Abstract
Purpose
Determine if hypoxia preconditioning can protect corneal endothelial cells from mechanical stress and perioperative procedures mimicking Descemet stripping automated endothelial keratoplasty (DSAEK).
Methods
Preconditioning was delivered by 2 hours of 0.5% oxygen incubation in a hypoxia chamber or by exposure to the prolylhydroxylase inhibitor FG-4592, which prevents Hypoxia inducible factor-1 alpha degradation. Damage to whole corneas was produced by brief sonication. To mimic use with DSAEK, FG-4592 preconditioned and control donor corneas were dissected with a microkeratome and the posterior donor button was pulled through a transplant insertion device (Busin glide). The area of endothelial damage was determined from trypan blue staining.
Results
In all cases, hypoxia preconditioning or incubation with FG-4592 protected corneal endothelial cells from death by mechanical stress. Hypoxia preconditioned human and rabbit corneas showed 19 and 29% less cell loss, respectively, relative to controls, which were both significant at p<0.05. FG-4592 preconditioning reduced endothelial cell loss associated with preparation and insertion of DSAEK grafts by 23% relative to control (p<0.01).
Conclusion
These results support the hypothesis that preconditioning by hypoxia or exposure to FG-4592 improves corneal endothelial cell survival and may also provide protection during surgical trauma.
Keywords: Endothelial keratoplasty, DSEK, DSAEK, hypoxia preconditioning, corneal endothelial cell loss, prolylhydroxylase inhibitor, Fiji segmentation software
Introduction
Endothelial keratoplasty (EK) is a surgical technique in which the damaged and depleted endothelial cells of the host cornea are selectively replaced with healthy donor endothelial cells.1 EK is the preferred surgical procedure for disease specifically affecting the corneal endothelium such as Fuchs endothelial corneal dystrophy (FECD) and pseudophakic bullous keratopathy.2 The advantages of EK over penetrating keratoplasty include rapid visual recovery, faster wound healing, reduced graft rejection and preservation of globe integrity and strength.2 Although EK has revolutionized keratoplasty techniques, it is not without its own set of difficulties and challenges.2 The surgical procedure of endothelial keratoplasty exerts a significant amount of stress on donor endothelium leading to perioperative cell loss which can be as high as 40% of the total endothelial cell density, and cell loss continues at an accelerated rate post keratoplasty.3-5 The exact pathophysiological mechanism for rapid endothelial cell loss post keratoplasty is not completely understood, with mechanical surgical trauma, inflammation, and sub-clinical immunologic rejection leading to endothelial cell apoptosis as the most probable explanations.6-9
Various strategies have been developed to decrease the dramatic post-surgical endothelial cell loss in EK. Most of the strategies are focused on reducing the mechanical surgical trauma by improving the surgical techniques.10 Alternative strategies include transfer of cultivated endothelial cells,11 reduction in oxidative stress induced apoptosis by genetic12, 13 or pharmacological intervention,14 promoting endothelial cell replication,15 and better eye banking techniques.16, 17
Hypoxia preconditioning, by exposure to a sub-lethal dose of hypoxia, can protect many cell types and organs from subsequent lethal trauma,18, 19 and it has been shown to protect cultured human corneal endothelial cells from lethal doses of oxidative stress.20 In addition to depressing mitochondrial function, hypoxia upregulates cell survival genes controlled by the nuclear transcription factor Hypoxia Inducible Factor-1α (HIF1α), increasing resistance to apoptotic cell damage.21 In the presence of oxygen, HIF1α is continually hydroxylated in the cell cytoplasm by prolylhydroxylases. Hydoxylated HIF1α is recognized by von Hippel Lindau factor, which directs HIF1α to the proteasome for degradation. When oxygen is limiting, HIF1α can migrate to the nucleus, bind to Hypoxia Response Elements (HREs) thereby activating hypoxia-inducible genes. Two prominent examples of HIF1α inducible genes are VEGF and Erythropoietin. FG-4592, which is currently in clinical trials for anemia,22 is a hypoxia inducible factor – prolyl hydroxylase inhibitor (HIF-PHI),23 thereby preventing HIF1α degradation leading to induction of HREs, indicating that FG-4592 could be used to induce protective factors without inhibiting mitochondrial function.
In this study, we examined the potential of hypoxia preconditioning to protect corneal endothelial cells from mechanical stress. We found that hypoxia or FG-4592 can significantly protect corneal endothelial cells in whole rabbit and human corneas that were subjected to sonication induced mechanical stress as well as protecting cultured human corneal endothelial cells subjected to oxidative stress. Moreover, FG-4592 protected endothelial cells subjected to perioperative procedures mimicking Descemet stripping automated endothelial keratoplasty (DSAEK). These results indicate that FG-4592 preconditioning has the potential to protect corneal endothelial cells during EK.
