Abstract
The etiology and clinical behavior of breast cancers vary by estrogen receptor (ER) expression, HER2 expression, and over time. Data from the U.S. and Denmark show rising incidence rates for ER+ and falling incidence rates for ER− breast cancers. Given that Ireland is a somewhat similar Western population, but with distinctive risk exposures (especially for lactation), we analyzed breast cancer trends by ER status; and for the first time, by the joint expression of ER±/HER2±. We assessed invasive breast cancers (n=24,845; 2004–2013) within the population-based National Cancer Registry of Ireland. The population at risk was obtained from the Irish Central Statistics Office (n=10,401,986). After accounting for missing ER and HER2 data, we assessed receptor-specific secular trends in age-standardized incidence rates (ASR) with the estimated annual percentage change (EAPC) and corresponding 95% confidence intervals (95%CI). Age-period cohort (APC) models were also fitted to further characterize trends accounting for age, calendar-period, and birth-cohort interactions. ASRs increased for ER+ (EAPC: 2.2%/year (95%CI: 0.97, 3.5%/year)) and decreased for ER− cancers (EAPC: −3.43%/year (95%CI: −5.05, −1.78%/year)), as well as for specific age groups at diagnosis (<30–49, 50–64 and ≥65 years). ER+/HER2− cancers rose, ER+/HER2+ cancers were statistically flat, and ER−/HER± cancers declined. Secular trends for ER± cancers in Ireland were like those previously observed. Stratification by HER2± expression did not substantively alter ER± trends. The divergence of ER± incidence rates among independent Western populations likely reflects calendar-period and/or risk factor changes with differential effects for ER+ and ER− breast cancers.
Keywords: breast cancer, estrogen receptor, HER2, secular trends, incidence rates
INTRODUCTION
Breast cancer is an etiologically and clinically heterogeneous disease with increasing numbers of both pre- and postmenopausal women being diagnosed annually in Europe [1]. At the same time, more women are surviving their diagnosis; these improvements are attributed to both earlier detection with screening and advances in treatment [2–4]. Estrogen receptor-positive (ER+) breast tumors account for up to 80% of newly diagnosed invasive cases in the United States (U.S.) and Europe. In contrast, estrogen receptor-negative (ER−) breast cancers are less common, however they are more aggressive and are more frequently diagnosed in younger women [4, 5].
In the U.S., incidence of invasive ER+ breast cancers increased by 1.2% per year between 1992 and 2008. In contrast, incidence of ER− breast cancers decreased by 2.4% per year during the same period [6]. To date few European countries have examined ER-specific age standardized trends, with similar patterns being observed [7, 8]. Understanding differences in ER breast cancer trends in other European countries may provide important clues to inform etiology and identify opportunities to explore potential drivers of the observed trends.
It is currently unknown whether divergent incidence trends by ER status observed in other European countries. In addition, current standards of clinical care include assessment of human epidermal growth factor receptor 2 (HER2) expression and treatment of HER2-positive (HER2+) cancers with Herceptin® (trastuzumab). However, there is only limited data on incidence patterns by HER2 status, and it remains unclear how HER2 patterns relate to ER incidence rates. Hence, assessment of national trends by joint ER and HER2 expression might provide additional clues in relation to breast cancer etiology.
Ireland provides a distinct research opportunity to investigate breast cancer trends using data from the National Cancer Registry of Ireland (NCRI). Whereas Ireland has experienced similar changes to the U.S. in some subtype specific breast cancer risk factors over time, e.g., parity and obesity [9], other breast cancer exposures such as lactation have a distinctly different distribution in Ireland than the U.S. and Denmark [10]. In addition, unlike many of European countries, Ireland is uniquely positioned to evaluate secular trends in breast cancer incidence as ER, PR and HER2 have long been captured by the NCRI, coinciding with Ireland’s participation in the early Herceptin® (trastuzumab) clinical trials [11].
METHODS
Breast cancer case and population data
The NCRI is a comprehensive population based tumor registry that has collected detailed records on all newly diagnosed cancers in the Republic of Ireland and collects information related to patient demographics, tumor pathology, medical and surgical treatment since 1994 [12]. Case ascertainment is estimated at 98%, at five years after diagnosis [13]. Approval for use of these data was granted by NCRI and the use of anonymized data provided by the NCRI is approved by the Health (Provision of Information) Act, 1997. The study population in this analysis included women between ages 20–84 years diagnosed with invasive breast cancer from 2004 through 2013. Covariate data included age at invasive breast cancer diagnosis, stage at diagnosis (TNM 5th edition), histological grade (Grade 1–4), estrogen receptor status (ER+, ER−, or unknown), human epidermal growth factor receptor 2 status (HER2-positive (+), negative (−), or unknown). Population-based denominators were obtained from the Irish Central Statistics Office online population estimation tool [14]. The general female population at risk in Ireland was 10,401,986 between 2004–2013.
