Abstract
Introduction
Little is known about the effect of breast cancers on health-related quality of life among women diagnosed between age 18 and 44 years. The goal of this study is to estimate the effect of breast cancer on health state utility by age at diagnosis (18–44 years versus ≥45 years) and by race/ethnicity.
Methods
The analytic sample, drawn from the 2009 and 2010 Behavioral Risk Factor Surveillance System and analyzed in 2013, included women diagnosed with breast cancer between age 18 and 44 years (n=1,389) and age ≥45 years (n=6,037). Health state utility values were estimated using Healthy Days variables and a published algorithm. Regression analysis was conducted separately by age at diagnosis and race/ethnicity.
Results
The breast cancer health state utility decrement within 1 year from date of diagnosis was larger for women diagnosed at age 18–44 years than for women diagnosed at age ≥45 years (−0.116 vs −0.070, p<0.05). Within the younger age-at-diagnosis group, Hispanic women 2–4 years after diagnosis had the largest health state utility decrement (−0.221, p<0.01), followed by non-Hispanic white women within 1 year of diagnosis (−0.126, p<0.01).
Conclusions
This study is the first to report estimates of health state utility values for breast cancer by age at diagnosis and race/ethnicity from a nationwide sample. The results highlight the need for separate quality of life adjustments for women by age at diagnosis and race/ethnicity when conducting cost-effectiveness analysis of breast cancer prevention, detection, and treatment.
Introduction
Approximately 12% of new breast cancer cases occur in women younger than age 45 years.1 Unlike for older women, the incidence of breast cancer with distant involvement (metastatic breast cancer) among younger women has increased significantly over the past 30 years.2 Breast cancer negatively affects women’s health-related quality of life (HRQoL), potentially more for younger women.3–16 Younger women with breast cancer commonly face chemotherapy-induced menopause, decreased sexual function, infertility, diminished body image, and other side effects.15–18 Owing to more-aggressive and less responsive tumors,12,19 breast cancer diagnosed at a younger age is also correlated with lower survival rates, higher recurrence rates, and negative prognostic variables.19–21
The HRQoL decrement attributable to breast cancer also varies by race/ethnicity. Hispanic breast cancer survivors report significantly lower HRQoL for all domains (mental, emotional, and physical) than any other racial/ethnic group,22–24 although differences are only significant among women older than age 50 years.14 Results for black women with breast cancer have been mixed, with some studies reporting low physical functioning among black women,25 and others reporting better emotional well-being and mental health compared with non-Hispanic white women.14,23 To the authors’ knowledge, the way in which racial/ethnic differences in HRQoL vary by age at diagnosis has not been explored in the literature.
Most HRQoL measures for breast cancer survivors use condition-specific instruments.26 Alternative instruments, such as the EuroQoL five-dimensions (EQ-5D) (www.euroqol.org/), capture broad measures of health-related well-being. Preference-based HRQoL measures elicit patient preferences over health states through visual analog scales, time trade-offs, and standard gambles.27 These choices are represented as preference-based health state utility (HSU) values, which are scaled to a single 0 (dead) to 1 (best health) cardinal index.28 HSU is a special HRQoL measure that represents global health-related well-being, is based on preference-based tradeoffs, and is used in economic evaluations to value improvements in morbidity and mortality from interventions (e.g., quality-adjusted life-years). The goal of this study is to estimate the effect of breast cancer on HSU by age at diagnosis (18–44 years versus ≥45 years) and by race/ethnicity. This study is the first to measure the impact of breast cancer on HSUs based on a preference-based measure of HRQoL by age at diagnosis and race/ethnicity from a population-based national sample.
Methods
Data
The Behavioral Risk Factor Surveillance System (BRFSS) is an annual, state-based, telephone health survey of non-institutionalized adults supported by CDC. The BRFSS sample is drawn from each U.S. state and some territories using random-digit-dial sampling methods.29 In 2009, four cancer survivorship questions were asked in all states. In 2010, a total of 13 states and territories (i.e., Alaska, California, Colorado, Connecticut, Indiana, Massachusetts, Missouri, New Mexico, Ohio, Oklahoma, South Dakota, Wisconsin, and Guam) asked the cancer survivorship questions in an optional cancer survivorship module. The BRFSS cancer survivorship questions measure whether an individual has ever been diagnosed with cancer, type of cancer, age at which cancer was diagnosed, and the number of cancers previously diagnosed.29 The authors pooled the 2009 data for all states and territories and the 2010 data for the 13 states and territories listed above to maximize available sample size. The median response rate for the entire BRFSS across included states was 52% in 2009 and 58% in 2010.
