Abstract
Purpose of Review
Type 2 diabetes (T2D) is a growing public health problem in youth, but conventional treatments are often insufficient to treat this disease and its comorbidities. We review evidence supporting an emerging role for bariatric surgery as a treatment for adolescent T2D.
Recent Findings
Paralleling what has been seen in adult patients, bariatric surgery dramatically improves glycemic control in patients with T2D. In fact, remission of T2D has been observed in as many as 95–100% of adolescents with diabetes after bariatric surgery, particularly vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB) surgery. This striking outcome may be due to both weight-dependent- and weight-independent factors and recent studies suggest T2D-related comorbidities may also improve after surgery.
Summary
Bariatric surgery including RYGB and VSG is a powerful therapeutic option for obese adolescents with T2D. Benefits must be weighed against risk for postoperative complications such as nutritional deficiencies, but earlier surgical intervention might lead to more complete metabolic remission in obese patients with T2D.
Keywords: Bariatric Surgery, Type 2 Diabetes, Obesity, Adolescent, Roux-en-Y Gastric Bypass Surgery, Sleeve Gastrectomy
Introduction
Type 2 diabetes (T2D) is increasingly recognized as a pediatric disease, and importantly, one that may be even more aggressive in younger patients. The American Diabetes Association(1) cites diabetes as the 7th leading cause of death in the U.S. in 2010 and deaths related to diabetes-related comorbidities are even greater. The SEARCH for Diabetes in Youth Study(2) estimated prevalence as 0.34 per 1,000 people under ages 10–19 years for the year 2001. One might speculate that these numbers may in fact grow in the coming decades. Based on a 30.4% increase in T2D cases observed between 2001 and 2009, it was estimated that T2D prevalence amongst youth in this age group might quadruple by the year 2050 (3).
A sense of urgency is borne out from data demonstrating accelerated development of diabetic nephropathy and cardiovascular disease amongst young adults with T2D, in comparison with individuals with type 1 diabetes with comparable glycemic control(4). In fact, in this study, almost half of patients with T2D were found to have albuminuria after an average of 11.6 years since diagnosis. Startlingly, many patients were found to have diabetes-related comorbidities present at diagnosis, including 13% of patients with microalbuminuria(5). This is particularly harrowing given the average age of youth-onset T2D diagnosis in this country is only 13.5 years(6). Furthermore, the TODAY study(7) highlights how challenging it can be to attain good glycemic control in these patients, finding higher rates of medication failure (in terms of both glycemic control and incidence of dyslipidemia, hypertension, and microalbuminuria) for younger patients. These startling statistics call for more effective treatments for young patients with T2D.
Obesity is the most important modifiable risk factor for T2D. Most youth with type 2 diabetes have at least one affected relative, but all youth with T2D are obese(5, 8). Therefore, early obesity treatment may be expected to slow or halt the progression to T2D in patients who do not yet have diabetes. A major problem faced by pediatricians, however, is that medical management for obesity in children is limited and often ineffective. Furthermore, obesity is common, affecting almost one-fifth of American children (9). Inspired by success as a treatment for adult obesity, bariatric surgery has recently emerged as a treatment for adolescent patients as well. Clinical studies (10–14) demonstrate a clear effect of bariatric surgery to improve glycemic control and often elicit complete remission of T2D, including in adolescents (15–18). This review will discuss the potential for bariatric surgery as a treatment for T2D in obese and overweight adolescents.
Nonsurgical treatment options for T2D
Medical management of T2D in pediatric patients centers primarily around metformin and/or insulin. Monotherapy with metformin is recommended as a first-line treatment, and may have additional benefit to reduce body weight. Guidelines from the American Academy of Pediatrics recommend starting insulin on all newly-diagnosed children with type 2 diabetes in whom ketones are present, HBA1c is greater than 9%, fasting blood glucose level is equal to or greater than 250 mg/dl, and/or if the clinician is unable to rule-out the possibility of T1D. Addition of metformin is an effective adjunct to insulin therapy, with the potential to improve HbA1c and allow for insulin discontinuation over a short period of time (19). In addition, at least 60 minutes of physical activity is recommended daily (20); even modest weight loss of <10% can improve glycemic control as assessed by HbA1c(21). However, lifestyle management alone is effective at achieving glycemic goals in less than 10% of pediatric patients(22). This fact is not surprising, particularly given that T2D disproportionately affects individuals of low-income and/or single-parent households(5, 8) where resources (time, money, availability of fresh produce) may be scarce.
Insulin and metformin are the only two FDA-approved medications for diabetes in patients younger than 18 years old, but other medications such as thiazolidindiones and incretins have been used with success as off-label agents in some patients(7, 23). The TODAY study (7) demonstrated that the addition of rosiglitazone enhances the efficacy of metformin, improving rates of glycemic control. However, combined therapy was still ineffective at maintaining durable control in 38.6% of patients, highlighting large gaps in our ability to reach this vulnerable population with current therapies. Also of note, addition of lifestyle intervention to metformin therapy did not significantly improve the rate of maintenance of durable glycemic control rates, stressing just how difficult it can be to implement meaningful diet and exercise changes for metabolic impact.
Bariatric surgery as a T2D treatment
Bariatric surgery has long been used as a therapy for severe obesity and in adults has been formally added to the arsenal for T2D treatment, by consensus of dozens of medical and surgical organizations internationally (24). The most commonly-performed bariatric procedures in both adolescents and adults are Roux-en-Y gastric bypass (RYGB), adjustable gastric banding (AGB), and vertical sleeve gastrectomy (VSG). Among these, RYGB and VSG appear to be the most effective both to reduce body weight and to improve metabolic health. Duodenal-jejunal bypass (DJB) surgery is also highly effective but due to its side effect profile is not currently used in the United States.
Not surprisingly, bariatric surgery induces weight loss that is superior to nonsurgical management(25). However, there is also a potent anti-diabetes effect. The Swedish Obesity Study(26) demonstrated that bariatric surgery was superior to lifestyle intervention to reduce the incidence of T2D in obese adults, reducing rates from 28.4 to 6.8 cases per 1,000 patients over 15 years. Improvement can occur very soon after surgery and, in fact, can precede weight loss(10–14, 27, 28). This anti-diabetic effect persists beyond the period of perioperative caloric restriction, indicating an independent effect of the surgery to affect glucose metabolism(14). In a 3-year longitudinal study(29), RYGB elicited T2D remission in 40% of patients, as compared with no patients treated with lifestyle intervention alone. Interestingly, T2D did remit in 29% of patients after AGB(29), but AGB is not thought to elicit any weight-independent improvement to glucose homeostasis(30). VSG appears to produce intermediate effects on T2D; the STAMPEDE trial(31) demonstrated remission in 38% of RYGB patients versus 24% of VSG patients. The 5-year follow-up to this study(32) showed comparable rates of diabetes remission after VSG and RYGB (23% and 29% of patients, respectively, at 5 years), versus only 5% of patients treated with intensive medical therapy. Other studies have reported much higher rates of T2D resolution after RYGB in (10, 13), but RYGB techniques can vary with respect to alimentary distance, pouch size, duration and completeness of follow-up, possibly leading to variability in reported response rates. Additionally, T2D severity may affect propensity for remission after bariatric surgery.
