Abstract
Many chronic human diseases, including multiple neurodegenerative diseases, are associated with deleterious protein aggregates, also called protein amyloids. One common therapeutic strategy is to develop protein aggregation inhibitors that can slow down, prevent, or remodel toxic amyloids. Natural products are a major class of amyloid inhibitors, and several dozens of natural product-based amyloid inhibitors have been identified and characterized in recent years. These plant- or microorganism-extracted compounds have shown significant therapeutic potential from in vitro studies as well as in vivo animal tests. Despite the technical challenges of intrinsic disordered or partially unfolded amyloid proteins that are less amenable to characterizations by structural biology, a significant amount of research has been performed, yielding biochemical and pharmacological insights into how inhibitors function. This review aims to summarize recent progress in natural product-based amyloid inhibitors and to analyze their mechanisms of inhibition in vitro. Major classes of natural product inhibitors and how they were identified are described. Our analyses comprehensively address the molecular interactions between the inhibitors and relevant amyloidogenic proteins. These interactions are delineated at molecular and atomic levels, which include covalent, non-covalent, and metal-mediated mechanisms. In vivo animal studies and clinical trials have been summarized as an extension. To enhance natural product bioavailability in vivo, emerging work using nanocarriers for delivery has also been described. Finally, issues and challenges as well as future development of such inhibitors are envisioned.
Keywords: Natural products, Amyloid inhibitor, Inhibition mechanisms, Non-covalent mechanisms, Covalent mechanisms
1. Introduction
Amyloidosis is associated with the largest class of protein misfolding diseases that includes a broad spectrum of neurological, metabolic and aging related diseases including Alzheimer’s disease (AD), prion disease, Parkinson’s disease, and type 2 diabetes (T2D). The pathological hallmarks of amyloidosis are structurally conserved intracellular and extracellular insoluble proteinaceous deposits termed amyloid fibrils [1–3]. Protein amyloid aggregation proceeds through a nucleation dependent process wherein monomeric and oligomeric aggregates form “seeds” that initiate an aggregation cascade that results in equilibrium between mature amyloid fibrils and their small precursor aggregates. Mature amyloid fibrils are comprised of several unbranched protofilament segments, which are in turn made up of β-sheet rich protein structures. These structures stack upon one another, forming the conserved amyloid “cross beta spine”, characterized by individual β-strand units being positioned perpendicular to the long axis of the protofilament [2]. Even though certain physiochemical properties conferred by amino acid sequence such as hydrophobicity, charge and β-sheet propensity can affect amyloidogenicity of natively unfolded proteins, extensive literature suggests that amyloid formation is facilitated by backbone interactions [2]. Over the last two decades, increasing evidence indicates that the primary pathological amyloid species are non-fibrillar precursor aggregates that range from unstructured oligomers (as small as dimers) to β-sheet rich aggregates termed protofibrils (as small as 20-mers) [2,4–9].
Generic mechanisms of amyloid induced cytotoxicity include cell membrane damage, organelle dysfunction, and impaired proteostasis that can ultimately lead to cell death [10–13]. Protein amyloid specific pathologies can also arise due to the cellular and physiological processes that are perturbed in specific tissues as well as the unique consequences linked to losing the native function of the aggregating proteins. For example, microtubule dysfunction as well as increased insulin resistance and reduced β-cell mass are manifestations of specific amyloid pathologies present in tauopathies and T2D, respectively. Currently amyloidosis can be classified based on if the amyloid deposits are localized or systemic and if the underlying pathologies are neuropathic. Using these criteria, Dobson and colleagues delineated amyloid diseases into three categories: neurodegenerative, non-neuropathic systemic and non-neuropathic localized amyloidosis [1,2]. Over fifty human protein misfolding diseases and their associated proteins and peptides have been described, including several physiologically important peptide hormones such as insulin [14] and amylin [15].
Due to a rapidly aging population and the modern sedentary lifestyle, we are witnessing rapidly growing numbers of people with chronic human diseases, including protein amyloid diseases. AD, for which currently there are no known cures, is reaching epidemic proportions. Progress towards managing protein misfolding diseases in general has been hampered by the failure to develop any effective disease-modifying drugs. This is in part due to our very limited mechanistic understanding of amyloidogenic protein – drug/small molecule interaction. Identification of effective therapeutic inhibitors is challenging because of intrinsic structural disorder of many protein targets of amyloid assembly. In this review we will primarily focus on natural product based amyloid inhibitors (Fig. 1) and in-depth analysis of their mechanisms of inhibition.
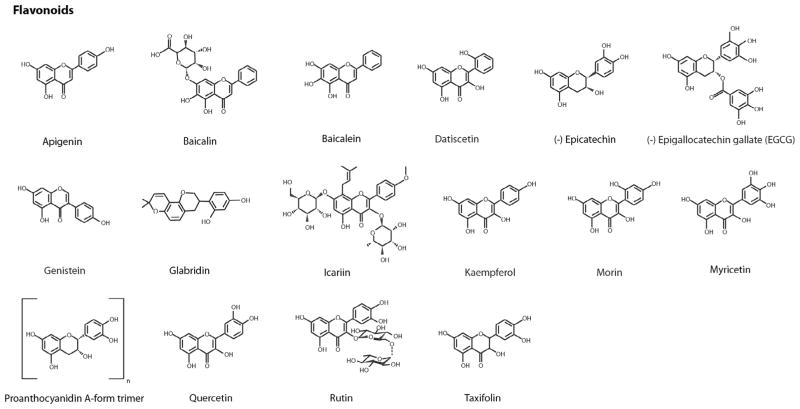
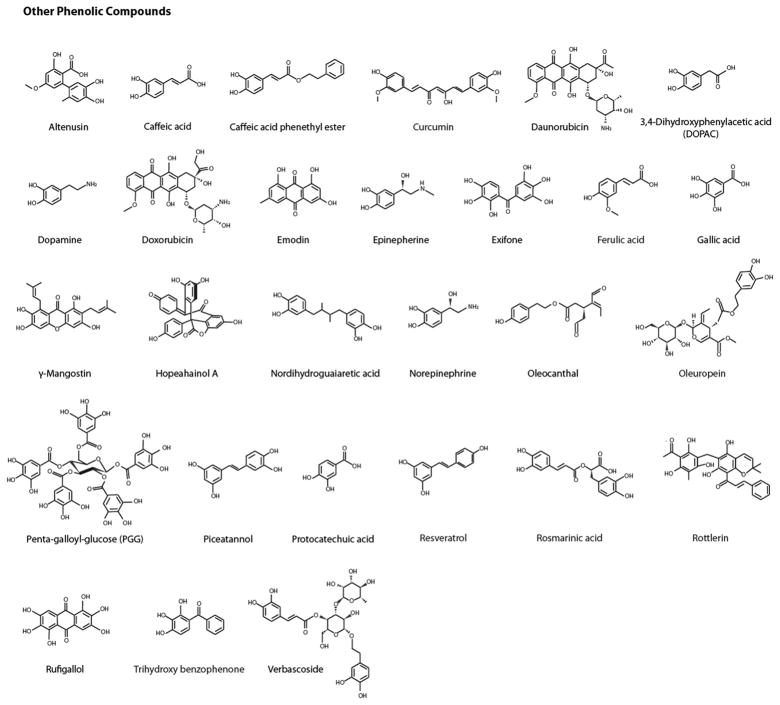
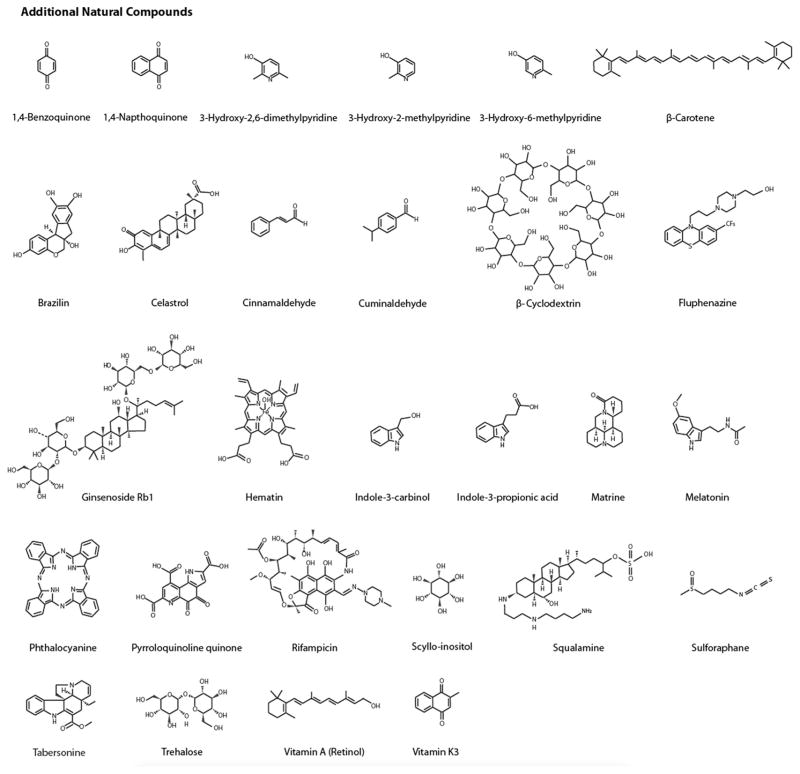
Fig. 1.
Chemical structures of identified natural product protein amyloid inhibitors. These inhibitors are classified as flavonoids, other phenolic compounds, and additional natural compounds including quinones, pyridines, aldehydes, sugar alcohols, terpenes, etc. In each category, compounds are listed alphabetically.
2. Drug discovery strategies against amyloidosis
There are multiple therapeutic strategies to identify disease-modifying agents against protein amyloidosis (for a recent review, see [16]). For natural compound identification, one source of information comes from epidemiological studies that suggest preventative effects against dementia, AD, or diabetes may be associated with the diets containing high intake of flavonoids and polyphenolic compounds [17]: The Mediterranean diet, featuring by a high intake of vegetables, fruits, cereals, and olive oil, was reported to be associated with reduced risk for AD and mild cognitive impairment in multiethnic community studies in New York [18,19]. Several cohort studies suggested that moderate intake of red wine (containing resveratrol) was associated with a reduction in risk of dementia, AD, or cognitive decline [20,21]. Curcumin, found in yellow curry spice turmeric in traditional Southeast Asian diets, and EGCG and myricetin, polyphenolic compounds present in green tea, have been associated with cognitive health [17]. However, the protective effects of diet as a whole are not the same with the specific effects of a single compound. How diet-specific natural compounds may provide healthy effects are not well known. Nevertheless, information from these epidemiological sources as well as information reported by alternative and complementary medicine led to testable hypotheses and experimental efforts that successfully identified numerous natural compound amyloid inhibitors [22–25].
One of the current strategies aimed at identifying therapeutic lead compounds for amyloidosis focuses on inhibiting amyloid aggregation by (i) inhibiting toxic amyloid formation and/or stabilizing its native form from aggregating and (ii) remodeling or degrading toxic amyloid oligomers and/or insoluble fibrils. Various approaches have been used. A variety of platforms, including in vitro ([26] and cell based approaches [27] have been used in a semi-to-high throughput capacity to screen for small molecules that prevent or modulate amyloid aggregation. One selection criterion used to choose the library of compounds for screening emphasizes the overall quantity and diversity of compounds rather than any specific underlying physicochemical features [26]. For instance, Chen and colleagues developed a high throughput small molecule microarray assay capable of identifying amyloid inhibitors by assessing binding affinity with amyloid β-peptide with ~11,000 different small molecule leads per array slide. Activities were assessed from a range of synthetic and natural compounds as well as compounds derived from diversity-oriented synthesis. Several high-resolution crystal structures of fragment sequences of amyloidogenic proteins [28,29] in concert with atomic structural analysis on small molecules that bind these structures [30–32] have revealed a variety of molecular scaffolds that either inhibit or modulate amyloid formation. These structures, some of which have been proposed as potential pharmacophores [30] that can presumably target the generic cross beta spine architecture common to all amyloids, are currently being used for structure-based drug design efforts. For example, Eisenberg’s group, utilizing Orange G, an amyloid binding dye, developed a high throughput screening platform that utilized iterative computational and experimental approaches, and investigated and fine tuned structure activity relationships for lead compounds with optimized activity against Aβ amyloid [33]. In addition, molecular docking and molecular dynamics simulation are commonly used approaches to screen small molecule libraries, to gain mechanistic insights into target – drug interactions, and to optimize lead compounds [33–35].