Measures
The BRFSS core module measures population HRQoL using the Healthy Days (HRQoL-4) module.29 This study focused on two questions that asked how many days in the past 30 the respondent’s health was not good—one question for physical health and another question for mental health. Two questions were used to define “overall healthy days” as 30 – (physically unhealthy days) – (mentally unhealthy days), with values <0 set to 0. This “overall healthy days” measure was then mapped, using estimates from the literature, to HSU (described below).
A binary variable was generated to indicate if a respondent has ever had breast cancer. Because respondents were asked to report age at diagnosis based on their first cancer and the cancer type only for their most recent cancer, respondents with more than one cancer were excluded. For the remaining respondents with a single cancer diagnosis, the years since diagnosis of breast cancer were calculated as the difference between the age at diagnosis and the respondent’s current age. Dummy variables were created to indicate individuals diagnosed with breast cancer 0–1 (index category); 2–4; 5–10; and >10 years ago. The American Cancer Society uses 5-year categories when they report cancer prevalence by years since diagnosis.30 To account for differences in HSU during the treatment phase in the first year after diagnosis, the authors divided the initial category (<5 years) into two categories (0–1 year and 2–4 years). Finally, each respondent’s race/ethnicity was classified as white, black, Hispanic, and other. The other category included respondents identified as multiracial, Asian, Native Hawaiian or other Pacific Islander, American Indian or Alaska Native, and other race.
The 2009 and 2010 BRFSS sample contains a total of 539,634 respondents with and without a history of cancer. This study excluded men, women with other cancers to enable a comparison of women with breast cancer to women without cancer, and respondents with missing values for any variable used in the analysis, resulting in a final analytic sample of 200,268.
Estimating Health State Utility
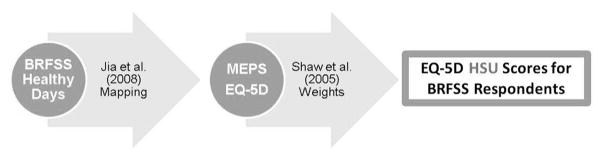
To derive preference-based HSU values using Healthy Days, this study used Jia and Lubetkin’s31 mapping. Jia and Lubetkin estimated the cumulative distributions of overall healthy days from the 2000–2002 BRFSS and the EQ-5D scores from the 2000–2002 U.S. Medical Expenditure Panel Survey. The cumulative distribution shows the percentage of the population whose scores were less than or equal to a given value. The two cumulative distributions were merged by matching their percentiles. For example, if 35% of U.S. adults reported having ≤25 overall healthy days in the past 30, a healthy day value of 25 would be matched with the EQ-5D score that was the 35th percentile of the EQ-5D distribution, which here turns out to be 0.815. Jia and Lubetkin merged the cumulative distributions separately for five age categories (18–24, 25–44, 45–64, 65–74, and ≥75 years). This study mapped the cumulative distribution of overall healthy days in the 2009–2010 BRFSS to EQ-5D-based HSU using their crosswalk (Figure 1). The mapping assumes that the Healthy Days measures correspond well to the range of health states described by the EQ-5D, for which preference weights for the U.S. population exist.32 The advantage of this particular mapping, relative to one other available approach,33 is that it predicts HSUs for all ages of adults in the BRFSS.
Figure 1.
Converting healthy days to health state utility scores.
BRFSS, Behavioral Risk Factor Surveillance System; EQ-5D, EuroQoL five-dimensions; HSU, health state utility; MEPS, Medical Expenditure Panel Survey.
Statistical Analysis
To examine means and proportions of continuous and categorical variables by age at diagnosis (<45 years, ≥45 years) for unadjusted differences by breast cancer status, t- and chi-square tests, respectively, were used. Next, linear regression (Stata, version 14.0) with robust variance adjustment was used to regress the mapped EQ-5D index scores on breast cancer, time since breast cancer diagnosis dummies (0–1 year [ref], 2–4 years, 5–10 years, >10 years), and the following covariates: indicators for chronic disease (diabetes, asthma, cardiovascular disease, stroke); education (less than high school graduate); race/ethnicity (for the pooled sample only, Hispanic, black, and other race); age; age squared; household income (<$35,000/year); currently married; smoking status (smokes some days, smokes every day); BMI; BMI squared; and a survey year indicator. Thus, the regressions included women with breast cancer and their controls. Regressions were run separately by age at diagnosis (<45 years, ≥45 years) and race/ethnicity (pooled races/ethnicities, white, black, and Hispanic). Because the estimation sample included a subset of states in 2010 and our primary population of interest (younger women with breast cancer) is a small subset of the population, this study did not use the BRFSS survey weights, which were not designed to be representative of the target population. All statistical tests were two-sided; this paper reports statistical significance at the 95% and 99% confidence levels.
