Abstract
Aims
Implantable cardioverter-defibrillator (ICD) treatment is beneficial in selected patients. However, it remains difficult to accurately predict which patients benefit most from ICD implantation. For this purpose, different risk models have been developed. The aim was to validate and compare the FADES, MADIT, and SHFM-D models.
Methods and results
All patients receiving a prophylactic ICD at the Leiden University Medical Center were evaluated. Individual model performance was evaluated by C-statistics. Model performances were compared using net reclassification improvement (NRI) and integrated differentiation improvement (IDI). The primary endpoint was non-benefit of ICD treatment, defined as mortality without prior ventricular arrhythmias requiring ICD intervention. A total of 1969 patients were included (age 63 ± 11 years; 79% male). During a median follow-up of 4.5 ± 3.9 years, 318 (16%) patients died without prior ICD intervention. All three risk models were predictive for event-free mortality (all: P < 0.001). The C-statistics were 0.66, 0.69, and 0.75, respectively, for FADES, MADIT, and SHFM-D (all: P < 0.001). Application of the SHFM-D resulted in an improved IDI of 4% and NRI of 26% compared with MADIT; IDI improved 11% with the use of SHFM-D instead of FADES (all: P < 0.001), but NRI remained unchanged (P = 0.71). Patients in the highest-risk category of the MADIT and SHFM-D models had 1.7 times higher risk to experience ICD non-benefit than receive appropriate ICD interventions [MADIT: mean difference (MD) 20% (95% CI: 7–33%), P = 0.001; SHFM-D: MD 16% (95% CI: 5–27%), P = 0.005]. Patients in the highest-risk category of FADES were as likely to experience ICD intervention as ICD non-benefit [MD 3% (95% CI: –8 to 14%), P = 0.60].
Conclusion
The predictive and discriminatory value of SHFM-D to predict non-benefit of ICD treatment is superior to FADES and MADIT in patients receiving prophylactic ICD treatment.
Keywords: Implantable cardioverter-defibrillator, Risk stratification, Primary prevention
What's new?
After 4.5-year follow-up, 24% of the primary-prevention implantable cardioverter-defibrillator (ICD) recipients had died, 68% of whom did not require any ICD intervention triggered by ventricular arrhythmias prior to death (non-benefit of ICD treatment).
This study validates and compares three pre-existing prediction models developed to predict patients' benefit from ICD treatment. The predictive and discriminatory value of SHFM-D to predict ICD non-benefit was superior to FADES and MADIT in patients receiving prophylactic ICD treatment.
Risk stratification and patient selection for ICD treatment using a multifactorial approach can identify patients at high risk for non-benefit of ICD treatment. Application of such ‘multifactorial’ risk models may be helpful for patient selection for primary-prevention ICD treatment in the future.
Introduction
Based on large randomized trials, current international guidelines advise prophylactic implantable cardioverter-defibrillator (ICD) implantation to prevent sudden cardiac death (SCD) in heart failure patients with reduced left ventricular ejection fraction (LVEF).1–5 This practice resulted in a significant increase in patients receiving an ICD in the past decades, yet under-treatment remains in patients qualifying for ICD treatment.6 Prophylactic ICD implantation in all eligible patients may, however, strain different healthcare systems and the pool of trained personnel.7 In routine clinical practice, 37% of primary-prevention ICD patients experience potentially lifesaving ICD intervention in the first 5 years after implantation, so the remaining 63% did not require ICD intervention.8 Therefore, better methods need to be developed to identify the patients who will actually benefit from ICD treatment. For this purpose, different risk models have been developed.
These prediction models may provide the ability to improve risk stratification or even patient selection for ICD implantation. However, the ability to identify patients' benefit of ICD treatment using these models has not been validated in large patient populations receiving an ICD in routine clinical practice, with the exception of one recent study validating a MADIT-II-like risk model.9
Therefore, the aim of the current study was (i) to validate the NYHA Functional class, Age, Diabetes, Ejection fraction, and history of Smoking (FADES) risk model, the Multicentre Autonomic Defibrillator Implantation Trial-II (MADIT) risk model, and the Seattle Heart Failure Model for Defibrillator recipients (SHFM-D) risk model10–12; and (ii) to analyse which model is most appropriate to predict death without prior ventricular arrhythmia requiring ICD intervention in patients receiving a prophylactic ICD in routine clinical practice.
Methods
Patient population
Patients undergoing ICD implantation at Leiden University Medical Center, The Netherlands, are consecutively registered in the departmental Cardiology Information System (EPD-vision®, Leiden University Medical Center) since 1996. The registry contains patient characteristics at the time of implantation. Thereafter, patients are followed, and all follow-up visits are documented. The current study includes patients receiving a transvenous defibrillator device for primary prevention of SCD. All device implantations were based on international guidelines; consequently, heart failure patients with reduced LVEF were included.1 The presence of congenital or monogenetic heart disease was considered as an exclusion criterion. Ischaemic heart disease was defined as coronary artery disease presenting with a stenosis >50% in at least one coronary artery.
Device implantation and programming
Implanted devices include single-chamber ICDs, dual-chamber ICDs, and cardiac resynchronization therapy-defibrillators (CRT-D). The defibrillators used were manufactured by Biotronik (Berlin, Germany), Boston Scientific [Natick, MA, USA; formerly CPI, Guidant (St Paul, MN, USA)], Medtronic (Minneapolis, MN, USA), or St Jude Medical/Ventritex (St Paul, MN, USA).