Statistical analysis
The primary objective of our analysis was to assess ER+ and ER− breast cancer incidence trends in Ireland; and specifically, to see whether Ireland has experienced a divergent pattern in the incidence of these subtypes, as observed previously in other populations. We allocated cases with unknown ER data to ER+ and ER− categories with a validated imputation method that was conditioned upon age and year of breast cancer diagnosis [6, 7]. We used a similar approach for unknown HER2 data. A bootstrap procedure was used as a sensitivity analysis for our imputation method to estimate variances accounting for random variations in the numbers of unknown cases as well as the uncertainty about the imputation. Accounting for the uncertainty about imputations had only a minor effect.
Receptor-specific breast cancer incidence rates were adjusted to the 2000 U.S. standard population by the direct method and expressed per 100,000 woman-years. The overall linear trend in the age-standardized incidence rate (ASR) was summarized with the estimated annual percentage change (EAPC) in the ASR, calculated by weighted log-linear regression under the assumption of a Poisson distribution. Age-period cohort (APC) models were fitted to further characterize the receptor-specific secular trends accounting for age, calendar-period, and birth-cohort interactions [6, 7]. Given the small number of breast cancer cases among women aged <30 years (n = 146), to facilitate APC analysis, we restricted the APC models to ages 30–84 years and combined single-year data into 2-year intervals. There were 27 two-year age groups (ages 31–32, 33–34, …, 83–84) and 5 two-year calendar-periods (2004–2005, 2006–2007, …, 2012–2013), spanning 31 partially overlapping 4-year birth-cohorts referred to by mid-year of birth (1921, 1923, …, 1977). APC parameters included net drift, local drifts, and cohort rate ratios (CRR). Net drift measures the sum of the log-linear trend in the calendar-period plus birth-cohort effects and is conceptually similar to the EAPC of the ASR. Local drifts provide corresponding EAPCs for each of the 27 two-year age groups. CRRs describe the incidence rates for a given birth-cohort relative to a reference cohort, a.k.a., the midpoint cohort year was 1951 in this NCRI dataset.
Finally, to account for the possible influence of Ireland’s National Breast Cancer Screening Program (BreastCheck) that was introduced between 2000 and 2007 among women aged 50–64 years [15], analysis of the receptor-specific subtypes were stratified according to three age groups (30–49 years, 50–64 years, 65+ years). Women aged 50–64 years were likely more heavily screened than women either 30–49 years or 65+ years. All statistical tests were two-sided and p values <0.05 were considered statistically significant. All analyses were carried out using MATLAB version 2016a.
RESULTS
Characteristics of study participants
NCRI data for women aged 20–84 years and diagnosed between 2004–2013 included 24,845 invasive breast cancers that accrued 15,878,592 woman-years (Table 1). A higher proportion of breast cancer cases were diagnosed after 2010 (43%), affected women aged 50 to 64 years (43%), were TNM stage 2 (45%) and grade 2 (50%). The highest proportions of cancers were ER+ (78%) and HER2− (70%), before the allocation of cases with unknown receptor status. The overall age-standardized incidence rate (ASR) was 163 per 100,000 woman years (for truncated range 20–84 years), representing the sum of the ASRs for ER+ (126 per 100,000), ER− (27 per 100,000), and ER-unknown cancers (9.5 per 100,000). Earlier stage and lower grade cancers were more likely among women with ER+ than ER− cancers (P-value <0.001 for both).
Table 1.
Descriptive characteristics of female breast cancer patients, ages 20–84 years at breast cancer diagnosis, in the National Cancer Registry of Ireland (NCRI), 2004–2013, with 15,878,592 woman-years in the general population at risk.