Materials and Methods
Rabbit Cornea Damage Model
Rabbit eyes were obtained from Pel-Freez Biologicals (Rogers, AR) within 24 hours of death and shipped overnight on ice. After trimming and sterilizing with 1% Iodine, each cornea was dissected and rinsed at least 6 times with sterile phosphate buffered saline (PBS) in a tissue culture hood. The cornea was put in a 12-well plate, immersed in 2 ml Dulbecco modified Eagle medium (DMEM) tissue culture medium with antibiotic-antimycotic and 200 uM glutamine, and maintained at 37°C for 27 hours.
Rabbit corneas were divided into four groups (Table 1). Group 1 corneas were placed in a 5% CO2 tissue culture incubator (balance room air) for 27 hours without sonication treatment. Group 2 corneas were placed in a hypoxia chamber (Coy Laboratory Products, Inc., Grass Lake, MI), 5% CO2, 0.5% O2, balance N2, for 2 hours,20 then moved to the Group 1 incubator for another 25 hours. Group 3 corneas were placed in the 5% CO2 incubator for 3 hours then taken out to damage the endothelial surface by sonication. The cornea, supported in the 12 well plate filled with culture medium, endothelial surface facing up, was placed under a Sonic Dismembrator Model 100 (Fisher Scientific, Pittsburgh, PA) with stainless steel tip (diameter 3 mm). The tip was lowered to just touching the fluid surface in the center of the cornea. A sonication pulse at Power setting 1 was released for 2 seconds. The cornea was then put back in the incubator for 24 hours. Group 4 corneas were first placed in the hypoxia chamber for 2 hours, then moved to the 5% CO2 incubator for 1 hour; in turn, their endothelial surfaces were damaged by sonication and put back in the 5% CO2 incubator for another 24 hours.
Table 1. Overview of the 4 experimental models used to test the potential protective effect of hypoxia pre-conditioning on corneal endothelial cell survival.
| Model | Description | Control: normoxia | Hypoxia pre-conditioning | |
|---|---|---|---|---|
| 0.5% O2 | FG-4592 | |||
| No. of replicates | No. of replicates | No. of replicates | ||
| 1 | Mechanical damage to rabbit corneas | |||
| Control: no sonication | 17 (Group 1) | 22 (Group 2) | -- | |
| Sonication | 22 (Group 3) | 23 (Group 4) | -- | |
| 2 | Exposure of cultured human corneal endothelial cells to tert-butyl hydroperoxide; | 12 | -- | 12 each |
| FG-4592 concentrations of 0 (control), 1, 5, 10, 50 and 100 micro-molar were tested | ||||
| 3 | Sonication damage to human donor corneas | 12 (Group 1) | 12 (Group 2) | 12 (Group 3) |
| 4 | In vitro keratoplasty (DSAEK) simulation with randomization of paired human donor corneas | 10 (Group 1) | -- | 10 (Group 2) |
Abbreviations: DSAEK = Descemet stripping automated endothelial keratoplasty
Measurement of endothelium damage was determined using a slightly modified procedure following Terry et al.10 After treatment and culturing, corneas were washed with PBS, the endothelial surface was stained with 0.2% trypan blue for 2 minutes and washed with PBS 3 times. The corneal image was taken under a dissection microscope (SMZ1500, Nikon USA, Melville, NY). Damaged endothelial surface was measured by quantitative analysis of the image in Adobe Photoshop CS5. Damaged area % = Pixels of blue stained area/Pixels of entire corneal area.
Human Corneal Endothelial Cell (HCEC) Model
To provide guidance for the dosage of FG-4592 (Sigma, St. Louis, MO), a human corneal endothelial cell line24 (HCEC) was used. Sonication was not considered appropriate for cells attached to a tissue culture plate, so tBHP (tert-Butyl hydroperoxide) was applied to damage cells. HCEC were seeded in 12-well plates at 2 × 105 cells/well in 1 ml Opti-MEM with 10% fetal calf serum (FBS). At approximately 60% confluence, the cells were changed to Opti-MEM with 0.5% FBS for 6 hours. The medium was then changed to include FG-4592 at 0, 1, 5, 10, 50 and 100 μM diluted in DMEM from a 50mM stock dissolved in dimethyl sulfoxide (DMSO). After maintaining for 2 hours, tertiary butyl hydroperoxide (tBHP) was added to the cells at the concentration of 1mM for another 2 hours.
Staining and counting damaged cells
The cells were dissociated with trypsin and collected by centrifugation. The cell pellet was suspended in 300 μl PBS. A 100 μl cell suspension was taken and mixed with 100 μl of 0.2% trypan blue. After staining for 2 minutes, cells were counted with a Cellometer Auto T4 (Nexcelom Bioscience LLC, Lawrence, MA) to determine the percent of trypan blue positive cells.