All device implantations were performed transvenously in the electrophysiology laboratory. During the procedure, sensing and pacing thresholds were tested and defibrillation threshold testing was performed.
With the exception of the early years the devices were programmed with three standard zones, zone limits were adjusted when clinically indicated. Ventricular arrhythmias from 150 to 188–190 bpm were observed in a monitoring zone in which no therapy was programmed. Faster ventricular arrhythmias from 188–190 to 220–231 bpm were detected in a ventricular tachycardia (VT) zone, which is programmed with two separate ATP bursts to initially attempt to terminate the arrhythmias and when unsuccessful a shock was delivered. Ventricular arrhythmias exceeding 210–231 bpm were primarily treated with shock in the fast VT and ventricular fibrillation (VF) zones. Finally, atrial arrhythmia detection was set to >170 bpm, and supraventricular tachycardia discriminators were enabled.
Follow-up
Periodical follow-up visits were performed every 3–6 months or more frequently when clinically indicated. Evaluations included clinical assessment and device interrogation, both under supervision of device cardiologists or electrophysiologists. Device interrogation included analysis and registration of the stored episodes and delivered ICD therapy. Device therapy was classified by printouts of intracardiac electrograms and was considered appropriate only when occurring in response to sustained VT or VF. Other triggers for ICD therapy were considered inappropriate (sinus or supraventricular tachycardia, non-sustained ventricular arrhythmias, T-wave over-sensing, or lead dysfunction). When follow-up visits were not performed for >6 months, follow-up was considered incomplete, and these patients were censored after their final follow-up visit still conforming to follow-up protocol. The primary endpoint of the current study was ICD non-benefit, defined as mortality without prior ventricular arrhythmias requiring ICD intervention, since these patients did not experience any benefit from implantation. Survival was obtained from municipal civil registries.
Risk models
The development of FADES, MADIT, and SHFM-D risk models is described elsewhere.10–12 An overview of the FADES, MADIT, and SHFM-D risk models is provided in Table 1.
Table 1.
Overview of development, calculation, and results of FADES, MADIT, and SHFM-D risk score models
| FADES10 | MADIT11,13 | SHFM-D12 | |||||
|---|---|---|---|---|---|---|---|
| Risk model development | |||||||
| Study design | Inclusion criteria | Study design | Inclusion criteria | Study design | Inclusion criteria | ||
| Single-centre cohort (Leiden University Medical Center, The Netherlands) | Primary-prevention ICD indication | Randomized controlled trial (MADIT-II) | Previous myocardial infarction | Developed in non-ICD patients. Validated in randomized controlled trial (SCD-HeFT) | NYHA Class II/III Ischaemic or non-ischaemic heart disease |
||
| Ischaemic heart disease | Ejection fraction ≤30% | Ejection fraction ≤35% | |||||
| Risk score calculation | |||||||
| Clinical characteristics | Score | Clinical characteristics | Score | Clinical characteristics | Score | ||
| NYHA functional class ≥3 Age 65–75 years Age ≥75 years Diabetes mellitus Ejection fraction ≤25% History of smoking |
1 0.5 2 1 1 1 |
NYHA functional class ≥3 Atrial fibrillation QRS >120 ms Age >70 years BUN >26 mg/dL BUN ≥50 mg/dL and/or serum creatinine ≥2.5 mg/dL |
1 1 1 1 1 VHR |
Age Gender Systolic blood pressure Ischaemic heart disease NYHA functional class Ejection fraction ACE-inhibitor/AT II antagonist use β-Blocker use Carvedilol use Statin use Digoxin use Furosemide equivalent daily dose, mg/kg Serum creatinine, mg/dL Serum sodium, mEq/dL |
Algorithm to calculate score was not made public | ||
| Endpoints | |||||||
| Death without appropriate ICD therapy | Relative reduction in 2-year mortality of ICD treatment compared with conventional treatment | Relative reduction in 4-year mortality of ICD treatment compared with conventional treatment | |||||
| Incidence of endpoints by risk scores | |||||||
|
Rank (score) n = 900 |
5-Year cumulative incidence |
Score n = 1191 |
2-Year mortality relative risk reduction |
Score n = 1191 |
8-Year mortality relative risk reduction |
Score by quintiles n = 2483 |
4-Year mortality relative (and absolute) risk reduction |
| 0 (0–1.5) | 11% | 0 | −13% | 0 | 40% | 1 | 54% (7%) |
| 1 (2–2.5) | 28% | 1 | 55% | 1–2 | 23% | 2 | 43% (9%) |
| 2 (3–5.5) | 61% | 2 | 44% | 3-VHR | 2% | 3 | 37% (11%) |
| ≥3 | 29% | 4 | 30% (14%) | ||||
| VHR | 9% | 5 | 0% (−5%) | ||||
Categorical variables are presented as n (%).
FADES, NYHA Functional class, Age, Diabetes, Ejection fraction, and history of Smoking; MADIT, Multicentre Automatic Defibrillator Implantation Trial; SHFM-D, Seattle Heart Failure Model for Defibrillator recipients; ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association functional class; BUN, blood urea nitrogen; VHR, very high risk; ACE, angiotensin-converting enzyme; AT, angiotensin; HD, heart disease.