3. Natural product-based amyloid inhibitors
3.1. Natural product inhibitors
Natural compounds that exhibit anti-amyloid effects have distinct advantages over other synthetic compounds: they are often naturally consumed as part of a healthy diet wherein they offer general nutraceutical benefits such as reduced risk for AD and T2D [36]. Several polyphenols including curcumin, resveratrol and epigallocatechin-3-gallate (EGCG), have progressed to clinical trials for AD treatment (See Section 3.3.). Moreover, based on their multiple functions including anti-oxidant, anti-inflammatory and metal chelating capacities, polyphenols are a rich source for a variety of different structural backbones that can be utilized in rational drug design efforts to find multifunctional anti-amyloid agents [37,38] (see Section 3.2.4.).
Using PubMed and other public databases, we conducted a general search for a comprehensive list of natural compound amyloid inhibitors. Because natural compounds could be identified based on a wide variety of beneficial activities against amyloid diseases such as inhibiting amyloid indirectly by attenuating amyloid protein expression levels or influencing other key biochemical targets associated with amyloid, only natural compounds that directly prevented or modulated amyloid aggregation are included in our list. Of the 72 compounds identified, 44 are phenolic compounds that include 16 flavonoids, 4 anthraquinones, 13 alkaloids (including 3 indoles, 3 pyridines, and 2 porphyrins), terpenes, and steroids. Fig. 1 provides the chemical structures of these compounds.
Many of the phenolic compounds identified from our search are present in the aforementioned diets that are epidemiologically linked with reduced risk of aging-associated amyloid pathologies [17,39,40]. Examples include oleuropein and oleocanthal found in olive oil, resveratrol found in fruit and red wine, curcumin found in turmeric, as well as EGCG and myricetin found in green tea. Additional polyphenols identified that are present in healthful foods include caffeic acid and rosmarinic acid found in culinary herbs, cinnamaldehyde found in cinnamon, and genistein found in legumes. In contrast to the flavonoids or phenolic acid derivatives that comprised the majority of structures found within polyphenol amyloid inhibitors, several inhibitors with strikingly different structures were identified: cyclodextrin, a cyclic carbohydrate byproduct formed from enzymatic starch breakdown; squalamine, an aminosterol isolated from dogfish with previously documented anti-viral and anti-bacterial activities [41,42]; vitamin A, a fat soluble vitamin [43]; hematin, a porphyrin employed as a therapeutic against porphyria [44]; rifampicin, an antibiotic for treating bacteria infections; and scyllo-inositol, a plant sugar alcohol found abundantly in coconut palm. Caution has to be taken that amyloid-inhibitory functions of the majority of these compounds have not been validated in vivo.
3.2. Mechanisms of inhibition
3.2.1. Chemical modes of action
The specific stages of aggregation and amyloid species that are targeted by natural product inhibitors, as well as the resulting biochemical and biomolecular processes that are linked to amyloid induced cytotoxicity are not yet fully understood. However, recent work has begun to elucidate a detailed understanding of the chemical mechanisms that underlie these processes. Anti-aggregation agents can exert their actions by forming covalent bonds [45–48] and/or non-covalent interactions (i.e. π-π interactions, hydrogen bonding, or charge–charge interactions between an inhibitor and the backbone or side chain residues of the target protein) [30,49–51] that may affect one or all stages of the aggregation processes. Eisenberg’s group has elucidated at least two different binding modes of amyloid pharmacophores whose non-covalent interactions can be delineated by relatively “tight” (i.e. co-crystal structure of Orange G bound to Aβ fibril fragment KLVFFA) or “less tight” binding (i.e., co-crystal structure of curcumin bound to Tau fibril fragment VQIVYK) within the cross beta spine of single crystal structures of amyloid fragment sequences from Aβ and Tau [30]. However, formation of a common amyloid pharmacophore mediated by predominantly non covalent bonding interactions fails to explain why in some cases these forces, which typify EGCG mediated anti-amyloid activities in many amyloid systems, are ineffective in others [52]. Accordingly, a series of papers have demonstrated that covalent adduct formation can occur between the nucleophilic side chain thiols and amines (as well as the amino terminal amine) and the electrophilic carbonyls within o-quinone intermediates and/or aldehyde moieties [45,46,48]. Covalent adduct formation significantly affects anti-aggregation effects of baicalein on α-synuclein [46,53,54] and likely also on amylin [25]. Adduct formation also affects EGCG’s binding affinity and remodeling of Aβ as well as amylin8-24 fibrils [47]. Such a mechanism appears to be essential to inhibiting phosphatase-cleaved amyloid precursor peptide (PAP)248–286 amyloid formation [52]; Similar mechanisms have been proposed in the observed anti-aggregation effects of taxifolin on Aβ aggregation as well as for cinnamaldehyde and oleocanthal on tau amyloid formation [45,55,56]. The following sections focus on numerous case studies to provide a detailed discussion of the non-covalent and covalent mechanisms that mediate natural compound-amyloidogenic protein interactions, and importantly, their association with observed inhibitory effects on amyloid induced cytotoxicity.
3.2.2. Non-covalent inhibition mechanisms
The type and specificity of the non-covalent interactions that mediate amyloid inhibitor activity can vary depending upon the protein or stage of aggregation that is targeted [49,52,57–60]. Thus, many of the non-covalent interactions described below are not meant to be a comprehensive description of all non-covalent interactions that may mediate the anti-amyloid activities of a particular compound. Rather it is a summary of some of the key interactions that have been shown to be important for each inhibitor within the specified context. We will use two extensively studied compounds, curcumin and EGCG, as showcase examples.
Curcumin has been documented to modulate amyloid assembly in various amyloid systems. Because curcumin has been identified as a potential pan-assay interference compound (PAINS) [61], it is especially important to have multiple orthogonal assays to validate the bioactivities of curcumin. Nonetheless, extensive literature shows that curcumin prevents amyloid formation, amyloid induced cytotoxicity, and provides beneficial in vivo effects including reduced plaque burden (for a recent review, see [62]; Table 1) via (i) inhibiting amyloid aggregation in the instances of amylin or Aβ [60,63–65] (ii) Accelerating α-synuclein aggregation that results in less toxic intermediates and insoluble aggregates [59,66]. Using experimental structure information, computational simulation work has provided insights into key non-covalent interactions between curcumin and the generic cross beta spine structure present in amyloid fibrillar structures as detailed below.
Table 1.
Natural Product Inhibitor in vivo Efficacy: Animal Studies and Clinical Trials.
| Natural Product | Targeted-Protein/Animal Model/Human Trial | Effects | Reference |
|---|---|---|---|
| In vivo Animal Studies | |||
| Curcumin | Aβ/Tg2576 mouse |
|
[129] |
| Curcumin | Aβ/Tg2576 mouse |
|
[65] |
| Curcumin | Aβ/Tg2576 mouse |
|
[130] |
| Epigallocatechin gallate (EGCG) | Aβ/Tg2576 mouse |
|
[131] |
| EGCG | Aβ and tau; Tg2576 mouse |
|
[132] |
| EGCG | α-synuclein/N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-and 6-hydroxydopamine-induced neurodegenerative C57-BL mice |
|
[133] |
| Ferulic acid | Aβ/Tg2576 mouse |
|
[130] |
| Ferulic acid | Aβ/APP/PS1 mouse |
|
[134] |
| Hopeahainol A | Aβ/APP/PS1 mouse |
|
[135] |
| Myricetin | Aβ/Tg2576 mouse |
|
[130] |
| Nordihydroguaiaretic acid | Aβ/Tg2576 mouse |
|
[130] |
| Oleuropein | Aβ/TgCRND8 mouse |
|
[136] |
| Oleuropein | Aβ/TgCRND8 mouse |
|
[137] |
| Oleuropein | Aβ/C. elegan CL2006 |
|
[108] |
| Scyllo-inositol | Amyloid β peptide (Aβ)/TgCRND8 mouse |
|
[105] |
| Quercetin | Aβ/C. elegan CL2006 |
|
[109] |
| Resveratrol | Aβ/Tg19959 mouse |
|
[110] |
| Resveratrol | Aβ/APP/PS1 mouse |
|
[138] |
| Resveratrol | Aβ/APP/PS1 mouse |
|
[139] |
| Rifampicin | Aβ and tau; APPOSK mouse, Tg2576 mouse, Tau609 mouse | APPOSK mouse
|
[104] |
Tg2576 mouse
| |||
Tau609 mouse
| |||
| Rosmarinic acid | Aβ/Tg2576 mouse |
|
[130] |
| Rutin | Aβ/APP/PS1 mouse |
|
[140] |
| Clinical Studies | |||
| Curcumin | Aβ/Clinical trial |
|
[141] |
| Curcumin | Aβ and tau; Clinical trial |
|
[111] |
| EGCG/Green Tea/Green Tea Extract | Transthyretin/Clinical trial |
|
[107] |
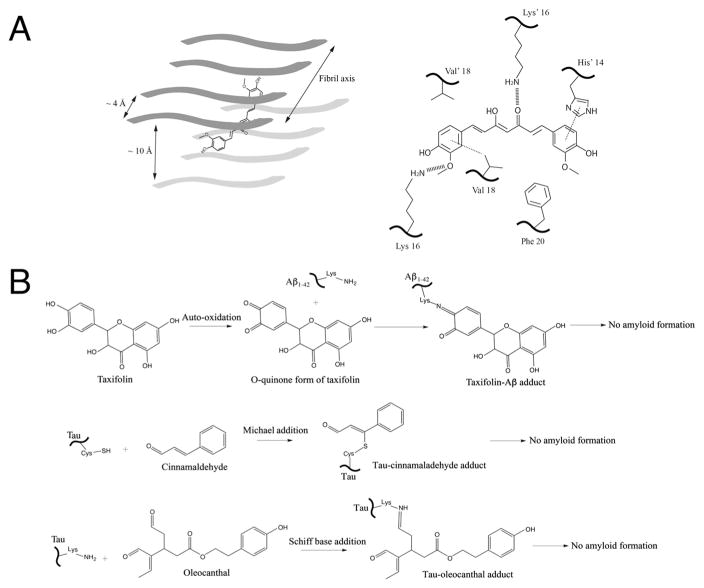
Docking simulations with amyloidogenic “steric zipper” hexapeptide amyloid beta fragment and full length Aβ1–40, showed that curcumin binds within the inter strand space (maintained at characteristic 10 Å distance of protein fibrils; Fig. 2A) in a planar fashion, with its phenyl heads oriented in parallel with the fibril axis [32]. Subsequent analysis revealed that curcumin formed key inter-residue side chain interactions that targeted the bolded residues within segment KLVFFA of the hexapeptide octamer assembly and residues HQKLVFFA in full-length amyloid β peptide [32]. In both cases, interatomic distances between these residues and specific regions of curcumin molecule were indicative of a variety of important non-covalent contacts that were mediated by hydrophobic and hydrogen bonding interactions (Fig. 2A). These include interactions between aromatic phenyl rings of curcumin and His14 (π stacking) and between the phenyl ring of curcumin and Val18 (π-alkyl stacking). Other interactions include hydrogen bonding between two separate flanking Lys16 residues (projected inward from opposite strands of the cross beta spine) [32]. Furthermore, data from molecular dynamics (MD) trajectories (at 1 ns duration) indicate much larger root mean square fluctuations for residues HQKLVF (1.4–3.6 Å) in full-length Aβ fibrils with curcumin bound than for controls with no curcumin bound (0.9–2.1 Å). Binding of curcumin results in β-sheet perturbations within the cross beta spine that may have important implications experimentally relevant to fibril inhibition [58,59,64–66]. Indeed, a similar mechanism of “binding and destabilization” has recently been described with curcumin-bound tau hexapeptide VQIVYK [31]. Eisenberg’s group recently solved curcumin-bound tau hexapeptide crystal structure that demonstrated curcumin intercalates within the inter-β-sheet pocket of four interacting oligomeric chains, with curcumin oriented in a planar-lengthwise fashion parallel to the fibril axis [30]. Using this complex structure as the starting point, a 20 ns MD trajectory was performed that showed curcumin-bound tau disrupts inter-strand beta sheet chain interactions that preclude ordered fibril assembly [31].
