Abstract
Changes in the structure and electrical behaviour of the left atrium are known to occur with conditions that predispose to atrial fibrillation (AF) and in response to prolonged periods of AF. We review the evidence that changes in myocardial thickness in the left atrium are an important part of this pathological remodelling process. Autopsy studies have demonstrated changes in the thickness of the atrial wall between patients with different clinical histories. Comparison of the reported tissue dimensions from pathological studies provides an indication of normal ranges for atrial wall thickness. Imaging studies, most commonly done using cardiac computed tomography, have demonstrated that these changes may be identified non-invasively. Experimental evidence using isolated tissue preparations, animal models of AF, and computer simulations proves that the three-dimensional tissue structure will be an important determinant of the electrical behaviour of atrial tissue. Accurately identifying the thickness of the atrial may have an important role in the non-invasive assessment of atrial structure. In combination with atrial tissue characterization, a comprehensive assessment of the atrial dimensions may allow prediction of atrial electrophysiological behaviour and in the future, guide radiofrequency delivery in regions based on their tissue thickness.
Keywords: Left atrial wall thickness, Atrial fibrillation, AF, Cardiac computed tomography, Cardiac CT
Introduction
Changes in the structure and electrical behaviour of the left atrium are known to occur with conditions that predispose to atrial fibrillation (AF) and in response to prolonged periods of AF. The temporal progression, as well as the relative importance of, structural and electrical remodelling remains obscure and is likely to vary among patients with AF. The human left atrial (LA) wall is a thin structure, which has made it difficult to assess in vivo until recently. Traditional assessment of the LA has been restricted to chamber size and flow measurement; however, as technology and experience with high-resolution cross-sectional imaging techniques have improved, the accurate assessment of the atrial wall has become a realistic prospect. Although AF can be effectively treated with radiofrequency catheter ablation (RFCA), for some groups outcomes remain suboptimal. The identification of structural parameters that may optimize patient selection and refine treatment delivery has therefore assumed greater importance. We review the published data regarding the thickness of the left atrial wall (LAWT) in pathological specimens and from imaging studies. We discuss the evidence that LAWT is a parameter that varies with clinical status, the potential for its use as a marker for pathological atrial remodelling and the evolving evidence emphasizing the importance of the three-dimensional structure of the atrial wall in arrhythmogenesis. Finally, we consider the role of LAWT in predicting the response to invasive treatment of AF and its potential for improving the safety of RFCA procedures.
Atrial wall thickness: pathological assessment and effect of cardiovascular comorbidities
Direct examination of atrial tissue in the ex vivo state has provided valuable information about atrial structure. Left atrial wall thickness has been systematically measured in a number of post-mortem studies, which are summarized in Table 1. The left atrium is a thin-walled structure whose shape and volume is likely to be highly sensitive to changes in loading conditions. This is relevant to any conclusions drawn about LA shape and wall thickness drawn from specimens examined in the ex vivo state. The tissue fixing process represents a potential source of error when attempting to establish the LA structure in the thorax from tissue specimens.
Table 1.
Pathological studies of left atrial wall characteristics
| Year | Author | Number of specimens | Age range of specimens | Co-morbidities | Methods of preparation | Methods of examination | Sites examined | Measured parameter | Measurements/mm |
Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1995 | Wang et al.33 | 9 | NR | NR | Fixed 10% formalin 1 week | NR | NR | LA wall thickness | Range 3–5 | |||
| 1999 | Ho et al.6 | 26 | 25–85 (mean 52 ± 18) | Bl | Fixed in 10% formalin | NR |
|
LA wall thickness in each location | Site | Median ± SD | Range | |
| Ant | 3.3 ± 2.1 | 1.5–4.8 | ||||||||||
| Post | 4.1 ± 0.7 | 2.5–5.3 | ||||||||||
| Sup | 4.5 ± 0.6) | 3.5–6.5 | ||||||||||
| Lat | 3.9 ± 0.7 | 2.5–4.9 | ||||||||||
| Vest | 2.3 ± 0.7 | 1.2–3.3 | ||||||||||
| 2003 | Hassink et al.75 | 20 | 46–88 | CV, NCV, AF | Specimens measured freshly at autopsy (and subsequent histology on formalin-fixed samples) | NR |
|
Site | Mean ± SE | Range | Variable extension of atrial myocardium into PV sleeves, which was greater in patients with AF. Hypertrophy, fibrosis, and disorganization greater in PV myocardium of AF patients | |
| Anterior wall | 1.6 ± 0.5 mm | 1.0–2.5 mm | ||||||||||
| Posterior wall | 1.7 ± 0.6 mm | 1.1–2.6 mm | ||||||||||
| 2004 | Becker1 | 20 | 27–93 (mean 63.5) | NCV, CV, CM, NCM | Through and through incision from left inferior pulmonary vein os to mitral valve annulus (shortest distance)—corresponding to mitral isthmus | Caliper on gross specimen |
|
Myocardial thickness | Site | Mean | Range | In addition, atrial myocardium extends onto the atrial aspect of MV leaflet |
| PV | 3.0 | 1.4–7.7 | ||||||||||
| Mid | 2.8 | 1.2–4.4 | ||||||||||
| MV | 1.2 | 0–3.2 | ||||||||||
| 2005 | Sanchez-Quintana et al.3 | 30 | Mean 54 ± 12 | CV, NCV | Isolated heart lung blocks fixed in 10% buffered formalin, sectioned in sagittal planes at regions: left VA junction, middle of posterior atrial wall, and right VA junction | Caliper on prepared sections |
|
Transmural thickness at these locations Myocardial thickness in addition (not included in table) |
Site | Mean ± SD | Range | |
| RVA Sup | 2.3 ± 0.5 | 1.1–4.8 | ||||||||||
| MidP Sup | 2.5 ± 0.5 | 1.1–5.3 | ||||||||||
| LVA Sup | 2.2 ± 0.3 | 1.2–4.5 | ||||||||||
| RVA Mid | 2.8 ± 0.5 | 1.5–5.0 | ||||||||||
| MidP Mid | 3.8 ± 0.6 | 3.1–5.0 | ||||||||||
| LVA Mid | 3.5 ± 1.2 | 1.7–5.0 | ||||||||||
| RVA Inf | 5.7 ± 2.5 | 2.7–10.0 | ||||||||||
| MidP Inf | 6.5 ± 2.5 | 2.8–12.0 | ||||||||||
| LVA Inf | 5.3 ± 2.0 | 2.5–9.0 | ||||||||||
| 2005 | Deneke et al.76 | 7 | 61–76 | All AF post-ablation | Tissue staining of paraffin blocks taken at autopsy | Direct measurement of tissue blocks |
|
Transmural thickness Percentage of wall ablated |
Site | Wall thickness range | ||
| LA isthmus | 4–10 mm | |||||||||||
| PV ostia | 1–3 mm | |||||||||||
| Posterior LA wall | 2–5 mm | |||||||||||
| 2006 | Hall et al.2 | 34 | NR | CV, NCV, CM, NCM, AA | Fixed in 10% formalin, bisected along the sagittal plane and parallel incisions made | Caliper on prepared specimens |
|
Transmural thickness | Site | Mean ± SD | Sig differences between thickness of roof compared with other areas, post wall sig thinner than sept, ant and isht, sept thickest sig thicker than other regions | |
| Ant | 1.86 ± 0.59 | |||||||||||
| Roof | 1.06 ± 0.49 | |||||||||||
| Post | 1.4 ± 0.46 | |||||||||||
| MI | 1.6 ± 0.48 | |||||||||||
| IAS | 2.2 ± 0.82 | |||||||||||
| 2008 | Cabrera et al.4 | 40 | Mean 49 ± 20 years | CV, NCV, AA | LA walls dissected to display lateral region LA between roof of atrium, left PVs and MV. Blocks of tissue encompassing left PVs, LLR, LAA, mitral vestibule and annulus serially sectioned at 12 or 15 µm in sagittal13 or frontal9 planes. Masson's trichrome stain at 1 mm intervals |
Caliper on gross specimens and then on22 histological specimens |
|
Myocardial thickness at superior level Myocardial thickness at inferior level |
Site | Mean ± SD | Range | |
| LLR Sup | 2.8 ± 1.1 | 1.5–4.2 | ||||||||||
| LLR Inf | 1.7 ± 0.8 | 0.5–3.5 | ||||||||||
| 2008 | Platonov et al.5 | 298 | Mean age 61 ± 17 | AF, no AF | ‘Routine autopsy’ on fresh specimens, not fixed in formalin | Calipers to measure tansmural thickness excluding fat |
|
Superior pulmonary veins (SPV) Centre of four pulmonary veins (CPV) Inferior pulmonary veins (IPV) |
Site | Mean ± SD (no AF group) |
Mean ± SD (AF group) |
|
| SPV | 2.3 ± 1.0 | 2.1 ± 0.9 | ||||||||||
| CPV | 2.6 ± 1.0 | 2.2 ± 1.0 | ||||||||||
| IPV | 2.9 ± 1.3 | 2.5 ± 1.3 | ||||||||||
| IAS | 3.1 ± 1.3 | |||||||||||
| Low post | 3.2 ± 1.4 | |||||||||||
| Mid post | 3.1 ± 1.5 | |||||||||||
| Roof | 4.6 ± 1.2 | |||||||||||
| 2009 | Wolf et al.8 | 53 (47 Fontan and 8 controls) | Fontan: 7.7 ± 9.2 Control: age <21 |
Fontan group Control group: NCD |
Fixed in 10% formalin | NR (Masson trichromeon formalin-fixed sections for fibrosis analysis) |
|
Fontan transmural thickness Control transmural thickness |
Group | Mean ± SD | Range | Significant difference in LAWT between Fontan and control groups |
| Control | 1.8 ± 0.5 | 1.0–2.3 | ||||||||||
| Fontan | 2.3 ± 0.6 | 1.0–4.0 | ||||||||||
| 2013 | Schwartzman et al.7 | 15 | 0–69 | NCV, (incidental discovery of SCV changes in 2) | Transmural sections of fresh cardiac tissue. LM: specimens fixed in formalin and embedded in paraffin blocks. Stained with Haematxylin and Eosin, Trichrome and Verhoeff-Van Gieson stains EM: ultrathin sections (60 nm) and stained in 4% uranyl-acetate and 1% lead citrate—transmission electron microscopy |
Calipers on light microscopy sections |
|
Transmural thickness ‘Atrial intimal thickness’ (AIT) (values not included) |
Site | Mean+/1SD | Demonstrated significant difference in AIT between age groups | |
NR, not reported; B, blinded; NCV, patients without a history of cardiovascular disease; CV, patients with cardiovascular disease (excluding atrial arrhythmias); AA, history of atrial arrhythmias; CM, significant (non-cardiac) co-morbidities; NCM, no significant co-morbidities; SCV, structural cardiovascular changes seen at autopsy; NCD, died from non-cardiac disease.