The FADES model was developed in a patient population suffering from ischaemic heart disease, receiving an ICD for primary prevention of SCD in routine clinical practice. The aim was to identify patients who died prior to requiring ICD interventions. Variables included in the model were NYHA functional class, age, diabetes mellitus, LVEF, and history of smoking. Patients were ranked into a low-risk group (Scores 0–1.5), intermediate-risk group (Scores 2–2.5), and a high-risk group (Scores 3–5.5). After 5 years, the cumulative incidence of death without ICD intervention was 61% in the high-risk group. The MADIT model was developed to analyse survival benefit of ICD treatment based on the MADIT-II study population, a large randomized controlled trial including patients with a previous myocardial infarction and LVEF ≤30%. The model included NYHA functional class, atrial fibrillation, QRS duration, age, and serum blood urea nitrogen. Patients with scores >2 were considered high risk. An additional very-high-risk group (VHR) was created, which included patients with severe kidney disease. In the MADIT-II study population, no long-term survival benefit of ICD implantation was observed in the (very) high-risk group.13 The final model, the SHFM-D risk model (a modified version of the original SHFM) was constructed in 10 038 patients without ICDs and validated using the SCD-HeFT study population. This randomized trial included both ischaemic and non-ischaemic patients with LVEF ≤35%. Variables included in the model were age, gender, systolic blood pressure, ischaemic heart disease, NYHA functional class, LVEF, ACE-inhibitor or angiotensin II (AT II) antagonist use, β-blocker use, carvedilol use, statin use, digoxin use, furosemide equivalent daily dose, serum creatinine, and serum sodium. Scores were ranked into five risk groups; no ICD survival benefit was observed in the highest-risk quintile, which included patients with an estimated annual mortality of >11.1%. The actual method to calculate the SHFM-D was never made public.
Notice that there were two important differences in the study populations used to develop the different risk models. First, the MADIT and SHFM-D models were developed specifically for patients receiving ICD-only treatment, whereas in the FADES study population 49% received additional CRT-D treatment. Second, both FADES and MADIT risk models were developed for patients with ischaemic heart disease, whereas SHFM-D was developed for patients with ischaemic and non-ischaemic heart diseases.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (Armonk, NY, USA; IBM Corp.). Continuous data are expressed as mean ± standard deviation (SD), or median and 25–75th percentile as appropriate, and categorical data as numbers (N) and percentages. Missing values were imputed using a single imputation model in order to produce accurate risk scores corresponding with the three models for every patient.14
Cumulative incidence curves of ICD non-benefit were calculated adjusting for the competing risk of potential ICD benefit (any appropriate ICD intervention).15 In the same way, the cumulative incidence of appropriate ICD therapy was adjusted for the competing risk of mortality. Additionally, risk ratios (RR) were calculated to analyse the risk of ICD non-benefit in contrast to the risk of appropriate ICD therapy.
Individual model performance was evaluated by calculating C-statistics. Hereafter, model performances of the three different models were compared by calculation of the net reclassification improvement (NRI) and the integrated differentiation improvement (IDI). Net reclassification improvement is based on reassignment of subjects into different risk categories when using a new risk model, which reassignment may be more (or less) appropriate for the subjects. Net reclassification improvement is estimated by [(n events reclassified higher − n events reclassified lower)/n events] + [n non-event reclassified lower − n non-events reclassified higher)/n non-events]. The IDI quantifies the improvement of sensitivity and specificity of a new model by estimating the difference in discrimination slopes of both models. Integrated differentiation improvement is calculated by [mean predicted probability (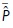 ) of event in subjects with event in model (i) −
) of event in subjects with event in model (i) − 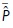 of event in subjects with event in model (ii)] − [
of event in subjects with event in model (ii)] − [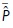 of event in subjects without event in model (i) −
of event in subjects without event in model (i) − 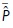 of event in subjects without event in model (ii)]. For more detailed explanation of these measures, we refer to a paper by Pencina et al.16 Since the FADES model was developed on the basis of a part of the current study population, these patients were excluded in the analysis of model performance of the FADES model. Finally, a P-value of <0.05 was considered as statistically significant.
of event in subjects without event in model (ii)]. For more detailed explanation of these measures, we refer to a paper by Pencina et al.16 Since the FADES model was developed on the basis of a part of the current study population, these patients were excluded in the analysis of model performance of the FADES model. Finally, a P-value of <0.05 was considered as statistically significant.
Results
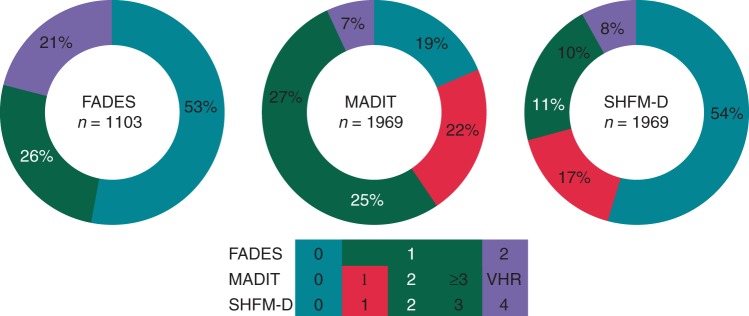
Since 1996, 2159 patients received an ICD for primary prevention of SCD. Hereof, 179 patients were excluded due to congenital or monogenetic disease, forming the current study population of 1969 patients [1150 CRT-D (58%)]. Table 2 demonstrates the clinical characteristics of the study population at implantation. Patients had a mean age of 63 ± 11 years, the majority was male (79%), and had ischaemic heart disease (66%). Additionally, most patients suffered from mild-to-moderate heart failure (NYHA 2–3: 78%) with reduced systolic heart function (LVEF 29 ± 11%) and intraventricular conduction delay (QRS: 132 ± 36 ms). The distribution of the risk scores in the study population is illustrated in Figure 1.