Human Cornea Damage Model
Human corneas deemed unsuitable for transplantation were obtained from VisionFirst eye bank, Indianapolis, IN. Unlike the rabbit corneas, the human corneas arrived with variable amounts of endothelial damage. To determine this starting damage, corneas were washed with PBS once, stained with 0.2% trypan blue for 2 minutes, washed with PBS 3 times, and an image was recorded. Corneas were put in a 6-well plate and immersed in 6 ml of DMEM with antibiotic-antimycotic and 200 uM glutamine.
All human corneas were divided into three groups (Table 1) and maintained at 37°C for a total of 27 hours. Corneas in Group 1 were placed in a 5% CO2 tissue culture incubator (balance room air) for 3 hours, then the endothelial surface was damaged by applying sonication, and culturing continued for 24 hours. Corneas in Group 2 were first put in a hypoxia chamber (5% CO2, 0.5% O2 balance N2) for 2 hours, then moved to the normoxic incubator for 1 hour. The endothelial surface was damaged by applying sonication, as described above, and culturing continued for 24 hours. Corneas in Group 3 were put in the tissue culture incubator with 5% CO2 (normoxia condition), but the medium contained 50 μM FG-4592 for the first 2 hours and then was changed to regular medium for 1 hour. After the endothelial surface was damaged by applying sonication, the cornea was maintained for another 24 hours. For evaluation of human corneas, net damage = final trypan blue staining minus initial staining using the same procedure as for rabbit corneas.
Perioperative Endothelial Keratoplasty Model
Ten pairs of human donor corneas (n = 20) were included in this phase of the study. Tissue having severe endothelial stress lines, significant Descemet folds, guttae, or endothelial cell density < 2000 cells/mm2 by specular microscopy was excluded. Each pair of excised corneoscleral rims was initially stored in Optisol – GS (Bausch & Lomb, Rochester, NY) and then randomized to one of two different groups (Table 1). Tissue in Group 1 (FG-positive group) was placed in 20 ml DMEM (HEPES buffered, 0 bicarbonate, Ref. # 12320-032, Gibco Laboratories, Gaithersburg, MD) with 50 μM FG-4592. Tissue in Group 2 (FG-negative group) was placed in plain DMEM without FG-4592. Both groups were incubated for 2 hours at 37°C in an incubator (Incucell Model LSIS B2V/IC 55, Czech Republic) in air. After two hours, tissues in both groups were transferred to plain DMEM and incubated for another one hour.
The corneoscleral rim was placed endothelial side up and stained with 0.06% trypan blue dye (concentration used by eye banks for endothelial keratoplasty preparation, C-Blue, Stephens Instruments, Lexington, KY) for one minute. The dye was washed with 0.9% balanced salt solution and a baseline preoperative image was taken. Tissue was mounted on an automated microkeratome (Moria, Antony, France) and a baseline ultrasonic pachymetry (Corneo-gage, Sonogage, Inc., Cleveland, OH) was measured. Epithelium was scraped off with a wet merocel sponge and a lamellar dissection was performed using 350-micron head. The central ultrasonic pachymetry reading of the posterior donor tissue was noted. Tissue was then placed on a teflon block with endothelial side up and punched with an 8 mm corneal trephine.
To mimic use with DSAEK, the donor button was placed in a graft insertion device (Busin donor glide, width 3.2 mm × length 2 mm, Cat #19098; Moria SA) and pulled through with microforceps to place it back in the Teflon block endothelial side up. The endothelium was stained again with the trypan blue dye (0.06%) for one minute, washed with balanced salt solution to remove extra stain and a final postoperative image was taken. Imaging was done using an operating microscope (Model M220, Wetzlar, Germany) and HD camera (Microcast HD pro-lite, Optronics, Goshen, IN). Tissue preparation, lamellar dissection and image analysis was done by two masked cornea surgeons.
The endothelial cell loss was calculated by using Fiji segmentation software as previously described.25,26 The endothelial cell loss difference (ECLD) was calculated by subtracting the endothelial cell loss of the baseline preoperative image from that of the final postoperative image.
Statistical Analysis
All statistical tests were 2-tailed, and p-values < 0.05 were considered significant. Cell loss differences between groups were assessed with the Student t-test for experiments with non-paired corneas; the paired t-test was used for experiments with paired donor corneas. Statistical analysis was done using Microsoft Excel 2010.