It has been hypothesized that earlier surgical intervention might improve long-term metabolic health among obese adolescents and, in particular, might prevent or improve the course of T2D. Bariatric surgery is, in fact, highly effective within the adolescent population and can result in loss of 58–73% of excess weight(18, 33–40) (Figure 1). Although fewer studies have been performed in this population and long-term follow-up data are only now evolving, the initial studies(15–18, 41) show that T2D remission mirrors that which is observed in older patients. In a large, recent 3-year longitudinal study of adolescent bariatric surgery(16) (Table 1), 95% of patients with T2D at baseline exhibited remission by 3 years; prevalence of remission did not differ after VSG versus RYGB, but this may reflect the relatively small numbers of patients with T2D in the study (13%). After an average of 8 years postoperatively, we recently showed that diabetes remission persisted in 8 of 9 patients with T2D at baseline(41) (Table 1). These data align with results of earlier, smaller studies, demonstrating improved fasting insulin and glucose levels, as well as better insulin sensitivity (as estimated by HOMA-IR) one year after RYGB(25). All measures fell from the pathologic to the normal range within this time period, despite the fact that patients were still obese (average BMI 35.8 kg/m2). Normalization of fasting insulin has been observed by 6 months postoperatively(42), corroborating evidence from adults that this effect occurs early. These biochemical observations do appear to translate to clinical benefit; in a study of 11 adolescents who underwent RYGB, 10 were able to discontinue oral hypoglycemics within one year after surgery(18).
Figure 1.
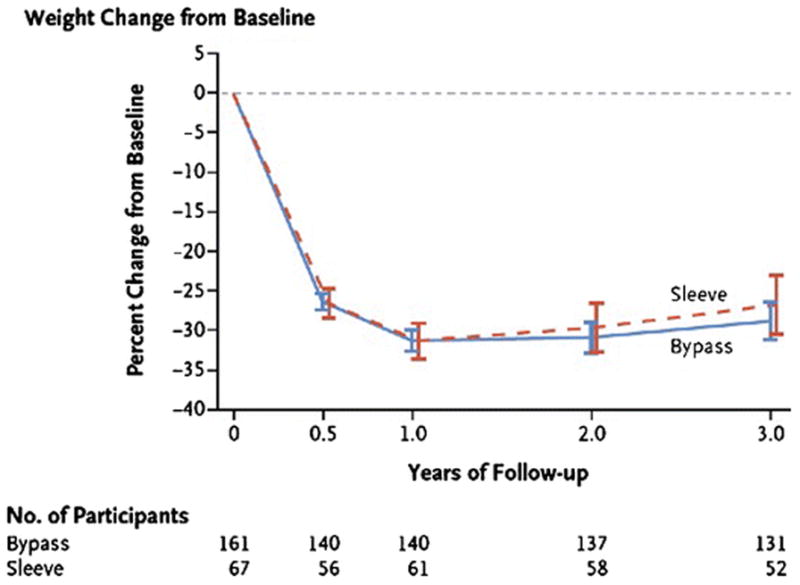
Weight change over time following adolescent bariatric surgery. Modeled least-squares mean percent changes in weight from baseline over time in adolescent patients after RYGB (“bypass”) and sleeve gastrectomy (“sleeve”) surgery in the Teen-LABS cohort. (From: Inge TH, et al. The New England Journal of Medicine. 2016;374(2):113–23, Copyright © (2016) Massachusetts Medical Society, reprinted with permission) [16••].
Table 1.
Resolution of T2D and related comorbidities in adolescents 3 and 5+ years after bariatric surgery
| 3-year follow-up (Teen-LABS study) | ||||
|---|---|---|---|---|
| Baseline prevalence of condition†-- % (95% CI) | 3-year follow-up prevalence of remission†-- % (95% CI) | |||
| Type 2 diabetes* | ||||
| Total | 13 (8–20) | 90 (65–98) | ||
| Gastric bypass | 14 (9–23) | 94 (66–99) | ||
| Sleeve gastrectomy | 9 (3–22) | 68 (7–99) | ||
| Prediabetes‡ | ||||
| Total | 10 (6–17) | 77 (48–92) | ||
| Gastric bypass | 13 (7–22) | 94 (66–99) | ||
| Sleeve gastrectomy | ∫ | ∫ | ||
| Dyslipidemiaβ | ||||
| Total | 76 (69–81) | 66 (56–74) | ||
| Gastric bypass | 79 (71–85) | 70 (59–79) | ||
| Sleeve gastrectomy | 69 (55–80) | 55 (36–73) | ||
| Elevated blood pressureα | ||||
| Total | 43 (35–51) | 73 (60–83) | ||
| Gastric bypass | 46 (37–55) | 78 (64–88) | ||
| Sleeve gastrectomy | 35 (23–50) | 53 (27–78) | ||
| Abnormal kidney functionψ | ||||
| Total | 17 (12–23) | 86 (63–90) | ||
| Gastric bypass | 19 (13–27) | 84 (59–95) | ||
| Sleeve gastrectomy | 12 (6–24) | ∫ | ||
| 5–12 year follow-up (FABS-5+ study; RYGB only) | ||||
| Baseline | Long-term follow-up | |||
| Prevalence of condition†-- % (95% CI) | Prevalence of condition†-- % (95% CI) | Prevalence of remission (observed) | Incidence (observed) | |
| Diabetes* | 16.1 (8.5–28.3) | 1.8 (0.2–12.0) | 7/8 (88%) | 0/45 (0%) |
| Dyslipidemiaβ | 85.7 (73.8–92.8) | 38.2 (26.3–51.7) | 29/45 (64%) | 4/8 (50.0%) |
| Elevated blood pressureα | 47.4 (34.7–60.4) | 16.4 (8.4–28.7) | 19/25 (76%) | 3/29 (10.3%) |
Generalized mixed models were used to calculate the modeled results.