Table 2.
Clinical characteristics of the study population
| n = 1969 | |
|---|---|
| Clinical parameters | |
| Age, years | 63 ± 11 |
| Male gender, n (%) | 1547 (79%) |
| BMI, kg/m2 | 26.5 ± 4.5 |
| Ischaemic heart disease, n (%) | 1305 (66%) |
| NYHA functional class | |
| I, n (%) | 355 (18%) |
| II, n (%) | 711 (36%) |
| III, n (%) | 822 (42%) |
| IV, n (%) | 81 (4%) |
| LVEF, % | 29 ± 11 |
| QRS duration, ms | 132 ± 36 |
| History of atrial fibrillation/flutter, n (%) | 544 (28%) |
| Creatinine clearance, mL/min | 80 ± 35 |
| Hypertension, n (%) | 862 (44%) |
| Diabetes, n (%) | 454 (23%) |
| History of smoking, n (%) | 961 (49%) |
| Device type | |
| Single chamber, n (%) | 84 (4%) |
| Dual chamber, n (%) | 734 (37%) |
| CRT-D, n (%) | 1150 (58%) |
| Medication | |
| ACE-inhibitors/AT II antagonists, n (%) | 1707 (87%) |
| Aldactone, n (%) | 748 (38%) |
| Amiodarone, n (%) | 239 (12%) |
| β-Blockers, n (%) | 1352 (69%) |
| Sotalol, n (%) | 203 (10%) |
| Calcium antagonists, n (%) | 153 (8%) |
| Diuretics, n (%) | 1366 (69%) |
| Statins, n (%) | 1279 (65%) |
Categorical variables are expressed by n (%), and continuous variables are presented as mean (SD).
NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; CRT-D, cardiac resynchronization therapy-defibrillator; ACE, angiotensin-converting enzyme; AT, angiotensin; SD, standard deviation.
Figure 1.
Distribution of the risk scores in the study population. Distribution of the risk scores of the FADES, MADIT, and SHFM-D risk models in the study population. FADES, NYHA Functional class, Age, Diabetes, Ejection fraction, and history of Smoking; MADIT, Multicentre Autonomic Defibrillator Implantation Trial; SHFM-D, Seattle Heart Failure Model for Defibrillator recipients.
During a median follow-up of 4.5 years (25th–75th percentile: 2.7–6.6 years), 466 (24%) ICD recipients died, of whom 318 (16%) did not experience ventricular arrhythmia, triggering appropriate ICD intervention. The annual mortality rate was 6%, yet 68% of the deceased died before receiving appropriate ICD therapy (ICD non-benefit).
Performance of FADES, MADIT, and SHFM-D risk models for implantable cardioverter-defibrillator non-benefit
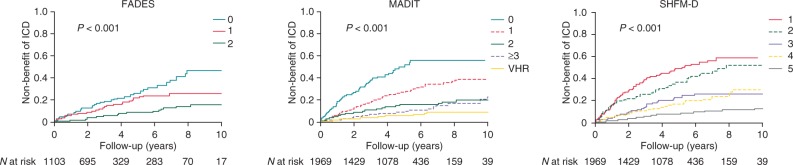
In patients with higher FADES, MADIT, or SHFM-D score, a higher cumulative incidence of ICD non-benefit was observed (Figure 2).
Figure 2.
Cumulative incidence curves for ICD non-benefit. Cumulative incidence curves of mortality without prior ventricular arrhythmia requiring ICD intervention per risk category of the FADES, MADIT, and SHFM-D risk models. FADES, NYHA Functional class, Age, Diabetes, Ejection fraction, and history of Smoking; MADIT, Multicentre Autonomic Defibrillator Implantation Trial; SHFM-D, Seattle Heart Failure Model for Defibrillator recipients.
As illustrated in Figure 3, the area under the ROC curves (AUC) were 0.66 (95% CI: 0.61–0.70; P < 0.001) for the FADES model, 0.69 (95% CI: 0.66–0.72; P < 0.001) for the MADIT model, and 0.75 (95% CI: 0.72–0.78; P < 0.001) for the SHFM-D model, indicating a poor discriminatory value of the FADES and MADIT models and a reasonable discriminatory value of SHFM-D.
Figure 3.