Fig. 2.
Schematic representations of several proposed mechanisms between inhibitors and amyloid proteins. (A) Non-covalent interaction mechanisms with curcumin as an example. Left panel: The planar curcumin molecule is depicted by a cartoon schematic within the cross beta spine of an octomeric fibrillar backbone. This representation is based on the structural model of curcumin bound to the VQIVYK segment from the tau protein as well as MD simulation results of curcumin docking onto Aβ hexapeptide KLVFFA [30]. Right panel: key non-covalent interactions occur within the cross beta spine of full-length amyloid β peptide and curcumin, as depicted from a recent MD simulation study [32]. His14 undergoes π–π stacking (dotted line) with one end of the aromatic heads of curcumin, which is also positioned in a hydrophobic area near Phe20 (bottom right). The central keto-enol functional groups and as well as the aromatic head (bottom left) undergo hydrogen bonding with lysine residues located on opposite sides of the cross beta spine (hashed lines). Additionally, π–alkyl interactions (depicted by dotted line) were seen between the aromatic head of curcumin (bottom left) and Val18 residues. (B) Covalent interaction mechanisms. Small molecule natural compounds containing electrophilic functional groups such as o-quinones and aldehydes form covalent adducts with amyloidogenic proteins and prevent amyloid formation. (Top panel) Taxifolin forms covalent adducts with the side chain amine group of lysine in Aβ1-42 via Schiff base formation via o-quinone intermediates. (Middle and Lower panels) Tau is covalently modified by the aldehyde functional groups in the case of cinnamaldehyde (middle panel) and oleocanthal (lower panel) via Michael addition and Schiff base respectively. Such conjugation prevents protein amyloid growth and formation.
Another recent study utilized MD simulations to investigate the molecular contacts that mediate amylin-curcumin interactions. MD simulations showed that multiple curcumin molecules self-associate, forming a nucleation site, characterized by exposed hydrophobic and hydrogen bonding contacts that bind to and stabilize small order amylin oligomeric “nano-assemblies” that attenuate higher order amyloid aggregation [63]. Helical structures have been proposed to play an important role in facilitating downstream β-sheet rich secondary changes that precede fibrillar amylin amyloid assembly [67–71]. Moreover, these data indicate that curcumin is capable of targeting more than just the cross beta spine motif. Residues in the amylin sequence that are targeted most frequently by curcumin included Leu12, Phe15, Phe23, Leu27 and Tyr37 (which includes all aromatic residues within amylin). Discrete MD simulations (DMD) data supported the presence of π – π stacking between these aromatic residues and the phenyl rings of curcumin. These simulation results are consistent with the hypothesis that π – π interactions are an important force mediating anti-amyloid effects of polyphenols [72,73]. DMD also suggested the presence of hydrogen bonding between both 4-hydroxy-3-methoxy phenyl substituents on curcumin and backbone/polar residues of amylin. Importantly, these curcumin-nucleated amylin “nano-assemblies” displayed fewer amylin-amylin contacts, which was attributed to formation of several smaller assemblies versus the one cluster seen in the control [63]. The possibility that curcumin nucleates and stabilizes small oligomeric assemblies that leads to fewer amylin-amylin interactions provides mechanistic insights into the experimentally confirmed ability of curcumin to significantly delay and prevent amylin amyloid formation in vitro [60].
A common phenomenon observed in each study was that curcumin intercalated within the hydrophobic core of all amyloid assemblies regardless of the type of peptide or the quaternary structure of each complex. The non-covalent interactions observed within these complexes give not only mechanistic insights into how curcumin exerts its anti-amyloid effects [58,59,65], but also show how the molecular scaffold of curcumin is capable of targeting the cross beta spine present in all amyloid fibrillar structures, as well as α-helical oligomers that may play an important role during the early events of amyloid aggregation [67,68].
EGCG exerts powerful anti-amyloid effects against a number of amyloidogenic proteins. It has the ability to prevent the formation of toxic prefibrillar oligomers (while stabilizing non-toxic off pathway oligomers), as well as inhibit amyloid fibril formation and remodel previously existing amyloid fibrils into less toxic insoluble aggregates [47,50,52,57,74–76]. Numerous studies have elucidated some of the key non-covalent binding events and interactions that mediate these effects. Multiple studies suggest that EGCG undergoes non-specific hydrophobic and hydrogen bonding interactions that can mediate its anti-amyloid activities: (i) Nitro blue tetrazolium (NBT) dye staining analysis as well as NMR data suggest that EGCG can bind to natively unstructured α-synuclein and amyloid β peptide or denatured bovine serum albumin but not other native globular proteins [57]. These data suggest that EGCG may have a propensity to target unfolded or natively unstructured proteins, presumably via non-specific backbone interactions [57]. (ii) EGCG can remodel preformed amyloid generated by the mutant form of acetylated fragment of yeast prion protein Sup35 (GNNQQNFQQF) but not the native fragment (GNNQQYQQY). Such differential effects may be due to the mutant fragment possessing more hydrophobic binding regions that can interact with EGCG [47]. (iii) Using a series of amylin mutants, Raleigh’s group investigated the importance of residue-specific aromatic/hydrophobic or covalent interactions that may mediate EGCG-induced amyloid inhibition and/or remodeling activities. They concluded that neither was critical, and that backbone hydrogen bonding/hydrophobic interactions likely mediate the effects of EGCG [49]. Other studies indicate that the ability of EGCG to inhibit amylin amyloid aggregation or remodel preformed amylin8-24 fibrils is attenuated in the presence of negatively charged lipid bilayers [47,76]. These data suggest that key polar and non-polar regions of amylin that mediate the non-specific hydrophobic interactions and hydrogen bonding as suggested by Raleigh and colleagues [49], are sequestered by both lipid bilayers and detergent, leading to fewer interactions with EGCG.
The anti-amyloid activities of EGCG may also be facilitated by protein specific regions and/or residue binding: (i) Data from biochemical assays, ion mobility mass spectrometry, 2D NMR spectroscopy, and computational simulations suggest that EGCG-Aβ binding results in compact monomeric and dimeric conformations, as well as higher order SDS-stable Aβ oligomers that are mediated by polar and non-polar interactions between EGCG and the aromatic hydrophobic core of Aβ [75,77]. Hyung and colleagues also suggested from molecular modeling studies that EGCG may interact with metal binding residues His6, His13, and His14 in the Aβ sequence [77]. (ii) NMR spectrometric data on prostatic acid phosphatase-cleaved amyloid precursor peptide (PAP248–286) with EGCG indicate that EGCG binding is selective for charged residues Lys, Arg, His along with Met but not its hydrophobic core [52]. Furthermore, EGCG did not exhibit anti-amyloid activity against acetylated PAP, indicating that non-specific hydrophobic interactions or hydrogen bonding cannot alone mediate the anti-amyloid activity of EGCG towards this amyloid peptide (See Section 3.2.3 for further discussion). MD simulations have also been applied in studying the interaction of EGCG with amyloidogenic peptides. In one study, MD simulations were used in combination with MM-PBSA calculations to examine interactions between EGCG and the Aβ42 monomer [78]. EGCG molecules were observed to expel water from the surface of the peptide and bind directly to it. Free energy decomposition revealed that the dominant terms contributing to binding involved nonpolar interactions, with polar interactions playing a minor role. In another study, MD simulations were used to examine the effect of EGCG on aggregation of amylin [79]. Replica exchange MD was used to study the conformation of amylin dimers. Initial conformations were extended and contained a three-stranded antiparallel β-sheet and the β-hairpin characteristic of cross-beta amyloid. When EGCG was present, the hydrophobic and interpeptide interactions that stabilized the extended β-sheet structures were blocked, resulting in the formation of conformations containing predominantly coil structure.
In summary, the anti-amyloid activities of EGCG are mediated by a broad spectrum of non-covalent interactions whose specificity and overall contribution to the observed anti-amyloid activity is determined by the interacting protein: in the case of amylin, non-specific interactions (i.e., hydrophobic/hydrogen bonding interactions) were sufficient to facilitate the anti-amyloid activity of EGCG; this contrasts sharply with its inhibition of PAP amyloid, wherein specific interactions are essential. This dichotomous behavior is also a departure from an inhibitory mechanism that is characterized by a common phenolic pharmacophore, as suggested by studies conducted with curcumin and Orange G [30]. To our knowledge, high-resolution structural information has not been obtained for EGCG in complex with an amyloid peptide.
3.2.3. Covalent interaction mechanisms
Multiple studies have suggested or confirmed the presence of o-quinone mediated covalent adduct formation from certain flavonoid or catechol-containing phenolic compounds with amyloid proteins [25,45–47,52,54]. Additional covalent mechanisms have also been reported between nucleophilic amines and thiols of amyloidogenic proteins and electrophilic reactive groups on inhibitors such as aldehydes (Fig. 2B, Middle and Lower Panels; [55,56]). Major covalent inhibition mechanisms and selected cases are presented below.
3.2.3.1. O-quinone mediated covalent mechanisms
We will use EGCG, taxifolin, baicalein, and catecholamines to showcase examples for o-quinone mediated covalent mechanisms. A series of NBT binding assays, transmission electron microscopy (TEM), and NMR analyses confirmed that site specific adduct formation to lysine residues within (PAP)248–286 is essential for EGCG-mediated anti-amyloid activity as well as SDS-stable insoluble aggregate formation of (PAP)248–286 [52]. Furthermore, mass spectrometry indicates that gallocatechin (GC), which lacks the gallic ester moiety presented in EGCG but retains the three contiguous hydroxyl groups on the B-ring, undergoes less conjugation with (PAP)248–286 and neither stabilizes low molecular weight SDS-stable PAP oligomers nor inhibits PAP amyloid formation. This suggests that the gallic ester moiety present in EGCG is essential for yielding a sufficient amount of adduct formation (i.e., 35% in EGCG-PAP samples versus 10% observed in GC-PAP samples) necessary to confer anti-amyloid activities. These effects may be attributed to higher stoichiometric amounts of gallol functional groups capable of o-quinone formation in EGCG (versus GC). Alternatively, the gallo ester may facilitate initial non-covalent EGCG-PAP interactions as suggested by NMR experiments that places EGCG in a more favorable position and orientation (as compared to GC) for covalent conjugation [52]. The latter explanation is similar to the idea of how aromatic/hydrophobic residues within the hydrophobic amyloid core may facilitate proper pharmacophore positioning of certain polyphenols along the cross beta spine of amyloid fibrils [72].