Sensitivity Analysis
The authors conducted several sensitivity analyses. First, the main analysis was repeated separately using physically healthy days, mentally healthy days, and overall healthy days as the dependent variables. For these analyses, unhealthy days were converted to healthy days by subtracting the number of unhealthy days from 30. Second, propensity score matching was used prior to regression to balance the differences in observed variables between women with and without a history of breast cancer. Five to one nearest-neighbor propensity score matching with replacement was used to match women with breast cancer to statistically similar women without breast cancer. The propensity score was estimated using logistic regression with the same covariates as the regression model. The match was executed on a pooled sample of all races/ethnicities and then separately for each race/ethnicity group. After the match, no women were outside the common support of the propensity score (i.e., the range of propensity scores among women with breast cancer and their matched controls was similar). Covariate balance was checked between women with breast cancer and their matched women without cancer. After matching, any unbalanced covariates (p<0.1) were included as covariates in regression analysis. Results for all sensitivity analyses are available in the Appendix (available online).
Results
Only 3.7% of the sample reported being diagnosed with breast cancer, and the mean number of years since breast cancer diagnosis among these women was 10.8 years (Table 1). Slightly less than one fifth (18.7%) of women with breast cancer were diagnosed between age 18 and 44 years, and 81.3% were diagnosed at age ≥45 years. Comparing women with breast cancer with women without cancer, the former had significantly lower unadjusted mapped HSU (0.793 vs 0.843); mean physically healthy days (24.2 vs 26.0); and overall healthy days (22.1 vs 23.2). However, mean mentally healthy days (26.6 vs 26.2) were statistically significantly higher among women with breast cancer than among women without cancer. Additionally, there were statistically significant differences in comorbidities, age, education, race/ethnicity, household income, smoking, and BMI between women with and without breast cancer.
Table 1.
BRFSS Descriptive Statistics for All Races/Ethnicities
| Variable | Breast cancer (n=7,426) | No cancer (n=192,842) |
|---|---|---|
| Diagnosed with | ||
| Breast cancer at age (years) | ||
| 18–44 | 18.7 | N/A |
| ≥45 | 81.3 | N/A |
| Diabetes | 15.1 | 10.1 |
| Asthma | 9.4 | 10.2 |
| Cardiovascular disease | 7.2 | 4.0 |
| Stroke | 5.0 | 3.1 |
| Years since breast cancer diagnosis, M (SD)a | 10.8 (9.6) | N/A |
| Age at breast cancer diagnosis | 55.8 (12.5) | N/A |
| Mapped health state utility (0.0–1.0), M (SD) | 0.793 (0.181) | 0.843 (0.170) |
| Healthy days (HRQoL-4) | ||
| Physically healthy days, M (SD) | 24.2 (9.9) | 26.0 (8.3) |
| Mentally healthy days, M (SD) | 26.6 (7.6) | 26.2 (7.9) |
| Days without activity limitations, M (SD) | 26.9 (7.6) | 27.6 (6.7) |
| Overall healthy days, M (SD)b | 22.1 (11.1) | 23.2 (10.2) |
| Age, M | 66.5 | 53.5 (16.0) |
| Education (less than high school graduate) | 6.5 | 7.7 |
| Race/ethnicity | ||
| White, non-Hispanic | 85.1 | 77.7 |
| African American, non-Hispanic | 6.6 | 9.3 |
| Hispanic, any race | 3.7 | 7.3 |
| Other, non-Hispanicc | 4.6 | 5.7 |
| Household income <$35,000/year | 49.0 | 42.7 |
| Currently married | 45.8 | 54.1 |
| Smokes some days | 3.4 | 4.4 |
| Smokes every day | 7.3 | 12.0 |
| BMI, M (SD) | 27.2 (5.7) | 27.6 (6.4) |
| 2009 BRFSS | 87.7 | 86.5 |
Note: Values are percentages unless otherwise noted. All differences between women with and without breast cancer are statistically significant (p<0.05).
Years since diagnosis for women with a breast cancer diagnosis.
Overall healthy days = 30 – (physically unhealthy days) – (mentally unhealthy days); values <0 are set to 0. This measure is used in the health state utility value from Jia and Lubetkin.34
Non-Hispanic Asian, American Indian, Pacific Islander, other race, or multiracial.
BRFSS, Behavioral Risk Factor Surveillance System; HRQoL, health-related quality of life; N/A, not applicable.