There is significant variation in LAWT between patients when considered in pathological studies. The majority of studies report average measurements of wall thickness between 1 and 4 mm1–4 with a range of reported measurements extending between 0.54 and 12 mm.3 Regional differences in atrial wall thickness are consistently identified in these studies. There is agreement among the tissue-based studies that the posterior wall thickness increases when moving from the superior aspect to the inferior aspect.3,5 Different conclusions are reached regarding comparative tissue thickness between the anterior and posterior wall.2,6 In addition to changes in wall thickness, an age-related increase in LA intimal thickness, fibrosis, and disorganization has been identified in pathological specimens of human atria.7
A group of pathological studies have compared LAWT measurements between groups of patients with different clinical profiles. Wolf et al.8 compared post-mortem atrial wall thickness in young patients who had undergone Fontan procedure palliation for univentricular physiology with controls who had died from non-cardiac disease. As expected the right atrium was significantly thicker in those treated with Fontan procedure. The mean LAWT was also 0.5 mm thicker in the Fontan group than in the control group. In the largest cohort of measurements of LAWT reported in a tissue-based study, Platonov et al.5 considered the posterior LA wall in 298 consecutive pathological specimens at routine autopsy. In this study, the posterior LA wall was significantly thinner in patients with a history of AF when compared with those without a history of AF. In this study, the mean posterior wall thickness was 2.6 and 2.9 mm, respectively, in the middle and inferior portion of the posterior wall in the no-AF group, and 0.4 mm lower at each level in the AF group.5
While atrial remodelling occurring in response to changes in atrial pressures resulting in increased atrial wall thickness in the context of a Fontan circulation is a plausible and likely sequence of events, establishing the temporal relationship between atrial structural changes and the development of atrial arrhythmias is more challenging. Structural changes (including changes in atrial wall thickness) are likely to result from pathological remodelling in response to altered haemodynamics occurring in conditions that predispose to AF. In addition, atrial structural remodelling is known to occur with prolonged episodes of AF9 itself and this may include changes in LAWT. Myocyte hypertrophy is seen in many different animal models of AF.10 Regardless of the temporal sequence of events, it is possible that if it could be assessed non-invasively, atrial wall thickness could become a useful marker of atrial pathological remodelling in patients at risk of, or already diagnosed with, AF.
Non-invasive assessment of left atrial wall thickness
Until recently, echocardiography was the only widely available tool available for cardiac structural assessment in AF patients. A very large evidence base has been collected that comprehensively demonstrates the value of echocardiographic atrial assessment. Two-dimensional estimates of atrial size were traditionally used to measure and follow atrial dilatation, and robust follow-up data document the value of this as a measure of atrial remodelling that has been shown independently predict progression of AF.11 Novel echocardiographic markers12 add additional value, and Doppler techniques allow non-invasive assessment of transmitral flow.13 These features will ensure that echocardiography remains a key part of the imaging assessment of AF patients. Despite these strengths, at present echocardiography is not well suited to the assessment of atrial tissue thickness. Transthoracic echocardiography does not routinely provide adequate spatial resolution to allow assessment of LAWT, although it has been reported for this indication.14 Transesophageal echocardiography (TEE) or intracardiac echocardiography (ICE) provides the highest spatial resolution. Transesophageal echocardiography was used to identify an increase in the interatrial septum thickness (IAST) in patients with a history of AF.15 Intracardiac echocardiography (with a maximum spatial resolution of 0.2–0.3 mm) has been demonstrated to accurately assess changes in right atrial wall thickness following application of radiofrequency (RF) energy in an experimental model.16 Changes in wall thickness were also demonstrated to correlate with depth of lesion formation. The invasive nature of these investigations and the limited extent of atria TEE can image17 means that in general these investigations have been limited to the peri-procedural period where their value is clearly established.
Cardiac magnetic resonance (CMR) imaging has emerged as the optimal modality for myocardial tissue characterization. Ventricular imaging using CMR is now acknowledged as the gold standard for the identification and localization of scar18 as well as for the assessment of ventricular function. More recently, atrial myocardial imaging has expanded and it now has a key role in the assessment of patients with atrial arrhythmias. Cardiac magnetic resonance has been used to identify pathological atrial remodelling through the identification of fibrosis.19 Accumulating evidence suggests that this may be useful in the prediction of disease progression. Cardiac magnetic resonance has been used in animal models20 and clinical studies21 to identify RF lesions. In some centres, CMR has been used to identify gaps in lesions to guide further invasive treatment of AF.22 Novel indices of atrial remodelling including shape assessment may offer additional value in prediction of response to invasive treatment of AF.23,24 Cardiac magnetic resonance will remain a critical tool for atrial assessment in pre- and post-ablation patients. The most effective way to harness the power of CMR to assess different groups of AF patients continues to be refined, tested, and debated.25,26 Cardiac magnetic resonance has been used in a small number of studies to assess LAWT. Hsing et al.27 measured LAWT at a single atrial site and demonstrated an increase in LAWT of around 4 mm following application of RF energy at a single site in the left atrium. In addition, Yokokawa et al.28 report that the magnitude of change in LAWT following application of RF energy at discrete anatomical sites is a predictor of early recurrence of AF. The mean measured wall thickness prior to application of RF energy in Hsing et al.'s study was 7 mm, a greater value than reported in the majority of pathological studies and other imaging studies. This is likely to reflect an averaging effect seen in which the spatial resolution of the imaging technique is below that of the structure being imaged (‘partial volume effect’). While CMR is the optimal modality for atrial tissue characterization, the ability of CMR to precisely identify atrial wall thickness in this case may be limited by the spatial resolution of currently available commercial CMR systems.
Computed tomography (CT) has emerged as the optimal modality for assessing the LA wall and, in addition, provides information about LA structure and shape under in vivo loading conditions. A number of studies have used cardiac CT to assess LAWT and these are summarized in Table 2.
Table 2.