Taxifolin does not inhibit monomeric or seeded amyloid beta fibril formation in anaerobic conditions or in the presence of a mild reducing reagent [45]. However, when incubated under aerobic conditions, taxifolin attenuates beta sheet rich secondary changes and prevents Aβ1–42 fibril formation, a phenomenon that was accelerated in the presence of an oxidizing reagent, sodium periodate [45]. Studies using mass spectrometry and site directed mutagenesis confirmed that the chemical mechanism responsible for taxifolin mediated anti-amyloid activity occurs via site specific covalent adduct formation, through Michael addition at residues Lys16 and Lys28 [45]. Further characterizations with catecholtype (myricetin and quercetin) and non-catechol type (morin, kaempferol and datiscetin) flavonoid compounds suggested that all compounds exhibited anti-amyloid activity against Aβ1-42. But in the presence of Lys16Nle, Lys28Nle, and Lys16Nle/Lys28Nle mutants, only non-catechol-type molecules showed anti-amyloid activity [45]. These results indicate that myricetin and quercetin direct their anti-amyloid activities through o-quinone-Lys covalent adduct formation similar as taxifolin but that such a mechanism may not be generalized to all flavonoid molecules.
In a similar case, baicalein prevents α-synuclein amyloid formation and the primary chemical mechanism responsible for this activity is believed to be via formation of baicalein-α-synuclein covalent adducts [46,53]. anti-amyloid activities of baicalein against α-synuclein aggregation are significantly enhanced when it is autoxidized into the quinone form of baicalein versus freshly prepared baicalein, but significantly weakened under anaerobic conditions [46,53]. Disrupting free radical cycling essential to o-quinone auto-oxidation via 5,5-dimethyl-1-pyrroline-N-oxide radical quenching reagent significantly reduced anti-amyloid effects of baicalein [53]. Finally, results from mass spectrometry confirmed that the o-quinone form of baicalein forms covalent adducts with α-synuclein [46,53]. Inhibitory effects of baicalein against amylin oligomerization, fibril formation and amylin amyloid-induced toxicity have also been characterized and validated using a variety of analytical approaches [25]. In addition, systematic structure activity studies using a series of baicalein analogues provided strong evidence for an essential role for its catechol moiety in mediating these effects. Importantly, Schiff-base mediated baicalein-amylin adducts were demonstrated by mass spectrometric evidence [25]. The latter mechanism can readily be explained by the ability of baicalein to undergo auto-oxidation to the o-quinone form and in turn to form covalent adducts with amylin.
Several studies suggest that numerous quinones and aminochromes derived from oxidized catecholamines undergo o-quinone mediated protein covalent adduct formation that leads to the dissolution of both preformed Aβ and α-synuclein amyloid fibrils [80–82]. Dopamine (DA), its precursor 3,4-dihydroxyphenylacetic acid (DOPAC), as well as other catecholamines such as norepinephrine and epinephrine were shown to bind to α–synuclein monomers and prevent β sheet secondary structural changes [81] and also inhibited fibril formation [81,82]. In agreement with spectroscopic based techniques that indicated dopamine could break up preformed Aβ and α-synuclein fibrils, elegant microscopy work tracked the time course of dopamine-mediated dissolution of a single fibrillar species of α-synuclein [81]. Further characterizations suggested that these effects are o-quinone mediated: administration of the anti-oxidant sodium metabisulifite prevented catecholamines from inhibiting fibril formation [82]; the oxidized products of catecholamines were significantly more effective versus the “fresh” parent catechol compounds in inhibiting fibril formation (albeit in this study, validation of polymerized oxidation products was not addressed); the importance for the catechol moiety was suggested by numerous related catecholamine analogues that did not confer anti-amyloid activities [81,82]; the presence of long wavelength fluorescence in samples containing DA and α-synuclein which indicates Tyr-derived radical coupling (i.e., a phenomenon that can serves as an indicator of potential DA-α-synuclein adduct formation at the Tyr residues) was noted [81,82]; the presence of SDS- and heat-treatment stable DA-α-synuclein monomers and oligomers suggests very stable DA-α-synuclein interactions, potentially mediated by covalent binding [80,82].
3.2.3.2. Non-catechol derived covalent mechanisms
Cinnamomum (tree) verum extract has been shown to inhibit amyloid fibril formation from tau and hen egg-white lysozyme [83,84]. Recently it was confirmed that cinnamaldehyde, the major component of cinnamon bark oil, prevents Tau187 amyloid formation without affecting tau mediated microtubule assembly [55]. Further characterizations confirmed that the unsaturated beta carbon on CA undergoes reversible nucleophilic attack by Tau187 cysteine thiols (Fig. 2B, Middle Panel; [55]). Amyloid formation from the double cysteine knock out Tau187 mutant C291S/C322S was not affected by cinnamaldehyde, suggesting that cysteine adduct formation was essential to anti-amyloid activity of cinnamomum.
Oleocanthal prevents tau from undergoing beta sheet secondary structure changes and inhibits tau amyloid formation in a concentration dependent manner [56]. Utilizing mass spectrometry (MS), further work established that oleocanthal forms a covalent adduct via Schiff base formation with the lysine residue of the hexapeptide VQIVYK sequence in the tau protein [56]. MS also suggested oleocanthal-tau (full length) adduct formation, but confirmation of the adduct was not possible due to inherent ambiguity related to oleocanthal-crosslinked products as well as because the MS spectra contained a low signal-to-noise ratio. Nonetheless, other data strongly suggests that covalent adduct formation is necessary for anti-amyloid activity of oleocanthal: systematic structure activity studies showed that the two aldehyde groups within oleocanthal are essential for its observed anti-amyloid activity (Fig. 2B, Lower Panel; [56]); Titration of lysine into the mixtures containing both tau and oleocanthal reduces tau amyloid formation, in a lysine concentration dependent manner. Such reduction is consistent with the ability of lysine to form adducts with the aldehyde reactive-functional groups in oleocanthal (maybe more readily than tau), which results in fewer oleocanthal molecules available to inhibit tau amyloid [56].
3.2.4. Natural product – Metal – Amyloid protein complexes
Transition metals, including copper, iron, and zinc are believed to play important roles in contributing to various amyloid diseases [85–88].While there are still debates about the inhibitory or accelerating effects these metals may have on amyloid formation in vitro [87,89,90], it is well known that metal dyshomeostasis is associated with neurodegenerative amyloid diseases [87,91]. Furthermore, metals have been shown to directly bind to and exacerbate amyloid plaque load and toxicity in vivo. A primary mechanism of toxicity has been attributed to redox active metals such as copper that are sequestered in the amyloid plaques and contribute to cytotoxic reactive oxygen species production in the presence of hydrogen peroxide and reducing agents [87]. Recent work showed that by combining molecular scaffolds or functional groups from individual compounds that chelate metals, interact with amyloid or both, it is possible to engineer multifunctional small molecules with improved activity and selectivity against metal-induced amyloid formation and toxicity [37].
Metal chelating activities of EGCG against various metals, including Zn(II), Cu(I/II), Fe(II/III), and Al(III), directly attenuate metal-catalyzed amyloid formation in vitro. For example, EGCG can form compact ternary complexes with Aβ and either Zn(II) or Cu(I/II) that lead to formation of SDS-resistant non-toxic oligomers [77]. Results from NMR and docking simulations showed that these ternary complexes are mechanistically related, at least in part, by the capacity of EGCG to chelate metals separately in solution, and, at the same time, compete (against metals) for binding with the metal binding His residues in amyloid beta peptide (Hyung et al. 2013 [77]). Importantly, these ternary complexes prevent metal-accelerated amyloid formation and are associated with EGCG-mediated cell rescue effects against metal-amyloid beta induced cytotoxicity [77]. Al(III) ion can accelerate amylin amyloid formation in vitro, but when EGCG and Al(III) are present at a 1:1 ratio in the presence of amylin, aggregation is more potently inhibited than in EGCG treated-metal free conditions [92].
Several studies indicate that curcumin can chelate various biologically active metals including redox-active Cu(I/II) and Fe(II/III) [93–96]. Curcumin can inhibit cytotoxic Cu(II)-triggered Aβ1-42 aggregation and remodel preformed Aβ fibrils in the presence of Cu(II) [94]. Mechanistically, these effects are associated with curcumin forming a ternary complex with Cu(II) and Aβ peptide that is facilitated by simultaneous Cu(II) chelation and peptide binding [95]. Curcumin binds Cu(II) and Fe(II) with similar μM-range affinity [93]. It appears that curcumin more readily binds the redoxactive metals copper and iron than redox-inactive zinc [93,97], which has been related to the role of curcumin in suppressing metal-induced oxidative neurotoxicity.
Verbascoside has similar structural and chemical properties as curcumin (Fig. 1). It was recently shown to inhibit Aβ fibrillation in vitro in metal-free conditions [98]. Recent biochemical and biophysical characterizations have further clarified how verbascoside and its esterified derivative, VPP, interact with freshly dissolved or preformed Aβ aggregates in the presence or absence of CuCl2 or ZnCl2 [38]. Verbascoside showed minimal effects on remodeling preformed aggregates in the absence of metal ions, but interestingly, it was selective for remodeling Aβ aggregates in the presence of CuCl2 but not ZnCl2. In contrast, VPP remodeled preformed Aβ aggregates formed in metal free or in conditions containing either ZnCl2 or CuCl2. In addition, metal binding for both compounds was supported by data from UV–Vis and NMR titration experiments and residue specific peptide binding was suggested [38]. The latter showed evidence that both compounds interacted with the metal binding region of Aβ1–42. Furthermore, saturation transfer difference (STD) NMR was utilized to elucidate the atomic details of which aspects of each compound interacted with preformed fibrils. These data suggested fibrillar Aβ1–42 interacted strongly with the glucose and rhamnose rings of verbascoside, in contrast to the ethyl ester moieties and hydrogens within the aromatic functional groups in VPP [38]. Future studies such as these, that provide atomic details regarding the nature of interactions for different molecular scaffolds on various amyloid targets, will enhance our understanding on the roles of metals in relation to inhibitors and target proteins and also our ability to utilize multifunctional molecular scaffolds to reduce metal-mediated amyloid toxicity.
3.2.5. Non-toxic off pathway aggregates
It has been shown that some natural compounds exert their anti-amyloid activities by inhibiting early oligomerization events as well as preventing mature fibril formation [25]. Others may actually accelerate amyloid formation that result in structural modulation and attenuated oligomer/fibril induced cytotoxicity [66]. Interestingly, it has also been confirmed in multiple cases that natural compounds exert their anti-amyloid effects by modulating amyloid formation and/or preformed fibrils towards the production of both inhibitor-bound soluble and insoluble aggregates [46,47,54,57,66,75,99,100]. These aggregates have been reported to display a wide range of size distributions that include soluble low molecular weight oligomers (as small as dimers) as well as high-molecular-weight insoluble amorphous aggregates (HMAA) that sediment under similar conditions as amyloid fibrils and are unable pass through 0.2 μM filters [47]. Several noteworthy biochemical and biophysical characteristics have been associated with these species. (i) Stability: both oligomers and HMAA remain stable in the presence of SDS denaturing conditions. Also, resistance to heat or high concentrations of denaturant have been observed in some cases [47,54,57,101] (ii) Off-(amyloid) pathway nature: the low molecular weight oligomers do not act as nucleation or template “seeds” for fibril elongation nor do they form amyloid fibrils themselves [54,57]. (iii) Non-toxic: both oligomers and high molecular weight (HMW) aggregates are inert in cell viability assays and are not recognized by conformation specific and toxic prefibrillar antibodies [57,66,99,100]. These characteristics contrast sharply with toxic prefibrillar intermediates that are believed to be one of the major (if not primary) contributors to amyloid induced toxicity [2,6,7,102]. We will discuss a few recent findings regarding inhibitor bound protein oligomeric or HMAA complexes, with an emphasis on the chemical mechanisms responsible for their formation.