| ASR per 100,000 | Total (n=24,845) 163.1 |
ER-positive (n=19,264) 126.1 |
ER-negative (n=4,161) 27.4 |
ER-unknown (n=1,420) 9.5 |
P value for heterogeneity* | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Age | <0.001 | ||||||||
| <50 years | 6,429 | 26 | 4,859 | 25 | 1,262 | 30 | 308 | 22 | |
| 50–64 years | 10,596 | 43 | 8,438 | 44 | 1,657 | 40 | 501 | 35 | |
| ≥65 years | 7,820 | 34 | 5,967 | 31 | 1,242 | 30 | 611 | 43 | |
| Mean (Std Dev) | 58 (12.7) | 58 (12.5) | 57 (13.1) | 61.53 (13.9) | |||||
| Median (Range) | 58 (20–84) | 58 (21–84) | 57 (21–84) | 62 (27–84) | |||||
| Year of Diagnosis | <0.001 | ||||||||
| 2004–2005 | 4,212 | 17 | 2,968 | 15 | 831 | 20 | 413 | 29 | |
| 2006–2009 | 9,835 | 40 | 7,517 | 39 | 1,728 | 42 | 590 | 42 | |
| 2010–2013 | 10,798 | 43 | 8,779 | 46 | 1,602 | 39 | 417 | 29 | |
| HER2 status | 0.362 | ||||||||
| Negative | 17,412 | 70 | 14,782 | 77 | 2,353 | 57 | 277 | 20 | |
| Positive | 3,926 | 16 | 2,390 | 12 | 1,439 | 35 | 97 | 7 | |
| Unknown | 3507 | 14 | 2,092 | 11 | 369 | 9 | 1,046 | 74 | |
| Stage | <0.001 | ||||||||
| Stage 0* | 114 | 0 | 34 | 0 | 35 | 1 | 45 | 3 | |
| Stage 1 | 7,949 | 32 | 3,580 | 34 | 988 | 24 | 381 | 27 | |
| Stage 2 | 11,074 | 45 | 8,688 | 45 | 1,926 | 46 | 460 | 32 | |
| Stage 3 | 3,032 | 12 | 2,228 | 12 | 678 | 16 | 126 | 9 | |
| Stage 4 | 1,715 | 7 | 1,162 | 6 | 355 | 9 | 198 | 14 | |
| TNM unavailable | 84 | 0 | 14 | 0 | 12 | 0 | 58 | 4 | |
| Unknown | 877 | 4 | 558 | 3 | 167 | 4 | 152 | 11 | |
| Grade | <0.001 | ||||||||
| Grade 1 | 2,613 | 11 | 2,432 | 13 | 42 | 1 | 139 | 10 | |
| Grade 2 | 12,426 | 50 | 11,056 | 57 | 899 | 22 | 471 | 33 | |
| Grade 3 | 8,374 | 34 | 5,007 | 26 | 2,991 | 72 | 376 | 26 | |
| Grade 4 | 26 | 0 | 11 | 0 | 12 | 0 | 3 | 0 | |
| Unknown | 1,406 | 6 | 758 | 4 | 217 | 5 | 431 | 30 | |
ASR; age-standardized incidence rate per 100,000 woman-years; Stage 0, including invasive Paget disease
P value for ER-positive vs. ER-negative comparison
Following correction of cases with unknown ER status, eighty-one percent of ER-unknown cases were allocated to ER+ cancers with the balance imputed to ER− cancers, resulting in 82% and 18% ER+ and ER− breast cancer cases for analysis (Table 2), respectively. The percentage change following the allocation of cases with unknown hormone receptor status is also shown in Table 2. All subsequent analyses refer to corrected ER and HER2 expression.
Table 2.
Percentage change following the allocation of the unknown ER and HER2 breast cancer cases to non-missing categories among patients diagnosed in Ireland, 2004–2013.
| Total (n=24,845) | |||||
|---|---|---|---|---|---|
| Before correction | After correction | ||||
| n | % | n | % | % change | |
| ER status | |||||
| Positive | 19,264 | 77.5 | 20,419 | 82.2 | 6.0 |
| Negative | 4,161 | 16.7 | 4426 | 17.8 | 6.4 |
| Unknown | 1,420 | 5.7 | – | – | – |
| ER/HER2 status | |||||
| ER+/HER2− | 14,782 | 59.5 | 17,502 | 70.4 | 18.4 |
| ER+/HER2+ | 2,390 | 9.6 | 2,892 | 11.6 | 21.0 |
| ER−/HER2+ | 1,439 | 5.8 | 1,707 | 6.9 | 18.6 |
| ER−/HER2− | 2,353 | 9.5 | 2,741 | 11.1 | 16.5 |
| Unknown | 3,881 | 15.6 | – | – | – |
Allocation of ER-unknow cases to either ER+ or ER− categories according to the observed distributions of ER+ and ER− breast cancer cases, conditional on age at diagnosis and year of diagnosis [6]
Age-adjusted incidence rates
ER+ and ER− incidence rates diverged over time (Figure 1, panel A), with EAPCs of +2.2% per year for ER+ breast cancers (95% CI: 0.97, 3. 5%/year) but −3.43% per year for ER− cancers (95% CI: −5.1, −1. 8%/year). Stratification by the joint distribution of ER and HER2 categories showed rising ER+/HER2− trends, with stable or falling rates for all three of the remaining ER/HER2 subtypes (Figure 1, panel B). Further stratification by three age groups for ER+ and ER− cancers is shown in Figure 2. Incidence rates tended to rise for all ER+ age groups and fall for all ER− cancers.
Figure 1.