The model failed because of the small sample size
Type 2 diabetes mellitus at baseline was defined by study investigators taking into consideration patient self-report of prior diagnosis, as well as prior medical records from referring physician, use of medications for DM, baseline HbA1c of ≥ 6.5%, or fasting glucose of at least 126 mg/dL, or oral glucose tolerance results in prior 6 months. Remission of T2D was defined as no use of medication for DM, and HbA1c < 6.5%, or, if HbA1c was not available, FBG <126 mg/dl.
Pre-diabetes at baseline was defined as no use of medications for T2D with HbA1c of ≥ 5.7% but < 6.5%, or, if HbA1c was not available, FBG 100 mg/dl to less than 126 mg/dl. Remission of pre-diabetes was defined as HBA1c <5.7%, or, if HBA1c was not available, FBG <100 mg/dl.
Dyslipidemia was defined for those <21 years of age as fasting triglycerides (TG) ≥130 mg/dl, or low density lipoprotein cholesterol (LDL-C) ≥ 130 mg/dl, or high density lipoprotein cholesterol (HDL-C) <40 mg/dl, or use of lipid lowering medications. If <21 years of age at follow-up, remission of dyslipidemia was defined as TG <130 mg/dl, and LDL-C <130 mg/dl, and HDL-C ≥ 40 mg/dl, and no use of lipid-lowering medications. If age was ≥ 21 years, resolution of dyslipidemia was defined as TG <200 mg/dl, and LDL-C <160 mg/dl, and HDL-C ≥ 40 mg/dl (males) or HDL-C ≥ 50 mg/dl (females), and no use of lipid-lowering medications.
Elevated BP is otherwise defined in a manner consistent with that used to define hypertension: use of BP medications or SBP ≥ 95th percentile or DBP ≥ 95th percentile (for age, sex, height) if 140 mmHg or DBP > 90 mmHg was used. Remission of elevated BP required that no medications for BP were used, and SBP and DBP were normal for age.
The presence of abnormal kidney function was determined using accepted criterial for chronic kidney disease (CKD) using glomerular filtration rate (GFR). For this study, abnormal kidney function was defined as any stage (1–5) of CKD. Resolution of abnormal kidney function was defined as attaining a GFR >60 with no evidence of kidney injury (urine albumin to creatinine ratio of ≤ 0.03).
Might earlier surgical intervention prevent progression to T2D in patients with prediabetes or in obese patients with normal glucose homeostasis? One might speculate that by improving insulin sensitivity earlier, one might also slow or halt the β-cell exhaustion that ultimately leads to full-blown T2D. In the Teen-LABS study(16), prediabetes remitted in 74% of patients at the 3-year follow-up visit, suggesting that this might be the case. Data do indicate that age at time of surgery, duration of diabetes, and severity of disease are all factors that influence the probability of postoperative diabetes remission(14, 43, 44). More data are needed to understand which patients may benefit most from earlier surgery, but the data to date are promising and highlight a potentially important tool especially for obese patients with insulin resistance who have not yet developed insulin dependence T2D.
Effect of early surgical intervention on T2D-related comorbidities
An important outcome of the TODAY study was the message that pediatric T2D, as compared with adult-onset T2D, may be a more aggressive disease with regard to the incidence and progression of certain T2D-related comorbidities. These include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, stroke risk, and renal disease. It has been speculated that early intervention with bariatric surgery during adolescence, before onset and/or progression of these comorbidities, may lead to more complete metabolic remission. Here, we highlight some of the potential benefits of early surgical intervention, although it should be emphasized that long-term outcomes (beyond 3 years) have rarely been reported and, thus, there is need for longer term data.
Cardiovascular disease
Cardiovascular disease is an important T2D-related comorbidity and is a significant public health problem as the leading cause of death in the U.S.(45). In adults, bariatric surgery can mitigate cardiovascular risk: RYGB improves Framingham 10-year cardiovascular risk scores by one year postoperatively in adults(46, 47) and both RYGB and VSG have been shown to improve New York Heart Association functional status(48). This may be related to potent reductions in dyslipidemia: lipid profiles are improved following bariatric surgery in adults(49, 50) and adolescents(16, 41), with comparable outcomes after VSG and RYGB(51, 52). In adolescents, reductions in total cholesterol, LDL, and triglyceride levels have been shown 1 year after RYGB(18, 42) and persist at 5 years postoperatively(41).
Reduced cardiovascular risk may also be related to other factors as well. Blood pressure is reduced in both adolescents(18, 41, 42) and adults after RYGB and VSG(53–55). Specifically, reductions in both systolic and diastolic blood pressure have been documented one year after RYGB in adolescents(18, 42) and are persistent after 5 years(41). Improvement is fairly consistent regardless of initial BMI, probably due to a comparable degree of weight lost by all patients(42).
An important open question is whether earlier surgical intervention has the potential for greater improvement in cardiovascular outcomes. No long-term studies are available yet, but preliminary data are promising(56). For example, in adolescents, left ventricular mass as well as thickness of the posterior wall and septum are reduced after RYGB(56), leading to improved diastolic function. Although left ventricular mass is improved in adults after RYGB, the thickness of neither the posterior wall nor septum are reduced(57). One might speculate that greater myocardial plasticity in younger patients may underlie this effect. Further study is needed to understand whether younger age at time of surgery may confer benefit in terms of cardiovascular risk reduction.
Polycystic ovarian syndrome (PCOS), gestational diabetes, and fertility
Insulin resistance and obesity are strongly linked with risk for polycystic ovarian syndrome (PCOS)(58), but the prevalence of PCOS as well as associated menstrual irregularity, hirsutism, and infertility are improved by bariatric surgery including VSG, RYGB, and AGB(59). A recent meta-analysis indicates that bariatric surgery reduced rates of PCOS from 45.6% preoperatively to only 7.1% postoperatively(59). In fact, infertility rates improve dramatically in women after bariatric surgery(60). Among adolescents after RYGB, pregnancy rates are higher than expected for this age group(61), possibly due to a combination of physiologic and psychosocial changes after surgery.
How might the potential for increased pregnancy rates after bariatric surgery relate to maternal and fetal health in this population? It is well-known that obese women have higher rates of gestational diabetes, as well as hypertension, fetal macrosomia, and cesarean section, compared with non-obese cohorts. Based on recently reviewed evidence(62), it is expected that bariatric surgery will improve both maternal health during pregnancy, as well as fetal outcomes for young women who do chose to become pregnant following adolescent bariatric surgery. In adolescents in particular, due to greater number of remaining reproductive years, it is critical to consider the potential risks that prior bariatric surgery may pose for both the mother and the infant. For example, bariatric surgery may increase risk for intrauterine growth restriction, small for gestational age babies, and nutritional deficiencies (specifically, for vitamin B12, folate, iron, and calcium) especially if individuals are not adherent with dietary and supplement recommendations(62). It is thus recommended that pregnancy be avoided for at least 12–18 months after surgery(63). On the other hand, bariatric surgery may reduce rates of childhood obesity in offspring(64), and uncomplicated pregnancies have been reported after adolescent bariatric surgery(25, 34). It is clear that we are only beginning to understand how bariatric surgery affects perinatal health outcomes. In the future, fetal and maternal outcomes should be systematically studied within the context of adolescent bariatric surgery in order to more completely understand the implications of performing these surgeries earlier in life.