As discussed earlier, EGCG inhibits PAP mediated fibril formation that results in the formation of insoluble SDS-resistant and EGCG-bound oligomeric PAP complexes [52]. EGCG directs similar formation of SDS- and heat-stable complexes in several other amyloid systems: (i) EGCG forms strong interactions with Aβ and α-synuclein that prevents amyloid formation and at the same time results in formation of EGCG-bound off-pathway complexes that were neither cytotoxic nor recognized by conformational-specific A11 antibody [57]; (ii) EGCG directs formation of high molecular weight insoluble light chain immunoglobulins (isolated and purified from nine different humans diagnosed with light chain amyloidosis or multiple myeloma) that are heat- and SDS-treatment stable [101]. It is unclear in these studies the chemical mechanism(s) that are responsible for the formation of these complexes. (iii) Using microscopic and filter retardation assays, both covalent and non-covalent mechanisms were shown to play a role in the ability of EGCG to remodel preformed amyloid fibrils from numerous amyloid systems (i.e., amylin8-24, Aβ or Sup35NM7–16) [47].
Baicalein can also stabilize an off pathway population of soluble α-synuclein oligomers that are robustly stable [46,54]: It took greater than 2 M guanidinium chloride (GuHCl) to cause noticeable changes in the secondary structure as determined by thermodynamic stability studies [54]; at the highest concentration tested (6 M GuHCl), baicalein-oligomers are not completely dissociated based on size exclusion chromatography data; at 37 °C baicalein-bound oligomers were shown to be stable for over a month (the longest duration investigated) [54]. Amazingly, these oligomers were determined not only to be “off pathway”, but also, in a concentration dependent manner, they inhibited monomeric α-synuclein amyloid fibril formation and showed only minor lipid membrane perturbation against unilamellar vesicles in vitro [54]. The unusual stability of these oligomers compounded with the previously confirmed adduct formation and its importance in mediating the anti-amyloid activity of baicalein against α-synuclein suggests that covalent modification plays a role in forming these species.
A recent study showed that nordihydroguaiaretic acid (NDGA), resveratrol, and myricetin remodeled both preformed soluble Aβ oligomers and fibrils towards SDS-stable aggregates that exhibited attenuated cytotoxicity compared to controls and were not recognized by prefibrillar oligomer and fibril conformation-specific antibodies [100]. Another study showed that resveratrol-remodeled fibril aggregates were off pathway and that surprisingly, resveratrol was able to remodel unstructured and toxic prefibrillar oligomers, as identified by circular dichroism and A11 antibodies respectively [99]. Curiously, it did not remodel unstructured, non-toxic soluble oligomers. Since NDGA-, resveratrol-, and myricetin-directed SDS-stable oligomers did not form when samples were boiled prior to SDS-PAGE, it is likely that the chemical mechanism conferring their SDS stability is non-covalent.
Following ultra centrifugation sedimentation assays, natural compound polyphenol derivatives of rufigallol, trihydroxy benzophenone and exifone, as well as the porphyrin, hematin, were shown to inhibit tau fibril assembly as determined by maintaining a significantly larger portion of tau in the soluble fraction compared to untreated controls (also confirmed by orthogonal TEM and ThT assays) [103]. At the same time, these compounds directed the formation of SDS-stable soluble and, to a lesser extent, SDS-stable insoluble high molecular weight oligomers. Other sedimentation assays showed that porphyrin phthalocyanine also was able to significantly disaggregate preformed tau filaments into SDS-stable soluble tau oligomers [103]. Further investigations regarding the chemical mechanisms responsible for formation of these soluble HMW oligomers were not performed.
3.3. In vivo efficacy and clinical studies
Our detailed analyses of “Mechanisms of Inhibition” in Section 3.2. are based primarily on in vitro biochemical and pharmacological studies. While mechanistic insights from in vitro studies may still be applicable to in vivo cases such as the interactions between directly injected inhibitor drugs with protein amyloid/oligomers in the circulation, such as hyperamylinemia [15]. To our knowledge, there are very few studies that address the mechanisms of amyloid inhibition by small molecules at cellular or in vivo levels. We envision a variety of confounding factors will come into play, such as cell membrane permeability/drug delivery, cellular environment, and drug metabolism. Furthermore, how amyloids induce cytotoxicity and lead to cell death are also not completely understood. For all the in vivo animal studies, most studies have been focused on examining relevant amyloid plaques using immunohistochemistry approach, quantifying amyloid oligomer levels, and testing basic neurobehavioral functions of the animals (Table 1). We expect future molecular mechanistic studies in the cellular or in vivo contexts will emerge as the field further develops.
Currently, there are about two dozens in vivo animal studies and only three human clinical trials that have been performed to test selected natural product inhibitors for their efficacy against amyloid diseases (Table 1). Several clinical trials have been registered in the U.S. National Institutes of Health (NIH) website (ClinicalTrials.gov), but either they are in progress or the final results of completed trials have not been reported [17]. Ongoing clinical trials include tests on resveratrol, genistein, and rosmarinic acid (RA) in AD or mild cognitive impairment patients [17]. Most of these compounds are well studied flavonoids or phenolic compounds, such as EGCG, quercetin, curcumin, oleuropein, and resveratrol, except for rifampicin [104], an antibiotic used to treat bacterial infections, and scyllo-inositol [105], a naturally occurring plant sugar alcohol. Almost all in vivo studies have targeted amyloid β peptide. However, EGCG has also been used to target α-synuclein [106] and transthyretin [107]. Most of the animal studies were conducted on transgenic mouse models, though several studies chose C. elegans worm as a model. C. elegans is known for its simplicity and short lifespan; additionally, deposition of Aβ amyloid in its muscles is age-dependent, as it is in humans, and leads to the paralysis of the worm, a phenotype that can be easily and clearly scored [108,109].
Typical effects observed by these primarily Aβ-targeted inhibitors are summarized in Table 1. Reduced Aβ plaque burden and lowered toxic oligomer levels were observed in the brain of the animal models. Furthermore, memory dysfunction symptoms were often ameliorated. Additional beneficial effects that were reported included reduced microglial activation, increased autophagic responses, reduced inflammatory cytokine levels, and better redox homeostasis. EGCG, curcumin, and rifampicin also targeted tau protein, and these compounds led to reduced phosphorylated tau. In contrast to the animal studies, very few human trials have been completed and reported. EGCG and curcumin had been tested to target Aβ, tau or transthyretin. However, no significant beneficial effects were observed in curcumin-based clinical trials. Noticeably, levels of two inhibitors (curcumin in a clinical trial and resveratrol in an animal study) were undetectable in the brain, suggesting limited bioavailability of some natural compounds [110,111]. This problem arises for some natural products relevant to their solubility and whether they can efficiently pass blood brain barrier, which will be further addressed in the next section. It is desirable that the bioavailability issue is addressed before more expensive and time-consuming in vivo trials are initiated. If a promising lead compound has unsatisfactory bioavailability to reach its target tissue, additional lead optimization steps may be required using medicinal chemistry and/or engineered drug delivery approaches.
3.4. Enhanced delivery for in vivo efficacy
A significant number of natural compound inhibitors, such as curcumin, resveratrol, and EGCG, are highly lipophilic [112], which are not ideal for drug delivery. These compounds have a low bioavailability in the plasma, which further limits their availability in the brain. For example, in one of the three clinical trials testing efficacy of curcumin [111], the authors were unable to demonstrate clinical or biochemical evidence of efficacy of curcumin in AD and preliminary data suggested limited bioavailability (undetectable in CSF and low in plasma at 7.32 ng/mL). In order to address low bioavailability issues, large quantities of the compounds must be administered in order to reach desired therapeutic effects. The large dose size of these compounds could lead to acute toxicity or low patient compliance [113].
Nanoparticle-based drug delivery approach has gained pace in recent years for both small molecules such as natural products as well as biomolecules such as peptides [112,114]. Nanoparticles can improve the effectiveness of natural compounds in disease treatment and prevention by increasing their bioavailability, including passing the BBB. In a recent study, the superior protective efficacy of resveratrol-loaded polysorbate 80-coated poly(lactide) nanoparticles compared to native resveratrol in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s Disease (PD) animal model was demonstrated, thus suggesting the advantage of nanoparticles over native drug in neuroprotection [115]. Another study used lipid-based nanoparticles (curcumin and piperine coloaded glyceryl monooleate nanoparticles). In vivo studies revealed that formulated dual drug (curcumin and piperine) loaded nanoparticles were able to cross the BBB, which rescued rotenone-induced motor coordination impairment, and restrained dopaminergic neuronal degeneration in a PD mouse model [116].
We and others recently reviewed and summarized multiple examples of nanoparticle formulations that increased the bioavailability (drug concentration in plasma) of selected natural compounds [112,117]. For examples, poor bioavailability is a major limitation to the therapeutic utility of curcumin in clinical trials [118,119]. Shen et al. compared the attenuation of morphine tolerance in mice induced by curcumin encapsulated in nanoparticles of PLGA and in nanoparticles of poly(ethylene glycol)-b-poly(lactic acid) (PEG-b-PLA)[119]. The mice that were given the curcumin-PLGA nanoparticles orally exhibited significantly greater attenuation of morphine tolerance than those mice given the curcumin-PEG-b-PLA nanoparticles, presumably due to greater absorption of the curcumin when formulated with PLGA. The mechanism behind PLGA nanoparticles increasing the bioavailability of curcumin has been investigated. Xie et al. showed data suggesting that the increase in bioavailability of curcumin was due to the inhibition of P-glycoprotein (P-gp)-mediated efflux. They concluded that the PLGA nanoparticles inhibit P-gp, which allows increased drug permeability and bioavailability [120]. Elucidation of the mechanisms of nanoparticle-based natural compound drug delivery will be important in further development of this promising technology. Moreover, these studies demonstrate the significant potential of nanoparticle-mediated drug delivery.
Liposomal encapsulation has also been used to improve bioavailability of hydrophobic compounds. Liposomes can better solubilize hydrophobic compounds and may also alter their pharmacokinetic properties. Multiple studies have been reported to use enhance the delivery of curcumin with liposome formulations [117]. In a study of rat oral administration of liposome-encapsulated curcumin (LEC), not only high bioavailability of curcumin but also a faster rate and better absorption of curcumin were observed as compared with other curcumin forms [121]. Oral LEC gave higher pharmacokinetic C(max) and shorter T(max) values, as well as a higher value for the area under the blood concentration-time curve (AUC), at the entire time points. In another case, a new type of liposome-propylene glycol liposomes (PGL) were engineered to facilitate the intracellular delivery of curcumin. From in vitro cell-based experiments, PGL showed the highest uptake of curcumin compared with that of conventional liposomes and free curcumin solution [122].
Another approach for increasing solubility and bioavailability of poorly soluble natural compounds and drugs is to form amorphous solid dispersions (ASDs) of the therapeutic compound with a polymer [123]. Most drugs and natural compounds tend to crystallize, which is a barrier to dissolution at physiological conditions. Those natural compounds that are also highly hydrophobic exhibit especially poor bioavailability due to the combination of hydrophobicity and crystallinity. ASDs are solid solutions of the therapeutic in an amorphous polymer carrier in which attractive interactions prevent crystallization. It is important to choose the polymer for biocompatibility and controlled release of the drug under physiological conditions and also for good storage stability. In particular, polysaccharides have recently been demonstrated to form ASDs that significantly enhance the solubility of natural compounds including ellagic acid, quercetin, curcumin, naringenin, and resveratrol [124–126]. These studies suggest that ASDs in nanoparticle form could potentially be very useful in improving bioavailability through improved solubility and transport across physiological barriers.
Even though nanoparticles have great potential to considerably improve the bioavailability of natural compounds and there has recently been significant progress in the development of such formulation, very few nanoparticle/natural compound drugs are currently being tested in clinical trials. More work needs to be performed to optimize these drug delivery systems and to better understand the mechanisms underlining the enhanced nanoparticle delivery.
3.5. Issues and perspectives
Natural product inhibitors are not without issues. These compounds examined thus far have relatively moderate inhibition potencies. Medicinal chemistry-based optimization steps (such as analogue synthesis and structure-function activity relationship characterizations) are often necessary. Another common issue is their solubility and bioavailability that has been addressed in the previous Section.