Studies in which computed tomography has been used to assess left atrial wall thickness
| Year | Author | No of patients/CM | Spatial resolution | Method of measuring LAWT | Sampling of measurements of LAWT | Measurements |
Histological studies of similar regions | Correlation with histological studies | Notes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | Lemola et al.29 | 50/all with AA (45pAF, 5 PsAF); NCV and CV | 0.58 mm in all three dimensions | Digital calipers to measure distance from contrast filled LA to fat pad (identified by a change in signal density). Not reported how many measurements were taken or how they chose points to measure |
Superior, middle, and inferior portions of LA posterior wall | Mean post LA wall thickness 2.2 ± 0.9 mm (range 0.9–7.4 mm) | 2 4 5 |
2—CT gives lower measurement 4—CT gives similar result at superior level, lower measurement at middle and inferior level and does not identify the variation seen on tissue sample 5—CT gives higher measurement |
||||
| 2007 | Imada et al.34 | 33 (16PsAF, 17pAF)/NCV and CV. Median age 68(PsAF) /62(pAF) | 0.625 or 1.25 mm slice thickness | Single measurement on anterior LA wall measured from left sagittal view. Method of measurement not reported | Single measurement taken, location of this measurement not reported | Mean anterior LAWT 2.6 mm (SD and range not given) | 2 | 2—CT gives lower measurement than histology | Also demonstrated neg correlation LAWT and LA diameter in PsAF only | |||
| 2007 | Hoffmeister et al.30 | 42 (26pAF, 16 no AA) | 1 mm slice thickness | No details about method of measuring LAWT | No details | Mean LAWT 2.4 ± 0.5 mm | Not comparable | |||||
| 2008 | Pan et al.32 | 180 NCV | Not reported | No details about method for measuring LAWT | Anterior and posterior LAWT on axial slices. No details about level or number of measurements (mean ± SD) | Age | Ant LAWT | Post LAWT | Difference | 2 4 5 7 |
2—CT identifies anterior LAWT as greater than posterior LAWT in contrast to pathological study. Posterior wall measurements are lower by CT than histology, anterior wall measurements are similar 4—CT gives lower measurements than those found at histology 5—Posterior wall measurements similar, CT gives higher measurements for anterior wall 7—CT gives lower measurements for post LAWT |
Change in LAWT with age Difference between ant and post LAWT Agree with Schwartzman et al. (number 8) in that age-related changes in the atrium are identified |
| <40 | 2.0 ± 0.9 | 0.7 ± 0.2 | 1.2 ± 0.4 | |||||||||
| 40–49 | 2.1 ± 0.5 | 1.1 ± 0.3 | 1.1 ± 0.5 | |||||||||
| 50–59 | 2.5 ± 0.7 | 1.5 ± 0.3 | 1.0 ± 0.7 | |||||||||
| 60–69 | 3.2 ± 0.2 | 1.8 ± 0.2 | 1.9 ± 1.1 | |||||||||
| 70–70 | 3.6 ± 0.4 | 1.9 ± 0.2 | 1.4 ± 0.5 | |||||||||
| >80 | 3.7 ± 0.9 | 2.4 ± 0.4 | 1.3 ± 1.2 | |||||||||
| 2011 | Nakamura et al.35 | 186 (62 each no AA, recurrent pAF, CAF) | 1.25 mm (16 slice) or 0.625 mm (64 slice) | No report on how measurements were made (assume manually), measurements taken on multiplanar reconstruction of section perpendicular to anterior LA wall | Anterior LA wall at level where anterior LA wall and aorta separate—given as mean value | Group | Mean ± SD | 2 | 2—CT gives lower measurement than histology in each group | Demonstrated pAF group had thicker anterior LAWT than no AA/CAF groups | ||
| CAF | 2.1 ± 0.2 | |||||||||||
| PAF | 2.4 ± 0.2 | |||||||||||
| No AA | 1.9 ± 0.2 | |||||||||||
| 2011 | Beinart et al.31 | 60 (all AA—PsAF) | 0.6 or 0.75 mm slice thickness | 12 pre-selected locations. Thickest measurable muscular segment (fat excluded on basis of HU) within 5 mm of reference point | 3 points on roof, 4 points on posterior wall, 3 points on floor, LLR and mitral isthmus origin | Site | Mean ± SD | Range | 2 3 4 5 6 7 8 |
2—CT gives lower measurement than histology [PW/roof (listed as ‘superior’ wall in Ho et al.)], other locations not obviously comparable 3—CT gives lower measurement at LLR than histology (taken representative measurement in Becker paper as middle measurement 4—CT gives lower measurements than histology on right, mid, and left sections [compare floor measurements (8, 9, 10 on Beinart paper) with mid PW measurements of Sanchez-Quintana] 5—CT gives higher measurement than histology at roof (middle), lower measurement on PW (middle), higher measurement on isthmus 6—CT gives lower measurement than histology (superior level) 7—CT gives lower measurement [compare mid sup PW (region 5) with SPV] on sup PW than histology (both AF and no AF groups), mid PW (AF/no AF groups) 8—CT gives lower measurements than histology at sup PW (region 5 with region 7), mid PW (6 with 6), low PW (9 with 5) |
Sig inter- and intra-patient variability in LAWT | |
| Roof right | 2.03 ± 0.53 | 0.90–3.00 | ||||||||||
| Roof middle | 2.15 ± 0.47 | 1.190–3.30 | ||||||||||
| Roof left | 2.00 ± 0.46 | 1.00–2.80 | ||||||||||
| Right PW | 1.94 ± 0.55 | 0.90–3.00 | ||||||||||
| Mid sup PW | 1.43 ± 0.44 | 0.70–2.60 | ||||||||||
| Mid PW | 1.87 ± 0.45 | 0.80–3.10 | ||||||||||
| Left PW | 1.80 ± 0.42 | 0.70–2.80 | ||||||||||
| Right floor | 1.78 ± 0.49 | 0.70–2.60 | ||||||||||
| Mid floor | 1.81 ± 0.44 | 1.00–3.00 | ||||||||||
| Left floor | 1.74 ± 0.42 | 1.00–2.70 | ||||||||||
| LLR | 2.10 ± 0.63 | 0.50–3.50 | ||||||||||
| MI | 2.05 ± 0.47 | 0.90–2.80 | ||||||||||
| 2013 | Suenari et al.65 | 54 (all undergoing AF ablation) (inc CV, NCV, CM, NCM) | 0.8 mm slice thickness at 0.4 mm intervals | No report on definition of wall borders, but measurements taken at middle of LA region on each sagittal section according to text (NB images show measurements on multiplanar reconstructions) | Superior LLR (Sup LLR), inferior left alteral ridge (Inf LLR) Bottom of LA Posterior wall Roof LA Also measured PV antra and carina |
Site | AF recurrence group—mean ± SD | No AF recurrence group—mean ± SD | 6 | 6—CT gives higher measurement than histology, CT does not demonstrate variability seen on tissue samples | Demonstrated fusion of CT and EAMS Demonstrated sup LLR thickness correlated with AF recurrence |
|
| Sup LLR | 4.20 ± 1.08 | 5.58 ± 1.67 | ||||||||||
| Inf LLR | 4.25 ± 1.09 | 5.06 ± 1.52 | ||||||||||
| 2013 | Dewland et al.38 | 187 (98 AA and 89 no AA) | 1.25 mm slice thickness | Measurements in axial plane using an automated algorithm (validated on carotid CT). Algorithm required borders of contrasting density. After all slices from a given region compared thinnest measurement was recorded. Note: imaging for AF at random time in cardiac cycle, just prior to atrial systole in control | Interatrial septum, below RIPV, LAA, ant LA wall: thinnest LAWT measured | AF | 0.7 mm (no range/SD) | Nil comparable | No directly comparable regions, but CT measurements are generally lower than reported tissue measurements | |||
| Control | 0.9 mm (no range/SD) | |||||||||||
| 2013 | Hayashi et al.77 | 51 (all AA undergoing ablation, CV, and NCV) | 0.67 mm slice thickness | Pericardial fat excluded on basis of HU. LAWT measured in 11 distinct locations. Multiplanar reconstructions taken to measure LAWT (oblique coronal to parallel to posterior wall or superior PV for LA roof, oblique axial perpendicular to LA posterior wall to measure mid posterior and infero-posterior walls, axial plane at level of mitral isthmus to measure MI and oblique reconstructed image perpendicular to sup LLR to measure LLR LAWT | 11 locations—right (R), middle (M), and left (L) of each of roof, mid posterior (mid post) and infero-posterior (inf-post) wall, then LLR and mitral isthmus | HCM | Control | 2 3 4 5 7 |