Unadjusted mean mapped utility was 0.037 lower (p<0.01) among women of all races/ethnicities diagnosed with breast cancer between age 18 and 44 years than among women without cancer (0.802 vs 0.839) (Table 2). This trend was consistent across all Healthy Days measures (Appendix Table 1, available online). HSU differences were also significant for white and Hispanic women (p<0.01). Comparing women of all races/ethnicities diagnosed with breast cancer at age ≥45 years with women without cancer, unadjusted mean utility was 0.049 lower (p<0.01) in the breast cancer group (0.790 vs 0.839). This trend was consistent across all Healthy Days measures except mentally healthy days (Appendix Table 1, available online). The HSU difference was also significant for women in each race/ethnicity group diagnosed at age ≥45 years (p<0.01).
Table 2.
Unadjusted, Mapped HSU by Age at Diagnosis, Breast Cancer, and Race/Ethnicity
| Race/ethnicity | Diagnosed at age 18–44 years | Diagnosed at age ≥45 years | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Breast cancer | No cancer | Difference | Breast cancer | No cancer | Difference | |
| All races/ethnicities | 0.802 | 0.839 | −0.037 | 0.790 | 0.839 | −0.049 |
|
| ||||||
| White | 0.811 | 0.844 | −0.033 | 0.795 | 0.844 | −0.049 |
|
| ||||||
| Black | 0.794 | 0.816 | −0.022 | 0.767 | 0.816 | −0.049 |
|
| ||||||
| Hispanic | 0.741 | 0.821 | −0.080 | 0.730 | 0.821 | −0.091 |
Note: Boldface indicates statistically significant difference (p<0.01) between women with and without breast cancer. HSU, health state utility.
After adjusting for covariates with multivariate regression, the HSU decrement between women with breast cancer and women without cancer for the younger age-at-diagnosis stratification was largest within 1 year of diagnosis (−0.116, p<0.01) and decreased, but was still significant, as years since diagnosis increased (Table 3). Similar analysis of the older age-at-diagnosis stratification showed the largest estimated effect on HSU within 1 year of diagnosis (−0.070, p<0.01) and smaller but still significant effects for each of the “years since diagnosis” categories. The HSU decrement associated with breast cancer within 1 year of diagnosis was significantly larger for women diagnosed before age 45 years (−0.116 vs −0.070, p<0.05). The full regressions are available in Appendix Table 2 (available online). Multivariate regression results using the Healthy Days measures were similar to those using HSU (Appendix Table 3, available online).
Table 3.
Marginal Effects of Breast Cancer on Mapped HSU from Regression Analysisa
| Variable | All races/ethnicities | White | ||
|---|---|---|---|---|
|
|
|
|||
| Diagnosed at age 18–44 years | Diagnosed at age ≥45 years | Diagnosed at age 18–44 years | Diagnosed at age ≥45 years | |
| Breast cancer + years since diagnosis | ||||
|
| ||||
| 0–1 | −0.116** (0.018) | −0.070**,b (0.007) | −0.126** (0.021) | −0.072**,b (0.008) |
|
| ||||
| 2–4 | −0.051** (0.013) | −0.032** (0.005) | −0.041** (0.014) | −0.031** (0.005) |
|
| ||||
| 5–10 | −0.037** (0.009) | −0.014**,b (0.004) | −0.036** (0.010) | −0.015**,b (0.004) |
|
| ||||
| >10 | −0.025** (0.006) | −0.021** (0.004) | −0.025** (0.006) | −0.023** (0.004) |
|
| ||||
| N (unweighted) | 194,230 | 198,880 | 150,948 | 155,022 |
|
| ||||
| R-squared | 0.194 | 0.194 | 0.191 | 0.191 |
|
| ||||
| African American | Hispanic | |||
|
| ||||
| Breast cancer + years since diagnosis | ||||
|
| ||||
| 0–1 | −0.057 (0.061) | −0.051* (0.023) | −0.096 (0.059) | −0.063* (0.031) |
|
| ||||
| 2–4 | 0.033 (0.027) | −0.035*,b (0.020) | −0.221** (0.064) | −0.058*,b (0.026) |
|
| ||||
| 5–10 | −0.032 (0.029) | −0.025 (0.013) | 0.027 (0.029) | −0.021 (0.021) |
|
| ||||
| >10 | −0.026 (0.020) | 0.020 (0.015) | −0.024 (0.029) | −0.036 (0.026) |
|
| ||||
| N (unweighted) | 17,997 | 18,252 | 14,188 | 14,341 |
|
| ||||
| R-squared | 0.188 | 0.188 | 0.195 | 0.197 |
Note: Boldface indicates statistical significance (*p<0.05; **p<0.01).