There are a significant number of in vivo animal studies, however, these studies focused on phenolic compounds and flavonoids that have similar physicochemical properties. Only a few in vivo studies test compounds that have significantly different chemical structures (hopeahainol A, rifampicin, and scyllo-inositol (Table 1)). Such limited diversity may hinder discovery of inhibitors that have novel structural scaffolds. To identify novel inhibitors, large library screens with diverse classes of compounds may be required, which in turn needs a high throughput platform. Lee and colleagues have implemented quantitative high throughput (qHTP) screening of approximately 292,000 compounds (not natural product focused) to identify drug-like inhibitors of tau assembly [127]. The Hecht group developed an Aβ-GFP fusion strategy and screened a synthetic triazine library containing ~1000 compounds [27]. Our group also developed a semi-high throughput platform using fluorescence based 384-well plate format, which can be readily expanded to 1596-well plate to achieve high throughput for large library screens, such as screening an NIH Clinical Collection library as part of our repurposing efforts to identify new inhibitors against multiple amyloidogenic proteins including Aβ, amylin, tau, and α-synuclein (data not shown). These examples indicate that technology is available to perform qHTP screens to identify novel natural product inhibitors from large natural product diversity libraries.
Besides natural product inhibitors, there are numerous non-natural product based inhibitors, including synthetic compounds, peptide mimics, and antibodies. In those cases, protein amyloid inhibition mechanisms are likely to be different, especially for peptide-based inhibitors and antibodies. These topics are beyond the scope of this review.
Fully understanding the mechanisms of inhibition is critical. Currently, a significant gap in knowledge remains in our understanding of the biochemical and pharmacological mechanisms of amyloid inhibition, such as differences in protein remodeling with different natural product inhibitors (see Section 3.2.5). One major reason for our incomplete mechanistic understanding is that many of these amyloidogenic protein targets are partially unfolded or intrinsically disordered, which limits high-resolution structural characterization of the protein-inhibitor interaction. However, recent developments in high-resolution mass spectrometry may provide a powerful tool to rapidly characterize protein-inhibitor covalent compounds. Furthermore, expanding crystal structural information of fragments of amyloidogenic proteins and/or fragments in complex with inhibitors will yield significant insights into inhibition mechanisms as well as provide a basis for structure-based inhibitor design [33,128].
It is clear that similar types of non-covalent and in some cases covalent interactions can facilitate anti-amyloid activities of numerous inhibitors. However, protein amyloid specificity may play an important role as well. For example, curcumin accelerates α-synuclein amyloid formation into less toxic species [66], whereas it delays and inhibits amylin amyloid formation [60]. Additionally, in many cases EGCG redirects amyloid formation from multiple different protein amyloid systems into off pathway non-toxic species, a common feature for many amyloid inhibitors. However, the observed anti-amyloid activities of EGCG can vary markedly according to the protein it interacts with: non-covalent mechanisms are only required for the activity of EGCG against amylin amyloid formation [49], whereas covalent interactions are essential for anti-amyloid activity of EGCG against (PAP)248–286 amyloid formation [52]. In this context, studies are needed to elucidate the chemical mechanisms by which specific molecular scaffolds inhibit specific amyloid proteins.
Due to bottlenecks in the therapeutic development process, developing a brand-new drug takes an enormous amount of time, money and effort. Therefore, it may be worthwhile to consider the concept of drug repurposing as a strategy to decrease costs and improve success rates: many drugs approved for other uses already have been tested in humans, so detailed information is available on their pharmacology, formulation, potential toxicity and side effects.
Acknowledgments
This work is in part supported by the Hatch Program of the National Institute of Food and Agriculture of the USDA, Project No. VA-135992 (B.X.), the Commonwealth Health Research Board Grant #208-1-16 (B.X., D.R.B, L.W., S.Z.), the Diabetes Action Research and Education Foundation Grant #462 (B.X.), and Award No. 16-1 from the Commonwealth of Virginia’s Alzheimer’s and Related Diseases Award Fund (B.X., D.R.B., L.W.), administered by the Virginia Center on Aging, School of Allied Health Professions, Virginia Commonwealth University, the NIA of the NIH under award number R01 AG041161 (S.Z.) and Alzheimer’s Drug Discovery Foundation, 20150601 (S.Z.).
Footnotes
Disclosure
The authors report no financial conflicts of interest in this work.
References
- 1.Knowles TPJ, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 2014;15(6):384–396. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- 2.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148(6):1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayed R, Pensalfini A, Margol L, Sokolov Y, Sarsoza F, Head E, et al. Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer. J Biol Chem. 2009;284(7):4230–4237. doi: 10.1074/jbc.M808591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CM, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300(5618):486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 8.Stroud JC, Liu C, Teng PK, Eisenberg D. Toxic fibrillar oligomers of amyloid-β have cross-β structure. Proc Natl Acad Sci USA. 2012;109(20):7717– 7722. doi: 10.1073/pnas.1203193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, et al. Atomic view of a toxic amyloid small oligomer. Science. 2012;335(6073):1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefani M, Dobson CM. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med. 2003;81(11):678–699. doi: 10.1007/s00109-003-0464-5. [DOI] [PubMed] [Google Scholar]
- 11.Rivera JF, Costes S, Gurlo T, Glabe CG, Butler PC. Autophagy defends pancreatic beta cells from human islet amyloid polypeptide-induced toxicity. J Clin Invest. 2014;124(8):3489–3500. doi: 10.1172/JCI71981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280(17):17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 13.Stroo E, Koopman M, Nollen EAA, Mata-Cabana A. Cellular regulation of amyloid formation in aging and disease. Front Neurosci. 2017;11(64) doi: 10.3389/fnins.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Petkova A, Huang K, Xu B, Hua QX, Ye IJ, et al. An Achilles’ heel in an amyloidogenic protein and its repair: insulin fibrillation and therapeutic design. J Biol Chem. 2010;285(14):10806–10821. doi: 10.1074/jbc.M109.067850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91(3):795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 16.Eisele YS, Monteiro C, Fearns C, Encalada SE, Wiseman RL, Powers ET, et al. Targeting protein aggregation for the treatment of degenerative diseases. Nat Rev Drug Discovery. 2015;14(11):759–780. doi: 10.1038/nrd4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada M, Ono K, Hamaguchi T, Noguchi-Shinohara M. Natural phenolic compounds as therapeutic and preventive agents for cerebral amyloidosis. Adv Exp Med Biol. 2015;863:79–94. doi: 10.1007/978-3-319-18365-7_4. [DOI] [PubMed] [Google Scholar]
- 18.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63(12):1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer’s disease. Lancet Neurol. 2004;3(10):579–587. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 21.Arntzen KA, Schirmer H, Wilsgaard T, Mathiesen EB. Moderate wine consumption is associated with better cognitive test results: a 7 year follow up of 5033 subjects in the Tromso Study. Acta Neurol Scand Suppl. 2010;190:23–29. doi: 10.1111/j.1600-0404.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- 22.Ardah MT, Paleologou KE, Lv G, Menon SA, Abul Khair SB, Lu JH, et al. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol Dis. 2015;74:89–101. doi: 10.1016/j.nbd.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigacci S, Guidotti V, Bucciantini M, Parri M, Nediani C, Cerbai E, et al. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J Nutr Biochem. 2010;21(8):726–735. doi: 10.1016/j.jnutbio.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Vitamin A exhibits potent antiamyloidogenic and fibril-destabilizing effects in vitro. Exp Neurol. 2004;189(2):380–392. doi: 10.1016/j.expneurol.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Velander P, Wu L, Ray WK, Helm RF, Xu B. Amylin amyloid inhibition by flavonoid Baicalein: key roles of its vicinal dihydroxyl groups of the catechol moiety. Biochemistry. 2016;55(31):4255–4258. doi: 10.1021/acs.biochem.6b00578. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Armstrong AH, Koehler AN, Hecht MH. Small molecule microarrays enable the discovery of compounds that bind the Alzheimer’s Abeta peptide and reduce its cytotoxicity. J Am Chem Soc. 2010;132(47):17015–17022. doi: 10.1021/ja107552s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim W, Kim Y, Min J, Kim DJ, Chang YT, Hecht MH. A high-throughput screen for compounds that inhibit aggregation of the Alzheimer’s peptide. ACS Chem Biol. 2006;1(7):461–469. doi: 10.1021/cb600135w. [DOI] [PubMed] [Google Scholar]
- 28.Wiltzius JJ, Sievers SA, Sawaya MR, Cascio D, Popov D, Riekel C, et al. Atomic structure of the cross-beta spine of islet amyloid polypeptide (amylin) Protein Sci. 2008;17(9):1467–1474. doi: 10.1110/ps.036509.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, et al. Structure of the cross-[beta] spine of amyloid-like fibrils. Nature. 2005;435(7043):773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landau M, Sawaya MR, Faull KF, Laganowsky A, Jiang L, Sievers SA, et al. Towards a pharmacophore for amyloid. PLoS Biol. 2011;9(6):e1001080. doi: 10.1371/journal.pbio.1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berhanu WM, Masunov AE. Atomistic mechanism of polyphenol amyloid aggregation inhibitors: molecular dynamics study of Curcumin, Exifone, and Myricetin interaction with the segment of tau peptide oligomer. J Biomol Struct Dyn. 2015;33(7):1399–1411. doi: 10.1080/07391102.2014.951689. [DOI] [PubMed] [Google Scholar]
- 32.Rao PP, Mohamed T, Teckwani K, Tin G. Curcumin binding to beta amyloid: a computational study. Chem Biol Drug Des. 2015;86(4):813–820. doi: 10.1111/cbdd.12552. [DOI] [PubMed] [Google Scholar]
- 33.Jiang L, Liu C, Leibly D, Landau M, Zhao M, Hughes MP, et al. Structure-based discovery of fiber-binding compounds that reduce the cytotoxicity of amyloid beta. eLife. 2013;2:e00857. doi: 10.7554/eLife.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemkul JA, Bevan DR. Morin inhibits the early stages of amyloid beta-peptide aggregation by altering tertiary and quaternary interactions to produce “off-pathway” structures. Biochemistry. 2012;51(30):5990–6009. doi: 10.1021/bi300113x. [DOI] [PubMed] [Google Scholar]
- 35.Lemkul JA, Bevan DR. The role of molecular simulations in the development of inhibitors of amyloid beta-peptide aggregation for the treatment of Alzheimer’s disease. ACS Chem Neurosci. 2012;3(11):845–856. doi: 10.1021/cn300091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stefani M, Rigacci S. Protein folding and aggregation into amyloid: the interference by natural phenolic compounds. Int J Mol Sci. 2013;14(6):12411–12457. doi: 10.3390/ijms140612411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savelieff MG, DeToma AS, Derrick JS, Lim MH. The ongoing search for small molecules to study metal-associated amyloid-beta species in Alzheimer’s disease. Acc Chem Res. 2014;47(8):2475–2482. doi: 10.1021/ar500152x. [DOI] [PubMed] [Google Scholar]