2—CT gives lower measurement on posterior wall than tissue specimens 4—CT gives lower measurement than tissue at mid posterior wall and mid infero-posterior wall 5—CT gives similar measurement mid posterior wall, higher value at MI compared with histology 7—CT measurements lower at mid posterior wall than tissue study In both groups |
LA wall thinner in HCM patients compared with controls at mid posterior wall and infero-posterior wall (other regions no difference) | ||
| R roof | 1.93 ± 0.49 | 1.95 ± 0.35 | ||||||||||
| M roof | 2.20 ± 0.51 | 2.23 ± 0.31 | ||||||||||
| L roof | 1.96 ± 0.34 | 1.98 ± 0.36 | ||||||||||
| R mid post |
1.90 ± 0.42 | 1.89 ± 0.21 | ||||||||||
| M post mid | 1.44 ± 0.17 | 1.58 ± 0.22 | ||||||||||
| L mid post | 1.85 ± 0.35 | 1.84 ± 0.22 | ||||||||||
| R inf-post | 1.66 ± 0.20 | 1.75 ± 0.27 | ||||||||||
| M inf-post mid | 1.64 ± 0.25 | 1.77 ± 0.20 | ||||||||||
| L inf-post | 1.62 ± 0.16 | 1.747 ± 0.18 | ||||||||||
| MI | 2.38 ± 0.36 | 2.21 ± 0.31 | ||||||||||
| LLR | 2.20 ± 0.55 | 2.14 ± 0.39 | ||||||||||
| 2014 | Park et al.78 | 33 (all AA undergoing ablation) | Inner and outer borders defined (no further info) and electronic calipers measured wall thickness in 31 pre-specified locations (identified on 3D volume rendered image and multiplanar reformatted images), measurements taken on multiplanar reformatted images—choice not further defined | Grouped from 31 locations into 7 areas: LAA, roof, anterior wall, posterior wall, floor, lateral wall, septum | Site | Median ± SD | 2 4 5 7 |
2—CT gives lower measurement on anterior, posterior and lateral wall 4—CT gives lower measurement than on posterior wall 5—CT gives similar measurements anterior wall and septum, higher measurements roof, posterior 7—CT gives lower measurement than histology |
Regions with CFAE had thicker walls | |||
| LAA | 2.2 ± 0.6 | |||||||||||
| Roof | 2.0 ± 0.6 | |||||||||||
| Ant | 1.8 ± 0.3 | |||||||||||
| Post | 1.7 ± 0.3 | |||||||||||
| Floor | 1.7 ± 0.3 | |||||||||||
| Lat | 1.8 ± 0.4 | |||||||||||
| Septum | 2.4 ± 0.8 | |||||||||||
| 2014 | Wi et al.37 | 31 (all AA undergoing ablation) | Reconstructed CT with 0.75 mm slice thickness | LAWT measurements obtained in 31 pre-specified locations. Surface divided on 3D VR image, point marked in centre of each region. To measure, defined a point on LA wall on 3D VR, axial, coronal, and sagittal images (to obtain perpendicular measurements). Measurements then made semi-automatically using software to identify transition inner and outer border of LA. Five measurements taken within 5 mm of each reference point and mean calculated | Grouped from locations to give mean (±SD) overall, at anterior appendage base and at lateral wall | Overall mean (±SD) | 2.4 (±0.4) mm (range 1.5–3.1 mm) |
2 | 2—CT gives lower measurements than tissue samples at lateral wall | |||
| Ant append | 3.4 (±1.2) | |||||||||||
| Lat wall | 1.5 (±0.4) | |||||||||||
| 2015 | Takahashi et al.63 | 50 AF (29 paroxysmal, 21 persistent) 25 SR | 0.5 mm slice thickness | Epicardial fat excluded by excluding voxels −50 to −200 HU. Measurements taken in coronal plane in LA roof and axial plane in other locations | LAWT thickness measurements acquired at five pre-selected locations on LA wall (anterior wall, posterior wall, roof and septum and LAA) and eight pre-selected locations at PV-LA junction. At each site in LA three measurements taken—within 5 mm of thickest part of LA and mean taken | Control group | AF group | Significantly thicker LA wall in AF patients (a stepwise increase which followed disease status—walls thinnest in control, then paroxysmal, then persistent). Also demonstrated thicker walls in areas of dormant conduction (as exposed by ATP provocation after PVI in AF patients) | ||||
| LA roof | 2.39 ± 0.5 mm | 2.46 ± 0.63 mm | ||||||||||
| Anterior walla | 1.65 ± 0.44 mm | 1.93 ± 0.44 mm | ||||||||||
| Posterior walla | 1.61 ± 0.31 mm | 1.93 ± 0.4 mm | ||||||||||
| Septuma | 1.17 ± 0.29 mm | 1.42 ± 0.34 mm | ||||||||||
| LA appendagea | 1.14 ± 0.4 mm | 1.41 ± 0.4 mm | ||||||||||
| 2015 | Park et al.64 | 71 patients all persistent AF | 0.76 mm slice thickness after reconstruction | Measurements taken at point of maximum thickness of interatrial septum 1 cm inferior to fossa ovalis. Plane not reported | Overall mean IAST | 6.75 mm | ||||||
| Data grouped according to tertiles and CFAE index calculated (100× area deemed to represent CFAE/total LA surface area). CFAE index was compared between groups according to tertiles (significant) and using linear regression model for LA volume and LA volume index (both significant) | ||||||||||||
NR, not reported; Bl, blinded; NCV, patients without a history of cardiovascular disease; CV, patients with cardiovascular disease (excluding atrial fibrillation); AF, history of atrial fibrillation; NCM, no significant co-morbidities; SCV, structural cardiovascular changes seen at autopsy; CM, significant (non-cardiac) co-morbidities; NCD, died from non-cardiac disease; LS Ps AF, long-standing persistent AF; CAF, chronic AF (resistant to electrical or chemical cardioversion or present for >1 year); PVI, pulmonary vein isolation.
aOnly measured in those in whom the posterior wall came into contact with the oesophagus.
In the first study to measure atrial wall thickness using cardiac CT, Lemola et al.29 took measurements of the thickness of the posterior LA wall at three different levels. In contrast to pathological studies, no significant differences in the LAWT at the superior, middle, and inferior level were identified. Hoffmeister et al.30 report a mean LAWT of 2.4 ± 0.5 mm without further details of the measurement locations, the method of defining the boundaries of the wall, or the orientation chosen for the measurement. Beinhart et al.31 examined CT scans from consecutive patients undergoing persistent AF ablation and recorded LAWT measurements from 12 pre-determined locations. Measurement was taken at the thickest measurable muscular segments and demonstrated significant inter- and intra-patient variability in LAWT measurements. When viewed alongside the results of tissue studies from similar locations, the CT measurements are generally lower than the tissue measurements.1,3–7
Association between left atrial wall thickness and clinical characteristics
Left atrial wall thickness has been demonstrated to increase between the ages of 50 and 79.32 In the same study, Pan et al.32 also observed the anterior LAWT was significantly greater than posterior LAWT in 180 patients without coronary or other known cardiac disease. These results are consistent with those results from several pathological studies2,33 while discrepant with the results from Ho et al.6
Imada et al.34 did not identify a significant difference between measurements of atrial wall thickness taken from the anterior wall between patients with paroxysmal AF (PAF) and non-PAF. In this study, the mean LAWT was 2.6 mm in both groups. Nakamura et al.35 found that the anterior LAWT was significantly thicker in patients with PAF (LAWT 2.4 ± 0.2 mm) when compared with both a group with no history of AF (LAWT 1.9 ± 0.2 mm) and a group with non-PAF (LAWT 2.1 ± 0.2 mm). This CT-based study indicates that LAWT is a variable that may change with the presence of type of AF. The identification of an increase in anterior LAWT with PAF that regresses with the transition to persistent AF is of great interest, suggesting a complex progression of changes in atrial anatomy in parallel with well-documented atrial dilatation (Figures 1 and 2).36
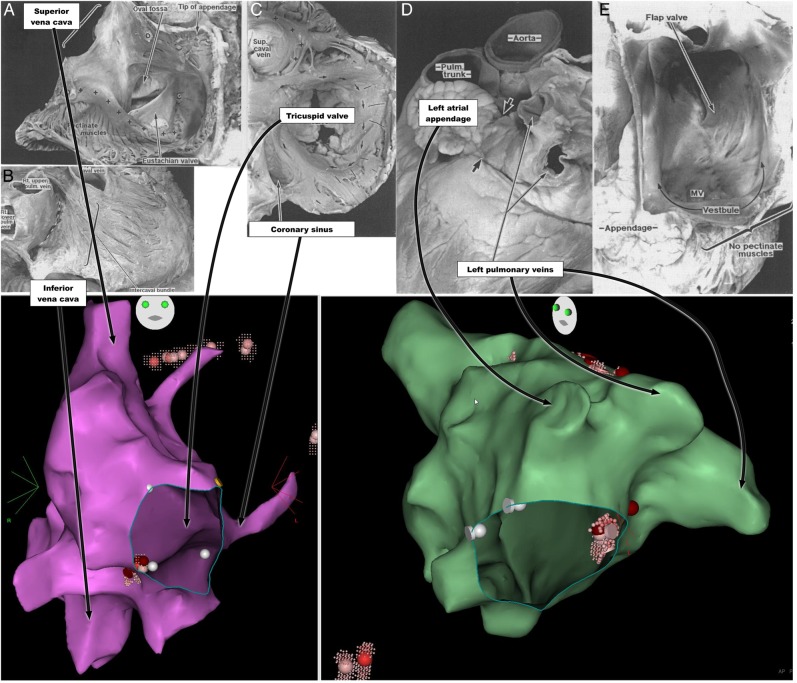
Figure 1.