Microvascular complications
Microvascular complications including retinopathy, nephropathy, and neuropathy are major causes of morbidity and mortality in patients with T2D. Thus, in order to consider bariatric surgery for primary treatment of T2D in adolescents, it is important to understand how surgery may affect incidence and progression of these comorbidities.
Diabetic nephropathy is a major cause of end-stage renal disease in the U.S.(65) and its presence greatly increases risk for mortality(66). Renal damage can be reversed, however, with diabetes remission(67). It appears that the anti-diabetic effect of bariatric surgery may also slow or reverse diabetes-related renal damage as indicated by presence of albuminuria(68–71) and by serum creatinine levels(70, 72). Not surprisingly, those patients with improved glycemic control 5 years after bariatric surgery exhibit lower urinary albumin-to-creatinine ratios(68). As hypertension, insulin resistance, and dyslipidemia are also risk factors for diabetic nephropathy, improvement to these obesity-related comorbidities after bariatric surgery may also play a role to improve renal disease(70).
In the Swedish Obesity Study, RYGB resulted in greater reductions in incidence of microalbuminuria than gastric banding(73). In this cohort, the hazard ratio for microalbuminuria was unaffected by risk factors including gender, age (above or below 47.9 years), BMI, serum insulin levels, and presence of impaired fasting blood glucose levels(73). However, overall microvascular risk reduction was greatest for patients with prediabetes(74), suggesting that intervention much earlier in the disease process may confer greater benefit in terms of preventing onset of diabetic nephropathy (and other microvascular complications). This is of particular importance for adolescent patients in whom T2D is associated with earlier and/or more aggressive comorbidity profiles. In adolescents 19 of 22 patients followed 3 years after RYGB or VSG had remission of abnormal kidney function(16); however, the number of patients with pre-existing renal dysfunction was too small to determine predictors of remission in this study.
Retinopathy is another microvascular complication of diabetes that can have devastating consequences on quality of life due to potential for reduced vision and/or blindness. Both RYGB and VSG appear to reduce incidence of diabetic retinopathy(75–77) in some patients. The principal risk factors for diabetic retinopathy are degree and duration of hyperglycemia and comorbid hypertension(78), both improved after bariatric surgery. However, risk for new-onset diabetic retinopathy is not eliminated by either surgery and progression of existing diabetic retinopathy can also occur(75, 76, 79). One meta-analysis(75) suggest that risk factors for progression include baseline severity and greater reduction in HBA1c. This paradoxic effect has also been observed in patients with type 1 and gestational diabetes and is hypothesized to be related to repeated hypoglycemia and resultant long-term retinal adaptations(75). As hypoglycemia can be common after RYGB, as a part of dumping syndrome, it is important to understand this relationship in order to understand which patients might be at most risk for new or progressive diabetic retinopathy after surgery. Furthermore, a closer look at which patients may be prone to onset or progression of diabetic retinopathy after bariatric surgery would prove helpful to risk-stratify and counsel patients considering surgery.
Less is known about the effect of bariatric surgery on risk for diabetic neuropathy, but a survey of patients after RYGB has indicated stability and/or improvement of diabetic neuropathy after RYGB(44). However, this has not been studied systematically. It is clear that larger studies are needed, particularly within the young adult and adolescent populations, in order to understand the effect of bariatric surgery on the natural history of diabetes-related microvascular complications. If effective in curbing progression and/or incidence of these problems in diabetic and prediabetic adolescents, bariatric surgery may be even more powerful than currently recognized to improve quality and length of life for obese adolescent patients.
Safety: weighing the risks and benefits of bariatric surgery for adolescent patients
Bariatric surgery may be a potent treatment for T2D, but it is important to take into consideration the safety of these procedures for the adolescent population. Serious complications related to surgery are uncommon but have been reported. Complications include staple line dehiscence(80, 81), marginal ucleration(82), minor bleeding, atelectasis, and superficial venous thrombosis(83). In adults, these risks are counterbalanced by an overall reduction in mortality rates(84). Long-term data have not been collected with regard to adolescent patients who have had bariatric surgery, but the preliminary data suggest similar complication rates(85). The Teen-LABS study reported a 30-day complication rate of 8%(86) and 3-year reoperation rates of 13%(16). Interestingly, in one study(85), perioperative hospital stays were shorter in adolescents as compared with adults, raising the question of whether better baseline health may contribute to lower perioperative risk within the adolescent population.
In addition to the risk of perioperative complications, patients may face long-term risk for micronutrient deficiencies due to gastric reduction and/or intestinal diversion. These deficiencies may pose unique risks to young patients in particular. Specifically, iron, calcium, vitamin D, vitamin B12, zinc, vitamin C, copper, and vitamin B1 deficiencies have been documented(87–89). Vitamin B12 deficiency is much more common after RYGB than VSG(87), likely owing to the intestinal diversion involved with the former. On the other hand, other lipid-soluble vitamins such as vitamins E, A, and K are infrequently a problem after RYGB(90), as it is not primarily a malabsorptive procedure. Recently, the Teen-LABS study(16) highlighted micronutrient deficiencies as a significant risk for adolescents after bariatric surgeries. In this study, 8% of patients had vitamin B12 deficiency at 3 years, as compared with <1% at the baseline visit. Iron deficiency was common, affecting 65% of RYGB patients and 34% of VSG patients. This corroborates adult studies highlighting iron deficiency as the one of the most common and earliest nutritional deficiencies observed following bariatric surgery(91). Other, less common nutritional deficiencies after bariatric surgery include vitamin B1 (thiamin) deficiency (87, 92) and folic acid deficiency(93), although both were rare in the Teen-LABS study and postoperatively were not more common than at baseline(16). Of note, protein-calorie malnutrition is uncommon beyond the immediate postoperative period(94) and the Teen-LABS study(16) reported no cases of hypoalbuminemia at the 3-year time point.