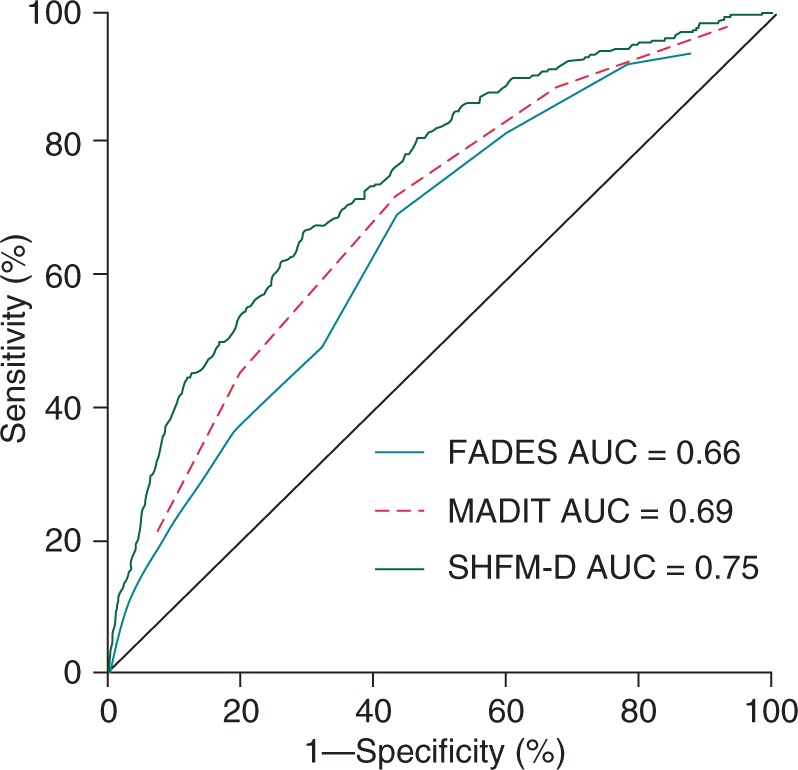
Receiver operating characteristic curves for ICD non-benefit. Receiver operating characteristic curves for mortality without prior ventricular arrhythmia requiring ICD intervention stratified for the FADES, MADIT, and SHFM-D risk models. FADES, NYHA Functional class, Age, Diabetes, Ejection fraction, and history of Smoking; MADIT, Multicentre Autonomic Defibrillator Implantation Trial; SHFM-D, Seattle Heart Failure Model for Defibrillator recipients.
Comparison of the SHFM-D with FADES resulted in an IDI of 11% (P < 0.001) and an IDI of 4% when comparing SHFM-D with MADIT (P < 0.001). This suggests that application of the SHFM-D instead of FADES or MADIT results in improved discrimination between patients with and without event by 4–11% (Table 3).
Table 3.
Evaluation of predictive ability of the FADES model vs. MADIT model vs. SHFM-D model for identifying patients at risk for non-benefit of ICD treatment using NRI and IDI
| IDI (%) | P-value | NRI (%) | P-value | Events correctly reclassified (%) | Non-events correctly reclassified (%) | |
|---|---|---|---|---|---|---|
| All patient | ||||||
| FADES vs. MADIT | 7 | <0.001 | −8 | 0.31 | 16 | −24 |
| FADES vs. SHFM-D | 11 | <0.001 | 3 | 0.71 | −13 | 16 |
| MADIT vs. SHFM-D | 4 | <0.001 | 26 | <0.001 | −26 | 52 |
IDI, integrated differentiation improvement; NRI, net reclassification improvement; FADES, NYHA Functional class, Age, Diabetes, Ejection fraction, and history of Smoking; MADIT, Multicentre Automatic Defibrillator Implantation Trial; SHFM-D, Seattle Heart Failure Model for Defibrillator recipients.
Net reclassification improvement was 3% when comparing FADES and SHFM-D (P = 0.71); however, comparing MADIT and SHFM-D resulted in a NRI of 26% (P < 0.001). This suggests that 26% of the patients were classified more appropriately in higher- or lower-risk groups by application of SHFM-D instead of MADIT. Classification was similar whether the FADES or SHFM-D was applied.
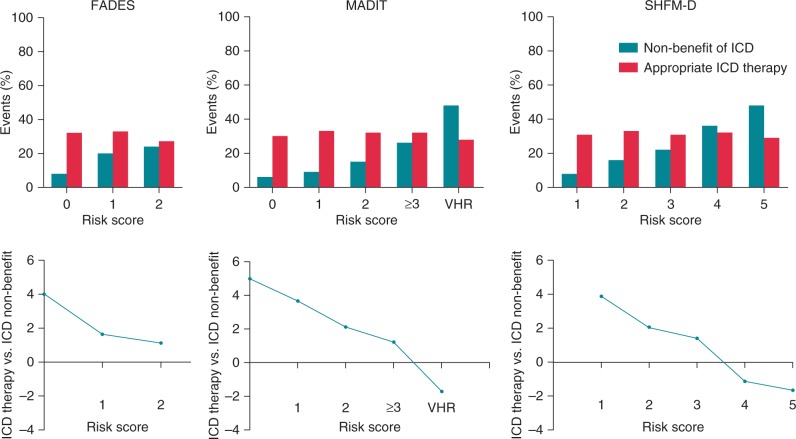
Figure 4 illustrates the occurrence of ICD non-benefit in contrast to the occurrence of appropriate ICD therapy. Although the occurrence of ICD non-benefit varies in all the models across the spectrum of risks, the incidence of appropriate ICD interventions did not differ. After 5-year follow-up, patients in the high-risk group of the FADES model were as likely to receive appropriate ICD therapy as to experience non-benefit of the ICD therapy [RR 1.1; mean difference (MD) 3% (95% CI: −8 to 14%), P = 0.60]. If the MADIT model was applied, the VHR group was 1.7 times more likely to experience ICD non-benefit than appropriate ICD therapy [MD 20% (95% CI: 7–33), P = 0.001]. Finally, application of the SHFM-D also demonstrated that patients in fifth quintile [RR: −1.7; MD 16% (95% CI: 5–27), P = 0.005] were more likely to experience non-benefit than appropriate ICD therapy.