Human atrial anatomy: pathological specimens with corresponding anatomical landmarks from Carto electro-anatomical map of right atrium under anteroposterior projection (purple) and left atrium under modified left anterior oblique projection (green). ( A–E) Reproduced with permission of BMJ publishing group from Wang et al.33 (A) Right atrium opened through an incision that passes parallel to the right coronary artery to the tip of the appendage and then through the terminal crest (O–O) into the superior vena cava. Pectinate muscles arise along the length of the terminal crest (+ +) towards the coronary sinus (*). There is a prominent Eustachian valve in this specimen. (B) Atria viewed from behind showing intercaval bundle after pericardium removed. (C) Tricuspid valve viewed from the right atrium with the endocardium removed to reveal the internal circumferential bundle (arrows) encircling the vestibule. The pectinate muscles (lines) are perpendicular to the circumferential bundle. ‘+’ indicates terminal crest. (D) External view of left atrium showing tubular appendage with arrow junction to pulmonary veins. (E) Internal aspect of left atrium demonstrating fossa ovalis on the septum. Pectinate muscles are confined to the appendage. The vestibule leading to the mitral valve (MV) is smooth.
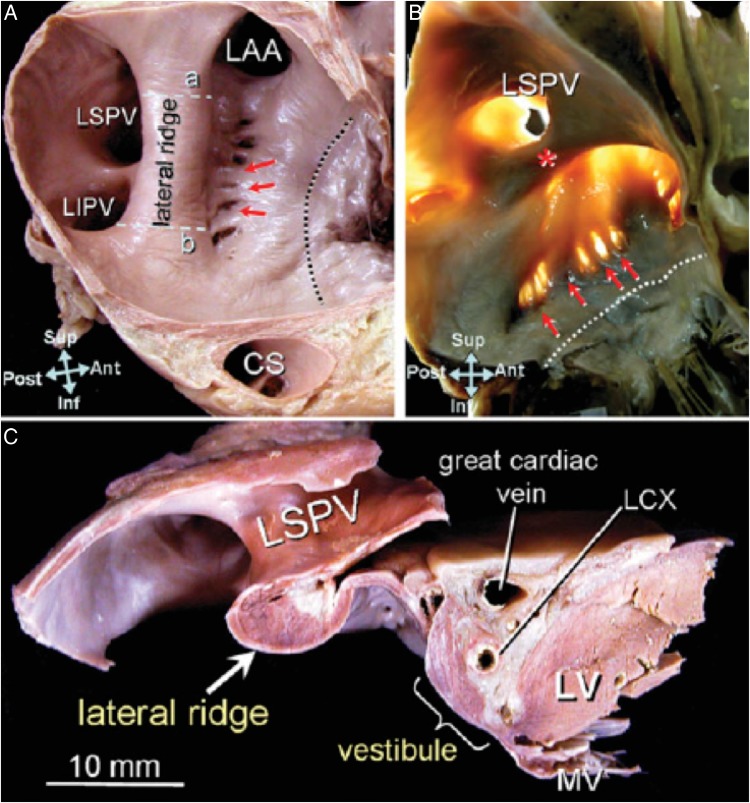
Figure 2.
Tissue section of human atria reproduced with permission from Cabrera et al.4 (A) Endocardial surface of the left lateral wall and (B) with translumination showing the prominent lateral ridge(*) interposed between the left atrial appendage (LAA) and the left pulmonary veins. Muscular trabeculations denoted by red arrows extend inferiorly from the ridge to the vestibule. The dotted line marks the mitral valve (MV) annulus. Lines ‘a’ and ‘b’ mark the sites where measurements were taken in this study. (C) is a section through line ‘a’ to demonstrate the thickness of the ridge. Also seen in the left circumflex coronary artery (LCX), coronary sinus (CS), and left ventricle (LV).
With the exception of a single tissue-based study, measurements of LAWT taken on CT are lower than those reported from ex vivo tissue samples.1,3–6 This may relate to the different loading conditions of the atria in the in vivo state when compared with the ex vivo state. It is also possible that there is a systematic underestimation of LAWT by CT. Computed tomography studies have not demonstrated the variation in LAWT seen at the different levels of the posterior wall that has been reported in pathological studies. It is not clear whether the variation in the posterior LAWT measured on pathological specimens reflects a change in atrial geometry between the in vivo and the ex vivo state, and is therefore an artefact from preparation of the tissue samples, or whether this represents an insensitivity of the imaging modality to identify this variation.
An important challenge in CT assessment of LAWT is the accurate identification of the epicardial and endocardial surfaces. This is an inherent limitation of the imaging modality as a result of the low tissue contrast at the epicardial boundary. In CT imaging, the Hounsfield unit (HU) intensity is used to discriminate between the tissue types. In some areas, the atrial wall will have similar HU intensity to neighbouring structures (e.g. the aortic wall and the oesophagus), which will make accurate identification of the epicardial surface challenging. There is no consensus on the optimal method for identifying the borders between which measurements are taken. As more experience is gained, it seems likely that there will be increased use of automated algorithms for assessing these boundaries, as has been seen in the most recent studies.37,38 In addition, all studies so far have assessed LAWT in a single dimension at a discrete number of locations and extrapolated these measurements as representative of either regional or global LAWT. Given the anatomical complexity of the LA, it may be helpful to exploit the full data set available in order to make an assessment of LAWT. A novel method designed to make a three-dimensional assessment of LAWT that has been validated in a LA phantom has recently been reported.39 Following identification of the epicardial and endocardial borders, non-intersecting field lines are derived between the two surfaces. This allows perpendicular measurements to be automatically calculated between these field lines, which provides wall thickness measurements throughout the atrium. If validated in a clinical setting, a global LAWT assessment will offer more detailed, accurate, and reproducible measurements to be identified from current imaging as well as offering the potential to derive estimates of atrial tissue mass.
Effect of atrial wall thickness on atrial electrophysiology
The ‘critical mass hypothesis’ was originally proposed in 191440 as one of several early theories to explain the pattern of electrical activity responsible for AF. Although in the intervening decades our understanding of AF has progressed, many contemporary models of the electrical patterns required to sustain fibrillation in atrial tissue also depend upon a critical mass of tissue being present, whether they depend upon the propagation of multiple wavelets41–44 or the existence of localized focal re-entrant mechanisms driving AF.45–48
Experimental evidence in support of a critical mass of atrial tissue being required to sustain AF comes from Byrd et al.49 and Lee et al.50 who demonstrated that the size of tissue sections correlated with the probability of being able to induce sustained AF within the sections These data are in keeping with the progressive atrial dilatation seen over time with AF, and its reconciliation with the long-held observation that ‘AF begets AF’.51
In addition to simple quantification of atrial mass impacting upon the ability of atrial tissue to sustain arrhythmias, investigators have considered the impact of the complex atrial architecture upon the electrophysiological mechanisms involved in atrial arrhythmias. Using high-density simultaneous electrode mapping of the epicardial and endocardial surface of the right atrium in isolated perfused canine myocardium, Schuessler et al.52 demonstrated differences in the timing of activation between the two surfaces. In addition, the voltage amplitude of the signals measured corresponded to the atrial tissue thickness. Finally, they demonstrated that the atrial wall is capable of harbouring re-entrant circuits that exist between the epicardial and endocardial surface, rather than existing on one or the other surface as had previously been modelled. Everett et al.53 measured the endocardial and epicardial activation sequences of experimental AF in five different canine models of AF. This study also demonstrated transmural differences in activation pattern seen independently in each model,53 providing further confirmation of the transmural patterns of electrical activation seen in fibrillating atria.
In Langendorf-perfused sheep hearts, Klos et al.54 further characterized the effect of tissue structure in experimentally induced AF. They identified abrupt changes in the myocardial thickness and changes in muscle fibre orientation as being the key structural determinants of wavebreak,54 that was often implicated in the initiation of AF. They explained this on the basis of a significant change in the source–sink relationship that a propagating wave of depolarization would meet at boundaries between thick and thin atrial wall, as previously identified in other anatomic sites55 and in vitro.56 Lines of functional conduction block have been identified during normal sinus rhythm and correlated with change in fibre geometry and wall thickness at comparable locations in the human atrium.57
Abrupt changes in tissue thickness are also implicated in the mechanisms sustaining periods of AF.58 In Langendorff-perfused sheep hearts, the activation sequences in normal hearts and those subjected to chronic rapid atrial pacing (RAP) were mapped optically when AF was induced. Computer-based stimulations, incorporating myocardial wall thickness, atrial stretch, and ion channel behaviour, based on the experimental results, demonstrated that transitions in myocardial thickness stabilize ‘atrial scroll waves’ (ASW, three-dimensional patterns of re-entry spanning the full thickness of the myocardium59), which may be considered as sources with the potential to drive AF.
High-density epicardial mapping of patients at cardiac surgery with AF of differing durations60 and in vivo high-resolution epi- and endocardial mapping in a goat model of AF with a range of durations61 demonstrated that the degree of dissociation between the epicardial and endocardial activation increased with longer duration of AF. This work has formed the basis for a new interpretation of the multiple wavelet hypothesis, known as the ‘double-layer’ hypothesis. While the double-layer hypothesis proposes ‘anarchical’ electrical activity as the basis for fibrillation in the atrium and is therefore a fundamentally different explanation to those based on ‘hierarchical’ electrical activity, the dissociation of activation between the epicardial and endocardial surfaces reinforces the concept that the three-dimensional structure of the atria will play a significant role in determining the electrical activity on which AF depends.