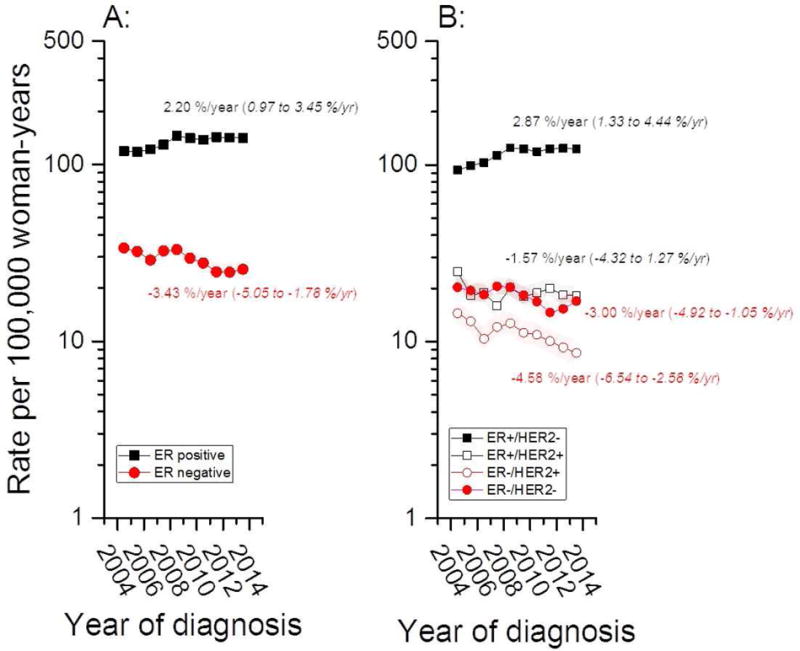
Age-standardized incidence rates (ASRs) for ER± (A) and ER±/HER2± (B) breast cancer among women aged 20–84 years at breast cancer diagnosis in Ireland from 2004 through 2013.
Figure 2.
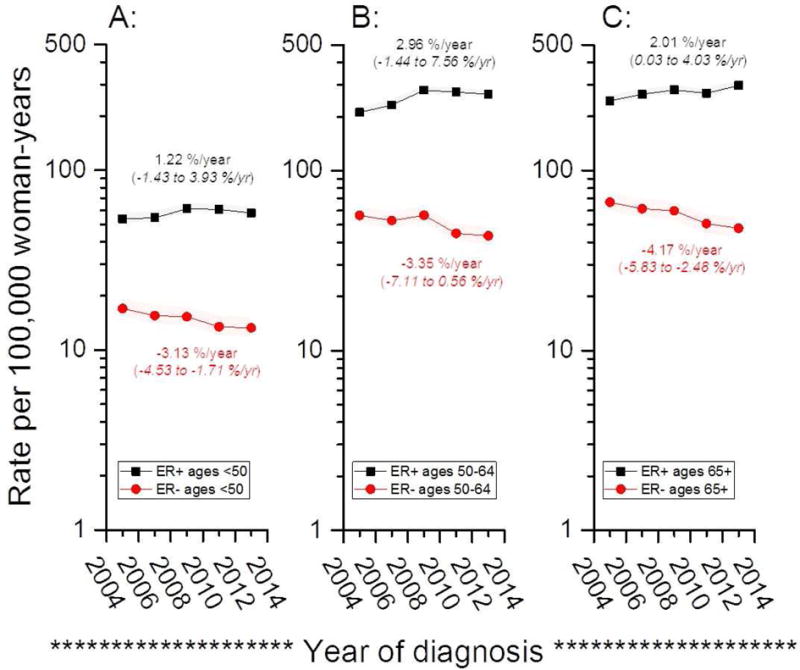
Receptor-specific age-standardized incidence rate (ASRs) for women aged 30–49 years (A), 50–64 years (B) and 65+ years (C) at breast cancer diagnosis among Irish women from 2004 through 2013.
Age-period-cohort models (APC)
Net drifts adjusted for calendar-period and birth-cohort deviations or changes, confirmed rising and falling linear trends for overall ER+ cancers (2.2 %/year) and ER− cancers (−3.5 %/year). Local drifts showed the largest changes among women near age 50 years and between ages 70–80 years (Figure 3). For example, among women aged 50 years, ER− breast cancers declined by 6.24 %/year (95% CI: −9.4 to −3.4 %/year). On the other hand, among women aged 52 years, ER+ breast cancers increased with an EAPC of 4.1 %/year (95% CI: 2.6 to 5.7 %/year). Compared to women born circa 1951 (Figure 4), ER+ cancer rose and ER− cancer declined over time with greater changes for ER− than ER+ cancers. ER− cancers fell from a CRR of 1.9 around 1930 to 0.56 around 1970; whereas, ER+ cancers rose from 0.6 to 1.3.
Figure 3.