Figure 4.
Implantable cardioverter-defibrillator non-benefit vs. ICD benefit by the different risk categories. Occurrence of ICD non-benefit in contrast to the occurrence of appropriate ICD therapy per risk category in the FADES, MADIT, and SHFM-D risk models. The upper panels illustrate the 5-year cumulative incidences of non-benefit of ICD therapy and appropriate ICD therapy for the different risk scores of the FADES, MADIT, and SHFM-D risk models. The lower panels illustrate the relative risk of appropriate ICD therapy vs. ICD non-benefit. FADES, NYHA Functional class, Age, Diabetes, Ejection fraction, and history of Smoking; MADIT, Multicentre Autonomic Defibrillator Implantation Trial; SHFM-D, Seattle Heart Failure Model for Defibrillator recipients.
Model performances in specified populations
Since the different risk models were originally developed in distinct patient population, model performance was also analysed separately in per device type and aetiology of heart failure. The distribution of risk scores and the model performance in these subgroups are demonstrated in the Supplementary material online, Figure S1 and Table S1.
In ICD-only patients (n = 819), the AUC was poor using MADIT (0.65; P < 0.001), but reasonable using FADES (0.73; P < 0.001) or SHFM-D (0.70; P < 0.001). There were no significant differences in IDI with application of all models. A trend was observed that application of the FADES model instead of SHFM-D resulted in a NRI of 29%, in favour of FADES (P = 0.05). Corresponding AUCs in CRT-D patients (n = 1150) were 0.61 for FADES, 0.69 for MADIT, and 0.76 for SHFM-D (all: P < 0.001). Integrated differentiation improvement was 4% (P < 0.001), and NRI was 41% in favour of SHFM-D when compared with MADIT. Comparison of FADES with SHFM-D resulted in IDI 10% (P < 0.001) in favour of SHFM-D, and NRI was not significant [5% (P = 0.09)].
In patients with ischaemic heart disease (n = 1305), AUCs were 0.69, 0.69, and 0.75, respectively, for FADES, MADIT, and SHFM-D (all: P < 0.001). Integrated differentiation improvement was 8% with application of SHFM-D compared with FADES (P < 0.001) and 4% compared with MADIT. Net reclassification improvement was 27% if applying SHFM-D instead of MADIT (P < 0.001), but was not significantly improved if compared with FADES [5% (P = 0.65)].
In patients with non-ischaemic heart disease (n = 664), AUC was 0.64 for FADES, 0.69 for MADIT, and 0.73 for SHFM-D (all: P < 0.001). Application of the SHFM-D resulted in an IDI of 3% (P = 0.01) compared with MADIT and 6% compared with FADES (P < 0.001), but NRI was not significantly in favour of any of the models.
Finally, Supplementary material online, Figure S2, illustrates the relative risks of ICD non-benefit vs. appropriate ICD therapy per risk group for all the above-mentioned specified populations.
Discussion
The main findings of the current study are as follows: (i) after a median follow-up of 4.5 years, 24% of the primary-prevention ICD recipients died, 68% of whom did not require any ICD intervention triggered by ventricular arrhythmias prior to death (non-benefit of ICD treatment); (ii) the discriminatory value and predictive performance of the SHFM-D to predict non-benefit of ICD treatment is superior to FADES and MADIT models, independently of patients' aetiology of heart disease (ischaemic or non-ischaemic heart disease); and (iii) with respect to patients' device type (ICD or CRT-D), the discriminatory and predictive performance of the FADES model was superior in ICD patients, but the SHFM-D was superior in CRT-D patients.
This study validates and compares three pre-existing prediction models developed to predict patients' benefit from ICD treatment. It aims to identify the model most effective for risk stratification of primary-prevention ICD recipients, which in addition may be helpful for patient selection for primary-prevention ICD treatment in the future.
Who benefits most from prophylactic implantable cardioverter-defibrillator treatment?
By applying the MADIT risk model to the MADIT-II study population, Goldenberg et al. observed a U-shaped pattern of ICD efficacy after 2-year follow-up, meaning that ICD treatment only resulted in significant mortality risk reduction in patients with intermediate risk (1–2 risk factors).11 After long-term follow-up, however, this ICD efficacy shifted: Barsheshet et al. demonstrated that after 8-year follow-up, ICD benefit was most pronounced in the low-risk population (0 risk factors) and moderate in the intermediate-risk population.13 Implantable cardioverter-defibrillator treatment was not associated with survival benefit in high-risk patients (3 risk factors or VHR). This suggests that ICD treatment is most beneficial in the less sick patients, and may not benefit the sickest patients. The current study demonstrated that the occurrence of non-benefit of ICD treatment was the highest in high-risk patients, which was the case for all risk models, again suggesting that the sickest patients benefit less or not at all of ICD treatment. This might be because their direct risk of non-SCD is disproportionally higher than their risk of SCD, resulting in a higher early mortality. The less sick patients, however, have longer life expectancy, and so they are exposed to the risk of SCD for a longer period of time.