Recent simultaneous epi- and endocardial optical mapping study62 in coronary perfused ex vivo human right atrial specimens has demonstrated the capacity of human atrial tissue to harbour stable intramural re-entry circuits. This work is the first demonstration of the ability of human atrial tissue to harbour intramural re-entry and contributes further evidence in support of the importance of the complex atrial microstructure in determining electrophysiological behaviour.
A number of clinical studies have reported evidence suggesting atrial tissue dimensions may predict the local atrial electrophysiological behaviour in patients undergoing AF ablation. Tissue thickness as measured on CT has been correlated with the presence of complex fractionated atrial electrograms (CFAEs),37 scar as defined by endocardial voltage amplitude63 and proportion of endocardium in which CFAEs may be identified.64 While the significance of CFAEs is uncertain, these studies suggest the possibility of predicting in vivo electrophysiological behaviour with anatomy assessed using cardiac CT (Figures 3 and 4).
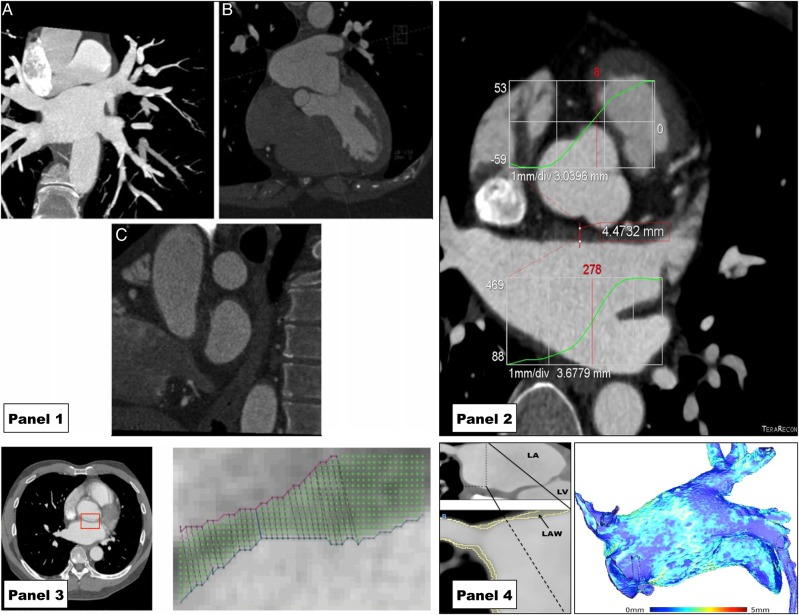
Figure 3.
Recent examples of the use of cardiac CT to assess LAWT demonstrating evolving methodology. Panel 1 reproduced with permission from Beinart et al.31 Standardized planes for measuring LAWT manually. (A) Oblique coronal plane parallel to posterior wall of LA (in maximum intensity projection). (B) Axial plane perpendicular to coronal plane at the level of the mitral isthmus. (C) Saggital plane orthogonal to the original plane near the ostium of the left pulmonary vein. Panel 2 reproduced with permission from Wi et al.37 Semi-automatic measurement of the LAWT based on histogram intensities (projected onto CT image) after manually selecting a region containing the LA wall. Panel 3 reproduced with permission from Dewland et al.38 An example of an automated, computer-based segmentation of the LA wall. (A) Axial plane of contrast-enhanced cardiac CT with anterior LA wall identified by red box. (B) An expanded illustration of this region with LA wall identified by computer algorithm on the basis of voxel intensity. Panel 4: a semi-automated approach to LA wall segmentation based on HU intensity that has been used to generate whole chamber LAWT maps as reported by Bishop et al.39 Left hand image demonstrates a multiplanar-reconstructed CT image of the left atrium. Expanded region shows the LA roof and right superior pulmonary vein with LA wall as identified by computer algorithm in yellow. Right image is a visual representation of the LAWT throughout the chamber calculated using the method reported by Bishop et al. based on generating field lines and solving the Laplace equation.
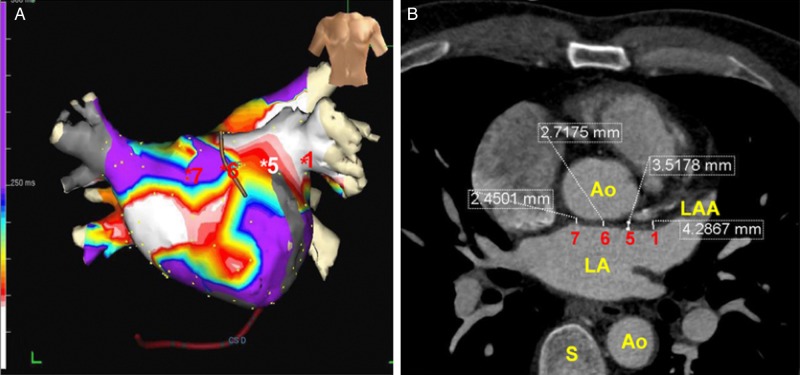
Figure 4.
The correlation of CT-measured LAWT with clinically measurable electrophysiological phenomena. Reproduced with permission from Wi et al.37 Left hand panel (A) is colour map representing the summation of CFAEs across the left atrium under an anteroposterior projection. On the left hand side (B) is the CT image from which LAWT measurements were made with representative measurements. In this report, CFAEs were observed more frequently in regions with increased atrial wall thickness (sites 1 and 5).
There remain many outstanding questions about the spatio-temporal organization of atrial electrical activity during human AF, but a crucial role for the three-dimensional characteristics of the tissue involved seems increasingly likely.
The impact of left atrial wall thickness on treatment: safety and efficacy considerations
Prior to invasive electrophysiology study and AF ablation, Suenari et al.65 compared CT-determined LAWT at a range of LA and pulmonary venous locations between those who experienced a recurrence of AF and those who did not. The study identified greater wall thickness at the superior left lateral ridge (LLR, the region defined as that between the left pulmonary veins and the LA appendage) in those who experienced a recurrence of AF after a single pulmonary vein isolation procedure, while in other areas there was no difference. As well as measuring increased LAWT in AF patients when compared with controls, Takahashi et al.63 found the wall of the left atrium–pulmonary vein junction to be significantly thicker at sites of adenosine-provoked pulmonary vein reconnection following pulmonary vein isolation (PVI), an observation that is consistent with failure to achieve a local transmural lesion. These studies indicate that atrial wall thickness may be a useful predictor of response to AF ablation and in the future may have a role in the prediction of response to and, ultimately, titrating delivery of, RF energy during AF ablation.
In addition to the use of CT for the assessment of LAWT, it has been used for the acute assessment of RF lesions. Girard et al.66 demonstrated the identification of ventricular RF lesions in porcine ventricular tissue using C-arm CT. The identification of RF lesions has been more extensively investigated using MRI67–69; however, the demonstration of CT's ability to identify acute RF lesions opens up the possibility of exploiting CT's higher spatial resolution in the atria to assess these lesions acutely as well.
The incidence of a significant complication associated with a catheter ablation procedure is 4–5%.70,71 The most serious complications of RFCA are due to perforation of the atrial wall.72,73 Risk of atrial perforation during ablation is likely to depend at least partly upon LAWT. It is recognized that RF application on the posterior LA wall is associated with a greater risk of serious complications than elsewhere because of oesophageal proximity, and current guidelines recommend the use of lower energy when RF is applied here.74 An imaging modality that could localize areas of decreased wall thickness more precisely may facilitate safer RF energy delivery. If a technique could simultaneously identify regions of increased wall thickness and it could be demonstrated that these required differential energy applications for successful transmural lesion creation, the assessment of LAWT would rapidly assume a central role by facilitating tissue tailored lesion delivery and the possibility of safety and efficacy dividends.
Conclusions
The left atrium is the critical cardiac chamber in the pathophysiology of AF, and the success of current treatments for AF is highly dependent upon the ability to create contiguous, transmural lesions. Cardiac CT is the optimal imaging modality to measure atrial wall thickness, and there has been a recent increase in the number of reports using CT for this indication. Electrophysiological behaviour appears to change with wall thickness profile both under experimental conditions and in vivo. A systematic examination of the effects of changes in wall thickness on local electrophysiology in vivo would likely contribute to this investigation. In addition, the accurate assessment of atrial wall thickness may provide important safety information prior to the delivery of RF energy and has the potential to improve the safety of AF ablation.
Funding
The research was funded/supported by the National Institute for Health Research (NIHR) Clinical Research Facility at Guy's & St Thomas’ NHS Foundation Trust and NIHR Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London. This work was supported by the Medical Research Council (grant number MR/N001877/1). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the MRC, or the Department of Health.
Conflict of interest: none declared.