Other covariates included in the regressions were race/ethnicity (pooled analysis); age; age squared; household income <$35,000/year; BMI; BMI squared; education (less than high school graduate); currently married; smokes some days; smokes every day; asthma; diabetes; cardiovascular disease; stroke; and an indicator for 2009 BRFSS. Full regression results are available in the Appendix (available online). SEs are robust.
Chow test indicates statistically significant differences (p<0.05) between women diagnosed at age 18–44 years and women diagnosed at age ≥45 years. No Chow test was significant at the p<0.01 level.
BRFSS, Behavioral Risk Factor Surveillance System; HSU, health state utility.
The HSU decrement between white women with breast cancer and white women without breast cancer was largest within 1 year of diagnosis (−0.126 for younger and −0.072 for older women, p<0.01), and smaller but still significant ≥2 years after diagnosis (Table 3). The HSU decrements associated with breast cancer within 1 year of diagnosis and 5–10 years since diagnosis were significantly larger for white women diagnosed at a younger age compared with white women diagnosed at an older age (p<0.05).
Among black women in the younger age-at-diagnosis group, none of the HSU decrements were significant. The HSU decrement within 1 year of diagnosis was significant among black women in the older age-at-diagnosis group (−0.051, p<0.05).
Among Hispanic women in the younger age-at-diagnosis group, the largest HSU decrement was for those with breast cancer at 2–4 years after diagnosis (−0.221, p<0.01), which was significantly larger than the similar decrement for Hispanic women diagnosed at age ≥45 years (−0.058). The HSU decrements within the first year and 2–4 years after diagnosis were significant among Hispanic women in the older age-at-diagnosis group (−0.063, p<0.05, and −0.058, p<0.05, respectively).
Discussion
This analysis generated three key findings. First, the HRQoL effects of breast cancer are larger among women diagnosed at younger ages. Second, the HRQoL effects of breast cancer are concentrated in the first year after diagnosis, with larger effects among women diagnosed at younger ages. Third, there are significant differences in the HRQoL effects of breast cancer by race/ethnicity.
Although the HSU decrements were largest in the year after diagnosis, breast cancer can have long-term effects on HRQoL regardless of the age at diagnosis. The estimated HSU decrements were small but statistically significant at 0–1, 2–4, 5–10, and >10 years after diagnosis for both age-at-diagnosis groups (Table 3, first panel). Previous studies of the EQ-5D instrument, including one focused on cancer, have shown minimally important differences of 0.06–0.07.34,35 The present estimated HSU decrements were smaller than those used in existing decision analysis models based on older populations.36 Previous studies have found no long-term negative HRQoL effects among most breast cancer survivors, except in women with recurrence or a history of chemotherapy.37
Consistent with prior literature on HRQoL, pooled across all races/ethnicities, the effect of breast cancer on HSU within 1 year of diagnosis was significantly larger on younger age-at-diagnosis women than on older age-at-diagnosis women.38–42 Relative to the minimally important difference thresholds of 0.06–0.07, the breast cancer decrements in the first year after diagnosis and the differences in those decrements between younger and older ages at diagnosis are clinically meaningful. The two age-at-diagnosis groups were also significantly different at 5–10 years after breast cancer diagnosis in the pooled data, but the decrements and difference in decrements were much smaller than in the first year after diagnosis. Thus, the larger HRQoL effects of breast cancer among women diagnosed at younger ages may be specific to the first year after diagnosis, during active treatment.