Left ventricular function as criterion for prophylactic implantable cardioverter-defibrillator implantation
Due to the design of clinical trials, left ventricular function (measured by LVEF) has developed into the key determinant for patient selection in prophylactic ICD treatment. However, a significant proportion of the SCD or life-threatening ventricular arrhythmias occur in patients with a LVEF of >40%, and only 37% of ICD recipients with severe left ventricular dysfunction have required intervention of the device in 5 years. This implies that LVEF is insufficient for the prediction of ventricular arrhythmia.8,17 Different cardiac and non-cardiac co-morbidities have been associated with survival of ICD recipients and the occurrence of SCD, warranting a multifactorial approach in patient selection for prophylactic ICD implantation.18
Multifactorial approach for prophylactic implantable cardioverter-defibrillator implantation
For this study, we evaluated the applicability of the FADES, MADIT, and SHFM-D multivariate risk models to predict non-benefit of ICD treatment in routine clinical practice. Naksuk et al. already validated the MADIT risk model in a smaller population (n = 382), and observed no differences in the occurrence of ICD shocks between the low-, intermediate-, and high-risk groups, but mainly a difference in mortality between the risk groups.19 The current study demonstrated that by using 14 simple clinical characteristics, namely age, gender, systolic blood pressure, aetiology of heart disease, NYHA functional class, LVEF, ACE-inhibitor/AT II antagonist use, β-blocker use, carvedilol use, statin use, furosemide equivalent daily dose, serum creatinine, and serum sodium, the SHFM-D is the most suitable model for prediction of non-benefit of ICD treatment in the entire study population (including ICD, CRT-D, ischaemic, and non-ischaemic patients). The performance of all risk models is better in patients with ischaemic than in patients with non-ischaemic heart disease. The non-ischaemic group includes a more heterogeneous patient group in which the substrate for ventricular arrhythmias is more diverse, whereas it might be more difficult to predict ICD benefit for the entire group. By using this model, 22% of patients with ischaemic and 14% of patients with non-ischaemic heart diseases are divided into the high-risk group, a subgroup that is likely to die without requiring ICD intervention. In addition, with the development of the SHFM-D, Levy et al. demonstrated that high-risk patients in the SCD-HeFT trial did not experience any survival benefit from ICD implantation, and 4-year mortality was 55% in the ICD group compared with 50% in the control group.12 In the subgroup including only ICD recipients (excluding patients with additional CRT options), the FADES model was most suited to predict ICD non-benefit. Thus, by analysing patients' NYHA functional class, age, history of diabetes, and smoking in addition to LVEF, 12% of ICD patients are divided into a high-risk group and are likely to experience non-benefit of ICD treatment. In CRT-D patients, the SHFM-D was the most suited model to predict ICD non-benefit. The SHFM-D might be superior to MADIT and FADES in CRT-D patients since it is originally developed to predict survival in heart failure patients, in contrast to the FADES and MADIT models which mostly include variables to predict arrhythmia risk. This study, however, demonstrates that the risk models are unable to differentiate between risks of appropriate ICD therapy but mostly differentiate by risk of death before first ICD intervention; therefore, accurate prediction of survival is of importance.
Of note, although high-risk patients may have experienced ICD non-benefit, this is independent of the effects these patients could have experienced from CRT implantation in terms of survival and reduction of heart failure symptoms.
Is there a pathway from bench to bedside?
Simplicity and accessibility of risk models are important factors that determine wide application in routine clinical practice. The three models examined in the current study all make use of easily obtainable clinical variables. A major pitfall, however, is that the actual algorithm to calculate the SHFM-D was never made public, which limits the accessibility of this risk model.
In addition, risk models such as CHA2DS2VASc or GRACE, which are implemented in routine clinical practice worldwide, differ from the risk models analysed in the current study by their identifiable endpoint. Whereas the occurrence of major cardiovascular events or mortality is easily ascertained without a control group, measuring actual ICD survival benefit in routine clinical practice is next to impossible. Approximately 30% of the appropriate ICD interventions are assumed to be lifesaving, but which?20 Additionally, inappropriate and appropriate ICD interventions have been associated with increased mortality.21,22 A surrogate for ICD survival benefit may be the ‘potential survival gain’ (time between first appropriate ICD intervention and death), which overestimates true survival gain. By estimating non-benefit of ICD treatment, i.e. death without prior ventricular arrhythmias requiring ICD intervention, patients are selected who certainly did not experience any survival benefit of the defibrillator, but this method underestimates the true occurrence of non-benefit of ICD treatment. In addition, potential harm due to ICD treatment (inappropriate shock or ICD-related complications) is not taken in consideration in the current methods to determine ICD benefit. Subsequently, in patients who may benefit from ICD treatment, the cons of ICD implantation should be considered still.23 Without an accurate measure to determine actual survival benefit of ICD treatment in patients receiving an ICD in routine clinical practice, it may be unsuited to change methods of risk stratification and patient selection only by validation of the SHFM-D or FADES model in routine clinical practice. Validation of the FADES and SHFM-D in study populations of previously performed randomized primary-prevention trials other than the SCD-HeFT may be insightful. However, the current study, as well as the study of the development of the FADES, SHFM-D, and even MADIT models, demonstrated that a multifactorial approach, such as SHFM-D or FADES, improves risk stratification for ICD treatment by identifying patients at high risk for non-benefit of ICD.12 Therefore, this approach may be helpful for the most appropriate allocation of limited ICDs.