References
- 1. Becker AE. Left atrial isthmus: anatomic aspects relevant for linear catheter ablation procedures in humans. J Cardiovasc Electrophysiol 2004;15:809–12. [DOI] [PubMed] [Google Scholar]
- 2. Hall B, Jeevanantham V, Simon R, Filippone J, Vorobiof G, Daubert J. Variation in left atrial transmural wall thickness at sites commonly targeted for ablation of atrial fibrillation. J Interv Card Electrophysiol 2006;17:127–32. [DOI] [PubMed] [Google Scholar]
- 3. Sánchez-Quintana D, Cabrera JA, Climent V, Farré J, De Mendonça MC, Ho SY. Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation 2005;112:1400–5. [DOI] [PubMed] [Google Scholar]
- 4. Cabrera JA, Ho SY, Climent V, Sánchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J 2008;29:356–62. [DOI] [PubMed] [Google Scholar]
- 5. Platonov PG, Ivanov V, Ho SY, Mitrofanova L. Left atrial posterior wall thickness in patients with and without atrial fibrillation: data from 298 consecutive autopsies. J Cardiovasc Electrophysiol 2008;19:689–92. [DOI] [PubMed] [Google Scholar]
- 6. Ho SYEN, Ph D, Sanchez-quintana D, Cabrera JA, Anderson RH. Anatomy of the left atrium : implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 1999;10:1525–34. [DOI] [PubMed] [Google Scholar]
- 7. Schwartzman D, Schoedel K, Stolz DB, Di Martino E. Morphological and mechanical examination of the atrial “intima”. Europace 2013;15:1557–61. [DOI] [PubMed] [Google Scholar]
- 8. Wolf C, Seslar S, Boer K, Juraszek A, MacGowan F, Cowan D et al. Atrial remodelling after the fontan operation. Am J Cardiol 2009;104:1737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 1990;82:792–7. [DOI] [PubMed] [Google Scholar]
- 10. Nishida K, Michael G, Dobrev D, Nattel S. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace 2010;12:160–72. [DOI] [PubMed] [Google Scholar]
- 11. Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J 2005;149:489–96. [DOI] [PubMed] [Google Scholar]
- 12. Yoon YE, Oh I-Y, Kim S-A, Park K-H, Kim SH, Park J-H et al. Echocardiographic predictors of progression to persistent or permanent atrial fibrillation in patients with paroxysmal atrial fibrillation (E6P Study). J Am Soc Echocardiogr 2015;28:709–17. [DOI] [PubMed] [Google Scholar]
- 13. Tiwari S, Schirmer H, Jacobsen BK, Hopstock LA, Nyrnes A, Heggelund G et al. Association between diastolic dysfunction and future atrial fibrillation in the Tromsø Study from 1994 to 2010. Heart 2015;101:1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. López-Candales A. Is the presence of interatrial septal hypertrophy a marker for atrial fibrillation in the elderly? Am J Geriatr Cardiol 2002;11:399–403. [DOI] [PubMed] [Google Scholar]
- 15. López-Candales A, Grewal H, Katz W. The importance of increased interatrial septal thickness in patients with atrial fibrillation: a transesophageal echocardiographic study. Echocardiography 2005;22:408–14. [DOI] [PubMed] [Google Scholar]
- 16. Ren J-F, Callans DJ, Schwartzman D, Michele JJ, Marchlinski FE. Changes in local wall thickness correlate with pathologic lesion size following radiofrequency catheter ablation: an intracardiac echocardiographic imaging study. Echocardiography 2001;18:503–7. [DOI] [PubMed] [Google Scholar]
- 17. Ren B, Mulder HW, Haak A, Van Stralen M, Szili-Torok T, Pluim JPW et al. A transoesophageal echocardiographic image acquisition protocol for wide-view fusion of three-dimensional datasets to support atrial fibrillation catheter ablation. J Interv Card Electrophysiol 2013;37:21–6. [DOI] [PubMed] [Google Scholar]
- 18. Morgan RB, Kwong R. Role of cardiac MRI in the assessment of cardiomyopathy. Curr Treat Options Cardiovasc Med 2015;17:53. [DOI] [PubMed] [Google Scholar]
- 19. McGann CJ, Kholmovski EG, Oakes RS, Blauer JJE, Daccarett M, Segerson N et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol 2008;52:1263–71. [DOI] [PubMed] [Google Scholar]
- 20. Harrison JL, Jensen HK, Peel SA, Chiribiri A, Grondal AK, Bloch LO et al. Cardiac magnetic resonance and electroanatomical mapping of acute and chronic atrial ablation injury: a histological validation study. Eur Heart J 2014;35:1486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mcgann C, Kholmovski E, Blauer J, Vijayakumar S, Haslam T, Cates J et al. Dark regions of no-reflow on late gadolinium enhancement magnetic resonance imaging result in scar formation after atrial fibrillation ablation. J Am Coll Cardiol 2011;58:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bisbal F, Guiu E, Cabanas-Grandío P, Berruezo A, Prat-Gonzalez S, Vidal B et al. CMR-guided approach to localize and ablate gaps in repeat AF ablation procedure. JACC Cardiovasc Imaging 2014;7:653–63. [DOI] [PubMed] [Google Scholar]
- 23. Bisbal F, Guiu E, Calvo N, Marin D, Berruezo A, Arbelo E et al. Left atrial sphericity: a new method to assess atrial remodeling. Impact on the outcome of atrial fibrillation ablation. J Cardiovasc Electrophysiol 2013;24:752–9. [DOI] [PubMed] [Google Scholar]
- 24. Bisbal F, Guiu E, Cabanas P, Calvo N, Berruezo A, Tolosana JM et al. Reversal of spherical remodelling of the left atrium after pulmonary vein isolation: incidence and predictors. Europace 2014;16:840–7. [DOI] [PubMed] [Google Scholar]
- 25. Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol 2015;65:2239–51. [DOI] [PubMed] [Google Scholar]
- 26. Walters TE, Ellims AH, Kalman JM. The role of left atrial imaging in the management of atrial fibrillation. Prog Cardiovasc Dis 2015;58:136–51. [DOI] [PubMed] [Google Scholar]
- 27. Hsing J, Peters DC, Knowles BR, Manning WJ, Josephson ME. Cardiovascular magnetic resonance imaging of scar development following pulmonary vein isolation: a prospective study. PLoS One 2014;9:e104844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yokokawa M, Tada H, Koyama K, Ino T, Naito S, Oshima S et al. Thickening of the left atrial wall shortly after radiofrequency ablation predicts early recurrence of atrial fibrillation. Circ J 2010;74:1538–46. [DOI] [PubMed] [Google Scholar]
- 29. Lemola K, Sneider M, Desjardins B, Case I, Han J, Good E et al. Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation 2004;110:3655–60. [DOI] [PubMed] [Google Scholar]
- 30. Hoffmeister PS, Chaudhry GM, Mendel J, Almasry I, Tahir S, Marchese T et al. Evaluation of left atrial and posterior mediastinal anatomy by multidetector helical computed tomography imaging: relevance to ablation. J Interv Card Electrophysiol 2007;18:217–23. [DOI] [PubMed] [Google Scholar]
- 31. Beinart R, Abbara S, Blum A, Ferencik M, Heist K, Ruskin J et al. Left atrial wall thickness variability measured by CT scans in patients undergoing pulmonary vein isolation. J Cardiovasc Electrophysiol 2011;22:1232–6. [DOI] [PubMed] [Google Scholar]
- 32. Pan N-H, Tsao H-M, Chang N-C, Chen Y-J, Chen S-A. Aging dilates atrium and pulmonary veins: implications for the genesis of atrial fibrillation. Chest 2008;133:190–6. [DOI] [PubMed] [Google Scholar]
- 33. Wang K, Ho SY, Gibson DG, Anderson RH. Architecture of atrial musculature in humans. Br Heart J 1995;73:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imada M, Funabashi N, Asano M, Uehara M, Ueda M, Komuro I. Anatomical remodeling of left atria in subjects with chronic and paroxysmal atrial fibrillation evaluated by multislice computed tomography. Int J Cardiol 2007;119:384–8. [DOI] [PubMed] [Google Scholar]
- 35. Nakamura K, Funabashi N, Uehara M, Ueda M, Murayama T, Takaoka H et al. Left atrial wall thickness in paroxysmal atrial fibrillation by multislice-CT is initial marker of structural remodeling and predictor of transition from paroxysmal to chronic form. Int J Cardiol 2011;148:139–47. [DOI] [PubMed] [Google Scholar]
- 36. Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT et al. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J 2014;35:1457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wi J, Lee H-J, Uhm J-S, Kim J-Y, Pak H-N, Lee M et al. Complex fractionated atrial electrograms related to left atrial wall thickness. J Cardiovasc Electrophysiol 2014;25:1141–9. [DOI] [PubMed] [Google Scholar]
- 38. Dewland TA, Wintermark M, Vaysman A, Smith L, Tong E, Eric V et al. Use of computed tomography to identify atrial fibrillation associated differences in left atrial wall thickness and density. Pacing Clin Electrophsyiology 2014;36:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bishop M, Rajani R, Plank G, Carr-White G, Wright M, O'Neill MD et al. A novel technique to measure atrial wall thickness using cardiac computed tomography. Europace 2015; Accepted for publication. [Google Scholar]