Of particular relevance to the adolescent population, bariatric surgeries including both RYGB and VSG have the potential to impair bone mineral density (BMD) in both adults(95–97) and adolescents(98). Even in adolescents who have reached adult height, adolescence is a critical period for bone mineral accretion(99). The impairment of BMD following bariatric surgery in adolescents may be related to impaired intestinal calcium absorption, particularly after RYGB(100), although other, non-nutritional factors may play a role as well. For example, reduced body weight might diminish mechanical load-induced bone mineralization. It has also been speculated that reduced ghrelin levels after either RYGB or VSG may be contributory, as ghrelin levels correlate with BMD in obese children(101). Although vitamin D supplementation is recommended for all RYGB patients postoperatively(90) it may not fully prevent BMD reduction(102). Despite interval reduction, above-average postoperative BMD has been demonstrated in adolescents after RYGB(98); however, it is unclear for how long BMD loss might continue. This is a particularly relevant question for adolescents.
Vitamin supplementation is critical after bariatric surgery, particularly after RYGB, to avoid dangerous consequences of micronutrient deficiencies. Most bariatric surgeons recommend long-term use of a multivitamin, calcium citrate, and supplemental vitamin D for all patients(103, 104). For RYGB patients, addition of elemental iron for all menstruating patients and vitamin B12 is also recommended. One concern is that adolescent patients may be less adherent to recommended supplementation(105). This may be an important individual consideration when weighing the potential risks and benefits of surgical versus medical management for adolescents with T2D, especially for those with history of poor adherence to prior medical therapies.
Conclusions: Interpretations and the future of adolescent bariatric surgery
At present, bariatric surgery is recommended for individuals with BMI of at least 40 kg/m2, or 35 kg/m2 with obesity-related comorbidities, including T2D. However, given the evidence reviewed here that bariatric surgery may reverse or postpone onset of T2D-related problems including microvascular complications and cardiovascular risk, it is important to consider whether earlier intervention might improve long-term outcomes for these patients. If so, then ideally, patients would be treated early after T2D diagnosis or even before diagnosis in those obese, pre-diabetic patients refractory to traditional medical therapy. For adults, it is debated as to whether the BMI cut-offs for bariatric surgery recommendation should be lowered. This requires special consideration for adolescents, in whom bariatric surgery may have the greatest potential but also the greatest risk.
With an average age of onset of youth-onset T2D in early adolescence in the U.S., intervening before T2D diagnosis or early in the disease process would mean performing surgery prior to the completion of growth and development in most males and in many females. Given the potential for reduced bone mineral density and for nutritional deficiencies that may compromise growth and development, surgery at this age has not yet been considered by pediatric surgeons. However, it is important to weigh the evidence and to determine the earliest age at which the benefits of surgery may outweigh the risks. An important aspect of this analysis will be to determine whether RYGB and/or VSG may have improved safety within the youngest cohort of patients in particular. Although this opinion may be viewed as extreme, it is a response to the potential for a more severe disease course in young patients who may have accelerated development of T2D-related comorbidities(5).
An important goal for future study should be to try to understand which patients might be best suited for medical versus surgical management. The TODAY study(106) cited higher BMI, presence of depression, black race, and higher baseline HbA1c levels as predictors of failure of medical T2D management. Perhaps these patients may be better suited for bariatric surgery. On the other hand, poor adherence to postoperative dietary and nutritional recommendations can be dangerous after bariatric surgery and it is important to consider what factors may predict non-adherence. In addition to these psychosocial variables, one should consider whether certain genetic variations or serum biomarkers might predict better outcomes, including more complete T2D remission, after bariatric surgery. Ongoing mechanistic studies in humans and rodents will be important for designing such assays, as well as for engineering less invasive T2D treatments for adolescents.
Acknowledgments
Dr. Stefater has received research grants from the Pediatric Endocrine Society and the Endocrine Fellows Foundation. Her training is also funded by an institutional T32 (DK007699) training grant.
Dr. Inge has received grants from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Dr. Stefater declares that she has no conflict of interest.
Dr. Inge has been a consultant for Standard Bariatric and UpToDate.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human participants or animals performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Association AD. Statistics About Diabetes. 2017 [cited 2017]. Available from: http://www.diabetes.org/diabetes-basics/statistics/?referrer=https://www.google.com/
- 2.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. Jama. 2014;311(17):1778–86. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes care. 2012;35(12):2515–20. doi: 10.2337/dc12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantino MI, Molyneaux L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes care. 2013;36(12):3863–9. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159–67. doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ, et al. International Society for P. ISPAD Clinical Practice Consensus Guidelines 2006–2007. Type 2 diabetes mellitus in the child and adolescent. Pediatric diabetes. 2008;9(5):512–26. doi: 10.1111/j.1399-5448.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- 7.Group TS, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. The New England journal of medicine. 2012;366(24):2247–56. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klingensmith GJ, Connor CG, Ruedy KJ, Beck RW, Kollman C, Haro H, et al. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatric diabetes. 2016;17(4):266–73. doi: 10.1111/pedi.12281. [DOI] [PubMed] [Google Scholar]
- 9.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. Jama. 2012;307(5):483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obesity surgery. 2005;15(4):474–81. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 11.Rizzello M, Abbatini F, Casella G, Alessandri G, Fantini A, Leonetti F, et al. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg. 20(1):50–5. doi: 10.1007/s11695-009-0017-2. [DOI] [PubMed] [Google Scholar]