Results
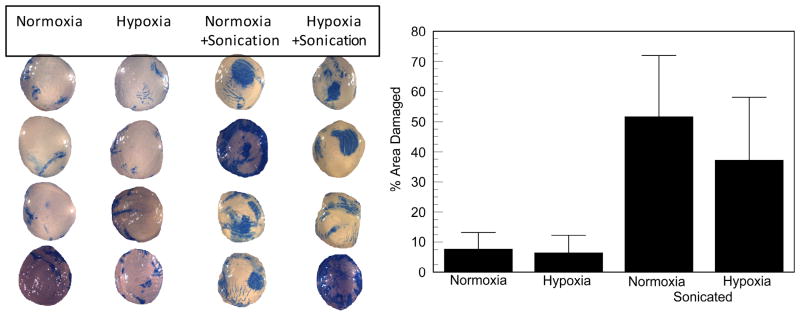
To determine if hypoxia preconditioning had any potential for protecting endothelial cells from mechanical damage we first used rabbit corneas that were exposed to 0.5% oxygen for two hours. Figure 1 shows that after collection of rabbit eyes, shipment overnight, dissection of corneas, and 27 hours of organ culture, 6-7% of the endothelial area was trypan blue positive, which was not significantly different between normoxia and two hours of hypoxia exposure, indicating that the hypoxia exposure itself was not damaging. For corneas subjected to mechanical stress via sonication, damage was 52 ± 20% (SD) for normoxia and 37 ± 21% (SD) for hypoxia pretreated corneas. The difference was modest but significant, p=0.024. This result shows that hypoxia preconditioning has potential to protect the endothelium from mechanical stress.
Figure 1.
Effect of hypoxia preconditioning on rabbit corneal endothelial mechanical damage. Left panel: representative images of trypan blue stained endothelium (sclera was masked). Right panel is mean ± SD % area trypan blue positive: Normoxia, n = 17, maintained in the tissue culture incubator, no sonication; Hypoxia, n = 22, 2 hours in the hypoxia chamber, no sonication; Normoxia + Sonication, n = 22; Hypoxia +Sonication, n = 23. *significantly different between hypoxia and no hypoxia with sonication, p=0.0238 (2-tailed).
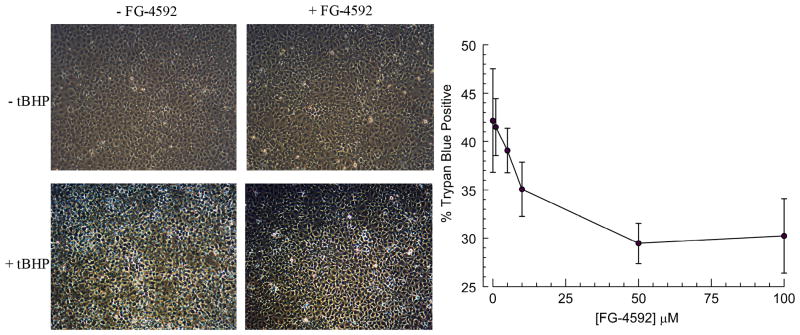
Because hypoxia chambers are unwieldy, we examined if a pseudo-hypoxic state by simple treatment with the prolylhydroxylase inhibitor FG-4592 could also provide protection. To get a sense of the dose of FG-4592 to use, we determined if FG-4592 could protect cultured human corneal endothelial cells from oxidative stress provided by two hours of 1 mM tBHP. Figure 2 shows representative phase contrast images of HCEC showing increased light-scattering after exposure to tBHP that is lessened by pre-conditioning with FG-4592. After collection of cells and trypan blue staining analysis, Figure 2 right panel shows that 50μM FG-4592 significantly reduced cell damage from 43% to 29%, p<0.001. These results indicate that FG-4592 also has potential to protect endothelial cells.
Figure 2.
Dose response of FG-4592. Human corneal endothelial cultures were subjected to tBHP with or without pre-treatment with FG-4592. Left panel: images of cultures just before trypsinization. Right panel % trypan blue positive cells vs. [FG-4592], ± SD, n=12 for each condition, *p<.0001.
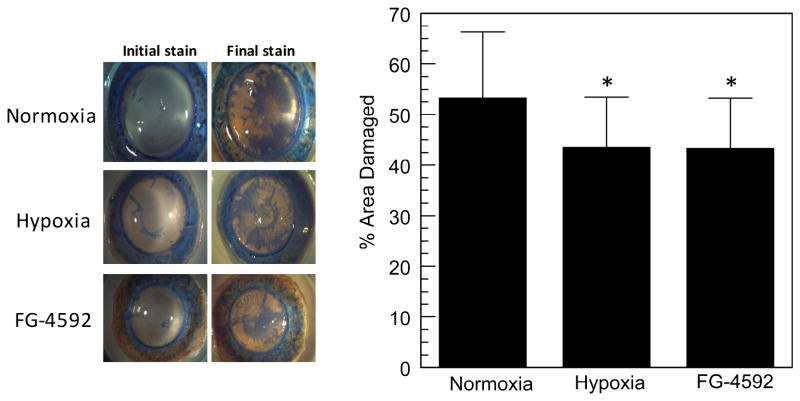
The sonication model was then repeated with human corneas and both hypoxia and FG-4592 were tested. Because the human corneas were delivered in variable conditions, initial staining was subtracted from the final staining to determine the % damage caused by each test condition. Figure 3 shows that again approximately 50% of the area was damaged by sonication, which was reduced to about 40% with either hypoxia or FG-4592 pretreatment. This ten percent difference was significant to just below a p-value of 0.05, n = 12 per condition.