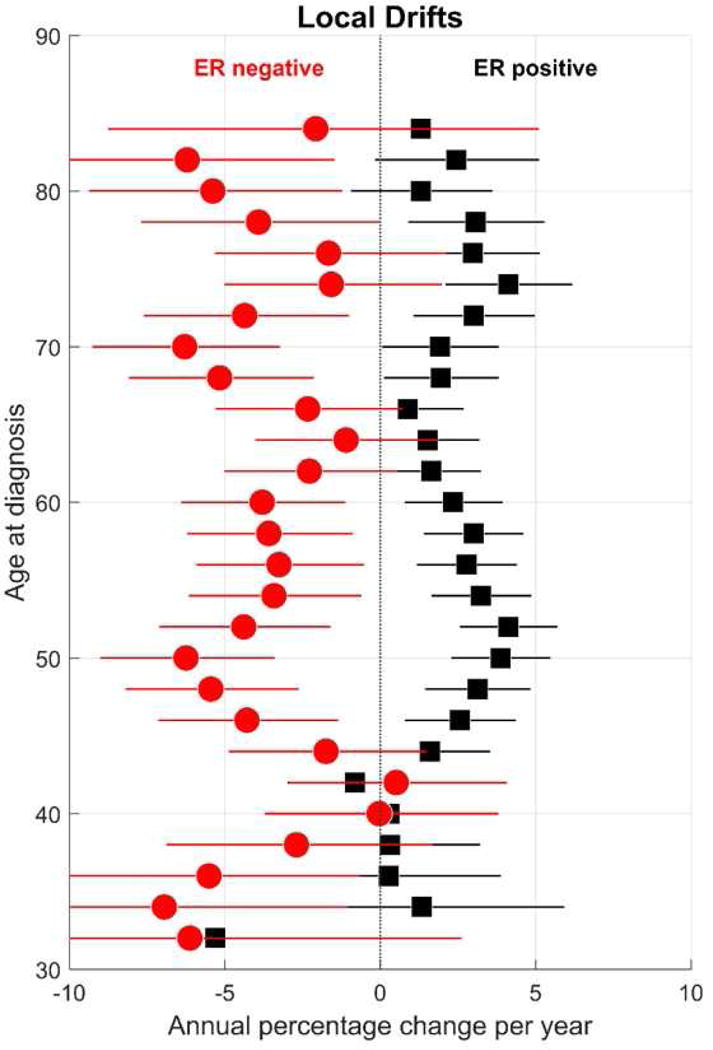
Receptor-specific ‘local drifts’ for breast cancer among Irish women aged 30–84 years at diagnosis from 2004 through 2013.
Figure 4.
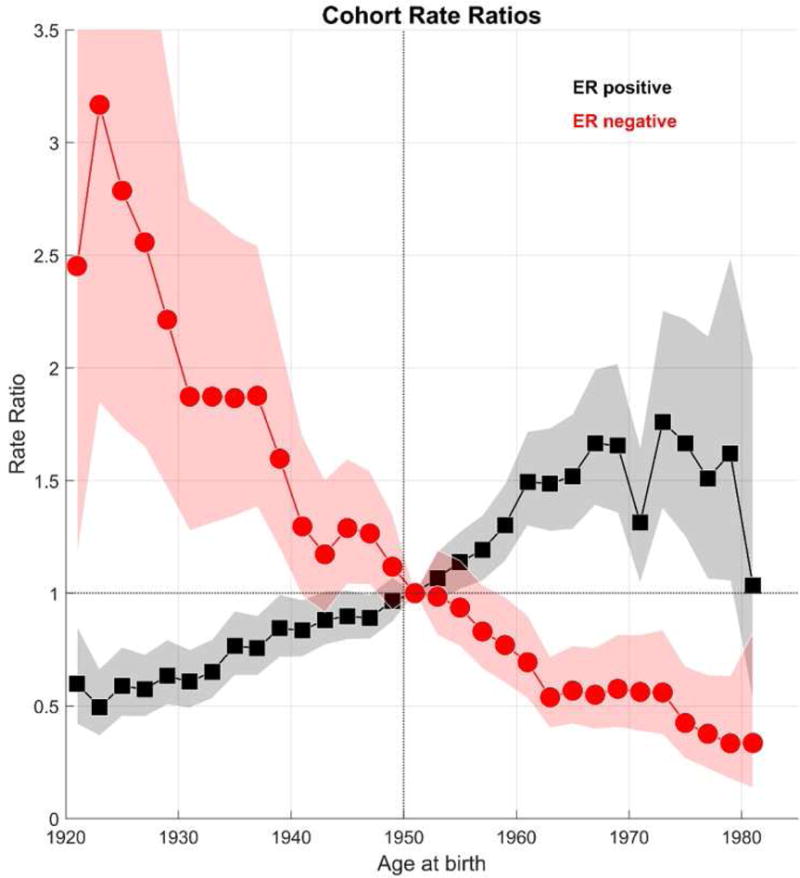
Receptor-specific cohort rate ratios (CRR) for breast cancer among Irish women aged 30–84 years at diagnosis from 2004 through 2013.
