research from the National Hospital Organization.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: National Hospital Organization Central Review Board for Clinical Trials.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Iwakiri K, Kinoshita Y, Habu Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol 2016;51:751–67. doi:10.1007/s00535-016-1227-8 [DOI] [PubMed] [Google Scholar]
- 2. Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2015;41:636–48. doi:10.1111/apt.13121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects--a randomised open-label cross-over study. Aliment Pharmacol Ther 2015;42:719–30. doi:10.1111/apt.13325 [DOI] [PubMed] [Google Scholar]
- 4. Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (Vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol 2015;6:e94 doi:10.1038/ctg.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savarino E, Martinucci I, Furnari M, et al. Vonoprazan for treatment of gastroesophageal reflux: pharmacodynamic and pharmacokinetic considerations. Expert Opin Drug Metab Toxicol 2016;8:1333–41. doi:10.1080/17425255.2016.1214714 [DOI] [PubMed] [Google Scholar]
- 6. Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther 2016;43:1048–59. doi:10.1111/apt.13588 [DOI] [PubMed] [Google Scholar]
- 7. Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther 2016;43:240–51. doi:10.1111/apt.13461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoshino S, Kawami N, Takenouchi N, et al. Efficacy of vonoprazan for proton pump inhibitor-resistant reflux esophagitis. Digestion 2017;95:156–61. doi:10.1159/000456072 [DOI] [PubMed] [Google Scholar]
- 9. Bruley des Varannes S, Coron E, Galmiche JP. Short and long-term PPI treatment for GERD. Do we need more-potent anti-secretory drugs? Best Pract Res Clin Gastroenterol 2010;24:905–21. doi:10.1016/j.bpg.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 10. Nagahara A, Hojo M, Asaoka D, et al. A randomized prospective study comparing the efficacy of on-demand therapy versus continuous therapy for 6 months for long-term maintenance with omeprazole 20 mg in patients with gastroesophageal reflux disease in Japan. Scand J Gastroenterol 2014;49:409–17. doi:10.3109/00365521.2013.878380 [DOI] [PMC free article] [PubMed] [Google Scholar]