Figure 3.
Effect of hypoxia or 50 μM FG-4592 pre-conditioning on human corneal endothelial mechanical damage. Left panel: representative images of trypan blue stained endothelium. Right panel: mean ± SD % area of endothelium that was trypan blue positive, n=12 for each condition, *p<0.05.
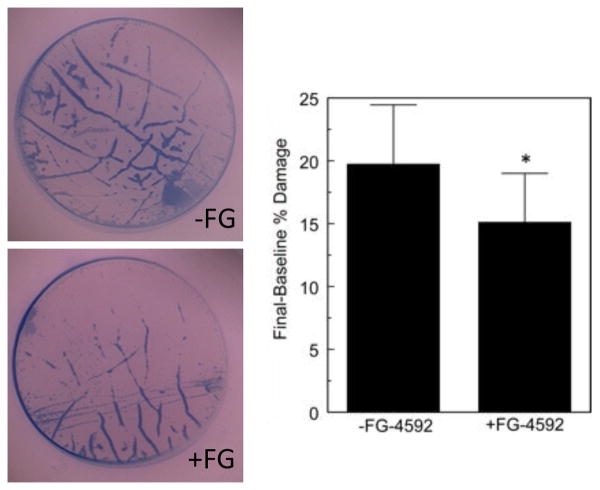
Lastly, we asked if human donor buttons prepared for EK and subjected to pulling through a Busin glide were also protected by FG-4592. Table 2 describes the data from the 10 donor pairs. The mean final endothelial cell loss was 21.1 ± 5.6% in the FG-negative group vs. 15.8 ± 4.2% in the FG-positive group (p = 0.00002). The mean endothelial cell loss difference (final – baseline endothelial cell loss) was 19.7 ± 4.7% in the FG-negative group vs. 15.1 ± 3.9% in FG-positive group (Figure 4, p = 0.000005).
Table 2. Perioperative endothelial keratoplasty model data for individual samples along with group means and standard deviations (SD).
| Donor Pair No. |
Donor age (years) |
Death to use (hours) |
Baseline cell density (cells/mm2) |
Baseline pachymetry (μm) |
Post-cut pachymetry (μm) |
Baseline cell loss (%) |
Final cell loss (%) |
Cell loss difference baseline to final (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FG - | FG + | FG - | FG+ | FG - | FG + | FG - | FG + | FG - | FG + | FG - | FG + | |||
| 1 | 66 | 282 | 2488 | 2725 | 639 | 624 | 267 | 276 | 4.38 | 2.45 | 32.3 | 24.5 | 27.9 | 22.0 |
| 2 | 46 | 184 | 3472 | 3448 | 654 | 611 | 242 | 251 | 4.89 | 2.28 | 24.1 | 15.7 | 19.2 | 13.4 |
| 3 | 70 | 72 | 2053 | 2066 | 562 | 655 | 239 | 201 | 0.59 | 0.9 | 20.7 | 18.0 | 20.1 | 17.9 |
| 4 | 50 | 48 | 2370 | 2415 | 479 | 485 | 180 | 186 | 0.02 | 0.17 | 14.9 | 11.8 | 14.9 | 11.6 |
| 5 | 50 | 114 | 2890 | 3096 | 608 | 600 | 214 | 215 | 0.03 | 0.09 | 18.8 | 14.9 | 18.8 | 14.8 |
| 6 | 54 | 100 | 2288 | 2342 | 559 | 630 | 242 | 190 | 0.57 | 0 | 26.7 | 19.3 | 26.2 | 19.3 |
| 7 | 41 | 183 | 2439 | 2494 | 489 | 496 | 95 | 55 | 0 | 0 | 22.6 | 16.5 | 22.6 | 16.5 |
| 8 | 71 | 54 | 2801 | 2967 | 908 | 797 | 491 | 470 | 0 | 0 | 19.1 | 15.2 | 19.1 | 15.2 |
| 9 | 62 | 132 | 3040 | 3236 | 917 | 869 | 579 | 368 | 3.37 | 1.59 | 18.6 | 11.9 | 15.2 | 10.3 |
| 10 | 66 | 55 | 2703 | 2833 | 586 | 581 | 201 | 205 | 0 | 0 | 13.4 | 10.2 | 13.4 | 10.2 |
| Mean ± SD | 58 ± 10 | 122 ± 71 | 2654 ± 414 | 2762 ± 435 | 640 ± 154 | 634 ± 119 | 275 ± 147 | 242 ± 112 | 1.4 ± 2.0 | 0.7 ± 1.0 | 21.1 ±5.6 | 15.8 ± 4.2 | 19.7 ± 4.7 | 15.1 ±3.9 |
| P value | 0.004 | 0.77 | 0.14 | 0.08 | 0.00002 | 0.000005 | ||||||||
Figure 4.