- 40. Garrey W. The nature of fibrilatory conduction of the heart: its relation to tissue mass and form. Am J Physiol 1914;33:397–414. [Google Scholar]
- 41. Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J 1959;58:59–70. [DOI] [PubMed] [Google Scholar]
- 42. Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964;67:200–20. [DOI] [PubMed] [Google Scholar]
- 43. Moe GK. A conceptual model of atrial fibrillation. J Electrocardiol 1968;1:145–6. [DOI] [PubMed] [Google Scholar]
- 44. Allessie M, Lammers WEJEP, Bonke F, Hollen J. Experimental evaluation of Moe's multiple wavelet hypothesis of atrial fibrillation. In: Zipes D, Jalife J, eds. Cardiac Electrophysiology and Arrhythmias. Orlando, FL: Grune and Stratton; 1985. p265–75. [Google Scholar]
- 45. Garrey W. Auricular fibrillation. Physiol Rev 1924;4:215–50. [Google Scholar]
- 46. Allessie MA, Bonke FIM, Schopman FJG. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. Circ Res 1973;33:54–62. [PubMed] [Google Scholar]
- 47. Clapham DE, Lechleiter JD, Girard S. Intracellular waves observed by confocal microscopy from Xenopus oocytes. Adv Second Messenger Phosphoprotein Res 1993;28:161–5. [PubMed] [Google Scholar]
- 48. Lechleiter JD, John LM, Camacho P. Ca2+ wave dispersion and spiral wave entrainment in Xenopus laevis oocytes overexpressing Ca2+ ATPases. Biophys Chem 1998;72:123–9. [DOI] [PubMed] [Google Scholar]
- 49. Byrd GD, Prasad SM, Ripplinger CM, Cassilly TR, Schuessler RB, Boineau JP et al. Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. Circulation 2005;112(Suppl.):I7–13. [DOI] [PubMed] [Google Scholar]
- 50. Lee AM, Aziz A, Didesch J, Clark KL, Schuessler RB, Damiano RJ. Importance of atrial surface area and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. J Thorac Cardiovasc Surg 2013;146:593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wijffels MCEF, Kirchhof CJHJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation : a study in awake chronically instrumented goats. Circulation 1995;92:1954–68. [DOI] [PubMed] [Google Scholar]
- 52. Schuessler RB, Kawamoto T, Hand DE, Mitsuno M, Bromberg BI, Cox JL et al. Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation 1993;88:250–63. [DOI] [PubMed] [Google Scholar]
- 53. Everett TH, Wilson EE, Hulley GS, Olgin JE. Transmural characteristics of atrial fibrillation in canine models of structural and electrical atrial remodeling assessed by simultaneous epicardial and endocardial mapping. Heart Rhythm 2010;7:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Klos M, Calvo D, Yamazaki M, Zlochiver S, Mironov S, Cabrera J-A et al. Atrial septopulmonary bundle of the posterior left atrium provides a substrate for atrial fibrillation initiation in a model of vagally mediated pulmonary vein tachycardia of the structurally normal heart. Circ Arrhythm Electrophysiol 2008;1:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moe GK, Mendez C. Functional block in the intraventricular conduction system. Circulation 1971;43:949–54. [DOI] [PubMed] [Google Scholar]
- 56. Fast VG, Klkber AG. Cardiac tissue geometry as a determinant of unidirectional conduction block: assessment of microscopic excitation spread by optical mapping in patterned cell cultures and in a computer model. Cardiovasc Res 1995;29:697–707. [PubMed] [Google Scholar]
- 57. Markides V. Characterization of left atrial activation in the intact human heart. Circulation 2003;107:733–9. [DOI] [PubMed] [Google Scholar]
- 58. Yamazaki M, Mironov S, Taravant C, Brec J, Vaquero LM, Bandaru K et al. Heterogeneous atrial wall thickness and stretch promote scroll waves anchoring during atrial fibrillation. Cardiovasc Res 2012;94:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction : a mechanism of atrial fibrillation. Cardiovasc Res 2002;54:204–16. [DOI] [PubMed] [Google Scholar]
- 60. Allessie MA, De Groot NMS, Houben RPM, Schotten U, Boersma E, Smeets JL et al. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease longitudinal dissociation. Circ Arrhythmia Electrophysiol 2010;3:606–15. [DOI] [PubMed] [Google Scholar]
- 61. Eckstein J, Zeemering S, Linz D, Maesen B, Verheule S, Van Hunnik A et al. Transmural conduction is the predominant mechanism of breakthrough during atrial fibrillation: evidence from simultaneous endo-epicardial high-density activation mapping. Circ Arrhythmia Electrophysiol 2013;6:334–41. [DOI] [PubMed] [Google Scholar]
- 62. Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J 2015;36:2390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takahashi K, Okumura Y, Watanabe I, Nagashima K, Sonoda K, Sasaki N et al. Relation between left atrial wall thickness in patients with atrial fibrillation and intracardiac electrogram characteristics and ATP-provoked dormant pulmonary vein conduction. J Cardiovasc Electrophysiol 2015;26:597–605. [DOI] [PubMed] [Google Scholar]
- 64. Park YM, Park HC, Ban J-E, Choi J-I, Lim HE, Park SW et al. Interatrial septal thickness is associated with the extent of left atrial complex fractionated atrial electrograms and acute procedural outcome in patients with persistent atrial fibrillation. Europace 2015;17:1700–7. [DOI] [PubMed] [Google Scholar]
- 65. Suenari K, Nakano Y, Hirai Y, Ogi H, Oda N, Makita Y et al. Left atrial thickness under the catheter ablation lines in patients with paroxysmal atrial fibrillation: insights from 64-slice multidetector computed tomography. Heart Vessels 2013;28:360–8. [DOI] [PubMed] [Google Scholar]
- 66. Girard EE, Al-Ahmad A, Rosenberg J, Luong R, Moore T, Lauritsch G et al. Contrast-enhanced C-arm CT evaluation of radiofrequency ablation lesions in the left ventricle. JACC Cardiovasc Imaging 2011;4:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peters DC, Wylie JV, Hauser TH, Kissinger KV, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: methods: results: conclusion. Radiology 2007;243:690–5. [DOI] [PubMed] [Google Scholar]
- 68. Arujuna A, Karim R, Caulfield D, Knowles B, Rhode K, Schaeffter T et al. Acute pulmonary vein isolation is achieved by a combination of reversible and irreversible atrial injury after catheter ablation: evidence from magnetic resonance imaging. Circ Arrhythm Electrophysiol 2012;5:691–700. [DOI] [PubMed] [Google Scholar]
- 69. Harrison JL, Jensen HK, Peel SA, Chiribiri A, Grøndal AK, Bloch LØ et al. Cardiac magnetic resonance and electroanatomical mapping of acute and chronic atrial ablation injury: a histological validation study. Eur Heart J 2014;35:1486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009;2:349–61. [DOI] [PubMed] [Google Scholar]
- 71. Cappato R, Calkins H, Chen S-A, Davies W, Iesaka Y, Kalman J et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–8. [DOI] [PubMed] [Google Scholar]
- 72. Cappato R, Calkins H, Chen S-A, Davies W, Iesaka Y, Kalman J et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol 2009;53:1798–803. [DOI] [PubMed] [Google Scholar]
- 73. Bunch TJ, Asirvatham SJ, Friedman PA, Monahan KH, Munger TM, Rea RF et al. Outcomes after cardiac perforation during radiofrequency ablation of the atrium. J Cardiovasc Electrophysiol 2005;16:1172–9. [DOI] [PubMed] [Google Scholar]
- 74. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen S-A et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 75. Hassink RJ, Aretz HT, Ruskin J, Keane D. Morphology of atrial myocardium in human pulmonary veins. J Am Coll Cardiol 2003;42:1108–14. [DOI] [PubMed] [Google Scholar]
- 76. Deneke T, Khargi K, Müller KM, Lemke B, Mügge A, Laczkovics A et al. Histopathology of intraoperatively induced linear radiofrequency ablation lesions in patients with chronic atrial fibrillation. Eur Heart J 2005;26:1797–803. [DOI] [PubMed] [Google Scholar]
- 77. Hayashi H, Hayashi M, Miyauchi Y, Takahashi K, Uetake S, Tsuboi I et al. Left atrial wall thickness and outcomes of catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Interv Card Electrophysiol 2014;40:153–60. [DOI] [PubMed] [Google Scholar]
- 78. Park J, Park CH, Lee H-J, Wi J, Uhm J-S, Pak H-N et al. Left atrial wall thickness rather than epicardial fat thickness is related to complex fractionated atrial electrogram. Int J Cardiol 2014;172:e411–3. [DOI] [PubMed] [Google Scholar]