For black women diagnosed at a younger age, the HSU change at 2–4 years after diagnosis was significantly higher than for women diagnosed at an older age. This could be because the sample of survivors did not include those who died earlier, leaving a relatively healthier subset of women in the analysis. African Americans have the lowest breast cancer survival rate of any racial/ethnic group: 77% versus 90% among white women. African Americans have reported lower breast cancer HRQoL decrements, especially in the emotional and mental health domains.14,23,25,41
The breast cancer decrement at 2–4 years after diagnosis for Hispanic women diagnosed between age 18 and 44 years was more than four times larger than the comparable decrement in the pooled sample and was significantly different from the comparable decrement for all other racial/ethnic groups. This is consistent with prior research.22–24 Many Hispanic women face unique difficulties after breast cancer diagnosis (e.g., challenges navigating the U.S. medical system, occupations requiring manual labor that are difficult to return to post-surgery, worries about recurrence).43–47 It follows that lower acculturated Hispanics may be at higher risk for poorer HRQoL from a breast cancer diagnosis.14,45,48 Hispanics are also more likely to be diagnosed with late-stage breast cancer than non-Hispanic whites, which would lower HRQoL.49–51
Limitations
This study is subject to a number of limitations. BRFSS is limited to the non-institutionalized population, and self-reports of cancer and low response rates could have resulted in a non-representative sample of respondents. BRFSS also had few breast cancer diagnoses at younger ages among black (n=136) and Hispanic (n=69) women, which limited the power to detect differences by race/ethnicity. Race/ethnicity is culturally complex and it is difficult to separate the effects of race/ethnicity from other concepts like SES. Because respondents were asked to report age at diagnosis based on their first cancer and only reported cancer type for their most recent cancer, individuals with multiple cancers were excluded from the analysis. BRFSS did not provide data on breast cancer stage, recurrence, or treatment. Differences in the types of cancers and treatment received by younger women could be driving the differences by age. However, the authors cannot test that hypothesis in these data. The authors believe a contribution of this paper is to generate these types of hypotheses by describing the differences in HSU by age at diagnosis. Finally, the mapping of Jia and Lubetkin31 adds additional uncertainty to the estimates, which are also subject to the limitations of the original mapping (e.g., mapping across two different data sets, overlap across physically and mentally unhealthy days).
Conclusions
The effect of breast cancer on HRQoL varies by age at diagnosis, time since diagnosis, and race/ethnicity. The results suggest that separate QoL adjustments for women by age at diagnosis and race/ethnicity would be important for conducting cost-effectiveness analysis of breast cancer prevention, detection, and treatment. This study provides HSU estimates for younger women with breast cancer by race/ethnicity that can be used to model downstream health states in secondary or observational models. However, the authors acknowledge that concerns about equity and fairness could arise if minorities experienced lower HSU decrements from breast cancer, which could lead to higher incremental cost-effectiveness ratios for interventions targeted at these groups. In the results, Hispanic women experienced higher, not lower, HSU decrements; the estimates for black women were generally imprecisely estimated. Breast cancer diagnosed in women younger than age 45 years may place a greater burden on their QoL than in older women, especially among younger Hispanic women.
Supplementary Material
Acknowledgments
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of CDC. This research was supported by contract number 200-2008-27958 Task Order 0026 from CDC to RTI International.
Appendix. Supplementary data
Supplementary data associated with this article can be found at http://dx.doi.org/10.1016/j.amepre.2015.09.026.
Footnotes
No financial disclosures were reported by the authors of this paper.
References
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER cancer statistics review, 1975–2010. Bethesda, MD: National Cancer Institute; 2012. http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, 2013. [Google Scholar]
- 2.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309(8):800–805. doi: 10.1001/jama.2013.776. http://dx.doi.org/10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
- 3.Hoyer M, Johansson B, Nordin K, et al. Health-related quality of life among women with breast cancer—a population-based study. Acta Oncol. 2011;50(7):1015–1026. doi: 10.3109/0284186X.2011.577446. http://dx.doi.org/10.3109/0284186X.2011.577446. [DOI] [PubMed] [Google Scholar]
- 4.Lee ES, Lee MK, Kim SH, et al. Health-related quality of life in survivors with breast cancer 1 year after diagnosis compared with the general population: a prospective cohort study. Ann Surg. 2011;253(1):101–108. doi: 10.1097/sla.0b013e3181f662ce. http://dx.doi.org/10.1097/SLA.0b013e3181f662ce. [DOI] [PubMed] [Google Scholar]