DISCUSSION
Our study has three main findings. First, as in the U.S. and Denmark [6, 7], age-adjusted and age-specific incidence rates for ER+ breast tumors are increasing over time, whereas rates for ER− cancers are decreasing. Second, there was a stronger age-specific association among women eligible for population-based screening mammography (age 50–64 years) than among younger or older women. Finally, HER2 expression had little influence upon the ER± incidence rate patterns, despite the well-acknowledged role of HER2 as a breast cancer biomarker for treatment and prognosis. The explanations for the divergent secular trends in rates by ER are not fully understood but likely reflect complex interactions between birth-cohort or generational effects (etiological risk factors) and/or calendar-period (e.g., screening or case ascertainment) effects. Furthermore, the comparability of ER status results in Ireland, the U.S., and Denmark suggests potential common driving mechanisms with dual or opposite effects for ER+ and/or ER− cancers.
A notable recent event to have influenced breast cancer incidence was the publication of findings of the Women’s Health Initiative (WHI) findings [16] and the Million Women’s studies [17] in 2002/2003, which showed that use of menopausal hormonal estrogen therapy (MHT) significantly increased breast cancer risk. The use of MHT is likely to partially account for increasing breast cancer trends prior to 2002, particularly ER+ breast cancers [18]. Similarly to other countries after the publication of these studies, prescribing patterns for MHT use declined [19]. However, despite declining use, increasing patterns of ER+ breast cancer are still observed, suggesting that other factors along with MHT use may influence the diverging trends. Limited observational evidence have shown largely null relationships between MHT use and risk of ER-negative breast cancer, thus it is unlikely that reduced ER− breast cancer incidence are due to declines in MHT use, however additional studies are needed to fully elucidate the relationship between declines in MHT use and changes in incidence patterns of ER− breast cancer.
Additional etiological risk factors with dual effects for ER+ and/or ER− breast cancers include but may not be limited to obesity and reproductive risk factors such as parity, age at first birth, and lactation [10, 20–22]. Obesity is a known driver of ER+ breast cancer, whereas among younger women ≤50 years, a large recent pooled analysis of 35,568 invasive breast cancer cases found that increased BMI was not associated with risk of ER− breast cancers [22]. In Ireland, between 1990 and 2011, the proportion of obese women has increased from 13 to 22%; and a recent pooled analysis of over 200 countries show that Irish women have the third highest body mass index (BMI) compared to the other European countries examined in that study [9, 23]. In addition to changing patterns of these factors, which would be anticipated to increase rates of ER+ tumors, increased screening and refinement of criteria for ER+ may also have increased rates of ER+ cancers [24, 25]. In relation to reproductive risk factors, epidemiologic studies, including the AMBER (African American Breast Cancer Epidemiology and Risk) consortium highlighted the influence of reproductive risk factors such as parity and lack of breastfeeding on ER− breast cancer risk [26, 27]. Prior studies within the European context have shown positive associations between rising breast cancer incidence overall and older age at first birth [28]. Further and more recent data from Denmark confirm an increased risk association for parity and early age at first birth for ER-cancers and protective association for ER+ cancers [29]. Notably Ireland has one of the lowest rates of breastfeeding worldwide [10] and therefore, it is unclear if this factor influences the declining ER− incidence rates in our study. To date, epidemiologic studies have found few risk factor associations that vary by HER2 status, apart from higher rates among Asian women [30].
Over the decade of our study, many calendar-period factors might also have influenced the divergence of ER± incidence rates. Screening mammography preferentially detects ER+ rather than ER− breast cancers [24, 25] and may have influenced the divergence of ER± incidence rates. Further, changes in standard clinical testing for ER± expression such as advances in antibodies for ER detection, development of standardized automated methods for ER immunohistochemistry, and refinements in clinical cut-offs for ER positivity [31, 32] may influence the trends in molecular subtype seen in this study. Currently, however, no study has shown that ER breast cancer subtype trends are changing as a direct result of changes in cut-offs for ER positivity. Studies with ER records dating to 1980, long before changing diagnostic tests and widespread screening mammography, show evidence of diverging trends in ER± breast cancer [33].