- 38.Korshavn KJ, Jang M, Kwak YJ, Kochi A, Vertuani S, Bhunia A, et al. Reactivity of metal-free and metal-associated amyloid-beta with glycosylated polyphenols and their esterified derivatives. Sci Rep. 2015;5:17842. doi: 10.1038/srep17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra S, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13–19. doi: 10.4103/0972-2327.40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rigacci S. Olive oil phenols as promising multi-targeting agents against Alzheimer’s disease. Adv Exp Med Biol. 2015;863:1–20. doi: 10.1007/978-3-319-18365-7_1. [DOI] [PubMed] [Google Scholar]
- 41.Moore KS, Wehrli S, Roder H, Rogers M, Forrest JN, McCrimmon D, et al. Squalamine: an aminosterol antibiotic from the shark. Proc Natl Acad Sci U SA. 1993;90(4):1354–1358. doi: 10.1073/pnas.90.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zasloff M, Adams AP, Beckerman B, Campbell A, Han Z, Luijten E, et al. Squalamine as a broad-spectrum systemic antiviral agent with therapeutic potential. Proc Natl Acad Sci USA. 2011;108(38):15978–15983. doi: 10.1073/pnas.1108558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takasaki J, Ono K, Yoshiike Y, Hirohata M, Ikeda M, Morinaga A, et al. Vitamin A has anti-oligomerization effects on amyloid-beta in vitro. J Alzheimer’s Dis. 2011;27(2):271–280. doi: 10.3233/JAD-2011-110455. [DOI] [PubMed] [Google Scholar]
- 44.D’Avola D, Lopez-Franco E, Sangro B, Paneda A, Grossios N, Gil-Farina I, et al. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J Hepatol. 2016;65(4):776–783. doi: 10.1016/j.jhep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Sato M, Murakami K, Uno M, Nakagawa Y, Katayama S, Akagi K, et al. Site-specific inhibitory mechanism for amyloid beta42 aggregation by catechol-type flavonoids targeting the Lys residues. J Biol Chem. 2013;288(32):23212–23224. doi: 10.1074/jbc.M113.464222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279(26):26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- 47.Palhano FL, Lee J, Grimster NP, Kelly JW. Toward the molecular mechanism(s) by which EGCG treatment remodels mature Amyloid Fibrils. J Am Chem Soc. 2013;135(20):7503–7510. doi: 10.1021/ja3115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishii T, Mori T, Tanaka T, Mizuno D, Yamaji R, Kumazawa S, et al. Covalent modification of proteins by green tea polyphenol (−)-epigallocatechin-3-gallate through autoxidation. Free Radical Biol Med. 2008;45(10):1384– 1394. doi: 10.1016/j.freeradbiomed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 49.Cao P, Raleigh DP. Analysis of the inhibition and remodeling of islet amyloid polypeptide amyloid fibers by flavanols. Biochemistry. 2012;51(13):2670– 2683. doi: 10.1021/bi2015162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tu LH, Young LM, Wong AG, Ashcroft AE, Radford SE, Raleigh DP. Mutational analysis of the ability of resveratrol to inhibit amyloid formation by islet amyloid polypeptide: critical evaluation of the importance of aromatic-inhibitor and histidine-inhibitor interactions. Biochemistry. 2015;54(3):666–676. doi: 10.1021/bi501016r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng B, Gong H, Xiao H, Petersen RB, Zheng L, Huang K. Inhibiting toxic aggregation of amyloidogenic proteins: a therapeutic strategy for protein misfolding diseases. Biochim Biophys Acta. 2013;1830(10):4860–4871. doi: 10.1016/j.bbagen.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 52.Popovych N, Brender JR, Soong R, Vivekanandan S, Hartman K, Basrur V, et al. Site specific interaction of the polyphenol EGCG with the SEVI amyloid precursor peptide PAP(248–286) J Phys Chem B. 2012;116(11):3650– 3658. doi: 10.1021/jp2121577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng X, Munishkina LA, Fink AL, Uversky VN. Molecular mechanisms underlying the flavonoid-induced inhibition of alpha-synuclein fibrillation. Biochemistry. 2009;48(34):8206–8224. doi: 10.1021/bi900506b. [DOI] [PubMed] [Google Scholar]
- 54.Hong DP, Fink AL, Uversky VN. Structural characteristics of alpha-synuclein oligomers stabilized by the flavonoid baicalein. J Mol Biol. 2008;383(1):214–223. doi: 10.1016/j.jmb.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.George RC, Lew J, Graves DJ. Interaction of cinnamaldehyde and epicatechin with tau: implications of beneficial effects in modulating Alzheimer’s disease pathogenesis. J Alzheimer’s Dis. 2013;36(1):21–40. doi: 10.3233/JAD-122113. [DOI] [PubMed] [Google Scholar]
- 56.Li W, Sperry JB, Crowe A, Trojanowski JQ, Smith AB, Lee VM. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J Neurochem. 2009;110(4):1339–1351. doi: 10.1111/j.1471-4159.2009.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, et al. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol. 2008;15(6):558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 58.Thapa A, Jett SD, Chi EY. Curcumin attenuates amyloid-beta aggregate toxicity and modulates amyloid-beta aggregation pathway. ACS Chem Neurosci. 2016;7(1):56–68. doi: 10.1021/acschemneuro.5b00214. [DOI] [PubMed] [Google Scholar]
- 59.Jha NN, Ghosh D, Das S, Anoop A, Jacob RS, Singh PK, et al. Effect of curcumin analogs onalpha-synuclein aggregation and cytotoxicity. Sci Rep. 2016;6:28511. doi: 10.1038/srep28511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu G, Gaines JC, Robbins KJ, Lazo ND. Kinetic profile of amyloid formation in the presence of an aromatic inhibitor by nuclear magnetic resonance. ACS Med Chem Lett. 2012;3(10):856–859. doi: 10.1021/ml300147m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem. 2017;60(5):1620– 1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guerrero-Muñoz MJ, Castillo-Carranza DL, Kayed R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem Pharmacol. 2014;88(4):468– 478. doi: 10.1016/j.bcp.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 63.Nedumpully-Govindan P, Kakinen A, Pilkington EH, Davis TP, Ke PC, Ding F. Stabilizing off-pathway oligomers by polyphenol nanoassemblies for IAPP aggregation inhibition. Sci Rep. 2016;6:19463. doi: 10.1038/srep19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75(6):742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 65.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 66.Singh PK, Kotia V, Ghosh D, Mohite GM, Kumar A, Maji SK. Curcumin modulates α-synuclein aggregation and toxicity. ACS Chem Neurosci. 2013;4(3):393–407. doi: 10.1021/cn3001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abedini A, Raleigh DP. A critical assessment of the role of helical intermediates in amyloid formation by natively unfolded proteins and polypeptides. Protein Eng Des Sel. 2009;22(8):453–459. doi: 10.1093/protein/gzp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knight JD, Hebda JA, Miranker AD. Conserved and cooperative assembly of membrane-bound alpha-helical states of islet amyloid polypeptide. Biochemistry. 2006;45(31):9496–9508. doi: 10.1021/bi060579z. [DOI] [PubMed] [Google Scholar]
- 69.Williamson JA, Loria JP, Miranker AD. Helix stabilization precedes aqueous and bilayer-catalyzed fiber formation in islet amyloid polypeptide. J Mol Biol. 2009;393(2):383–396. doi: 10.1016/j.jmb.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hebda JA, Saraogi I, Magzoub M, Hamilton AD, Miranker AD. A peptidomimetic approach to targeting pre-amyloidogenic states in type II diabetes. Chem Biol. 2009;16(9):943–950. doi: 10.1016/j.chembiol.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saraogi I, Hebda JA, Becerril J, Estroff LA, Miranker AD, Hamilton AD. Synthetic α-helix mimetics as agonists and antagonists of IAPP amyloid formation. Angew Chem Int Ed Engl. 2010;49(4):736–739. doi: 10.1002/anie.200901694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 2006;67(1):27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 73.Gazit E. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002;16(1):77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 74.Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, et al. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc Natl Acad Sci USA. 2010;107(17):7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopez del Amo JM, Fink U, Dasari M, Grelle G, Wanker EE, Bieschke J, et al. Structural properties of EGCG-induced, nontoxic Alzheimer’s disease Abeta oligomers. J Mol Biol. 2012;421(4–5):517–524. doi: 10.1016/j.jmb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 76.Engel MF, VandenAkker CC, Schleeger M, Velikov KP, Koenderink GH, Bonn M. The polyphenol EGCG inhibits amyloid formation less efficiently at phospholipid interfaces than in bulk solution. J Am Chem Soc. 2012;134(36):14781–14788. doi: 10.1021/ja3031664. [DOI] [PubMed] [Google Scholar]
- 77.Hyung SJ, DeToma AS, Brender JR, Lee S, Vivekanandan S, Kochi A, et al. Insights into antiamyloidogenic properties of the green tea extract (−)-epigallocatechin-3-gallate toward metal-associated amyloid-beta species. Proc Natl Acad Sci USA. 2013;110(10):3743–3748. doi: 10.1073/pnas.1220326110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu FF, Dong XY, He L, Middelberg AP, Sun Y. Molecular insight into conformational transition of amyloid beta-peptide 42 inhibited by (−)-epigallocatechin-3-gallate probed by molecular simulations. J Phys Chem B. 2011;115(41):11879–11887. doi: 10.1021/jp202640b. [DOI] [PubMed] [Google Scholar]
- 79.Mo Y, Lei J, Sun Y, Zhang Q, Wei G. Conformational ensemble of hIAPP dimer: insight into the molecular mechanism by which a green tea extract inhibits hIAPP aggregation. Sci Rep. 2016;6:33076. doi: 10.1038/srep33076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazzulli JR, Burbulla LF, Krainc D, Ischiropoulos H. Detection of free and protein-bound ortho-quinones by near-infrared fluorescence. Anal Chem. 2016;88(4):2399–2405. doi: 10.1021/acs.analchem.5b04420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J, Zhu M, Manning-Bog AB, Di Monte DA, Fink AL. Dopamine and L-dopa disaggregate amyloid fibrils: implications for Parkinson’s and Alzheimer’s disease. FASEB J. 2004;18(9):962–964. doi: 10.1096/fj.03-0770fje. [DOI] [PubMed] [Google Scholar]
- 82.Conway KA, Rochet JC, Bieganski RM, Lansbury PT. Kinetic stabilization of the α-synuclein protofibril by a dopamine-α-synuclein adduct. Science. 2001;294(5545):1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 83.Ramshini H, Ebrahim-Habibi A, Aryanejad S, Rad A. Effect of cinnamomum verum extract on the amyloid formation of hen egg-white lysozyme and study of its possible role in Alzheimer’s disease. Basic Clin Neurosci. 2015;6(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 84.Peterson DW, George RC, Scaramozzino F, LaPointe NE, Anderson RA, Graves DJ, et al. Cinnamon extract inhibits tau aggregation associated with Alzheimer’s disease in vitro. J Alzheimer’s Dis. 2009;17(3):585–597. doi: 10.3233/JAD-2009-1083. [DOI] [PubMed] [Google Scholar]
- 85.Plascencia-Villa G, Ponce A, Collingwood JF, Arellano-Jimenez MJ, Zhu X, Rogers JT, et al. High-resolution analytical imaging and electron holography of magnetite particles in amyloid cores of Alzheimer’s disease. Sci Rep. 2016;6:24873. doi: 10.1038/srep24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tiiman A, Luo J, Wallin C, Olsson L, Lindgren J, Jarvet J, et al. Specific binding of Cu(II) ions to amyloid-beta peptides bound to aggregation-inhibiting molecules or SDS micelles creates complexes that generate radical oxygen species. J Alzheimer’s Dis. 2016;54(3):971–982. doi: 10.3233/JAD-160427. [DOI] [PubMed] [Google Scholar]
- 87.Tougu V, Tiiman A, Palumaa P. Interactions of Zn(II) and Cu(II) ions with Alzheimer’s amyloid-beta peptide. Metal ion binding, contribution to fibrillization and toxicity. Metallomics. 2011;3(3):250–261. doi: 10.1039/c0mt00073f. [DOI] [PubMed] [Google Scholar]