- 5.Lidgren M, Wilking N, Jonsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16(6):1073–1081. doi: 10.1007/s11136-007-9202-8. http://dx.doi.org/10.1007/s11136-007-9202-8. [DOI] [PubMed] [Google Scholar]
- 6.Lovrics PJ, Cornacchi SD, Barnabi F, Whelan T, Goldsmith CH. The feasibility and responsiveness of the health utilities index in patients with early-stage breast cancer: a prospective longitudinal study. Qual Life Res. 2008;17(2):333–345. doi: 10.1007/s11136-007-9305-2. http://dx.doi.org/10.1007/s11136-007-9305-2. [DOI] [PubMed] [Google Scholar]
- 7.Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:32. doi: 10.1186/1756-9966-27-32. http://dx.doi.org/10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peasgood T, Ward SE, Brazier J. Health-state utility values in breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2010;10(5):553–566. doi: 10.1586/erp.10.65. http://dx.doi.org/10.1586/erp.10.65. [DOI] [PubMed] [Google Scholar]
- 9.Trentham-Dietz A, Sprague BL, Klein R, et al. Health-related quality of life before and after a breast cancer diagnosis. Breast Cancer Res Treat. 2008;109(2):379–387. doi: 10.1007/s10549-007-9653-1. http://dx.doi.org/10.1007/s10549-007-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch L, Jansen L, Herrmann A, et al. Quality of life in long-term breast cancer survivors—a 10-year longitudinal population-based study. Acta Oncol. 2013;52(6):1119–1128. doi: 10.3109/0284186X.2013.774461. http://dx.doi.org/10.3109/0284186X.2013.774461. [DOI] [PubMed] [Google Scholar]
- 11.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405. doi: 10.1093/jnci/djr541. http://dx.doi.org/10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 12.Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001;30:44–51. doi: 10.1093/oxfordjournals.jncimonographs.a003459. http://dx.doi.org/10.1093/oxford-journals.jncimonographs.a003459. [DOI] [PubMed] [Google Scholar]
- 13.Kwan ML, Ergas IJ, Somkin CP, et al. Quality of life among women recently diagnosed with invasive breast cancer: the Pathways Study. Breast Cancer Res Treat. 2010;123(2):507–524. doi: 10.1007/s10549-010-0764-8. http://dx.doi.org/10.1007/s10549-010-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janz NK, Mujahid MS, Hawley ST, et al. Racial/ethnic differences in quality of life after diagnosis of breast cancer. J Cancer Surviv. 2009;3(4):212–222. doi: 10.1007/s11764-009-0097-y. http://dx.doi.org/10.1007/s11764-009-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pritchard KI. Adjuvant therapy of the very young woman. Breast. 2007;16:S136–S146. doi: 10.1016/j.breast.2007.07.023. http://dx.doi.org/10.1016/j.breast.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Arora NK, Gustafson DH, Hawkins RP, et al. Impact of surgery and chemotherapy on the quality of life of younger women with breast carcinoma—a prospective study. Cancer. 2001;92(5):1288–1298. doi: 10.1002/1097-0142(20010901)92:5<1288::aid-cncr1450>3.0.co;2-e. http://dx.doi.org/10.1002/1097-0142(20010901)92:5<1288::AID-CNCR1450>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 17.Baucom DH, Porter LS, Kirby JS, Gremore TM, Keefe FJ. Psychosocial issues confronting young women with breast cancer. Breast Dis. 2005;23:103–113. doi: 10.3233/bd-2006-23114. [DOI] [PubMed] [Google Scholar]
- 18.Kornblith AB, Powell M, Regan MM, et al. Long-term psychosocial adjustment of older vs younger survivors of breast and endometrial cancer. Psychooncology. 2007;16(10):895–903. doi: 10.1002/pon.1146. http://dx.doi.org/10.1002/pon.1146. [DOI] [PubMed] [Google Scholar]
- 19.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26(20):3324–3330. doi: 10.1200/JCO.2007.14.2471. http://dx.doi.org/10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 20.El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. http://dx.doi.org/10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartley MC, McKinley BP, Rogers EA, et al. Differential expression of prognostic factors and effect on survival in young (< or =40) breast cancer patients: a case-control study. Am Surg. 2006;72(12):1189–1194. discussion 94–95. [PubMed] [Google Scholar]
- 22.Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413–428. doi: 10.1007/s11136-006-9138-4. http://dx.doi.org/10.1007/s11136-006-9138-4. [DOI] [PubMed] [Google Scholar]
- 23.Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85–95. doi: 10.1007/s10549-006-9479-2. http://dx.doi.org/10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanez B, Thompson EH, Stanton AL. Quality of life among Latina breast cancer patients: a systematic review of the literature. J Cancer Surviv. 2011;5(2):191–207. doi: 10.1007/s11764-011-0171-0. http://dx.doi.org/10.1007/s11764-011-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Ah DM, Russell KM, Carpenter J, et al. Health-related quality of life of African American breast cancer survivors compared with healthy African American women. Cancer Nurs. 2012;35(5):337–346. doi: 10.1097/NCC.0b013e3182393de3. http://dx.doi.org/10.1097/NCC.0b013e3182393de3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pusic AL, Cemal Y, Albornoz C, et al. Quality of life among breast cancer patients with lymphedema: a systematic review of patient-reported outcome instruments and outcomes. J Cancer Surviv. 2013;7(1):83–92. doi: 10.1007/s11764-012-0247-5. http://dx.doi.org/10.1007/s11764-012-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torrance GW. Preferences for health outcomes and cost-utility analysis. Am J Manag Care. 1997;3(suppl):S8–S20. [PubMed] [Google Scholar]