Our current analyses focused on the breast cancer population in the Republic of Ireland. Additional analyses are needed to determine if the results observed are reflective of other populations in Europe. To date in Europe, ER-subtype age standardized trends have only been examined to our knowledge in Scotland and Denmark [7, 8]. Limited data for a registry report from Northern Ireland show rising ER+ and declining ER− frequency distributions, based on 3, 875 breast cancer patients over four selected calendar years (1996, 2001, 2006 and 2012) [34]; these findings are consistent with divergent ER+ and ER− incidence rates in the NCRI. While similar patterns have been observed, it is important to consider differences in patterns of breast cancer risk factors among these countries, particularly in relation to reproductive patterns. At the present time, there are very few cancer registries worldwide with long-term ER data and even fewer with HER2 data. Thus, future studies will be needed to assess the findings observed in Ireland in a larger European context.
The strengths of this study include the low proportion of missing ER subtype data and the ability to examine joint expression of ER±/HER2± in a population-based cancer registry with nearly complete coverage of the Irish population. Our primary limitation is the lack of information on individual-level breast cancer risk factors. We also relied upon statistical models to adjust for missing receptor data. Further our analysis assessed clinical ER and HER2 data collected within the cancer registry. Future analysis is needed to assess other breast tumor markers including mitotic activity index (MAI) that may also be important for understanding etiology [35]. However, we applied an assumption for missingness that has been validated for ER expression, and then extended to HER2 expression in this study and the proportions of unknown receptor status was comparatively low. Our limited study period and follow-up time precluded the ability to examine complementary patterns of breast cancer mortality and we were unable to examine breast cancer mortality in depth. Further to comprehensively assess mortality, an ER± incidence-based mortality (IBM) analysis would be required for which ER-specific mortality data is needed but not collected in most cancer registries. However, as additional data becomes available over time, future studies will aim to determine ER/HER2-specific incidence-based mortality trends in Ireland, and indeed in other populations throughout Europe.
In conclusion, this study highlights increasing incidence of ER+ with decreasing ER− breast cancer in Ireland, a pattern that has been observed in other populations. These findings highlight the increased need for focused adoption of breast cancer prevention strategies based on ER status and the increased effort to further establish modifiable causal risk factors that are driving the development of these tumors. Furthermore, a clearer understanding of the mechanisms behind the decline in ER− breast cancers is needed to inform opportunities for prevention of this aggressive breast cancer subtype for women in Ireland and throughout the world.
KEY MESSAGES.
We observed rising ER+ and falling ER− age standardized breast cancer incidence rates in Ireland,.
HER2 status did not substantively impact receptor specific secular trends, with the possible exception of ER+/HER2+ incidence rates, which remained stable.
Divergent ER± incidence rates across different Western populations, including the US and Denmark, suggests similar calendar-period and/or risk factor changes with dual (opposite or different) effects on ER+ and ER− breast cancer.
Highlights.
We observed rising ER+ and falling ER− age standardized breast cancer incidence rates in Ireland.
HER2 status did not substantively impact receptor specific secular trends, with the possible exception of ER+/HER2+ incidence rates, which remained stable.
Divergent ER± incidence rates across different Western populations including the US and Denmark, suggests similar calendar-period and/or risk factor changes with dual (opposite or different) effects on ER+ and ER− breast cancer.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Financial support: This study was supported by the Cancer Prevention Fellowship Program of the Division of Cancer Prevention and Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
None declared, the authors have no conflicts of interest to declare.
Study Presentation: This study has been submitted for presentation at the Irish Association for Cancer Research Annual Meeting 2017 and the American Association of Cancer Research Annual Meeting 2017.
Disclaimer: The authors have no disclosures to declare.
References
- 1.Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer. 2015;51(9):1164–87. doi: 10.1016/j.ejca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1495–506. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- 3.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–36. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME. How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst. 2014;106(8) doi: 10.1093/jnci/dju165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson WF, Rosenberg PS, Katki HA. Tracking and evaluating molecular tumor markers with cancer registry data: HER2 and breast cancer. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst. 2011;103(18):1397–402. doi: 10.1093/jnci/djr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson WF, Rosenberg PS, Petito L, Katki HA, Ejlertsen B, Ewertz M, et al. Divergent estrogen receptor-positive and -negative breast cancer trends and etiologic heterogeneity in Denmark. Int J Cancer. 2013;133(9):2201–6. doi: 10.1002/ijc.28222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpe KH, McClements P, Clark DI, Collins J, Springbett A, Brewster DH. Reduced risk of oestrogen receptor positive breast cancer among peri- and post-menopausal women in Scotland following a striking decrease in use of hormone replacement therapy. Eur J Cancer. 2010;46(5):937–43. doi: 10.1016/j.ejca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Collaboration, N.C.D.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islami F, Liu Y, Jemal A, Zhou J, Weiderpass E, Colditz G, et al. Breastfeeding and breast cancer risk by receptor status–a systematic review and meta-analysis. Ann Oncol. 2015;26(12):2398–407. doi: 10.1093/annonc/mdv379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.http://www.cancertrials.ie/news-events/cancerwise-icorg-article-march-20th-2002. Accessed on: February 9th 2017.