- 12.Basso N, Capoccia D, Rizzello M, Abbatini F, Mariani P, Maglio C, et al. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: the gastric hypothesis. Surg Endosc. 2011 doi: 10.1007/s00464-011-1755-5. [DOI] [PubMed] [Google Scholar]
- 13.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Annals of surgery. 2004;240(2):236–42. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–50. doi: 10.1097/00000658-199509000-00011. discussion 50–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Sabah SK, Almazeedi SM, Dashti SA, Al-Mulla AY, Ali DA, Jumaa TH. The efficacy of laparoscopic sleeve gastrectomy in treating adolescent obesity. Obesity surgery. 2015;25(1):50–4. doi: 10.1007/s11695-014-1340-9. [DOI] [PubMed] [Google Scholar]
- 16••.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. The New England Journal of Medicine. 2016;374(2):113–23. doi: 10.1056/NEJMoa1506699. A part of the Teen-LABS study, this was the first large (242 patients), prospective study of outcomes 3 years after sleeve gastrectomy or RYGB in adolescents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vilallonga R, Himpens J, van de Vrande S. Long-Term (7 Years) Follow-Up of Roux-en-Y Gastric Bypass on Obese Adolescent Patients (<18 Years) Obesity facts. 2016;9(2):91–100. doi: 10.1159/000442758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inge TH, Miyano G, Bean J, Helmrath M, Courcoulas A, Harmon CM, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009;123(1):214–22. doi: 10.1542/peds.2008-0522. [DOI] [PubMed] [Google Scholar]
- 19.Kelsey MM, Geffner ME, Guandalini C, Pyle L, Tamborlane WV, Zeitler PS, et al. Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatric diabetes. 2016;17(3):212–21. doi: 10.1111/pedi.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Copeland KC, Silverstein J, Moore KR, Prazar GE, Raymer T, Shiffman RN, et al. Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364–82. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 21.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George MM, Copeland KC. Current treatment options for type 2 diabetes mellitus in youth: today’s realities and lessons from the TODAY study. Current diabetes reports. 2013;13(1):72–80. doi: 10.1007/s11892-012-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JD, Mills E, Carlisle SE. Treatment of Pediatric Type 2 Diabetes. The Annals of pharmacotherapy. 2016;50(9):768–77. doi: 10.1177/1060028016655179. [DOI] [PubMed] [Google Scholar]
- 24.Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2016;12(6):1144–62. doi: 10.1016/j.soard.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Lawson ML, Kirk S, Mitchell T, Chen MK, Loux TJ, Daniels SR, et al. One-year outcomes of Roux-en-Y gastric bypass for morbidly obese adolescents: a multicenter study from the Pediatric Bariatric Study Group. J Pediatr Surg. 2006;41(1):137–43. doi: 10.1016/j.jpedsurg.2005.10.017. discussion -43. [DOI] [PubMed] [Google Scholar]
- 26.Carlsson LM, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson B, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. The New England journal of medicine. 2012;367(8):695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 27.Rubino F, R’Bibo SL, del Genio F, Mazumdar M, McGraw TE. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 6(2):102–9. doi: 10.1038/nrendo.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Courcoulas AP, Belle SH, Neiberg RH, Pierson SK, Eagleton JK, Kalarchian MA, et al. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA surgery. 2015;150(10):931–40. doi: 10.1001/jamasurg.2015.1534. A part of the LABS study, the adult correlate to the Teen-LABS study, this was a large, prospective study examining the impact of RYGB and AGB versus lifestyle intervention in adult diabetic patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14(9):1553–61. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 31.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. The New England journal of medicine. 2014;370(21):2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. The New England journal of medicine. 2017;376(7):641–51. doi: 10.1056/NEJMoa1600869. A prospective study of 134 adult patients followed 5 years after bariatric surgery showed superior rates of diabetes remission amongst patients treated with VSG or RYGB, as compared with intensive medical therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capella JF, Capella RF. Bariatric surgery in adolescence is this the best age to operate? Obes Surg. 2003;13(6):826–32. doi: 10.1381/096089203322618597. [DOI] [PubMed] [Google Scholar]
- 34.Strauss RS, Bradley LJ, Brolin RE. Gastric bypass surgery in adolescents with morbid obesity. The Journal of pediatrics. 2001;138(4):499–504. doi: 10.1067/mpd.2001.113043. [DOI] [PubMed] [Google Scholar]
- 35.Inge T, Wilson KA, Gamm K, Kirk S, Garcia VF, Daniels SR. Preferential loss of central (trunk) adiposity in adolescents and young adults after laparoscopic gastric bypass. Surg Obes Relat Dis. 2007;3(2):153–8. doi: 10.1016/j.soard.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Collins J, Mattar S, Qureshi F, Warman J, Ramanathan R, Schauer P, et al. Initial outcomes of laparoscopic Roux-en-Y gastric bypass in morbidly obese adolescents. Surg Obes Relat Dis. 2007;3(2):147–52. doi: 10.1016/j.soard.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Stanford A, Glascock JM, Eid GM, Kane T, Ford HR, Ikramuddin S, et al. Laparoscopic Roux-en-Y gastric bypass in morbidly obese adolescents. J Pediatr Surg. 2003;38(3):430–3. doi: 10.1053/jpsu.2003.50074. [DOI] [PubMed] [Google Scholar]
- 38.Dolan K, Creighton L, Hopkins G, Fielding G. Laparoscopic gastric banding in morbidly obese adolescents. Obes Surg. 2003;13(1):101–4. doi: 10.1381/096089203321136674. [DOI] [PubMed] [Google Scholar]
- 39.Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7(1):102–7. doi: 10.1016/S1091-255X(02)00125-7. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien PE, Sawyer SM, Laurie C, Brown WA, Skinner S, Veit F, et al. Laparoscopic adjustable gastric banding in severely obese adolescents: a randomized trial. Jama. 2010;303(6):519–26. doi: 10.1001/jama.2010.81. [DOI] [PubMed] [Google Scholar]
- 41••.Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. The lancet Diabetes & endocrinology. 2017;5(3):165–73. doi: 10.1016/S2213-8587(16)30315-1. Five-year outcomes of the Teen-LABS study, a large prospective study of adolescent bariatric surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inge TH, Jenkins TM, Zeller M, Dolan L, Daniels SR, Garcia VF, et al. Baseline BMI is a strong predictor of nadir BMI after adolescent gastric bypass. J Pediatr. 2010;156(1):103–8. e1. doi: 10.1016/j.jpeds.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. Jama. 2008;299(3):316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 44.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Annals of surgery. 2003;238(4):467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14):1–134. [PubMed] [Google Scholar]