Effect of 50 μM FG-4592 pre-treatment on human corneal endothelium undergoing EK preparation. Left panel: representative trypan blue stained images of donor buttons. Right panel: mean ± SD Final-Baseline % Trypan Blue area. Note that the endothelial cell loss was concentrated in the area of mechanical stress lines and corneal folds.
Discussion
The results of this in vitro pilot study suggest that hypoxia preconditioning, either by direct hypoxic incubation or pseudo-hypoxia via FG-4592 incubation can reduce corneal endothelial cell loss during mechanical stress. Hypoxia preconditioned human and rabbit corneas showed 19 and 29% less cell loss, respectively, relative to controls (Table 3), which were both significant at p<0.05. FG-4592 reduced endothelial cell loss associated with preparation and insertion of DSAEK grafts by 23% relative to control (Table 3). This supports the hypothesis that exposure to FG-4592 increases corneal endothelial cell resistance to apoptosis and may also provide protection during surgical trauma as well.
Table 3. Mean and standard deviation of corneal endothelial cell loss (%) in 4 experimental models.
| Model | Description | Control: normoxia | Hypoxia methods | |
|---|---|---|---|---|
| 0.5% O2 | FG-4592 50μM | |||
| 1 | Sonication damage to rabbit corneas | 52 ± 20 | 37 ± 21 | -- |
| 2 | Human corneal endothelial cell cultures: exposure to tert-butyl hydroperoxide | 43 ± 11 | -- | 29 ± 8 |
| 3 | Sonication damage to human donor corneas | 53 ± 13 | 43 ± 10 | 43 ± 10 |
| 4 | In vitro keratoplasty (DSAEK) simulation | 20 ± 4.7 | -- | 15 ± 3.9 |
Abbreviations: DSAEK = Descemet stripping automated endothelial keratoplasty
FG-4592 is an orally available drug currently under clinical trial for treating anemia in chronic kidney disease patients,22,23 targeted to increase erythropoietin availability. FG-4592 inhibits the enzyme prolyl hydroxylase which leads to stabilization and increased activity of HIF-1α, which enters the nucleus and increases the expression of anti-apoptotic genes responsible for enhanced survival, growth and metabolism of the cells.23,27 FG-4592 acts as a hypoxia mimetic agent leading to a cellular environment like hypoxia. A major difference is that oxygen is available, so mitochondrial function is not impaired, as it would be during hypoxia.
The effect of hypoxia preconditioning and its protective role has been extensively studied in the central nervous system and ischemic stroke.28-30 Recent studies have also demonstrated the protective role of hypoxia in ocular tissues such as retina and corneal endothelial cells to different forms of injury.31-33 Cheng et al 20 studied the effect of hypoxia preconditioning on corneal endothelial cells. They used two different reactive oxygen species (ROS) generating cytotoxins, tertiary butyl hydroperoxide and paraquat to induce oxidative damage to corneal endothelium. Hypoxia preconditioning was achieved by keeping the corneas in a hypoxia glove box. They assessed cell death by flow cytometric analysis using Annexin V and propidium iodide double staining. They concluded that 2 hours of hypoxia pre-conditioning was sufficient to mobilize HIF1a and protect human corneal endothelial cells from ROS-producing cytotoxins. Iron is also a cofactor in the hydroxylation of HIF1α. A study by Zhu et al showed that desferrioxamine, an iron chelator and “hypoxia mimetic”, had neuroprotective properties in the retina against lethal ischemic injury.31
Trypan blue dye used in the current study stains the area of bare Descemet's membrane with denuded corneal endothelium, as well as devitalized, broken cells, that remain attached to Descemet membrane. Thus trypan blue staining is labelling areas where cells were lost and where cells have entered end-stage apoptosis. Also, we noted that the trypan blue staining was more prominent in areas of mechanical stress lines and corneal folds created while the DSAEK button was passed through the Busin glide. Albon et al 7 found more apoptotic cell loss in areas of corneal folds. Another study done by Gain et al 32 showed the absence of cytoprotective (anti-apoptotic) factors such as Bcl-2 and heat shock proteins characteristically absent in the endothelial cells located in corneal folds. Thus, mechanical stress is a powerful contributor to endothelial cells loss, and strategies such as hypoxia preconditioning may be helpful, but cannot totally overcome these physical forces.