- 12.National Cancer Regirstry of Ireland. http://www.ncri.ie/.
- 13.National Cancer Registry Ireland (NCRI) Cancer in Ireland 2013 - Annual Report of the National Cancer Registry. 2013 Available at: http://www.thehealthwell.info/node/553481.
- 14.Central Statistics Office. http://www.cso.ie/px/pxeirestat/Statire/SelectVarVal/Define.asp?maintable=PEA11&PLanguage=0.
- 15.BreastCheck The National Breast Screening Programme History [Internet] Available from: http://www.breastcheck.ie/content/history.
- 16.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Beral V, C. Million Women Study Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 18.Chen WY, Hankinson SE, Schnitt SJ, Rosner BA, Holmes MD, Colditz GA. Association of hormone replacement therapy to estrogen and progesterone receptor status in invasive breast carcinoma. Cancer. 2004;101(7):1490–500. doi: 10.1002/cncr.20499. [DOI] [PubMed] [Google Scholar]
- 19.Usher C, Teeling M, Bennett K, Feely J. Effect of clinical trial publicity on HRT prescribing in Ireland. Eur J Clin Pharmacol. 2006;62(4):307–10. doi: 10.1007/s00228-005-0083-x. [DOI] [PubMed] [Google Scholar]
- 20.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96(3):218–28. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 21.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8(4):R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250–63. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boylan EA, McNulty BA, Walton J, Flynn A, Nugent AP, Gibney MJ. The prevalence and trends in overweight and obesity in Irish adults between 1990 and 2011. Public Health Nutr. 2014;17(11):2389–97. doi: 10.1017/S1368980014000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilliland FD, Joste N, Stauber PM, Hunt WC, Rosenberg R, Redlich G, et al. Biologic characteristics of interval and screen-detected breast cancers. J Natl Cancer Inst. 2000;92(9):743–9. doi: 10.1093/jnci/92.9.743. [DOI] [PubMed] [Google Scholar]
- 25.Porter PL, El-Bastawissi AY, Mandelson MT, Lin MG, Khalid N, Watney EA, et al. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 1999;91(23):2020–8. doi: 10.1093/jnci/91.23.2020. [DOI] [PubMed] [Google Scholar]
- 26.Palmer JR, Boggs DA, Wise LA, Ambrosone CB, Adams-Campbell LL, Rosenberg L. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1883–91. doi: 10.1158/1055-9965.EPI-11-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambrosone CB, Zirpoli G, Hong CC, Yao S, Troester MA, Bandera EV, et al. Important Role of Menarche in Development of Estrogen Receptor-Negative Breast Cancer in African American Women. J Natl Cancer Inst. 2015;107(9) doi: 10.1093/jnci/djv172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soerjomataram I, Pukkala E, Brenner H, Coebergh JW. On the avoidability of breast cancer in industrialized societies: older mean age at first birth as an indicator of excess breast cancer risk. Breast Cancer Res Treat. 2008;111(2):297–302. doi: 10.1007/s10549-007-9778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson WF, Pfeiffer RM, Wohlfahrt J, Ejlertsen B, Jensen MB, Kroman N. Associations of parity-related reproductive histories with ER+/− and HER2+/− receptor-specific breast cancer aetiology. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Telli ML, Chang ET, Kurian AW, Keegan TH, McClure LA, Lichtensztajn D, et al. Asian ethnicity and breast cancer subtypes: a study from the California Cancer Registry. Breast Cancer Res Treat. 2011;127(2):471–8. doi: 10.1007/s10549-010-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnitt SJ. Estrogen receptor testing of breast cancer in current clinical practice: what’s the question? J Clin Oncol. 2006;24(12):1797–9. doi: 10.1200/JCO.2005.05.0666. [DOI] [PubMed] [Google Scholar]
- 32.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass AG, Lacey JV, Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99(15):1152–61. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 34.Cairnduff V, Fitzpatrick D, Gavin AT. Monitoring care of female breast cancer patients in N. Ireland diagnosed 2012 with comparisons 1996, 2001 and 2006. N Ireland Cancer Registry; 2015. Available at http://www.qub.ac.uk/nicr2015. [Google Scholar]
- 35.Louwman WJ, van Diest PJ, van Beek MW, Schapers RF, Nolthenius-Puylaert MB, Baak JP, et al. Trends in breast cancer aggressiveness before the introduction of mass screening in southeastern Netherlands 1975–1989. Breast Cancer Res Treat. 2002;73(3):199–206. doi: 10.1023/a:1015842720190. [DOI] [PubMed] [Google Scholar]