- 88.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci. 1998;158(1):47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 89.Mold M, Ouro-Gnao L, Wieckowski BM, Exley C. Copper prevents amyloid-beta(1–42) from forming amyloid fibrils under near-physiological conditions in vitro. Sci Rep. 2013;3:1256. doi: 10.1038/srep01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atwood Cs, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, et al. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273(21):12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- 91.Myhre O, Utkilen H, Duale N, Brunborg G, Hofer T. Metal dyshomeostasis and inflammation in Alzheimer’s and Parkinson’s diseases: possible impact of environmental exposures. Oxid Med Cell Longevity. 2013;2013:726954. doi: 10.1155/2013/726954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu ZX, Zhang Q, Ma GL, Chen CH, He YM, Xu LH, et al. Influence of aluminium and EGCG on fibrillation and aggregation of human islet amyloid polypeptide. J Diabetes Res. 2016;2016:1867059. doi: 10.1155/2016/1867059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J Alzheimer’s Dis. 2004;6(4):367–377. doi: 10.3233/jad-2004-6403. discussion 443–9. [DOI] [PubMed] [Google Scholar]
- 94.Kochi A, Lee HJ, Vithanarachchi SM, Padmini V, Allen MJ, Lim MH. Inhibitory activity of curcumin derivatives towards metal-free and metal-induced amyloid-beta aggregation. Curr Alzheimer Res. 2015;12(5):415– 423. doi: 10.2174/1567205012666150504150125. [DOI] [PubMed] [Google Scholar]
- 95.Picciano AL, Vaden TD. Complexation between Cu(II) and curcumin in the presence of two different segments of amyloid beta. Biophys Chem. 2013;184:62–67. doi: 10.1016/j.bpc.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 96.Zhao XZ, Jiang T, Wang L, Yang H, Zhang S, Zhou P. Interaction of curcumin with Zn(II) and Cu(II) ions based on experiment and theoretical calculation. J Mol Struct. 2010;984(1–3):316–325. [Google Scholar]
- 97.Liu K, Gandhi R, Chen J, Zhang S. Bivalent ligands targeting multiple pathological factors involved in Alzheimer’s disease. ACS Med Chem Lett. 2012;3(11):942–946. doi: 10.1021/ml300229y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kurisu M, Miyamae Y, Murakami K, Han J, Isoda H, Irie K, et al. Inhibition of amyloid β aggregation by acteoside, a phenylethanoid glycoside. Biosci Biotechnol Biochem. 2013;77(6):1329–1332. doi: 10.1271/bbb.130101. [DOI] [PubMed] [Google Scholar]
- 99.Ladiwala AR, Lin JC, Bale SS, Marcelino-Cruz AM, Bhattacharya M, Dordick JS, et al. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Abeta into off-pathway conformers. J Biol Chem. 2010;285(31):24228–24237. doi: 10.1074/jbc.M110.133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ladiwala AR, Dordick JS, Tessier PM. Aromatic small molecules remodel toxic soluble oligomers of amyloid beta through three independent pathways. J Biol Chem. 2011;286(5):3209–3218. doi: 10.1074/jbc.M110.173856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Andrich K, Hegenbart U, Kimmich C, Kedia N, Bergen HR, Schonland S, et al. Aggregation of full length immunoglobulin light chains from al amyloidosis patients is remodeled by epigallocatechin-3-gallate. J Biol Chem. 2016;292(6):2328–2344. doi: 10.1074/jbc.M116.750323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283(44):29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, et al. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J Biol Chem. 2005;280(9):7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 104.Umeda T, Ono K, Sakai A, Yamashita M, Mizuguchi M, Klein WL, et al. Rifampicin is a candidate preventive medicine against amyloid-beta and tau oligomers. Brain. 2016;139(Pt 5):1568–1586. doi: 10.1093/brain/aww042. [DOI] [PubMed] [Google Scholar]
- 105.McLaurin J, Kierstead ME, Brown ME, Hawkes CA, Lambermon MH, Phinney AL, et al. Cyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med. 2006;12(7):801–808. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- 106.Weinreb O, Mandel S, Youdim MB, Amit T. Targeting dysregulation of brain iron homeostasis in Parkinson’s disease by iron chelators. Free Radical Biol Med. 2013;62:52–64. doi: 10.1016/j.freeradbiomed.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 107.Kristen AV, Lehrke S, Buss S, Mereles D, Steen H, Ehlermann P, et al. Green tea halts progression of cardiac transthyretin amyloidosis: an observational report. Clin Res Cardiol. 2012;101(10):805–813. doi: 10.1007/s00392-012-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Diomede L, Rigacci S, Romeo M, Stefani M, Salmona M. Oleuropein aglycone protects transgenic C. elegans strains expressing Abeta42 by reducing plaque load and motor deficit. PLoS One. 2013;8(3):e58893. doi: 10.1371/journal.pone.0058893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Regitz C, Dussling LM, Wenzel U. Amyloid-beta (Abeta(1)(-)(4)(2))-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol Nutr Food Res. 2014;58(10):1931–1940. doi: 10.1002/mnfr.201400014. [DOI] [PubMed] [Google Scholar]
- 110.Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54(2):111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ringman JM, Frautschy SA, Teng E, Begum AN, Bardens J, Beigi M, et al. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res Ther. 2012;4(5):43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Watkins R, Wu L, Zhang C, Davis RM, Xu B. Natural product-based nanomedicine: recent advances and issues. Int J Nanomed. 2015;10:6055– 6074. doi: 10.2147/IJN.S92162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muqbil I, Masood A, Sarkar FH, Mohammad RM, Azmi AS. Progress in nanotechnology based approaches to enhance the potential of chemopreventive agents. Cancers. 2011;3(1):428–445. doi: 10.3390/cancers3010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang L, Qiu W, Crooke S, Li Y, Abid A, Xu B, et al. Development of autologous C5 vaccine nanoparticles to reduce intravascular hemolysis in vivo. ACS Chem Biol. 2017;12(2):539–547. doi: 10.1021/acschembio.6b00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lindner G, da Rocha D, Santos Bonfanti, Colle D, Gasnhar Moreira EL, Daniel Prediger R, Farina M, et al. Improved neuroprotective effects of resveratrol-loaded polysorbate 80-coated poly(lactide) nanoparticles in MPTP-induced Parkinsonism. Nanomedicine. 2015;10(7):1127–1138. doi: 10.2217/nnm.14.165. [DOI] [PubMed] [Google Scholar]
- 116.Kundu P, Das M, Tripathy K, Sahoo SK. Delivery of dual drug loaded lipid based nanoparticles across the blood-brain barrier impart enhanced neuroprotection in a rotenone induced mouse model of Parkinson’s disease. ACS Chem Neurosci. 2016;7(12):1658–1670. doi: 10.1021/acschemneuro.6b00207. [DOI] [PubMed] [Google Scholar]
- 117.Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46(1):2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shen H, Hu X, Szymusiak M, Wang ZJ, Liu Y. Orally administered nanocurcumin to attenuate morphine tolerance: comparison between negatively charged PLGA and partially and fully PEGylated nanoparticles. Mol Pharm. 2013;10(12):4546–4551. doi: 10.1021/mp400358z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xie X, Tao Q, Zou Y, Zhang F, Guo M, Wang Y, et al. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011;59(17):9280–9289. doi: 10.1021/jf202135j. [DOI] [PubMed] [Google Scholar]
- 121.Takahashi M, Uechi S, Takara K, Asikin Y, Wada K. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J Agric Food Chem. 2009;57(19):9141–9146. doi: 10.1021/jf9013923. [DOI] [PubMed] [Google Scholar]
- 122.Zhang L, Lu CT, Li WF, Cheng JG, Tian XQ, Zhao YZ, et al. Physical characterization and cellular uptake of propylene glycol liposomes in vitro. Drug Dev Ind Pharm. 2012;38(3):365–371. doi: 10.3109/03639045.2011.604331. [DOI] [PubMed] [Google Scholar]
- 123.Lee TW, Boersen NA, Yang G, Hui HW. Evaluation of different screening methods to understand the dissolution behaviors of amorphous solid dispersions. Drug Dev Ind Pharm. 2014;40(8):1072–1083. doi: 10.3109/03639045.2013.807279. [DOI] [PubMed] [Google Scholar]
- 124.Li B, Konecke S, Wegiel LA, Taylor LS, Edgar KJ. Both solubility and chemical stability of curcumin are enhanced by solid dispersion in cellulose derivative matrices. Carbohydr Polym. 2013;98(1):1108–1116. doi: 10.1016/j.carbpol.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 125.Li B, Harich K, Wegiel L, Taylor LS, Edgar KJ. Stability and solubility enhancement of ellagic acid in cellulose ester solid dispersions. Carbohydr Polym. 2013;92(2):1443–1450. doi: 10.1016/j.carbpol.2012.10.051. [DOI] [PubMed] [Google Scholar]
- 126.Li B, Konecke S, Harich K, Wegiel L, Taylor LS, Edgar KJ. Solid dispersion of quercetin in cellulose derivative matrices influences both solubility and stability. Carbohydr Polym. 2013;92(2):2033–2040. doi: 10.1016/j.carbpol.2012.11.073. [DOI] [PubMed] [Google Scholar]
- 127.Crowe A, Huang W, Ballatore C, Johnson RL, Hogan AM, Huang R, et al. Identification of aminothienopyridazine inhibitors of tau assembly by quantitative high-throughput screening. Biochemistry. 2009;48(32):7732–7745. doi: 10.1021/bi9006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Krotee P, Rodriguez JA, Sawaya MR, Cascio D, Reyes FE, Shi D, et al. Atomic structures of fibrillar segments of hIAPP suggest tightly mated beta-sheets are important for cytotoxicity. eLife. 2017;6 doi: 10.7554/eLife.19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21(21):8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hamaguchi T, Ono K, Murase A, Yamada M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-β aggregation pathway. Am J Pathol. 2009;175(6):2557–2565. doi: 10.2353/ajpath.2009.090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25(38):8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, et al. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–187. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 133.Mandel S, Weinreb O, Amit T, Youdim MB. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (−)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem. 2004;88(6):1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- 134.Mori T, Koyama N, Guillot-Sestier MV, Tan J, Town T. Ferulic acid is a nutraceutical beta-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS One. 2013;8(2):e55774. doi: 10.1371/journal.pone.0055774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu X, Ye L, Ge H, Chen L, Jiang N, Qian L, et al. Hopeahainol A attenuates memory deficits by targeting beta-amyloid in APP/PS1 transgenic mice. Aging Cell. 2013;12(1):85–92. doi: 10.1111/acel.12022. [DOI] [PubMed] [Google Scholar]
- 136.Grossi C, Rigacci S, Ambrosini S, Ed Dami T, Luccarini I, Traini C, et al. The polyphenol oleuropein aglycone protects TgCRND8 mice against Ass plaque pathology. PLoS One. 2013;8(8):e71702. doi: 10.1371/journal.pone.0071702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grossi C, Ed Dami T, Rigacci S, Stefani M, Luccarini I, Casamenti F. Employing Alzheimer disease animal models for translational research: focus on dietary components. Neuro-degenerative Dis. 2014;13(2–3):131–134. doi: 10.1159/000355461. [DOI] [PubMed] [Google Scholar]
- 138.Solberg NO, Chamberlin R, Vigil JR, Deck LM, Heidrich JE, Brown DC, et al. Optical and SPION-enhanced MR imaging shows that trans-stilbene inhibitors of NF-kappaB concomitantly lower Alzheimer’s disease plaque formation and microglial activation in AbetaPP/PS-1 transgenic mouse brain. J Alzheimer’s Dis. 2014;40(1):191–212. doi: 10.3233/JAD-131031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Porquet D, Grinan-Ferre C, Ferrer I, Camins A, Sanfeliu C, Del Valle J, et al. Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’s disease. J Alzheimer’s Dis. 2014;42(4):1209–1220. doi: 10.3233/JAD-140444. [DOI] [PubMed] [Google Scholar]
- 140.Xu PX, Wang SW, Yu XL, Su YJ, Wang T, Zhou WW, et al. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Abeta oligomer level and attenuating oxidative stress and neuroinflammation. Behav Brain Res. 2014;264:173–180. doi: 10.1016/j.bbr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 141.Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28(1):110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]