- 28.Gold M, Siegel J, Russell L, Weinstein M, editors. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 29.CDC. Behavioral Risk Factor Surveillance System Survey Data, 2009–2010. Atlanta, GA: USDHHS, CDC; 2012. [Google Scholar]
- 30.American Cancer Society. Cancer Treatment and Survivorship Facts & Figures 2014–2015. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 31.Jia H, Lubetkin EI. Estimating EuroQol EQ-5D scores from Population Healthy Days data. Med Decis Making. 2008;28(4):491–499. doi: 10.1177/0272989X07312708. http://dx.doi.org/10.1177/0272989X07312708. [DOI] [PubMed] [Google Scholar]
- 32.Shaw JW, Johnson JA, Coons SJ. U.S. valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. http://dx.doi.org/10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Jia H, Zack MM, Moriarty DG, Fryback DG. Predicting the EuroQol Group’s EQ-5D index from CDC’s “Healthy Days” in a U.S. sample. Med Decis Making. 2011;31(1):174–185. doi: 10.1177/0272989X10364845. http://dx.doi.org/10.1177/0272989X10364845. [DOI] [PubMed] [Google Scholar]
- 34.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14(6):1523–1532. doi: 10.1007/s11136-004-7713-0. http://dx.doi.org/10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 35.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. http://dx.doi.org/10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Glick HA, Hayman JA, Solin LJ. Decision-analytic model and cost-effectiveness evaluation of postmastectomy radiation therapy in high-risk premenopausal breast cancer patients. J Clin Oncol. 2002;20(11):2713–2725. doi: 10.1200/JCO.2002.07.008. http://dx.doi.org/10.1200/JCO.2002.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. http://dx.doi.org/10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 38.Ziner KW, Sledge GW, Bell CJ, Johns S, Miller KD, Champion VL. Predicting fear of breast cancer recurrence and self-efficacy in survivors by age at diagnosis. Oncol Nurs Forum. 2012;39(3):287–295. doi: 10.1188/12.ONF.287-295. http://dx.doi.org/10.1188/12.ONF.287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 40.Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. J Clin Oncol. 2004;22(10):1849–1856. doi: 10.1200/JCO.2004.04.173. http://dx.doi.org/10.1200/JCO.2004.04.173. [DOI] [PubMed] [Google Scholar]
- 41.Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21(22):4184–4193. doi: 10.1200/JCO.2003.04.196. http://dx.doi.org/10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 42.Cimprich B, Ronis DL, Martinez-Ramos G. Age at diagnosis and quality of life in breast cancer survivors. Cancer Pract. 2002;10(2):85–93. doi: 10.1046/j.1523-5394.2002.102006.x. http://dx.doi.org/10.1046/j.1523-5394.2002.102006.x. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724–733. doi: 10.1007/s13187-011-0249-4. http://dx.doi.org/10.1007/s13187-011-0249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graves KD, Jensen RE, Canar J, et al. Through the lens of culture: quality of life among Latina breast cancer survivors. Breast Cancer Res Treat. 2012;136(2):603–613. doi: 10.1007/s10549-012-2291-2. http://dx.doi.org/10.1007/s10549-012-2291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058–1067. doi: 10.1002/cncr.23660. http://dx.doi.org/10.1002/cncr.23660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janz NK, Hawley ST, Mujahid MS, et al. Correlates of worry about recurrence in a multiethnic population-based sample of women with breast cancer. Cancer. 2011;117(9):1827–1836. doi: 10.1002/cncr.25740. http://dx.doi.org/10.1002/cncr.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664–1674. doi: 10.1002/cncr.26478. http://dx.doi.org/10.1002/cncr.26478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fatone AM, Moadel AB, Foley FW, Fleming M, Jandorf L. Urban voices: the quality-of-life experience among women of color with breast cancer. Palliat Support Care. 2007;5(2):115–125. doi: 10.1017/s1478951507070186. http://dx.doi.org/10.1017/S1478951507070186. [DOI] [PubMed] [Google Scholar]
- 49.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97(1):134–147. doi: 10.1002/cncr.11051. http://dx.doi.org/10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 50.Lantz PM, Mujahid M, Schwartz K, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96(12):2173–2178. doi: 10.2105/AJPH.2005.072132. http://dx.doi.org/10.2105/AJPH.2005.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen MS., Jr Cancer health disparities among Asian Americans: what we do and what we need to do. Cancer. 2005;104(12 Suppl):2895–2902. doi: 10.1002/cncr.21501. http://dx.doi.org/10.1002/cncr.21501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.