The fellow-eye design utilized in the endothelial keratoplasty damage model minimized confounding factors for endothelial cell loss between the two groups. The baseline endothelial cell density measured by specular microscopy was higher in the FG-positive group (P = 0.004). However, the specular image represents a very small area of the endothelium. The baseline images of the entire corneal endothelial surface did not show a statistically significant difference in the baseline endothelial cell loss between the two groups (p = 0.08, Table 2).
The range of final endothelial cell loss in the keratoplasty model, as calculated by Fiji software, was 10 - 32%. This was comparable to the 14 – 34% range of endothelial cell loss reported by Jardine et al using the Fiji software with a Descemet membrane endothelial keratoplasty model.26 We agree with their assessment that the Fiji segmentation software is an accurate, efficient and reproducible means for quantifying endothelial cell loss and that it could be a very helpful tool for the eye banks to assess the percentage of corneal endothelial cell damage during endothelial graft preparation.
The present study has several limitations. This was an ex-vivo, experimental pilot study. The long-term side effects of FG-4592 on intraocular tissues are unknown. Utilization of FG-4592 requires incubation in a cell culture media at 37°C to achieve the hypoxia mimetic effect. The study was not able to differentiate between endothelial cell loss caused by direct mechanical trauma and endothelial cell loss resulting from storage conditions. Larger follow up studies are needed to verify the findings.
In summary, surgically induced trauma from a combination of donor preparation and surgical implantation is an important cause of endothelial cell loss with endothelial keratoplasty. FG-4592, a prolylhydroxylase inhibitor that creates a pseudo-hypoxia has the potential to be one of the strategies used to reduce endothelial cell loss during donor preparation and implantation of EK grafts and thus improve the number of viable corneal endothelial cells transferred to the graft recipient.
Acknowledgments
Funding: this project was partially funded by a research grant from the Eye Bank Association of America and NIH RO1 EY008834
Footnotes
Financial Disclosures: the authors report no financial conflicts of interest.
References
- 1.Melles GR. Posterior lamellar keratoplasty: DLEK to DSEK to DMEK. Cornea. 2006;25:879–81. doi: 10.1097/01.ico.0000243962.60392.4f. [DOI] [PubMed] [Google Scholar]
- 2.Anshu A, Price MO, Tan DTH, et al. Endothelial keratoplasty: a revolution in evolution. Surv Ophthalmol. 2012;57:236–252. doi: 10.1016/j.survophthal.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Koenig SB, Covert DJ, Dupps WJ, et al. Visual acuity, refractive error, and endothelial cell density six months after Descemet stripping and automated endothelial keratoplasty (DSAEK) Cornea. 2007;26:670–674. doi: 10.1097/ICO.0b013e3180544902. [DOI] [PubMed] [Google Scholar]
- 4.Price MO, Fairchild KM, Price DA, et al. Descemet's stripping endothelial keratoplasty: Five-year graft survival and endothelial cell loss. Ophthalmology. 2011;118:725–729. doi: 10.1016/j.ophtha.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Price MO, Calhoun P, Kollman C, et al. Descemet stripping endothelial keratoplasty ten-year endothelial cell loss compared with penetrating keratoplasty. Ophthalmology. 2016;123:1421–1427. doi: 10.1016/j.ophtha.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Ishii N, Yamaguchi T, Yazu H, et al. Factors associated with graft survival and endothelial cell density after Descemet's stripping automated endothelial keratoplasty. Sci Rep. 2016;6:25276. doi: 10.1038/srep25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albon J, Tullo AB, Aktar S, et al. Apoptosis in the endothelium of human corneas for transplantation. Invest Ophthalmol Vis Sci. 2000;41:2887–2893. [PubMed] [Google Scholar]
- 8.Komuro A, Hodge DO, Gores GJ, et al. Cell death during corneal storage at 4 degrees C. Invest Ophthalmol Vis Sci. 1999;40:2827–2832. [PubMed] [Google Scholar]
- 9.Armitage WJ. Predicting endothelial cell loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci. 2003;44:3326–3331. doi: 10.1167/iovs.02-1255. [DOI] [PubMed] [Google Scholar]
- 10.Terry MA, Saad HA, Shamie N, et al. Endothelial keratoplasty: the influence of insertion techniques and incision size on donor endothelial survival. Cornea. 2009;28:24–31. doi: 10.1097/ICO.0b013e318182a4d3. [DOI] [PubMed] [Google Scholar]