- 46.Arterburn D, Schauer DP, Wise RE, Gersin KS, Fischer DR, Selwyn CA, Jr, et al. Change in predicted 10-year cardiovascular risk following laparoscopic Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19(2):184–9. doi: 10.1007/s11695-008-9534-7. [DOI] [PubMed] [Google Scholar]
- 47.Torquati A, Wright K, Melvin W, Richards W. Effect of gastric bypass operation on Framingham and actual risk of cardiovascular events in class II to III obesity. J Am Coll Surg. 2007;204(5):776–82. doi: 10.1016/j.jamcollsurg.2006.12.038. discussion 82–3. [DOI] [PubMed] [Google Scholar]
- 48.McCloskey CA, Ramani GV, Mathier MA, Schauer PR, Eid GM, Mattar SG, et al. Bariatric surgery improves cardiac function in morbidly obese patients with severe cardiomyopathy. Surg Obes Relat Dis. 2007;3(5):503–7. doi: 10.1016/j.soard.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Zlabek JA, Grimm MS, Larson CJ, Mathiason MA, Lambert PJ, Kothari SN. The effect of laparoscopic gastric bypass surgery on dyslipidemia in severely obese patients. Surg Obes Relat Dis. 2005;1(6):537–42. doi: 10.1016/j.soard.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401–7. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 51.Benaiges D, Goday A, Ramon JM, Hernandez E, Pera M, Cano JF. Laparoscopic sleeve gastrectomy and laparoscopic gastric bypass are equally effective for reduction of cardiovascular risk in severely obese patients at one year of follow-up. Surg Obes Relat Dis. 2011 doi: 10.1016/j.soard.2011.03.002. in press. [DOI] [PubMed] [Google Scholar]
- 52.Woelnerhanssen B, Peterli R, Steinert RE, Peters T, Borbely Y, Beglinger C. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy-a prospective randomized trial. Surg Obes Relat Dis. 2011 doi: 10.1016/j.soard.2011.01.044. in press. [DOI] [PubMed] [Google Scholar]
- 53.Iannelli A, Dainese R, Piche T, Facchiano E, Gugenheim J. Laparoscopic sleeve gastrectomy for morbid obesity. World J Gastroenterol. 2008;14(6):821–7. doi: 10.3748/wjg.14.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 55.Vidal J, Ibarzabal A, Romero F, Delgado S, Momblan D, Flores L, et al. Type 2 diabetes mellitus and the metabolic syndrome following sleeve gastrectomy in severely obese subjects. Obes Surg. 2008;18(9):1077–82. doi: 10.1007/s11695-008-9547-2. [DOI] [PubMed] [Google Scholar]
- 56.Ippisch HM, Inge TH, Daniels SR, Wang B, Khoury PR, Witt SA, et al. Reversibility of cardiac abnormalities in morbidly obese adolescents. Journal of the American College of Cardiology. 2008;51(14):1342–8. doi: 10.1016/j.jacc.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 57.Willens HJ, Chakko SC, Byers P, Chirinos JA, Labrador E, Castrillon JC, et al. Effects of weight loss after gastric bypass on right and left ventricular function assessed by tissue Doppler imaging. Am J Cardiol. 2005;95(12):1521–4. doi: 10.1016/j.amjcard.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 58.Ehrmann DA. Polycystic ovary syndrome. The New England journal of medicine. 2005;352(12):1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 59.Skubleny D, Switzer NJ, Gill RS, Dykstra M, Shi X, Sagle MA, et al. The Impact of Bariatric Surgery on Polycystic Ovary Syndrome: a Systematic Review and Meta-analysis. Obesity surgery. 2016;26(1):169–76. doi: 10.1007/s11695-015-1902-5. [DOI] [PubMed] [Google Scholar]
- 60.Milone M, De Placido G, Musella M, Sosa Fernandez LM, Sosa Fernandez LV, Campana G, et al. Incidence of Successful Pregnancy After Weight Loss Interventions in Infertile Women: a Systematic Review and Meta-Analysis of the Literature. Obesity surgery. 2016;26(2):443–51. doi: 10.1007/s11695-015-1998-7. [DOI] [PubMed] [Google Scholar]
- 61.Roehrig HR, Xanthakos SA, Sweeney J, Zeller MH, Inge TH. Pregnancy after gastric bypass surgery in adolescents. Obes Surg. 2007;17(7):873–7. doi: 10.1007/s11695-007-9162-7. [DOI] [PubMed] [Google Scholar]
- 62.Magdaleno R, Jr, Pereira BG, Chaim EA, Turato ER. Pregnancy after bariatric surgery: a current view of maternal, obstetrical and perinatal challenges. Arch Gynecol Obstet. 2012;285(3):559–66. doi: 10.1007/s00404-011-2187-0. [DOI] [PubMed] [Google Scholar]
- 63.Kominiarek MA. Preparing for and managing a pregnancy after bariatric surgery. Seminars in perinatology. 2011;35(6):356–61. doi: 10.1053/j.semperi.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dalfra MG, Busetto L, Chilelli NC, Lapolla A. Pregnancy and foetal outcome after bariatric surgery: a review of recent studies. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(9):1537–43. doi: 10.3109/14767058.2012.663829. [DOI] [PubMed] [Google Scholar]
- 65.US Renal Data System. USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States [press release] Bethesda, MD: National Institute of Diabetes and Digestive Kidney Diseases; 2014. [Google Scholar]
- 66.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality risk in type 2 diabetes. Journal of the American Society of Nephrology: JASN. 2013;24(2):302–8. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. The New England journal of medicine. 1998;339(2):69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 68.Heneghan HM, Cetin D, Navaneethan SD, Orzech N, Brethauer SA, Schauer PR. Effects of bariatric surgery on diabetic nephropathy after 5 years of follow-up. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2013;9(1):7–14. doi: 10.1016/j.soard.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 69.Stephenson DT, Jandeleit-Dahm K, Balkau B, Cohen N. Improvement in albuminuria in patients with type 2 diabetes after laparoscopic adjustable gastric banding. Diabetes & vascular disease research. 2013;10(6):514–9. doi: 10.1177/1479164113498083. [DOI] [PubMed] [Google Scholar]
- 70.Friedman AN, Wolfe B. Is Bariatric Surgery an Effective Treatment for Type II Diabetic Kidney Disease? Clinical journal of the American Society of Nephrology: CJASN. 2016;11(3):528–35. doi: 10.2215/CJN.07670715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Upala S, Wijarnpreecha K, Congrete S, Rattanawong P, Sanguankeo A. Bariatric surgery reduces urinary albumin excretion in diabetic nephropathy: a systematic review and meta-analysis. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2016;12(5):1037–44. doi: 10.1016/j.soard.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 72.Zakaria AS, Rossetti L, Cristina M, Veronelli A, Lombardi F, Saibene A, et al. Effects of gastric banding on glucose tolerance, cardiovascular and renal function, and diabetic complications: a 13-year study of the morbidly obese. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2016;12(3):587–95. doi: 10.1016/j.soard.2015.10.062. [DOI] [PubMed] [Google Scholar]