Limitations
This observational study in a large population of patient receiving prophylactic ICD treatment might be limited by the non-randomized design; however, results are representative for patients receiving prophylactic ICD treatment in routine clinical practice. Patients were enrolled during a long follow-up period while clinical guidelines were changed, which may have created a heterogeneous population. The current study only includes patients who received an ICD; however, high-risk patients may have died without or before ICD implantation, which may have influenced the endpoint. Patients at high risk, who are otherwise candidates for a CRT device (LBBB or QRS ≥ 150 ms), may benefit from CRT alone rather than CRT-D. In addition, device interrogations were performed under supervision of device cardiologists or electrophysiologists; there was, however, no external independent adjudication for ventricular arrhythmias.
Conclusion
In routine clinical practice, the annual mortality rate of patients receiving a prophylactic ICD or CRT-D was 6%; 68% of the deceased died without prior ventricular arrhythmias requiring ICD intervention (non-benefit of ICD treatment). Risk stratification and patient selection for ICD treatment using a multifactorial approach can identify patients at high risk for non-benefit of ICD treatment. Of the tested models, the SHFM-D risk model is the most qualified risk model to identify non-benefit of prophylactic ICD treatment, whereas the FADES model was most suited in a specific subgroup (ICD-only patients). These models may be useful in patient selection for prophylactic ICD implantation in the future.
Supplementary material
Funding
This study is funded by Department of Cardiology, Leiden University Medical Center, The Netherlands.
Conflict of interest: The Department of Cardiology of the Leiden University Medical Center receives unrestricted research grants from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and St Jude Medical. W.C.L. has received research grants from Medtronic, Thoratec, Heart Ware, and National Institutes of Health. The University of Washington holds the copyright to the SHFM-D.
Supplementary Material
References
- 1. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2006;8:746–837. [DOI] [PubMed] [Google Scholar]
- 2. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 3. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151–8. [DOI] [PubMed] [Google Scholar]
- 4. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 5. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 6. Camm AJ, Nisam S. European utilization of the implantable defibrillator: has 10 years changed the ‘enigma’? Europace 2010;12:1063–9. [DOI] [PubMed] [Google Scholar]
- 7. Hlatky MA, Mark DB. The high cost of implantable defibrillators. Eur Heart J 2007;28:388–91. [DOI] [PubMed] [Google Scholar]
- 8. van Welsenes GH, van Rees JB, Borleffs CJ, Cannegieter SC, Bax JJ, van Erven L, et al. Long-term follow-up of primary and secondary prevention implantable cardioverter defibrillator patients. Europace 2011;13:389–94. [DOI] [PubMed] [Google Scholar]
- 9. Providência I, Boveda S, Lambiase P, Defaye P, Algalarrondo V, Sadoul N, et al. Prediction of nonarrhythmic mortality in primary prevention implantable cardioverter-defibrillator patients with ischemic and nonischemic cardiomyopathy. J Am Coll Cardiol Clin Electrophysiol 2015;1:29–37. [DOI] [PubMed] [Google Scholar]
- 10. van Rees JB, Borleffs CJ, van Welsenes GH, van der Velde ET, Bax JJ, van Erven L, et al. Clinical prediction model for death prior to appropriate therapy in primary prevention implantable cardioverter defibrillator patients with ischaemic heart disease: the FADES risk score. Heart 2012;98:872–7. [DOI] [PubMed] [Google Scholar]
- 11. Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 2008;51:288–96. [DOI] [PubMed] [Google Scholar]
- 12. Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation 2009;120:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012;59:2075–9. [DOI] [PubMed] [Google Scholar]
- 14. Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 2006;59:1087–91. [DOI] [PubMed] [Google Scholar]
- 15. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–430. [DOI] [PubMed] [Google Scholar]
- 16. Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 17. de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, et al. Out-of-hospital cardiac arrest in the 1990's: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol 1997;30:1500–5. [DOI] [PubMed] [Google Scholar]
- 18. Lee DS, Tu JV, Austin PC, Dorian P, Yee R, Chong A, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol 2007;49:2408–15. [DOI] [PubMed] [Google Scholar]
- 19. Naksuk N, Akkaya M, Adabag S. Application of the multicenter automatic defibrillator implantation trial II risk score in a nontrial setting. Am J Cardiol 2013;112:530–2. [DOI] [PubMed] [Google Scholar]
- 20. Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol 2008;52:1111–21. [DOI] [PubMed] [Google Scholar]
- 21. van Rees JB, Borleffs CJ, de Bie MK, Stijnen T, van Erven L, Bax JJ, et al. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol 2011;57:556–62. [DOI] [PubMed] [Google Scholar]
- 22. Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008;359:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olde Nordkamp LR, Wilde AA, Tijssen JG, Knops RE, van Dessel PF, de Groot JR. The ICD for primary prevention in patients with inherited cardiac diseases: indications, use, and outcome: a comparison with secondary prevention. Circ Arrhythm Electrophysiol 2013;6:91–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.