- 11.Okumura N, Kinoshita S, Koizumi N. Cell-based approach for treatment of corneal endothelial dysfunction. Cornea. 2014;33:S37–41. doi: 10.1097/ICO.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 12.Fuchsluger TA, Jurkunas U, Kazlauskas A, et al. Anti-apoptotic gene therapy prolongs survival of corneal endothelial cells during storage. Gene Ther. 2011;18:778–787. doi: 10.1038/gt.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcia RN, Dana MR, Kazlauskas A. Corneal graft rejection is accompanied by apoptosis of the endothelium and is prevented by gene therapy with Bcl-xL. Am J Transplant. 2007;7:2082–2089. doi: 10.1111/j.1600-6143.2007.01897.x. [DOI] [PubMed] [Google Scholar]
- 14.Shin YJ, Seo JM, Chung TY, et al. Effect of cysteamine on oxidative stress-induced cell death of human corneal endothelial cells. Curr Eye Res. 2011;36:910–917. doi: 10.3109/02713683.2011.593726. [DOI] [PubMed] [Google Scholar]
- 15.Okumura N, Koizumi N, Ueno M, et al. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol. 2012;181:268–277. doi: 10.1016/j.ajpath.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Koh SWM, Gloria D, Molloy J. Corneal endothelial autocrine VIP enhances its integrity in stored human donor corneoscleral explant. Invest Ophthalmol Vis Sci. 2011;52:5632–5640. doi: 10.1167/iovs.10-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh SW. Corneal endothelial autocrine trophic factor VIP in a mechanism-based strategy to enhance human donor cornea preservation for transplantation. Exp Eye Res. 2012;95:48–53. doi: 10.1016/j.exer.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piret JP, Lecocq C, Toffoli S, et al. Hypoxia and CoCl2 protect HepG2 cells against serum deprivation- and t-BHP-induced apoptosis: A possible anti-apoptotic role for HIF-1. Exp Cell Res. 2004;295:340–349. doi: 10.1016/j.yexcr.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Narasimhan P, Yu F, et al. Neuroprotection by hypoxic preconditioning involves oxidative stress-mediated expression of hypoxia-inducible factor and erythropoietin. Stroke. 2005;36:1264–1269. doi: 10.1161/01.STR.0000166180.91042.02. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Q, Nguyen T, Song H, et al. Hypoxia protects human corneal endothelium from tertiary butyl hydroperoxide and paraquat-induced cell death in vitro. Exp Biol Med (Maywood) 2007;232:445–453. [PMC free article] [PubMed] [Google Scholar]
- 21.Seta KA, Yuan Y, Spicer Z, et al. The role of calcium in hypoxia-induced signal transduction and gene expression. Cell Calcium. 2004;36:331–340. doi: 10.1016/j.ceca.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Besarab A, Chernyavskaya E, Motylev I, et al. Roxadustat (FG-4592): Correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27:1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker K, Saad M. A new approach to the management of anemia in CKD patients: a review on Roxadustat. Adv Ther. 2017;34:848–853. doi: 10.1007/s12325-017-0508-9. [DOI] [PubMed] [Google Scholar]
- 24.Schmedt T, Chen Y, Nguyen TT, et al. Telomerase immortalization of human corneal endothelial cells yields functional hexagonal monolayers. PLoS One. 2012;7:e51427. doi: 10.1371/journal.pone.0051427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jardine GJ, Holiman JD, Stoeger CG, et al. Imaging and quantification of endothelial cell loss in eye bank prepared DMEK grafts using trainable segmentation software. Curr Eye Res. 2014;39:894–901. doi: 10.3109/02713683.2014.887120. [DOI] [PubMed] [Google Scholar]
- 27.Webb JD, Coleman ML, Pugh CW. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell Mol Life Sci. 2009;66:3539–3554. doi: 10.1007/s00018-009-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Simon R. Ischemic tolerance in the brain. Neurology. 1997;48:306–311. doi: 10.1212/wnl.48.2.306. [DOI] [PubMed] [Google Scholar]
- 29.Simon RP, Niiro M, Gwinn R. Prior ischemic stress protects against experimental stroke. Neurosci Lett. 1993;163:135–137. doi: 10.1016/0304-3940(93)90364-q. [DOI] [PubMed] [Google Scholar]
- 30.Roth S, Li B, Rosenbaum PS, et al. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest Ophthalmol Vis Sci. 1998;39:777–785. [PubMed] [Google Scholar]
- 31.Zhu Y, Zhang L, Gidday JM. Deferroxamine Preconditioning Promotes Long-Lasting Retinal Ischemic Tolerance. J Ocul Pharmacol Ther. 2008;24:527–536. doi: 10.1089/jop.2008.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gain P, Thuret G, Chiquet C, et al. In situ immunohistochemical study of Bcl-2 and heat shock proteins in human corneal endothelial cells during corneal storage. Br J Ophthalmol. 2001;85:996–1000. doi: 10.1136/bjo.85.8.996. [DOI] [PMC free article] [PubMed] [Google Scholar]