- 73.Carlsson LM, Romeo S, Jacobson P, Burza MA, Maglio C, Sjoholm K, et al. The incidence of albuminuria after bariatric surgery and usual care in Swedish Obese Subjects (SOS): a prospective controlled intervention trial. Int J Obes (Lond) 2015;39(1):169–75. doi: 10.1038/ijo.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlsson LM, Sjoholm K, Karlsson C, Jacobson P, Andersson-Assarsson JC, Svensson PA, et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. The lancet Diabetes & endocrinology. 2017 doi: 10.1016/S2213-8587(17)30061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorman DM, le Roux CW, Docherty NG. The Effect of Bariatric Surgery on Diabetic Retinopathy: Good, Bad, or Both? Diabetes & metabolism journal. 2016;40(5):354–64. doi: 10.4093/dmj.2016.40.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merlotti C, Ceriani V, Morabito A, Pontiroli AE. Bariatric surgery and diabetic retinopathy: a systematic review and meta-analysis of controlled clinical studies. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2017;18(3):309–16. doi: 10.1111/obr.12490. [DOI] [PubMed] [Google Scholar]
- 77.Kim YJ, Kim BH, Choi BM, Sun HJ, Lee SJ, Choi KS. Bariatric surgery is associated with less progression of diabetic retinopathy: A systematic review and meta-analysis. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2017;13(2):352–60. doi: 10.1016/j.soard.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes care. 2012;35(3):556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amin AM, Wharton H, Clarke M, Syed A, Dodson P, Tahrani AA. The impact of bariatric surgery on retinopathy in patients with type 2 diabetes: a retrospective cohort study. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2016;12(3):606–12. doi: 10.1016/j.soard.2015.08.508. [DOI] [PubMed] [Google Scholar]
- 80.Arteaga JR, Huerta S, Livingston EH. Management of gastrojejunal anastomotic leaks after Roux-en-Y gastric bypass. Am Surg. 2002;68(12):1061–5. [PubMed] [Google Scholar]
- 81.Burgos AM, Braghetto I, Csendes A, Maluenda F, Korn O, Yarmuch J, et al. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg. 2009;19(12):1672–7. doi: 10.1007/s11695-009-9884-9. [DOI] [PubMed] [Google Scholar]
- 82.Rasmussen JJ, Fuller W, Ali MR. Marginal ulceration after laparoscopic gastric bypass: an analysis of predisposing factors in 260 patients. Surgical endoscopy. 2007;21(7):1090–4. doi: 10.1007/s00464-007-9285-x. [DOI] [PubMed] [Google Scholar]
- 83.Chouillard EK, Karaa A, Elkhoury M, Greco VJ Intercontinental Society of Natural Orifice E, Laparoscopic S. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for morbid obesity: case-control study. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2011;7(4):500–5. doi: 10.1016/j.soard.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 84.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 85.Tsai WS, Inge TH, Burd RS. Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome. Arch Pediatr Adolesc Med. 2007;161(3):217–21. doi: 10.1001/archpedi.161.3.217. [DOI] [PubMed] [Google Scholar]
- 86.Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA pediatrics. 2014;168(1):47–53. doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)-a prospective study. Obes Surg. 2010;20(4):447–53. doi: 10.1007/s11695-009-0068-4. [DOI] [PubMed] [Google Scholar]
- 88.Clements RH, Katasani VG, Palepu R, Leeth RR, Leath TD, Roy BP, et al. Incidence of vitamin deficiency after laparoscopic Roux-en-Y gastric bypass in a university hospital setting. Am Surg. 2006;72(12):1196–202. doi: 10.1177/000313480607201209. discussion 203–4. [DOI] [PubMed] [Google Scholar]
- 89.Griffith DP, Liff DA, Ziegler TR, Esper GJ, Winton EF. Acquired copper deficiency: a potentially serious and preventable complication following gastric bypass surgery. Obesity (Silver Spring) 2009;17(4):827–31. doi: 10.1038/oby.2008.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33(1):13–24. doi: 10.1016/j.diabet.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 91.Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am. 2009;56(5):1105–21. doi: 10.1016/j.pcl.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Towbin A, Inge TH, Garcia VF, Roehrig HR, Clements RH, Harmon CM, et al. Beriberi after gastric bypass surgery in adolescence. J Pediatr. 2004;145(2):263–7. doi: 10.1016/j.jpeds.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 93.Mallory GN, Macgregor AM. Folate Status Following Gastric Bypass Surgery (The Great Folate Mystery) Obes Surg. 1991;1(1):69–72. doi: 10.1381/096089291765561493. [DOI] [PubMed] [Google Scholar]
- 94.Jeffreys RM, Hrovat K, Woo JG, Schmidt M, Inge TH, Xanthakos SA. Dietary assessment of adolescents undergoing laparoscopic Roux-en-Y gastric bypass surgery: macro- and micronutrient, fiber, and supplement intake. Surg Obes Relat Dis. 2011 doi: 10.1016/j.soard.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89(3):1061–5. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- 96.Mahdy T, Atia S, Farid M, Adulatif A. Effect of Roux-en Y gastric bypass on bone metabolism in patients with morbid obesity: Mansoura experiences. Obes Surg. 2008;18(12):1526–31. doi: 10.1007/s11695-008-9653-1. [DOI] [PubMed] [Google Scholar]
- 97.von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53(7):918–21. doi: 10.1016/j.metabol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 98.Kaulfers AM, Bean JA, Inge TH, Dolan LM, Kalkwarf HJ. Bone loss in adolescents after bariatric surgery. Pediatrics. 2011;127(4):e956–61. doi: 10.1542/peds.2010-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schafer AL. Vitamin D and intestinal calcium transport after bariatric surgery. The Journal of steroid biochemistry and molecular biology. 2016 doi: 10.1016/j.jsbmb.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pacifico L, Anania C, Poggiogalle E, Osborn JF, Prossomariti G, Martino F, et al. Relationships of acylated and des-acyl ghrelin levels to bone mineralization in obese children and adolescents. Bone. 2009;45(2):274–9. doi: 10.1016/j.bone.2009.04.204. [DOI] [PubMed] [Google Scholar]
- 102.Carlin AM, Rao DS, Yager KM, Parikh NJ, Kapke A. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: a randomized prospective clinical trial. Surg Obes Relat Dis. 2009;5(4):444–9. doi: 10.1016/j.soard.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 103.Xanthakos SA, Inge TH. Nutritional consequences of bariatric surgery. Curr Opin Clin Nutr Metab Care. 2006;9(4):489–96. doi: 10.1097/01.mco.0000232913.07355.cf. [DOI] [PubMed] [Google Scholar]
- 104.Fullmer MA, Abrams SH, Hrovat K, Mooney L, Scheimann AO, Hillman JB, et al. Nutritional strategy for adolescents undergoing bariatric surgery: report of a working group of the Nutrition Committee of NASPGHAN/NACHRI. J Pediatr Gastroenterol Nutr. 2012;54(1):125–35. doi: 10.1097/MPG.0b013e318231db79. [DOI] [PubMed] [Google Scholar]
- 105.Rand CS, Macgregor AM. Adolescents having obesity surgery: a 6-year follow-up. Southern medical journal. 1994;87(12):1208–13. doi: 10.1097/00007611-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 106.Zeitler P, Hirst K, Copeland KC, El Ghormli L, Levitt Katz L, Levitsky LL, et al. HbA1c After a Short Period of Monotherapy With Metformin Identifies Durable Glycemic Control Among Adolescents With Type 2 Diabetes. Diabetes care. 2015;38(12):2285–92. doi: 10.2337/dc15-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]


